Abstract
The objective of this work is to confirm the asymmetry in non-linguistic auditory perception, as well as the influence of anxiety-depressive disorders on it. Eighty-six people were recruited in the emotional well-being group, fifty-six in the anxiety group, fourteen in the depression group, and seventy-seven in the mixed group. In each group, audiograms were obtained from both ears and the differences were statistically analyzed. Differences in hearing sensitivity were found between both ears in the general population, such differences increased in people with anxiety-depressive disorders. When faced with anxiety-depressive disorders, the right ear suffered greater hearing loss than the left, showing peaks of hyper-hearing at the frequency of 4000 Hz in the anxiety subgroup, and hearing loss in the depression subgroup. In relation to anxiety, the appearance of the 4:8 pattern was observed in the right ear when the person had suffered acute stress in the 2 days prior to the audiometry, and in both ears if they had suffered stress in the 3–30 days before said stress. In conclusion, the advantage of the left ear in auditory perception was increased with these disorders, showing a hyperaudition peak in anxiety and a hearing loss in depression.
1. Introduction
Though some researchers had considered hemispheric lateralization at the beginning of the 19th century, there is a general consensus that the study of functional asymmetry of the cerebral hemispheres began in 1861 [1]. For a long time, many researchers thought that asymmetry was limited to language, and therefore to the human species. However, it has been observed that such asymmetry between hemispheres not only occurs in mammals [2], but also in amphibians and reptiles [3], bony fishes [4], and birds [5], as well as cartilaginous and jawless fishes [6]. Recent evidence for asymmetrical organization has been shown in invertebrate species, such as Octopus vulgaris [7], honey bee Apis mellifera [8] and nematode Caenorhabditis elegans [9], which showed that lateralization is not restricted to humans, but constitutes a fundamental principle of nervous system organization [10]. Furthermore, it has been seen that this asymmetry is not limited to language but encompasses specific cognitive tasks and behavioral characteristics, such as handedness, auditory preference, and physical exercise [11,12].
After the initial anatomical findings, functional observations have been found that corroborate them [13,14]. Regarding the recognition of sounds related to language, a clear superiority of the left hemisphere is observed. Thus, the dichotic listening test using verbal auditory stimuli showed the so-called Right Ear Advantage effect (REA) in processing language sounds when two different stimuli are presented simultaneously in both ears [13]. This effect has been subsequently confirmed by neuroimaging techniques [15,16] and its deviation used as a labeler of alterations in language [17] and in the psychiatric sphere [17,18,19]. Even an imagery REA (with an imagined voice or a sound input) has been confirmed in more recent studies [20,21] and its alteration found in patients with hallucinations [22].
However, the superiority of the right hemisphere is debatable when it comes to processing non-speech sounds (tones and music), since the investigations show controversial results. While Sininger and Bathara found a clear advantage of the right hemisphere when it comes to detecting tonal stimulation of different spectrum [23], Brancucci et al. circumscribed this advantage for high frequencies [24]. The results are much more disparate when the hemispheric laterality is measured through orienting biases [25] or in specific high-level motor-related and multisensory cortical regions [26].
The latest evidence focuses on the parameters of sound, rather than on the language vs. music domains. In this way, the right hemisphere would be specialized in discriminating the intensity variations [27] and sound frequency [23,28,29], while the left hemisphere would do so for variations in its duration [28,29,30]. More specifically, studies based on animal models [31,32] and in humans [33,34] suggest that the underlying difference in auditory asymmetry has to do with the time windows over which the left versus the right auditory cortex processes sounds (asymmetric-sampling-in-time).
Recent medical literature displays some associations among otological pathology and mental stress. The most significant one is anxiety and depression related to hearing loss, a fact mostly explained by the social isolation linked to deafness [35,36,37,38,39,40]. In addition, a constant noise seems to induce anxious-depressive states which at the same time decreases tolerance thresholds [41,42]. Anxiety and depression are disorders of the emotional sphere that affect 10% of the global population [43], with a high rate of comorbidity between these two entities both in primary-care-only patients [44] and patients referred to a mental health specialist [45]. Functional magnetic resonance imaging (fMRI), dichotic listening tests, and electroencephalographic (EEG) tests have been extensively used to analyze hemispheric laterality on these mental disorders [46,47,48,49]. Thus, it has been reported that major depression is associated with a subtle loss of the thickness of the right superior temporal cortex compared to that observed in healthy subjects [46]. From the point of view of function, major depression has shown hypofunction of different structures in both hemispheres [47,48], but with an apparent right hemispheric dominance [49].
Anxiety disorders, on the other hand, present a relatively greater right parietotemporal activity than the left one. Additionally, part of the left hemispheric advantage is lost when it comes to recognizing stimuli from language [48,50]. These facts seem to also indicate that anxiety presents dysfunctions in both hemispheres, when compared with healthy subjects, although with relative hyperfunction in the right.
There is less scientific evidence on what happens when anxiety and depressive symptoms coexist. It has been observed, so far, a greater activity of the right amygdala and its interconnectivity with the right superior temporal gyrus when compared with healthy subjects [51,52].
We wonder if the hemispheric asymmetry in terms of hearing and emotional processing could be reflected in the classic tonal audiograms that are performed for the detection of hearing loss. Only a few studies have looked at the impact of anxiety or depression on hearing thresholds on an audiogram. Some of them have reported higher hearing thresholds in these disorders, specifically at low frequencies in the case of anxiety [53]. On the contrary, a marked hearing loss at higher frequencies has been found in depression. However, other studies have found contradictory results [54].
The goal of this study is to determine whether there are significant hearing differences between the right and the left ear as measured by classical tonal audiometry and the influence on it of anxiety-depressive disorders. In this way, interaural hearing differences and auditory thresholds were used to compare people with symptoms of anxiety and/or depression and people without these symptoms, looking for which part of the audiogram is the most affected. It would allow orienting hearing loss detected by classical tonal audiometry towards a specific neurotic picture.
2. Materials and Methods
2.1. Study Type
A cross-sectional study comparing pure-tone audiometry (air conduction) in subjects with a significant degree of anxiety and/or depression compared with subjects without a significant degree of these symptoms.
The study was approved by the Clinical Research Ethics Committee of the University Hospital La Princesa (Madrid, Spain) and later on by the Southeast Local Research Commission of Madrid.
2.2. Subjects
For this study, 327 people who sought care at the Health Centre Mejorada del Campo (Madrid, Spain) were selected by systematic sampling (1 in 5) from the lists of people with on-demand appointment at 6 family medicine consultations (3 in the morning and 3 in the afternoon). All the subjects who agreed to participate were informed about the study and what it involved. Subjects were then asked to sign the informed consent form and complete an initial questionnaire. Those subjects with significant hearing loss, acute otological process, psychotic disease, epilepsy, serious illness, pregnancy, or alcohol or drug use were excluded. From 327 subjects, 58 refused informed consent and 36 presented some exclusion criteria, so that 233 participants were finally included in the study.
Included participants underwent an ear examination and any earwax blockages were removed. Pure-tone audiometry (air conduction) was then carried out in a room where silence was guaranteed thanks to its location away from other rooms or passageways. A soundproof room was renounced as the aim was to measure airway hearing in conditions closer to reality. Later, participants underwent screening for anxiety and depression (Goldberg Anxiety and Depression Scale—GADS). Participants without significant scores for anxiety and depression on the GADS were included in the emotional well-being group, whereas participants with significant scores for anxiety and/or depression were then asked to take the Hamilton Rating Scale questionnaires. Participants were included in the anxiety, depression or mixed group, depending on whether their scores were significant for anxious symptoms, depressive symptoms or both.
2.3. Study Variables
2.3.1. Pure-Tone Audiometry (Air Conduction)
The instrument used was a basic Maico MA40 clinical audiometer with a CE-0124 calibration certificate in accordance with the ANSI S3.1996 calibration standard, made in Eden Prairie (Maico, Eden Prairie, MN, USA). Signals were frequency-modulated, pulsed pure tones. Frequency accuracy was ±1% for each frequency.
The lower threshold for air conduction pure tones was measured for the following frequencies in each ear: 125, 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000 and 8000 Hz. Each threshold was collected after checking it at least three times.
2.3.2. Goldberg Anxiety and Depression Scale (GADS)
This scale was developed by Goldberg [55] in 1988 from a modified version of the Psychiatric Assessment Schedule. It is a screening tool. In the present work a validated Spanish version was used [56]. The physician asks the patient questions about the symptoms contained in the scales and occurring in the last 15 days, ignoring any symptoms that are no longer present or present only in mild degree. As well as supporting a diagnosis of anxiety or depression (or both, in mixed cases), the GADS is able to discriminate between these two entities. The following cut-off points were used: a total anxiety score > 4 detects 73% of all cases of anxiety; a total depression score > 3 detects 82% of all cases of depression.
2.3.3. Hamilton Anxiety Rating Scale (HAM-A)
The Hamilton Anxiety Rating Scale used was the abridged 14-item version in its validated Spanish-language version [57]. It is a clinician-administered scale on which each item is rated from 0 to 4. Patients are asked about how they have felt in the last 3 weeks. It yields a score in the range of 0 to 56. The following cut-off points were used: 0–5 points (no anxiety), >5 points (anxiety).
2.3.4. Hamilton Depression Rating Scale (HAM-D)
The Hamilton Depression Rating Scale is a clinician-administered scale that aims to evaluate symptom severity from a quantitative perspective. For this assessment, the validated Spanish version was used [58]. Each item is rated on a three- or five-point scale, with a score from 0 to 2 or 0 to 4, respectively. It yields a total score in the range of 0 to 52. The following cut-off points were used to assess depression: no depression: 0–7; depression: >7.
2.3.5. Sociodemographic Variables
Information was collected on age (in years), gender, nationality, education, working status, noise exposure, use of ear protection, and present or past use of any analgesic, anxiolytic or antidepressant for longer than a week.
The data were collected in a database (see Supplementary Document S1).
2.4. Statistical Analysis
2.4.1. Sample Characteristics
A descriptive analysis of the sample was carried out to know the characteristics of the members of the sample.
2.4.2. Audiogram Analysis
Right- and left-ear audiograms were first compared in each group (emotional well-being, anxiety, depression and mixed). By means of the Kolmogorov–Smirnoff test, it was shown that the values of the hearing thresholds did not conform to a normal distribution, so a logarithmic transformation was applied. At this point, the data met the criteria of normal distribution and homogeneity of variance (test de Bartlett), which allowed the application of a two-factor ANOVA: ear (2 levels; right and left) and frequencies (11 levels; 0.125, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6 and 8 kHz). When significant effects were obtained that allowed further comparisons, the Bonferroni multiple comparison test was applied.
The different groups were indicated with their acronyms, for the emotional well-being (EWB), anxiety (ANX), depressive symptoms (DEP), and mixed group (MIX).
The magnitude of the interaural difference was compared by pairs of groups using a two-factor ANOVA. Group (2 levels; EWB-ANX/EWB-MIX/EWB-DEP/ANX-MIX/ANX-DEP/MIX-DEP) and frequencies (11 levels; 0.125, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6 and 8 kHz). When significant effects allowed further comparisons, the Bonferroni multiple comparison test was applied.
Subsequently, the hearing thresholds of both ears were compared between the different groups. The comparison was quantitative (mean and standard deviation of the hearing thresholds) and qualitative (relative frequencies of 6 auditory patterns) for each frequency of the audiogram. For the quantitative comparison, a three-factor ANOVA was applied (frequency and ear as intra-subject factors and group as inter-subject factors). The significant effects allowed a later post hoc comparison by means of Tukey’s correction. For the qualitative assessment, the different auditory patterns (major Peak, minor peak, major valley, minor valley, major plateau, minor plateau) were coded from 0 to 6. The frequency of appearance of said auditory patterns at each frequency of the audiogram was compared between groups by creating contingency tables and applying Pearson’s chi-square analysis for frequency comparison.
The chosen significance level was 0.05. Data analysis was carried out using version 9.0.0. of the GraphPad Prism software for Windows (GraphPad Software, San Diego, CA, USA). See Supplementary Document with statistical data.
3. Results
The sample consisted of 233 participants; of these, 86 made up the emotional well-being group, 77 the mixed group, 56 the anxiety group and 14 the depression group.
The proportion of women was similar for the three pathological groups (around 75%), although higher than in the emotional well-being group (48%). Regarding age, the mean for all groups was around 45 years, except for the depression group, which had a higher mean of almost 10 years. Again, the depression group differed by presenting a higher proportion of people who were unemployed (57%) and by a greater active consumption of analgesics (42.86%). The rest of the sociodemographic variables did not show notable differences between groups.
Table 1 shows the hearing thresholds for each frequency in the right and left ear in the various study groups.

Table 1.
Auditory thresholds of the right and left ear for each group.
3.1. Analysis in Subjects without Psychological Alterations
Audiometry in a group of 86 subjects without psychological alterations (emotional well-being group) showed that there was a statistically significant difference between the two ears, although no specific frequency showed significant differences, that could be observed through the post-test (Figure 1). The different frequencies for the two ears (Figure 1A) and the interaural difference (Figure 1B) are represented for this study group.
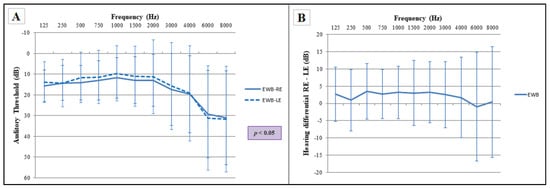
Figure 1.
Comparison of auditory frequencies between both ears for the emotional well-being group (EWB). (A) Audiogram of both ears, RE: right ear, LE: left ear. Statistical significance using two-way ANOVA (p ≤ 0.05), Bonferroni post hoc comparisons (p > 0.05); (B) plotting of the interaural difference in the emotional well-being group for the different frequencies. Sample size: n = 86. The error bars depict standard deviations.
3.2. Comparison of Interaural Hearing Differences
First, the interaural difference of the emotional well-being group was compared with the other groups (anxiety, mixed and depression), (Figure 2A–C). All these comparisons were statistically significant (two-way ANOVA). In addition, the interaural differences that had been observed in the emotional well-being subjects were enlarged by the psychological alteration they suffered.
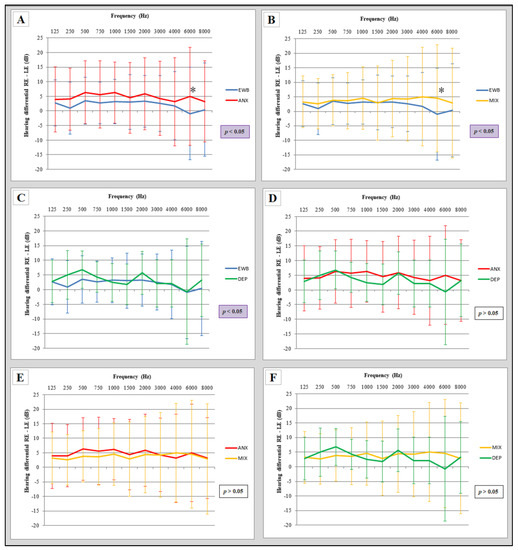
Figure 2.
Comparison of interaural hearing differences between groups. (A) Comparison of interaural differences between the emotional well-being group and the anxiety group; (B) comparison of interaural differences between the emotional well-being group and the mixed group; (C) comparison of interaural differences between the emotional well-being group and the depression group; (D) comparison of interaural differences between the anxiety group and the depression group; (E) comparison of interaural differences between the anxiety group and the mixed group; (F) comparison of interaural differences between the mixed group and the depression group. EWB: emotional wellbeing group (n = 86), ANX: anxiety group (n = 56), MIX: mixed (n = 77), DEP: depression group (n = 14). RE: right ear, LE: left ear. Statistical significance using two-way ANOVA (p ≤ 0.05), Bonferroni post hoc comparisons (* p < 0.05). The error bars depict standard deviations.
However, when comparing these interaural differences in the pathology groups (Figure 2D–F), no statistically significant difference was observed (two-way ANOVA).
3.3. Specific Location of Interaural Differences in the Different Groups
The comparison of the audiogram of both ears in the emotional well-being group showed that, although statistically significant differences were found between both ears, no significant differences were observed in any specific frequency (two-way ANOVA, Bonferroni post-test) (Figure 3A). However, such differences were found in the rest of the groups, except in the depression group (Figure 3D). It is striking that the interaural differences in the anxiety group are located at the frequency of 1000 and 2000 Hz (Figure 3B), while in the mixed group, specific differences were situated at higher frequencies (3000 and 4000 Hz) (Figure 3C).
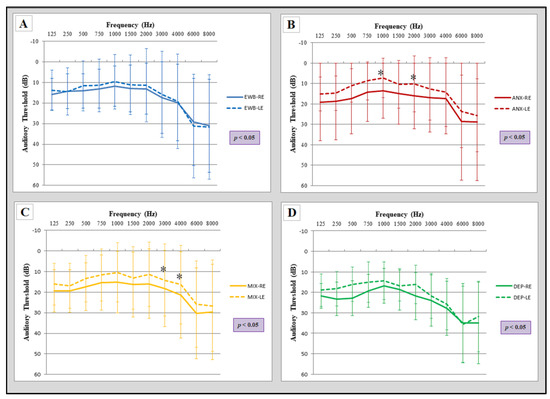
Figure 3.
Comparison of audiograms between both ears for each group. All groups showed statistically significant differences between both ears (two-way ANOVA). (A) EWB: emotional well-being group (n = 86); (B) ANX: anxiety group (n = 56) showed significant differences in the frequency of 1000 and 2000 Hz; (C) MIX: mixed group (n = 77) showed differences in the frequency of 3000 and 4000 Hz; (D) DEP: depression group (n = 14). RE: right ear, LE: left ear. Statistical significance, two-way ANOVA (p ≤ 0.05) and Bonferroni post hoc comparisons (* p < 0.05). The error bars depict standard deviations.
3.4. Comparison of Hearing Thresholds between Groups
Statistically significant differences were found in hearing thresholds of specific ears (right or left), when comparing the emotional well-being group to other groups. The emotional well-being group presented a lower threshold in the right ear (heard better in the right ear) for the frequencies of 125 and 250 Hz compared to the mixed group, and for the frequencies of 125, 250, 500, 750 and 2000 Hz compared to the depression group (Figure 4).
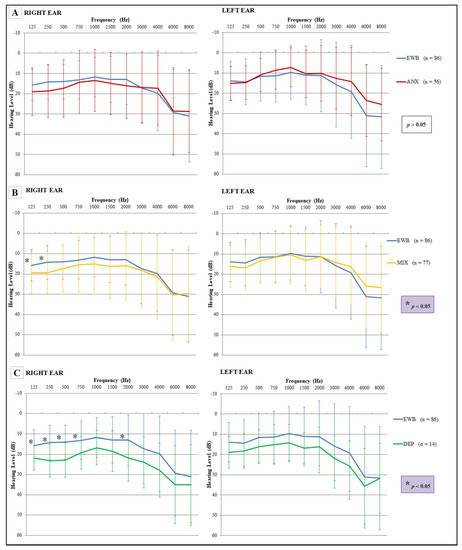
Figure 4.
Comparison of hearing thresholds, for both ears, between the emotional well-being groups with mental dysfunction groups. (A) The comparison between emotional well-being and anxiety did not show significant differences; (B) the comparison between emotional well-being and mixed group showed significant differences, specifically localized in the right ear at the frequencies of 125 and 250 Hz; (C) the comparison between emotional well-being and the group with depression group showed significant differences, specifically localized in the right ear at the frequencies of 125, 250, 500, 750 and 2000 Hz. Three-way ANOVA and Tukey post hoc test (* p < 0.05). The error bars depict standard deviations.
When the hearing thresholds for each ear were compared among the pathology groups, it was observed that the anxiety group showed a lower threshold than the depression group in the frequency of 4000 Hz of the right ear, while in left ear differences were found in the frequency of 3000 and 4000 Hz. In general, the thresholds for the group with depressive symptoms increased for all frequencies analyzed (Figure 5).
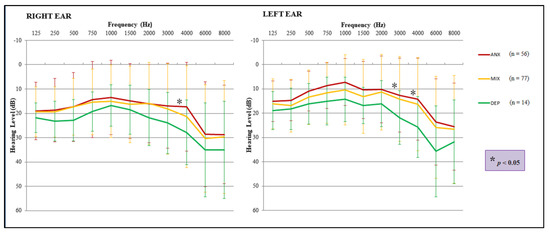
Figure 5.
Comparison of hearing thresholds, for both ears, among the mental dysfunction groups. Hearing thresholds were significantly higher in the depression group than in the anxiety group, showing differences in the right ear (4000 Hz) and in the left ear (3000 and 4000 Hz). Three-way ANOVA and Tukey post hoc test (* p < 0.05). The error bars depict standard deviations.
3.5. Modifications in the Shape of the Audiogram as a Function of Changes in Hearing Sensitivity
The change in hearing sensitivity in the pathology groups conditioned the significant appearance (Pearson’s chi-square test) of specific patterns in the left ear for certain frequencies of the audiogram with respect to the emotional well-being group. It was observed that the anxiety group presented a minor peak pattern at the frequency of 4000 Hz with a higher frequency than the emotional well-being group, while the depression group showed a lower frequency of said minor peak at 4000 Hz and a lower frequency of the minor valley pattern at 3000 Hz (Figure 6).
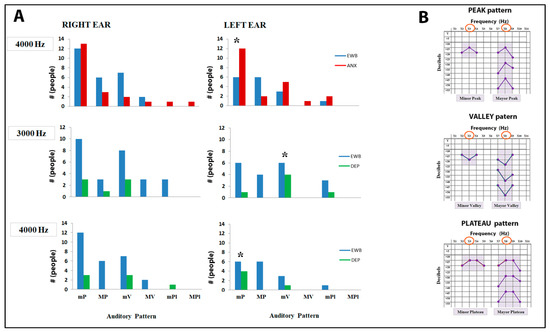
Figure 6.
Differences in the presence of auditory patterns between the emotional well-being group with the anxiety group and the depression group. (A) Possible differences in patterns were analyzed for all frequencies of the audiogram, but significant differences were only found in the left ear between emotional well-being and the anxiety group (4000 Hz), and between emotional well-being and the depression group (3000 and 4000 Hz); (B) the types of patterns that were analyzed are shown on the right. EWB: emotional well-being group (n = 86), ANX: anxiety group (n = 56), MIX: mixed group (n = 77), DEP: depression group (n = 14). mP: minor peak, MP: major peak, mV: minor valley, MV: major valley, mPl: minor plateau, MPl: major plateau. Χ2 Pearson test, (* p < 0.05).
Finally, there is a specific pattern (4:8 pattern, that is, hyperaudition at 4000 and 8000 Hz with decreased hearing at 6000 Hz) that appears to be temporarily linked to stress. This relationship shows an asymmetric presentation between both ears as a function of the time that said stress occurred. When the person had suffered stress in the last two days, the right ear showed the statistically significant presence of this pattern compared to those who had not, while the left ear did not show this pattern. However, over time (when 3–30 days had elapsed after the stressful event) this pattern extended to the left ear (Fisher’s exact test) (Figure 7).
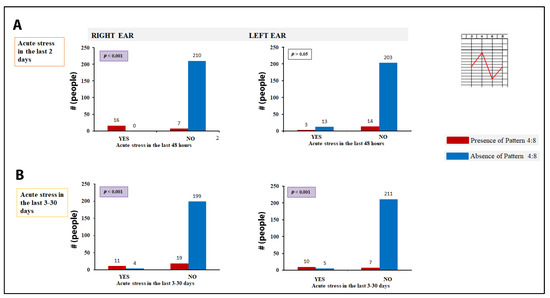
Figure 7.
Repercussion of acute stress on hyperaudition peaks, 4:8 auditory pattern. (A) The statistical association between the presence of stress in the last two days and the appearance of the 4:8 pattern in the right ear is shown; (B) the study in subjects who have suffered stress between the previous 3 and 30 days shows that the 4:8 pattern appears in both ears. Fisher’s exact test.
4. Discussion
In the present work, significant differences have been found in hearing sensitivity between both ears in the general population. These differences have been observed to increase in people suffering from anxiety disorders, depression, or both. However, no differences were observed between these groups. Likewise, the specific frequencies where the interaural differences in the affected groups were significantly different have been determined and have been compared between the different study groups. It was also found that these differences in specific frequencies caused the appearance of specific patterns in the audiogram of the left ear, both in the anxiety and depression groups, with respect to the emotional well-being group.
All this together suggests that anxiety would cause an increase in the interaural difference due to hyperaudition in the left ear, reflecting a dominance of the right cerebral hemisphere. If the process progresses to a state of depression, hearing in both ears would worsen, but the interaural difference would remain significant, mainly at the expense of greater loss in the right ear, that is, of the left hemisphere.
There are several factors that seem to influence lateralization. Better tonal hearing through the left ear is consistent with an apparent dominance of the right hemisphere when processing non-verbal auditory information (tones and music) [59,60]. Similar results have been previously obtained by analyzing the asymmetry of basic auditory perception using pure tones in healthy individuals with age ranges between 3–5 years [61] and 18–32 years [23,62], finding a specific and statistically significant difference for 1 kHz [23]. In this last study, only the frequencies 500, 1000 and 4000 Hz were tested. In our study, from eleven analyzed frequencies, no specific differences were found, but a global one with a prioritization of the central frequencies. In addition, the right hemisphere has shown superiority when it comes to better discriminating variations in the intensity of tones [27]. In addition, it is reported that the right hemisphere is more influenced by the degree of activation or emotionality of the individual [63,64]. Currently, there are two theories to explain emotional processing and hemispheric laterality, the Right Hemisphere Hypothesis (RHH) [65,66] and the Valence Hypothesis (VH) [67,68]. The first one postulates that the right hemisphere is specialized in processing all kinds of emotions. The second one suggests a specialization of the left/right hemisphere to process positive/negative emotions, respectively. More recent results support a coexistence of both theories, where the activity of the right hemisphere would be superior for all emotional processing, being more evident when a positive emotional stimulus is presented to the left hemisphere or a negative emotional stimulus is presented to the right hemisphere [63,69].
In addition, a certain degree of relative arousal or alertness is required for day-to-day activities. For an activity that demands the subject’s attention, such as the classic tonal audiogram, even more. It is possible that this aspect also contributed to the subjects of the emotional well-being group hearing better through the left ear. Likewise, it seems to contribute greatly in the case of the anxiety and mixed groups. In this way, it is reported a clear hyperfunction of the right hemisphere in anxiety disorders [50,51]. This hyperfunction would be responsible for better hearing of medium and high frequencies (1 and 2 kHz) in the case of pure anxiety, swinging towards higher frequencies (3 and 4 kHz) when anxiety symptoms coexist with depressive symptoms, and low frequencies in cases of depression. This suggests that more intense and prolonged levels of anxiety would prioritize increasingly higher frequencies in the left ear. The reason for prioritizing these frequencies could be related to the ability to detect threatening sounds, normally belonging to the treble scale. This tonal prioritization is probably carried out at the cochlear level through the descending cortifugal auditory system [70,71], by a lower inhibition of these frequencies in the left cochlea and/or a higher inhibition of their homonyms in the right cochlea. It is known that other levels of tonal selection throughout the auditory nervous system cannot be ruled out. Regarding the group with depressive symptoms, the tonal auditory superiority of the left ear is consistent with the right hemispheric dominance attributed to depression [49]. However, in the depressed patient, instead of an alert state, there is an exhaustion that would cause greater hearing loss, so it would not activate the system in the same way.
Since there are no differences when the specific frequencies for each ear are compared between well-being and anxiety, but there are differences when comparing the well-being group with the mixed group or the depression group, it could be thought that the anxiety group would be an intermediate state between the emotional well-being group and the other pathological groups. When comparing the well-being group with the mixed group, an increase in the auditory threshold was observed in the latter at the frequencies of 125 and 250 Hz in the right ear. When comparing the well-being group with the depression group, 500, 750 and 2000 Hz were added to the above frequencies, with which the depression group showed a higher hearing threshold for a greater number of frequencies. However, the comparison between the groups with anxiety-depressive disorders only showed differences between the anxiety and depression groups, with a higher hearing threshold being observed in the depression group in the right ear at the frequency of 4000 Hz and in the left ear at frequencies of 3000 and 4000 Hz. All these inhibitions and stimulations would be mediated by a series of neurotransmitters in the auditory system.
Serotonin enhances neuronal excitability in the dorsal cochlear nucleus, which is sensitive to high frequencies, in contrast with its inhibition of other auditory regions [72,73]. Acetylcholine, on the other hand, is the main neurotransmitter in the olivocochlear efferent system, which provides feedback to (inhibits) cochlear hair cells and sensory neurons [74,75]. This inhibiting effect is stronger on the right side than on the left one [76,77]. There is also evidence for noradrenergic, dopaminergic and histaminic innervation at various points of the auditory pathway, although its effect is not sufficiently well known [78,79,80,81]. In summary, the generalized activation of hearing in the left ear in anxiety could be due to serotoninergic and noradrenergic dominance with a gradual increase in serotonin turnover (reduction) at intermediate stages [82]. Persistent anxiety eventually decreases noradrenergic function and increases cholinergic function, leading to pure depressive symptoms [83]. The neurotransmitter hypothesis connects with the asymmetric sampling in time model of asymmetry [33]. The asymmetric results found for the ears (and therefore for the hemispheres) could be explained by modifications in temporal processing, as anxiety can modify the temporal processing of sound information. Studies with a larger sample and with a better definition in terms of intensity and duration of anxiety-depressive symptoms would be needed to confirm this point.
In cases of anxiety and mixed symptoms, the increase in the interauricular difference, is maximum at 6 kHz, which suggests that such selection is made at a certain level of the cochlea by the mechanism described above. However, it cannot be ruled out the role of amygdala and its connections with the cortex in the right–left asymmetries of auditory processing [84,85]. Thus, it has been reported that the activation of the right and left amygdala can vary depending on states of rest, anxiety or stress [86]. Our data suggest that from the emotional well-being one could evolve to develop anxiety symptoms, showing a greater left tonal auditory advantage in all frequencies of the audiogram (being maximum at 6 kHz level), continuing with mixed symptoms moving to focus on high frequencies (maximum in 6 kHz), and ending by focusing on the low frequencies, keeping an interauricular difference at the frequency of 2 kHz, in the case of pure depressive symptoms. These findings are consistent with current knowledge regarding the effect of different neurotransmitters on the auditory system [72,74,76].
Very few studies have been carried out on laterality of hearing thresholds in people with anxiety-depressive symptoms. In patients with major depression, according to our data, a significantly higher air conduction hearing threshold was reported. However, some of them have shown higher left hearing thresholds [87], some in the low frequencies [88]. This discordance may be explained by the fact that our study includes a small sample of 14 participants with depressive symptoms (based on their scores on the GADS ± Hamilton scales at a single point in time), not with a diagnosis of depression. Its interest lies in the fact that reflects the usual approach in primary care consultations [89,90]. It is possible that more intense and evolved symptoms of depression end up reversing the left hearing advantage in the low frequencies. However, a larger sample is needed in the depression group to be able to corroborate the results.
The peak and valley patterns found at some frequencies in the left ear when comparing the audiograms of healthy people and people with anxiety or depression suggest a very precise regulatory mechanism. This mechanism mainly affects the 4000 Hz frequency, enhancing its perception in the case of anxiety or decreasing it in the case of depression. These data are consistent with those obtained when comparing the audiogram between pathological groups: anxiety and depression present a differentiated hearing at the 4000 Hz frequency of both ears and at the 3000 Hz frequency of the left ear, anxiety being the one that perceives them significantly better. This fact is not trivial: the frequency of 4000 Hz is collected in the first curve of the cochlea, where its privileged location, promptly and directly, would allow the body alert mechanisms to be activated more quickly in the face of threatening sounds (the treble ones). In addition, certain levels of anxiety will favor the hearing of this frequency through brain feedback mechanisms. The greater involvement of the left ear due to its relationship with the right hemisphere (more emotional) would allow the recruitment of adjacent frequencies, as is the case of the 3000 Hz frequency. On the contrary, in depression this activation capacity would be lost in the face of threatening stimuli, translating into a worse hearing of the alert frequencies. The underlying mechanisms could be found in a depletion of the hypersensitivity system of the frequency 4000 Hz. Up to now, only one relationship between a certain frequency of the audiogram and a nosological entity had been reported, the scotoma at 4000 Hz caused by acoustic trauma [91]. In addition, its appearance is more frequent in the left ear. The fact that this frequency is the most affected by exposure to noise supports the idea that it is a frequency especially sensitive to threatening stimuli and alert situations. Its greater presence in the left ear would be justified by a greater sensitivity of this ear to threatening situations.
The association between the 4:8 pattern and having experienced acute stress reinforces the idea that anxiety (in this case reactive to a recent stressful situation) enhances the sensitivity of the 4000 Hz frequency. Scientific evidence supports that moderate anxiety shows an increase in activity in the frontal and lower region of the left cerebral hemisphere, while that of extreme anxiety does so in the temporal and lower region of the right [92,93]. Our results suggest that acute stress would be reflected first in the right ear and later in the left one. It is possible that short or moderate intensity acute stresses only affect the hearing of the right ear, while prolonged or higher intensity ones end up affecting both or only the left ear. This fact agrees with the finding of other studies in which it is observed in rats that prolonged stress during the early postnatal days leads to atypical leftward turning behavior [94]. Human studies also suggest a greater blood flow to the right hemisphere than to the left hemisphere in children who had a smaller weight at birth [95]. Regarding exposure to acute stress, differences have been observed for emotion processing in such a way that stressed participants responded faster to angry faces presented to the left visual hemifield but responded faster to happy faces presented to the right one [96]. The results of our study could be explained by a predominant effect on right hearing of the early pathophysiological mechanisms of anxiety (mainly the autonomic nervous system [97]), while the late ones (neurotransmitters [72,73,98], endogenous opioids [99,100], hypothalamic–pituitary–adrenal axis [101] would affect both sides or, above all, the left hearing.
In conclusion, there is a physiological interaural asymmetry for pure tones in favor of the left ear. This asymmetry is accentuated in processes of anxiety and/or depression, being more evident in high frequencies when anxiety symptoms are present. These findings support the use of the classic tonal audiogram as a diagnostic, accessible and inexpensive approach tool to address possible emotional disorders, especially in the primary care setting. They could also provide new insights into the hemispheric laterality of auditory perception and how it relates to anxiety and depression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym14010024/s1, S1: Database. S2: Statistical data
Author Contributions
Conceptualization, B.E.-G., M.J.Á.-P. and F.G.; Data curation, B.E.-G. and F.G.; Formal analysis, B.E.-G. and F.G.; Investigation, B.E.-G.; Methodology, B.E.-G., M.J.Á.-P. and F.G.; Project administration, B.E.-G.; Resources, B.E.-G.; Software, B.E.-G.; Supervision, F.G.; Validation, M.J.Á.-P. and F.G.; Visualization, B.E.-G., M.J.Á.-P. and F.G.; Writing—original draft, B.E.-G. and F.G.; Writing—review and editing, M.J.Á.-P. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Hospital Universitario La Princesa (Madrid) (protocol code 05/11, 8 March 2012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data is available in Document S1 in the Supplementary Section; S1. Original Data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Broca, P. Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bull. Mem. Soc. Anat. Paris 1861, 6, 330–357. [Google Scholar]
- Corballis, M.C. The evolution and genetics of cerebral asymmetry. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Cantalupo, C.; Capocchiano, M.; Vallortigara, G. Population lateralisation and social behaviour: A study with 16 species of fish. Laterality 2000, 5, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J. survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008, 76, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.L.; Wilson, S.W. Asymmetry in the epithalamus of vertebrates. J. Anat. 2001, 199, 63–84. [Google Scholar] [CrossRef]
- Byrne, R.A.; Kuba, M.; Griebel, U. Lateral asymmetry of eye use in Octopus vulgaris. Anim. Behav. 2002, 64, 461–468. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G. From Antenna to Antenna: Lateral Shift of Olfactory Memory Recall by Honeybees. PLoS ONE 2008, 3, e2340. [Google Scholar] [CrossRef]
- Taylor, R.W.; Hsieh, Y.-W.; Gamse, J.T.; Chuang, C.-F. Making a difference together: Reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development 2010, 137, 681–691. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Güntürkün, O. Hemispheric Asymmetries: The Comparative View. Front. Psychol. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Roediger, D.; Kucukboyaci, N.E.; Carlson, C.; Devinsky, O.; Kuzniecky, R.; Halgren, E.; Thesen, T. Hemispheric asymmetries of cortical volume in the human brain. Cortex J. Devoted Study Nerv. Syst. Behav. 2011, 49, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Frässle, S.; Paulus, F.M.; Krach, S.; Schweinberger, S.; Stephan, K.E.; Jansen, A. Mechanisms of hemispheric lateralization: Asymmetric interhemispheric recruitment in the face perception network. NeuroImage 2016, 124, 977–988. [Google Scholar] [CrossRef]
- Kimura, D. Functional Asymmetry of the Brain in Dichotic Listening. Cortex 1967, 3, 163–178. [Google Scholar] [CrossRef]
- Bidelman, G.M.; Bhagat, S.P. Right-ear advantage drives the link between olivocochlear efferent ‘antimasking’ and speech-in-noise listening benefits. NeuroReport 2015, 26, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Eichele, T.; Nordby, H.; Rimol, L.M.; Hugdahl, K. Asymmetry of evoked potential latency to speech sounds predicts the ear advantage in dichotic listening. Cogn. Brain Res. 2005, 24, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hirnstein, M.; Westerhausen, R.; Korsnes, M.S.; Hugdahl, K. Sex differences in language asymmetry are age-dependent and small: A large-scale, consonant–vowel dichotic listening study with behavioral and fMRI data. Cortex J. Devoted Study Nerv. Syst. Behav. 2013, 49, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Løberg, E.-M.; Jørgensen, H.A.; Lundervold, A.; Lund, A.; Green, M.F.; Rund, B. Left hemisphere lateralisation of auditory hallucinations in schizophrenia: A dichotic listening study. Cogn. Neuropsychiatry 2008, 13, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Løberg, E.-M.; Falkenberg, L.E.; Johnsen, E.; Kompus, K.; Kroken, R.A.; Nygård, M.; Westerhausen, R.; Alptekin, K.; Özgören, M. Auditory verbal hallucinations in schizophrenia as aberrant lateralized speech perception: Evidence from dichotic listening. Schizophr. Res. 2012, 140, 59–64. [Google Scholar] [CrossRef]
- Hugdahl, K.; Rund, B.R.; Lund, A.; Asbjørnsen, A.; Egeland, J.; Landrø, N.I.; Roness, A.; Stordal, K.I.; Sundet, K. Attentional and executive dysfunctions in schizophrenia and depression: Evidence from dichotic listening performance. Biol. Psychiatry 2003, 53, 609–616. [Google Scholar] [CrossRef]
- Prete, G.; Marzoli, D.; Brancucci, A.; Tommasi, L.; Prete, G.; Marzoli, D.; Brancucci, A.; Tommasi, L. Hearing it right: Evidence of hemispheric lateralization in auditory imagery. Hear. Res. 2016, 332, 80–86. [Google Scholar] [CrossRef]
- Prete, G.; D’Anselmo, A.; Brancucci, A.; Tommasi, L. Evidence of a Right Ear Advantage in the absence of auditory targets. Sci. Rep. 2018, 8, 15569. [Google Scholar] [CrossRef]
- Altamura, M.; Prete, G.; Elia, A.; Angelini, E.; Padalino, F.A.; Bellomo, A.; Tommasi, L.; Fairfield, B. Do patients with hallucinations imagine speech right? Neuropsychologia 2020, 146, 107567. [Google Scholar] [CrossRef]
- Sininger, Y.S.; Bhatara, A. Laterality of basic auditory perception. Laterality Asymmetries Body Brain Cogn. 2012, 17, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Brancucci, A.; Prete, G.; Meraglia, E.; Di Domenico, A.; Lugli, V.; Penolazzi, B.; Tommasi, L. Asymmetric Cortical Adaptation Effects during Alternating Auditory Stimulation. PLoS ONE 2012, 7, e34367. [Google Scholar] [CrossRef]
- Fischer, J.; Teufel, C.; Drolet, M.; Patzelt, A.; Rübsamen, R.; Von Cramon, D.Y.; Schubotz, R.I. Orienting asymmetries and lateralized processing of sounds in humans. BMC Neurosci. 2009, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.W.; Phinney, R.E.; Brefczynski-Lewis, J.A.; DeYoe, E.A. Lefties Get It “Right” When Hearing Tool Sounds. J. Cogn. Neurosci. 2006, 18, 1314–1330. [Google Scholar] [CrossRef]
- Brancucci, A.; Babiloni, C.; Rossini, P.M.; Romani, G.L. Right hemisphere specialization for intensity discrimination of musical and speech sounds. Neuropsychologia 2005, 43, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Stracke, H.; Draganova, R.; Pantev, C. Hemispheric Asymmetry of Auditory Evoked Fields Elicited by Spectral versus Temporal Stimulus Change. Cereb. Cortex 2009, 19, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Kakigi, R. Hemispheric asymmetry of auditory mismatch negativity elicited by spectral and temporal deviants: A magnetoencephalographic study. Brain Topogr. 2015, 28, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Brancucci, A.; D’Anselmo, A.; Martello, F.; Tommasi, L. Left hemisphere specialization for duration discrimination of musical and speech sounds. Neuropsychologia 2008, 46, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, J.S. Right-left asymmetry in the cortical processing of sounds for social communication vs. navigation in mustached bats. Eur. J. Neurosci. 2012, 35, 257–270. [Google Scholar] [CrossRef]
- Washington, S.D.; Kanwal, J.S. Sex-dependent hemispheric asymmetries for processing frequency-modulated sounds in the primary auditory cortex of the mustached bat. J. Neurophysiol. 2012, 108, 1548–1566. [Google Scholar] [CrossRef]
- Poeppel, D. The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time’. Speech Commun. 2003, 41, 245–255. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Belin, P. Spectral and Temporal Processing in Human Auditory Cortex. Cereb. Cortex 2001, 11, 946–953. [Google Scholar] [CrossRef]
- Contrera, K.J.; Betz, J.; Deal, J.A.; Choi, J.S.; Ayonayon, H.N.; Harris, T.; Helzner, E.; Martin, K.R.; Mehta, K.; Pratt, S.; et al. Association of Hearing Impairment and Emotional Vitality in Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2016, 71, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Stam, M.; Smit, J.H.; Twisk, J.W.R.; Lemke, U.; Smits, C.; Festen, J.M.; Kramer, S.E. Change in Psychosocial Health Status Over 5 Years in Relation to Adults’ Hearing Ability in Noise. Ear Hear. 2016, 37, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, M.; Kitahara, T.; Okayasu, T.; Yamashita, A.; Hasukawa, A.; Ota, I.; Yamanaka, T. Negative prognostic factors for psychological conditions in patients with audiovestibular diseases. Auris Nasus Larynx 2016, 43, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, K.; Lewandowska, M.; Skarżyński, H. Health-related quality of life and mental distress in patients with partial deafness: Preliminary findings. Eur. Arch. Otorhinolaryngol. 2015, 273, 767–776. [Google Scholar] [CrossRef]
- Pronk, M.; Deeg, D.J.H.; Kramer, S.E. Hearing Status in Older Persons: A Significant Determinant of Depression and Loneliness? Results from the Longitudinal Aging Study Amsterdam. Am. J. Audiol. 2013, 22, 316–320. [Google Scholar] [CrossRef]
- Garnefski, N.; Kraaij, V. Cognitive coping and goal adjustment are associated with symptoms of depression and anxiety in people with acquired hearing loss. Int. J. Audiol. 2012, 51, 545–550. [Google Scholar] [CrossRef]
- Durai, M.; Searchfield, G. Anxiety and depression, personality traits relevant to tinnitus: A scoping review. Int. J. Audiol. 2016, 55, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeon, Y.J.; Lee, J.-Y.; Kim, Y.H. Characteristics of tinnitus in adolescents and association with psychoemotional factors. Laryngoscope 2017, 127, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- WHO. Comprehensive Mental Health Action Plan 2013–2020. Available online: http://www.who.int/entity/mental_health/action_plan_2013/en/index.html (accessed on 13 January 2019).
- Wiegner, L.; Hange, D.; Björkelund, C.; Ahlborg, G. Prevalence of perceived stress and associations to symptoms of exhaustion, depression and anxiety in a working age population seeking primary care-an observational study. BMC Fam. Pract. 2015, 16, 38. [Google Scholar] [CrossRef]
- Goldberg, D.P.; Reed, G.M.; Robles, R.; Minhas, F.; Razzaque, B.; Fortes, S.; Mari, J.D.J.; Lam, T.P.; Garcia, J.Á.; Gask, L.; et al. Screening for anxiety, depression, and anxious depression in primary care: A field study for ICD-11 PHC. J. Affect. Disord. 2017, 213, 199–206. [Google Scholar] [CrossRef] [PubMed]
- De Kovel, C.G.; Aftanas, L.; Aleman, A.; Alexander-Bloch, A.F.; Baune, B.T.; Brack, I.; Bülow, R.; Filho, G.B.; Carballedo, A.; Connolly, C.G.; et al. No Alterations of Brain Structural Asymmetry in Major Depressive Disorder: An ENIGMA Consortium Analysis. Am. J. Psychiatry 2019, 176, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, N.; Hirano, Y.; Nakamura, I.; Hirano, S.; Sato, J.; Oribe, N.; Ueno, T.; Kanba, S.; Onitsuka, T. Right hemisphere pitch-mismatch negativity reduction in patients with major depression: An MEG study. J. Affect. Disord. 2017, 215, 225–229. [Google Scholar] [CrossRef]
- Bruder, G.E.; Stewart, J.W.; McGrath, P.J. Right brain, left brain in depressive disorders: Clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci. Biobehav. Rev. 2017, 78, 178–191. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Lu, S. Neural Basis of Depression Related to a Dominant Right Hemisphere: A Resting-State fMRI Study. Behav. Neurol. 2018, 2018, 5024520. [Google Scholar] [CrossRef] [PubMed]
- Bruder, G.E.; Alvarenga, J.; Abraham, K.; Skipper, J.; Warner, V.; Voyer, D.; Peterson, B.S.; Weissman, M.M. Brain laterality, depression and anxiety disorders: New findings for emotional and verbal dichotic listening in individuals at risk for depression. Laterality 2016, 21, 525–548. [Google Scholar] [CrossRef]
- Jung, Y.-H.; Shin, J.E.; Lee, Y.I.; Jang, J.H.; Jo, H.J.; Choi, S.-H. Altered Amygdala Resting-State Functional Connectivity and Hemispheric Asymmetry in Patients with Social Anxiety Disorder. Front. Psychiatry 2018, 9, 164. [Google Scholar] [CrossRef]
- Peng, X.; Lau, W.K.W.; Wang, C.; Ning, L.; Zhang, R. Impaired left amygdala resting state functional connectivity in subthreshold depression individuals. Sci. Rep. 2020, 10, 17207. [Google Scholar] [CrossRef] [PubMed]
- Guest, M.; Boggess, M.; D’Este, C.; Attia, J.; Brown, A. An Observed Relationship between Vestibular Function and Auditory Thresholds in Aircraft-Maintenance Workers. J. Occup. Environ. Med. 2011, 53, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.V.; Carney, L.; Bishop, C.E. Auditory measures in clinically depressed individuals. I. Basic measures and transient otoacoustic emissions. Int. J. Audiol. 2004, 43, 493–498. [Google Scholar] [CrossRef]
- Goldberg, D.; Bridges, K.; Duncan-Jones, P.; Grayson, D. Detecting anxiety and depression in general medical settings. BMJ 1988, 297, 897–899. [Google Scholar] [CrossRef]
- Montón, C.; Pérez Echeverría, M.J.; Campos, R.; García Campayo, J.; Lobo, A. Escalas de ansiedad y depresión de Goldberg: Una guía de entrevista eficaz para la detección del malestar psíquico. Aten. Primaria Soc. Esp. Med. Fam. Comunitaria 1993, 12, 345–349. [Google Scholar]
- Carrobles, J.; Costa, M.; Del Ser, T.; Bartolomé, P. La Práctica de la Terapia de Conducta; Promolibro: Valencia, Spain, 1986. [Google Scholar]
- Ramos-Brieva, J.A.; Cordero Villafáfila, A. Validación de la versión castellana de la escala de Hamilton para la depresión. Actas Luso-Esp. Neurol. Psiquiatr. Cienc. Afines 1986, 14, 324–334. [Google Scholar]
- Andoh, J.; Matsushita, R.; Zatorre, R.J. Asymmetric Interhemispheric Transfer in the Auditory Network: Evidence from TMS, Resting-State fMRI, and Diffusion Imaging. J. Neurosci. 2015, 35, 14602–14611. [Google Scholar] [CrossRef] [PubMed]
- Zatorre, R.J.; Gandour, J.T. Neural specializations for speech and pitch: Moving beyond the dichotomies. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1087–1104. [Google Scholar] [CrossRef]
- Kimura, D. Speech lateralization in young children as determined by an auditory test. J. Comp. Physiol. Psychol. 1963, 56, 899–902. [Google Scholar] [CrossRef] [PubMed]
- King, F.L.; Kimura, D. Left-ear superiority in dichotic perception of vocal nonverbal sounds. Can. J. Psychol. 1972, 26, 111–116. [Google Scholar] [CrossRef]
- Wyczesany, M.; Capotosto, P.; Zappasodi, F.; Prete, G. Hemispheric asymmetries and emotions: Evidence from effective connectivity. Neuropsychologia 2018, 121, 98–105. [Google Scholar] [CrossRef]
- Todd, W.V.; Douglas, J.G. Cerebral cortex. In Nolte’s the Human Brain; Elsevier: Amsterdam, The Netherlands, 2016; pp. 541–578. [Google Scholar]
- Gainotti, G. Emotional Behavior and Hemispheric Side of the Lesion. Cortex J. Devoted Study Nerv. Syst. Behav. 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia 2012, 50, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Mednick, D.; Moss, E.; Saron, C.; Schaffer, C.E. Ratings of emotion in faces are influenced by the visual field to which stimuli are presented. Brain Cogn. 1987, 6, 403–411. [Google Scholar] [CrossRef]
- Baijal, S.; Srinivasan, N. Emotional and hemispheric asymmetries in shifts of attention: An ERP study. Cogn. Emot. 2011, 25, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2018, 25, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.A. Decoding the auditory corticofugal systems. Hear. Res. 2006, 212, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Straka, M.; Hughes, R.; Lee, P.; Lim, H. Descending and tonotopic projection patterns from the auditory cortex to the inferior colliculus. Neuroscience 2015, 300, 325–337. [Google Scholar] [CrossRef]
- Papesh, M.A.; Hurley, L. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hear. Res. 2016, 332, 121–136. [Google Scholar] [CrossRef]
- Felix, R.A.; Elde, C.J.; Nevue, A.A.; Portfors, C.V. Serotonin modulates response properties of neurons in the dorsal cochlear nucleus of the mouse. Hear. Res. 2017, 344, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Maison, S.F.; Liu, X.-P.; Vetter, D.; Eatock, R.A.; Nathanson, N.M.; Wess, J.; Liberman, M.C. Muscarinic Signaling in the Cochlea: Presynaptic and Postsynaptic Effects on Efferent Feedback and Afferent Excitability. J. Neurosci. 2010, 30, 6751–6762. [Google Scholar] [CrossRef] [PubMed]
- Schofield, B.R.; Motts, S.D.; Mellott, J.G. Cholinergic cells of the pontomesencephalic tegmentum: Connections with auditory structures from cochlear nucleus to cortex. Hear. Res. 2011, 279, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, S.; Collet, L. Functional asymmetry of medial olivocochlear system in humans. Towards a peripheral auditory lateralization. NeuroReport 1996, 7, 993–996. [Google Scholar] [CrossRef]
- Philibert, B.; Veuillet, E.; Collet, L. Functional asymmetries of crossed and uncrossed medial olivocochlear efferent pathways in humans. Neurosci. Lett. 1998, 253, 99–102. [Google Scholar] [CrossRef]
- Giraudet, F.; Horner, K.C.; Cazals, Y. Similar Half-Octave TTS Protection of the Cochlea by Xylazine/Ketamine or Sympa-thectomy. Hear. Res. 2002, 174, 239–248. [Google Scholar] [CrossRef]
- Maison, S.F.; Le, M.; Larsen, E.; Lee, S.-K.; Rosowski, J.J.; Thomas, S.A.; Liberman, M.C. Mice Lacking Adrenergic Signaling Have Normal Cochlear Responses and Normal Resistance to Acoustic Injury but Enhanced Susceptibility to Middle-Ear Infection. J. Assoc. Res. Otolaryngol. 2010, 11, 449–461. [Google Scholar] [CrossRef][Green Version]
- Nevue, A.A.; Felix, R.A.; Portfors, C.V. Dopaminergic projections of the subparafascicular thalamic nucleus to the auditory brainstem. Hear. Res. 2016, 341, 202–209. [Google Scholar] [CrossRef]
- Ji, W.; Suga, N. Histaminergic modulation of nonspecific plasticity of the auditory system and differential gating. J. Neurophysiol. 2013, 109, 792–802. [Google Scholar] [CrossRef][Green Version]
- Sadock, V.A.; Sadock, B.J.; Ruiz, P. Anxiety disorders. In Kaplan and Sadock’s Synopsis of Psychiatry; Behavioral Sciences/Clinical Psychiatry; Wolters Kluwer: Philadelphia, PA, USA, 2015; pp. e19290–e19567. [Google Scholar]
- Sadock, V.A.; Sadock, B.J.; Ruiz, P. Mood disorders. In Kaplan and Sadock’s Synopsis of Psychiatry; Behavioral Sciences/Clinical Psychiatry; Wolters Kluwer: Philadelphia, PA, USA, 2015; pp. e17600–e19258. [Google Scholar]
- Sander, D.; Grafman, J.H.; Zalla, T. The Human Amygdala: An Evolved System for Relevance Detection. Rev. Neurosci. 2003, 14, 303–316. [Google Scholar] [CrossRef]
- Frühholz, S.; Trost, W.; Grandjean, D. The role of the medial temporal limbic system in processing emotions in voice and music. Prog. Neurobiol. 2014, 123, 1–17. [Google Scholar] [CrossRef]
- Tetereva, A.O.; Balaev, V.V.; Kartashov, S.I.; Ushakov, V.L.; Ivanitsky, A.M.; Martynova, O.V. Asymmetry of amygdala resting-state functional connectivity in healthy human brain. NeuroReport 2020, 31, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Khalfa, S.; Granier, J.-P.; Reynaud, E.; El Khoury, M.; Grosse, E.-M.; Samuelian, J.-C.; Blin, O. Pure-tone auditory thresholds are decreased in depressed people with post-traumatic stress disorder. J. Affect. Disord. 2010, 127, 169–176. [Google Scholar] [CrossRef]
- Yovell, Y.; Sackeim, H.A.; Epstein, D.G.; Prudic, J.; Devanand, D.P.; McElhiney, M.C.; Settembrino, J.M.; Bruder, G.E. Hearing loss and asymmetry in major depression. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 82–89. [Google Scholar] [CrossRef]
- Pérez-Franco, B.; Turabián-Fernández, J. ¿Es válido el abordaje ortodoxo de la depresión en atención primaria? Aten. Primaria 2006, 37, 37–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aragonès, E. Desacuerdos diagnósticos entre médicos generales y psiquiatras. Aten. Primaria 2008, 40, 644. [Google Scholar] [CrossRef]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol.-Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef]
- Engels, A.S.; Heller, W.; Mohanty, A.; Herrington, J.D.; Banich, M.; Webb, A.G.; Miller, G.A. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology 2007, 44, 352–363. [Google Scholar] [CrossRef]
- Nitschke, J.B.; Heller, W.; Palmieri, P.A.; Miller, G.A. Contrasting Patterns of Brain Activity in Anxious Apprehension and Anxious Arousal. Psychophysiology 1999, 36, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Mundorf, A.; Matsui, H.; Ocklenburg, S.; Freund, N. Asymmetry of turning behavior in rats is modulated by early life stress. Behav. Brain Res. 2020, 393, 112807. [Google Scholar] [CrossRef]
- Jones, A.; Osmond, C.; Godfrey, K.M.; Phillips, D.I.W. Evidence for Developmental Programming of Cerebral Laterality in Humans. PLoS ONE 2011, 6, e17071. [Google Scholar] [CrossRef]
- Brüne, M.; Nadolny, N.; Güntürkün, O.; Wolf, O.T. Stress induces a functional asymmetry in an emotional attention task. Cogn. Emot. 2013, 27, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Frizzo, A.C.F.; Chagas, E.F.B.; Garner, D.M.; Raimundo, R.D.; Sousa, L.V.D.A.; Valenti, V.E. A relationship between brainstem auditory evoked potential and vagal control of heart rate in adult women. Acta Neurobiol. Exp. 2018, 78, 305–314. [Google Scholar] [CrossRef]
- Hurley, L.; Hall, I. Context-dependent modulation of auditory processing by serotonin. Hear. Res. 2011, 279, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Hughes, L.F.; Bledsoe, S.C. Dynorphin release by the lateral olivocochlear efferents may inhibit auditory nerve activity: A cochlear drug delivery study. Neurosci. Lett. 2014, 571, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sahley, T.L.; Anderson, D.J.; Chernicky, C.L. Bi-phasic intensity-dependent opioid-mediated neural amplitude changes in the chinchilla cochlea: Partial blockade by an N-Methyl-d-Aspartate (NMDA)-receptor antagonist. Eur. J. Pharmacol. 2008, 580, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Gadea, M.; Gómez, C.; González-Bono, E.; Espert, R.; Salvador, A. Increased cortisol and decreased right ear advantage (REA) in dichotic listening following a negative mood induction. Psychoneuroendocrinology 2005, 30, 129–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).