Abstract
The origin of chiral asymmetry in biology has attracted the attention of the research community throughout the years. In this paper we discuss the role of chirality and chirality sign alternation (L–D–L–D in proteins and D–L–D–L in DNA) in promoting self-organization in biology, starting at the level of single molecules and continuing to the level of supramolecular assemblies. In addition, we also discuss chiral assemblies in solutions of homochiral organic molecules. Sign-alternating chiral hierarchies created by proteins and nucleic acids are suggested to create the structural basis for the existence of selected mechanical degrees of freedom required for conformational dynamics in enzymes and macromolecular machines.
1. Introduction
The origin and potential role of chiral asymmetry in biology have attracted the attention of the research community throughout the years [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Although, at the macroscopic level, nonbiological structures with a homochiral molecular basis are not known, living organisms have been engaged in a unique experiment of creating stable homochiral systems. In our opinion, an understanding of the role of chirality in biology requires a more general approach to the observed interconnectivity of all the involved systems, starting at the level of 1) homochirality in the primary structure of proteins and the (deoxy)ribose backbone of nucleic acids; followed by 2) macromolecular hierarchies of higher-order structures; and 3) considering functional aspects and the role of chirality in the mechanical aspects of function, e.g., of enzymes and macromolecular machines. Using this approach, in this work we reformulate the question on the origin of chirality and instead ask, why did the chiral asymmetry emerge and what are its consequences in biology? The second question within this context: could a chiral asymmetry of nonbiological origin have been transferred to and integrated into biology? To answer these questions, we discuss the physical and chemical consequences of the presence of chirality and their role in the self-organization and formation of structural hierarchies in cells. Eventually the same principles can be applied even to artificial systems. A unifying biophysical basis of these phenomena was discussed earlier by the authors [19,20,21,22]. Here we propose that homochirality may serve as an essential factor that invokes the physical and chemical mechanisms required to control the formation of discrete structural hierarchies in macromolecules and macromolecular assemblies, even determining to a large extent their function, like in the case of macromolecular machines.
2. The Hierarchies of Chiral Structures in Proteins and Nucleic Acids
For macromolecular structures and their assemblies in cells we define the basic principle of organization, which starts from the lowest level of an asymmetric carbon atom in a sp3-hybridization state and progresses to superhelices and supramolecular structures, as with the creation of hierarchies of sign-alternating chiral entities. In other words, the hierarchies of structural levels of organization of proteins and nucleic acids in cells, normally described qualitatively, may instead be represented as a regular interconnected chiral system. Starting from the level of an asymmetric carbon atom in the amino acids of the primary structure of a protein and in DNA deoxyribose, we earlier noted that there is a tendency to switch the chirality sign at subsequent structural levels, resulting in sequences of the types L–D–L–D in proteins and D–L–D–L in DNA [19,20,21,22].
An interesting manifestation of this principle is found in helical structures. This type of structure can be organized into four chiral levels in the cases of both proteins and DNA (RNA). A block diagram illustrating the levels of organization is shown in Figure 1.
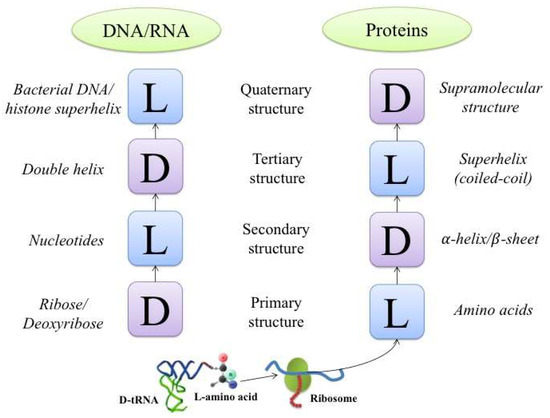
Figure 1.
A schematic presentation showing the sequence of alternating types of chiral structures with alternating chirality signs starting from the primary structure up to the level of quaternary structure organization for DNA (or RNA, left column) and proteins (right column). L, left configuration of enantiomer or helix; D, right configuration of enantiomer or helix.
In this example, the primary protein structure is represented by a sequence of L-amino acids. When organized into secondary structures, they are twisted into right-handed α-helices, which in turn may be folded into a bundle of left-handed superhelices, or coiled-coils (Figure 2).
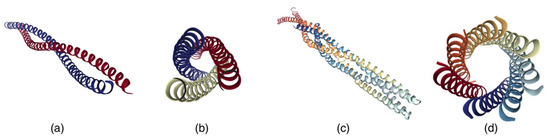
Figure 2.
Protein structures containing left-handed coiled-coils: (a) PDB ID: 1D7M [23] (based on two right-handed α-helices), (b) PDB ID: 1WT6 [24] (based on three right-handed α-helices), (c) PDB ID: 1QU7 [25] (based on four right-handed α-helices), and (d) PDB ID: 2HY6 [26] (based on seven right-handed α-helices) [27].
A quaternary/supramolecular protein structure can be assembled from independent superhelices, leading to the formation of a right-handed twisted structure (like the F-actin double helix [28], Figure 3).

Figure 3.
Tropomyosin left-handed coiled-coil (green) twists on the F-actin right-handed double helix (purple) in the right-handed direction.
In the case of DNA and RNA, alternation of the chirality sign is observed in A- and B-forms of DNA and the A-form of RNA during the transition to higher-order structures. Thus, basic single-chain DNA is composed of D-deoxyribose. The nucleobases within a single chain are connected in the left-handed gauche conformation, while two chains form the well-known right-handed DNA double helix. In bacteria, a circular right-handed helix is twisted into a left-handed superhelix. In eukaryotes, a right-handed DNA double helix wraps around the histone octamer in the left-handed direction. It is important to emphasize here that we only describe the central motives leading to the formation of a sequence of hierarchical structures. This does not exclude the possibility of the formation of structures and structural assemblies outside the frame of this scheme.
It should be mentioned that the designations of “left-handedness” and “right-handedness” are used here in accordance with the current nomenclature [29]. Thus, according to the Cahn–Ingold–Prelog rules, the configuration of a compound is designated as R (“right”) if the sense of rotation of a curve passing through the substituents is clockwise and as S (“left”) if the sense of rotation is counterclockwise. This is reflected in the designation of right-handed and left-handed helices. These definitions are closely associated with the definitions of the sign of chirality, which makes them universal and convenient for the analysis presented here. In essence, it is not that important whether it is a right- or left-handed configuration, since the basis of the presented concept is the switch of the chirality sign during transition to a new level in the hierarchy: a D or L chirality sign at the lower structural level is switched to L or D, respectively, at the next higher level.
The ranking of structures based on sign-alternating chirality does not always coincide exactly with the traditional classification of the structural levels, revealing an essential difference between these classifications. In proteins, L/D alternation of the chirality sign in structural hierarchies is not necessarily absolute, since deviations may have physical justification and serve certain biological purposefulness. An example is β-sheet structures, which have a right-handed β-propeller twist [30]. In this case, left-twisted strands are linked by hydrogen bonds that stabilize the right-twisted surface of the sheet. Thus, in this case, while the left-twisted structure is formed by L-amino acids, the right-twisted next level is stabilized by hydrogen bonds. In the case of a polyproline helix, a left-handed structure is stabilized by van der Waals interactions and formed by a sequence of L-amino acids due to the steric properties of proline. The next level of the right-handed superhelix contributes to stabilizing the first level. In both cases of a β-propeller structure and the polyproline helix, a stable higher level stabilizes a “wrong” left-handed level of the lower-order structure.
There are other examples of chirality sign alternations in cells. A single chain of F-actin in muscles or in the actin microfilaments of the cytoskeleton forms a left-handed motif, while a double chain is right-handed [28]. Another example is that of the left-handed tubulin microtubules of the cytoskeleton, which are formed by an asymmetrical pair of α- and β-tubulins, which together form a left-handed helix [31]. In the functional context, the chirality signs of actin and tubulin microfilaments in turn serve in the recognition of interaction partners which have opposite chirality sign: left-handed membrane phospholipids for actin, and right-handed nucleic acid chains for tubulin microfilaments.
3. Structure Formation in Homochiral Systems of Nonbiological Origin
The formation of chiral sign-alternating hierarchical structures in initially homochiral systems is not an exclusive property of biological organisms; it can be reliably reproduced in various polymeric and liquid-crystalline systems [20,22]. For example, a thermodynamically governed process of racemization of any macroscopic homochiral system leads to the elimination of chiral polarization of substances in solutions of compounds of nonbiological origin, which in turn results in suppression of the spontaneous formation of a succession of chiral structures [32]. On the other hand, it has been observed that artificially created homochiral systems may undergo the same process of spontaneous formation of hierarchies of molecular and supramolecular structures with an alternating sign of chirality.
A well-known example from the field of mechanics is the twisting of elastic threads. When a long rope is twisted in any direction, it leads to the formation of reverse turns of the helix. By increasing the tension, longer coils having the same direction will emerge, after which hairpins will appear, and finally, a double superhelix will be formed. In this case, a helix of one sign of chirality forms hairpins and superhelices of opposite chirality sign. During the transition to the next level of the structural hierarchy, the system changes its scale and symmetry, leading to the formation of new degrees of freedom, redistribution of energy, and, as a result, a lower elastic tension. In other words, the system lowers its free energy level by forming a new structure. Note that in this example the energy decreases primarily due to the formation of elastic interactions, i.e., due to an enthalpic component, while such rearrangements in the hierarchical structure formation of (bio)polymers are related to the entropic factor. This idea is one of the key points in the concept of the formation of chiral sign-alternating hierarchical structures.
Cited below are examples of physicochemical systems with the ability to form sign-alternating chiral levels. The authors showed that synthetic C3 symmetric tris[3(3’-carbamoylamino)-2,2’-bipyridyl]-benzene-1,3,5-tricarbonamide derivatives containing three chiral bis[(R) or (S)-2-methylbutylthio]-tetrathiafulvalenyl units at the periphery can assemble into twisted fibers with a right-handed helix assembled from left enantiomers and a left-handed helix from right enantiomers [33]. The formation of helical structures of polyacetylene after the addition of chiral amines has also been demonstrated [34]. In this case, when left (L/S) enantiomers were added, a right-handed helix was formed, while right (D/R) enantiomers promoted the formation of a left-handed helix. These are clear examples of chirality sign switch during the transition from the primary level to a secondary one.
There are also examples of systems that show chirality sign switch during transition from the secondary to the tertiary level—the formation of superhelices with chirality sign opposite to the sign of the initial helices. Thus, super-amphiphiles consisting of hydrophilic isocyanopeptides and more-hydrophobic polystyrene have been shown to assemble in water into rod micelles, vesicles, and helical aggregates [35]. Micellar rods built up by a polystyrene core and with a surface formed by right-handed polyisocyanides self-assemble into left-handed supercoiled fibers.
The self-assembly of hierarchical disk-shaped molecules with chiral tails into single helical structures has also been demonstrated [36]. In this case, a crown ether appended phthalocyanine with chiral aliphatic tails had the ability to self-assemble into right-handed helical stacks in chloroform, which in turn formed left-handed coiled-coil structures.
The formation of characteristically stiff helical structures (so-called strings) in homochiral solutions of trifluoroacetylated amino alcohols was observed in several solvents [37]. Right enantiomers formed a left-handed chiral string that, in turn, twisted into a right-handed superhelix.
The authors showed that naturally self-assembled diphenylalanine peptide nanotubes based on L-diphenylalanine formed right-handed helical structures, and diphenylalanine peptide nanotubes based on D-diphenylalanine formed left-handed helical structures [38].
The successful fabrication of flower-like structures using an achiral porphyrin and chiral amphiphilic histidine has been demonstrated [39]. Curved nanosheets were arranged in a clockwise manner or counterclockwise manner depending on the absolute configuration of histidine—the L- or D-enantiomer, respectively.
As for helical structures in two-dimensional mono- and bilayers of some amphiphiles that contain at least one chiral center (in particular, modified phospholipids), helicoidal structures were shown to form under compression when one of the enantiomers (L or D) was present in abundance. In this case, the chirality of the helix was always related to the chirality of the enantiomer and changed its sign to the opposite when the chirality sign of the enantiomer was changed [40]. These examples clearly illustrate that the relationships between the chirality sign, the levels of the structural hierarchy, and spontaneous assembly of new chiral levels can be easily reproduced in model systems, which may have had implications during the early stages of biological evolution. In particular, a homochiral system or a system in which one of the enantiomers may have been in slight abundance would have had a certain evolutionary advantage in creating the stable structures which were required during the early period of transition from nonliving to living matter with entities able to support life as we know it.
4. Physical Bases and Biological Functionality of Sign-Alternating Chiral Hierarchical Structures
Questions related to the origin of mirror asymmetry in the world of carbon compounds in nature are continuously discussed in the scientific literature [1,2,3,4,5,6,7,8,9,10]. Here we focus on the analysis of the central role played by chirality in the self-organization of living matter. The insights obtained through this analysis allow us to consider chirality as an essential physical criterion, to be used in a more general ranking of the hierarchy of macromolecular organization, instead of the commonly used descriptive gradation of the structural levels in proteins, nucleic acids, and their assemblies. These insights can be used for a better understanding of the mechanisms of some well-known biological processes like folding, the formation of macromolecular assemblies, and the function and dynamics of molecular machines.
4.1. Protein Folding
The formation of sign-alternating chiral hierarchies in macromolecular structures and their assemblies is driven by the necessity for a system to lower the initial level of free energy. This is achieved during energy-dependent selection of homochiral monomers of the primary structures of macromolecules from racemized mixtures.
In 1968, Cyrus Levinthal formulated the well-known paradox: “In nature, proteins apparently do not sample all of these possible configurations since they fold in a few seconds, and even postulating a minimum time for going from one conformation to another, the proteins would have time to try on the order of 108 different conformations at most before reaching their final state. We feel that protein folding is speeded and guided by the rapid formation of local interactions which then determine the further folding of the peptide. This suggests local amino acid sequences which form stable interactions and serve as nucleation points in the folding process” [41]. Currently, protein folding models are based on the assumption of the existence of a funnel in the configuration space on a potential energy surface with a complex landscape, which directs the folding process into the native conformation. It is assumed that this funnel, characterized by a minimum of free energy, sets the direction of the folding trajectory in the configuration space of the macromolecule, passing through a chain of local energy minima. However, this representation is not physically specified, because it does not provide a mechanism for selecting the optimal trajectory.
The observations described above suggest that during folding, optimal-energy sign-alternating chiral sequences (with structural levels L–D–L–D) may provide the “Ariadne’s thread” that helps direct the folding of macromolecules along a certain trajectory in Levinthal’s trap. From the thermodynamic point of view, it is known that a homochiral molecular substance, whether it is a solution of amino acids or carbohydrates, undergoes racemization in order to equalize concentrations of enantiomers, maximizing entropy of the system while lowering its free energy level. In contrast, a linear homochiral polymer has the option to lower its free energy not only by racemization of monomers (which we call “horizontal” racemization), but also by “vertical” racemization: by creating higher-level structures with different chirality sign. In this approach, the building blocks are not “asymmetric carbons” but right- and left-handed units of different levels of organization. Thus, a system, by distributing its initial homochirality, lowers its free energy. This process leads to the folding and formation of parts and, eventually, a complete macromolecular structure, with a more stable (long-lived) backbone conformation, as compared to the initial structures of an unfolded polypeptide.
The half-period shift of the chirality signs of the structural levels of proteins and nucleic acids may serve as a "friend or foe" recognition code for these two important classes of biomolecules. In this case, the entire field of matching interactions in cells can be represented in the form of a table—a chessboard where chiral chemical compounds move and interact. It seems logical to consider that within one class of compounds, for example, proteins, an interaction develops in the space of left enantiomers, whereas in the family of nucleic acids, it develops in the space of right enantiomers. The same consideration may apply to all classes of chiral macromolecules, including right carbohydrates and left (in eukaryotes) phospholipids [42].
For many decades, the fundamental problem has remained unsolved: why does an enzyme need a large globule with complex hierarchical organization. We provide a general answer: an enzyme is a molecular machine, a construction. The fact that the final native structure of a protein is formed exclusively by a fully synthesized chain indicates the fundamental role of the integral entropy factor, which, in our opinion, is associated with symmetries, whereas the enthalpy factor works in local processes of structure formation.
4.2. Molecular Machines
From the physical point of view, the principal difference between living and nonliving matter is that living entities use molecular and macroscopic machines as active elements that convert different types of energy, substances, or information [20,43]. We call a machine a structure or a device that can convert, in a cyclic manner, different forms of energy and perform useful work due to the availability of selected mechanical, including quantum mechanical, degrees of freedom (translational, rotational) that kinetically separate work and dissipation. We consider all molecular machines to be isothermal due to their small size. Machines do not exist in nonliving nature; however, energy converters that are not capable of performing useful work do exist. From a physical point of view, the biological purpose of each molecular machine is to lower the symmetry of the system, supporting or increasing its complexity. In nonbiological systems, spontaneous nonconjugated processes will, on average, increase the level of symmetry [44].
The structural elements of a molecular machine are usually a rigid framework, as well as leverage systems and hinges. These are linear elements, and inside the structure each element has two options: to transfer or not to transfer force (energy) along the selected degrees of freedom. A helix is a unique (and the simplest) nonlinear element of the construction of molecular machines. Helices as rigid structures are capable of realizing translational and rotational degrees of freedom. However, for translational and rotational motion in a compressed spring, a direction of movement is essential. Symmetry is broken in a transfer of force or in "forward–backward" or "right–left" displacements. A discrete valve element—a ratchet device—can be used as an analogy [43,45]. However, this valve element cannot actually be realized in living systems, whereas a helix can be realized.
5. Discussion
We have described the regularity of the spontaneous formation of L–D sign-alternating structural hierarchies in biological macromolecules, which initially exist in a nonequilibrium state due to the homochirality of the primary structures. The change of the chirality sign in the hierarchy of intramolecular and supramolecular protein and nucleic acid structures constitutes the physical symmetrical invariant of molecular biology. In general, molecular biology can be considered as a type of periodic system of hierarchies of sign-alternating chiral structures, where the central block consists of four structural levels of proteins and DNA, forming a closed achiral invariant. The level of molecular biology ends here, and the cytological level with its symmetrical characteristics begins.
These hierarchical structures reflect the discrete character of macromolecular structures of cells, from primary asymmetric to supramolecular. Molecular chirality and chiral molecular self-assembly can be studied by circular dichroism spectroscopy [46,47,48,49], and while convincing qualitative evaluations prove our concept, it is of immense importance to obtain quantitative estimates of the proportions and chirality signs of structures at various hierarchic levels. The problem still lacks a solution, although many studies have been performed in this field [50,51,52,53,54]. In essence, the problem includes two questions that are related to introducing a measure of chirality and that mutually complement each other: how the degree of chirality and the chirality sign can be quantitatively estimated for molecular structures with the same symmetry type, and how the degrees of chirality can be quantitatively compared for structures with different types of symmetry.
In the present work, it was not our goal to discuss the nature of global symmetry breaking at the stage of the origin of life on Earth. Perhaps this problem does not exist at all. The paradoxical outcome of this study may be a new look at an old hypothesis about the relationship between the chiral polarization of biomolecules and a spontaneous break of electroweak symmetry [55,56,57,58]. In other words, the initial cause of the chiral polarization of biomolecules could lie not in local symmetry breaking at the level of chemical compounds—probionts—but in the conjugate sequence of chiral symmetry breaking, leading into the depths of the microworld.
In addition to its general fundamental significance, this concept can be further developed in the fields of pharmacology and biotechnology (analysis of chiral drug–target interaction, mirror image aptamer design, etc.).
Author Contributions
Conceptualization, V.A.T.; investigation, E.V.M.; writing—original draft preparation, V.A.T. and E.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Russian Science Foundation, grant No. 19-74-00082 (to E.V.M.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwartz, A.W. Origin of life. The origin of macromolecular chirality. Curr. Biol. 1994, 4, 758–760. [Google Scholar] [CrossRef]
- Kojić-Prodić, B.; Štefanić, Z. Symmetry versus Asymmetry in the Molecules of Life: Homomeric Protein Assemblies. Symmetry 2010, 2, 884–906. [Google Scholar] [CrossRef]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left-right asymmetric development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef] [PubMed]
- Podlech, J. Origin of organic molecules and biomolecular homochirality. Cell Mol. Life Sci. 2001, 58, 44–60. [Google Scholar] [CrossRef]
- Hein, J.E.; Blackmond, D.G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef]
- Dorta-Urra, A.; Bargueño, P. Homochirality: A Perspective from Fundamental Physics. Symmetry 2019, 11, 661. [Google Scholar] [CrossRef]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2019, 11, a032540. [Google Scholar] [CrossRef]
- Famiano, M.; Boyd, R.; Kajino, T.; Onaka, T.; Mo, Y. Astrophysical Sites that Can Produce Enantiomeric Amino Acids. Symmetry 2019, 11, 23. [Google Scholar] [CrossRef]
- Suzuki, N.; Itabashi, Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry 2019, 11, 966. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Kobayashi, K. Origin of Terrestrial Bioorganic Homochirality and Symmetry Breaking in the Universe. Symmetry 2019, 11, 919. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Symmetry Breaking in Self-Assembled Nanoassemblies. Symmetry 2019, 11, 950. [Google Scholar] [CrossRef]
- Aav, R.; Mishra, K.A. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Yakovenko, L.V. Physical Aspects of the Emergence of Living Cell Precursors: The Ion and Chiral Asymmetries as Two Fundamental Asymmetry Types. Mosc. Univ. Phys. Bull. 2008, 63, 151–163. [Google Scholar] [CrossRef]
- Zlenko, D.; Zanin, A.; Skoblin, A.; Tverdislov, V.; Stovbun, S. Spontaneous resolution in racemic solutions of N-trifluoroacetylated α-aminoalcohols. J. Mol. Struct. 2019, 1183, 8–13. [Google Scholar] [CrossRef]
- Hirose, K.; Ukimi, M.; Ueda, S.; Onoda, C.; Kano, R.; Tsuda, K.; Hinohara, Y.; Tobe, Y. The Asymmetry is Derived from Mechanical Interlocking of Achiral Axle and Achiral Ring Components—Syntheses and Properties of Optically Pure [2]Rotaxanes. Symmetry 2018, 10, 20. [Google Scholar] [CrossRef]
- Ustrnul, L.; Kaabel, S.; Burankova, T.; Martõnova, J.; Adamson, J.; Konrad, N.; Burk, P.; Borovkov, V.; Aav, R. Supramolecular chirogenesis in zinc porphyrins by enantiopure hemicucurbit[n]urils (n = 6, 8). Chem. Commun. 2019, 55, 14434–14437. [Google Scholar] [CrossRef]
- Rickhaus, M.; Mayor, M.; Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef]
- Chen, Z.; Choi, C.K.K.; Wang, Q. Origin of the Plasmonic Chirality of Gold Nanorod Trimers Templated by DNA Origami. ACS Appl. Mater. Interfaces 2018, 10, 26835–26840. [Google Scholar] [CrossRef]
- Tverdislov, V.A. Chirality as an Instrument of Stratification of Hierarchical Systems in Animate and Inanimate Nature. 2012. Available online: https://arxiv.org/abs/1212.1677 (accessed on 7 January 2020).
- Tverdislov, V.A. Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 2013, 58, 128–132. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V.; Ilchenko, S.A.; Zhulyabina, O.A.; Yakovenko, L.V. A periodic system of chiral structures in molecular biology. Biophysics 2017, 62, 421–432. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V. On regularities of spontaneous formation of structural hierarchies in chiral systems of non-living and living nature. Phys. Uspekhi 2019, 189, 375–385. [Google Scholar] [CrossRef]
- Burkhard, P.; Kammerer, R.A.; Steinmetz, M.O.; Bourenkov, G.P.; Aebi, U. The coiled-coil trigger site of the rod domain of cortexillin I unveils a distinct network of interhelical and intrahelical salt bridges. Structure 2000, 8, 223–230. [Google Scholar] [CrossRef]
- Garcia, P.; Ucurum, Z.; Bucher, R.; Svergun, D.I.; Huber, T.; Lustig, A.; Konarev, P.V.; Marino, M.; Mayans, O. Molecular insights into the self-assembly mechanism of dystrophia myotonica kinase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Yokota, H.; Kim, S.H. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 1999, 400, 787–792. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Q.; Deng, Y.; Cheng, C.S.; Kallenbach, N.R.; Lu, M. A seven-helix coiled coil. Proc. Natl. Acad. Sci. USA 2006, 103, 15457–15462. [Google Scholar] [CrossRef]
- RCSB PDB. Available online: http://www.rcsb.org (accessed on 15 January 2020).
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Cross, L.C.; Klyne, W. Rules for the Nomenclature of Organic Chemistry: Section E: Stereochemistry. Pure Appl. Chem. 1974, 45, 11–30. [Google Scholar]
- Chothia, C. Conformation of twisted beta-pleated sheets in proteins. J. Mol. Biol. 1973, 75, 295–302. [Google Scholar] [CrossRef]
- Mandelkow, E.-M.; Schultheiss, R.; Rapp, R.; Müller, M.; Mandelkow, E. On the surface lattice of microtubules: Helix starts, protofilament number, seam, and handedness. J. Cell Biol. 1986, 102, 1067–1073. [Google Scholar] [CrossRef]
- Amabilino, D. Chirality at the Nanoscale, Nanoparticles, Surfaces, Materials and More; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; 440p. [Google Scholar] [CrossRef]
- Danila, I.; Riobé, F.; Piron, F.; Puigmartí-Luis, J.; Wallis, J.D.; Linares, M.; Ågren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical chiral expression from the nano- to mesoscale in synthetic supramolecular helical fibers of a nonamphiphilic C3-symmetrical π-functional molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.; Fischer, M.; Sommerdijk, N.A.J.M.; Nolte, R.J.M. Helical superstructures from charged Poly(styrene)-Poly(isocyanodipeptide) block copolymers. Science 1998, 280, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.; Rowan, A.E.; Nolte, R.J.M. Mastering Molecular Matter. Supramolecular Architectures by Hierarchical Self-Assembly. J. Mater. Chem. 2003, 13, 2661–2670. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Zanin, A.M.; Skoblin, A.A.; Mikhaleva, M.G.; Zlenko, D.V.; Tverdislov, V.A. Self Assembly of Supramolecular Homochiral Structures in Solutions of Chiral Biomimetics. Mosc. Univ. Phys. Bull. 2015, 70, 51–56. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Zhulyabina, O.A.; Tverdislov, V.A. Molecular modeling and computational study of the chiral-dependent structures and properties of self-assembling diphenylalanine peptide nanotubes. J. Mol. Model 2019, 25, 199. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Chen, J.; Liu, M. Hierarchical Self-Assembly of a Porphyrin into Chiral Macroscopic Flowers with Superhydrophobic and Enantioselective Property. ACS Nano. 2017, 11, 12453–12460. [Google Scholar] [CrossRef]
- Nandi, N.; Vollhardt, D. Effect of Molecular Chirality on the Morphology of Biomimetic Langmuir Monolayers. Chem. Rev. 2003, 103, 4033–4076. [Google Scholar] [CrossRef]
- Levinthal, C. How to Fold Graciously. In Mossbauer Spectroscopy in Biological Systems: Proceedings of the meeting held at Allerton House, Monticello, IL, USA; DeBrunner, J.T.P., Munck, E., Eds.; University of Illinois: Champaign, IL, USA, 1969; pp. 22–24. [Google Scholar]
- Wächtershäuser, G. From pre-cells to Eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef]
- Blumenfeld, L.A. Problems of Biological Physics; Springer: New York, NY, USA, 1981; 222p. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; 552p. [Google Scholar]
- Feynman, R. The Feynman Lectures on Physics. Volume I; Basic Books; California Institute of Technology: Pasadena, CA, USA, 2010; 968p. [Google Scholar]
- Gottarelli, G.; Lena, S.; Masiero, S.; Pieraccini, S.; Spada, G.P. The use of circular dichroism spectroscopy for studying the chiral molecular self-assembly: An overview. Chirality 2008, 20, 471–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alù, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.T. Circular dichroism and its use in protein-folding studies. Methods Mol. Biol. 2011, 752, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, M. Chirality and Symmetry Measures: A Transdisciplinary Review. Entropy 2003, 5, 271–312. [Google Scholar] [CrossRef]
- Randić, M.; Razinger, M. Molecular shapes and chirality. J. Chem. Inf. Comput. Sci. 1996, 36, 429–441. [Google Scholar] [CrossRef]
- Yewande, E.O.; Neal, M.P.; Low, R. The Hausdorff chirality measure and a proposed Hausdorff structure measure. Mol. Phys. 2009, 107, 281–291. [Google Scholar] [CrossRef]
- Raos, G. Degrees of chirality in helical structures. Macromol. Theory Simul. 2002, 11, 739–750. [Google Scholar] [CrossRef]
- Dryzun, C.; Zait, A.; Avnir, D. Quantitative symmetry and chirality—A fast computational algorithm for large structures: Proteins, macromolecules, nanotubes, and unit cells. J. Comput. Chem. 2011, 32, 2526–2538. [Google Scholar] [CrossRef]
- Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on earth. J. Theor. Biol. 1966, 11, 495–498. [Google Scholar] [CrossRef]
- Letokhov, V.S. Difference of energy-levels of left and right molecules due to weak interactions. Phys. Lett. A 1975, 53, 275–276. [Google Scholar] [CrossRef]
- Berger, R.; Quack, M.; Tschumper, G.S. Electroweak quantum chemistry for possible precursor molecules in the evolution of biomolecular homochirality. Helv. Chim. Acta 2000, 83, 1919–1950. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).