Abstract
A key aspect of modern drug research is the development of delivery methods that ensure the possibility of implementing targeted therapy for a specific biological target. The use of nanocarriers enables to achieve this objective, also allowing to reduce the toxicity of used substances and often extending their bioavailability. Through the application of docking methods, the possibility of using cube rhombellanes as potential carriers for two oxindole derivatives was analyzed. In the studies, compounds identified as inhibitors of the CDK2 enzyme and a set of nanostructures proposed by the Topo Cluj Group were used. The popular fullerene molecule C60 was used as the reference system. The estimated binding affinities and structures of obtained complexes show that use of functionalized cube rhombellanes containing hydrogen bond donors and acceptors in their external molecular shell significantly increases ligand affinity toward considered nanocariers, compared to classic fullerenes. The presented values also allow to state that an important factor determining the mutual affinity of the tested ligands and nanostructures is the symmetry of the analyzed nanocarriers and its influence on the distribution of binding groups (aromatic systems, donors and acceptors of hydrogen bonds) on the surface of nanoparticles.
1. Introduction
In recent medicinal chemistry one of the most important research goals is to find ways for effective drug delivery. In numerous works the subject matter was taken up using natural substances such as albumin [1], chitosan [2,3], gelatin [4], or synthetic compounds as gold complexes [5,6], hydrogels [7], magnetic iron oxides [8] or polymers [9,10,11,12,13], as one of the drug carriers, however, one of the most dynamically developing groups of compounds are nanoparticles based on fullerene compounds [14,15,16,17,18]. Application of considered drug carriers not only improves the way of their delivery to the biological target, but also increases the bioavailability and significantly reduces the occurrence of side effects associated with the toxicity of substances with pharmacological potential [15,19]. In the case of many new nanocarriers, a significant extension in the time of drug bioactivity is observed [20], often also related with an increase in the observed pharmacological effect [21]. Among the compounds belonging to the fullerenes group, numerous studies have focused on C60 molecule which exhibits biological activity towards cells [14]. Its chemical structure enables for penetration of cell membrane [22,23,24], however in low concentrations it is non-toxic for living organisms [15,16,25,26]. The characteristic spherical surface of this nanomolecule created by conjugated carbon rings allows for π-stacking interactions with biological targets containing aromatic systems like aminoacids, vitamins, nucleic acid bases or drugs [27]. The numerous research show that C60 is a good carrier for drugs containing extended aromatic clusters in their structure like doxorubicin used in chemotherapy [18], however, there is a large group of compounds which chemical structure does not provide such significant possibilities in terms of staking impacts. The presence of numerous donors and acceptors of hydrogen bonds in such compounds indicates on the second potential stabilizing factor, which can occur through the selection of an appropriate nanocarrier. Such group of compounds encompasses for example oxindole derivatives which are well known as competitive inhibitors of many enzymes included in the group of kinases. The two compounds presented in Figure 1 namely CHEMBL272026 (Lig1) [28] and CHEMBL410072 (Lig2) [29] represent such group of compounds. This molecules were identified as inhibitors of Cyclin dependent kinase 2 (cdk2) and potential drugs in therapy of cancer diseases [29,30,31,32,33,34]. In the chemical structure of these compounds, except for the oxindole core containing two condensed rings, many donors and acceptors of hydrogen bond can be found which are localized in the molecule core and both side chains of considered molecules. Such chemical structure of immobilized compounds requires appropriate nanostructures ensuring the possibility of occurrence of both types of impacts, namely π-stacking and hydrogen bonds. The presented requirements are met by the structures proposed by Diudea [35,36], namely the cube rhombellanes and functionalized structures obtained by addition of second layer to the molecule core connected by ester or amide bonds. The Cube rhombellane (Cube-rbl) homeomorphs (functionalized systems) contain a core created by a system of cyclic molecules connected by ether bonds, e.g., hydroxyl derivatives of cyclohexane or benzene and examples of such structures are presented in Figure 2. Often in such systems the hyper-adamantane motif is found (1, 6) created by system of tri- and di-hydroxy benzene derivatives, however in many systems an internal core is created only by cyklohexane derivatives, like for 156 molecule (2) and 308a4 core (4). The Cube-rbl homeomorphs contain also external layer created by addition of eight subunits connected by three bonds of different type, e.g., ester for 308 (3) and 396 (7) or amide 372 (5) see Figure 2, the chemical definition of all Cube-rbl homeomorphs used in this work is presented in Table 1.
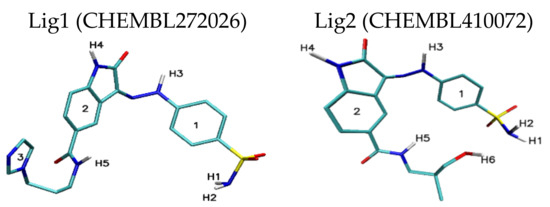
Figure 1.
Graphic representation of Lig1 and Lig2 molecules. The following colors are assigned to the chemical elements: azure—carbon; dark blue—nitrogen; red—oxygen; white—hydrogen; yellow—sulphur.
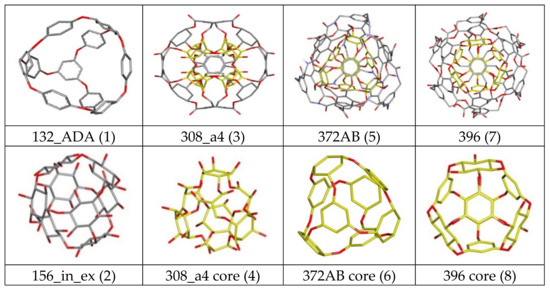
Figure 2.
Graphic representation of the cube rhombellanes (1,2) and functionalized systems (3,5,7). Systems presented in yellow colour (4,6,7) correspond to the molecular cores of functionalized systems.

Table 1.
Description of cube rhombellanes (Core) and functionalized structures with external layer (C-rbl). Value chem. type define chemical bond type characteristic for specific layer of considered molecule (core—molecular core; ex. shell—external layer of the nanomolecule).
2. Methods
The structures of ligand molecules used during docking stage, namely CHEMBL272026 ((3Z)-N-[3-(1H-Imidazol-1-yl)propyl]-2-oxo-3-[(4-sulfamoylphenyl)hydrazono]-5-indolinecarboxamide) (Lig1) and CHEMBL410072 ((3Z)-N-(3-Hydroxy-2,2-dimethylpropyl)-2-oxo-3-[(4-sulfamoylphenyl)hydrazono]-5-indolinecarboxamide) (Lig2), were obtained from CHEMBL Database [37]. The structures of nanomolecules, namely Cube Rhombellane homeomorphs, were made by Topo Cluj Group [35,36], the C60 structure was downloaded from Brookhaven Protein Database PDB [38]. The docking procedure was realized with use of AutoDockVina [39]. All structures used during docking stage, specifically ligands and nanoparticles, contain only polar hydrogen atoms which can participate in hydrogen bond creation. For all considered nanocarriers there were established the grid box dimensions equal to 26 × 26 × 26 Å and the chosen values ensure free interactions of ligand molecules with each fragment of nanostructure surface. All molecules used during docking procedure were processed with use of AutoDock Tools package [40]. During calculations the scoring function was applied with exhaustiveness parameter equal 20, further increase of this value did not contribute to the increase of the reproducibility of the results obtained for calculations realized with different values of random seed. The structural analysis of obtained complexes, related with identification of hydrogen bonds and distances between aromatic systems, were realized with use of VMD package [41].
3. Results and Discussion
The affinities of considered oxindole derivatives namely Lig1 and Lig2 toward chosen nanostructures were estimated with use of docking procedure. All obtained values are presented in Table 2 and Table 3. Both considered ligand structures exhibit similar affinity relative to reference structure fullerene C60, expressed by values equal to −5.6 and −5.3 kcal/mol. The observed difference in presented values correlates with the number of cyclic systems interacting with fullerene surfaces. All ligands conformations obtained for this nanostructure are characterized by high energetic and structural similarity, what confirms slight differences of binding affinity values (ΔGmax/ΔGmin/ΔGavrage) characterizing considered population of analyzed complexes. The values obtained for the C60 structure were used to assess the suitability of other nanostructures as nanocarriers for the considered ligands. In the case of both used inhibitors, it was noticed that seven nanostructures exhibit similar or significantly higher affinity compared to the reference system. Among all complexes formed by Lig1, the systems containing 360a, 372AB, 308a4, 308b4 and ADA_132 nanocarriers deserve special attention. For all mentioned complexes there is observed a noticeable increase of binding affinity values and the best manifestation of this phenomenon are the differences in values of binding constant the increase of which is in the range from 40 to 175% Table 2 relative to reference system. The complexes of C60 fullerene with molecules exhibiting pharmacological potential are mainly stabilized by stacking interactions between aromatic systems of both molecules. In the case of Lig1 molecule its structure contains three cyclic systems which exhibit affinity relative to the aromatic surface of fullerene. The graphic representation Figure 3 and values presented in Table 4 confirm that all cyclic systems of ligand molecule preserve planar orientation relative to C60 fullerene, also relatively small distances oscillating around 3.6 Å indicate on a dominant role of stacking interactions in complex stabilization. The non-homoatomic structure of the rest of nanostructures used during docking stage provides a wider range of impacts including the potential of hydrogen bonds formation. The data presented in Table 4 unambiguously show that such type of interactions plays an important role in stabilization of complexes obtained during docking procedure. The complex of Lig1 molecule and 360a nanomolecule, characterized by highest values of binding affinity (−6.20 kcal/mol), is stabilized by four hydrogen bonds involving all hydrogen bond donors present in the considered molecule, their lengths (from 2.07 to 2.73 Å) indicate on medium or weak strength of such interactions. The ligand conformation also supports the presence of stacking interactions between aromatic systems of oxindole core of molecule and fullerene nanocarrier Figure 3, which primarily confirms their mutual planar orientation and relatively short distance equal to 3.68 Å. In the case of the rest of chosen complexes, there is observed a much higher share of stacking interactions involved in their structure stabilization, which manifests through the involvement of at least two cyclic systems in interactions with rhombllane structures. The conformations of Lig1 molecules characterized by planar or quasi planar orientation of two rings distant from nanocarriers in the range from 3.41 to 4.43, caused a much smaller activity in creation of hydrogen bonds by considered molecule, what confirms not only their quantity but also measured distances, placed near the border limit of hydrogen bond length. Such situation can be observed even in the case of the second complex (372AB) characterized by binding affinity lower only by 0.1 kcal/mol relative to 360a system. The important factor describing the quality of binding with nanocarrier is the difference between energy values characterizing all system conformations obtained during docking stage. In the case of best structure, the difference between best and worst conformation does not exceed 0.3 [kcal/mol] (0.18 relative to the average), while in the case of the second system this difference increases to 0.6 [kcal/mol] (0.43 relative to the average). In the case of Lig2 molecule, the systems formed with 360a, 308a4, 308b4, 372AB and 396 nanocarriers are of particular interest. All mentioned systems exhibit large increase of binding constant relative to reference system placed in the range from 132 to 440%. The highest affinity, analogically like in the case of first group of complexes, was found for 360a molecule. All complexes of this nanomolecule obtained during docking stage, regardless of the ligand molecule conformation, are characterized by significantly higher energy values compared to the rest of considered systems (ΔGmax = −6.30; ΔGAVERAGE = −6.08 kcal/mol). The Lig2 inhibitor interacts with this nanocarrier through hydrogen bonds and stacking interactions. The data presented in Table 4 shows that four hydrogen atoms from Lig2 molecule are involved in creation of bonds and two of them exhibit the possibility of interaction with two different hydrogen bond acceptors. The presented distances, placed in the range from 2.09 to 2.48 Å, allow to classify them as medium and weak strength interactions. The Lig2 conformation presented in Figure 4 confirms the planar orientation of aromatic ring from oxindole core relative to the aromatic system of 360a subunit. The quite short distance between cyclic systems equals 3.66 Å and theirs mutual orientation indicates an important share of stacking interaction in complex stability. In the case of Lig2 complexes with the other four nanostructures, all best obtained systems are characterized by similar affinity value equal to −5.80 kcal/mol However, the analysis of minimal and average values presented in Table 3 allows to conclude that population of conformations obtained for 308b4 molecule is characterized by the highest cohesion of energy values. Such observation could point to better affinity of the Lig2 ligand to the surface of this nanostructure. All four complexes are stabilized by stacking interactions of one of aromatic systems, in the case of 308a4 and 396 systems from the oxindole core of molecule, while in the case of 308b4 and 372AB from the side chain. The quantity and quality of hydrogen bonds created by ligand molecule in complexes is also quite similar, however some discrepancies are observed. The Lig2 complex with 308b4 molecule is maintained by four medium strength impacts, while in the case of 396 and 372AB there are observed three and two hydrogen bonds for 308a4. In the case of 396 and 308a4 systems, there was found also one potential impact slightly exceeding border limit of hydrogen bond.

Table 2.
Values of binding affinity [kcal/mol] of Lig1 molecule relative to nanosystems obtained during docking stage.

Table 3.
Values of binding affinity [kcal/mol] of Lig2 molecule relative to nanosystems obtained during docking stage.
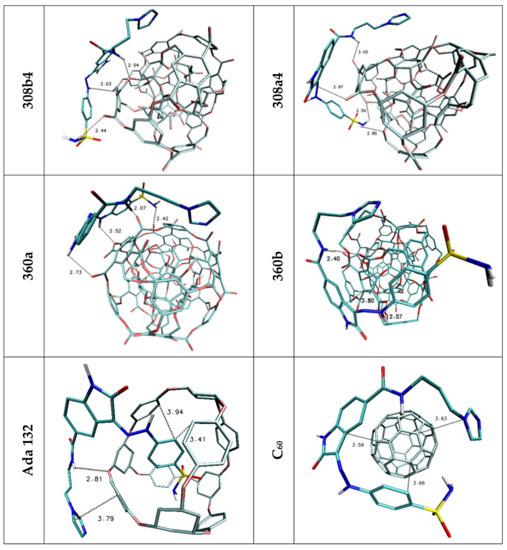
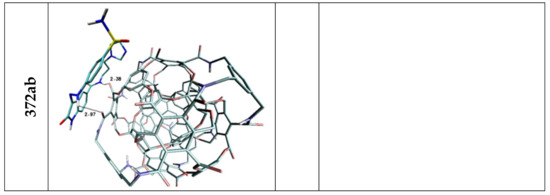
Figure 3.
Graphic representations of Lig1 complexes with nanomolecules characterized by highest values od binding enthalpy. The following colors are assigned to the chemical elements: azure—carbon; dark blue—nitrogen; red—oxygen; white—hydrogen; yellow—sulphur.

Table 4.
Distances characterizing interactions involved in stabilization of Lig1 and Lig2 complexes.
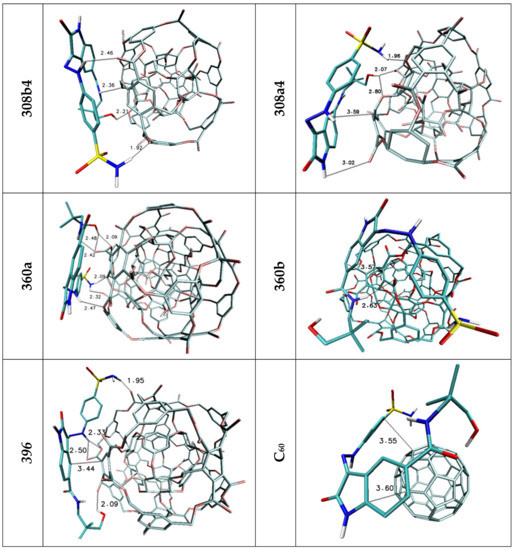
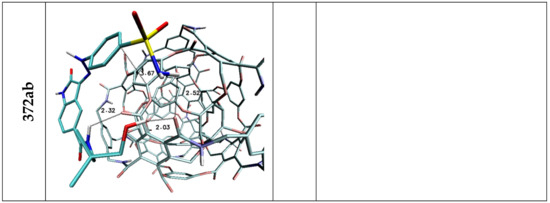
Figure 4.
Graphic representations of Lig2 complexes with nanomolecules characterized by highest values od binding entalphy. The following colors are assigned to the chemical elements: azure—carbon; dark blue—nitrogen; red—oxygen; white—hydrogen; yellow—Sulphur.
The Binding Affinity and Conformational Diversity
The cube rhombellanes and its homeomorphs exihibit conformational diversity strictly related with structure of molecule core and its symmetry obtained by use of different structural isomers of cyclohexane or changes of dihedral angles describing its mutual orientation. In Figure 5 there are presented exemplary structures of nanosystems exhibiting properties of structural isomers. The molecule 144 has two structural isomers (“ex_ex” and “in_ex”), i.e., the change of one of cyclohexane form causes the occurrence of two nanostructures exhibiting different symmetry what can be observed in Figure 5a–d. The example of structural diversity of more complex molecules are systems 360 a/b, in this case the different mutual orientation of benzene and cyclohexane rings causes the structural change of the molecular core (Figure 5f,h), which also contributed to the change of the topology of external shell of considered molecules (Figure 5e,g). Table 1 and Table 2 contain also data representing ligand affinities toward different structural conformers of considered nanocarriers. Among all considered nanostructures, two different structural forms related with changes of molecular symmetry may be found in the case 144, 156, 308 and 360 molecules. Only in one case both considered structural forms of the nanocarrier exhibit similar binding affinity towards considered ligand molecules, namely both complexes of 308 isomers are characterized by similar maximal, minimal and average values of ΔG and observed differences do not exceed −0.13 kcal/mol. Another situation is observed for conformers 144, 156 and 360. In the case of the first two molecules different symmetry (see Figure 5a–d) caused slight but noticeable changes of their affinities towards both ligands, observed differences in values are placed in the range from 0.3 to 0.5 kcal/mol and for both systems “in_ex” form is preferred. The most significant impact of structural changes related with symmetry of central core of nanomolecule is observed in the case of 360 system. The structural diversities of compared systems presented in Figure 5e,h significantly affected binding properties of these two nanocarriers toward considered ligands. All values characterizing these systems, including maximal, average and minimal binding affinities, unambiguously show belter binding properties of 360a structure and the observed differences from 0.7 to 1.2 kcal/mol indicate a low binding efficiency of ligands with the 306b structure, lower even than in the reference system C60.
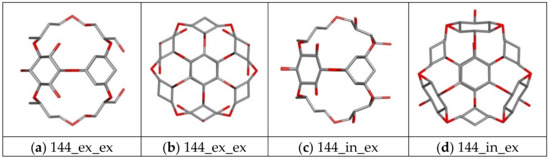
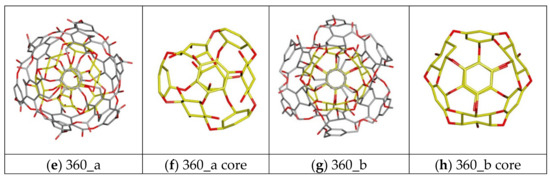
Figure 5.
Graphic representation of different symmetry of chosen nanomolecules used during docking stage. Systems presented in yellow color correspond to the different structural isomers of the molecular core of the 360 particle.
4. Conclusions
The ligands molecules considered in this work contain aromatic systems, hydrogen bond donors and acceptors. Such chemical structures indicate that the most appropriate nanocarrier should use both types of potential impacts during creation of nanocomplexes. Presented data show that properly designed nanomolecules can improve binding of molecules with pharmacological potential relative to commonly used fullerenes. Through the use of benzene or cyclohexane rings linked by ester and amide bonds, such structures of nanoparticles can be obtained that provide better affinity to the analyzed inhibitors than in the case of other fullerene systems. The confirmation of this are the complexes of both analyzed ligands with 360a molecule, characterized by up to 4–5 fold higher value of binding constant than in the case of reference structure, fullerene C60. However, not always the presence of hydrogen bond donors and acceptors in nanomolecule surface contributes to an increase of binding affinity and durability of complexes, because an equally important factor determining these features is the symmetry of the analyzed nanosystems defining the mutual distribution of aromaticity centers, as well as hydrogen donors and acceptors on the surface of the molecule. The most extreme example of such dependence are the isomers of the 360 molecule, i.e., the “a” form of this molecule proved to be the most effective in interacting with the tested ligands (−6.2/6.3 kcal/mol), while the “b” form showed worse properties than the referred fullerene (−5.5/−5.1 kcal/mol).
Author Contributions
Conceptualization, P.C.; methodology, P.C.; software, P.C.; validation, P.C.; formal analysis, P.C.; investigation, P.C.; resources, P.C. and B.S.; data curation, P.C.; writing-original draft preparation, P.C.; writing-review & editing, P.C. and B.S.; visualization, P.C.; supervision, P.C.; project administration, P.C.; funding acquisition, P.C.
Funding
This research received no external funding.
Acknowledgments
Thanks to the Professor MV Diudea for sharing the structures. This research was supported by PL-Grid Infrastructure (http://www.plgrid.pl/en).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Damascelli, B.; Patelli, G.L.; Lanocita, R.; Di Tolla, G.; Frigerio, L.F.; Marchianò, A.; Garbagnati, F.; Spreafico, C.; Tichà, V.; Gladin, C.R.; et al. A Novel Intraarterial Chemotherapy Using Paclitaxel in Albumin Nanoparticles to Treat Advanced Squamous Cell Carcinoma of the Tongue: Preliminary Findings. Am. J. Roentgenol. 2003, 181, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Cascone, M.G.; Lazzeri, L.; Carmignani, C.; Zhu, Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J. Mater. Sci. Mater. Med. 2002, 13, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, A.K. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J. Control. Release 2004, 99, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.M.; Baik, D.J.; Kang, J.S. Toxic characteristics of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials 2003, 24, 55–63. [Google Scholar] [CrossRef]
- Alyautdin, R.N.; Petrov, V.E.; Langer, K.; Berthold, A.; Kharkevich, D.A.; Kreuter, J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated poly(butylcyanoacrylate) nanoparticles. Pharm. Res. 1997, 14, 325–328. [Google Scholar] [CrossRef]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, A.; Wirth, M.; Gabor, F. WGA-grafted PLGA-nanospheres: Preparation and association with Caco-2 single cells. J. Control. Release 2004, 99, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Da Ros, T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Berlin, Germany, 2008; ISBN 9781402068454. [Google Scholar]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Andrievsky, G.; Klochkov, V.; Derevyanchenko, L. Is the C60 Fullerene Molecule Toxic?! Fuller. Nanotub. Carbon Nanostructures 2005, 13, 363–376. [Google Scholar] [CrossRef]
- Szefler, B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int. J. Nanomed. 2018, 13, 6143–6176. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, R.R.; Prylutska, S.V.; Chumakl, V.V.; Skorokhyd, N.R.; Lehka, L.V.; Evstigneev, M.P.; Prylutskyy, Y.I.; Berger, W.; Heffeter, P.; Scharff, P.; et al. Application of C60 Fullerene-Doxorubicin Complex for Tumor Cell Treatment In Vitro and In Vivo. J. Biomed. Nanotechnol. 2015, 11, 1139–1152. [Google Scholar] [CrossRef]
- Morgen, M.; Bloom, C.; Beyerinck, R.; Bello, A.; Song, W.; Wilkinson, K.; Steenwyk, R.; Shamblin, S. Polymeric Nanoparticles for Increased Oral Bioavailability and Rapid Absorption Using Celecoxib as a Model of a Low-Solubility, High-Permeability Drug. Pharm. Res. 2012, 29, 427–440. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef]
- Turov, V.V.; Chehun, V.F.; Barvinchenko, V.N.; Krupskaya, T.V.; Prylutskyy, Y.I.; Scharff, P.; Ritter, U. Low-temperature 1H-NMR spectroscopic study of doxorubicin influence on the hydrated properties of nanosilica modified by DNA. J. Mater. Sci. Mater. Med. 2011, 22, 525–532. [Google Scholar] [CrossRef]
- Schuetze, C.; Ritter, U.; Scharff, P.; Fernekorn, U.; Prylutska, S.; Bychko, A.; Rybalchenko, V.; Prylutskyy, Y. Interaction of N-fluorescein-5-isothiocyanate pyrrolidine-C60 with a bimolecular lipid model membrane. Mater. Sci. Eng. C 2011, 31, 1148–1150. [Google Scholar] [CrossRef]
- Qiao, R.; Roberts, A.P.; Mount, A.S.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Bilyy, R.; Overchuk, M.; Bychko, A.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.G.; Ritter, U. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J. Biomed. Nanotechnol. 2012, 8, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grynyuk, I.I.; Grebinyk, S.M.; Matyshevska, O.P.; Prylutskyy, Y.I.; Ritter, U.; Siegmund, C.; Scharff, P. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materwiss. Werksttech. 2009, 40, 238–241. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The Biological Mechanisms and Physicochemical Characteristics Responsible for Driving Fullerene Toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [CrossRef]
- Evstigneev, M.P.; Buchelnikov, A.S.; Voronin, D.P.; Rubin, Y.V.; Belous, L.F.; Prylutskyy, Y.I.; Ritter, U. Complexation of C60 Fullerene with Aromatic Drugs. Chem Phys Chem 2013, 14, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Czeleń, P. Molecular dynamics study on inhibition mechanism of CDK-2 and GSK-3β by CHEMBL272026 molecule. Struct. Chem. 2016, 27, 1807–1818. [Google Scholar] [CrossRef]
- Czeleń, P. Inhibition mechanism of CDK-2 and GSK-3β by a sulfamoylphenyl derivative of indoline—A molecular dynamics study. J. Mol. Model. 2017, 23, 230. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Canavese, M.; Santo, L.; Raje, N. Cyclin dependent kinases in cancer. Cancer Biol. Ther. 2012, 13, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Child, E.S.; Hendrychov, T.; McCague, K.; Futreal, A.; Otyepka, M.; Mann, D.J. A cancer-derived mutation in the PSTAIRE helix of cyclin-dependent kinase 2 alters the stability of cyclin binding. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Diudea, M.V.; Lungu, C.N.; Nagy, C.L.; Diudea, M.V.; Lungu, C.N.; Nagy, C.L. Cube-Rhombellane Related Structures: A Drug Perspective. Molecules 2018, 23, 2533. [Google Scholar] [CrossRef] [PubMed]
- Szefler, B.; Czeleń, P.; Diudea, M. V Docking of indolizine derivatives on cube rhombellane functionalized homeomorphs. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 7–18. [Google Scholar] [CrossRef]
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 1 March 2016).
- Kim, K.H.; Ko, D.K.; Kim, Y.T.; Kim, N.H.; Paul, J.; Zhang, S.Q.; Murray, C.B.; Acharya, R.; Kim, Y.H.; DeGrado, W.F.; et al. Protein-directed self-assembly of a fullerene crystal. Nat. Commun. 2016, 7, 11429. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Potemkin, V.A.; Grishina, M.A.; Belik, A.V. A Method for Multiconformational Modeling of the Three-Dimensional Shape of a Molecule. J. Struct. Chem. 2002, 43, 1033–1039. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).