Abstract
Classic Cu–O coordination bonds in 1 or elongated semi-coordination ones in 2 and 3 were applied to construct CuII–4f complexes composed of trinuclear subunits linked through μ-NO3− ions with formulae given as [Cu2Tm(H2tehy)2]2(NO3)6·H2O, (1), {[Cu2Ho(H2tehy)2(NO3)2][Cu2Ho(H2tehy)2(H2O)2]}(NO3)4·2H2O, (2), and {[Cu2Er(H2tehy)2(H2O)]2([Cu2Er(H2tehy)2(NO3)]2}(NO3)10·2H2O·4CH3OH, (3), where H2tehy = C19H20N2O4 is a tetrahydroxy Schiff base ligand. Topological analysis showed that the same characteristic motif of coordination accompanied by hydrogen bonds involving the uncoordinated nitrate oxygen atom and ligand’s phenoxy O atoms is responsible for linking trinuclear subunits into a hexanuclear one as well as for bridging the hexanuclear coordination units in 3 into a 1D supramolecular polymer, with the Cu–O distance being 3.19(1) Å, much longer than the limit of a semi-coordination bond (3.07 Å). The Cambridge Structural Database was used to discuss issues of crystallographic criteria (distance and angular preferences) for the assessment of the stabilizing or destabilizing effect of hydrogen bonding on coordination. The presented results show that the symmetrically repeated arrangement of molecules may provide a useful tool for identifying higher order non-covalently bonded supramolecular aggregates. The complexes 1–3 have been characterized by X-ray diffraction, FTIR, and thermal analysis. The magnetic studies indicated the ferromagnetic interaction between CuII and HoIII ions.
1. Introduction
Symmetry and asymmetry are the fundamental features of mater in solids and liquids that help in the understanding of many physical phenomena and chemical reactions. Symmetry is often a useful clue for the recognition of supramolecular synthons.
The application of non-covalent interactions in crystal engineering is a thoroughly studied research area, among them hydrogen bonds [1,2,3,4,5,6,7], halogen bonds [8,9,10], dipole⋯dipole [11,12,13,14], and stacking [15,16,17,18,19,20] interactions should be mentioned. Such non-covalent interactions provide directional and stabilizing contacts that can be used successfully in the design of coordination compounds [21]. Additionally, there are also reports showing that hydrogen bonding can act as a pathway for magnetic interaction between metal ions [1,22,23,24,25,26].
The coordination sphere plasticity for hexacoordinated copper(II) ions is known as the Jahn–Teller effect. This distortion from ideal symmetry usually exhibits as an elongation of two axial bonds in the tetragonal bipyramid polyhedron of the complex. The Jahn–Teller deformations are believed to be stabilized in the solid state by, e.g., hydrogen bonds. The observed crystal elongation has led to the introduction of a term of a “semi-coordination bond” by Brown et al. in 1967 [27]. The theoretical studies performed by Valach et al. resulted in the calculation of the limit of a semi-coordination bond being of 3.07 or 3.04 Å for the Cu–O bond located at the elongated out-of-plane axis [28,29]. Valach also found that transition from the bonded to non-bonded state in the elongated metal–ligand direction occurs discontinuously [28].
There are some interesting reports on semi-coordinated bonds found in crystal structures of CuII cations staying within this Cu–O distance limits [29,30,31,32]. The strength of metal–ligand bonds depends on the bond length [33]. However, Nelyubina et al. proved that even very long and weak interatomic contacts (Cu⋯O 3.6 Å, 0.5 kcal/mol) may mediate magnetic super-exchange pathways [34]. On the other hand, non-covalent interactions have proved to be effective in the design of supramolecular polymers of exceptional properties [35,36,37,38].
The nitrate anion is often used in crystal engineering as a counterion since it has three oxygen atoms ready to form both coordinative and/or hydrogen bonds. However, the NO3− anion generally links the neighboring copper centers by two oxygen donors independently [24]. The hydrogen bonds are usually formed with the non-coordinated nitrate oxygen. A recurrent structural motif based on nitrate ions being the interplay between a coordination or semi-coordination bond and bifurcated hydrogen bonds has been found in the presented structures 1–3 ([Cu2Tm(H2tehy)2]2(NO3)6·H2O, (1), {[Cu2Ho(H2tehy)2(NO3)2][Cu2Ho(H2tehy)2(H2O)2]}(NO3)4·2H2O, (2), and {[Cu2Er(H2tehy)2(H2O)]2([Cu2Er(H2tehy)2(NO3)]2}(NO3)10·2H2O·4CH3OH, (3), where H2tehy = C19H20N2O4 is a doubly deprotonated tetrahydroxy Schiff base ligand and in previously reported ones [39,40] (Scheme 1). The topologically analogous supramolecular arrangement has been found in 3, but this time linking not only the trinuclear subunits into hexanuclear ones but also the hexanuclear ones into a linear supramolecular aggregate, with a much longer Cu⋯Onitrate distance (3.19(1) Å) which is above the limit of a semi-coordination bond (3.07 Å) [28] but still shorter than the distance where a very weak interaction has been reported by Nelyubina et al. (3.6 Å) [34].

Scheme 1.
(a) Structure of H4tehy ligand and (b) a building motif found in 1–3; green dotted lines—out-of-plane Cu–O semi-coordination bonds at the apical positions; blue dashed lines—hydrogen bonds.
The series of CuII–4f complexes presented here formed by a tetrahydroxy compartmental Schiff base ligand H4tehy (where H4tehy = C19H22N2O4) and nitrate anions offers an interesting object to study the influence of hydrogen bonding on the coordination and formation of a supramolecular network.
We present here the synthesis, crystal structure, FTIR spectra, and thermal and magnetic properties of complexes 1–3 where topological similarities are discussed in terms of crystallographic distance limit of a Cu–O semi-coordination bond and the cooperative or competitive nature of coexisting coordination and hydrogen bonds.
2. Materials and Methods
2.1. Materials
Starting materials: 2,3-dihydroxybenzaldehyde, 2,2-dimethyl-1,3-propanediamine, Cu(CH3COO)2·H2O, Ho(NO3)3·5H2O, Er(NO3)3·5H2O, Tm(NO3)3·5H2O, and CH3OH were purchased from commercially available sources (Sigma Aldrich) and were used without further purification. The Schiff base ligand 3-[[3-[(2,3-dihydroxyphenyl)methylideneamino]-2,2-dimethylpropyl]iminomethyl]benzene-1,2-diol (H2tehy); C19H22N2O4) was prepared as described in the literature [41,42].
2.2. Synthesis of Complexes
The heteronuclear compounds 1–3 were synthesized as follows: To 30 mL of methanolic solution of 0.1368 g Schiff base ligand (0.4 mmol) was added dropwise 10 mL of methanolic solution of 0.0799 g Cu(CH3COO)2·H2O (0.4 mmol,) giving a green mixture. After 30 minutes the freshly prepared solution (5 mL) of Ho(NO3)3·5H2O (0.2 mmol, 0.0882 g), Er(NO3)3·5H2O (0.2 mmol, 0.0887 g) or Tm(NO3)3·5H2O (0.2 mmol, 0.0890 g) was slowly added to the constantly stirred suspension. The resulting deep green solution was stirred for another 30 min. A small amount of precipitate was filtered off, and the reaction mixture was left undisturbed at 4 °C. Slow evaporation yielded green crystals suitable for X-ray crystal structure analysis.
[Cu2Tm(H2tehy)2]2(NO3)6·H2O (1): empirical formula C76H82N14O35Cu4Tm2, molecular weight 2343.57 g/mol. Yield 42%. Analytical data (%), Calcd: C, 38.95; H, 3.53; N, 8.37; Cu, 10.85; Tm, 14.42. Found: C, 38.50; H, 3.20; N, 8.40; Cu, 10.40; Tm, 14.00.
{[Cu2Ho(H2tehy)2(NO3)2][Cu2Ho(H2tehy)2(H2O)2]}(NO3)4·2H2O (2): empirical formula C76H88N14O38Cu4Ho2, molecular weight 2389.62 g/mol. Yield 38%. Analytical data (%), Calcd: C, 38.20; H, 3.71; N, 8.21; Cu, 10.64; Ho, 13.80. Found: C, 38.00; H, 3.50; N, 8.00; Cu, 10.30; Ho, 13.50.
{[Cu2Er(H2tehy)2(H2O)]2([Cu2Er(H2tehy)2(NO3)]2}(NO3)10·2H2O·4CH3OH (3): empirical formula C156H184Cu8Er4N28O76, molecular weight 4844.66 g/mol. Yield 30%. Analytical data (%), Calcd: C, 38.67; H, 3.83; N, 8.10; Cu, 10.49; Er, 13.81. Found: C, 39.00; H, 3.20; N, 8.40; Cu, 10.00; Er, 13.40.
2.3. Methods
The elemental CHN analysis was performed using a CHN 2400 Perkin Elmer analyser. The contents of metals (copper, holmium, erbium, and thulium have been determined using ED XRF spectrophotometer (Canberra−Packard). The FTIR spectra of compounds in KBr pellets were recorded in the range of 4000 to 400 cm−1 on the M–80 spectrophotometer (Carl Zeiss Jena). Thermal analyses of 1–3 and H2tehy were conducted under air flow in the temperature range of 20 to 1000°C (1–3) and 20 to 700°C (H2tehy) at a heating rate of 10 °C·min−1 by the thermogravimetric (TG) and differential scanning calorimetry (DSC) methods with the use of the SETSYS 16/18 analyser (Setaram). The samples of 7.72 mg (1), 7.88 mg (2), 5.76 mg (3) were heated in Al2O3 crucibles. The XRD powder diffractograms of the decomposition products were collected at room temperature on an Empyrean PANanalytical automated powder diffractometer (CuKα radiation λ = 1.54187 Å) in the 2θ range of 20 to 90°. The magnetic susceptibility for finely ground crystalline samples was measured over the temperature range of 1.8 to 300 K at magnetic field 0.1 T using a Quantum Design SQUID-VSM magnetometer. The field dependences of magnetization were investigated at 2 K in the applied field up to 5 T and were corrected by subtracting the sample–holder signal and contribution χD estimated from Pascal’s constants [43].
Diffraction intensities for 1–3 were measured on SuperNova X-ray diffractometer (with Atlas S2 CCD detector and the mirror-monochromatized CuKα radiation (λ = 1.54184 Å) at 294 K for 1 and at 120 K for 2–3, using the ω scan technique. The CrysAlis CCD and CrysAlis Red programs [44] have been applied for data collection, cell refinement, and data reduction. The molecular models were found by direct methods using SHELXS-97 and refined on F2 by the full-matrix least-squares using the SHELXL-97 implemented in OLEX2 [45,46]. Non-hydrogen atoms (except the disordered part of the ligand molecule in 3 and selected nitrate and water molecules in 1–3) were refined anisotropically. The 2,2-dimethylpropyl bridge in 3 is disordered over two positions with site occupation factors (sof’s) of the major part being of 0.6. The structure of 3 has been refined in a noncentrosymmetric space group as a two-component twin with the following twin law (−1 0 0, 0 1 0, 0 0 −1) (two-fold rotation about b axis with inversion twinning). The refined twin population parameter BASF (fractional contribution of twin domain calculated on batch scale factors) was 0.42. The conditions for the data collection and the crystal structure refinement parameters are shown in Table 1. The drawings were made in Mercury and Diamond software [47,48]. The experimental details and final atomic parameters for 1–3 were deposited with the Cambridge Crystallographic Data Centre as supplementary material (CCDC ID 1901698–1901700). Copies of the data can be obtained free of charge on request via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk.

Table 1.
Crystal data and structure refinement details for 1–3.
3. Results and Discussion
3.1. Molecular and Crystal Structure of Complexes 1–3
Compounds 1–3 were obtained by the same method of synthesis giving, however, crystal structures of different symmetry. The complexes show subtle differences in the coordination architecture and in the degree of Jahn–Teller distortion of CuII ions.
3.2. Hexanuclear Complex of 1
Compound 1 form crystals in the monoclinic space group (C2/c) with half of the coordination unit being symmetrically independent (Table 1). The asymmetric part consists of a trinuclear CuII–TmIII–CuII core linked by a bridging nitrate anion (lying on the two-fold axis) to the other half resulting in a hexanuclear coordination entity with the Cu2–O10 coordination bond length being 2.594(5) Å (Figure 1 and Figure 2; Table 2 and Table 3). This characteristic motif is supplemented by two O4–H4⋯O9 hydrogen bonds with a bifurcated acceptor O atom (Table 4). A similar characteristic arrangement of two trinuclear subunits linked by a bridging NO3− ion and hydrogen bonds has been discussed in structures of 2 and 3.

Figure 1.
View at the asymmetric unit in 1 (left) and view at the hexanuclear unit repeated by a two-fold axis (right) linked by semi-coordination bond Cu2–O10 and hydrogen bonds (marked with dashed lines).

Figure 2.
Scheme of coordination unit in complex 1 with Cu–O and O⋯O distances in angstrom (Å), the position of two-fold axis is marked, the two halves are related by a two-fold axis, green dotted lines—semi-coordination Cu–O bonds at the apical positions, red dash–dotted lines—contact behind the limit of a semi-coordination bond but topologically important, blue dashed lines—hydrogen bonds, * nitrate anion disordered other three positions (sof’s 0.33), ** disorder of water and nitrate anion (sof’s 0.5).

Table 2.
Selected bond lengths and Cu⋯O contacts in angstrom (Å) for crystals 1–3.

Table 3.
Selected bond angles in degrees for 1–3.

Table 4.
Selected hydrogen bonds for 1–3.
The smaller N2O2 compartments are occupied by CuII cations. Cu1 ion has the coordination number of five or six because one of the monodentate NO3− anions is disordered over three positions. The Cu1⋯O16/O16A/O16B distance varies from 2.66(1) to 3.57(1) Å (Table 2), which differentiates the character of the interatomic contact (semi-coordination or non-bonding). The nitrate ion may be bonded directly to Cu1 cation by a coordinative bond, or it can act as a hydrogen bond acceptor in O8–H8⋯O14 interaction (Figure 1 and Figure 2; Table 4). Cu2 cation is penta-coordinated. The apical position of the polyhedron is occupied by a bridging nitrato ion with Cu2–O10 bonds being of 2.594(5) Å. The Cu2 ion has also a long contact, above the limit of a semi-coordination bond (Cu2⋯O11 3.28(2) Å), to a nitrate anion hydrogen bonded through the same O11 atom to the phenoxy group (O3–H3⋯O11 interaction). The TmIII ion is octa-coordinated by two O4 cavities of the perpendicularly oriented Schiff base ligands. In the crystal structure, there is only one uncoordinated solvent molecule—water linked strongly by a hydrogen bond to the H2tehy ligand (Table 4).
3.3. Dimer of Trinuclear Cores Linked by a Semi-Coordination Bond in 2
The − coordination unit in the monoclinic crystal 2 (P21/c) is built of two symmetrically independent trinuclear CuII–HoIII–CuII coordination moieties linked by a bidentate nitrate ligand into one hexanuclear entity (Figure 3 and Figure 4). However, the distances between the O nitrate atom and CuII centers are different: 2.488(5) and 3.031(5) Å for Cu2–O24 and Cu3–O25 pairs of atoms, respectively. The first distance may be regarded as a classic coordination bond, whereas, the other one is very long but still within the limit of a semi-coordination bond (3.07 Å). Additionally, the coordination motif is again accompanied by two bifurcated hydrogen bonds O3–H3⋯O23 and O16–H16⋯O23 to the non-coordinated nitrate O atom, similar to 1. The hydrogen bonding seems to compete with the coordination bond causing the elongation of the Cu3–O25 distance above the conventional coordinative bond limit, but it seems to stabilize the whole motif. Because of the topological similarities between structures 1 and 2, the Cu3–O25 bond has been recognized as a semi-coordinative one, and the structure of 2 as a hexanuclear one.
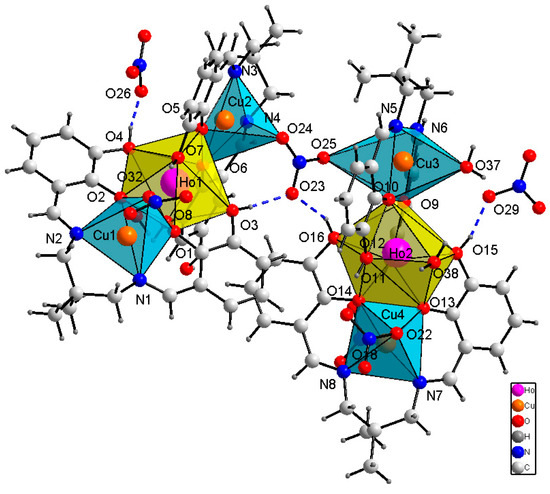
Figure 3.
Coordination polyhedra and selected hydrogen bonds (dashed lines) in 2.
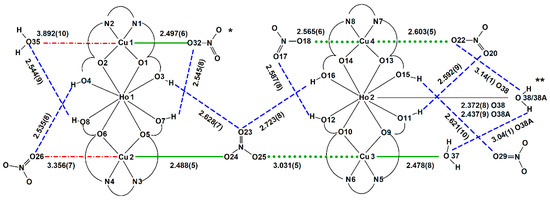
Figure 4.
Scheme of coordination in complex 2 with Cu–O and O⋯O distances in angstrom (Å), green bold lines—coordination bonds Cu–O at the apical positions, green dotted lines—Cu–O semi-coordination bonds at the apical positions, red dash–dotted lines—contacts behind the limit of a semi-coordination bond but topologically important, blue dashed lines—hydrogen bonds, * nitrate anion disordered over two positions (sof’s 0.5); ** water molecule disordered other two positions (sof’s 0.6:0.4).
The CuII ions occupy the N2O2 cavities of the doubly deprotonated H2tehy ligand. Cu1 and Cu2 cations have a coordination number (CN) of five. The apical position of tetragonal pyramids occupies NO3− ions. The contacts between atoms Cu1⋯O35 (3.89(1) Å) and Cu2⋯O26 (3.356(7) Å) are much longer than coordinative ones. Additionally, the same nitrate O atom is involved in hydrogen bonding (Figure 4, Table 4). Cu2 is linked by the nitrato bridge to Cu3 ion. The non-bonding nitrate O23 atom accepts two bifurcated hydrogen bonds closing the characteristic building motif. Cu3 (CN = 6) supplements its coordination sphere with a water molecule (Cu3–O37 2.475(8) Å). Cu4 (CN = 6) coordinates two monodentate NO3− ions. The Ho1 ion has the same coordination environment as Tm1 in 1, whereas, Ho2 is nona-coordinated. Except for two perpendicularly located H2tehy ligands, there are two other positions which are disordered water molecules. This results in the change in conformation of the Schiff base ligands, which adopt a bent conformation instead of nearly planar as in 1. The remaining uncoordinated nitrate anions and water molecules interact through many hydrogen bonds (Table 4).
3.4. Supramolecular Polymer Built of Hexanuclear Monomers in 3
Compound 3 crystallizes in the orthorhombic Pnc2 space group with two types of symmetrically independent hexanuclear coordination units composed of trinuclear subunits A and B linked by the NO3− ions into AA and BB entities (Figure 5 and Figure 6).
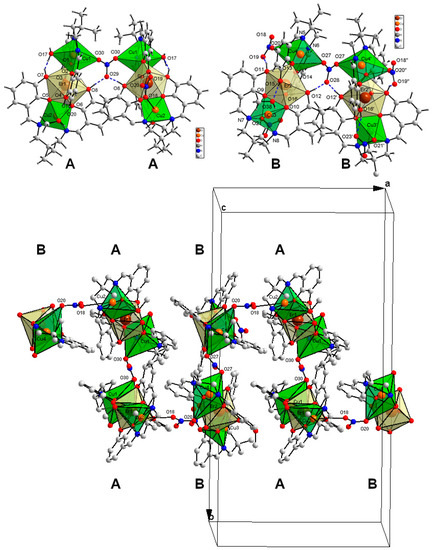
Figure 5.
Hexanuclear coordination units composed of trinuclear subunits A and B in 3 (top) and the suggested supramolecular polymeric chain AA–BB–AA–BB in 3 (bottom); symmetry codes: ’ −x, 1 − y, z; ’’ 1 − x, 1 − y, z.
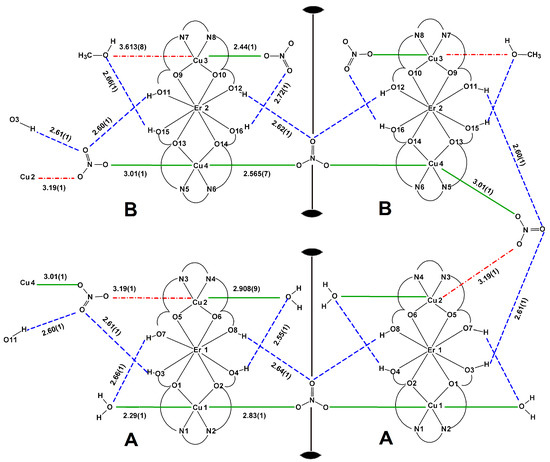
Figure 6.
Scheme of coordination in complex 3 with Cu–O and O⋯O distances in Å, the position of two-fold axes is marked, green bold lines—coordination bonds Cu–O at the apical positions, green dotted lines—Cu–O semi-coordination bonds at the apical positions, red dash–dotted lines—contacts behind the limit of a semi-coordination bond but topologically important, blue dashed lines—hydrogen bonds.
In a topological sense, compound 3 may be regarded as a 1D supramolecular polymer AA–BB–AA–BB running as a folded chain along the a axis with hexanuclear entities AA and BB linked by an elongated semi-coordinative bond (Cu4–O20 3.01(1) Å) and by a long contact of the same topology but probably of a different nature of interaction (Cu2–O18 3.19(1) Å). Each of these semi-coordination bonds or contacts is accompanied by a specific array of hydrogen bonds forming the characteristic motif found in 1 and 2 (Figure 6).
The assumption of an attractive character of this long contact is based on topological similarities of the recurrent supramolecular motif found in 1–3 and on the results of studies performed by Nelyubina et al. [34]. The stabilizing effect is provided by the accompanying hydrogen bonds O3–H3⋯O19, O11–H11⋯O19, and O11–H11⋯O20 (Table 4), wherein it is important that the hydrogen bonding occurs through the non-coordinated O nitrate atoms. The ErIII cation is octa-coordinated as TmIII in 1. Cu1 (CN = 6) has H2O molecule in the apical position and μ-NO3− ion at the other apex of a tetragonal bipyramid. The nitrate anion links by a semi-coordination bond (Cu1–O30 2.83(1) Å) two related by a two-fold rotation Cu1 ions into a hexanuclear unit. The apical ligands of Cu2 ion (CN = 6) are one methanol molecule and one NO3− anion with a very long Cu2–O18 contact distance (3.19(1) Å) bridging to Cu4 cation. The Cu4 (CN = 6) is linked further to a repeated by a two-fold axis to another Cu4 ion. Cu3 (CN = 5) ion coordinates one monodentate nitrate ion. The Cu3⋯O38 (3.613(8) Å) contact was not identified as a coordinative because of the long distance and a competitive influence of O15–H15⋯O38 hydrogen bond (interaction to the same Onitrate atom).
3.5. The Geometric Criteria for a Semi-Coordination Bond on the Base of CSD Search
For the search of Cambridge Structural Database (CSD, ver. 5.40 Nov. 2018) the Conquest software (ver. 2.0.0.) was used, the restrictions were as follows R values <5%, no disorder, no powder structures [47]. The search for Cu⋯O–NO2 distances up to 4 Å without determining the type of bonding or contact gave 1068 results. The number of occurrences of a given Cu–O distance (Figure 7) clearly confirm the Jahn–Teller distortion: 1) the first sharp peak with the maximum at ca. 1.9 Å corresponds to the shorter in-plane coordination bonds, 2) the next population of longer Cu–O distances has a wider range with a lower maximum at ca. 2.5 Å (Cu–O distance to the out-of-plane ligands). Next, the number of occurrence of Cu⋯O contacts seems to be nearly constant from three until ca. 3.5 Å, where it starts to grow with a small increase at ca. 3.1 Å. From the crystallographic point of view, this plot does not indicate any sharp border between coordination and semi-coordination bonding nor the sharp border of the second one.
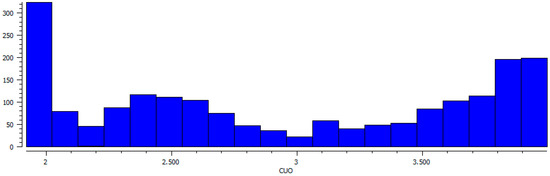
Figure 7.
Distribution of Cu⋯O distance values up to 4 Å found in Cambridge Structural Database (CSD).
The plot of Cu⋯O–N(O2) angle shows three maxima at ca. 7, 75, and 115° (Figure 8). The highest maximum at ca. 115° corresponds to the optimal orientation of oxygen lobes toward the positive charge of the Cu center in coordination bonding, the peaks at ca. 7 and 75° are related to the angles between Cu and the non-interacting nitrate oxygen atoms.
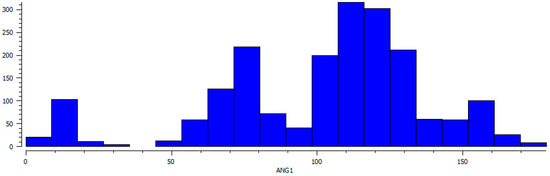
Figure 8.
Distribution of Cu⋯O–N(O2) angle values (ANG1 in °) found in CSD.
It shows a clear directional preference in the orientation of nitrate ions toward the Cu metal centers (semi-coordination Cu⋯O–N(O2) angle in the range of ca. 100 to 140°). It is even more pronounced at the scatter plot of Cu⋯O–N(O2) angle vs Cu⋯O distance values (Figure 9). The directionality is sustained until ca. 3.65 Å and only above this distance limit does the distribution of Cu⋯O–N(O2) valence angles start to be more random.
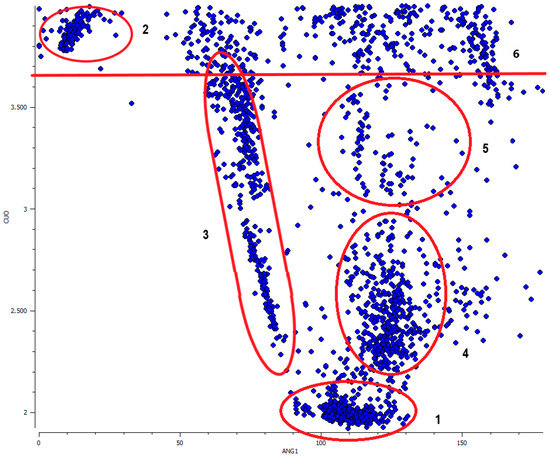
Figure 9.
Scatter plot of Cu⋯O–N(O2) angle (ANG1 in °) vs Cu⋯O distance (CUO in Å) up to 4 Å with marked six regions described in the text.
At the scatter plot (Figure 9) six groups of data can be distinguished: (1) the group of classic coordination bond to the in-plane ligands with short Cu⋯O distance (up to ca. 2.2 Å) and Cu⋯O–N(O2) angle being in the range of 90 to 130°; (2) and (3) are clearly distinctive groups with low valence angles 5 to 10° and 60 to 90° and ranges of Cu⋯O distances above 3.65 Å and 2.3–3.7 Å, respectively. They correspond to the non-bonding contacts to the neighborly located oxygen atoms within the same coordinated NO3− molecule; (4) the elongated coordination or semi-coordination bond to the out-of-plane ligands (Cu–O bond ca. 2.3–3 Å, Cu⋯O–N(O2) angle ca. 110–140°); (5) the extended area for very long interactions of a different character but still with a high degree of directionality (Cu⋯O distance ca. 3–3.65 Å, Cu⋯O–N(O2) angle ca. 100–150°), could be probably hydrogen bond assisted coordination bonds. Additionally, in this group, if the regarded Cu⋯O–N(O2) contact was not included as a coordination, nearly all of the copper(II) centers were tetra- or penta-coordinated and the nitrate anion was located at the “free” non-coordinated side of the central ion closing, at least in a topological sense, the coordination sphere of copper; 6) Group of contacts above 3.65 Å showing no specific directional preferences. A similar distribution of Cu–O distances and Cu–O–X(O2) angles was observed in the group of other oxo complexes, where X was C or N; 7226 data, Figure S1 in Supplementary Materials).
3.6. Infrared Spectra
The obtained CuII–4f complexes show similar FTIR spectral features (Figure 10 and Figures S2–S4). A strong band at 1620 cm−1 from ν(C=Nimine) is red-shifted by 20 cm−1 in comparison to the free H4tehy ligand indicating a decrease of the C=N bond order because of the formation of the coordination bond to the copper(II). The broad band with a maximum at ca. 3400 cm−1 comes from ν(O–H) stretching vibrations of coordinated and/or solvated water/methanol molecules and from the undeprotonated hydroxyl groups of the H2tehy ligand. The strong phenolic ν(C–O) stretching vibration band was observed at 1240 cm−1 for free H4tehy ligand, whereas, in complexes there was a doublet with peaks at 1252 and 1220 cm−1, confirming the coordination through these groups [49,50,51,52,53].
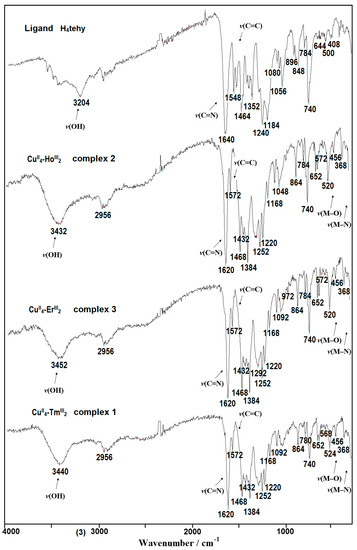
Figure 10.
FTIR spectra of the ligand H4tehy and its complexes 1–3.
3.7. Thermal Properties
The TG, DTG, and DSC curves of 1–3 (Figure 11 and Figures S5–S7) show that the CuII–4f compounds are stable at room temperature. During heating to ca. 100 °C the complexes 1–3 desolvate with a mass loss found 1.20% (1), 2.00% (2), 3.60% (3); calculated 0.80% (1), 1.50% (2), 3.40% (3).

Figure 11.
Thermogravimetric (TG) curves of complexes 1–3 in air.
The DSC curves indicate a small endothermic effect. The decomposition process of the CuII–4f compounds was intricate, and the intermediate solid products were hard to distinguish. The final decomposition products of 1–3 were mixtures of metal oxides CuO and Ho2O3/Er2O3/Tm2O3 (calculated from TG curves and verified experimentally by powder XRD patterns (Figure S8). The calculated TG curves percentages (29.40% (1), 31.00% (2), 31.50% (3)) coincided with the theoretical values 30.00% (1), 28.90% (2), 30.00% (3).
3.8. Magnetic Properties
The plots of temperature-dependent molar susceptibility (χMT versus T) were shown in Figure 12, where χM is the molar magnetic susceptibility, and T is the absolute temperature. The magnetic properties of the complexes result from the interplay between three factors: the CuII⋯CuII and CuII–LnIII interactions, as well as the thermal population of the Stark components of the lanthanide(III) ions. The χMT values of the complexes studied at room temperature were calculated theoretically by the Equation (1) assuming four CuII and two magnetically isolated LnIII ions.
where N is Avogadro constant, β is the Bohr magneton, and k is Boltzman’s constant. In this equation gLn is the g factor of the ground J terms of LnIII and is expressed as in Equation (2):
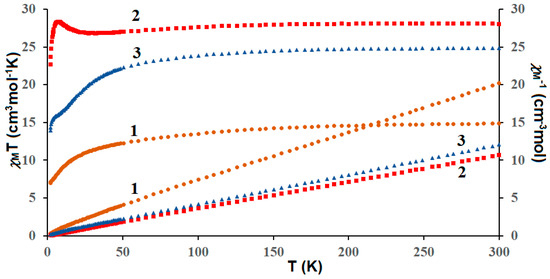
Figure 12.
Temperature dependence of experimental χMT and χM−1 versus T for CuII–4f complexes 1–3.
In CuII4–TmIII2 (1) complex the χMT value of 14.85 cm3Kmol−1 observed at 300 K corresponds to the value of 15.80 cm3Kmol−1 expected for two TmIII (3H6, S = 1, L = 5, J = 6, g = 3/2) and four CuII (S = 1/2, g = 2) magnetically isolated ions. This value steadily decreased as T was lowered to reach 6.99 cm3Kmol−1 at 1.8 K. For CuII4–HoIII2 (2) the χMT value experimentally determined at 300 K (28.04 cm3Kmol−1) is slightly lower than the value of 29.63 cm3Kmol−1 expected for two HoIII (5I8, J = 8, L = 6, S = 2, g = 5/4) and four CuII (S = 1/2, g = 2) noninteracting metal ions. With the lowering of the temperature the χmT remained constant until 120 K, then it decreased to 27.00 cm3Kmol−1 at 19 K and next increased to reach a value of 28.48 cm3Kmol−1 at 6.6 K. Finally, it showed a small decrease to 22.07 cm3Kmol−1 at 1.8 K. For the compound Cu4II–Er2III (3) the experimental value of χMT at room temperature was equal to ca. 24.83 cm3Kmol−1 corresponding to the calculated value of 24.45 cm3Kmol−1 for two uncoupled ErIII (4I15/2, S = 3/2, L = 6, J = 15/2, g = 6/5) and four uncoupled CuII ions (S = 1/2, g = 2). As shown in Figure 12, this value decreased with the lowering of the temperature to 13.93 cm3Kmol−1 at 1.8 K.
The lowering of χMT at low temperature in 1 and 3, is caused most probably by the crystal field splitting of LnIII ion, and/or a combination of the contribution of the overall antiferromagnetic interactions of metal ions. The profile of the χMT vs T curve in 2 strongly suggests the existence of two competitive phenomena. The decrease of χMT on temperature lowering was most probably caused by the depopulation of the Ho Stark sublevels, or the presence of magnetic anisotropy, or the antiferromagnetic coupling between ions, whereas, the increase of the χMT at lower temperatures may arise because of the ferromagnetic CuII−HoIII interaction.
4. Conclusions
The nuclearity of the presented complexes 1–3 was tuned by the interplay between coordination and hydrogen bonds involving nitrate anions. The recurrent array of coordination or semi-coordination bonds and hydrogen bonding presented here can be added to the crystal engineering library as a building motif. The coordination bond may be weakened and elongated until it transforms into a non-covalent interaction when a competitive influence of hydrogen bonding will occur. It is worth to underline that even at long distances (above the limit of semi-coordination bond 3.07 Å) the directionality of the Cu⋯O–NO2 contact (100–150°) may be sustained up to ca. 3.65 Å, which also correlates well with the reports of Nelyubina et al. about weak but still attractive in nature interactions (Cu⋯O 3.6 Å, 0.5 kcal/mol), which was involved in the magnetic super-exchange [34]. These may be regarded as crystallographic criteria for the searching of elongated semi-coordination bonds when assisted by hydrogen bonding. On the basis of these results, the following conclusions can be drawn: That depending on the supramolecular topology and symmetry of the analyzed system, the hydrogen bonding can stabilize the coordination, or it can disrupt it. It seems that the stabilizing effect on semi-coordination bonding is observed when the accompanying hydrogen bond is directed to the non-coordinated O nitrate atom (see sets of contacts in crystal 3: O3–H3⋯O19 and Cu2⋯O18; bifurcated O11–H11⋯O19/O20 and Cu4⋯O20). The destabilizing effect occurs when coordination and hydrogen bonding compete for the same O atom (see the pairs of contacts in 1: O13–H13⋯O11 and Cu2⋯O11; in 2: O8–H8⋯O35 and Cu1⋯O35; O4–H4⋯O26 and Cu2⋯O26). Additionally, the border of 3.07 Å for a semi-coordination bond can be probably extended when the “hydrogen bond assisted” coordination occurs, but additional angular preference factor must be considered.
The presented results show that hydrogen bonding has a significant influence on the coordination bonds being responsible for the weakening and the elongation of the axial out of plane bonds in structures disturbed by the Jahn–Teller effect. Irrespective of the semi-coordination bond distance limit, topological analysis of crystal structures should always be performed since even minor structural features may help in better understanding of physicochemical properties of coordination compounds, as well as in designing of self-assembling materials or in protein–ligand docking methods.
Supplementary Materials
The following are available online: https://www.mdpi.com/2073-8994/11/4/460/s1, Figure S1. Scatter plot of Cu⋯O–X(O2) angle where X = C or N (ANG1 in °) vs Cu⋯O distance (CUO in Å) up to 4 Å with marked six regions described in the text; Figure S2. FTIR spectra of the H4tehy ligand and complex 1; Figure S3. FTIR spectra of the H4tehy ligand and complex 2; Figure S4. FTIR spectra of the H4tehy ligand and complex 3; Figure S5. TG and DSC curves of 1 in air; Figure S6. TG and DSC curves of 2 in air; Figure S7. TG and DSC curves of 3 in air; Figure S8. The powder XRD diffractograms of the final products of decomposition in air of complexes 1–3.
Author Contributions
Conceptualization, B.M.; funding acquisition, B.M.; investigation, B.M., D.O., B.C., H.G.; methodology, B.M., B.C.; software, B.M.; visualization, B.M., D.O., B.C.; writing—original draft preparation, B.M., B.C.; writing—review and editing, B.M., B.C.
Funding
The research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Operational Program Development of Eastern Poland 2007–2013 (Contract No. POPW.01.03.00-06-009/11-00, equipping the laboratories of the Faculties of Biology and Biotechnology, Mathematics, Physics and Informatics, and Chemistry for studies of biologically active substances and environmental samples).
Acknowledgments
We kindly thank Prof. Keiji Hirose for the invitation to participate in this special issue on Symmetry and Asymmetry in Host-Guest Chemistry and Supramolecular Chemistry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sundberg, M.R. Effect of hydrogen bonding on coordinate bond. Semi-coordination in Werner-type copper(II) complexes revisited. Rev. Inorg. Chem. 2000, 20, 195–218. [Google Scholar] [CrossRef]
- Grabowski, S.; Grabowski, J.S. Analysis of Hydrogen Bonds in Crystals. Crystals 2016, 6, 59. [Google Scholar] [CrossRef]
- Videnova-Adrabińska, V. The hydrogen bond as a design element in crystal engineering. Two- and three-dimensional building blocks of crystal architecture. J. Mol. Struct. 1996, 374, 199–222. [Google Scholar]
- Aakeröy, C.B.; Seddon, K.R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar] [CrossRef]
- Desiraju, G.R. Reflections on the Hydrogen Bond in Crystal Engineering. Cryst. Growth Des. 2011, 11, 896–898. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Miroslaw, B.; Koziol, A.E.; Bielenica, A.; Dziuba, K.; Struga, M. Substituent effect on supramolecular motifs in series of succinimide polycyclic keto derivatives—Spectroscopic, theoretical and crystallographic studies. J. Mol. Struct. 2014, 1074, 695–702. [Google Scholar] [CrossRef]
- Ding, X.; Tuikka, M.; Haukk, M. Halogen Bonding in Crystal Engineering. In Recent Advances in Crystallography; Benedict, J.B., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Miroslaw, B.; Plech, T.; Wujec, M. Halogen bonding in the antibacterial 1,2,4-triazole-3-thione derivative - Spectroscopic properties, crystal structure and conformational analysis. J. Mol. Struct. 2015, 1083, 187–193. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Struga, M.; Miroslaw, B.; Pakosinska-Parys, M.; Drzewiecka, A.; Borowski, P.; Kossakowski, J.; Koziol, A.E. Synthesis, characterization and supramolecular synthons in crystals of new derivatives of 10-oxa-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. J. Mol. Struct. 2010, 965. [Google Scholar] [CrossRef]
- Madura, I.D.; Czerwińska, K.; Jakubczyk, M.; Pawełko, A.; Adamczyk-Woźniak, A.; Sporzyński, A. Weak C–H⋯O and Dipole–Dipole Interactions as Driving Forces in Crystals of Fluorosubstituted Phenylboronic Catechol Esters. Cryst. Growth Des. 2013, 13, 5344–5352. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Sarma, J.A.R.P.; Krishna, T.S.R. Dipole-dipole interactions and the inversion motif in the crystal structures of planar chloro aromatics: The unusual packings of 1,2,3-trichlorobenzene and 1,2,3,7,8,9-hexachlorodibenzo-p-dioxin. Chem. Phys. Lett. 1986, 131, 124–128. [Google Scholar] [CrossRef]
- Newman, W.D.; Cortes, C.L.; Afshar, A.; Cadien, K.; Meldrum, A.; Fedosejevs, R.; Jacob, Z. Observation of long-range dipole-dipole interactions in hyperbolic metamaterials. Sci. Adv. 2018, 4, eaar5278. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Bauzá, A.; Gurbanov, A.V.; Zubkov, F.I.; Maniukiewicz, W.; Rodríguez-Diéguez, A.; López-Torres, E.; Frontera, A. The role of unconventional stacking interactions in the supramolecular assemblies of Hg(ii) coordination compounds. CrystEngComm 2016, 18, 9056–9066. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Zaręba, J.K.; Bauzá, A.; Kubicki, M.; Bartyzel, A.; Keramidas, A.D.; Butusov, L.; Mirosław, B.; Frontera, A. Recurrent supramolecular motifs in discrete complexes and coordination polymers based on mercury halides: prevalence of chelate ring stacking and substituent effects. CrystEngComm 2018, 20, 1065–1076. [Google Scholar] [CrossRef]
- James, S. Pi-Pi-Stacking as a Crystal Engineering Tool. In Encyclopedia of Supramolecular Chemistry; Atwood, J.L., Steed, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 1093–1099. [Google Scholar]
- Yao, Z.-F.; Wang, J.-Y.; Pei, J. Control of π–π Stacking via Crystal Engineering in Organic Conjugated Small Molecule Crystals. Cryst. Growth Des. 2018, 18, 7–15. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Blagojević Filipović, J.P.; Hall, M.B.; Zarić, S.D. Stacking interaction potential energy surfaces of square-planar metal complexes containing chelate rings. Adv. Inorg. Chem. 2019, 73, 159–186. [Google Scholar]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Doyle, R.P.; Julve, M.; Lloret, F.; Nieuwenhuyzen, M.; Kruger, P.E. Hydrogen-bond tuning of ferromagnetic interactions: synthesis, structure and magnetic properties of polynuclear copper(ii) complexes incorporating p-block oxo-anions. Dalt. Trans. 2006, 0, 2081–2088. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Golobic, A.; Kozlevcar, B.; Robertazzi, A.; Vargiu, A.V.; Gamez, P.; Reedijk, J. Magnetic coupling between copper(II) ions mediated by hydrogen-bonded (neutral) water molecules. Inorg. Chem. 2009, 48, 5473–5479. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.M.; Srivastava, A.K.; Mathur, P.; Lahiri, G.K. Varying structural motifs in oxyanions (NO3−, CO32−) and phenoxyacetate (PhOAc−) bridged coordination polymers derived from alkoxo-bridged dicopper building blocks with {Cu2O2} core. RSC Adv. 2011, 1, 893–902. [Google Scholar] [CrossRef]
- Haldar, S.; Vijaykumar, G.; Carrella, L.; Musie, G.T.; Bera, M. Structure and properties of a novel staircase-like decanuclear [CuII10] cluster supported by carbonate and carboxylate bridges. New J. Chem. 2018, 42, 1276–1283. [Google Scholar] [CrossRef]
- Takeda, S.; Watanabe, A.; Maruta, G.; Matsuo, T. Magnetic Interactions through Short Hydrogen Bond in Hydrogen-Bonded Basic Copper (II) Salt; Cu 2 Na(D 3 O 2 )(SO 4 ) 2. Mol. Cryst. Liq. Cryst. 2002, 376, 443–448. [Google Scholar] [CrossRef]
- Brown, D.S.; Lee, J.D.; Melsom, B.G.A.; Hathaway, B.J.; Procter, I.M.; Tomlinson, A.A.G. The structure of Cu(en)2(BF4)2 and an infrared spectral criterion for “semi-co-ordinated” polyanions. Chem. Commun. (London) 1967, 0, 369–371. [Google Scholar] [CrossRef]
- Valach, F. A bond-valence approach to the semicoordination of copper-oxygen and copper–nitrogen complexes. Polyhedron 1999, 18, 699–706. [Google Scholar] [CrossRef]
- Valach, F.; Rohlíček, J.; Lukeš, V.; Kožíšek, J.; Jorík, V. Manifestation of copper coordination sphere plasticity in [Cu2(2-bromopropionato)4]n and [Cu2(3-bromopropionato)4(H2O)2]. Inorganica Chim. Acta 2018, 479, 106–112. [Google Scholar] [CrossRef]
- Choi, J.-H.; Joshi, T.; Spiccia, L. Syntheses, Structural, and Spectroscopic Properties of Copper(II) Complexes of Constrained Macrocyclic Ligands. Zeitschrift für Anorg. und Allg. Chemie 2012, 638, 146–151. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni⋯I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef]
- Valach, F.; Grobelny, R.; Glowiak, T.; Mrozinski, J.; Lukeš, V.; Blahová, Z. Structural study of semi-coordination in a seven-coordinate copper(II) complex: distortion isomerism of [Cu(CH3COO)2 (4-aminopyridine)2 (H2O)]. J. Coord. Chem. 2010, 63, 1645–1651. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-ligand bond lengths and strengths: are they correlated? A detailed CSD analysis. Zeitschrift für Krist. - Cryst. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Korlyukov, A.A.; Fedyanin, I.V.; Lyssenko, K.A. Extremely long Cu⋯O contact as a possible pathway for magnetic interactions in Na2Cu(CO3)2. Inorg. Chem. 2013, 52, 14355–14363. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Hager, M.D.; Schubert, U.S. Supramolecular Polymers. Polym. Sci. A Compr. Ref. 2012, 269–310. [Google Scholar]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular Polymers in Aqueous Media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, B.; Miroslaw, B.; Bartyzel, A. Hexanuclear [Cu4IILn2III] compounds incorporating N,O-donor ligands—Synthesis, crystal structures and physicochemical properties. Inorganica Chim. Acta 2017, 466, 160–165. [Google Scholar] [CrossRef]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Syntheses, crystal structures, thermal and magnetic properties of new heterotrinuclear CuII–LnIII–CuII complexes incorporating N2O4-donor Schiff base ligands. Polyhedron 2018, 144, 225–233. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernández, M.I.; Gómez-Fórneas, E.; González-Noya, A.; Maneiro, M.; Pedrido, R.; Rodríguez, M.J. Self-Assembly of Dimeric MnIII–Schiff-Base Complexes Tuned by Perchlorate Anions. Eur. J. Inorg. Chem. 2007, 2007, 3789–3797. [Google Scholar] [CrossRef]
- Aguiari, A.; Bullita, E.; Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P.A. Macrocyclic and macroacyclic compartmental Schiff bases: synthesis, characterization, X-ray structure and interaction with metal ions. Inorganica Chim. Acta 1992, 202, 157–171. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Rigaku Oxford Diffraction Ltd. Crysalis-Pro Software System; ver. 1.171.38.46; Rigaku Corporation Ltd.: Oxford, UK, 2016. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. IUCr New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Li, G.-B.; Hong, X.-J.; Gu, Z.-G.; Zheng, Z.-P.; Wu, Y.-Y.; Jia, H.-Y.; Liu, J.; Cai, Y.-P. Efficient synthesis and characterization of the low dimensional heteronuclear complexes with a N2O2-donor Schiff base ligand. Inorganica Chim. Acta 2012, 392, 177–183. [Google Scholar] [CrossRef]
- Alaghaz, A.-N.M.A.; El-Sayed, B.A.; El-Henawy, A.A.; Ammar, R.A.A. Synthesis, spectroscopic characterization, potentiometric studies, cytotoxic studies and molecular docking studies of DNA binding of transition metal complexes with 1,1-diaminopropane–Schiff base. J. Mol. Struct. 2013, 1035, 83–93. [Google Scholar] [CrossRef]
- Dubey, R.K.; Baranwal, P.; Jha, A.K. Zinc(II) and mercury(II) complexes with Schiff bases: syntheses, spectral, and structural characterization. J. Coord. Chem. 2012, 65, 2645–2656. [Google Scholar] [CrossRef]
- Bartyzel, A. Synthesis, crystal structure and characterization of manganese(III) complex containing a tetradentate Schiff base. J. Coord. Chem. 2013, 66, 4292–4303. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Perpiñán, M.F.; Sánchez, A.E.; Torralba, M.C.; González, V. Water inclusion mediated structural diversity and the role of H-bonds in molecular assemblies of manganese(III) bicompartmental Schiff-base complexes. Inorganica Chim. Acta 2016, 453, 169–178. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).