Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy)
Abstract
1. Introduction
2. Study Site
3. Materials and Methods
4. Results
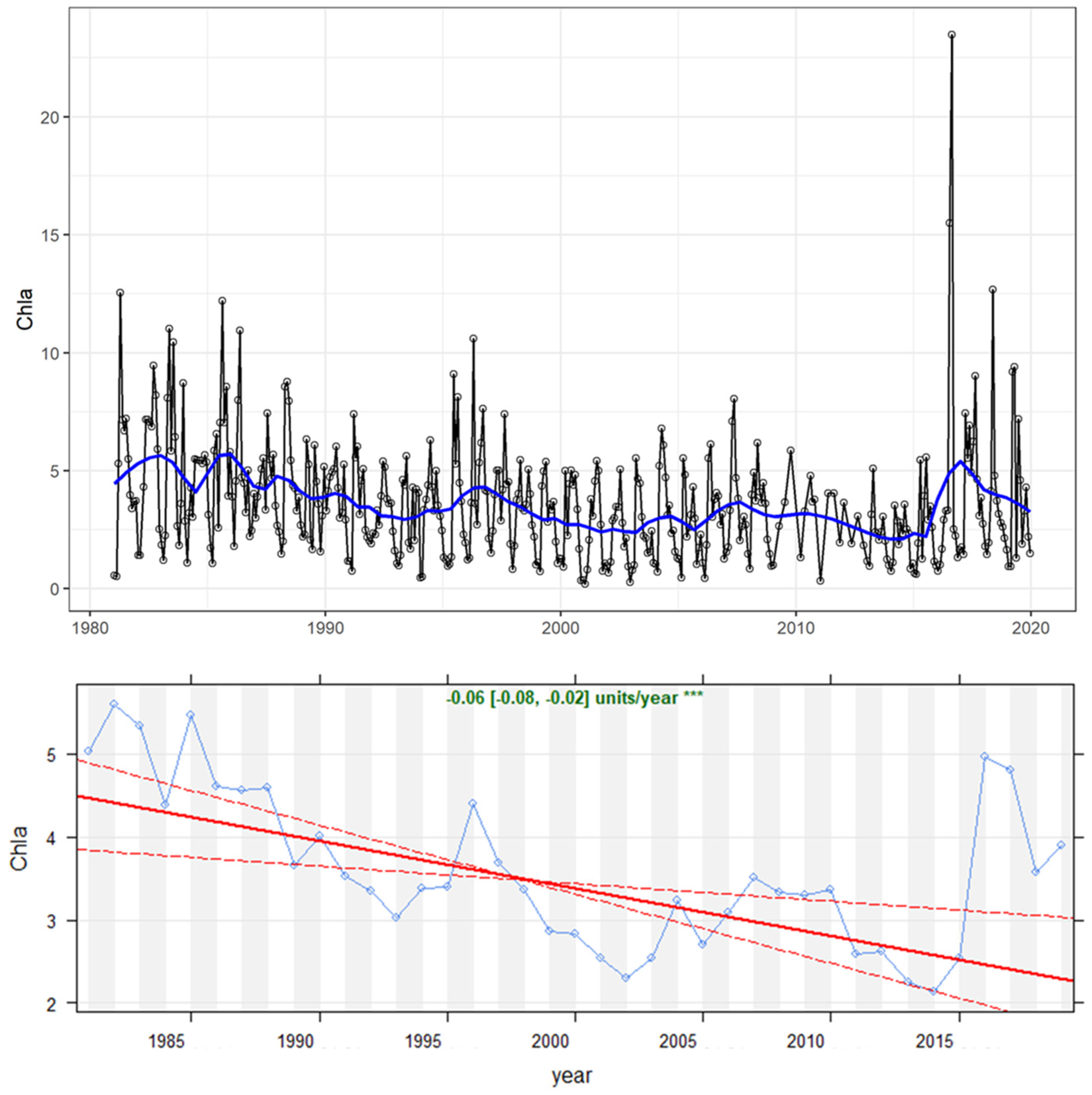
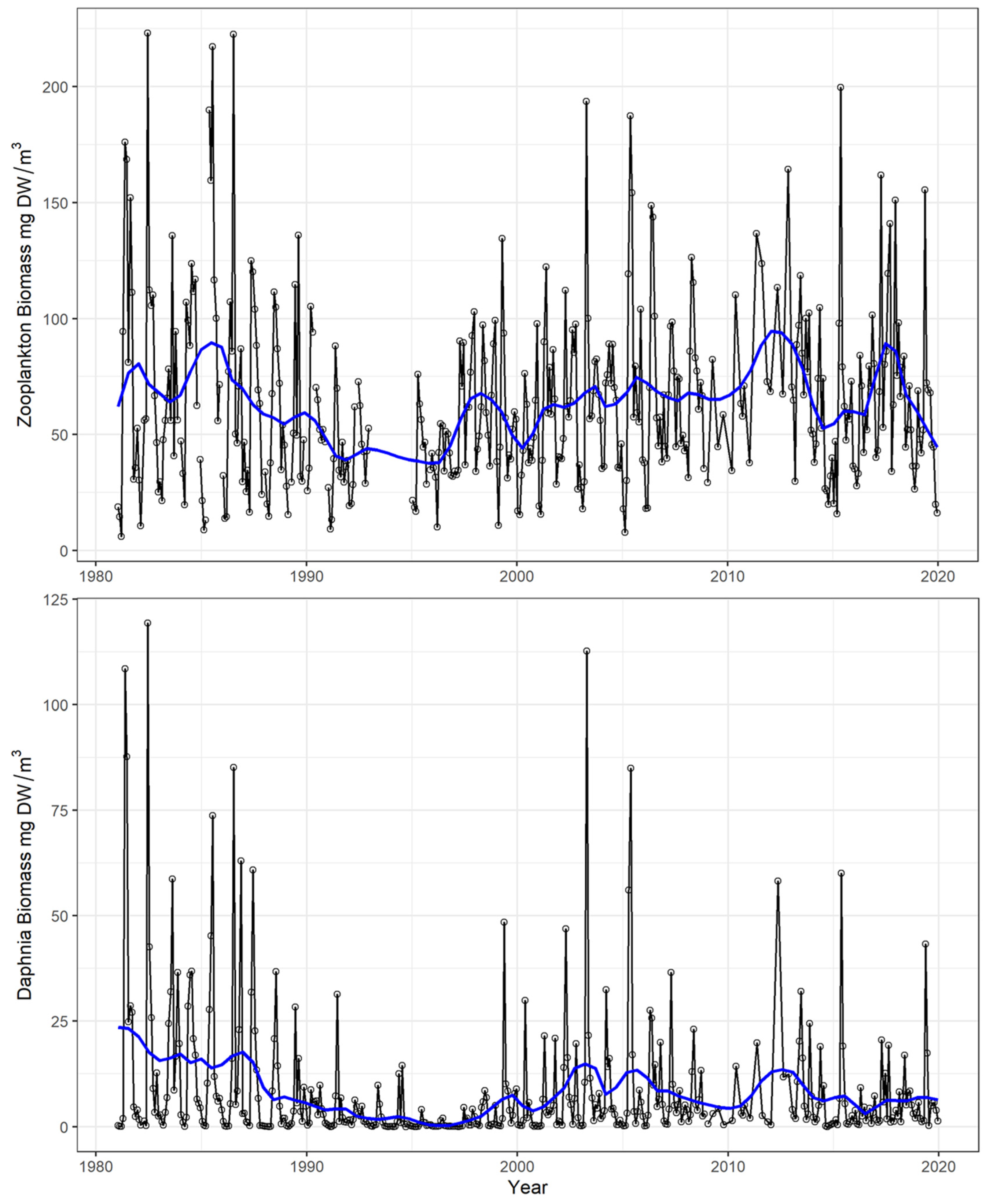
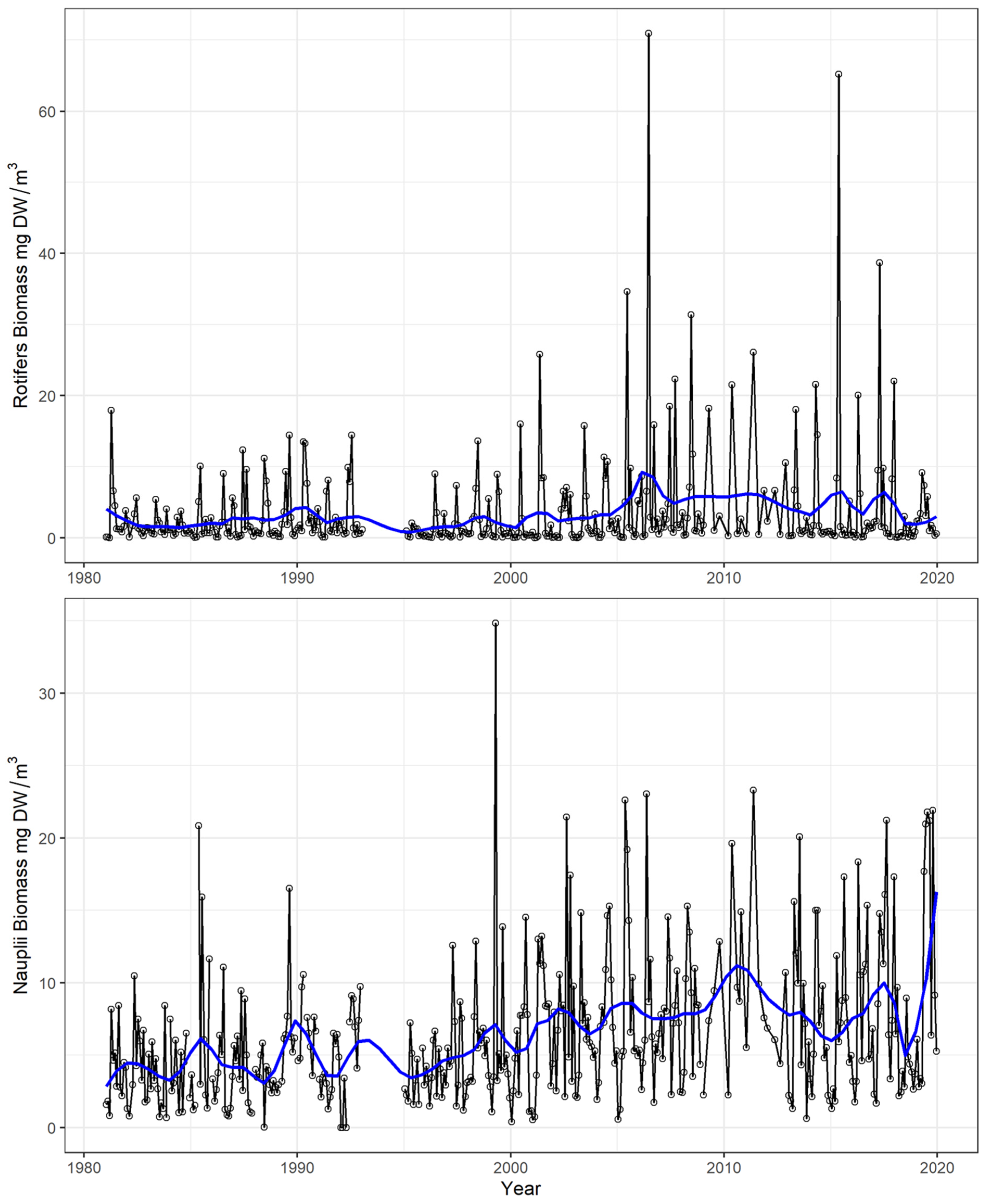
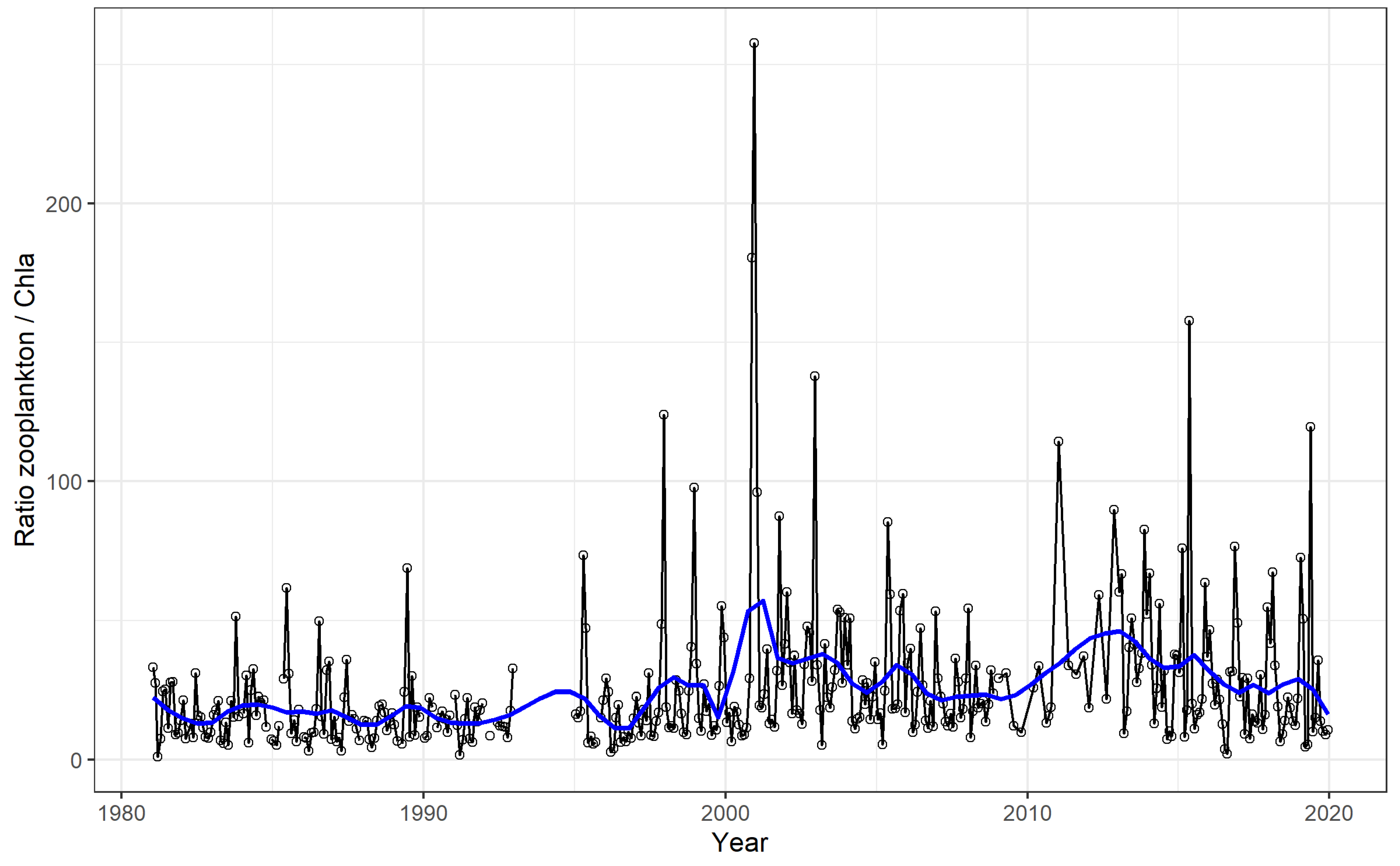
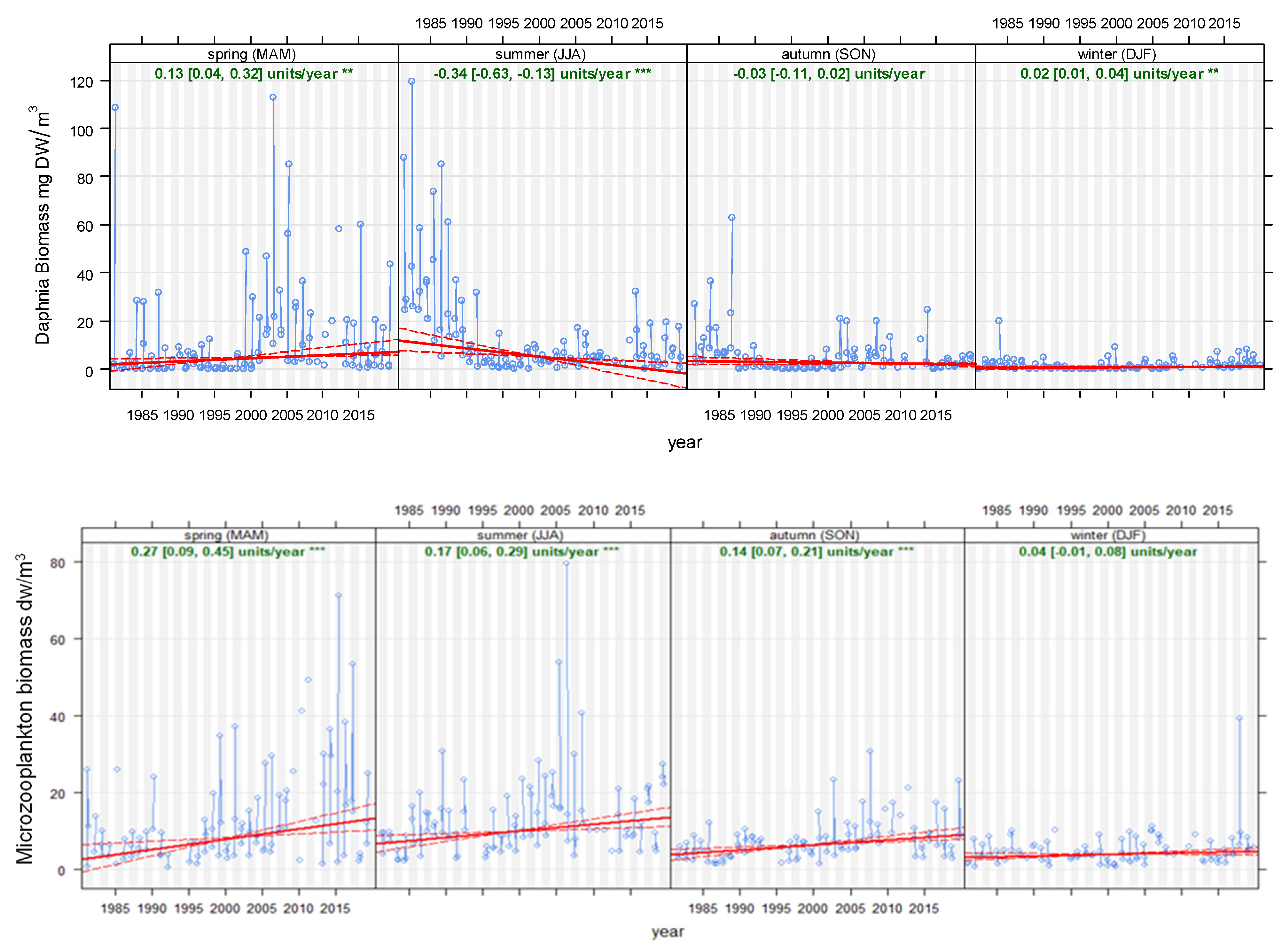
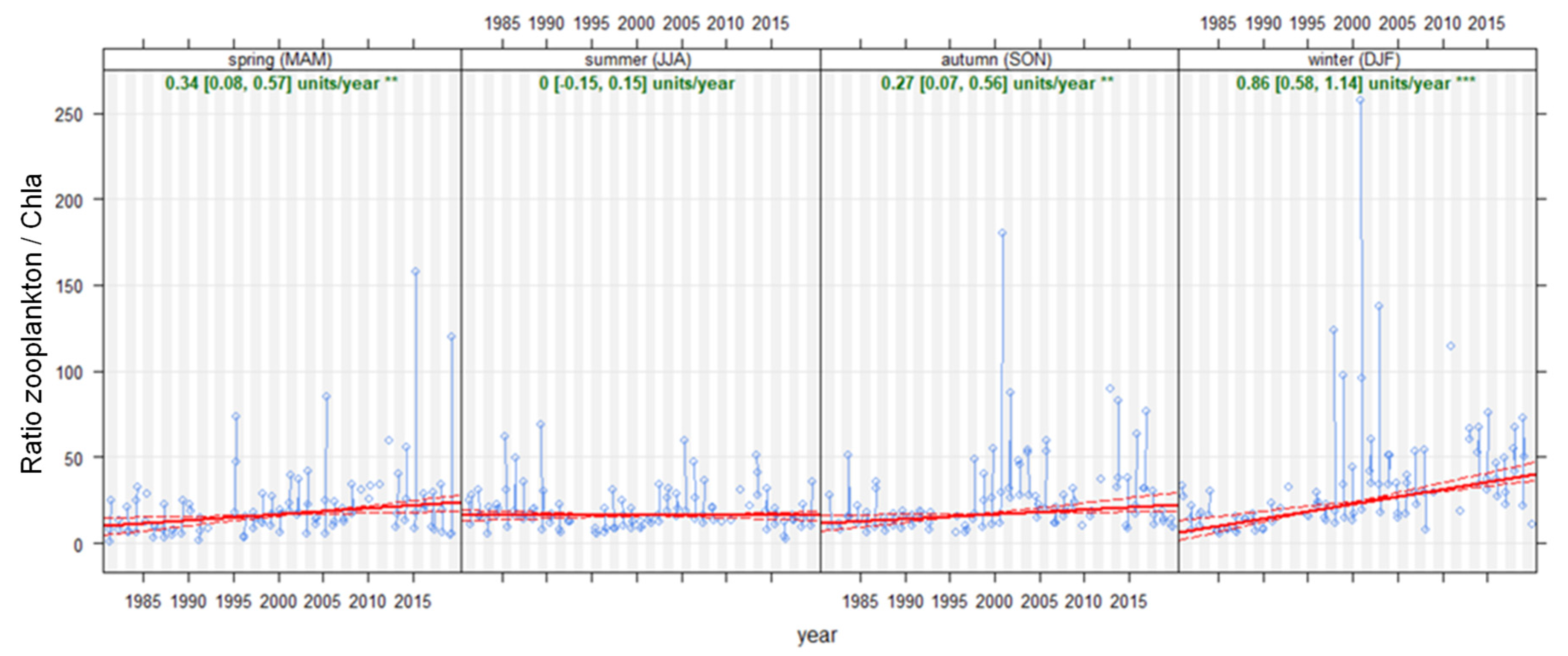
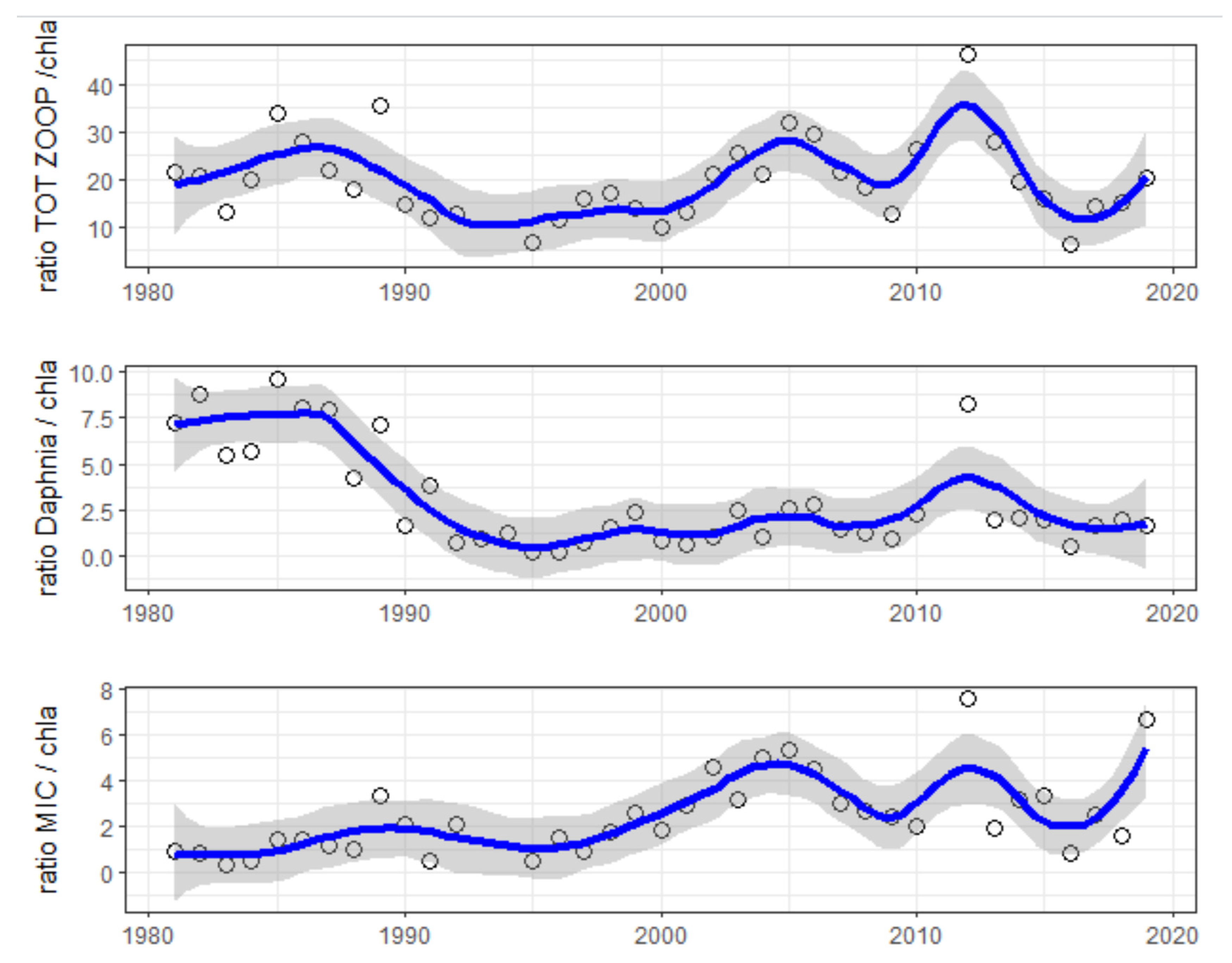
4.1. Time Series and Long-Term Trends
4.2. Seasonality
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williamson, C.E.; Dodds, W.; Kratz, T.K.; Palmer, M.A. Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front. Ecol. Environ. 2008, 6, 247–254. [Google Scholar] [CrossRef]
- Schindler, D.W. Lakes as Sentinels and Integrators for the Effects of Climate Change on Watersheds, Airsheds, and Landscapes. Limnol. Oceanogr. 2009, 54, 2349–2358. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Nõges, P.; Argillier, C.; Borja, Á.; Garmendia, J.M.; Hanganu, J.; Kodeš, V.; Pletterbauer, F.; Sagouis, A.; Birk, S. Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Sci. Total Environ. 2016, 540, 43–52. [Google Scholar] [CrossRef]
- Voigt, W.; Perner, J.; Davis, A.J.; Eggers, T.; Schumacher, J.; Bährmann, R.; Fabian, B.; Heinrich, W.; Köhler, G.; Lichter, D.; et al. Trophic levels are differentially sensitive to climate. Ecology 2003, 84, 2444–2453. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Montoya, J.M.; Trimmer, M.; Woodward, G. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol. 2011, 17, 1681–1694. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Nat. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Bazely, D.R.; Koh, S.; Timciska, M.; Haggith, E.G.; Carleton, T.J.; Coomes, D.A. Seeing the forest for the deer: Do reductions in deer-disturbance lead to forest recovery? Biol. Conserv. 2011, 144, 376–382. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Jones, I.D.; Maberly, S.C. Long-term change in the phenology of spring phytoplankton: Species-specific responses to nutrient enrichment and climatic change. J. Ecol. 2008, 96, 523–535. [Google Scholar] [CrossRef]
- Stich, H.B.; Brinker, A. Oligotrophication outweighs effects of global warming in a large, deep, stratified lake ecosystem. Glob. Change Biol. 2010, 16, 877–888. [Google Scholar] [CrossRef]
- Adrian, R.; O’Reilly, C.M.; Zagarese, H.; Baines, S.B.; Hessen, D.O.; Keller, W.; Livingstone, D.M.; Sommaruga, R.; Straile, D.; Van Donk, E.; et al. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009, 54, 2283–2297. [Google Scholar] [CrossRef]
- Straile, D. The North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc. R. Soc. B Biol. 2002, 269, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Blenckner, T.; Adrian, R.; Livingstone, D.M.; Jennings, E.; Weyhenmeyer, G.A.; George, D.G.; Jankowski, T.; Järvinen, M.; Aonghusa, C.N.; Nõges, T.; et al. Large-scale climatic signatures in lakes across Europe: A meta-analysis. Glob. Change Biol. 2007, 13, 1314–1326. [Google Scholar] [CrossRef]
- Rusak, J.A.; Yan, N.D.; Somers, K.M. Regional climatic drivers of synchronous zooplankton dynamics in north-temperate lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 878–889. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- WFD; European Community. Directive 2000/60/EC of October 23 2000 of the European Parliament and of the Council establishing a framework for community action in the field of water policy. Off. J. 2000, L327, 1–72. [Google Scholar]
- Moss, B. The Water Framework Directive: Total environment or political compromise? Stoten 2008, 400, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Caroni, R.; Irvine, K. The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? Biol. Environ. Proc. R. Ir. Acad. 2010, 110, 35–53. [Google Scholar] [CrossRef]
- O’Connor, R.J.; Walls, T.E.; Hughes, R.M. Using multiple taxonomic groups to index the ecological condition of lakes. Environ. Monit. Assess. 2000, 61, 207–229. [Google Scholar] [CrossRef]
- García-Reyes, M.; Largier, J.L.; Sydeman, W.J. Synoptic-scale upwelling indices and predictions of phyto-and zooplankton populations. Prog. Oceanogr. 2014, 120, 177–188. [Google Scholar] [CrossRef]
- Manca, M.; DeMott, W.R. Response of the invertebrate predator Bythotrephes to a climate-linked increase in the duration of a refuge from fish predation. Limnol. Oceanogr. 2009, 54, 2506–2512. [Google Scholar] [CrossRef]
- Arfè, A.; Quatto, P.; Zambon, A.; MacIsaac, H.J.; Manca, M. Long-term changes in the zooplankton community of Lake Maggiore in response to multiple stressors: A functional principal components analysis. Water 2019, 11, 962. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Morabito, G.; Volta, P.; Rogora, M.; Yan, N.D.; Manca, M. Climate warming restructures an aquatic food web over 28 years. Glob. Change Biol. 2020, 26, 6852–6866. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Rogora, M.; Cancellario, T.; Caroni, R.; Kamburska, L.; Manca, D.; Musazzi, S.; Tiberti, R.; Lami, A. High-frequency monitoring through in-situ fluorometric sensors: A supporting tool to long-term ecological research on lakes. Front. Environ. Sci. 2023, 10, 1058515. [Google Scholar] [CrossRef]
- Manca, M.; Ruggiu, D. Consequences of pelagic food-web changes during a long-term lake oligotrophication process. Limnol. Oceanogr. 1998, 43, 1368–1373. [Google Scholar] [CrossRef]
- Ambrosetti, W.; Barbanti, L. Deep water warming in lakes: An indicator of climatic change. J. Limnol. 1999, 58, 1–9. [Google Scholar] [CrossRef]
- Obertegger, U.; Smith, H.A.; Flaim, G.; Wallace, R.L. Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia 2011, 662, 157–162. [Google Scholar] [CrossRef]
- Obertegger, U.; Manca, M. Response of rotifer functional groups to changing trophic state and crustacean community. J. Limnol. 2011, 70, 231–238. [Google Scholar] [CrossRef]
- Rogora, M.; Bertoni, R.; Callieri, C.; Ciampittiello, M.; Dresti, C.; Marchetto, A.; Fenocchi, A. Long-term nutrient and oxygen dynamics in a deep large subalpine lake (Lake Maggiore, Italy): Climate change as the main driver of trophic and ecological status. In Proceedings of the ELLS-IAGLR-2018 “Big Lakes–Small World”, Evian, France, 23–28 September 2018. [Google Scholar]
- de Bernardi, R.; Giussani, G.; Manca, M.; Ruggiu, D. Trophic status and the pelagic system in Lago Maggiore. In Trophic Relationships in Inland Waters: Proceedings of an International Symposium, Tihany, Hungary, 1–4 September 1987; Springer: Dordrecht, The Netherlands, 1990; pp. 1–8. [Google Scholar]
- Bertoni, R.; Callieri, C.; Morabito, G.; Pinolini, M.L.; Pugnetti, A. Quali-quantitative changes in organic carbon production during the oligotrophication of Lake Maggiore, Italy. Int. Ver. Theor. Angew. Limnol. 1997, 26, 300–304. [Google Scholar] [CrossRef]
- Manca, M.; Cavicchioni, N.; Morabito, G. First observations on the effect of a complete, exceptional overturn of Lake Maggiore on plankton and primary productivity. Internat. Rev. Hydrobiol. 2000, 85, 209–222. [Google Scholar] [CrossRef]
- Rogora, M.; Marchetto, A.; Mosello, R. Trends in the chemistry of atmospheric deposition and surface waters in the Lake Maggiore catchment. Hydrol. Earth Syst. Sci. 2001, 5, 379–390. [Google Scholar] [CrossRef]
- Marchetto, A.; Musazzi, S. Comparison between sedimentary and living diatoms in Lago Maggiore (N. Italy): Implications of using transfer functions. J. Limnol. 2001, 60, 19–26. [Google Scholar] [CrossRef]
- Fenocchi, A.; Rogora, M.; Sibilla, S.; Dresti, C. Relevance of inflows on the thermodynamic structure and on the modeling of a deep subalpine lake (Lake Maggiore, Northern Italy/Southern Switzerland). Limnologica 2017, 63, 42–56. [Google Scholar] [CrossRef]
- Dresti, C.; Rogora, M.; Fenocchi, A. Hypolimnetic oxygen depletion in a deep oligomictic lake under climate change. Aquat. Sci. 2023, 85, 4. [Google Scholar] [CrossRef]
- Caroni, R.; Free, G.; Visconti, A.; Manca, M. Phytoplankton functional traits and seston stable isotopes signature: A functional-based approach in a deep, subalpine lake, Lake Maggiore (N. Italy). J. Limnol. 2012, 71, 84–94. [Google Scholar] [CrossRef]
- de Bernardi, R.; Giussani, G.; Manca, M.; Ruggiu, D. Long-term dynamics of plankton communities in Lago Maggiore (N. Italy). Int. Ver. Theor. Angew. Limnol. Verh. 1988, 23, 729–733. [Google Scholar] [CrossRef]
- Ambrosetti, W.; Barbanti, L.; Carrara, E.A. Mechanisms of hypolimnion erosion in a deep lake (Lago Maggiore, N. Italy). J. Limnol. 2010, 69, 3. [Google Scholar] [CrossRef]
- Callieri, C.; Piscia, R. Photosynthetic efficiency and seasonality of autotrophic picoplankton in Lago Maggiore after its recovery. Freshwat. Biol. 2002, 47, 941–956. [Google Scholar] [CrossRef]
- Morabito, G.; Oggioni, A.; Austoni, M. Resource ratio and human impact: How diatom assemblages in Lake Maggiore responded to oligotrophication and climatic variability. In Phytoplankton Responses to Human Impacts at Different Scales; Salmaso, N., Naselli-Flores, L., Cerasino, L., Flaim, G., Tolotti, M., Padisák, J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germay; New York, NY, USA; London, UK, 2012; pp. 47–60. [Google Scholar]
- Manca, M.M.; Portogallo, M.; Brown, M.E. Shifts in phenology of Bythotrephes longimanus and its modern success in Lake Maggiore as a result of changes in climate and trophy. J. Plank. Res. 2007, 29, 515–525. [Google Scholar] [CrossRef]
- Tonolli, L. Holomixy and oligomixy in Lake Maggiore: Inference on the vertical distribution of zooplankton. Int. Ver. Theor. Angew. Limnol. Verh. 1969, 17, 231–236. [Google Scholar] [CrossRef]
- Kiefer, F. Versuch einer Revision der Gattung Eudiaptomus Kiefer (Copepoda, Calanoida). Mem. Ist. Ital. Idrobiol. 1968, 24, 9–160. [Google Scholar]
- Kiefer, F. Freilebend Copepoda—Die Binnengewässer; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1978; Volume 26, 343p. [Google Scholar]
- Einsle, U. Crustacea: Copepoda: Calanoida und Cyclopoida; Subwasserfauna bon Mitteleuropa. Bd.8/Heft 4/Teil 1:1–209; Gustav Fischer: Stutgart, Germany, 1993; 209p. [Google Scholar]
- Margaritora, F.G. Fauna d’Italia. Cladocera. In Guide per il Riconoscimento Delle Specie Animali Delle Acque Interne Italiane. Collana del Progetto Finalizzato “Promozione Della Qualità Dell’ambiente”; Consiglio Nazionale delle Ricerche: Bologna, Italia, 1981. [Google Scholar]
- McCauley, E. The estimation of the abundance and biomass of zooplankton in samples. In A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; Downing, J.A., Rigler, F.H., Eds.; Blackwell Scientific Pubblication: Oxford, UK, 1984; Volume 17, pp. 228–265. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Waste Water, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APAT/IRSA-CNR. Metodi Analitici per le Acque. 29/2003, 3020: Determinazione di Elementi Chimici Mediante Spettroscopia di Emissione con Sorgente al Plasma (ICP-OES); APAT/IRSA-CNR: Roma, Italy, 2003. [Google Scholar]
- Wickham, H.; Wickham, H. Data Analysis; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Wilke, C.O. ggridges: Ridgeline Plots in ‘ggplot2’. R Package Version 0.5.6. 2024. Available online: https://wilkelab.org/ggridges/ (accessed on 15 November 2024).
- Carslaw, D.C.; Ropkins, K. Openair—An R package for air quality data analysis. Environ. Modelling Softw. 2012, 27, 52–61. [Google Scholar] [CrossRef]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofmann, H. GGally: Extension to ‘ggplot2’. R Package Version 2.2.1. 2024. Available online: https://CRAN.R-project.org/package=GGally (accessed on 7 January 2025).
- Hyndman, R.J.; Khandakar, Y. Automatic time series forecasting: The forecast package for R. J. Stat. Softw. 2008, 27, 1–22. [Google Scholar] [CrossRef]
- Moritz, S.; Bartz-Beielstein, T. imputeTS: Time Series Missing Value Imputation in R. R J. 2017, 9, 1. [Google Scholar] [CrossRef]
- Moss, B.; McKee, D.; Atkinson, D.; Collings, S.E.; Eaton, J.W.; Gill, A.B.; Harvey, I.; Hatton, K.; Heyes, T.; Wilson, D. How important is climate? Effects of warming, nutrient addition and fish on phytoplankton in shallow lake microcosms. J. Appl. Ecol. 2003, 40, 782–792. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Søndergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]
- Jeppesen, E.; Canfield, D.E.; Bachmann, R.W.; Søndergaard, M.; Havens, K.E.; Johansson, L.S.; Lauridsen, T.L.; Sh, T.; Rutter, R.P.; Warren, G.; et al. Toward predicting climate change effects on lakes: A comparison of 1656 shallow lakes from Florida and Denmark reveals substantial differences in nutrient dynamics, metabolism, trophic structure, and top-down control. Inland Wat. 2020, 10, 197–211. [Google Scholar] [CrossRef]
- Piscia, R.; Mazzoni, M.; Bettinetti, R.; Caroni, R.; Cicala, D.; Manca, M.M. Stable Isotope Analysis and persistent organic pollutants in crustacean zooplankton: The role of size and seasonality. Water 2019, 11, 1490. [Google Scholar] [CrossRef]
- DeMott, W.R.; Pape, B.J. Stoichiometry in an ecological context: Testing for links between Daphnia P-content, growth rate and habitat preference. Oecologia 2005, 142, 20–27. [Google Scholar] [CrossRef]
- Brede, N.; Sandrock, C.; Straile, D.; Spaak, P.; Jankowski, T.; Streit, B.; Schwenk, K. The impact of human-made ecological changes on the genetic architecture of Daphnia species. Proc. Nat. Acad. Sci. USA 2009, 106, 4758–4763. [Google Scholar] [CrossRef]
- Karpowicz, M.; Sługocki, Ł.; Kozłowska, J.; Ochocka, A.; López, C. Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecol. Indic. 2020, 117, 106585. [Google Scholar] [CrossRef]
- Caroni, R.; Piscia, R.; Free, G.; Manca, M. Interpreting Seasonal Patterns and Long-Term Changes of Zooplankton in a Deep Subalpine Lake Using Stable Isotope Analysis. Water 2023, 15, 3143. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Nat. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.L.; Dodson, S.I. Predation, body size, and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Straile, D.; Jöhnk, K.; Henno, R. Complex effects of winter warming on the physicochemical characteristics of a deep lake. Limnol. Oceanogr. 2003, 48, 1432–1438. [Google Scholar] [CrossRef]
- Schwefel, R.; Müller, B.; Boisgontier, H.; Wüest, A. Global warming affects nutrient upwelling in deep lakes. Aquat. Sci. 2019, 81, 50. [Google Scholar] [CrossRef]
- Woolway, R.I.; Sharma, S.; Weyhenmeyer, G.A.; Debolskiy, A.; Golub, M.; Mercado-Bettín, D.; Perroud, M.; Stepanenko, V.; Tan, Z.; Grant, L.; et al. Phenological shifts in lake stratification under climate change. Nat. Commun. 2021, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Jane, S.F.; Mincer, J.L.; Lau, M.P.; Lewis, A.S.; Stetler, J.T.; Rose, K.C. Longer duration of seasonal stratification contributes to widespread increases in lake hypoxia and anoxia. Glob. Change Biol. 2023, 29, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, H.H. Generation time, length of life, instar duration and frequency of moulting, and their relationship to temperature in eight species of Cladocera from the River Thames, Reading. Oecologia 1975, 19, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Grover, J.P. Algal growth in warm temperate reservoirs: Kinetic examination of nitrogen, temperature, light, and other nutrients. Wat. Res. 1998, 32, 3539–3548. [Google Scholar] [CrossRef]
- Manca, M.; Ramoni, C.; Comoli, P. The decline of Daphnia hyalina galeata in Lago Maggiore: A comparison of the population dynamics before and after oligotrophication. Aquat. Sci. 2000, 62, 142–153. [Google Scholar] [CrossRef]
- Gauthier, J.; Prairie, Y.T.; Beisner, B.E. Thermocline deepening and mixing alter zooplankton phenology, biomass and body size in a whole-lake experiment. Freshwat. Biol. 2014, 59, 998–1011. [Google Scholar] [CrossRef]
- Hansson, L.A.; Nicolle, A.; Granéli, W.; Hallgren, P.; Kritzberg, E.; Persson, A.; Björk, J.; Nilsson, P.A.; Brönmark, C. Food-chain length alters community responses to global change in aquatic systems. Nat. Climat. Change 2013, 3, 228–233. [Google Scholar] [CrossRef]
- Shurin, J.B.; Clasen, J.L.; Greig, H.S.; Kratina, P.; Thompson, P.L. Warming shifts top-down and bottom-up control of pond food web structure and function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, D.; Morabito, G.; Panzani, P.; Pugnetti, A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy). In Phytoplankton and Trophic Gradients, Proceedings of the 10th Workshop of the International Association of Phytoplankton Taxonomy & Ecology (IAP), Granada, Spain, 21–29 June 1996; Springer: Dordrecht, The Netherlands, 1998; pp. 243–257. [Google Scholar]
- Vijverberg, J.; Koelewijn, H.P. Size dependent mortality and production of Diaphanosoma brachyurum (Lieven) in an eutrophic lake. Int. Ver. Theor. Angew. Limnol. Verh. 1991, 24, 2768–2771. [Google Scholar]
- Burford, M.A.; Johnson, S.A.; Cook, A.J.; Packer, T.V.; Taylor, B.M.; Townsley, E.R. Correlations between watershed and reservoir characteristics, and algal blooms in subtropical reservoirs. Wat. Res. 2007, 41, 4105–4114. [Google Scholar] [CrossRef]
- Morabito, G.; Rogora, M.; Austoni, M.; Ciampittiello, M. Could the extreme meteorological events in Lake Maggiore watershed determine a climate-driven eutrophication process? Hydrobiologia 2018, 824, 163–175. [Google Scholar] [CrossRef]
- Tapolczai, K.; Anneville, O.; Padisák, J.; Salmaso, N.; Morabito, G.; Zohary, T.; Tadonléké, R.D.; Rimet, F. Occurrence and mass development of Mougeotia spp. (Zygnemataceae) in large, deep lakes. Hydrobiologia 2015, 745, 17–29. [Google Scholar] [CrossRef]
- Rogora, M.; Austoni, M.; Caroni, R.; Giacomotti, P.; Kamburska, L.; Marchetto, A.; Mosello, R.; Orru’, A.; Tartari, G.; Dresti, C. Temporal changes in nutrients in a deep oligomictic lake: The role of external loads versus climate change. J. Limnol. 2021, 80, 2051. [Google Scholar] [CrossRef]
- Jeppesen, E.; Lauridsen, T.; Mitchell, S.F.; Burns, C.W. Do planktivorous fish structure the zooplankton communities in New Zealand lakes? N. Z. J. Mar. Freshw. Res. 1997, 31, 163–173. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Fenger-Grøn, M.; Bramm, M.E.; Sandby, K.; Møller, P.H.; Rasmussen, H.U. Impact of fish predation on cladoceran body weight distribution and zooplankton grazing in lakes during winter. Freshwat. Biol. 2004, 49, 432–447. [Google Scholar] [CrossRef]
- Jensen, E.; Brucet, S.; Meerhoff, M.; Nathansen, L.; Jeppesen, E. Community structure and diel migration of zooplankton in shallow brackish lakes: Role of salinity and predators. Hydrobiologia 2010, 646, 215–229. [Google Scholar] [CrossRef]
- Keller, W.; Conlon, M. Crustacean zooplankton communities and lake morphometry in Precambrian Shield lakes. Can. J. Fish. Aquat. Sci. 1994, 51, 2424–2434. [Google Scholar] [CrossRef]
- Gyllström, M.; Hansson, L.A.; Jeppesen, E.; Criado, F.G.; Gross, E.; Irvine, K.; Kairesalo, T.; Kornijow, R.; Miracle, M.R.; Nykänen, M.; et al. The role of climate in shaping zooplankton communities of shallow lakes. Limnol. Oceanogr. 2005, 50, 2008–2021. [Google Scholar] [CrossRef]
- Gerten, D.; Adrian, R. Effects of climate warming, North Atlantic Oscillation and El Niño on thermal conditions and plankton dynamics in European and North American lakes. Sci. World J. 2000, 2, 586–606. [Google Scholar] [CrossRef] [PubMed]
- Strecker, A.L.; Cobb, T.P.; Vinebrooke, R.D. Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnol. Oceanogr. 2004, 49, 1182–1190. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; Quintana, X.D.; Jensen, E.; Nathansen, L.W.; Trochine, C.; Meerhoff, M.; Gascón, S.; Jeppesena, E. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: Implications for effects of climate change. Limnol. Oceanogr. 2010, 55, 1697–1711. [Google Scholar] [CrossRef]
- Zingel, P.; Cremona, F.; Nõges, T.; Cao, Y.; Neif, É.M.; Coppens, J.; Işkın, U.; Lauridsen, T.L.; Davidson, T.A.; Søndergaard, M.; et al. Effects of warming and nutrients on the microbial food web in shallow lake mesocosms. Eur. J. Protistol. 2018, 64, 1–12. [Google Scholar] [CrossRef]
- Cremona, F.; Agasild, H.; Haberman, J.; Zingel, P.; Nõges, P.; Nõges, T.; Laas, A. How warming and other stressors affect zooplankton abundance, biomass and community composition in shallow eutrophic lakes. Clim. Change 2020, 159, 565–580. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Meerhoff, M.; Lacerot, G.; Clemente, J.M.; Scasso, F.; Kruk, C.; Goyenola, G.; García-Alonso, J.; Amsinck, S.L.; et al. High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: Evidence from lakes, fish exclosures and surface sediments. Hydrobiologia 2011, 667, 133–147. [Google Scholar] [CrossRef]
- Rasconi, S.; Gall, A.; Winter, K.; Kainz, M.J. Increasing water temperature triggers dominance of small freshwater plankton. PLoS ONE 2015, 10, e0140449. [Google Scholar] [CrossRef]
- Moore, M.; Folt, C. Zooplankton body size and community structure: Effects of thermal and toxicant stress. Trends Ecol. Evol. 1993, 8, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, L.; Luoto, T.P.; Manca, M.; Weisse, T. A paleolimnological perspective on aquatic biodiversity in Austrian mountain lakes. Aquat. Sci. 2015, 77, 59–69. [Google Scholar] [CrossRef]
- Ohlberger, J. Climate warming and ectotherm body size–from individual physiology to community ecology. Func. Ecol. 2003, 27, 991–1001. [Google Scholar] [CrossRef]
- Razlutskij, V.I.; Feniova, I.Y.; Ejsmont-Karabin, J.; Palash, A.L.; Tunowski, J.; Sysova, E.; Zilitinkevich, N.S. Impact of enhanced summer temperatures on the distribution and structure of zooplankton communities in the heated stratified lakes: Implications for climate change. Limnologica 2018, 73, 1–11. [Google Scholar] [CrossRef]


| Coefficient | Estimate | StdError | ZScore | p-Value |
|---|---|---|---|---|
| Moving Average 1 | −0.877 | 0.050 | −17.443 | ≤0.001 |
| Moving Average 2 | −0.098 | 0.050 | −1.982 | 0.047 |
| Seasonal AR 1 | 0.894 | 0.338 | 2.640 | 0.008 |
| Seasonal AR 2 | −0.141 | 0.077 | −1.826 | 0.068 |
| Seasonal Moving Average 1 | −1.510 | 0.338 | −4.462 | ≤0.001 |
| Seasonal Moving Average 2 | 0.606 | 0.231 | 2.617 | 0.009 |
| Intercept | 0.005 | 0.006 | 0.841 | 0.400 |
| Chl-a | −0.696 | 0.274 | −2.539 | 0.011 |
| TP | 0.326 | 0.302 | 1.078 | 0.281 |
| Water temperature | −0.161 | 0.429 | −0.375 | 0.708 |
| Coefficient | Estimate | StdError | ZScore | p-Value |
|---|---|---|---|---|
| Moving Average 1 | −0.843 | 0.048 | −17.493 | ≤0.001 |
| Moving Average 2 | −0.135 | 0.048 | −2.797 | 0.005 |
| Seasonal Moving Average 1 | −0.710 | 0.048 | −14.755 | ≤0.001 |
| Seasonal Moving Average 2 | −0.112 | 0.052 | −2.140 | 0.032 |
| Chl-a | −0.214 | 0.174 | −1.229 | 0.219 |
| TP | −0.202 | 0.180 | −1.117 | 0.264 |
| Water temperature | 0.360 | 0.266 | 1.353 | 0.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caroni, R.; Piscia, R.; Manca, M. Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy). Water 2025, 17, 511. https://doi.org/10.3390/w17040511
Caroni R, Piscia R, Manca M. Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy). Water. 2025; 17(4):511. https://doi.org/10.3390/w17040511
Chicago/Turabian StyleCaroni, Rossana, Roberta Piscia, and Marina Manca. 2025. "Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy)" Water 17, no. 4: 511. https://doi.org/10.3390/w17040511
APA StyleCaroni, R., Piscia, R., & Manca, M. (2025). Indicators of Climate-Driven Change in Long-Term Zooplankton Composition: Insights from Lake Maggiore (Italy). Water, 17(4), 511. https://doi.org/10.3390/w17040511







