Abstract
The Lower Passaic River (LPR), located within the New York/New Jersey Harbor Estuarine System, has experienced long-term industrial activities, resulting in elevated concentrations of trace metals in sediment and water. This study aims to assess the bioaccumulation behavior, potential human health risks, and sources of copper (Cu), lead (Pb), and mercury (Hg) in the LPR. Trace metal concentrations were measured in water, sediment, and seven edible aquatic species. Data were analyzed using statistical approaches, and evaluated by bioaccumulation factors (BAFs) and human health risk assessments based on U.S. Environmental Protection Agency (USEPA) guidelines. Results showed that Hg exhibited the highest bioaccumulation potential among the studied metals, except for Cu in Callinectes sapidus. Non-carcinogenic risks from the consumption of aquatic species followed the order Cu > Hg > Pb, with total target hazard quotient (TTHQ) values below 1, suggesting the non-carcinogenic health risk is negligible for adults and for most species in children, except C. sapidus and Morone americana. Carcinogenic risks for all species were within the acceptable threshold (Target Risk < 1 × 10−4). Sensitivity analysis indicated that body weight and exposure duration primarily influenced children’s carcinogenic risk, whereas trace metal concentrations were more significant for adults. Overall, this study provides insight into contaminant dynamics and health implications in a legacy-contaminated urban river system.
1. Introduction
The rapid increase in trace metal pollution has become a pressing environmental issue in recent decades due to the alarming accumulation in various ecosystems and the resulting adverse effects on the ecosystem integrity and human health [1,2,3,4]. Trace metals like chromium (Cr), copper (Cu), lead (Pb), manganese (Mn), mercury (Hg), nickel (Ni), and zinc (Zn) are pervasive in the environment. Trace metals are naturally derived from sources (e.g., weathering of parent rocks and volcanic activity). However, trace metal pollution can arise from anthropogenic sources (e.g., inadequate waste management in industrial facilities, unregulated industrialization, and hazardous agricultural practices) [5].
In addition to the harmful effects that trace metals have on organisms, understanding the mechanisms of their accumulation and mobility in aquatic ecosystems is essential. Although trace metals in sediments are typically stable, they can become mobile and be reintroduced into the overlying water column due to sediment resuspension once sediment is altered or disturbed [6,7]. Although only a small amount of trace metals can accumulate in living species from sediment, these accumulated metals can then be transported to higher trophic levels, which undergo magnification within the food chain [8]. Generally, aquatic species accumulate higher trace metal concentrations from surface water [9], through the ingestion of food materials and suspended solids and adsorption via skin or tissues, and particularly in lipophilic tissues such as gills [10].
Trace metals can readily accumulate in aquatic species, ultimately entering the human body through the food chain due to human consumption [11]. This bioaccumulation poses risks not only to aquatic ecosystems but also to human health [12,13]. Copper (Cu) enters the environment through industrial emissions, fossil fuel combustion, and natural events like volcanic activity. Elevated Cu levels can lead to liver and kidney damage, chronic hepatitis, and biliary cirrhosis [14]. Lead (Pb), though naturally occurring, is primarily introduced by mining, industrial processes, and fuel combustion. It impairs neurological development, especially in children, and damages multiple organs and systems [15,16,17]. Mercury (Hg) is released from sources such as coal combustion, waste incineration, and industrial discharge. It affects the nervous system, causing cognitive deficits, tremors, and, in severe cases, Minamata disease [18].
These concerns are particularly evident in some specific industrialized locations like the Passaic River in the United States, known for its high levels of contamination stemming from historical industrial activities such as chemical manufacturing and waste disposal practices [19]. This pollution has resulted in the deposition of legacy contaminants in the river’s sediments, making it a significant concern of environmental and public health [19,20]. Over time, these contaminants can be mobilized, posing risks to both aquatic ecosystems and human populations [20]. Despite ongoing remediation efforts, critical knowledge gaps remain regarding the bioaccumulation of trace metals in edible aquatic species native to the Passaic River [21,22]. The Lower Passaic River (LPR), a 17-mile stretch from Dundee Dam to Newark Bay, is one of the most contaminated sections and has been designated a Superfund Site [20]. Yet, recent peer-reviewed research on trace metal accumulation and associated human health risks is lacking—particularly in light of New Jersey Department of Environmental Protection’s (NJDEP) advisories against fish consumption and the ban on commercial fishing in the area [23]. This lack of up-to-date data limits the ability to assess current exposure risks and inform effective public health interventions.
This research seeks to address existing gaps in knowledge through a comprehensive assessment of Cu, Pb, and Hg accumulation in commonly consumed aquatic species from the Lower Passaic River, using multiple analytical and modeling approaches. To achieve this, we developed a novel hybrid methodology that moves beyond traditional assessments by combining a variety of multivariate statistical analyses and Artificial Intelligence (AI)-based modeling (Artificial Neural Network (ANN)) and probabilistic risk analysis (Monte Carlo simulation and sensitivity analysis). The probabilistic framework captured uncertainty and helped differentiate between key determinants of carcinogenic risk. These approaches can add novel components to an integrative methodology and improve the existing knowledge focused on sediment or water characterization methods that allow for an assessment of sources of metals to the river system, bioaccumulation dynamics, and human health risk assessments associated with metal exposures within a historically contaminated urban river system.
Despite the health significance of trace metal contamination, comprehensive studies on their distribution in food sources and associated human health risks remain limited. The complexity and diversity of contamination sources further complicate efforts to trace their origins accurately [24,25]. While advanced analytical and computational tools are available, their effectiveness is often limited by incomplete datasets [26]. As a result, there is a pressing need for more integrated and data-driven investigations to better understand trace metal exposure risks in ecosystems like the Passaic River. This study aims to (i) analyze the concentration of trace metals (Cu, Pb, and Hg) in the water and sediment of the Lower Passaic River, (ii) evaluate their bioaccumulation in edible aquatic species, (iii) assess the potential health risks related to the ingestion of trace metals through the consumption of aquatic species, and (iv) determine the potential origins of the trace metals present in these species.
Our hypothesis is that (1) trace metal concentrations will significantly differ among species, based on their habitat and feeding behavior; (2) Hg will show the highest potential for bioaccumulation as compared to Cu and Pb based on its known mobility and persistence; and (3) industrial and urban activities will be the dominant sources of trace metals in the Lower Passaic River. We anticipate that while almost all metal concentrations will be in compliance with regulatory safety limits, some species, particularly those with higher trophic positions, may show elevated accumulation, which would begin to highlight the need for continued monitoring and risk assessment.
2. Methodology
2.1. Study Area
The Passaic River, located in northeastern New Jersey, spans approximately 130 km (80 miles) and flows through diverse landscapes, ranging from rural and suburban areas to highly urbanized regions. Originating from the Great Swamp in Morris County, New Jersey, the river meanders through seven counties, including Passaic, Essex, and Bergen, before discharging into Newark Bay.
The upper reaches of the Passaic River are characterized by a relatively pristine environment with significant ecological value, including wetland habitats that support a variety of flora and fauna. However, as the river flows downstream, it passes through urban and industrial areas, particularly in the Lower Passaic River (LPR), where historical and ongoing industrial activities have resulted in significant environmental contamination. The LPR covers 17 miles from the Dundee Dam to Newark Bay and has been subject to extensive pollution from hazardous substances such as dioxins, trace metals, and polychlorinated biphenyls (PCBs) [19,20] leading to its designation as a Superfund site by the U.S. Environmental Protection Agency (USEPA) [20]. This study quantified trace metal concentrations in sediment, water, and selected edible aquatic species from the Dundee Dam to river mile 8.3 (Figure 1).
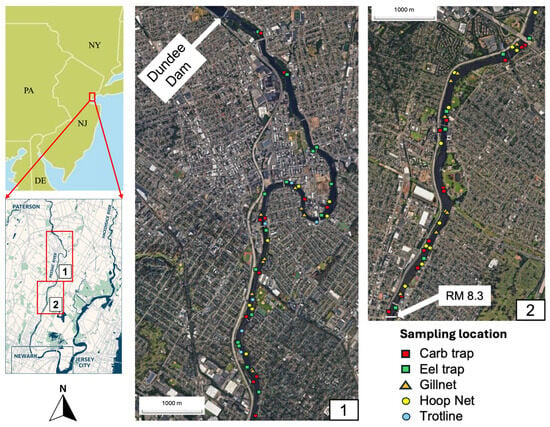
Figure 1.
Map of the study area: Dundee Dam to river mile 8.5 in the Lower Passaic River.
2.2. Sample Collection, Analytical Methods and Data Analysis
Samples were collected using various fishing methods, including beach seine, crab trap, eel trap, electrofishing (backpack and boat), gillnet, hoop net, minnow trap, and trotline [27]. To ensure consistent sampling effort, crab traps, eel traps, minnow traps, and trotlines were each deployed in sets of three at every sampling location. These methods were employed during two distinct sampling periods: from 9 to 28 September 2019, and from 2 to 21 August 2021 [27]. The specified effort levels for the different sampling methodologies are comprehensively outlined in the Fish/Decapod Quality Assurance Project Plan (QAPP) [28], as well as in its subsequent addenda, including Addendum No. 7 [28], and Addendum No. 8 [29].
Trace metal concentrations (Cu, Pb, and Hg) in the whole bodies (calc.) of seven common edible aquatic species (Anguilla rostrata, Micropterus salmoides, Callinectes sapidus, Cyprinus carpio, Ictalurus punctatus, Lepomis auritus, and Morone americana) were obtained for the years 2019 and 2021 [27]. Water column data (2019–2021) were collected from the Passaic River Public Digital Library [30], and sediment trace metal data from Soetan et al. [31] were used to assess bioaccumulation in edible aquatic species. The water sampling points map is presented in Figure S1. Copper and lead concentrations were determined using the U.S. Environmental Protection Agency (USEPA) method SW-846 6020 (USEPA, Washington, DC, USA, 2002) with inductively coupled plasma mass spectrometry (ICP-MS) employing argon plasma. Mercury (Hg) levels were measured following USEPA method 1631 (USEPA, Washington, DC, USA, 2014), utilizing cold vapor atomic fluorescence spectrometry (CVAFS). The detection frequencies and reporting limits (RLs) for these trace metals in tissue samples are summarized in Table S1.
While the data were collected in two discrete years (2019 and 2021), the same sampling protocols, analytical procedures, and reporting standards provided valid comparisons across the years. Sampling sites for water and sediment were selected to be geographically congruent with the sites where aquatic species were sampled to ensure that bioaccumulation factors (BCFW and BCFS) correspond with local environmental conditions [28,30].
Microsoft Excel (V.16.85.2; Microsoft Corporation, Redmond, WA, USA), JMP Pro (V.16.2.0; SAS Institute Inc., Cary, NC, USA), and RStudio (V.2024.04.1 + 748; Posit Software, PBC, Boston, MA, USA) were utilized for data analysis in this study. Principal component analysis (PCA), Pearson’s correlation matrix (PCM), and cluster analysis (CA) are three examples of multivariate statistical techniques that were used to analyze the dataset in detail and identify the distribution patterns of specific trace metals among various edible aquatic species based on their similarities, dissimilarities, and concentrations.
2.3. Water Bioaccumulation Factor (BCFW) and Sediment Bioaccumulation Factor (BCFS)
The accumulation tendencies from water and sediment were assessed using the bioaccumulation factor (BCF). Equations (1) and (2) were employed to calculate the BCFw and BCFs, respectively [10,32].
where C is the average trace metal concentration in edible aquatic species (mg/kg ww), Cfactor is a conversion factor with a value of 0.085 to transform fresh weight to dry weight, as established in earlier research [33,34], this factor is linked to an average moisture level of approximately 85 percent in fish muscle tissue, which consistent with results from Ahmed et al. [35], TMCW denotes the mean dissolved trace element’s concentration in water (mg/L), and TMCS indicates average trace element concentration in sediment (mg/kg dw). The sample sizes reported are for individual specimens, and trace metal concentrations that did inform the BCF calculations were averaged by species per site to limit variability and enhance the statistical reliability.
The following is the categorization for trace metal bioaccumulation: A low chance of accumulation is indicated by BCFW values below 1000 L/kg, accumulation is indicated by values between 1000 L/kg and 5000 L/kg, and high accumulation is indicated by values equal to or beyond 5000 L/kg [36,37], and bioaccumulation is indicated by BCFS greater than 1 [38]. Although secondary datasets were used, uncertainties arising from differences in sampling effort, detection limits, or analytical variability are minimized through standardized protocols [27,28,29] and averaging of concentrations for BCF calculations.
2.4. Artificial Neural Network Modeling
Artificial Neural Networks (ANNs) were used to model the nonlinear relationships between trace metal concentrations and bioaccumulation factors in aquatic organisms, proving effective under diverse conditions [39,40,41]. The input variables to the ANN were the concentrations of individual trace metals measured in water and sediment, while the output variables were the corresponding bioaccumulation factors in water (BCFW) and sediment (BCFS).
Three learning algorithms—Levenberg–Marquardt (LM), Gradient Descent (GD), and Scaled Conjugate Gradient (SCG)—were evaluated, along with log-sigmoidal and tan-sigmoidal transfer functions. A multilevel feed-forward neural network was structured into input, hidden, and output layers. The number of hidden layer neurons was varied from 2 to 8.
In order to avoid overfitting and guarantee robust model generalization, the dataset was randomly divided into 60% for training, 20% for testing, and 20% for validation. The training of the model was tracked using both training and validation errors, and early stopping was applied to terminate training when the validation error began to increase. The simplest network architecture was selected that performed well in predictive accuracy and had low mean squared error (MSE) in all subsets. Model development, training, and evaluation were performed in MATLAB (R2024a; The MathWorks Inc., Natick, MA, USA).
2.5. The Average Pollution Load Evaluation Technique
To investigate the potential detrimental effects of the target trace metals on exposed aquatic species, the average pollution index method (APLI) is utilized; APLI reflects the level of trace metal (TM) contamination in aquatic species samples by applying the following formula:
where n is the total number of trace metals, Ci is the mean concentration of trace metals in the samples, and Si is the maximum allowable concentration (MAC) of trace metals (FAO/WHO 2014). A higher APLI value indicates that the aquatic species are unsuitable for consumption [42,43]. The pollution status established on trace metal concentration in aquatic species is classified as follows: unpolluted (<0.1), micro polluted (0.1–0.2), mildly polluted (0.2–0.5), moderately polluted (0.5–0.7), strongly polluted (0.7–1.0), and severely polluted (>1.0) [44].
2.6. Human Health Risk Assessment Method
Through direct oral intake, humans are exposed to trace metals found in aquatic species, which carry both non-carcinogenic and carcinogenic dangers. This study evaluated the risk of trace metal exposure using the human health risk assessment approach suggested by the USEPA [45], where the target populations were adults and children. The assessment was conducted in three sequential parts: (i) estimating the exposure dose from oral intake on a daily basis (EDI), (ii) calculating non-carcinogenic risk [hazard quotient (HQ) and target hazard quotient (THQ)], and (iii) determining carcinogenic risk (TCR) from life-time exposure.
To avoid redundancy, the equations and parameters used for both non-carcinogenic and carcinogenic assessment are presented together, as they have the same exposure pathways and share similar input variables (Tables S2–S4).
The revised USEPA [46] recommendations, which include a cancer slope factor (CSF) and reference dose (RfD) for lead (Pb), were followed. As the studied aquatic species are frequently consumed, the assessment focused on the oral ingestion route. Tables S2–S4 offer detailed health risk assessment models, reference dosages, and carcinogenic slope factors, respectively.
2.7. Monte Carlo Simulation
The assessment of potential carcinogenic risk was conducted using a Monte Carlo Simulation to evaluate the ingestion of carcinogenic trace metals by consuming aquatic species [47,48]. In risk-based computations, this approach is frequently employed to account for variability and uncertainty [49]. This probabilistic method was employed consistently for hazard quotient (HQ)/total target hazard quotient (THQ) and TCR calculations, facilitating uncertainty quantification through a single risk assessment process rather than individual analyses. Ten thousand random iterations of the input data were used in each simulation to guarantee the accuracy of the outcomes. For this study, the average, 5th, and 95th percentiles of Pb cancer risks were obtained from the TCR probability distribution. Additionally, a sensitivity analysis was conducted to ascertain whether input factors significantly affected the risk estimates. Crystal Ball (V.11.1.3; Oracle Corporation, Austin, TX, USA) was used to perform the evaluations.
2.8. Positive Matrix Factorization (PMF)
This study used a mathematical receptor model called EPA PMF (V.5.0; U.S. Environmental Protection Agency, Washington, DC, USA) to determine the sources of trace metals in aquatic edible species. This model was chosen because it incorporates uncertainty estimates into the factorization process, effectively handles missing and below-detection-limit data, and minimizes rotational ambiguity, ensuring more reliable source identification compared to other receptor models such as PCA or UNMIX. Moreover, EPA PMF 5.0 is widely validated and recommended by the U.S. Environmental Protection Agency for environmental source apportionment studies [50].
This model effectively calculates source contributions and summaries using robust factorization algorithms. Mathematically, it is represented by Equation (4):
where Xiy is the ith species’ value in the yth sample; gia is the ith species’ value from the yth source; the ath source’s contribution to the yth sample is indicated by fay; and the systematic error is denoted by eiy. In order to best replicate the observed value xiy, our model sought to identify gia and fay values. The data refinement process proceeded until the ultimate Q value, which is determined by Equation (5), was reached.
where αiy denotes the “uncertainty” in the ith species of sample number y. Data below the minimum detection limit (MDL) are substituted using the methods described in Equations (6) and (7).
Here, the ith species of sample number y’s uncertainty is shown by αiy. Equations (6) and (7) show how data below the minimal detection limit (MDL) are replaced.
where all the parameters in the equations are the same as defined before. To ensure consistent outcomes, the model was run 20 times with varying random seeds and different numbers of factors. The model was considered robust when lower Q values, greater R2 values, and distinct factor interpretability were achieved.
2.9. Analytical Workflow and Integration of Models
This study employed a hierarchical analytical workflow to integrate the methodological components. The workflow began with multivariate statistical techniques (PCA and CA) to characterize correlations and clustering. Subsequently, the PMF model provided quantitative source apportionment, while the SOM model visualized nonlinear relationships and validated clustering patterns. Finally, the HQ, TTHQ, TCR, and Monte Carlo simulation translated concentration data into exposure and risk estimates, enabling an integrated assessment of contamination sources and associated health risks.
3. Results and Discussion
3.1. Concentrations of Trace Metals
Trace metal concentrations in the study area were as follows: in surface water, Cu ranged from 1.66 × 10−3 to 7.61× 10−3 mg/L, Pb from 1.03 × 10−4 to 6.28 × 10−3 mg/L, and Hg from 5.6 × 10−7 to 1.22 × 10−4 mg/L; in sediment, Cu ranged from 15.4 to 253 mg/kg, Pb from 19.2 to 306 mg/kg, and Hg from 0.23 to 4.7 mg/kg (Table S5). Cu and Pb concentrations were found to be higher in the study than in the Earth’s shale, as reported by Taylor and McLennan [51], and the Upper Continental Crust (UCC) levels given by JECFA [52].
Table 1 lists the concentrations of trace elements [mg/kg wet weight (ww)] in seven aquatic species. In general, the average concentration of Cu, Pb and Hg in these species’ decreased in the following order: Cu (5.03 ± 1.16) > Pb (0.33 ± 0.15) > Hg (0.16 ± 0.03). These values are below the recommended guidelines of the maximum allowable concentration (MAC) established by the Food and Agricultural Organization and the World Health Organization (Table 1) [53].

Table 1.
Trace metal concentration (mg/kg wet weight) in edible aquatic species with corresponding weight, length, feeding habits, and habitat.
In general, copper (Cu) concentrations surpassed all other trace metals across all species. The Cu content in the analyzed samples ranged from 0.52 mg/kg in M. salmoides to 23.9 mg/kg in C. sapidus (Table 1). The average Cu concentration across all edible aquatic samples was 5.03 mg/kg, lower than the maximum allowable concentration (MAC). The elevated Cu levels likely result from its adsorption to organic matter and particulate matter in sediments, as well as ongoing contributions from industrial and urban runoff. In aquatic species, Cu bioavailability is moderated by metallothionein binding, which limits excessive accumulation in edible tissues [54,55].
Lead (Pb) concentrations showed minimal variation among the tested species. Among the edible aquatic species analyzed, M. salmoides exhibited the lowest mean concentration of 0.09 mg/kg, while A. rostrata demonstrated the highest mean concentration of 0.37 mg/kg. The mean Pb concentration in the analyzed samples was 0.34 mg/kg, within the maximum allowable concentration (MAC) of 1 mg/kg (Table 1). Lead is relatively insoluble and strongly binds to carbonates, sulfides, and organic matter in sediments, which reduces its bioavailability for aquatic species. Consequently, Pb tends to accumulate in inert tissues like bones or exoskeletons rather than soft edible tissues [56].
Concentrations of mercury were relatively constant between species, with C. sapidus having the lowest concentration (0.08 mg/kg) and I. punctatus the highest (0.29 mg/kg). Therefore, the Hg concentrations measured in this study were below the prescribed guideline values (Table 1). Mercury, especially as methylmercury, is highly bioavailable and transfuses biology through biological membranes. Microbial methylation can increase Hg mobility and trophic transfer, especially under low oxygen and high organic matter conditions in sediments. Several environmental factors influence Hg methylation and the subsequent uptake of Hg by aquatic species, including pH, dissolved oxygen and sediment organic content [57,58].
3.2. Trace Metal Accumulation in Edible Aquatic Species
The sediment trace metals were ranked by their calculated bioaccumulation factors (BCFS) as follows: Hg > Cu > Pb. The mean BCFS for Cu, Pb, and Hg were 0.00298, 0.0002, and 0.00741, respectively (Table S6). Figure 2a and Figure 2b illustrate the combined bioaccumulation factors of these trace metals (Cu, Pb, and Hg) for individual aquatic edible species from sediment and water, respectively. A similar bioaccumulation pattern was observed in water (BCFW), with the ranking of Hg > Cu > Pb. The average BCFW values for Cu, Pb, and Hg were 129.34 L/kg, 41.87 L/kg, and 2224.46 L/kg, respectively (Table S6).
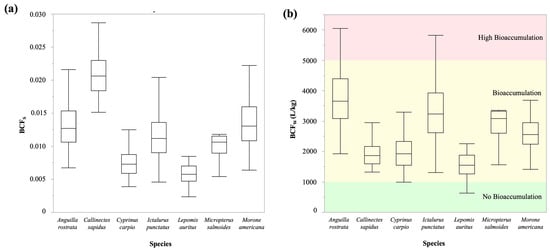
Figure 2.
Trace metals’ bioaccumulation in edible aquatic species from sediment (a) and water (b).
Bioconcentration factors (BCFS) values indicate bioaccumulation patterns, with aquatic species potentially accumulating metals. Although BCFS > 1 suggests a bioaccumulation, significant accumulation is only considered when BCFs exceed 100 [59]. In this study, the average total BCFW of all trace metals in water was 2396, indicating a significant amount of bioaccumulation from water. On the other hand, BCFS for every species was less than 1, which suggests that aquatic species do not accumulate much trace metal from sediment. These results suggest that the primary source of trace metal bioaccumulation in the studied species is surface water, rather than sediment. This likely reflects the relative solubility and bioavailability of dissolved metals from the water column in contrast to particulate metals that are frequently immobilized when adsorbed to oxides, clay minerals and organic matter [60].
The established order (Hg > Cu > Pb) is consistent with established ecological and biochemical processes that affect the behaviors of trace metals. Mercury (Hg), particularly in the form of methylmercury, undergoes biotransformation during more anoxic and suboxic conditions facilitated by sulfate- and iron-reducing bacteria [58]. Biotransformation via methylation significantly enhances the lipid solubility and bioavailability of Hg, making it more likely to partition biological membranes and bind tightly to sulfhydryl groups within proteins, thus promoting biomagnification through trophic levels [61]. In contrast, copper (Cu) is an essential micronutrient that is stringently regulated in aquatic organisms by a number of homeostatic mechanisms, including binding to metallothionein and enzymatic control, which limit excess accumulation [62]. In contrast, lead (Pb) readily forms stable organic matter and iron/manganese oxides complexes that reduce its dissolved fraction [56] and bioavailability and preferentially bioaccumulates in inert tissues [63].
Other factors, such as pH, dissolved oxygen (DO), and organic matter also have an influence on the process of transformation and accumulation. A decrease in pH can promote metal solubility, while decreased levels of DO within sediments support Hg methylation [64]. In addition, the presence of organic matter can complex with Cu and Pb, reducing their free-ion activity and, therefore, decreasing bioavailability [65]. However, reductive dissolution of iron/manganese (Fe/Mn) oxides in low-oxygen conditions may also release bound metals back into the water column, which could potentially increase exposure [66]. The geochemical interactions provide insights in explanation of the higher Hg accumulation and moderate Cu enrichment compared to Pb [61].
The feeding behavior and living habits of species also affect bioaccumulation patterns. The benthic, omnivorous C. sapidus accumulates more sediment-derived Cu, while the carnivorous, bottom feeding A. rostrata and I. punctatus show fairly high Hg accumulation, likely due in part to trophic transfer, and benthic prey consumption (Table S6). Overall, both biochemical regulation and ecological variables—trophic level, as well as preferred habitat—may play important roles in trace metal bioaccumulation differentials [67,68].
3.3. Artificial Neural Network Modeling for BCFW and BCFS
The ANN predictions identified an optimal network topology of 3:5:1 for modeling both BCFW and BCFS (Figure 3). Among the algorithms tested, the Levenberg–Marquardt algorithm yielded the highest correlation coefficients (R-values) and the lowest mean squared error (MSE) values, as shown in Table S7. While increasing the number of hidden neurons generally improved the model’s predictive ability, the coefficient of determination (R) did not always improve, indicating a risk of overfitting with excessive complexity [40,69].
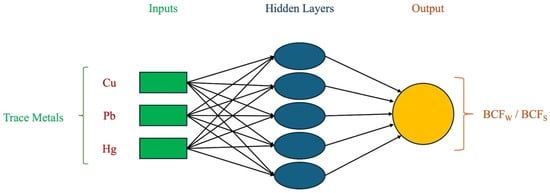
Figure 3.
The artificial neural network of BCFW and BCFS with topology.
To minimize this impact, the complexity of the network was deliberately restricted, and generalization of the model was established through validation data which yielded high R-values and low MSE similar to training. Use of an independent validation subset and stopping criteria ensured that the final ANN model adequately fit nonlinear structural relationships without fitting noise. The close agreement between experimental and predicted values (Figure 4) continues to support the model robustness and reliability for describing trace metal bioaccumulation.
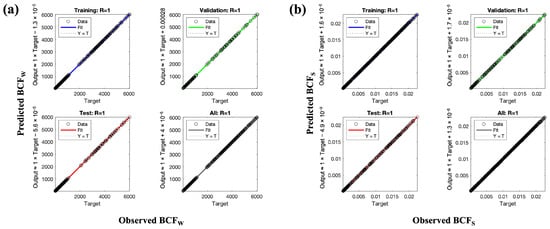
Figure 4.
Correlation between experimental and ANN-predicted BCF values: (a) BCFW and (b) BCFS.
Mechanistically, the ANN’s robust predictive performance for Hg is consistent with its well-documented nonlinear bioaccumulation behavior due to methylation, lipid solubility, and trophic transfer while Cu and Pb show regulated and/or particle-bound behavior [67]. This suggests that the ANN was able to reflect biological uptake processes driven by physiological control and geochemical availability [70].
3.4. Pollution Evaluation Indices
In this study, APLI was used to determine the degree of contamination in the aquatic edible species. It was found that the APLI values in the examined samples varied from 0.16 to 0.43 (Figure S2). Based on the pollution classification, these values correspond to micro to mild contamination levels, indicating generally low but detectable trace metal accumulation in the studied species.
The highest APLI value was found in C. sapidus (0.43) and C. carpio (0.35), followed by M. salmodies (0.16), which shows moderate pollution by these trace metals. Various feeding behavior and living habitats are known to result in the accumulation of elevated levels of trace metals in aquatic species [71,72]. Different dietary preferences among the species can influence the degree to which they accumulate these trace metals, leading to significant variability in contamination levels. This variability is consistent with studies reporting high contamination levels in various foodstuffs [13,73], highlighting trace metals pollution’s persistent and widespread nature in the food chain.
3.5. Human Health Risk Assessment
3.5.1. Estimated Daily Intake (EDI)
Table 2 presents the EDIs of the evaluated trace metals from adult and children’s ingestion of edible aquatic species. The mean EDI values in aquatic species were in the descending sequence of Cu > Pb > Hg for both children and adults. According to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [52], the estimated daily intakes of copper (Cu) from the edible species samples were found to be below the Maximum Daily Tolerable Intake (MDTI). However, the mean estimated daily intake (EDI) of lead (Pb) in A. rostrata, C. sapidus, C. carpio, and L. auritus exceeded the MTDI for adults, as reported by FAO/WHO [74]. Furthermore, all species exhibited EDI values for children that were higher than those for adults, reflecting their greater exposure potential relative to body weight.

Table 2.
The estimated daily intake (EDI) of trace metals from the diet of aquatic species is expressed in mg/kg-bw/day.
3.5.2. Non-Carcinogenic Risk
Non-carcinogenic health risks for adults and children caused by each trace metal were evaluated using the hazard quotient (HQ) (Table S8). According to the USEPA [75], HQ values greater than 1 (HQ > 1) implies a possible non-carcinogenic health risk from exposure. In the present study, HQ values for each individual trace metal were less than 1 (i.e., HQ < 1), indicating no expected non-carcinogenic risk in adults and children, with the exception of Cu in C. sapidus, where the total hazard quotient (THQ) reached 1.78 ± 0.24 for children (Table S8). Cu contributed most to TTHQ across all metals in both cohorts.
The TTHQ values for the seven aquatic edible species consumed by children and adults are depicted in Figure 5a. The TTHQ values listed in descending order are as follows: C. sapidus > M. americana > C. carpio > A. rostrata > → I. punctatus > L. auritus > M. salmoides, respectively. The TTHQ value of children consuming C. sapidus and M. americana were both above 1 indicating potential non-carcinogenic health risk; all adult values were below 1.
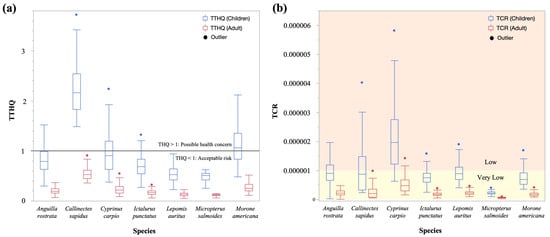
Figure 5.
Target Hazard Quotients (THQ) in children and adults and their contributions: (a) three trace metals’ Total Target Hazard Quotients (TTHQ), (b) Carcinogenic Risk (CR) for Pb.
Children are more susceptible to trace metal exposure than adults due to physiological and behavioral differences. In terms of physiology, children have higher metabolic rates and gastrointestinal absorption, as well as lower body mass and immature detoxification and excretion systems, leading to higher internal doses of metals [76,77]. Children are also known to consume more food per unit body weight for active development and growth, which makes them more exposed and therefore susceptible to toxicological effects [76,78]. Therefore, even trace levels of contaminants in aquatic foods pose lesser health risk to adults comparatively [77,78].
3.5.3. Carcinogenic Risk
In this study, lead (Pb) was included in the target carcinogenic risk (TCR) calculation, acknowledging that this trace metal can induce both non-carcinogenic and carcinogenic health risks in children and adults, depending on the exposure level. Based on animal research, the USEPA has classified lead (Pb) as a Group B2 carcinogen. Table S9 presents the TCR values for Pb exposure in children and adults by consuming the examined aquatic species. Among the seven species, C. carpio has the highest TCR value for both children (2.08 × 10−6 ± 1.10 × 10−6), indicating a low Pb-related cancer risk, and adults (5.07 × 10−7 ± 2.68 × 10−7), indicating a very low Pb-related cancer risk (Table S9). According to the TCR, the species are ranked in descending order of Pb-related cancer risk as follows: C. carpio > C. sapidus > A. rostrata > L. auritus > I. punctatus > M. americana > M. salmoides (Figure 5b).
The TCR assessment indicates that the potential carcinogenic health risks to population in the study area from trace metal exposure through the consumption of studied species are within the recommended limit (1 × 10−6 ± 1 × 10−4) (Figure 5b). Comparable findings have been reported in several other studies. For instance, Habib et al. [79] investigated trace metal bioaccumulation in wild fish species from groundwater sources in Egypt, highlighting potential health risks associated with heavy metal contamination. Sadighara et al. [80] assessed heavy metal concentrations in aquatic animals, emphasizing the implications for human health. Similarly, Taghavi et al. [81] evaluated trace metal levels in fish from Iranian coastal waters, discussing the environmental conditions influencing metal accumulation.
All these results also have planning implications for local public health. As outlined in the New Jersey Department of Environmental Protection (NJDEP) fish consumption advisories, the consumption of many species from the LPR, including species such as catfish and American eel, have already been listed as limited or prohibited because of elevated metal concentrations [82]. The findings presented in this study align with those potential risks, particularly for the Pb-associated risks for common carp (C. carpio) and American eel (A. rostrata) and for the Cu-associated risks of blue crabs (C. sapidus). While the calculated values for both TCR and TTHQ pertaining to the consumption of those fish were largely risk-free, with the exception of TTHQ > 1 for children, highlight continued fish consumption monitoring and adherence to NJDEP guidelines to help avoid long-term exposure. As such, this research captures the reality of exposure risks to certain populations, the need for continuous risk communication, and ongoing regulation of consuming aquatic species.
3.5.4. Probabilistic Evaluation of Health Risks and Sensitivity Analysis
The carcinogenic risks associated with lead (Pb) were evaluated using the Monte Carlo Simulation method. The average probability of TCR for lead found in this investigation was 9.28 × 10−7 ± 2.32 × 10−7 for children and 9.29 × 10−7 ± 2.30 × 10−7 for adults (Figure 6a,b). TCR’s 5th and 95th percentile values were 4.92 × 10−7 and 1.81 × 10−6 for children, and 5.05 × 10−7 and 1.77 × 10−6 for adults, respectively. These values indicate that the cancer risk from Pb exposure is within an acceptable range (very low to low) across the mean, 5th, and 95th percentiles. Sensitivity analysis was employed to assess the importance of the input factors in relation to the TCR computation, revealing that Pb concentration is the most critical factor influencing TCR values [83]. For children, the variables C (17.0%), Ep (16.9%), Fintake (16.3%), and Ed (15.0%) showed positive effects on Pb-induced TCR. Conversely, BW (−17.5%) and MT (−17.3%) had negative effects. On the other hand, for adults, C (17.3%), Fintake (17.2%), Ed (17.0%), and Ep (16.9%) showed positive effects on Pb-induced TCR, contributing 17.3%, 17.2%, 17.0%, and 16.9%, respectively, while BW and MT showed negative effects at −15.8% each (Figure 6c,d). These results indicate that metal concentration is a significant determinant of carcinogenic risk. In evaluating cancer risk for children, body weight and exposure duration are also critical factors due to their heightened vulnerability. However, other researchers have found that contaminant concentration remains the primary determinant of cancer risk [84], highlighting the multifactorial nature of exposure assessment.
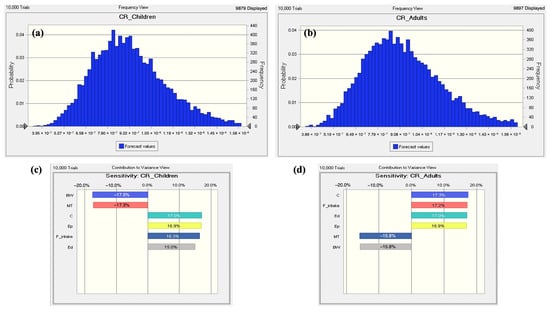
Figure 6.
Predicted probability distribution [(a) children and (b) adults] and sensitivity analysis [(c) children and (d) adults] results of the TCR for the aquatic edible species.
3.6. Multivariate Analysis
Trace metal distributions were further assessed using cluster analysis (CA), Pearson’s correlation matrix (PCM) and principal component analysis (PCA) (Figure S3). Hierarchical clustering with Ward’s method (Figure S3a) revealed two separate clusters: Cu formed its own group, and Pb and Hg clustered together representing the same variance. This implies Cu shows a different concentration and is likely from different sources compared to Pb and Hg.
The Pearson’s correlation matrix (Figure S3b) showed weak positive correlations between Cu-Pb (0.13) and Pb-Hg (0.12) and a weak negative correlation between Cu-Hg (−0.27), which again suggests that these trace metals are either from different sources or effect different processes in the environment. The PCA biplot (Figure S3c) indicated Component 1 and Component 2 explained 42.5% and 36.1% of the total variance, respectively, with Cu loading strongly along Component 1 and Pb and Hg loading along Component 2, similar to our cluster analysis and suggesting some separation in origin and behavior.
Ecologically, this implies that Cu contamination in fish and sediments is probably attributed to anthropogenic inputs such as industrial effluents, antifouling paints, and agricultural runoff [62,85]. In contrast, Pb and Hg exhibit co-association, implying similar diffuse sources such as atmospheric deposition and fossil fuel combustion and accumulation over prolonged time scales [12,17,48]. From a mechanistic perspective, the observed clustering may reflect differences in geochemical speciation: Cu is likely to form stable organic complexes and adsorb to particulates while Hg and Pb are more strongly associated with sulfide or organic-sulfur ligands under low redox conditions, contributing to their long-term persistence and biological availability [17,63]. The distinctiveness of Cu, with respect to Pb and Hg, also reflects fundamentally different geochemical pathway and bioavailability, and sources of each metal species in aquatic systems.
3.7. Self-Organizing Map
The contamination patterns and classifications induced by trace metals were evaluated using Self-Organizing Map (SOM) analysis, with the results illustrated in Figure 7. Color gradients are utilized to demonstrate the significance of the variables presented for every SOM unit, where similar and different colors indicate positive and negative associations between variables, respectively. Elevated concentrations of Cu were identified in the upper right region of the maps, while Pb concentrations were prominent in the upper left region, and Hg exhibited high positive concentrations on the left side of the map (Figure 7a). The SOM analysis also delineated two clusters of sampling points: cluster 1 with 1500 samples and cluster 2 with 536 samples (Figure 7b).
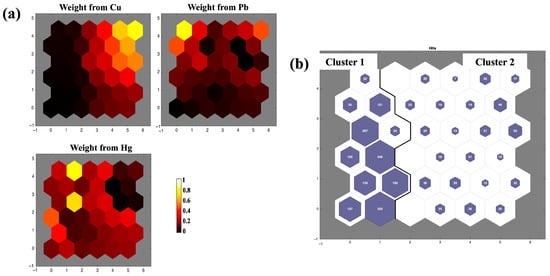
Figure 7.
SOM projection [(a) trace metal concentrations, and (b) cluster map] in the edible aquatic species.
The spatial separation observed in the SOM output indicates different environmental and physiological controls on metal accumulation. Hg accumulation in identified neurons reflects regions of high bioavailability where methylation processes and trophic transfer are key, whereas Cu and Pb clusters co-occur in areas of particulate adsorption and redox-sensitive sediment–water interactions [60,66]. These findings align with those of Bhuiyan et al. [24], who also employed SOM to assess trace metal contamination. In their study, high Pb concentrations were found in the lower left neurons of the SOM, and Cd was concentrated in the upper left corner, indicating spatially distinct patterns of metal accumulation and variable associations across the SOM grid.
3.8. Positive Matrix Factorization (PMF) Model
The study utilized the EPA PMF (V.5.0) model to assess relative contributions of trace metals in aquatic species. The PMF model was evaluated by ensuring residuals were within the range of –0.5 to +0.5 and by minimizing the Q-value. The PMF model was run 20 times with factor solutions ranging from 2–5 in order to find the best solution for the model and observed and predicted values were strongly correlated (R2 = 0.99 for Hg; R2 = 1 for Cu and Pb), indicating reliability to the model (Table S10).
Factor 1 found 100% of Hg (Figure 8) that exceeded the sediment concentration in the sediment (1.8 mg/kg), which was above the Earth’s shale value (Table S5), indicating strong anthropogenic influence. Likely sources of Hg include coal combustion, industrial discharge, metal processing, waste incineration, and atmospheric deposition [20,86,87,88], which have been identified in previous studies in similar aquatic systems.
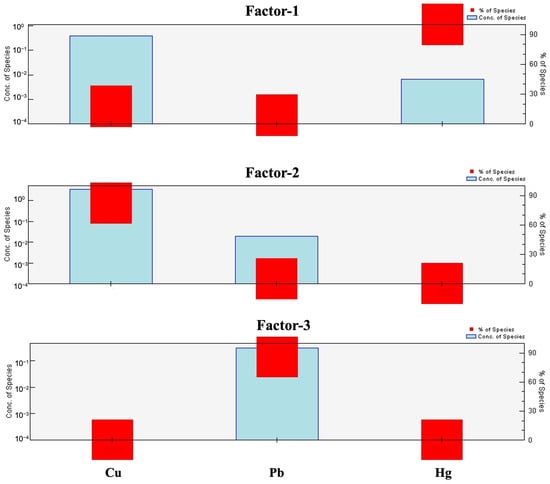
Figure 8.
Source profiles and contributions of trace metals in aquatic species samples are derived.
Copper (Cu) with 82.14% loading was the main determinant of the factor 2. The input of Cu can be attributed to industrial emissions (e.g., metal processing and manufacturing), landfill leachate, combustion of fossil fuels, wood processing, fertilizer production, and natural influences from soil dust, volcanic eruptions, as well as forest fires [89,90]. Minor contributions of Cu into the aquatic matrix can also occur from vehicular emissions via atmospheric deposition and runoff.
The factor 3 was characterized by Pb (85.91% loading). The high concentrations of Pb have been attributed to the use of Pb-containing compounds during industrial activities, metal mining, coal, and oil combustion, waste incineration, and atmospheric deposition. Although the earth possesses some natural sources of Pb in soils and sediments, these anthropogenic activities are mainly responsible for contamination measurements [91,92].
In conclusion, the results of the PMF analysis indicate that Hg receives a considerable influence from industrial and atmospheric activity. Cu has contributions from both industrial and natural activities, while Pb is mainly from industrial, mining, and combustion sources. These results indicate that mitigating trace metal pollution requires management strategies targeting specific anthropogenic activities.
The results from this study collectively demonstrate a clear pathway from contaminant sources through ecological accumulation to potential human exposure. Through the PMF model, it was identified that anthropogenic sources (particularly industrial discharge and atmospheric deposition), were the main driving force of anthropogenic trace metal loading to the LPR. These anthropogenic trace metal consequently bioaccumulated into biota (Hg > Cu > Pb), as supported by BCF and ANN modeling. Bioaccumulated trace metal transferred and confirmed as exposure values in human risk values based upon dietary intake exposures (EDI, HQ and TCR). These scientific findings demonstrate a pathway between anthropogenic sources, to ecological contamination, trophic transfer, and finally were risk to human health. These findings provide evidence for the need for an integrated monitoring and mitigation approaches in contaminated river systems.
3.9. Limitations
This study offers valuable insights into trace metal contamination in edible aquatic species from the LPR, focusing on noncarcinogenic and carcinogenic risks using hazard quotient (HQ), hazard index (HI), and carcinogenic risk (CR) metrics. However, the analysis was limited to three trace metals (Cu, Pb, and Hg) across seven species due to data constraints. The inclusion of cadmium (Cd), arsenic (As), and antimony (Sb) in future studies is essential because they are extremely toxic, resistant to degradation, and can bioaccumulate in aquatic food webs. Cadmium and arsenic have long been known to be carcinogenic and result in systemic health effects, even at relatively low levels of exposure, while antimony is gaining attention as an industrial pollutant. The omission of these elements therefore likely underestimates overall health risks and could obscure possible mixed or interactive effects among trace metals. First, the dataset used in this study comprised data from 2019 and 2021 and lacked both seasonal and temporal variability, which may affect metal concentrations and bioaccumulation tendencies. Second, this study did not address food processing, cooking, or other dietary practices that may alter human exposure to trace metals. Finally, a limitation of using secondary data is the potential for uncertainty due to sampling bias, analytical variability, and incomplete spatial coverage. Future studies should consider assessing a wider range of metals, the temporality and seasonality of metals, potential seasonality of metal concentrations, and the impact of food processing to provide a fuller assessment of human health risk.
4. Conclusions
This study evaluated the concentrations and sources of three trace metals (Cu, Pb, and Hg) in commonly consumed aquatic species, and the potential human health risks in the Lower Passaic River, New Jersey. The findings indicate significant variability in trace metal concentrations among aquatic species, likely due to dietary behavior and habitat differences. Among the trace metals, Hg exhibited the highest mobility, while Cu and Pb showed lower mobility. While both sediment and water have implications for trace metal bioavailability, the current results suggest that, under the current conditions, dissolved-phase metals in submerged water may have a larger contribution to bioaccumulation, but this should be interpreted cautiously, as sediment–water interactions and Fe/Mn oxides bound metal were not tested directly. Compared to other investigated species, A. rostrata and I. punctatus showed considerable metal accumulation and greater metal pollution indices.
Multivariate analyses (CA, PCA, PMF, and SOM) suggest that trace metals in aquatic species originated from diverse sources and/or manufacturing systems. The target hazard quotient (THQ) for children from individual trace metals (except Cu in C. sapidus) and the total target hazard quotient (TTHQ) for combined trace metals (except in C. sapidus and M. americana) indicated no significant non-carcinogenic health risks. The TTHQ values for children in certain species exceeded the threshold of 1, implying potential non-carcinogenic risks for this sensitive population that warrant further investigation. The Pb-associated total carcinogenic risk (TCR) from aquatic species consumption ranged from very low to low, remaining within tolerable limits (TCR < 1 × 10−4); nonetheless, the cumulative and chronic carcinogenic potential associated with Pb signifies the necessity of continued monitoring. Thus, while current metal levels may not create immediate and overall health risks for adults, the heightened risk indices for children, coupled with the long-term survivability of Pb, necessitate scientific circumspection when constructing health risk implications.
From the standpoint of environmental management and policy, these results underscore the essential need for continual monitoring of the trace metals, management of industrial discharges and urban runoff, and the applicability of aquatic specie contamination data to regional water quality management programs. Further, regulators can consider the addition of even more bioaccumulation indicators within their frameworks, as well as carrying out campaigns in public awareness regarding safe aquatic specie consumption, specifically for sensitive populations. In general, support for these actions can enhance evidence-based environmental management and protect the human and ecosystem health of the Lower Passaic River and urban waterways in the future. This work concentrated more on human health risks than on ecological risk assessment, but the combined analytical framework lays a strong foundation for future research that integrates human exposure modeling with ecological risk indicators (e.g., ERI/PERI) or advances (e.g., ANN, RF) in machine learning. Integrating human health risk modeling and ecological risk indicators will provide more insights into source–risk connections and broaden the applicability of this research design for integrated environmental risk management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17223254/s1, Figure S1. Map of the water sampling points: Dundee Dam to river mile 8.3 in the Lower Passaic River.; Figure S2. APLI value in edible aquatic species in the study area.; Figure S3. Multivariate analysis results [(a) cluster analysis (CA), (b) Pearson correlation matrix (PCM), and (c) principal component analysis (PCA)] for trace metals in seven edible aquatic species in LPR.; Table S1. Summary of the detection frequency and reporting limits (RLs) for trace metals in tissue samples collected from LPR RM 8.3 to Dundee Dam (2019 & 2021).; Table S2. Human health risk assessment methods based on USEPA guidelines.; Table S3. Exposure parameters and their values based on USEPA guidelines.; Table S4. Reference doses (RfD) and cancer slope factors (CSF) for the studied trace metals based on USEPA guidelines.; Table S5. Trace metal concentrations in surface waters (n = 198) and sediments (n = 200), based on samples collected between 2019 and 2021.; Table S6. Bioaccumulation of trace metals from sediment and water in aquatic species.; Table S7. Data file for BCFW and BCFS derived from the artificial neural network (ANN).; Table S8. Estimate the target hazard quotient (THQ) and total target hazard quotient (TTHQ) of trace metals from the consumption of aquatic species; Table S9. Target Carcinogenic Risk (TCR) of Pb from trace metals in consumed aquatic species; Table S10. Accuracy metrics of the positive matrix factorization (PMF) model for trace metal analysis in edible aquatic species
Author Contributions
M.S.I.—conceptualization; formal analysis; visualization; methodology; software; writing of original draft. S.M.—review and editing. H.F.—supervision, review and editing. T.K.C.—review and editing. Y.Q.—review and editing. S.Y.—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Montclair State University Doctoral Assistantship, the Anchor QEA Scholarship, and the ERI Scholarship.
Data Availability Statement
The original data presented in the study are openly available in Passaic River Public Digital Library at https://sharepoint.ourpassaic.org/SitePages/Home.aspx (accessed on 1 October 2024).
Acknowledgments
The authors would like to thank the two anonymous reviewers for their constructive comments and suggestions, which have improved the quality of an early version of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mansour, S.A.; Belal, M.H.; Abou-Arab, A.A.K.; Gad, M.F. Monitoring of Pesticides and Heavy Metals in Cucumber Fruits Produced from Different Farming Systems. Chemosphere 2009, 75, 601–609. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of Heavy Metals in Fruits and Vegetables: Health Risk Implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological Risk Assessment of Heavy Metals in Sediment and Human Health Risk Assessment of Heavy Metals in Fishes in the Middle and Lower Reaches of the Yangtze River Basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Islam, M.S.; Ghosh, G.C.; Ghosh, P.; Zaman, S.; Hossain, M.R.; Habib, A.; Nice, M.S.; Rahman, M.S.; Islam, K.R.; et al. Receptor Model-Based Sources and Risks Appraisal of Potentially Toxic Elements in the Urban Soils of Bangladesh. Toxicol. Rep. 2023, 10, 308–319. [Google Scholar] [CrossRef]
- Zakir, H.M.; Quadir, Q.F.; Mollah, M.Z.I. Human Health Risk Assessment of Heavy Metals Through the Consumption of Common Foodstuffs Collected from Two Divisional Cities of Bangladesh. Expo. Health 2021, 13, 253–268. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M. Assessment of Heavy Metals Mobility and Toxicity in Contaminated Sediments by Sequential Extraction and a Battery of Bioassays. Ecotoxicology 2015, 24, 1279–1293. [Google Scholar] [CrossRef]
- Ye, S.; Lin, M.; Li, L.; Liu, J.; Song, L.; Li, Z. Abundance and Spatial Variability of Invasive Fishes Related to Environmental Factors in a Eutrophic Yunnan Plateau Lake, Lake Dianchi, Southwestern China. Environ. Biol. Fishes 2015, 98, 209–224. [Google Scholar] [CrossRef]
- Revenga, J.E.; Campbell, L.M.; Arribére, M.A.; Ribeiro Guevara, S. Arsenic, Cobalt and Chromium Food Web Biodilution in a Patagonia Mountain Lake. Ecotoxicol. Environ. Saf. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Mataba, G.R.; Verhaert, V.; Blust, R.; Bervoets, L. Distribution of Trace Elements in the Aquatic Ecosystem of the Thigithe River and the Fish Labeo Victorianus in Tanzania and Possible Risks for Human Consumption. Sci. Total Environ. 2016, 547, 48–59. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Rahman, M.; Sultana, S.; Babu, S.M.O.F.; Sarker, M.S.I. Bioaccumulation and Heavy Metal Concentration in Tissues of Some Commercial Fishes from the Meghna River Estuary in Bangladesh and Human Health Implications. Mar. Pollut. Bull. 2019, 145, 436–447. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, G.C.; Ghosh, P.; Jahan, I.; Zaman, S.; Islam, M.S.; Hossain, M.R.; Habib, A.; Biswas, B.; Sultana, N.; et al. Arsenic, Iron, and Manganese in Groundwater and Its Associated Human Health Risk Assessment in the Rural Area of Jashore, Bangladesh. J. Water Health 2022, 20, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Gobeille, A.K.; Morland, K.B.; Bopp, R.F.; Godbold, J.H.; Landrigan, P.J. Body Burdens of Mercury in Lower Hudson River Area Anglers. Environ. Res. 2006, 101, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M. Determination of Heavy Metals in Fish and Vegetables in Bangladesh and Health Implications. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 986–1006. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Islam, M.S.; Ghosh, G.C.; Ghosh, P.; Zaman, S.; Habib, A.; Hossain, M.R.; Bosu, H.; Islam, M.R.; Imran, M.A.; et al. Human Health Risk and Hydro-Geochemical Appraisal of Groundwater in the Southwest Part of Bangladesh Using GIS, Water Quality Indices, and Multivariate Statistical Approaches. Toxin. Rev. 2023, 42, 285–299. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, G.C.; Hossain, M.R.; Islam, M.S.; Habib, A.; Zaman, S.; Bosu, H.; Nice, M.S.; Haldar, M.; Khan, A.S. Human Health Risk and Receptor Model-Oriented Sources of Heavy Metal Pollution in Commonly Consume Vegetable and Fish Species of High Ganges River Floodplain Agro-Ecological Area, Bangladesh. Heliyon 2022, 8, e11172. [Google Scholar] [CrossRef]
- Javed, M.B.; Cuss, C.W.; Grant-Weaver, I.; Shotyk, W. Size-Resolved Pb Distribution in the Athabasca River Shows Snowmelt in the Bituminous Sands Region an Insignificant Source of Dissolved Pb. Sci. Rep. 2017, 7, 43622. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef]
- Fernandes Azevedo, B.; Barros Furieri, L.; Peçanha, F.M.; Wiggers, G.A.; Frizera Vassallo, P.; Ronacher Simões, M.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar] [CrossRef]
- Iannuzzi, T.J.; Ludwig, D.F. Historical and Current Ecology of the Lower Passaic River. Urban Habitats 2004, 2, 147–173. [Google Scholar]
- Olson, K.R.; Tharp, M. How Did the Passaic River, a Superfund Site near Newark, New Jersey, Become an Agent Orange Dioxin TCDD Hotspot? J. Soil Water Conserv. 2020, 75, 33A–37A. [Google Scholar] [CrossRef]
- Aluthgun Hewage, S.; Batagoda, J.H.; Meegoda, J.N. Remediation of Contaminated Sediments Containing Both Organic and Inorganic Chemicals Using Ultrasound and Ozone Nanobubbles. Environ. Pollut. 2021, 274, 116538. [Google Scholar] [CrossRef] [PubMed]
- Wirgin, I.; Chambers, R.C.; Waldman, J.R.; Roy, N.K.; Witting, D.A.; Mattson, M.T. Effects of Hudson River Stressors on Atlantic Tomcod: Contaminants and a Warming Environment. Rev. Fish. Sci. Aquac. 2023, 31, 342–371. [Google Scholar] [CrossRef]
- Olson, K.R.; Cihacek, L. How United States Agricultural Herbicides Became Military and Environmental Chemical Weapons: Historical and Residual Effects. Open J. Soil Sci. 2022, 12, 13–81. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Karmaker, S.C.; Bodrud-Doza, M.; Rakib, M.A.; Saha, B.B. Enrichment, Sources and Ecological Risk Mapping of Heavy Metals in Agricultural Soils of Dhaka District Employing SOM, PMF and GIS Methods. Chemosphere 2021, 263, 128339. [Google Scholar] [CrossRef]
- Kuerban, M.; Maihemuti, B.; Waili, Y.; Tuerhong, T. Ecological Risk Assessment and Source Identification of Heavy Metal Pollution in Vegetable Bases of Urumqi, China, Using the Positive Matrix Factorization (PMF) Method. PLoS ONE 2020, 15, e0230191. [Google Scholar] [CrossRef]
- Shi, L.; Li, J.; Palansooriya, K.N.; Chen, Y.; Hou, D.; Meers, E.; Tsang, D.C.W.; Wang, X.; Ok, Y.S. Modeling Phytoremediation of Heavy Metal Contaminated Soils through Machine Learning. J. Hazard Mater. 2023, 441, 129904. [Google Scholar] [CrossRef]
- Windward, 2019 and 2021 Biota Data Summary Report. 2023. Available online: https://www.windwardenv.com/publications-and-presentations/ (accessed on 12 December 2024).
- Windward. Quality Assurance Project Plan: Fish and Decapod Crustacean Tissue Collection for Chemical Analysis and Fish Community Survey. 2009. Available online: https://sharepoint.ourpassaic.org/Public%20Documents/2009-08-06%20FINAL%20Fish%20Tissue%20Collection%20QAPP%20CPG.pdf (accessed on 22 May 2025).
- Windward. Current Conditions Addendum to the Quality Assurance Project Plan. Fish and Crab Tissue Collection for Chemical Analysis. Lower Passaic River Restoration Project Lower Passaic River Study Area RI/FS. 2021. Available online: https://www.windwardenv.com/publications-and-presentations/ (accessed on 12 December 2024).
- AECOM. Small Volume Chemical Water Column Monitoring—Data Summary Report Phase 1. 2022. Available online: https://sharepoint.ourpassaic.org/Datasets/20170130_Crosswalk_to_PassaicRiver-NewarkBay_Public_Datasets.xlsx (accessed on 15 February 2025).
- Soetan, O.; Nie, J.; Feng, H. Preliminary Environmental Assessment of Metal-Contaminated Sediment Dredging in an Urban River, New Jersey, USA. Mar. Pollut. Bull. 2022, 184, 114212. [Google Scholar] [CrossRef]
- Streit, B. Bioaccumulation of Contaminants in Fish. In Fish Ecotoxicology; Birkhäuser: Basel, Switzerland, 1998; pp. 353–387. [Google Scholar]
- Sobhanardakani, S. Tuna Fish and Common Kilka: Health Risk Assessment of Metal Pollution through Consumption of Canned Fish in Iran. J. Consum. Prot. Food Saf. 2017, 12, 157–163. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Tayebi, L.; Hosseini, S.V. Health Risk Assessment of Arsenic and Heavy Metals (Cd, Cu, Co, Pb, and Sn) through Consumption of Caviar of Acipenser Persicus from Southern Caspian Sea. Environ. Sci. Pollut. Res. 2018, 25, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle Proximate Composition of Various Food Fish Species and Their Nutritional Significance: A Review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Arnot, J.A.; Gobas, F.A. A Review of Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Assessments for Organic Chemicals in Aquatic Organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.D.; Russell, M.H.; Buck, R.C. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Liang, Y.; Wang, H.; Dong, Y.H.; Leung, S.Y.; Wong, M.H. Bioaccumulation of Heavy Metals in Fish and Ardeid at Pearl River Estuary, China. Ecotoxicol. Environ. Saf. 2014, 106, 62–67. [Google Scholar] [CrossRef]
- Bertato, L.; Chirico, N.; Papa, E. Predicting the Bioconcentration Factor in Fish from Molecular Structures. Toxics 2022, 10, 581. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Mobaswara, M.Z.; Nice, M.S.; Islam, K.R.; Netema, B.N.; Rahman, M.S.; Habib, A.; Zaman, S.; Ghosh, G.C.; Tul-Coubra, K.; et al. Application of Machine Learning and Multivariate Approaches for Source Apportionment and Risks of Hazardous Elements in the Cropland Soils near Industrial Areas in Bangladesh. Ecol. Indic. 2023, 154, 110856. [Google Scholar] [CrossRef]
- Elzwayie, A.; El-shafie, A.; Yaseen, Z.M.; Afan, H.A.; Allawi, M.F. RBFNN-Based Model for Heavy Metal Prediction for Different Climatic and Pollution Conditions. Neural Comput. Appl. 2017, 28, 1991–2003. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Lu, J.; Lai, Z.; Tong, Y.; Wang, M. Trace Metals (As, Cd, Cr, Cu, Hg, Ni, Pb, Zn) and Stable Isotope Ratios (Δ13C and Δ15N) in Fish from Wulungu Lake, Xinjiang, China. Int. J. Environ. Res. Public Health 2021, 18, 9007. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Ai, S.; Yi, J.; Wu, F.; Li, J.; Liu, Z.; Zhang, J. Response of Aquatic Ecosystems Multi-Trophic Biological Communities under Multiple Pollutants Stress. Sci. Total Environ. 2024, 955, 177001. [Google Scholar] [CrossRef]
- Wang, J.N.; Ma, P.C.; Zhang, L.J.; Chen, M.B.; Huang, C.S.; Liu, X.L.; Hu, G.C.; Xu, Z.C. Accumulation Characteristics and Health Risk Assessment of Heavy Metals in Wild Fish Species from Diaojiang River, Guangxi. Environ. Sci. 2017, 38, 2600–2606. [Google Scholar] [CrossRef]
- USEPA. Screening Level Ecological Risk Assessment Protocol for Hazardous Waste Combustion Facilities. Appendix E: Toxicity Reference Values. 1999. Available online: https://lmpublicsearch.lm.doe.gov/sitedocs/sw-a-005922.pdf (accessed on 14 April 2025).
- USEPA Regional Screening Levels (RSLs)—Generic Tables. 2025. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 6 July 2025).
- Eid, M.H.; Eissa, M.; Mohamed, E.A.; Ramadan, H.S.; Tamás, M.; Kovács, A.; Szűcs, P. New Approach into Human Health Risk Assessment Associated with Heavy Metals in Surface Water and Groundwater Using Monte Carlo Method. Sci. Rep. 2024, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lučić, M.; Momčilović, M.; Marković, J.; Jović, M.; Smičiklas, I.; Onjia, A. Monte Carlo Simulation of Health Risk from Cadmium, Lead, and Nickel in Cigarettes. Toxicol. Environ. Chem. 2023, 105, 92–110. [Google Scholar] [CrossRef]
- USEPA. Guiding Principles for Monte Carlo Analysis. 1997. Available online: https://www.epa.gov/risk/guiding-principles-monte-carlo-analysis (accessed on 14 April 2025).
- USEPA. EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide. 2014. Available online: https://www.epa.gov/air-research/epa-positive-matrix-factorization-50-fundamentals-and-user-guide (accessed on 13 May 2025).
- Taylor, S.R.; McLennan, S.M. The Continental Crust, Its Composition and Evolution: An Examination of the Geochemical Record Preserved in Sedimentary Rocks; Blackwell Scientific Publications: Oxford, UK, 1985; ISBN 0632011483. [Google Scholar]
- JECFA. Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 922; World Health Organization: Geneva, Switzerland, 2004; ISBN 92-4-120922-4. [Google Scholar]
- FAO/WHO. Report of the Eighth Session of the Codex Committee on Contaminants in Foods; Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission; Food and Agriculture Organization of the United Nations/World Health Organization: Rome, Italy, 2014. [Google Scholar]
- Amiard, J.; Amiardtriquet, C.; Barka, S.; Pellerin, J.; Rainbow, P. Metallothioneins in Aquatic Invertebrates: Their Role in Metal Detoxification and Their Use as Biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Warren, L.A.; Haack, E.A. Biogeochemical Controls on Metal Behaviour in Freshwater Environments. Earth Sci. Rev. 2001, 54, 261–320. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C. Partitioning of Trace Metals in Sediments: Relationships with Bioavailability. Hydrobiologia 1987, 149, 43–52. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate Stimulation of Mercury Methylation in Freshwater Sediments. Environ. Sci. Technol. 1992, 26, 2281–2287. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms Regulating Mercury Bioavailability for Methylating Microorganisms in the Aquatic Environment: A Critical Review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef]
- USEPA. Technical Support Document For Water Quality-Based Toxics Control. 1991. Available online: https://www.epa.gov/system/files/documents/2024-07/technical-support-document-for-water-quality-based-toxics-control.pdf (accessed on 12 July 2025).
- Schwab, L.; Gallati, N.; Reiter, S.M.; Kimber, R.L.; Kumar, N.; McLagan, D.S.; Biester, H.; Kraemer, S.M.; Wiederhold, J.G. Mercury Isotope Fractionation during Dark Abiotic Reduction of Hg(II) by Dissolved, Surface-Bound, and Structural Fe(II). Environ. Sci. Technol. 2023, 57, 15243–15254. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Zhang, Y. Climate-Driven Changes of Global Marine Mercury Cycles in 2100. Proc. Natl. Acad. Sci. USA 2023, 120, e2202488120. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M. Copper. In Homeostasis and Toxicology of Essential Metals; Academic Press: Cambridge, MA, USA, 2011; pp. 53–133. [Google Scholar]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic Effects of Lead Exposure on Bioaccumulation, Oxidative Stress, Neurotoxicity, and Immune Responses in Fish: A Review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Eckley, C.S.; Achá, D.; Feng, X.; Gilmour, C.C.; Jonsson, S.; Mitchell, C.P.J. Challenges and Opportunities for Managing Aquatic Mercury Pollution in Altered Landscapes. Ambio 2018, 47, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of Dissolved Organic Matter (DOM) Characteristics on Dissolved Mercury (Hg) Species Composition in Sediment Porewater of Lakes from Southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Zeng, K.; Liu, L.; Zheng, N.; Yu, Y.; Xu, S.; Yao, H. Iron at the Helm: Steering Arsenic Speciation through Redox Processes in Soils. Environ. Res. 2025, 274, 121327. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Wang, W.-X.; Rainbow, P.S. Comparative Approaches to Understand Metal Bioaccumulation in Aquatic Animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 315–323. [Google Scholar] [CrossRef]
- Netema, B.N.; Chakraborty, T.K.; Nice, M.S.; Islam, K.R.; Debnath, P.C.; Chowdhury, P.; Rahman, M.S.; Halder, M.; Zaman, S.; Ghosh, G.C.; et al. Appraisal of Microplastic Pollution and Its Related Risks for Urban Indoor Environment in Bangladesh Using Machine Learning and Diverse Risk Evolution Indices. Environ. Pollut. 2024, 360, 124631. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.M.; Batista, J.E.; Mariano, P.; Fonseca, V.; Duarte, B.; Silva, S. Artificial Intelligence Meets Marine Ecotoxicology: Applying Deep Learning to Bio-Optical Data from Marine Diatoms Exposed to Legacy and Emerging Contaminants. Biology 2021, 10, 932. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, L.; Cao, J.; Li, S.; Yang, Z. Trace Elements in Four Freshwater Fish from a Mine-Impacted River: Spatial Distribution, Species-Specific Accumulation, and Risk Assessment. Environ. Sci. Pollut. Res. 2018, 25, 8861–8870. [Google Scholar] [CrossRef] [PubMed]
- Nyeste, K.; Zulkipli, N.; Uzochukwu, I.E.; Somogyi, D.; Nagy, L.; Czeglédi, I.; Harangi, S.; Baranyai, E.; Simon, E.; Nagy, S.A.; et al. Assessment of Trace and Macroelement Accumulation in Cyprinid Juveniles as Bioindicators of Aquatic Pollution: Effects of Diets and Habitat Preferences. Sci. Rep. 2024, 14, 11288. [Google Scholar] [CrossRef]
- Hasan, M.K.; Shahriar, A.; Hossain, N.; Shovon, I.K.; Hossain, A.; Jolly, Y.N.; Begum, B.A. Trace Metals Contamination in Riverine Captured Fish and Prawn of Bangladesh and Associated Health Risk. Expo. Health 2021, 13, 237–251. [Google Scholar] [CrossRef]
- FAO/WHO. General Standard for Contaminants and Toxins in Food and Feed. 1995. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 14 May 2025).
- USEPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). 1989. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed on 12 April 2025).
- USEPA. Exposure Factors Handbook: 2011 Edition. 2011. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-2011-edition (accessed on 12 May 2025).
- Faustman, E.M.; Silbernagel, S.M.; Fenske, R.A.; Burbacher, T.M.; Ponce, R.A. Mechanisms Underlying Children’s Susceptibility to Environmental Toxicants. Environ. Health Perspect. 2000, 108, 13. [Google Scholar] [CrossRef]
- NCR. Pesticides in the Diets of Infants and Children; National Academies Press: Washington, DC, USA, 1993; ISBN 978-0-309-04875-0. [Google Scholar]
- Habib, S.S.; Naz, S.; Fazio, F.; Cravana, C.; Ullah, M.; Rind, K.H.; Attaullah, S.; Filiciotto, F.; Khayyam, K. Assessment and Bioaccumulation of Heavy Metals in Water, Fish (Wild and Farmed) and Associated Human Health Risk. Biol. Trace Elem. Res. 2024, 202, 725–735. [Google Scholar] [CrossRef]
- Sadighara, P.; Mofid, V.; Mahmudiono, T.; Rahmani, A.; Tajdar-Oranj, B.; Peivasteh-Roudsari, L.; Farhangfar, A.; Moradi, M.; Fakhri, Y. Concentration of Heavy Metals in Canned Tuna Fish and Probabilistic Health Risk Assessment in Iran. Int. J. Environ. Anal. Chem. 2024, 104, 1719–1729. [Google Scholar] [CrossRef]
- Taghavi, M.; Shadboorestan, A.; Kalankesh, L.R.; Mohammadi-Bardbori, A.; Ghaffari, H.R.; Safa, O.; Farshidfar, G.; Omidi, M. Health Risk Assessment of Heavy Metal Toxicity in the Aquatic Environment of the Persian Gulf. Mar. Pollut Bull. 2024, 202, 116360. [Google Scholar] [CrossRef] [PubMed]
- NJDEP. Fish Smart, Eat Smart: A Guide to Healthier Fish Consumption. 2015. Available online: https://dep.nj.gov/dsr/fish-advisories-studies/ (accessed on 11 February 2025).
- Bodrud-Doza, M.; Islam, S.M.D.-U.; Hasan, M.T.; Alam, F.; Haque, M.M.; Rakib, M.A.; Asad, M.A.; Rahman, M.A. Groundwater Pollution by Trace Metals and Human Health Risk Assessment in Central West Part of Bangladesh. Groundw. Sustain. Dev. 2019, 9, 100219. [Google Scholar] [CrossRef]
- Haque, M.M.; Niloy, N.M.; Khirul, M.A.; Alam, M.F.; Tareq, S.M. Appraisal of Probabilistic Human Health Risks of Heavy Metals in Vegetables from Industrial, Non-Industrial and Arsenic Contaminated Areas of Bangladesh. Heliyon 2021, 7, e06309. [Google Scholar] [CrossRef]
- Apte, S.C. Biogeochemistry of Copper in the Fly River. In Developments in Earth and Environmental Sciences; Bolton, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 9, pp. 321–373. ISBN 978-0-444-52964-0. [Google Scholar]
- Kruge, M.A.; Lara-Gonzalo, A.; Gallego, J.L.R. Environmental Forensics of Complexly Contaminated Sites: A Complimentary Fingerprinting Approach. Environ. Pollut. 2020, 263, 114645. [Google Scholar] [CrossRef]
- Sakizadeh, M. Spatial Distribution and Source Identification Together with Environmental Health Risk Assessment of PAHs along the Coastal Zones of the USA. Environ. Geochem. Health 2020, 42, 3333–3350. [Google Scholar] [CrossRef]
- Yildiz, Y.; Karadag, R.; Cheema, M.; Sayedahmed, M. Ion Selective Electrode Determination of Ammonia Nitrogen in Passaic River Waste Water in New Jersey Essex County Area. Am. J. Analyt. Chem. 2022, 13, 96–107. [Google Scholar] [CrossRef]
- Batagoda, J.H.; Hewage, S.D.A.; Meegoda, J.N. Remediation of Heavy-Metal-Contaminated Sediments in USA Using Ultrasound and Ozone Nanobubbles. J. Environ. Eng. Sci. 2019, 14, 130–138. [Google Scholar] [CrossRef]
- USEPA. Reregistration Eligibility Decision (RED) for Coppers. 2009. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1004513.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000008%5CP1004513.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 12 February 2025).
- Walker, W.J.; McNutt, R.P.; Maslanka, C.K. The Potential Contribution of Urban Runoff to Surface Sediments of the Passaic River: Sources and Chemical Characteristics. Chemosphere 1999, 38, 363–377. [Google Scholar] [CrossRef]
- Jaglal, K. Contaminated Aquatic Sediments. Water Environ. Res. 2020, 92, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).