Who Ate Whom—Competition and Predation in a Freshwater Microcosm
Abstract
1. Introduction
2. Materials and Methods
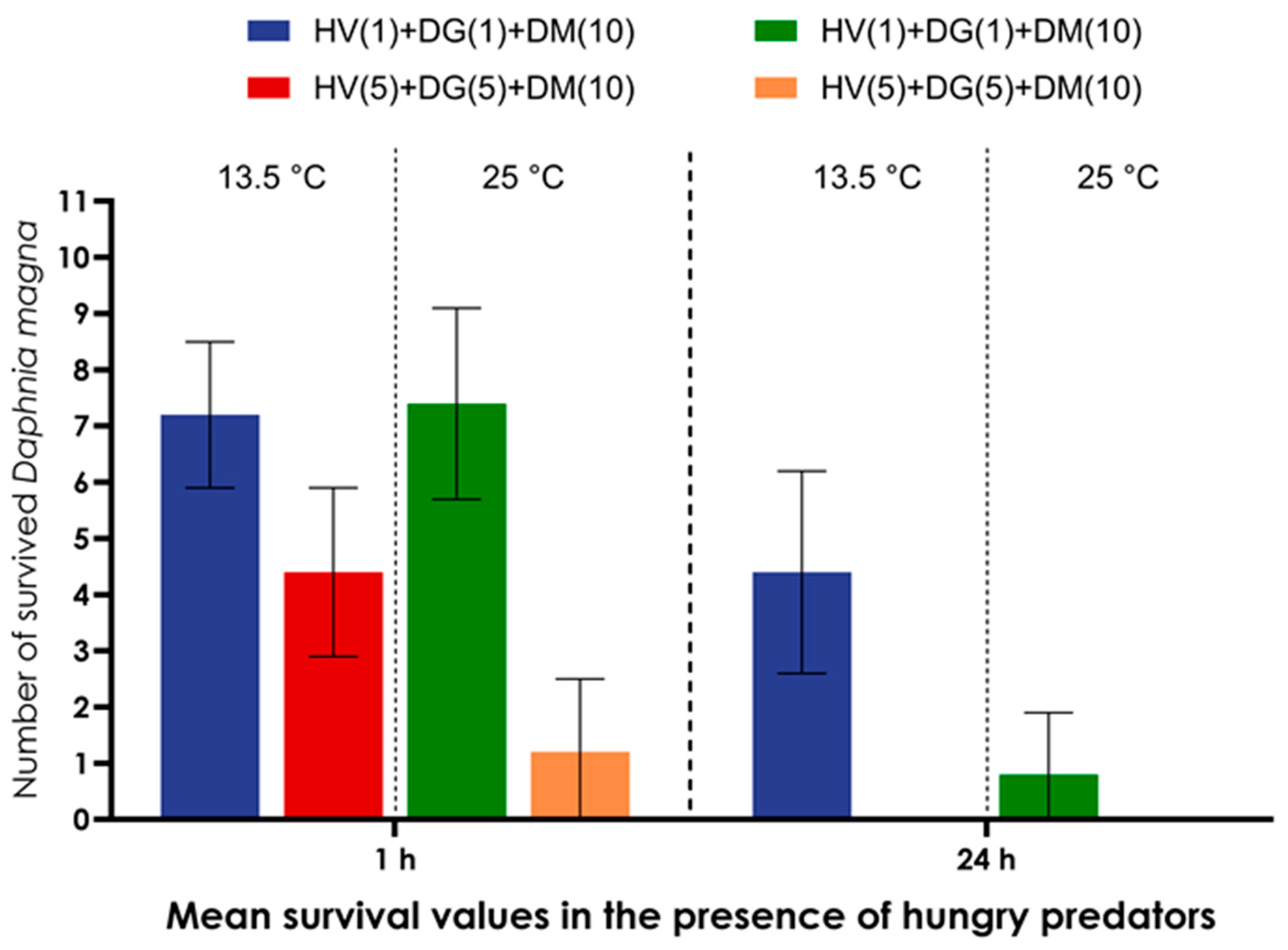
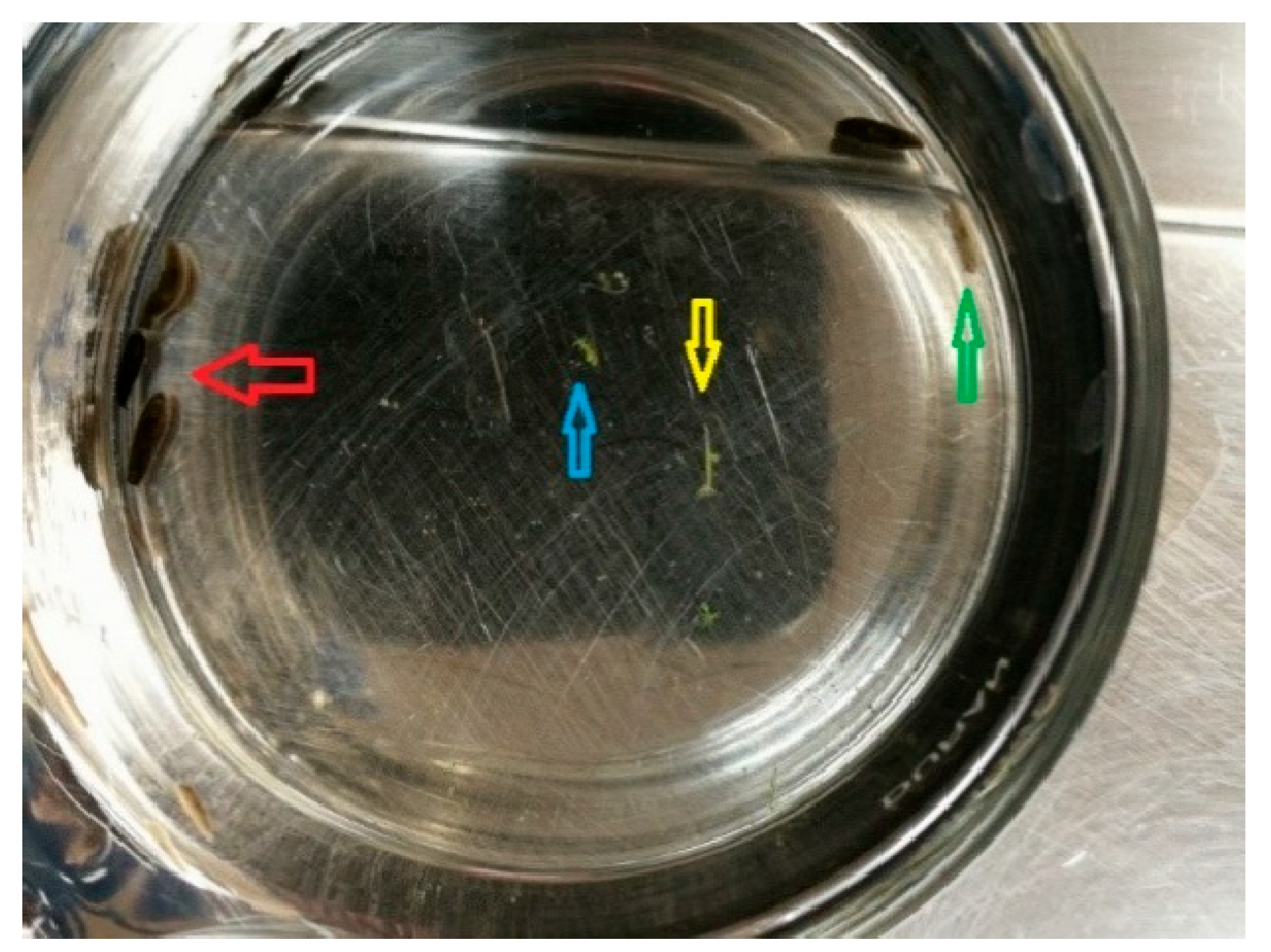
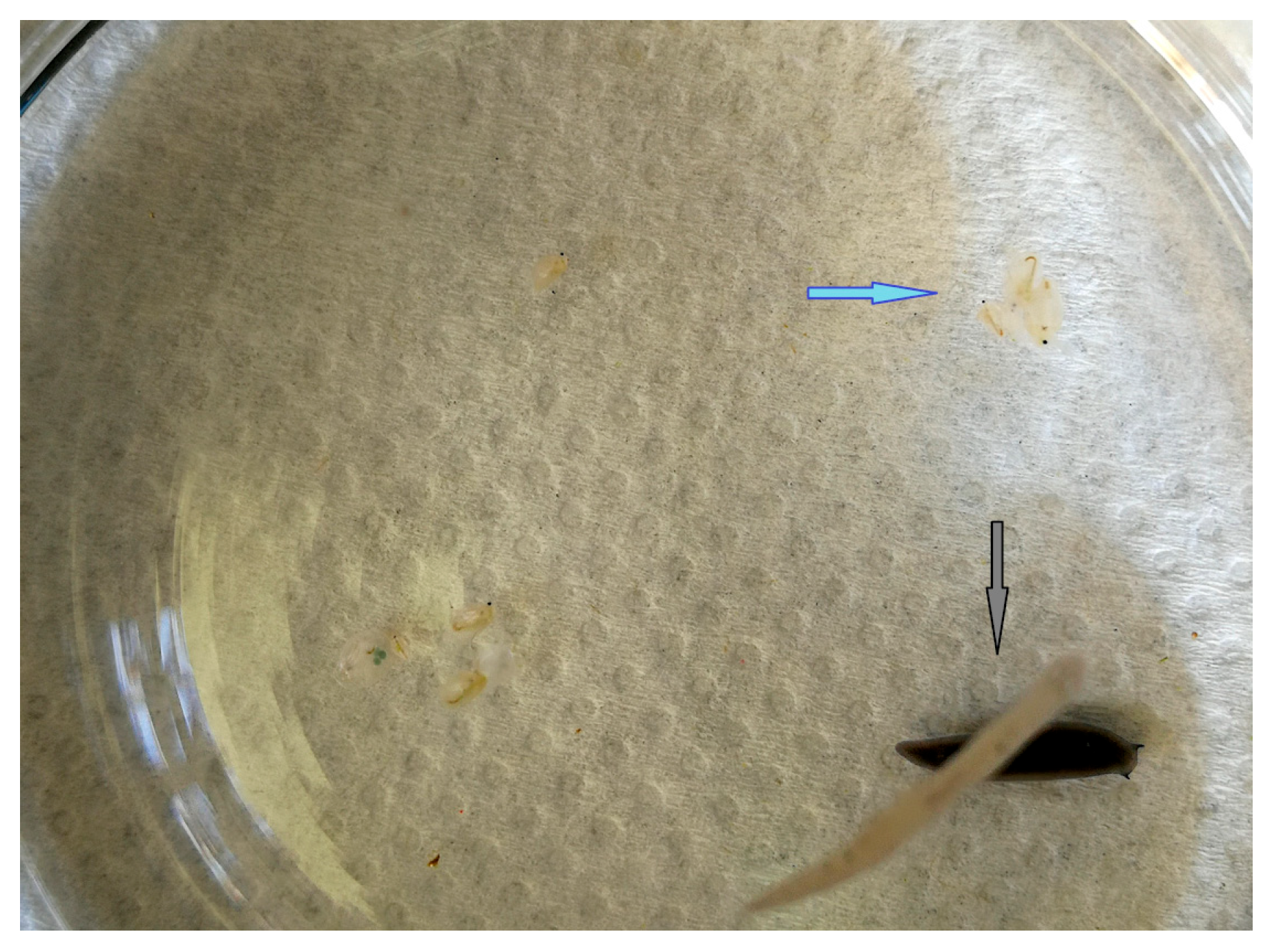
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira de Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Meehan, M.L.; Turnbull, K.F.; Sinclair, B.J.; Lindo, Z. Predators minimize energy costs, rather than maximize energy gains under warming: Evidence from a microcosm feeding experiment. Funct. Ecol. 2022, 36, 2279–2288. [Google Scholar] [CrossRef]
- Sertić Perić, M.; Dražina, T.; Žutinić, P.; Rubinić, J.; Kuczyńska-Kippen, N.; Zhang, C.; Špoljar, M. Ecological Dynamics and Conservation Strategies for Mediterranean Salt Marshes: Insights from a Pilot Study of Biodiversity and Environmental Drivers in the Palud Marsh, Croatia. Sustainability 2024, 16, 10523. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Palkovacs, E.P.; Wasserman, B.A.; Kinnison, M.T. Eco-evolutionary trophic dynamics: Loss of top predators drives trophic evolution and ecology of prey. PLoS ONE 2011, 6, e18879. [Google Scholar] [CrossRef]
- Price, E.L.; Sertić Perić, M.; Romero, G.Q.; Kratina, P. Land use alters trophic redundancy and resource flow through stream food webs. J. Anim. Ecol. 2019, 88, 677–689. [Google Scholar] [CrossRef]
- Lytle, D.A. Life-history and behavioural adaptations to flow regime in aquatic insects. In Aquatic Insects: Challenges to Populations; Royal Entomological Society: St Albans, UK, 2008; pp. 122–138. [Google Scholar] [CrossRef]
- Duarte, R.C.; Flores, A.A.V.; Stevens, M. Camouflage through colour change: Mechanisms, adaptive value and ecological significance. Phil. Trans. R. Soc. B 2017, 372, 20160342. [Google Scholar] [CrossRef]
- Blewett, T.A.; Binning, S.A.; Weinrauch, A.M.; Ivy, C.M.; Rossi, G.S.; Borowiec, B.G.; Lau, G.Y.; Overduin, S.L.; Aragao, I.; Norin, T. Physiological and behavioural strategies of aquatic animals living in fluctuating environments. J. Exp. Biol. 2022, 225, jeb242503. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, C.E.; Tessier, A.J. To sink or swim: Variable diapause strategies among Daphnia species. Limnol. Oceanogr. 2004, 49, 1333–1340. [Google Scholar] [CrossRef]
- Heo, Y.M.; Oh, S.-Y.; Kim, K.; Han, S.-I.; Kwon, S.L.; Yoo, Y.; Kim, D.; Khim, J.S.; Kang, S.; Lee, H.; et al. Comparative Genomics and Transcriptomics Depict Marine Algicolous Arthrinium Species as Endosymbionts That Help Regulate Oxidative Stress in Brown Algae. Front. Mar. Sci. 2021, 8, 753222. [Google Scholar] [CrossRef]
- Vacant, S.; Benites, L.F.; Salmeron, C.; Intertaglia, L.; Norest, M.; Cadoudal, A.; Sanchez, F.; Caceres, C.; Piganeau, G. Long-Term Stability of Bacterial Associations in a Microcosm of Ostreococcus tauri (Chlorophyta, Mamiellophyceae). Front. Plant Sci. 2022, 13, 814386. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, W.; Stoks, R.; Duvivier, C.; Bednarska, A.; De Meester, L. Population dynamics determine genetic adaptation to temperature in daphnia. Evolution 2009, 63, 1867–1878. [Google Scholar] [CrossRef]
- Peck, L.S. Organisms and responses to environmental change. Mar. Genom. 2011, 4, 237–243. [Google Scholar] [CrossRef]
- Stoks, R.; Geerts, A.N.; De Meester, L. Evolutionary and plastic responses of freshwater invertebrates to climate change:realized patterns and future potential. Evol. Appl. 2014, 7, 42–55. [Google Scholar] [CrossRef]
- Abrams, P.A. The Evolution of Predator-Prey Interactions: Theory and Evidence. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 79–105. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Allen, W.L.; Sherratt, T.N.; Speed, M.P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry; Oxford University Press: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Minelli, A. Predation. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2008; pp. 2923–2929. [Google Scholar] [CrossRef]
- Burns, C.W. The relationship between body size of filter-feeding Cladocera and the maximum size of particle ingested. Limnol. Oceanogr. 1968, 13, 675–678. [Google Scholar] [CrossRef]
- Anderson, R.S. Predator relationships and predation rates for crustacean zooplankters from some lakes in western Canada. Can. J. Zool. 1970, 48, 1229–1240. [Google Scholar] [CrossRef]
- Allan, J.D. Competition and the relative abundance of two cladocerans. Ecology 1973, 54, 484–498. [Google Scholar] [CrossRef]
- Dodson, S.I. Zooplankton competition and predation: An experimental test of the size efficiency hypothesis. Ecology 1974, 55, 605–613. [Google Scholar] [CrossRef]
- Muscatine, L.; Lenhoff, H.M. Symbiosis of hydra and algae. II. Effects of limited food and starvation on growth of symbiotic and aposymbiotic hydra. Biol. Bull. 1965, 123, 316–328. [Google Scholar] [CrossRef]
- Weber, J.; Klug, M.; Tardent, P. Some physical and chemical properties of purified nematocysts of Hydra attenuata pall. (Hydrozoa, cnidaria). Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 88, 855–862. [Google Scholar] [CrossRef]
- Calevro, F.; Filippi, C.; Deri, P.; Albertosi, C.; Batistoni, R. Toxic effects of aluminium, chromium and cadmium in intact and regenerating freshwater planarians. Chemosphere 1998, 37, 651–659. [Google Scholar] [CrossRef]
- Kovačević, G.; Gregorović, G.; Kalafatić, M.; Jaklinović, I. The Effect of Aluminium on the Planarian Polycelis felina (Daly.). Water Air Soil Pollut. 2009, 196, 333–344. [Google Scholar] [CrossRef]
- Roca, J.R.; Ribas, M.; Baguñà, J. Distribution, ecology, mode of reproduction and karyology of freshwater planarians (Platyhelminthes; Turbellaria; Tricladida) in the springs of the central Pyrenees. Ecography 1992, 15, 373–384. [Google Scholar] [CrossRef]
- Beier, S.; Bolley, M.; Traunspurger, W. Predator-prey interactions between Dugesia gonocephala and free-living nematodes. Freshw. Biol. 2004, 49, 77–86. [Google Scholar] [CrossRef]
- Miner, B.E.; De Meester, L.; Pfrender, M.E.; Lampert, W.; Hairston, N.G. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B Biol. Sci. 2012, 279, 1873–1882. [Google Scholar] [CrossRef]
- Lynch, M. The evolution of cladoceran life histories. Q. Rev. Biol. 1980, 55, 23–42. [Google Scholar] [CrossRef]
- Ten Berge, W.F. Breeding Daphnia magna. Hydrobiologia 1978, 59, 121–123. [Google Scholar] [CrossRef]
- Bettinetti, R.; Croce, V.; Noè, F.; Ponti, B.; Quadroni, S.; Galassi, S. Ecotoxicity of pp’DDE to Daphnia magna. Ecotoxicology 2013, 22, 1255–1263. [Google Scholar] [CrossRef]
- Nagel, A.H.; Cuss, C.W.; Goss, G.G.; Shotyk, W.; Glover, C.N. Chronic toxicity of waterborne thallium to Daphnia magna. Environ. Pollut. 2021, 268, 115776. [Google Scholar] [CrossRef]
- Rivera-De La Parra, L.; Sarma, S.S.S.; Nandini, S. Effects of predation by Hydra (Cnidaria) on cladocerans (Crustacea: Cladocera). J. Limnol. 2016, 75, 39–47. [Google Scholar] [CrossRef]
- Walsh, E.J. Habitat-specific predation susceptibilities of a littoral rotifer to two invertebrate predators. Hydrobiologia 1995, 313, 205–211. [Google Scholar] [CrossRef]
- Kovačević, G.; Petrinec, D.; Tramontana Ljubičić, P.; Reipert, S.; Sirovina, D.; Špoljar, M.; Peharec Štefanić, P.; Želježić, D. Formation of Microalgal Hunting Nets in Freshwater Microcosm Food Web: Microscopic Evidence. Water 2023, 15, 3448. [Google Scholar] [CrossRef]
- Kovačević, G.; Tramontana Ljubičić, P.; Petrinec, D.; Sirovina, D.; Novosel, M.; Želježić, D. How Daphnia magna Defends Itself against Predators: Mechanisms and Adaptations in a Freshwater Microcosm. Water 2024, 16, 398. [Google Scholar] [CrossRef]
- Wilden, B.; Majdi, N.; Kuhlicke, U.; Neu, T.R.; Traunspurger, W. Flatworm mucus as the base of a food web. BMC Ecol. 2019, 19, 15. [Google Scholar] [CrossRef]
- Klintworth, S.; von Elert, E. Inducible morphological defense in Daphnia pulex: Food quantity effects revised. Aquat. Ecol. 2021, 55, 47–57. [Google Scholar] [CrossRef]
- Blaustein, L.; Dumont, H.J. Typhloplanid flatworms (Mesostoma and related genera): Mechanisms of predation and evidence that they structure aquatic invertebrate communities. Hydrobiologia 1990, 198, 61–77. [Google Scholar] [CrossRef]
- Thielicke, M.; Sluys, R. Prey capture and feeding behaviour in an endemic land flatworm from São Tomé Island. J. Nat. Hist. 2019, 53, 1385–1393. [Google Scholar] [CrossRef]
- Dumont, H.J.; Carets, I. Flatworm predator (Mesostoma cf. lingua) releases a toxin to catch planktonic prey (Daphnia magna). Limnol. Oceanogr. 1987, 32, 699–702. [Google Scholar] [CrossRef]
- Lass, S.; Spaak, P. Chemically induced anti-predator defences in plankton: A review. Hydrobiologia 2003, 491, 221–239. [Google Scholar] [CrossRef]
- Chakri, K.; Touati, L.; Alfarhan, A.H.; Al-Rasheid, K.A.S.; Samraoui, B. Effect of vertebrate and invertebrate kairomones on the life history of Daphnia magna Straus (Crustacea: Branchiopoda). Comptes Rendus Biologies 2010, 333, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Petrinec, D. Green Hydra (Hydra viridissima Pallas, 1766) as a Competitor in Freshwater Microcosm and the Possibility of Application in the Teaching Process (Original in Croatian). Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2020. [Google Scholar]
- Archer, L.C.; Sohlström, E.H.; Gallo, B.; Jochum, M.; Woodward, G.; Kordas, R.L.; Rall, B.C.; O’Gorman, E.J. Consistent temperature dependence of functional response parameters and their use in predicting population abundance. J. Anim. Ecol. 2019, 88, 1670–1683. [Google Scholar] [CrossRef]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Series: Monographs in Behavior and Ecology; Princeton University Press: Princeton, NJ, USA, 1986; Volume 1. [Google Scholar] [CrossRef]
- Sura, S.A.; Mahon, H.K. Effects of competition and predation on the feeding rate of the freshwater snail, Helisoma trivolvis. Am. Midl. Nat. 2011, 166, 358–368. [Google Scholar] [CrossRef]
- Scharf, I. The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 2016, 119, 37–48. [Google Scholar] [CrossRef]
- Belenioti, M.; Chaniotakis, N. Aggressive Behaviour of Drosophila suzukii in Relation to Environmental and Social Factors. Sci. Rep. 2020, 10, 7898. [Google Scholar] [CrossRef]
- Klug, M.; Weber, J.; Tardent, P. Hemolytic and toxic properties of Hydra attenuata nematocysts. Toxicon 1989, 27, 325–339. [Google Scholar] [CrossRef]
- Sher, D.; Zlotkin, E. A hydra with many heads: Protein and polypeptide toxins from hydra and their biological roles. Toxicon 2009, 54, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Habdija, I.; Primc Habdija, B.; Radanović, I.; Špoljar, M.; Matoničkin Kepčija, R.; Vujčić Karlo, S.; Miliša, M.; Ostojić, A.; Sertić Perić, M. Protista-Protozoa i Metazoa- Invertebrata. Strukture i funkcije; Alfa d.d.: Zagreb, Croatia, 2011; p. 584. [Google Scholar]
- Massaro, F.C.; Negreiros, N.F.; Rocha, O. A search for predators and food selectivity of two native species of Hydra (Cnidaria: Hydrozoa) from Brazil. Biota Neotrop. 2013, 13, 35–40. [Google Scholar] [CrossRef][Green Version]
- Sher, D.; Fishman, Y.; Zhang, M.; Melamed-Book, N.; Zlotkin, E. Osmotically-driven prey disintegration in the gastro-vascular cavity of the green hydra by a pore-forming protein. FASEB J. 2008, 22, 207–214. [Google Scholar] [CrossRef]
- Mews, L.K.; Smith, D.C. The green hydra symbiosis. VI. What is the role of maltose transfer from alga to animal? Proc. R. Soc. London. Ser. B Biol. Sci. 1982, 216, 397–413. [Google Scholar] [CrossRef]
- Luiselli, L.; Pacini, N. Microcosms and Mesocosms: Small-Scale Experiments, Big Impacts for Tropical Ecology. Afr. J. Ecol. 2025, 63, e70076. [Google Scholar] [CrossRef]
- Özkan, K.; Korkmaz, M.; Amorim, C.A.; Yılmaz, G.; Koru, M.; Can, Y.; Pacheco, J.P.; Acar, V.; Çolak, M.A.; Yavuz, G.C.; et al. Mesocosm Design and Implementation of Two Synchronized Case Study Experiments to Determine the Impacts of Salinization and Climate Change on the Structure and Functioning of Shallow Lakes. Water 2023, 15, 2611. [Google Scholar] [CrossRef]
- Liboriussen, L.; Landkildehus, F.; Meerhof, M.; Bramm, M.E.; Sondegaard, M.; Christoffersen, K.; Richardson, K.; Sondegaard, M.; Lauridsen, T.L.; Jeppesen, E. Global warming: Design of a flow through shallow lake mesocosm climate experiment. Limnol. Oceanogr. Methods 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Stewart, R.I.A.; Dossena, M.; Bohan, D.A.; Jeppesen, E.; Kordas, R.L.; Ledger, M.E.; Meerhoff, M.; Moss, B.; Mulder, C.; Shurin, J.B.; et al. Mesocosm Experiments as a Tool for Ecological Climate-Change Research. In Advances in Ecological Research; Woodward, G., O’Gorman, E.J., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2013; Volume 48, pp. 71–181. ISBN 978-0-12-417199-2. [Google Scholar]
- Šorf, M.; Davidson, T.A.; Brucet, S.; Menezes, R.F.; Søndergaard, N.; Lauridsen, T.L.; Landkildehus, F.; Liboriussen, L.; Jeppesen, E. Zooplankton response to climate warming: A mesocosm experiment at contrasting temperatures and nutrient levels. Hydrobiologia 2015, 745, 185–203. [Google Scholar] [CrossRef]
- Ersoy, Z.; Scharfenberger, U.; Baho, D.L.; Bucak, T.; Feldmann, T.; Hejzlar, J.; Levi, E.E.; Mahdy, A.; Nõges, T.; Papastergiadou, E.; et al. Impact of nutrients and water level changes on submerged macrophytes along a temperature gradient: A pan-European mesocosm experiment. Glob. Chang. Biol. 2020, 26, 6831–6851. [Google Scholar] [CrossRef]
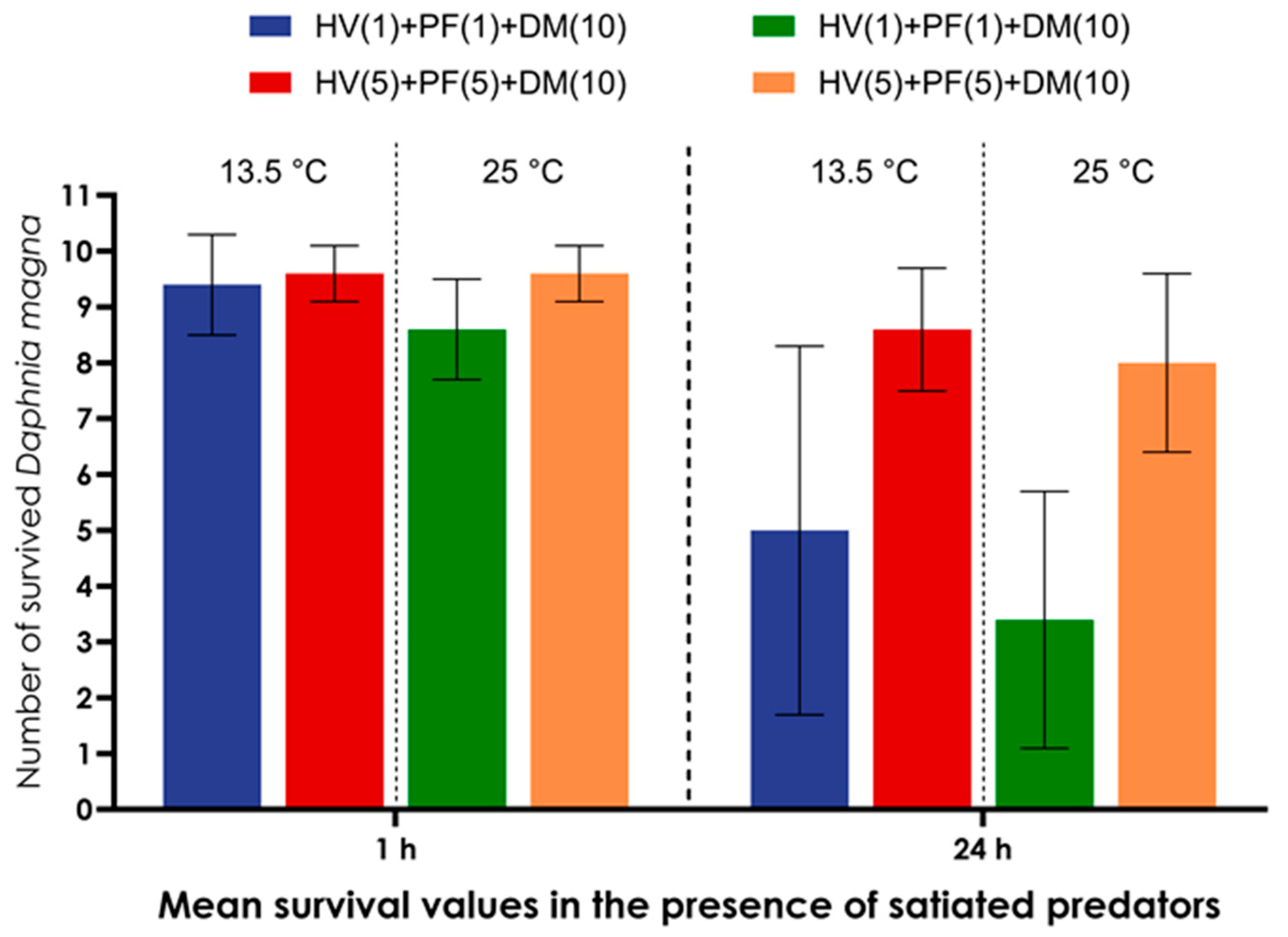
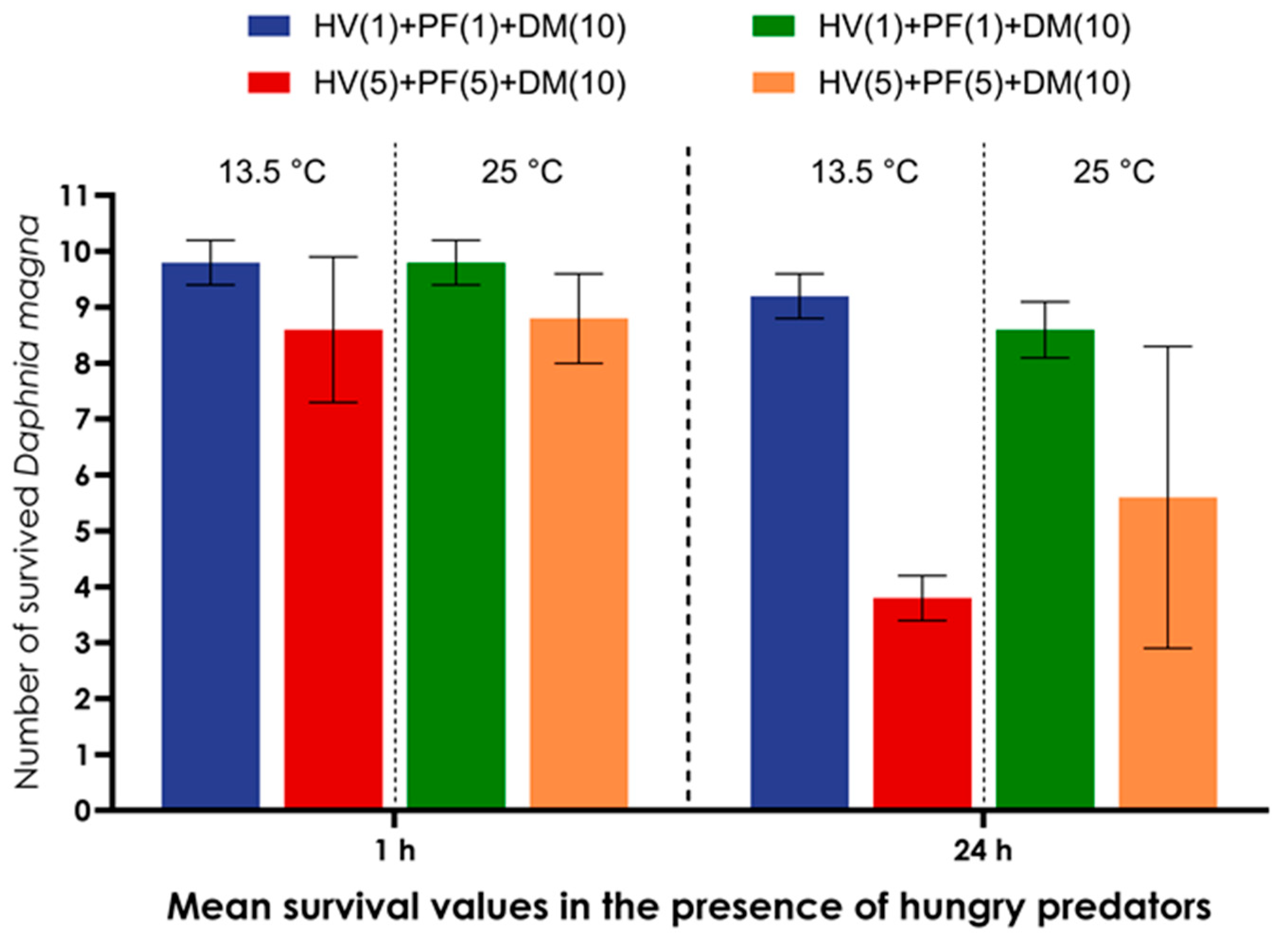
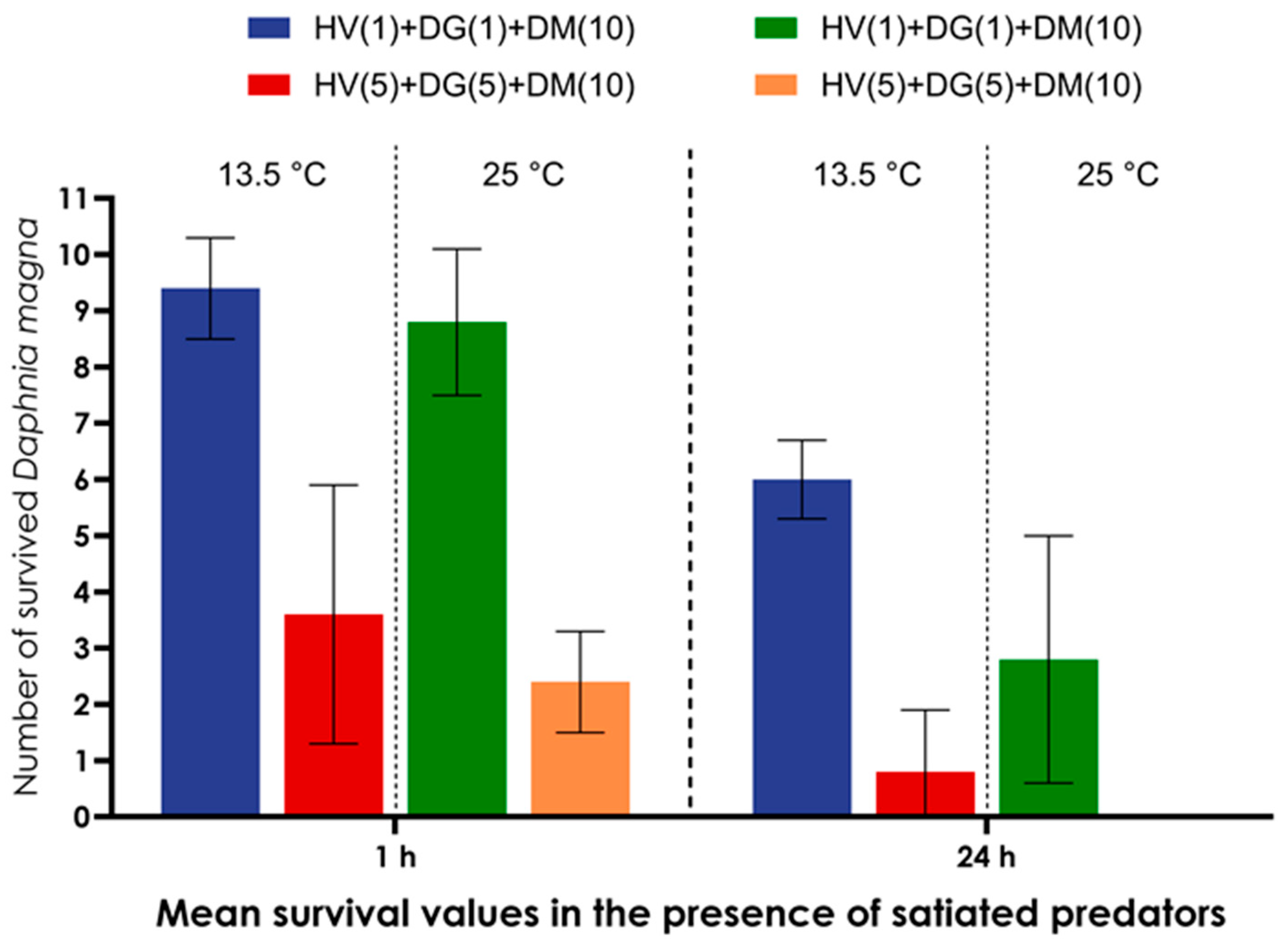
| Groups of Model Organisms | Model Organisms Combined in a Microcosmos | Number of Individuals of Model Organisms Combined in Each Microcosmos |
|---|---|---|
| Experimental groups | ||
| With DM | ||
| DM with turbellarians | DM + PF + DG | 10 DM + 1 PF + 1 DG; 10 DM + 5 PF + 5 DG |
| DM with hydrozoan and turbellarians | DM + HV + PF | 10 DM + 1 HV + 1 PF; 10 DM + 5 HV + 5 PF |
| DM + HV + DG | 10 DM + 1 HV + 1 DG; 10 DM + 5 HV + 5 DG | |
| Without DM | HV + PF + DG PF + DG | 1 HV + 1 PF + 1 DG; 5 HV + 5 PF + 5 DG 1 PF + 1 DG; 5 PF + 5 DG |
| Control group | DM | 10 DM |
| Microcosm | 1 h | 24 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13.5 °C | 25 °C | 13.5 °C | 25 °C | |||||||||
| PF | DG | HV | PF | DG | HV | PF | DG | HV | PF | DG | HV | |
| PFS(1) + DGS(1) + HVS(1) | - | - | - | - | - | - | - | - | - | half-eaten, scraps, bitten | C | - |
| PFS(5) + DGS(5) + HVS(5) | - | - | - | - | - | - | - | - | - | scrap | C | - |
| PFH(1) + DGH(1) + HVH(1) | - | - | - | - | - | - | - | C | - | scraps | C | - |
| PFH(5) + DGH(5) + HVH(5) | - | - | - | - | - | - | scraps | C | - | bitten, scraps | C | - |
| Microcosm | 1 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|---|
| 13.5 °C | 25 °C | 13.5 °C | 25 °C | |||||
| PF | DG | PF | DG | PF | DG | PF | DG | |
| PFS(1) + DGS(1) | - | - | - | - | - | - | eaten bitten | C |
| PFS(5) + DGS(5) | eaten | C | eaten | C | eaten | C | eaten, predation in progress | C |
| PFH(1) + DGH(1) | - | - | - | - | - | - | half-eaten bitten | C |
| PFH(5) + DGH(5) | - | - | - | - | - | - | eaten | C |
| Experimental Setup | Temperature | Exposure | PF Grouping | |
|---|---|---|---|---|
| DG + PF | Satiated | 13.5 °C | 1 h | + |
| 24 h | - | |||
| 25 °C | 1 h | - | ||
| 24 h | - | |||
| Hungry | 13.5 °C | 1 h | + | |
| 24 h | + | |||
| 25 °C | 1 h | + | ||
| 24 h | - | |||
| DG + PF + DM | Satiated | 13.5 °C | 1 h | + |
| 24 h | + | |||
| 25 °C | 1 h | - | ||
| 24 h | - | |||
| Hungry | 13.5 °C | 1 h | - | |
| 24 h | - | |||
| 25 °C | 1 h | + | ||
| 24 h | - | |||
| DG + PF + HV | Satiated | 13.5 °C | 1 h | + |
| 24 h | - | |||
| 25 °C | 1 h | + | ||
| 24 h | + | |||
| Hungry | 13.5 °C | 1 h | - | |
| 24 h | - | |||
| 25 °C | 1 h | + | ||
| 24 h | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, G.; Sirovina, D.; Tramontana Ljubičić, P.; Petrinec, D.; Sertić Perić, M.; Želježić, D.; Novosel, M.; Špoljar, M. Who Ate Whom—Competition and Predation in a Freshwater Microcosm. Water 2025, 17, 3166. https://doi.org/10.3390/w17213166
Kovačević G, Sirovina D, Tramontana Ljubičić P, Petrinec D, Sertić Perić M, Želježić D, Novosel M, Špoljar M. Who Ate Whom—Competition and Predation in a Freshwater Microcosm. Water. 2025; 17(21):3166. https://doi.org/10.3390/w17213166
Chicago/Turabian StyleKovačević, Goran, Damir Sirovina, Petra Tramontana Ljubičić, Daniela Petrinec, Mirela Sertić Perić, Davor Želježić, Maja Novosel, and Maria Špoljar. 2025. "Who Ate Whom—Competition and Predation in a Freshwater Microcosm" Water 17, no. 21: 3166. https://doi.org/10.3390/w17213166
APA StyleKovačević, G., Sirovina, D., Tramontana Ljubičić, P., Petrinec, D., Sertić Perić, M., Želježić, D., Novosel, M., & Špoljar, M. (2025). Who Ate Whom—Competition and Predation in a Freshwater Microcosm. Water, 17(21), 3166. https://doi.org/10.3390/w17213166







