Hydrogeochemical Evolution and Ecological Irrigation Evaluation of Mine Water in an Arid Coal Region: A Case Study from Northwest China
Abstract
1. Introduction
2. Materials and Methods
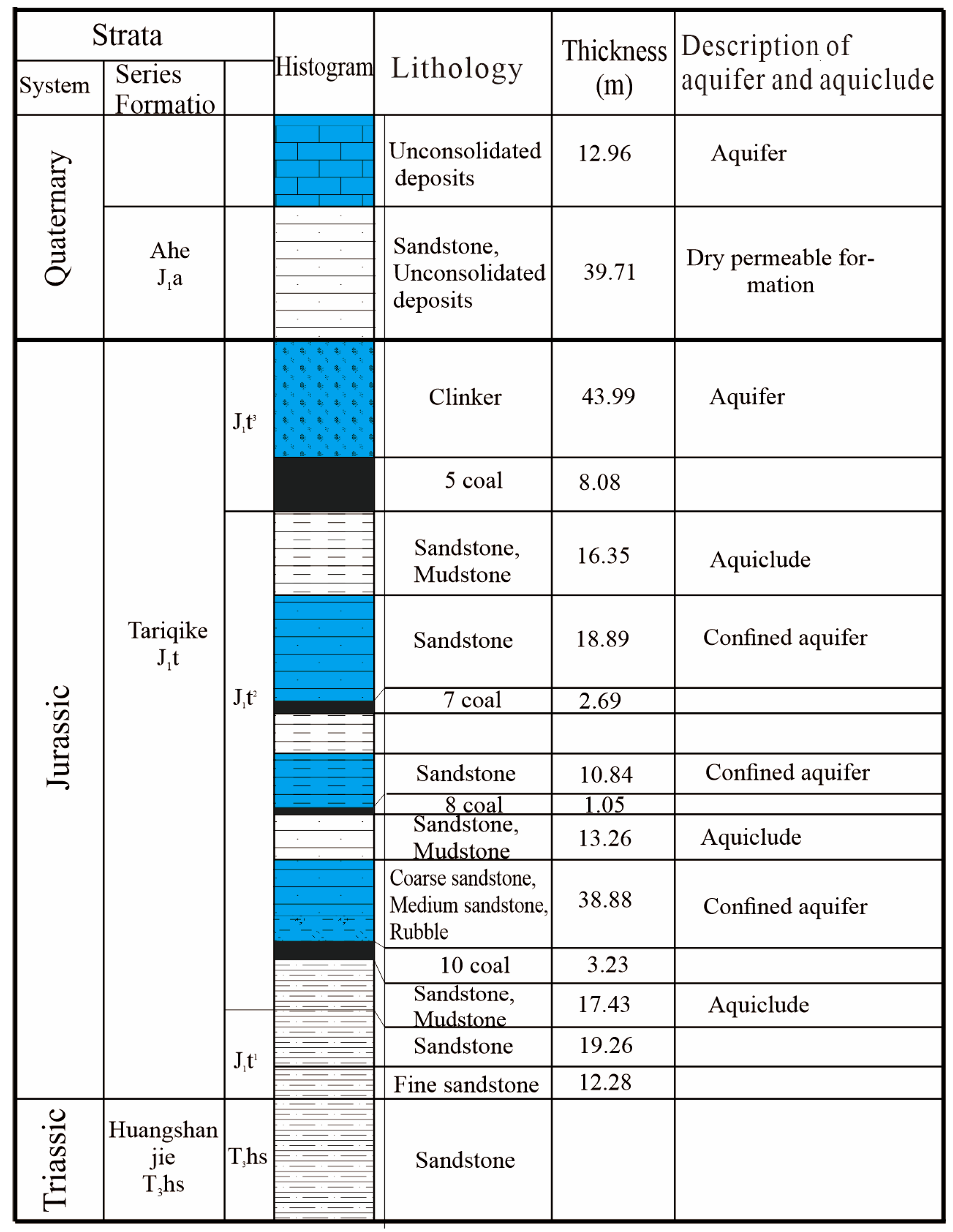
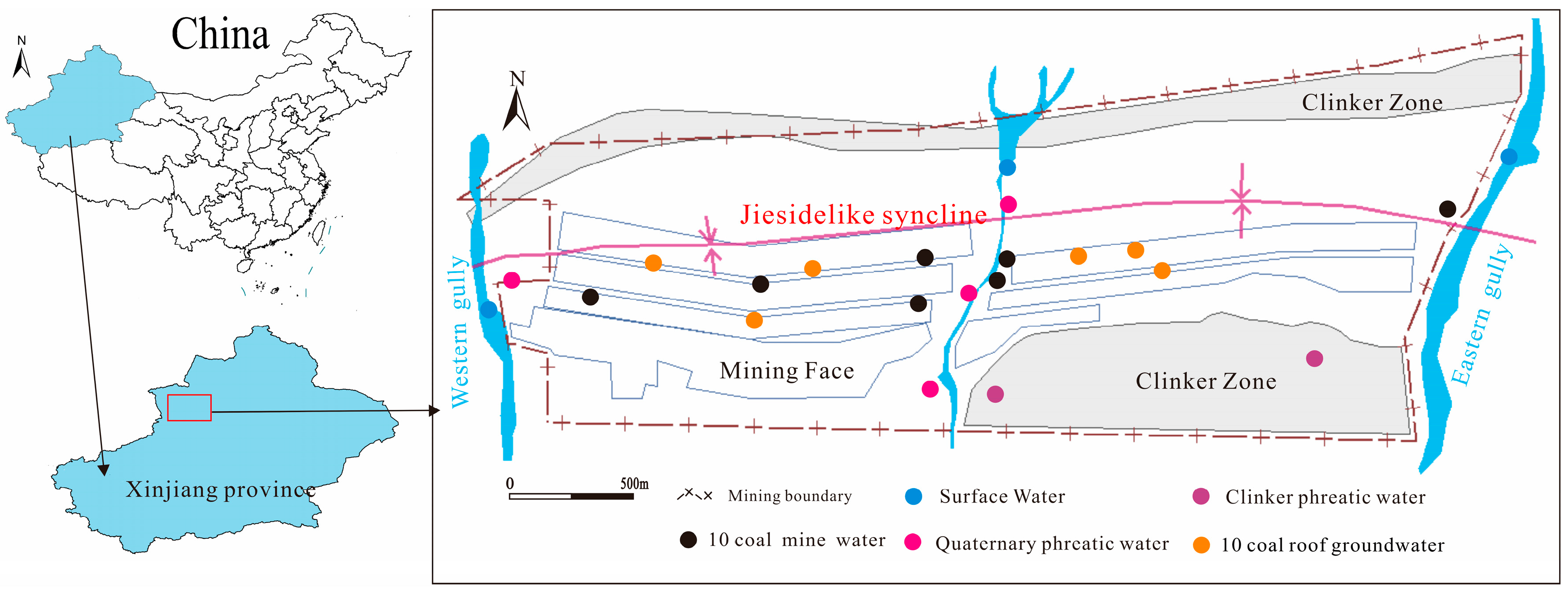
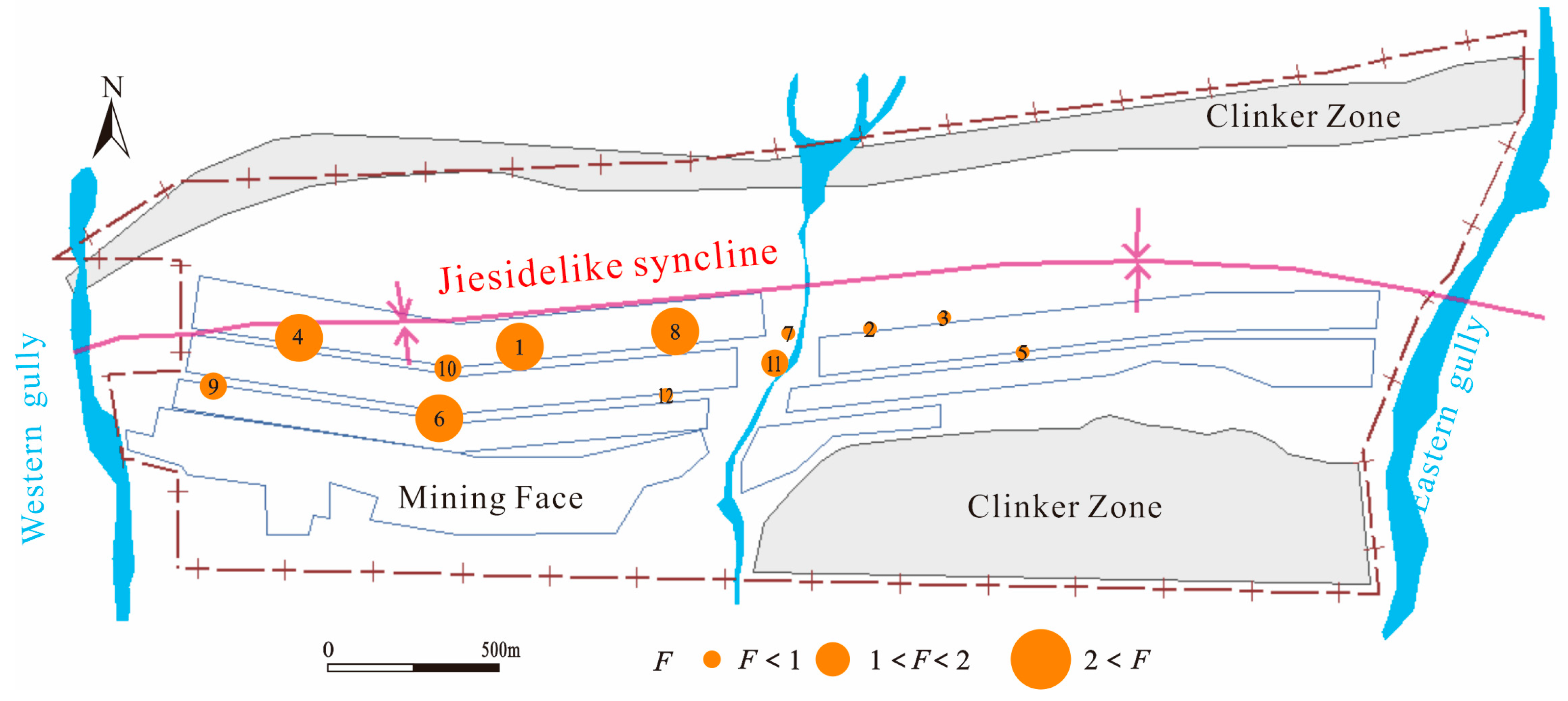
2.1. Study Area
2.2. Sample Collection and Analysis
3. Results and Discussion
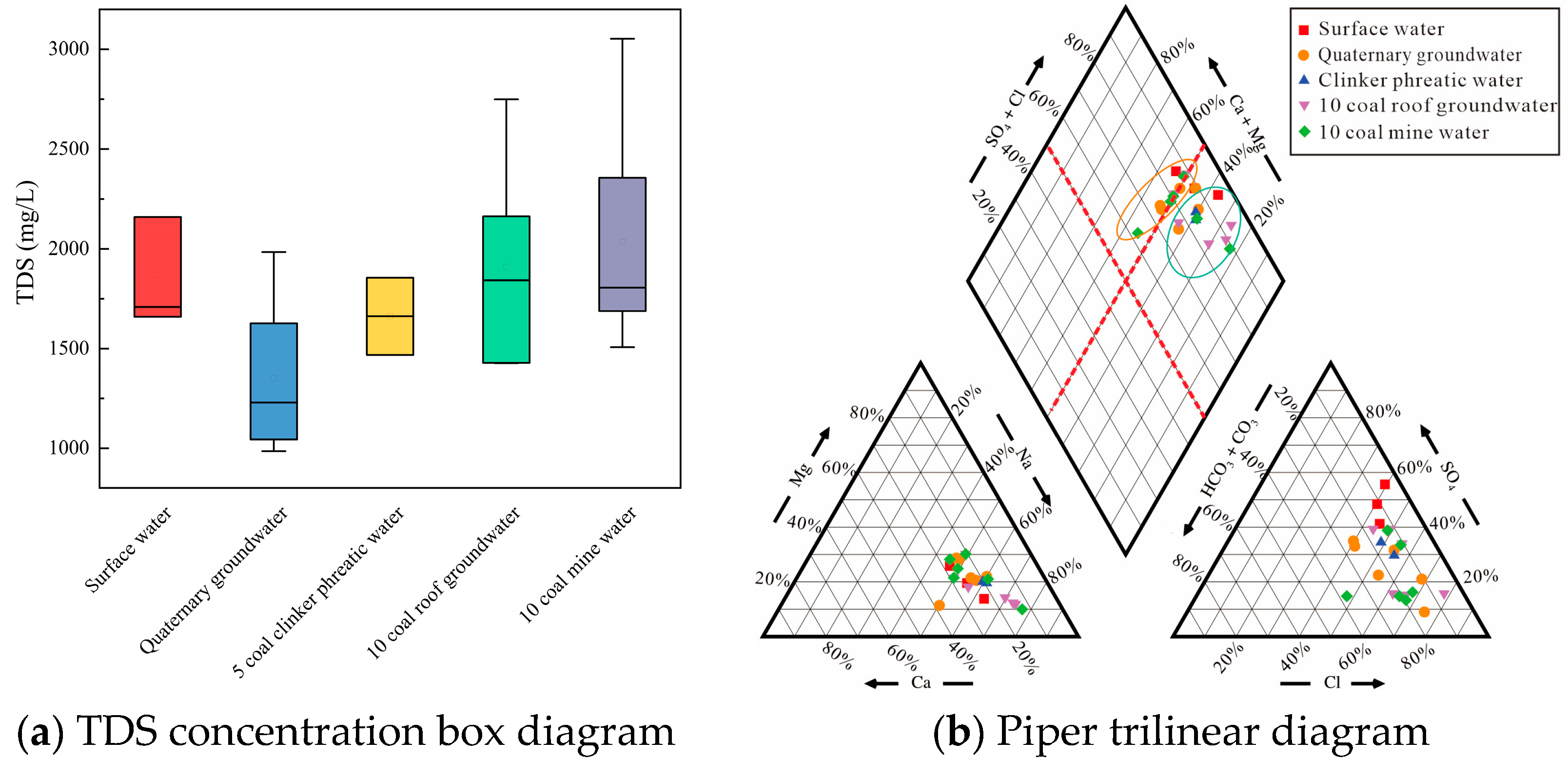
3.1. Hydrochemistry of Mine Water
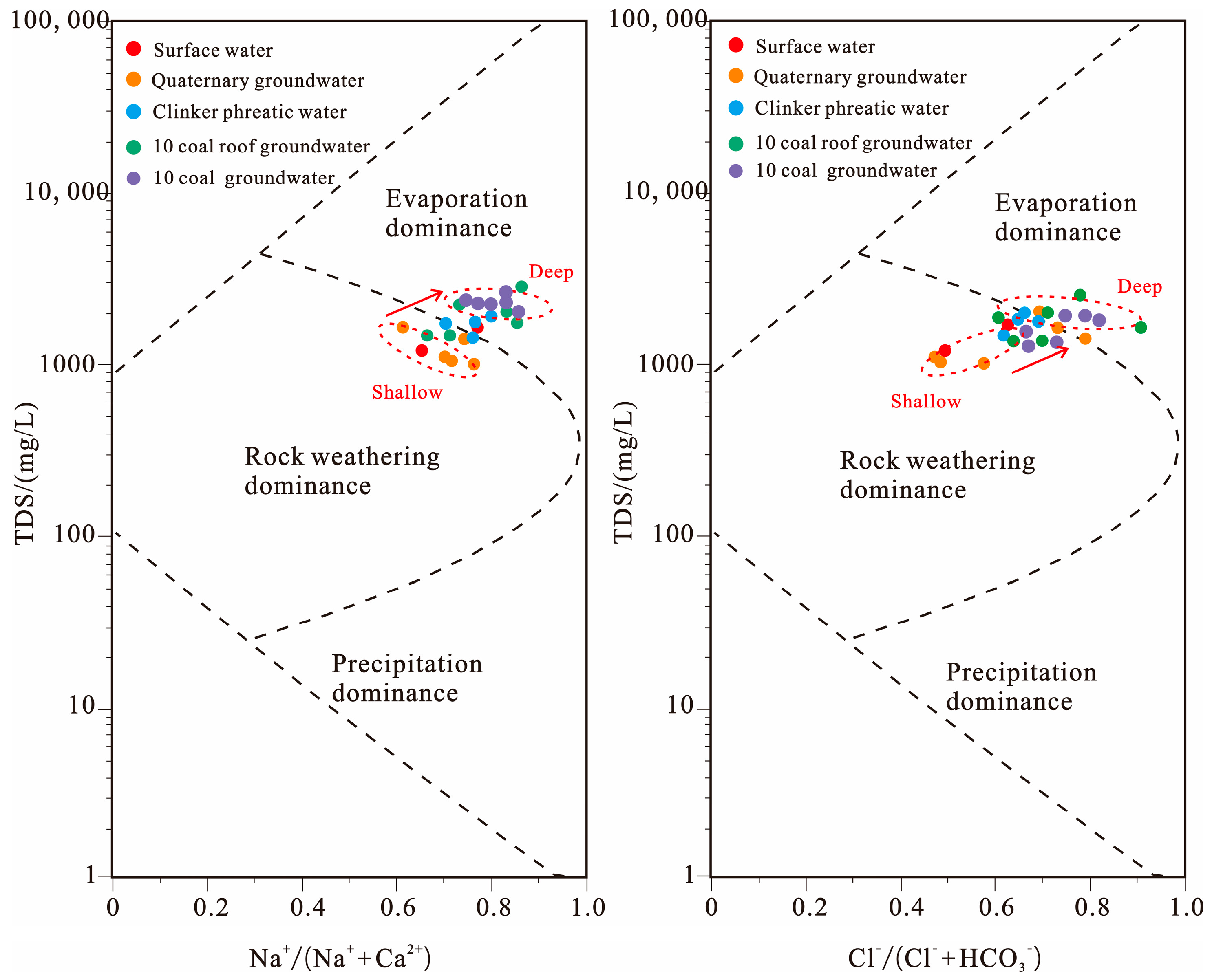
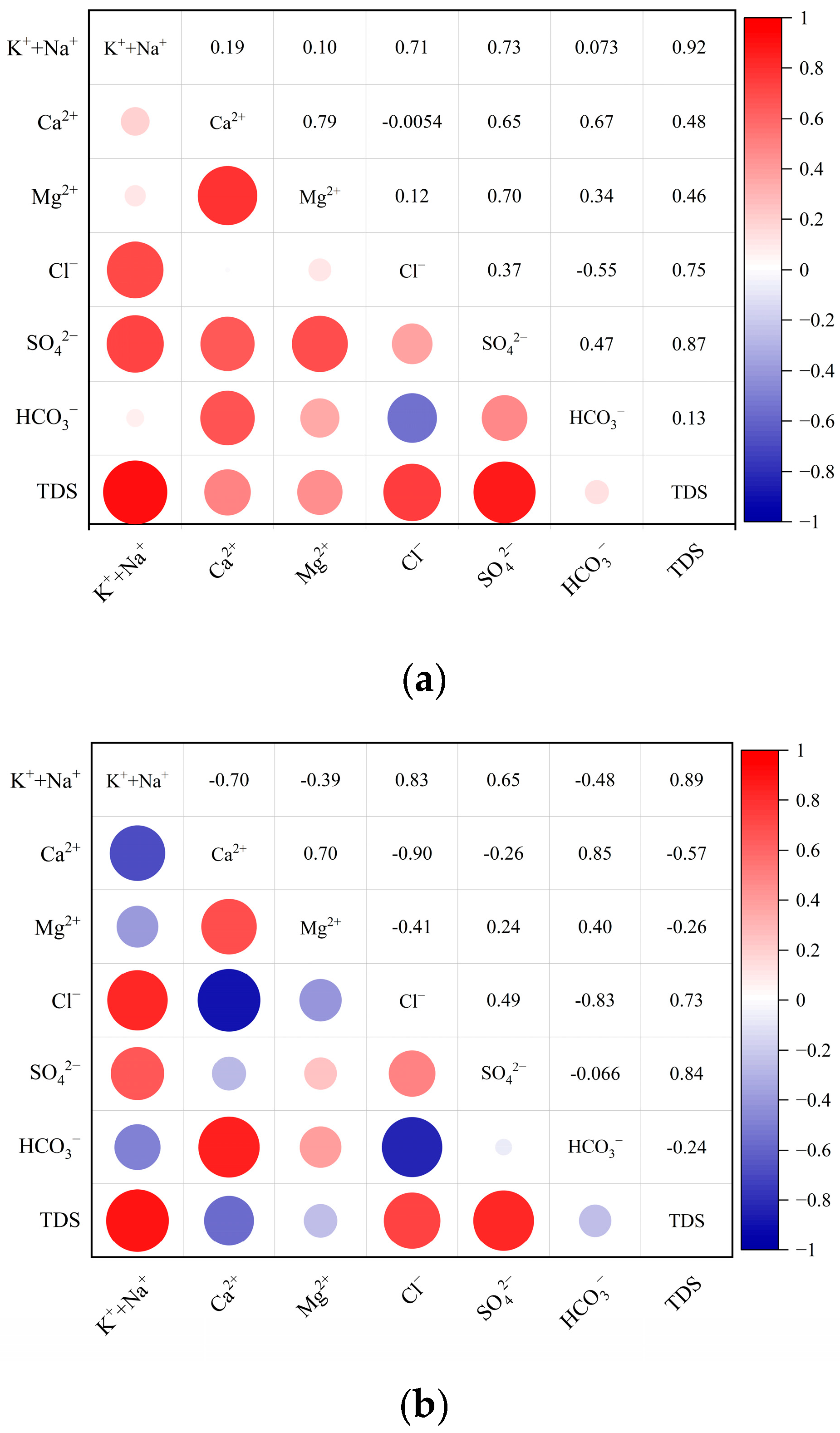
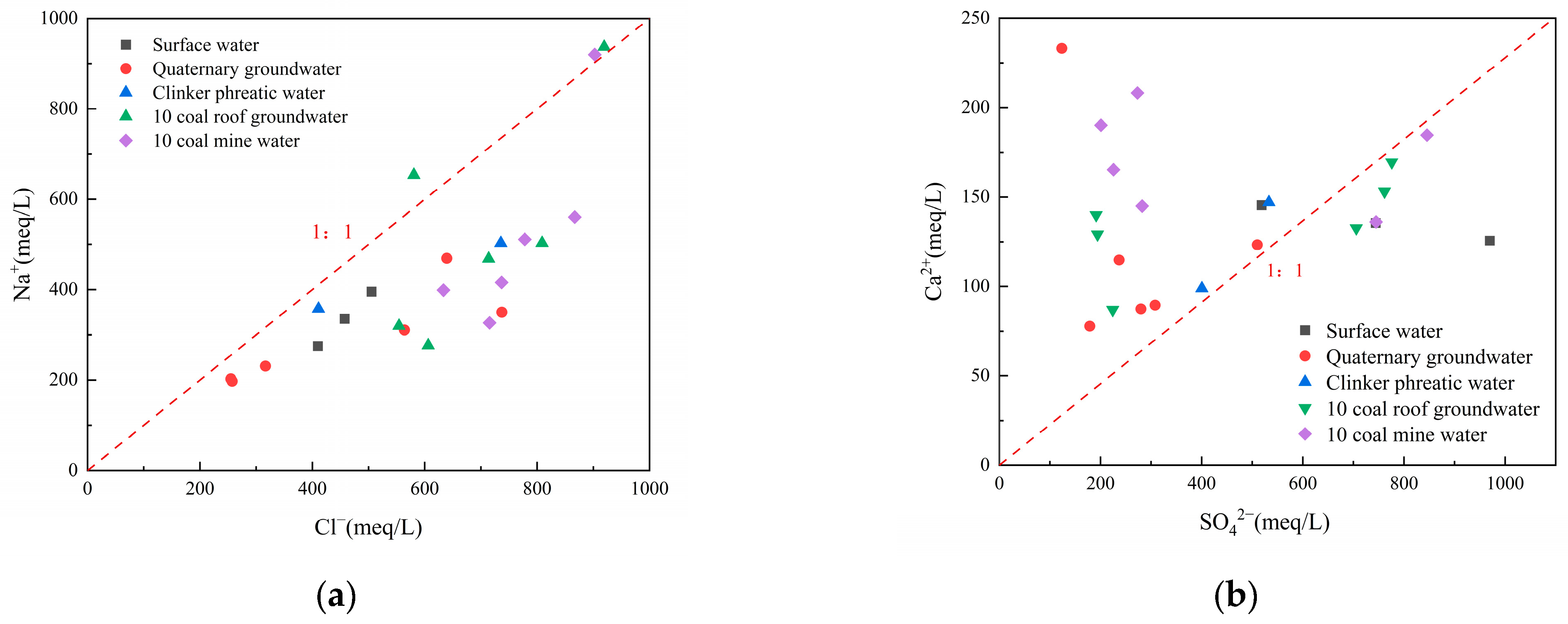
3.2. Hydrogeochemical Evolution of Mine Water
3.3. Ecological Irrigation Risk Evaluation
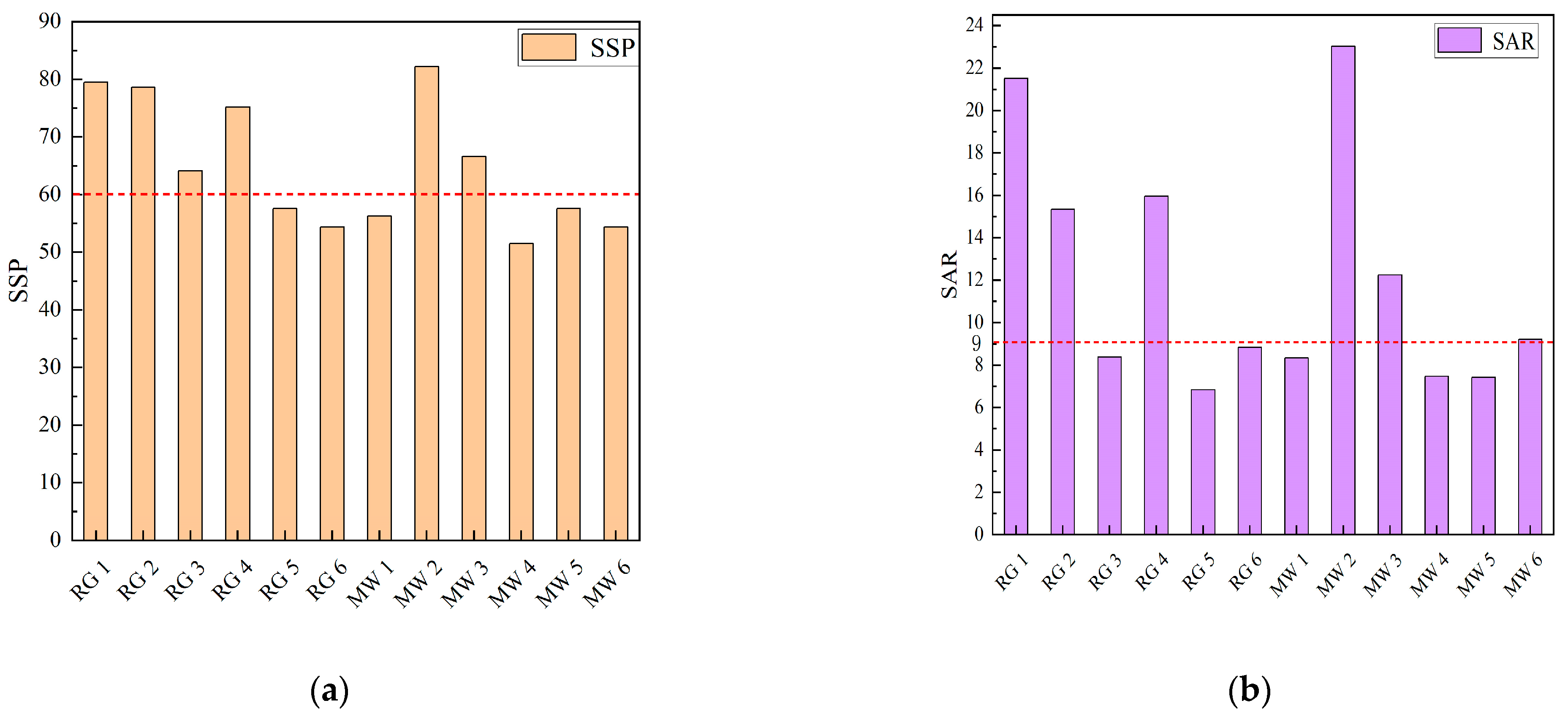
3.3.1. Risks to Soil Safety Induced by Mine Water Ecological Irrigation
- (1)
- Soluble Sodium Percentage (SSP)
- (2)
- Sodium Adsorption Ratio (SAR)
- (3)
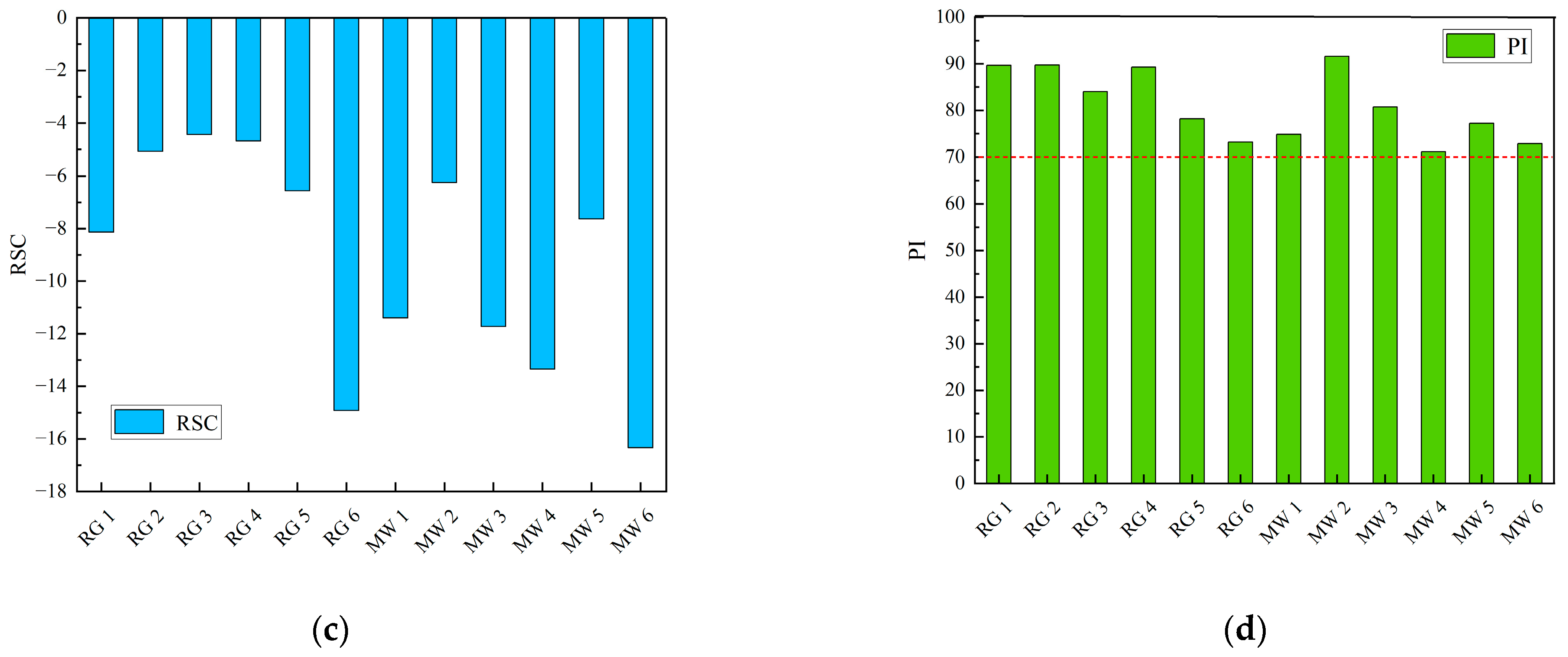
- Residual Sodium Carbonate (RSC)
- (4)
- Permeability Index (PI)
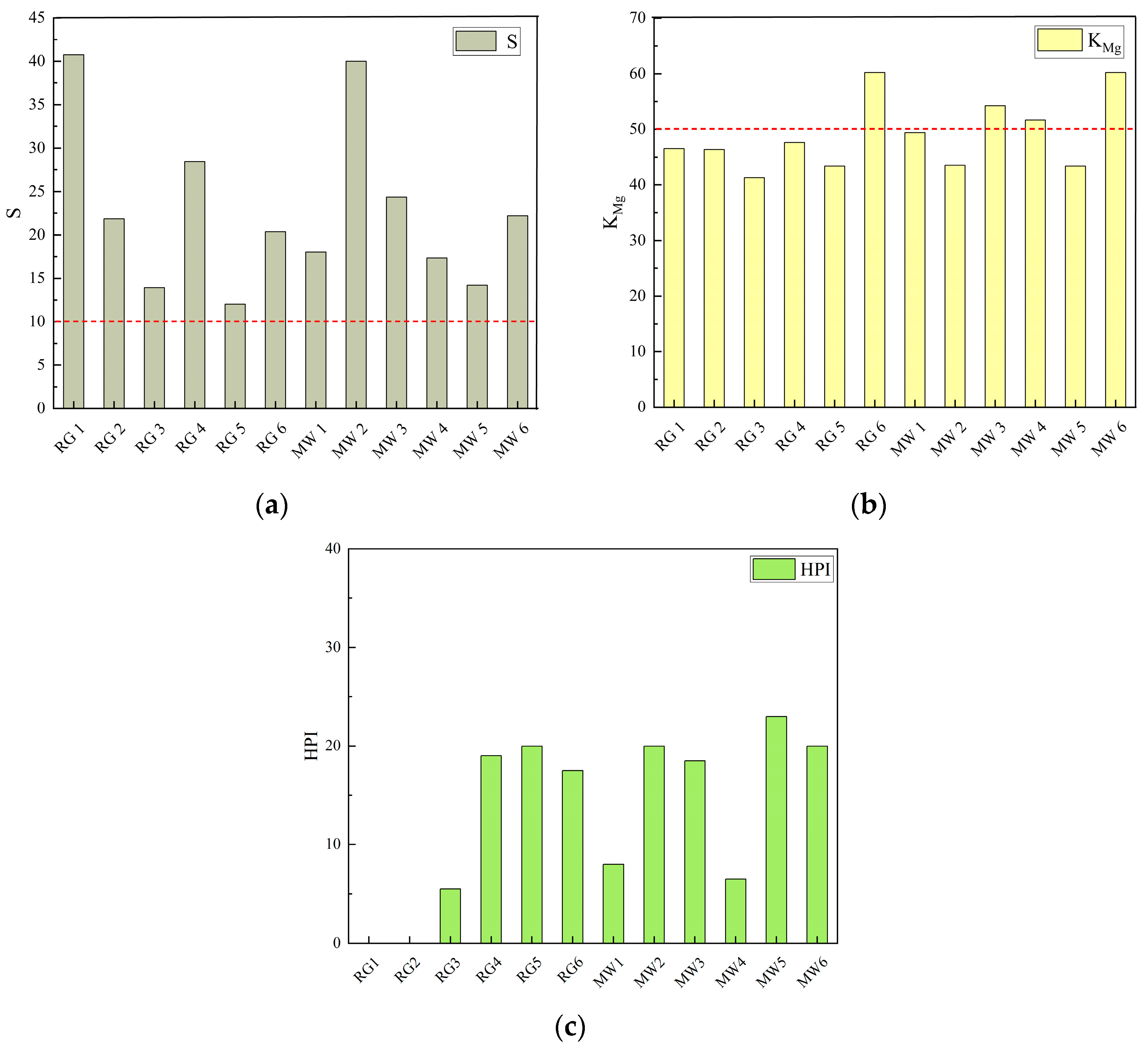
3.3.2. Risks to Plant Growth Induced by Mine Water Ecological Irrigation
- (1)
- Salinity Hazard (S)
- (2)
- Magnesium Adsorption Ratio (KMg)
- (3)
- Heavy Metal Pollution Index (HPI)
3.3.3. Potential Risks to the Groundwater Induced by Mine Water Ecological Irrigation
3.4. Management and Preventive Measures for Using Mine Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Zhang, D.S.; Li, W.P.; Lai, X.P.; Fan, G.W.; Liu, W.Q. Development on the basic theory of water protection during coal mining in northwest of China. J. China Coal Soc. 2017, 42, 36–43. [Google Scholar]
- Wang, S.M.; Shen, Y.J.; Sun, Q.; Hou, E.K. Scientific issues of coal detraction mining geological assurance and their technology expectations in ecologically fragile mining areas of Western China. J. Min. Strat. Control Eng. 2020, 2, 043531. [Google Scholar]
- Fan, L.M.; Ma, X.D.; Ji, R.J. Progress in engineering practice of water-preserved coal mining inwestern eco-environment frangible area. J. China Coal Soc. 2015, 40, 1711–1717. [Google Scholar]
- Yang, J.; Wang, H.; Wang, T.T.; Wang, Q.; Liu, J. Removal law of typical pollution components during underground storage of mine water: Taking Mindong No.1 Mine Inner Mongolia as an example. J. China Coal Soc. 2020, 45, 2918–2925. [Google Scholar]
- Gu, D.Z.; Li, J.F.; Cao, Z.G.; Wu, B.Y.; Jiang, B.Y.; Yang, Y.; Yang, J.; Chen, Y. Technology and engineering development strategy of water protection and utilization of coal mine in China. J. China Coal Soc. 2021, 46, 3079–3089. [Google Scholar]
- Guo, Y.N. Spatial distribution characteristics of suita-bility of mine water for irrigation in the Shendong mining area. J. Min. Sci. Technol. 2024, 9, 561–572. [Google Scholar]
- Merritt, P.; Power, C. Assessing the long-term evolution of mine water quality in abandoned underground mine workings using first-flush based models. Sci. Total Environ. 2022, 846, 157390. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.W.; Chen, Y.F.; Ge, R.T.; Ma, L.; Zhou, K.D.; Shi, X.P. Discrimination of water-inrush source and evolution analysis of hydrochemical environment under mining in Renlou coal mine, Anhui Province, China. Environ. Earth Sci. 2020, 79, 61. [Google Scholar] [CrossRef]
- Chen, L.W.; Xie, W.P.; Feng, X.Q.; Zhang, N.; Yin, X. Formation of hydrochemical composition and spatio-temporal evolution mechanism under mining-induced disturbance in the Linhuan coal-mining district. Arab. J. Geosci. 2017, 10, 57. [Google Scholar] [CrossRef]
- Gui, H.R.; Song, X.M.; Lin, M.L. Water-inrush mechanism research mining above karst confined aquifer and applications in North China coalmines. Arab. J. Geosci. 2017, 10, 180. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, W.; Wang, Z.H.; Zhou, Z.; Yang, J.; Xu, F.; Xue, J.; Li, G. Occurrence, Main Source and Health Risks of Fluorine in Mine Water. Expo Health 2025, 17, 279–292. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhang, L.; Xu, Z.M.; Chen, G.; Zhao, X.; Li, X.; Gao, Y.; Zhang, S.; Zhu, L. Mutifield action mechanism and research progress of coal mine water quality formation and evolution. J. China Coal Soc. 2022, 47, 423–437. [Google Scholar]
- Roisenberg, C.; Loubet, M.; Formoso, M.L.; Berger, G.; Munoz, M.; Dani, N. Tracing the Origin and Evolution of Geochemical Characteristics of Waters from the Candiota Coal Mine Area (Southern Brazil): Part I. Mine Water Environ. 2016, 35, 29–43. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Cui, B.Y.; Wang, Y.N.; Zhang, S.; Feng, G.; Zhang, Z. Evolution Law of Shallow Water in Multi-Face Mining Based on Partition Characteristics of Catastrophe Theory. Fractal Fract. 2023, 7, 779. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Batarseh, M.; Imreizeeq, E.; Tilev, S.; Al Alaween, M.; Suleiman, W.; Al Remeithi, A.M.; Al Tamimi, M.K.; Al Alawneh, M. Assessment of groundwater quality for irrigation in the arid regions using irrigation water quality index (IWQI) and GIS-Zoning maps: Case study from Abu Dhabi Emirate, UAE. Groundw. Sustain. Dev. 2021, 14, 100611. [Google Scholar] [CrossRef]
- Yıldız, S.; Karakuş, C.B. Estimation of irrigation water quality index with development of an optimum model: A case study. Environ. Dev. Sustain. 2020, 22, 4771–4786. [Google Scholar] [CrossRef]
- Shi, J.F.; Wu, P.; Zhang, R.X.; Li, X.X.; Cha, X.F. Water quality characteristics and irrigation suitability evaluation under the influence of coal mine drainage–a case study of the river watershed in Zhijin, Guizhou. Water Sav. Irrig. 2016, 11, 71–76. [Google Scholar]
- Ni, S.H.; Wang, H.L. Risk assessment and control of farmland irrigation with coal mine water. Coal Econ. Res. 2021, 41, 48–53. [Google Scholar]
- Chen, S.; Gui, H.R. Hydrogeochemical characteristics of groundwater in the limestone aquifers of the Taiyuan Group and its geological significance in the Suxian mining area. Hydrogeol. Eng. Geol. 2016, 43, 33–41. [Google Scholar]
- Wang, T.T.; Yang, J.; Jin, D.W.; Li, G.; Zhou, Z.; Xue, J.; Shang, H. The hydrogeochemical characteristics and formation mechanism of high-fluoride mine water. J. Clean. Prod. 2023, 430, 139671. [Google Scholar] [CrossRef]
- Piper, A.M. A graphical procedure in the geochemical interpretation of wateranalysis. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 795–840. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Jothibasu, A. Modeling water quality index to assess shallow groundwater quality for sustainable utilization in Southern India. Int. J. Adv. Geosci. 2014, 2, 122. [Google Scholar] [CrossRef]
- Mao, M.; Zhu, X.Q. Chemical characteristics of groundwater in Xuanhua basin and assessment of irrigation applicability. J. Arid Land Resour. Environ. 2020, 34, 142–149. [Google Scholar]
- GB/T 25499-2010; Water Quality Standard of Green Land Irrigation for Urban Sewage Reclamation. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standards Press of China: Beijing, China, 2010.
- Jandu, A.; Malik, A.; Dhull, S.B. Fluoride and nitrate in groundwater of rural habitations of semiarid region of northern Rajasthan, India: A hydrogeochemical, multivariate statistical, and human health risk assessment perspective. Environ. Geochem. Health 2021, 43, 3997–4026. [Google Scholar] [CrossRef] [PubMed]
- Xie, F. The Groundwater Quality Assessment and Response of Different Irrigation Water Sources to Groundwater in Jinghuiqu Irrigation District; Northwest A&F University: Xianyang, China, 2016; pp. 32–36. [Google Scholar]
- Yidana, M.S. Groundwater classification using multivariate statistical methods: SouthernGhana. J. Afr. Earth Sci. 2010, 57, 455–469. [Google Scholar] [CrossRef]
- Raju, N.J.; Shukla, U.K.; Ram, P. Hydrogeochemistry for the assessment of groundwater quality in Varanasi:a fast-urbanizing center in Uttar Pradesh, India. Environ. Monit. Assess. 2011, 173, 279–300. [Google Scholar] [CrossRef]
- Nag, S.K.; Das, S. Assessment of groundwater quality from Bankura I and II Blocks, Bankura District, West Bengal, India. Appl. Water Sci. 2017, 7, 2787–2802. [Google Scholar] [CrossRef]
- Amrani, S.; Hinaje, S.; El Fartati, M.; Gharmane, Y.; Yaagoub, D. Assessment of groundwater quality for drinking and irrigation in the Timahdite-Almis Guigou Area (Middle Atlas, Morocco). Appl. Water Sci. 2022, 12, 82. [Google Scholar] [CrossRef]
- Wang, T.T.; Jin, D.W.; Yang, J. Heavy metal pollution characteristics and source analysis of water drainage from a mine in Inner Mongolia. Coal Geol. Explor. 2021, 49, 45–51. [Google Scholar]
- Singha, S.; Pasupuleti, S.; Singha, S.S.; Kumar, S. Effectiveness of groundwater heavy metal pollution indices studies by deep-learning. J. Contam. Hydrol. 2020, 235, 103718. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848-2017; Standard for Groundwater Quality. National Standard of the People’s Republic of China; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standards Press of China: Beijing, China, 2017.
- Gao, M.X. Effects of Sulfur Supply on Cadmium up Take and Accumulation in Rice Seedlings; Northwest A&F University: Xianyang, China, 2009. [Google Scholar]
| Sample Types | Samples Number | Statistical Analyses | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | pH | TDS (mg/L) | Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface water | 3 | minimum | 275.47 | 125.65 | 46.30 | 409.92 | 518.49 | 116.25 | 7.70 | 1658.43 | 31.2 |
| maximum | 395.58 | 145.53 | 82.94 | 505.30 | 969.69 | 226.08 | 7.87 | 2158.77 | 35.5 | ||
| mean | 335.53 | 135.59 | 64.62 | 457.61 | 744.09 | 189.47 | 7.79 | 1842.22 | 33.4 | ||
| variance | 60.05 | 9.94 | 18.32 | 47.69 | 225.60 | 63.41 | 0.09 | 275.33 | 47.2 | ||
| CV | 0.02 | 0.02 | 0.07 | 0.02 | 0.02 | 0.04 | 0.04 | 0.01 | 0.21 | ||
| Quaternary groundwater | 6 | minimum | 197.60 | 77.94 | 41.76 | 255.20 | 123.54 | 151.09 | 7.82 | 985.42 | 30.4 |
| maximum | 469.45 | 233.30 | 92.36 | 737.33 | 510.00 | 290.90 | 8.40 | 1984.22 | 35.4 | ||
| mean | 293.53 | 121.05 | 61.45 | 461.65 | 272.87 | 253.83 | 8.04 | 1349.53 | 32.8 | ||
| variance | 105.66 | 57.66 | 18.20 | 211.35 | 134.13 | 53.75 | 0.25 | 395.37 | 86.5 | ||
| CV | 0.04 | 0.06 | 0.07 | 0.03 | 0.04 | 0.03 | 0.06 | 0.01 | 0.28 | ||
| Clinker phreatic water | 2 | minimum | 357.68 | 98.89 | 61.58 | 411.00 | 400.58 | 251.63 | 7.15 | 1468.09 | 30.6 |
| maximum | 502.75 | 147.13 | 91.13 | 735.53 | 533.08 | 392.82 | 7.84 | 1854.34 | 35.2 | ||
| mean | 430.22 | 123.01 | 76.36 | 573.27 | 466.83 | 322.23 | 7.50 | 1661.22 | 32.9 | ||
| variance | 102.58 | 34.11 | 20.90 | 229.48 | 93.69 | 99.84 | 0.49 | 273.12 | 92.3 | ||
| CV | 0.02 | 0.05 | 0.06 | 0.03 | 0.02 | 0.03 | 0.09 | 0.01 | 0.29 | ||
| 10 coal roof groundwater | 6 | minimum | 276.72 | 87.02 | 45.16 | 554.29 | 191.46 | 117.85 | 7.10 | 1428.27 | 30.8 |
| maximum | 937.13 | 169.46 | 154.04 | 919.41 | 775.87 | 415.48 | 8.13 | 2748.88 | 34.8 | ||
| mean | 526.56 | 135.25 | 78.42 | 697.23 | 475.58 | 302.20 | 7.72 | 1908.78 | 32.7 | ||
| variance | 242.42 | 27.87 | 39.07 | 144.50 | 299.37 | 99.98 | 0.40 | 505.22 | 96.8 | ||
| CV | 0.03 | 0.04 | 0.08 | 0.02 | 0.04 | 0.03 | 0.08 | 0.01 | 0.30 | ||
| 10 coal mine water | 6 | minimum | 326.53 | 136.16 | 63.05 | 633.29 | 201.05 | 175.85 | 7.73 | 1507.27 | 31.2 |
| maximum | 920.13 | 208.20 | 167.90 | 902.41 | 845.69 | 428.30 | 9.40 | 3051.66 | 35.2 | ||
| mean | 522.19 | 171.59 | 109.16 | 772.16 | 428.83 | 329.80 | 8.46 | 2035.66 | 32.4 | ||
| variance | 212.03 | 27.79 | 38.26 | 99.73 | 287.16 | 88.47 | 0.67 | 573.62 | 76.7 | ||
| CV | 0.03 | 0.03 | 0.06 | 0.01 | 0.04 | 0.03 | 0.10 | 0.01 | 0.27 |
| Serial Number | Sample Types | Sample Name | Fe | Mn | Cu | Zn | Hg | As | Cd | Cr6+ | Pb | HPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 coal roof groundwater | 10 coal roof groundwater 1 | <0.08 | <0.05 | <0.001 | <0.005 | <0.00005 | 0.002 | <0.0005 | <0.005 | <0.001 | / |
| 2 | 10 coal roof groundwater 2 | <0.08 | <0.05 | <0.001 | <0.005 | <0.00005 | <0.001 | <0.0005 | <0.005 | <0.001 | / | |
| 3 | 10 coal roof groundwater 3 | <0.08 | 0.10 | <0.001 | <0.005 | <0.00005 | 0.005 | <0.0005 | <0.005 | <0.001 | 5.50 | |
| 4 | 10 coal roof groundwater 4 | 0.13 | 0.06 | <0.001 | <0.005 | <0.00005 | 0.005 | <0.0005 | <0.005 | <0.001 | 19.00 | |
| 5 | 10 coal roof groundwater 5 | 0.11 | 0.08 | <0.001 | <0.005 | <0.00005 | 0.004 | <0.0005 | <0.005 | <0.001 | 20.00 | |
| 6 | 10 coal roof groundwater 6 | 0.13 | 0.09 | <0.001 | <0.005 | <0.00005 | 0.001 | <0.0005 | <0.005 | <0.001 | 17.50 | |
| 7 | 10 coal mine water | 10 coal mine water 1 | 0.22 | <0.05 | <0.001 | <0.005 | <0.00005 | <0.001 | <0.0005 | <0.005 | <0.001 | 8.00 |
| 8 | 10 coal mine water 2 | 0.10 | <0.05 | <0.001 | <0.005 | <0.00005 | 0.008 | <0.0005 | <0.005 | <0.001 | 20.00 | |
| 9 | 10 coal mine water 3 | 0.15 | 0.03 | <0.001 | <0.005 | <0.00005 | <0.001 | <0.0005 | <0.005 | <0.001 | 18.50 | |
| 10 | 10 coal mine water 4 | 0.24 | 0.09 | <0.001 | <0.005 | <0.00005 | 0.002 | <0.0005 | <0.005 | <0.001 | 6.50 | |
| 11 | 10 coal mine water 5 | 0.09 | 0.06 | <0.001 | <0.005 | <0.00005 | 0.002 | <0.0005 | <0.005 | <0.001 | 23.00 | |
| 12 | 10 coal mine water 6 | 0.12 | 0.06 | <0.001 | <0.005 | <0.00005 | 0.002 | <0.0005 | <0.005 | <0.001 | 20.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Shang, H.; Wang, T.; Xue, J.; Wang, X.; Zhou, Z.; Wang, Q. Hydrogeochemical Evolution and Ecological Irrigation Evaluation of Mine Water in an Arid Coal Region: A Case Study from Northwest China. Water 2025, 17, 3132. https://doi.org/10.3390/w17213132
Wang H, Shang H, Wang T, Xue J, Wang X, Zhou Z, Wang Q. Hydrogeochemical Evolution and Ecological Irrigation Evaluation of Mine Water in an Arid Coal Region: A Case Study from Northwest China. Water. 2025; 17(21):3132. https://doi.org/10.3390/w17213132
Chicago/Turabian StyleWang, Hao, Hongbo Shang, Tiantian Wang, Jiankun Xue, Xiaodong Wang, Zhenfang Zhou, and Qiangmin Wang. 2025. "Hydrogeochemical Evolution and Ecological Irrigation Evaluation of Mine Water in an Arid Coal Region: A Case Study from Northwest China" Water 17, no. 21: 3132. https://doi.org/10.3390/w17213132
APA StyleWang, H., Shang, H., Wang, T., Xue, J., Wang, X., Zhou, Z., & Wang, Q. (2025). Hydrogeochemical Evolution and Ecological Irrigation Evaluation of Mine Water in an Arid Coal Region: A Case Study from Northwest China. Water, 17(21), 3132. https://doi.org/10.3390/w17213132






