Abstract
Arsenic contamination in aquifers poses a significant global health risk due to its toxicity and widespread presence in groundwater used for drinking. Although several approaches for arsenic removal exist, many are either expensive or logistically difficult. This study assesses the efficacy of native limestones from two arsenic-contaminated regions in Mexico as a sustainable treatment alternative. Tested in batch and column experiments using synthetic solutions, as well as natural and arsenic-enriched groundwater, the limestones were characterized mineralogically (XRD) and chemically (XRF). Surface area, particle size, average pore volume in rocks, and competing anions (i.e., bicarbonate and sulfate) in groundwaters played important roles in removal performance. The results show that smaller particle sizes improve arsenic retention. Up to 87.6% of the arsenic was removed from groundwater containing 1.29 mg/L of arsenic when treated with rock particles smaller than 0.062 mm. Natural groundwater, however, in general, exhibited lower efficiency than synthetic solutions due to anion interference. Although site-specific evaluations are essential, these results indicate that limestone may be a cost-effective and locally accessible solution for addressing arsenic (As) contamination in regions with abundant limestone outcrops.
Keywords:
arsenic; limestones; Zimapán; groundwater; Mexico; treatment options; environment; sustainability 1. Introduction
Arsenic (As) is a well-known human carcinogen that produces diverse health effects through the chronic ingestion of As-rich water. Non-cancer health effects include peripheral vascular disease, skin lesions, neurological effects, respiratory and cardiovascular diseases, diabetes, and high blood pressure, among other pathologies. On the other hand, skin, bladder, lung, liver, prostate, and kidney cancer have been related to As exposure [1]. It is also associated with pregnancy-related problems and alterations in the innate immune function of children, potentially affecting their cardiovascular system at a young age [2,3]. Millions of people worldwide have been reported to drink water containing arsenic (As) concentrations above the World Health Organization (WHO) guideline of 0.010 mg/L [4]. Recent modeling applying random forest machine learning estimated that 94 to 220 million people are potentially exposed to high arsenic concentrations in groundwater [5]. Health effects due to chronic As exposure to water and food have occurred in many countries, like Bangladesh, India, Argentina, Chile, Mexico, China, Pakistan, and Vietnam, among others [6,7], also affecting the economic and welfare of people, especially in low-income countries.
The toxicity and widespread occurrence of arsenic-rich water have driven the development of water treatment methods to remove arsenic. The state of the art on arsenic removal has evolved significantly in recent decades; however, significant challenges remain. Various procedures are currently employed to treat contaminated water, including coagulation–flocculation, precipitation processes, membrane separation, and adsorption. However, the most commonly used methods imply high costs or operation difficulties that hinder their application in low-income and isolated communities. For instance, coagulation–flocculation has been used for many years to remove arsenic in various countries; however, it generates solid waste that must be disposed of and necessitates operational adjustments and control to achieve optimal results. Membrane separation is also widely used; however, its application poses challenges in areas with water shortages due to the waste associated with the volume of arsenic-enriched water produced in the brine and the costs of replacing the membranes. Other methods, such as bioremediation, electrochemical technologies, ion exchange resins, and photochemical systems, have also been employed, albeit with limited application to specific locations [8,9,10]. Each method has advantages, drawbacks, and limitations depending on local conditions. Adsorption may render good results and be applied with low-cost materials. A review of current methods for removing arsenic, including their limitations and suitability for specific regions, can be found in Sadee et al. (2025) [11], Ghosh et al. (2025) [12], and Kumar et al. (2019) [13]; in Baig et al. for adsorption using different materials (2015) [9]; and in Pryadko et al. (2023) for various iron-containing adsorbents [14]. Here, we include some examples of the materials reported in those articles, focusing on their adsorption capacity and percentage removal of arsenic, organized according to the material type (Table 1). Low-cost materials such as iron oxides and natural compounds have proven effective in removing As [9,15]. However, its efficiency can be compromised at extreme pH levels or in the presence of competing compounds. For inorganic materials, a variety of results have been reported. Although these studies yield the most robust results, in some cases, they are also low-cost and straightforward to execute. Compared to other, more recent methods (nanomaterials), they may have a lower capacity. In the case of natural compounds, they can be inexpensive, and their capacity is variable [11]. Some of them may yield good results without any treatment, such as rice husk cellulose [16]; however, others may require pretreatments, ranging from simple washing with water to acidic or alkali solutions, or chemical modifications, like metal impregnation, to enhance their efficiency [16,17]. In specific cases, natural adsorbents complement other methods or are combined with them. When considering plants and biomass, a lower capacity is generally observed than in nanomaterials or hybrids, as ionic competition occurs under specific environmental conditions. In the case of nanomaterials, hybrid materials, and clays, they possess a high and selective specific surface area and may act synergistically with other materials, such as metals, thereby improving their capacity and stability. Their limitations may include high costs (such as carbon nanotubes) and the complexity of their synthesis [18]. In this study, we evaluated native limestones collected from two groundwater arsenic-polluted zones as a potential option for arsenic removal based on their abundance and accessibility in the areas, with almost no cost and straightforward preparation and operation for the removal process.

Table 1.
Adsorbents, adsorption capacity, concentrations of As treated, and removal percentages (from Baig et al. (2015), Sadee et al. (2025), Ghosh et al. (2025), Pryadko et al. (2023), and Kumar et al. (2019) [9,11,12,13,14]). Specific references for each adsorbent are included in the articles.
In Mexico, arsenic above national and international standards has been detected in many aquifers [6,19]. The reported occurrence of high As concentrations in water affects areas in the states of Guanajuato [20], Durango [19], Jalisco, Chihuahua, Zacatecas, Coahuila, Sonora, Hidalgo, and Oaxaca, encompassing locations in approximately 25 States [19,21].
The first health problems related to the ingestion of As-rich water in Mexico were identified in the northcentral “Comarca Lagunera” area [22] and later in other sites, such as Zimapán, central Mexico [23]. Arsenic in the Zimapán aquifer is associated with mineralization, specifically the oxidation of arsenopyrite in the deep limestone aquifer and mine waste [24]. However, the natural source is responsible for the presence of arsenic (As) in the wells used for drinking water. Seeking options to remove arsenic in this low-income area, which lacks surface water bodies, we identified limestone as a potential adsorption medium due to the widespread outcrops of this type of rock in the area. Adsorption-based removal methods have been developed with a focus on developing countries, using natural and manufactured materials, including plant waste, iron oxide-coated sand, activated carbon, iron oxide, zeolites, gibbsite, and goethite [10]. An overview of natural materials applied to remove arsenic, indicating their advantages and limitations, was reported by Yeo et al. (2021) [16]. While that paper includes a diverse range of natural adsorbents, including biomaterials, minerals, rocks, and soils, the use of calcite is not explicitly addressed.
Several studies have been conducted to evaluate the effectiveness of limestone in removing arsenic from water. Limestone rocks collected in Minnekahta, USA, were reported to decrease the groundwater As concentration from 0.036 mg/L to 0.006 mg/L. The removal was attributed to the formation of calcium arsenate hydroxide, Ca5(AsO4)3OH [25]. Limestones that have been merged with other materials have also been tested for their capacity to remove arsenic. Shan et al. (2013) [26] used a mixture of limestone, hematite, and goethite in batch, column, and field experiments, reporting a reduction in As concentrations in groundwater from 400 μg/L to less than 10 μg/L. Devi et al. (2014) [27] demonstrated that iron oxide-coated sand was effective in removing arsenic (As), reducing the concentration from 0.200 mg/L to 0.010 mg/L in column experiments. Cederkvist et al. (2010) [28] assessed the performance of a dual-porosity filtration system comprising limestone and waste ochreous sludge. Their results showed the system to be a promising, low-cost method for treating water containing copper, chromate, and arsenate.
Here, we will evaluate the advantages and drawbacks of using native limestones without treatment, except for crushing and grinding, as a sustainable treatment alternative based on studies conducted at two sites in Mexico with groundwater arsenic (As) concentrations exceeding up to 120 times the World Health Organization (WHO) drinking water guideline. The evaluation will consider the removal capacity of rocks in relation to particle size, mineralogy, calcium carbonate content, and surface area, aiming to provide a sustainable alternative in Mexico and other sites with similar problems with arsenic (As) presence in groundwater and limestone outcrops located near or within polluted zones.
2. Methodology
2.1. Sampling and Analysis
Limestone samples were collected from outcrops at two locations in central Mexico, Guanajuato and Zimapán (Figure 1), with groundwater arsenic concentrations exceeding the Mexican and WHO drinking water standards [4,29]. One of the sites, Zimapán, is a historical mining zone where the highest groundwater arsenic concentrations (up to 1.2 mg/L) are related to mineralization in deep wells tapping a limestone aquifer. In the other zone in Guanajuato state, the presence of arsenic originates from the dissolution of volcanic glass and possibly the dissolution and desorption of Fe minerals and clays in a low-temperature geothermal system [20]. Previous studies have evaluated the efficiency of arsenic removal using different rock types collected in the Zimapán zone [30,31]. Those studies showed that limestones had the best capacity for As removal. The evaluation presented here includes only reported research on As removal using native limestones from the abovementioned two contaminated zones. The rocks were mineralogically characterized by X-ray diffraction (XRD) using an EMPYREAN diffractometer (Malvern Panalytical, Malvern, UK) with a nickel (Ni) filter, a fine focus copper tube, and a PIXcel3D detector and chemically characterized by X-ray fluorescence (XRF) with a RIGAKU ZSX Primus II spectrometer (Rigaku, Tokyo, Japan) [31,32,33,34,35,36].
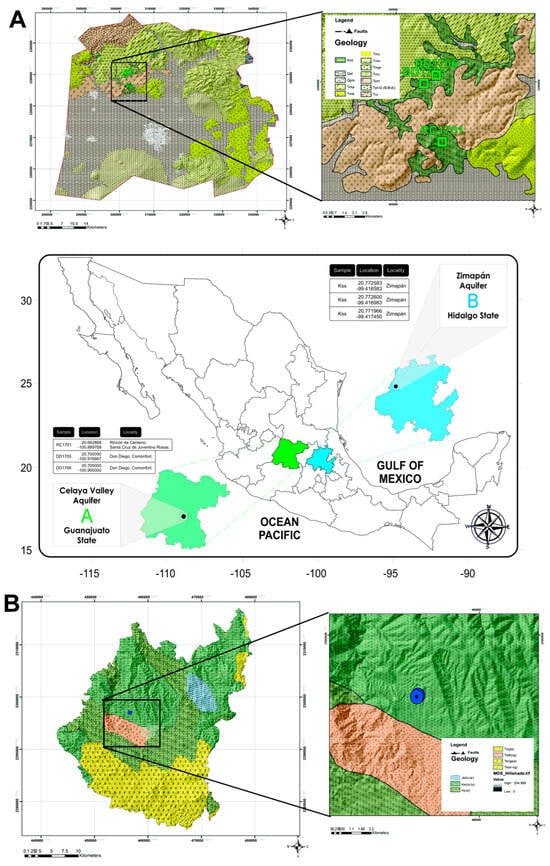
Figure 1.
Location of the sampled rocks used in the removal experiments. (A) Sampling sites of rocks collected in Guanajuato State (upper); map of Mexico showing the location of arsenic-polluted aquifers (lower), (B) Sampling site of rocks collected in Hidalgo State.
2.2. Experiments
2.2.1. Batch Tests
The arsenic (As) removal capacity was determined using batch experiments; however, column experiments were also conducted in one study. The rocks were crushed and sieved into particles of different sizes, which were then placed in contact with synthetic solutions with varying arsenic (As) concentrations and groundwater collected from the polluted wells in each area. The specific surface area of the various-sized rock particles was determined using the BET (Brunauer–Emmett–Teller) method. A 1:20 rock-to-water ratio was agitated for various periods and then centrifuged and filtered. Hydride generation atomic absorption spectrometry was used to measure the arsenic concentrations in the initial and final solutions. Groundwater samples were collected in wells with arsenic (As) presence at Zimapán, Hidalgo (samples Zimapán V, ZIM1801 (MUHI), and ZIM1804 (Pb-18)), and in Guanajuato state (samples Praderas de la Venta (PV03) and San José Merino (SJMer)). The physicochemical parameters of the groundwater were determined for each well. Field measures included temperature, pH, and conductivity. Preservation methods and chemical analyses followed standard procedures and complied with analytical quality controls as informed by each study. The Analytical Chemistry Laboratory of the Institute of Geophysics, UNAM, Mexico, analyzed ions, arsenic, and silica by APHA-AWWA methods (2005) [37]. Bicarbonate was determined by volumetry (HCl titration); chloride (Cl−) was analyzed by potentiometry with selective electrodes; sulfate (SO42−) was determined by turbidimetry; Ca2+ and Mg2+ were analyzed by volumetry (titrating with EDTA); Na+ and K+ were determined by atomic emission spectrophotometry; silica (SiO2) was determined by UV–visible spectroscopy (molybdosilicic acid method); and arsenic (As) was determined with a Perkin Elmer AAnalyst 200 atomic absorption spectrometer and FIAS 100 system (Perkin Elmer, Springfield, IL, USA). The analytical quality was assessed through ionic balance (less than 10%) and certified (NIST-traceable) reference solutions (hps, North Charleston, SC, USA).
Additionally, to further evaluate the influence of other ions on arsenic (As) removal, batch experiments were conducted using limestones and water collected from arsenic-free springs (Axocopan and Atlimeyaya, in the state of Puebla, central Mexico), supplemented with various arsenic concentrations (Axocopan with 1.5 mg/L and Atlimeyaya with 1.399 mg/L) [35].
The arsenic removal efficiency was compared by considering distinct rock characteristics, agitation times, particle sizes, surface area, pH, and concentrations of major ions using statistical methods and accounting for expected geochemical processes.
2.2.2. Column Tests
Experiments were conducted in percolation columns packed with limestone rocks from the Soyatal Formation (Zimapán, Mexico) to evaluate arsenic (As) removal in the presence of common groundwater ions, including Cl−, SO42−, and HCO3−. Acrylic columns were filled with limestone particles (0.5–1.41 mm) and fed with solutions (0.5 L/day downflow) simulating the concentrations of MUHI Well (1.2 mg/L As coexisting with another anion). Over 18 weeks, physicochemical parameters (pH, conductivity, and oxidation–reduction potential) and analyte concentrations were monitored. Subsequently, the rocks were analyzed via XRF, XRD, and Scanning Electron Microscopy (SEM) to identify mineralogical and chemical changes [36]. A schematic diagram of the experiments is depicted in Figure 2.
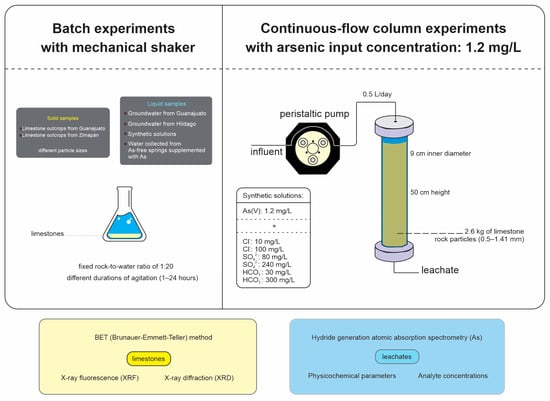
Figure 2.
Schematic diagram of batch (left) and column experiments (right) to evaluate the removal of arsenic from water.
3. Results and Discussion
3.1. Mineralogy
Calcite was the primary mineral identified in the studied rocks, with reported percentages ranging from 38 to 89%, followed by quartz, which accounted for 4 to 36% (Table 2). It qualified as abundant and predominant in M1 and M3, respectively. Some samples also presented other minerals, such as micas, hematite, fluorite, phyllosilicates, and clays (including kaolinite, chlorite, and smectite). The Soyatal rocks (KSS, M2, and M3) had the highest calcite content, while samples from Guanajuato exhibited the highest quartz concentration, reaching up to 36%. The mineralogy indicates that calcite, hematite, goethite, and clays are potential minerals capable of retaining arsenic in rocks [38,39].

Table 2.
Mineralogical composition of samples.
3.2. Rock Chemistry
Table 3 presents the analysis of major oxides, as determined by XRF. The concentrations of major elements present significant variability in the analyzed samples. CaO is the dominant component, with an average of 36.38% and maximum values in M2 (50.89%) and RC1701 (44.72%), indicating a composition rich in carbonates, primarily calcite. Silica (SiO2) varies widely, ranging from 6.91% to 46.56%, with M1 (46.56%) and DD1706 (35.39%) exhibiting the most significant presence of quartz or silicates. Iron oxide (reported as Fe2O3) exhibits high values in M1 (17.52%), indicating the presence of iron minerals such as hematite or goethite, whereas in the other samples, it is considerably lower (average of 3.68%). Potassium oxide (K2O) exhibits high variability, ranging from 0.20% to 8.57%. M1 is the most enriched sample, possibly due to feldspars or micas. Sodium oxide (Na2O) and phosphorus oxide (P2O5) are in low concentrations in all samples, with means of 0.163% and 0.101%, respectively. The samples are generally predominantly calcareous, with variations in silica and iron oxides, highlighting M1 as an atypical sample with high SiO2, Fe2O3, and K2O concentrations.

Table 3.
Concentrations (mg/kg) of major elements (as oxides) in rock samples.
Minor elements (Table 4) also reflect significant differences between the samples, which allows for the inference of alteration processes and geochemical enrichment. Barium (Ba) reaches its highest concentration in M1 (2111 mg/kg), suggesting the possible presence of barite or other barium minerals. Strontium (Sr) is elevated in RC1701 (430 mg/kg) and KSS (543 mg/kg), indicating an association with calcite, consistent with the high CaO content in these samples. Regarding transition elements, vanadium (V) is highest in M1 (261 mg/kg), while chromium (Cr) is highest in DD1706 (69 mg/kg), suggesting differences in mineralogical composition and alteration processes. Nickel (Ni) and cobalt (Co) are low in most samples, except in DD1706, where Ni reaches 41 mg/kg, which may indicate the presence of ultramafic or sulfide minerals.

Table 4.
Concentrations of trace elements in rock samples (mg/kg).
The main characteristics of the rock particles used in the experiments are presented in Table 5.

Table 5.
Grain size, specific surface area, and average pore size of rock samples used in the experiments.
As expected, the specific surface area increases with the smaller grain size for all samples. However, differences were observed between the samples from Guanajuato (RC1701, DD1706, and DD1705) and those from Zimapán (KSS01, KSS02, KSS03, and KSS04), which corresponded to the same rock sample (KSS) but with different particle sizes, exhibiting more than double the specific surface area compared to those from Guanajuato. Additionally, the average pore size was higher in Zimapán rocks compared to Guanajuato, but with more minor differences than in the area.
3.3. Groundwater Hydrogeochemistry
Table 6 shows that the groundwater pH of the samples used in the experiments ranges from slightly acidic to alkaline values (5.57 to 8.18) with conductivity from 184 to 661 µS/cm, indicating low mineralization. Temperatures range from 16.6 to 45.7 °C, with the highest temperatures measured in SJMer and PV03, collected in Guanajuato state. Bicarbonates (HCO3−) are the main anions in all samples, while the predominance of cations shows evident differences. Arsenic concentrations span a wide range, from non-detectable (Axocopan and Atlimeyaya) to more than 129 times the WHO drinking water guidelines (1.297 mg/L in El Muhi well). Essential variations were observed in fluoride concentrations, ranging from 0.5 mg/L to 7.2 mg/L, with three samples (ZIM1801, PV03, and SJMer) exceeding the WHO guideline of 1.5 mg/L and the Mexican standard of 1.0 mg/L. The high concentration of arsenic in ZIM1801 can be attributed to the interaction of water with arsenic minerals, specifically the oxidation of arsenopyrite and the dissolution of scorodite [24]. The origin of fluoride is possibly related to the interaction of groundwater with fluorite occurring in veins near the well.

Table 6.
Physicochemical parameters and concentrations of major and minor species in groundwater samples used in the experiments.
The hydrogeochemical characteristics of the waters were plotted in Piper, Schoeller, and Stiff diagrams to evaluate the similarities and differences between the groundwater samples used in the experiments.
The Piper diagram (Figure 3) reveals substantial differences in cation predominance between the samples from Zimapán and Guanajuato and the springs. Calcium is predominant in Zimapán water, while sodium is predominant in the Guanajuato samples; however, there is no cation predominance in the springs. Bicarbonate is the primary anion in all sampled waters. However, PV03 has a higher chloride proportion, and sulfate levels are lower in both samples from Guanajuato (PV03 and SJMer) than in those from Zimapán. These differences are evident in the central diamond, where the Guanajuato waters separate from the rest. The location of most samples in the bicarbonate zone reflects water–rock interaction with limestones or possibly with basalts. The waters from Zimapán, classified as the bicarbonate calcium type, reflect interaction with a limestone matrix. In contrast, PV03 and SJMerAgre, with higher Na + K concentrations, are classified as the bicarbonate sodium type, whose composition may be related to evaporation or ionic exchange.
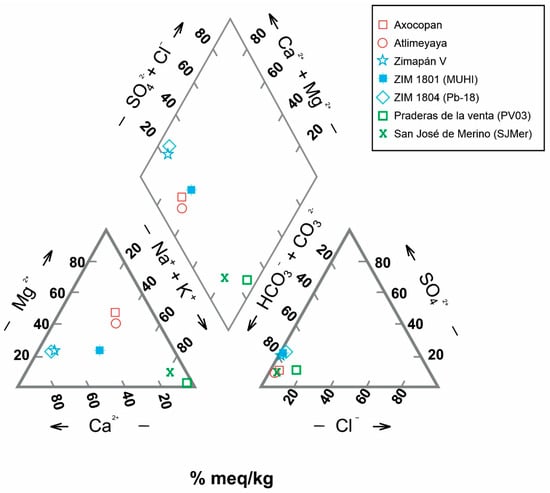
Figure 3.
Piper diagram of groundwater samples.
The Schoeller diagram (Figure 4) also reveals the differences in cation predominance between all samples, indicating higher calcium concentrations in the Zimapán samples than the others. A similar anion pattern can be observed between San José de Merino and Atlimeyaya, but with higher concentrations in San José de Merino. Lower sulfate and calcium concentrations in the Guanajuato samples, compared to those of Zimapán, are also evident in the diagram. Sulfate enrichment may be related to interaction with sulfate minerals or sulfide oxidation. This last process has been reported to occur in the Zimapán zone, specifically concerning arsenic, due to the presence of arsenopyrite [24]. The highest chloride concentration of Praderas de la Venta is also evidenced in the diagram. Groundwater samples from Guanajuato exhibit a predominance of Na+ + K+ and HCO3−, which, in conjunction with higher temperatures, suggests a hydrochemical system influenced by water–rock interactions with igneous materials or sodium-rich sediments, such as feldspars or volcanic ash.
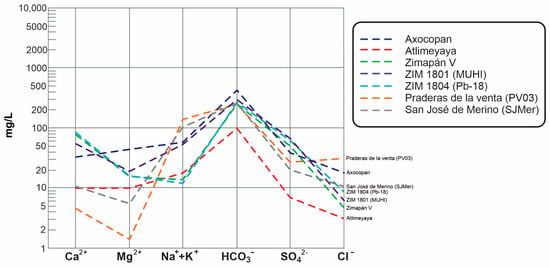
Figure 4.
Schoeller diagram of the groundwater samples used in the experiments.
3.4. Removal Experiments
An overview of arsenic (As) removal efficiency in batch treatment experiments with various rock types, particle sizes, agitation times, groundwater, and synthetic waters, as reported in different studies, is presented below.
Table 7 shows that the final arsenic concentrations decrease significantly with increasing limestone quantity. With 1 g of rock, the final concentrations vary between 0.0452 mg/L and 0.052 mg/L; with 5 g, they are reduced to a range of 0.0183 mg/L to 0.026 mg/L; and with 10 g, they reach the lowest concentrations, between 0.014 mg/L and 0.016 mg/L. Despite these reductions, only 10 g of limestone and 5 h of contact time achieve a final concentration close to the permissible limit (0.014 mg/L). The standard deviation is low in all cases, suggesting consistent and reproducible results. However, greater variability is observed with 1 g of limestone because this method is less robust than using larger amounts of adsorbent.

Table 7.
Arsenic removal at different agitation times and rock amounts (<0.5 mm particle size KSS rocks) in batch experiments with 100 mL Zimapán V water sample [32].
Sorption isotherms were constructed (Figure 5 and Figure 6) to understand the processes involved in arsenic (As) retention based on the concentrations obtained from batch tests. Sorption isotherms allow for the interpretation of phenomena involved in the retention of a sorbate by a sorbent [40]. In the specific case of arsenic (As) retention using limestones from the Soyatal Formation (originating from the state of Hidalgo, Mexico), it has been demonstrated that the essential constituent mineral, namely, calcite, is the primary sorbent [41]. For As sorption by calcite, two main removal mechanisms have been identified: (1) adsorption and (2) coprecipitation [42].
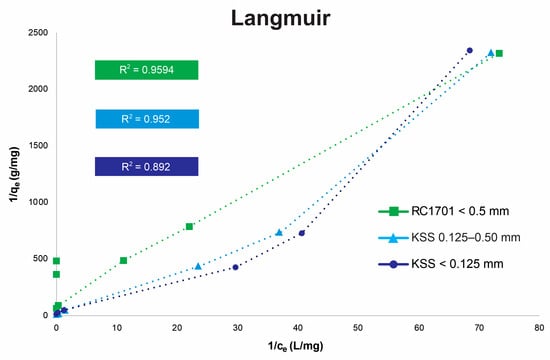
Figure 5.
Plot of 1/qe versus 1/Ce Langmuir sorption isotherm (using 20 g of limestone and 100 mL of solution with 60 min of agitation time).
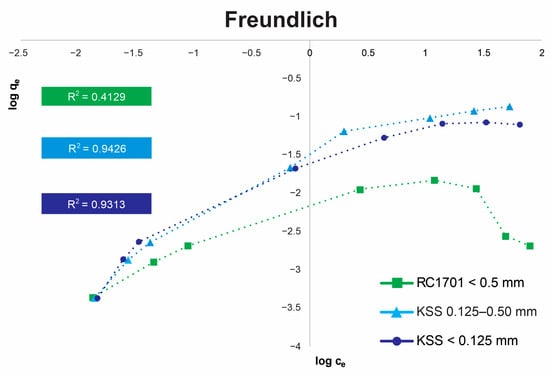
Figure 6.
Plot of log Ce versus log qe Freundlich sorption isotherm (using 20 g of limestone and 100 mL of solution with 60 min of agitation time).
Figure 5 and Figure 6 present the Langmuir and Freundlich isotherms for the following samples: (1) rock RC1701 (Guanajuato) with a particle size < 0.5 mm, (2) rock KSS (Hidalgo) with a particle size of 0.125–0.5 mm, and (3) rock KSS (Hidalgo) with a particle size < 0.125 mm. The determination coefficients (R2) for rock RC1701 (Guanajuato) indicate that the Langmuir isotherm best describes its sorption behavior (R2: 0.9594). This rock exhibits lower solubility due to its CaCO3 content (66%) [43] and a smaller average pore size (see Table 5) compared to the KSS rock (Hidalgo). Its adherence to the Langmuir model suggests homogeneous arsenic adsorption via monolayer coverage at fixed adsorption sites until saturation is achieved. For the KSS limestone (Hidalgo) with a smaller particle size (<0.125 mm), a better fit (R2 = 0.9313) is obtained with the Freundlich model, indicating multilayer sorption in a heterogeneous system [44]. Kinetic experiments presented by Alexandratos et al. (2007) [42] reveal dual As(V) uptake by calcite, characterized by an initial rapid uptake, interpreted as chemisorption on readily accessible surface sites, followed by a secondary sorption process. The latter, leading to significant additional As(V) uptake over extended timescales, has been attributed to (1) surface precipitation, (2) coprecipitation, (3) diffusion into less accessible sites within pores and fractures, and/or (4) adsorption onto sites with slower reaction kinetics due to lower affinity [42]. The experimental results indicate that the more soluble rock, based on its CaCO3 concentration (89%) and findings reported by Sosa (2019) [45], along with its larger average pore size (see Table 5), better meets the necessary conditions for slow removal mechanisms. It should be noted that the data points in the isotherms in Figure 5 and Figure 6 are based on concentrations obtained from batch tests with agitation for 60 min. The Freundlich model for the KSS limestone with a smaller particle size reflects the behavior of a natural material where no evident saturation can be observed, likely due to As (co)precipitation processes, and where the sorbent surface is non-uniform, both in terms of porosity and the presence of sorption sites with varying interaction energies [42]. For KSS limestone with a larger particle size (0.125 mm to 0.5 mm), the determination coefficients (R2) are similar (0.952 for Langmuir and 0.9426 for Freundlich), indicating that this natural material exhibits the intermediate characteristics of a complex system, bridging homogeneous/heterogeneous behavior, monolayer/multilayer sorption, and saturation/continuous growth in removal. Langmuir and Freundlich isotherms have been reported for the calcite–As(V) system [46,47].
The effect of using different rocks and grain sizes collected in Guanajuato on As removal from synthetic solutions and groundwater samples is reported in Table 8. Rock grain size has a significant impact on removal efficiency. Fine-grained particles (<0.05 mm) are more efficient in both groundwater matrices; however, their removal is less efficient than that achieved with synthetic solutions. For example, RC1701 achieves an efficiency of 95.39% in treating synthetic solutions, while with groundwater, it is 50.27%. Similarly, DD1706 achieves an efficiency of 97.80% in synthetic solutions and 52.08% in groundwater. This tendency is because fine-grained particles have a larger surface area, which favors arsenic adsorption.

Table 8.
Removal efficiency for synthetic solutions and groundwater in experiments with different rocks from the Guanajuato area [34,48].
On the other hand, coarse grains (0.50–1.41 mm) exhibit reduced efficiency for rock RC1701, with values dropping to 85.16% in synthetic solutions and up to 19.18% in groundwater due to their lower surface area, which limits interaction with dissolved arsenic. However, the effect of grain size is not as evident with rock DD1706. Still, a longer interaction time (5 h) is required to achieve the highest removal with the larger grain size compared to the treatment with the smaller one (1 h) [48].
When comparing the rocks RC1701 and DD1706, both exhibit similar performance in synthetic solutions, with DD1706 showing a slight advantage (up to 97.80% compared to 95.39%). However, in groundwater, RC1701 is more efficient in some cases, such as when the initial arsenic concentration is 0.039 mg/L, which reaches an efficiency of 56.67% compared to 52.08% for DD1706. This behavior suggests that RC1701 may be more effective in natural water conditions, possibly due to its mineralogy, which has a higher calcite content than DD1706.
The initial arsenic concentration also influences the removal efficiency. In synthetic solutions, the efficiency does not vary dramatically with the initial concentration, whereas in groundwater, the efficiency decreases as the arsenic concentration increases. For example, with RC1701, the efficiency drops from 56.67% to 50.27% as the initial concentration increases from 0.039 mg/L to 0.076 mg/L, suggesting a possible saturation of the adsorption sites at higher concentrations.
Synthetic waters exhibit high removal efficiencies, reaching up to 97.80%. In contrast, the efficiency decreases to 19.18% in groundwater from Guanajuato, suggesting that the methods are more effective in controlled conditions than in natural waters with complex matrices.
Table 9 reports the results of batch removal experiments conducted using the same procedure with rock collected in Zimapán (KSS) and arsenic-rich groundwater samples (ZIM1801 (MUHI) and ZIM1804 (Pb-18)), as well as non-polluted groundwater samples supplemented with arsenic (Axocopan and Atlimeyaya). The results compiled in the table indicate similar removal efficiencies, ranging from 75.6% to 87.2%, with the highest percentage observed in the groundwater sample with the lowest arsenic content. In addition, the similar removal of arsenic from natural waters and samples added with arsenic shows that the rocks possess ample capacity to retain arsenic by treating distinct water types. The highest removal from Atlimeyaya compared with Axocopan may correspond to its lower bicarbonate concentration and ionic strength (approximately four times lower), which favors As retention, as reported by Sosa et al. (2020) [36]. Additionally, a higher As removal was achieved with arsenic-enriched spring waters, reaching up to 1.158 mg/L for Atlimeyaya.

Table 9.
Arsenic removal results from experiments using groundwater samples and KSS rock.
The initial concentrations exceed the permissible limit for drinking water (0.01 mg/L according to the WHO and NOM-127-SSA1-2021 [4,29]) in all cases. After treatment, final concentrations range between 0.078 mg/L and 0.366 mg/L, indicating that arsenic levels remain a health risk. However, the high removal percentage highlights the evident As removal capacity of the rocks.
Experiments conducted using the same rock (KSS) and water from two polluted wells (Pb-18 and MUHI), as shown in Figure 7, provide additional information on the influence of the water matrix, agitation times, and particle size on arsenic (As) removal.
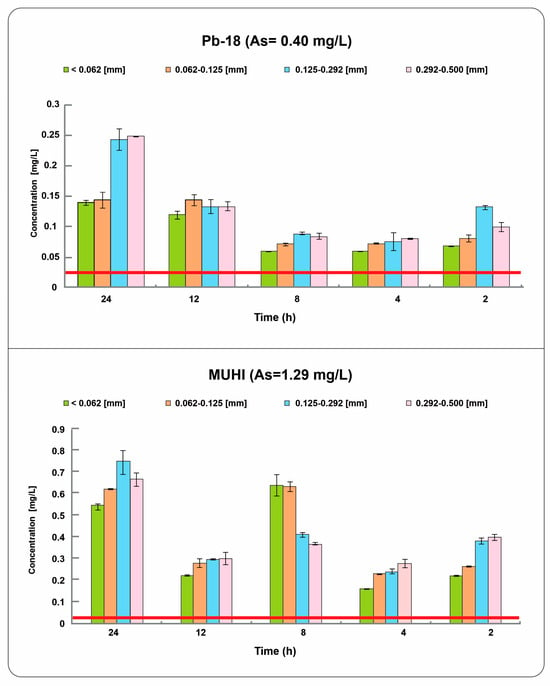
Figure 7.
Final concentrations resulting from batch experiments with KSS rock of different particle sizes after 2 h, 4 h, 8 h, 12 h, and 24 h of agitation time. The red line indicates the Mexican drinking water standard for populations of fewer than 50,000 inhabitants.
The highest removal was achieved with the smallest particle size and after 4 h of treatment for both wells. However, differences in behavior, removal efficiency, and the stability of concentrations are evident between MUHI and Pb-18, as shown in Figure 7. In the MUHI well water, arsenic concentrations after the interaction with the rocks range from 0.1568 mg/L to 0.7381 mg/L, which are considerably higher than those observed in well Pb-18, where concentrations range from 0.0589 mg/L to 0.2485 mg/L. This difference may be related to the high arsenic contamination at MUHI, which is more than three times that of Pb-18. Regarding removal efficiency, MUHI shows percentages ranging from 41.83% to 87.64%. On the other hand, Pb-18 records removal percentages between 48.29% and 87.74%, with higher efficiency in arsenic reduction considering the identical particle size, especially at lower final concentrations. Concentration variations with time also differ between the two samples, with Pb-18 exhibiting an increase in the final concentrations at 8 h of treatment and only a slight rise in MUHI. This difference suggests that the method may remove arsenic from water with a distinct chemical composition yet exhibit specific behavior over time. However, the similar removal percentages may reflect that both waters have the same hydrogeochemical classification (bicarbonate-calcium type) and contain similar sulfate and bicarbonate concentrations, which are the anions identified as the main interferents on As removal [36].
The results of the column experiments (Figure 8) indicated that chloride (Cl−) did not interfere with the removal of As. Although a peak appeared in As concentration at week 7, the outlet concentrations decreased again over time, which could be explained by the heterogeneity of the geological material or by preferential flows within the percolation column; therefore, this increase in chloride ions at week 7 is not decisive in considering it an interfering ion since from week 9 to 18, it remained at values below 0.025 mg/L. In the case of the anions SO42−, HCO3−, and CO32−, competition was observed in removing arsenic As(V) since a gradual increase was observed over time. In the last few weeks, the removal capacity was considerably reduced. The experiments demonstrated that native limestone rocks from Zimapán are effective for arsenic (As) removal, although their efficiency critically depends on the ionic composition of the water [36].
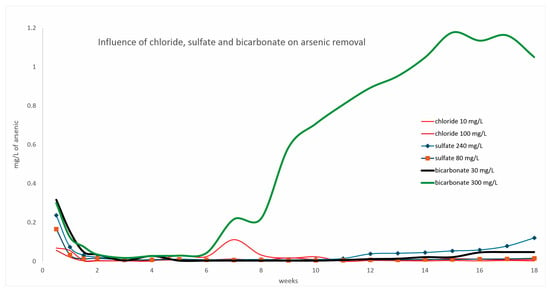
Figure 8.
Results from column experiments showing the As concentration in the output over 18 weeks, showing the influence of anions on As removal. Initial As concentration = 1.2 mg/L.
3.5. General Discussion
According to the results, the arsenic (As) removal efficiency in natural systems depends on the mineralogy, chemistry, and surface area of the rocks used and the characteristics of the water and ions that may interfere with As retention.
In mineralogy, the presence of calcite (CaCO3) significantly influences arsenic (As) removal capacity. The samples with high CaO content, such as RC1701 (44.72% CaO) and KSS (48.67% CaO), demonstrated greater efficiencies in groundwaters. The retention of As can be attributed to the presence of CaCO3 in calcite through coprecipitation or surface adsorption [41]. However, calcite may not be the sole mineral engaged in As removal, as iron oxide minerals identified in some samples show a higher affinity for this element. Iron oxides (hematite and goethite) might contribute to the adsorption in samples DD1706 and M1, forming stable complexes on their surfaces [38]. Clay minerals like smectite, kaolinite, and chlorite, with high surface areas and ion exchange capacities [49], could also aid in arsenic (As) retention in some rocks. Surface area plays a critical role in As removal efficiency, as indicated by experiments where smaller particles with greater surface areas yielded higher percentages. Synthetic solutions treated with rocks of grain sizes less than 0.05 mm showed over 95% As removal efficiencies, while lower efficiencies were observed with grains ranging from 0.5 to 1.41 mm. The specific surface area measured by the BET method reinforces this correlation: rocks with larger surface area values (i.e., KSS with 10.79 m2/g) exhibit better As removal performance compared to those with smaller values (i.e., RC1701 with 2.34 m2/g). Additionally, pore size may influence the accessibility of adsorption sites for As species, potentially enhancing performance. However, this factor was not clearly shown in our studies, as comparable high removal percentages were achieved with rocks from Guanajuato and Zimapán, possibly due to the more significant influence of other rock characteristics.
The lower removal efficiency obtained with natural waters, compared to synthetic ones with certain rocks, relates to the presence of other anions and cations that may affect arsenic (As) adsorption. Column experiments with synthetic solutions revealed carbonate (CO32−), bicarbonate (HCO3−), and sulfate (SO42−) as the primary interfering anions affecting As retention by calcite [36]. Those anions in groundwater may thus reduce the removal efficiency of arsenic (As) in natural waters compared to synthetic solutions. Ionic strength may also influence As removal by limestones, with the highest removal corresponding to lower ionic strength [36].
On the other hand, the removal efficiency of As decreases as its initial concentration in water increases. In synthetic solutions, the arsenic removal efficiency is high (~95%) with the smallest particle size, and does not show substantial variations with the rocks from Guanajuato. However, in natural waters, the efficiency decreases from 56.67% to 50.27% when the initial concentration increases from 0.039 mg/L to 0.076 mg/L in Guanajuato waters. Nevertheless, with Zimapán rocks, the removal ranges were similar between natural and synthetic waters but with the highest As concentration decrease (1.158 mg/L) in the latter ones.
An attempt to summarize adsorption results and compare rock characteristics concerning removal efficiency is presented in Table 10, considering experiments within similar conditions. However, it is complicated to compare the whole tests since, as described in this manuscript, different parameters were evaluated in each experiment (i.e., As concentrations and water characteristics). Nevertheless, some information may be gathered from the table, such as the lower removal efficiency treating groundwater compared to synthetic solutions and the higher removal achieved with the smaller particle size for each rock. Regarding mineralogy, while all the rocks are classified as limestones, the calcite percentage was not directly related to the As removal since DD1706, with the lowest percentage, rendered the highest removal (although slightly higher than KSS with the highest calcite content), possibly due to the presence of iron oxides, as discussed before in this manuscript. Surface area also seems to play an important role, mainly with groundwater, since the highest removal (87.2%) was reached with KSS having the highest superficial area (10.79 m2/g). A similar consideration may be ascribed to the average pore volume.

Table 10.
Effect of rock parameters on As removal efficiency in 20 g rock/100 mL water experiments.
According to the results presented by Sosa-Islas et al. (2023) [41], when using columns packed with limestone from the Soyatal Formation (Upper Cretaceous) to treat aqueous solutions with arsenic (As) in coexistence with certain ions (chlorides, sulfates, and bicarbonates), calcite retains As(V) mainly through two mechanisms: adsorption and coprecipitation. Geochemical modeling was carried out to calculate the saturation indices of the mineral phases by measuring the physicochemical parameters (pH, conductivity, and redox potential) and determining the concentrations present in the leachates. Moreover, for the solid waste from the limestone filtration system, X-ray absorption spectroscopy (XAS) was used: XANES (X-ray absorption near-edge structure) to identify As speciation and EXAFS (Extended X-ray absorption fine structure) to determine the local environment of As in calcite, analyzing interatomic distances and coordination numbers. The mechanisms of arsenic removal by calcite can be described as follows. Adsorption occurs by forming inner-sphere surface complexes by corner-sharing between the AsO43− tetrahedron and the CaO6 octahedra on the calcite surface. The two reported As-Ca distances (~3.4–3.6 Å), confirmed by EXAFS, indicate the formation of a chemical bond. It is worth mentioning that this chemisorption proposal is supported by the difficulty of arsenate desorption when leaching with anions occurs, such as chloride (Cl−), and by the persistence of arsenate adsorption at pH > point of zero charge (PZC). Coprecipitation occurs by replacing the carbonate group (CO32−) with AsO43− in the calcite crystal structure during its formation. The AsO43− has a tetrahedral geometry vs. the trigonal planar molecular geometry of CO32−, but the flexibility of calcite allows for its incorporation. EXAFS showed similar coprecipitation and adsorption environments, suggesting the coexistence of both mechanisms. Finally, secondary mechanisms (<10%) of As removal were reported, such as the precipitation of phases with Ca and Na, and the retained As associated with iron (Fe).
The experiments included in the tables were performed without modifying the pH of the solutions; however, other tests with rocks M1, M2, and M3, as well as pH values between 7.0 and 11.3, showed differences in As removal with varying pH levels. The experiments reported retention ranging from 63% to 92.2% for M1, with a maximum at pH 8; from 75.4% to 98.3% for M2, with a maximum at pH 10.8; and from 37.3% to 97.7% for M3, with a maximum at pH 10.5 [31]. These results indicate that pH has a significant influence on the removal process, with a basic pH being more favorable. However, the influence of the pH of the treated waters, ranging from slightly acidic (5.57) to basic (8.18), was not reflected in the arsenic removal of the experiments reported in this manuscript.
After using limestone rocks to remove arsenic from aqueous solutions until saturation, we do not plan to regenerate the adsorbent; instead, we aim to find a secondary use for these residues. The rocks will no longer be effective for the removal of As present in water; however, they can be used in mine tailings as geochemical barriers to (1) neutralize the pH and (2) retain contaminants through precipitation processes. Although limestone rocks may not have available sorption sites, they can still participate by retaining contaminants through dissolution and (co)precipitation processes, thereby mitigating the impact of mine tailings (more than four deposits at Zimapán) and reducing the mobility of pollutants. One already studied option [50] is to integrate limestone rocks into permeable reactive barriers to treat acid mine drainage (AMD). The advantages of this approach include the following: (1) the circular economy, with the transformation of spent adsorbents into a resource for mine waste stabilization; (2) environmental safety, reducing AMD toxicity by immobilizing multiple contaminants simultaneously. This dual-phase strategy aligns with sustainable water treatment practices, leveraging limestone’s inherent geochemical properties beyond its primary adsorption lifecycle.
4. Conclusions
This study demonstrates that native limestones from Mexico are effective for arsenic (As) removal from groundwater, with performance governed by rock characteristics, water chemistry, and operational parameters.
Experimental results with synthetic water and arsenic-rich groundwater showed that limestones are a promising alternative for removing arsenic from contaminated waters. Specifically, native limestones with calcite content (e.g., KSS:89% CaCO3) achieved up to 87.6% As removal efficiency using fine particles (<0.062 mm), as demonstrated in Zimapán groundwater (with As content of 1.29 mg/L); this can be attributed to the increase in specific surface area and pore volume in smaller particles.
Although limestones from different outcrops have the potential to retain arsenic, their performance depends on their specific characteristics. Additionally, the particle size and rock-to-water ratios determined efficiencies in batch experiments. Finer particles consistently enhanced the removal due to their larger surface area and improved accessibility of adsorption sites. This influence of the area was more evident in arsenic removal from groundwaters. The calcite content shows a link with As retention in groundwater. However, the presence of iron oxides additionally contributes to the elimination.
The chemical composition of groundwater also influenced arsenic (As) removal, with bicarbonate and sulfate reducing efficiency in natural waters due to competitive adsorption (Figure 6). Ionic strength and pH (optimal range: 8–10.8) were additional factors to consider. Due to competitive anion interference, groundwater showed lower removal efficiencies than synthetic solutions. Column tests verified that sulfate, carbonate, and bicarbonate reduced As(V) sorption.
Using finer particles, adjusting water chemistry, and assessing rock mineralogical properties are recommended to maximize arsenic removal. Furthermore, it is crucial to implement a continuous monitoring program to ensure that arsenic concentrations remain within safe limits; final concentrations from 0.078 to 0.261 mg/L after treating Zimapán groundwaters still exceeded WHO guidelines, highlighting the need for iterative optimization, that is, refining and optimizing the result of each iteration. In summary, although synthetic solutions and fine rock particle sizes offer promising results, further research and adjustments are needed to enhance efficiency in groundwater.
The abundance of limestone outcrops in Mexico, accounting for approximately 30% of the territory, underscores the significance of this type of rock for arsenic removal in the country. However, challenges remain in translating laboratory results to field applications, particularly due to variable groundwater chemistry and competing ions, as well as the heterogeneity of the geological material. The next steps for development include (1) the optimization of adsorption parameters (conducting long-term column experiments under real groundwater conditions), (2) evaluating the impact of seasonal hydrochemical variations, (3) evaluating the impact of the heterogeneity of the geological material, (4) testing the repurposing of spent limestone as geochemical barriers in acid mine drainage (AMD) systems and assessing long-term stability of precipitates to ensure contaminant immobilization, and (5) preserving permeability hybrid systems by integrating limestone with iron-based adsorbents or another processes to obtain As-free drinking water.
Our roadmap for developing and implementing a large-scale treatment system for arsenic removal is as follows. We will conduct pilot-scale trials in affected regions to validate scalability, particularly given hydrogeochemical variations and geological heterogeneity. We will deploy packed-bed columns in arsenic-affected communities to validate scalability. We will monitor breakthrough curves and adjust operational parameters, such as flow rate and the frequency of adsorbent replacement. We will develop predictive models to optimize adsorbent lifetime and treatment efficiency. It is worth mentioning that the initial laboratory work has already been carried out to theoretically scale up limestone filters to a pilot plant [51,52]. Secondly, local crushing/sieving facilities should be established to produce cost-effective adsorbents. Thirdly, community engagement and training involve collaborating with local stakeholders to design decentralized treatment units and training operators in system maintenance, effluent monitoring, and the safe handling of saturated limestone.
Author Contributions
Conceptualization, M.A.A.-H.; Formal analysis, M.A.A.-H., A.S.S.-I., J.I.M.-A., M.M.-G., D.E.S., A.A., O.C., and O.N.; Funding acquisition, M.A.A.-H. and J.I.M.-A.; Investigation, M.A.A.-H., A.S.S.-I., J.I.M.-A., M.M.-G., and D.E.S.; Methodology, A.S.S.-I., M.M.-G., D.E.S., A.A., O.C., and O.N.; Visualization, A.S.S.-I. and J.I.M.-A.; Writing—original draft, M.A.A.-H.; Writing—review and editing, M.A.A.-H., A.S.S.-I., and J.I.M.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Dirección General de Asuntos del Personal Académico, UNAM, PAPIIT grant numbers IN106121, IN106918, and IN105023.
Data Availability Statement
The data will be made available upon request.
Acknowledgments
The authors are indebted to two anonymous reviewers for their significant observations and suggestions. We thank Patricia Girón for XRF analyses (Laboratorio de Fluorescencia de Rayos X, Geology Institute, UNAM), Teresa Pi Puig for DRX analyses (Laboratorio de Difracción de Rayos X, Geology Institute, UNAM), Teodoro Hernández for the use of crushing and milling equipment (Laboratorio Universitario de Geoquímica Isotópica, UNAM), and Viridiana Maturano Rojas for BET determinations (Instituto de Ciencias Aplicadas y Tecnología, UNAM).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| As | Arsenic |
| BET | Brunauer-Emmett-Teller |
| EXAFS | Extended X-ray absorption fine structure |
| PZC | Point of Zero Charge |
| PV03 | Praderas de la Venta |
| SJMer | San José de Merino |
| SEM | Scanning Electron Microscopy |
| XAS | X-ray absorption spectroscopy |
| XANES | X-ray absorption near-edge structure |
| XRD | X-ray Diffraction |
| XRF | X-ray Fluorescence |
| WHO | World Health Organization |
References
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245. [Google Scholar] [CrossRef] [PubMed]
- Parvez, F.; Akhtar, E.; Khan, L.; Haq, M.A.; Islam, T.; Ahmed, D.; Raqib, R. Exposure to low-dose arsenic in early life alters innate immune function in children. J. Immunotoxicol. 2019, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arellano, J.M.; Osorio-Yáñez, C.; Sánchez-Peña, L.C.; Ayllon-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid, G.; Del Razo, L.M. Natriuretic peptides and echocardiographic parameters in Mexican children environmentally exposed to arsenic. Toxicol. Appl. Pharmacol. 2020, 403, 115164. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Litter, M.I.; Alarcón-Herrera, M.T.; Arenas, M.J.; Armienta, M.A.; Avilés, M.; Cáceres, R.E.; Cipriani, H.N.; Cornejo, L.; Dias, L.E.; Cirelli, A.F.; et al. Small-scale and household methods to remove arsenic from water for drinking purposes in Latin America. Sci. Total Environ. 2012, 429, 107–122. [Google Scholar] [CrossRef]
- Baig, S.A.; Sheng, T.; Hu, Y.; Xu, J.; Xu, X. Arsenic removal from natural water using low cost granulated adsorbents: A review. CLEAN Soil Air Water. 2015, 43, 13–26. [Google Scholar] [CrossRef]
- Kabir, F.; Chowdhury, S. Arsenic removal methods for drinking water in the developing countries: Technological developments and research needs. Environ. Sci. Pollut. Res. 2017, 24, 24102–24120. [Google Scholar] [CrossRef]
- Sadee, B.A.; Zebari, M.S.; Galali, Y.; Saleem, M.A. A review on arsenic contamination in drinking water: Sources, health impacts, and remediation approaches. RSC Adv. 2025, 15, 2684–2703. [Google Scholar] [CrossRef]
- Ghosh, S.; Igwegbe, C.A.; Malloum, A.; Elmakki, M.A.E.; Onyeaka, H.; Fahmy, A.H.; Osim, N.; Ahmadi, S.; Alameri, B.M.; Ghosh, S.; et al. Sustainable technologies for removal of arsenic from water and wastewater: A comprehensive review. J. Mol. Liq. 2025, 427, 127412. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Pryadko, A.; Mukhortova, Y.R.; Botvin, V.V.; Grubova, I.Y.; Galstenkova, M.R.; Wagner, D.V.; Gerasimov, E.Y.; Sukhinina, E.V.; Pershina, A.G.; Kholkin, A.L.; et al. A comprehensive study on in situ synthesis of a magnetic nanocomposite of magnetite and reduced graphene oxide and its effectiveness at removing arsenic from water. Nano-Struct. Nano-Objects 2023, 36, 101028. [Google Scholar] [CrossRef]
- Gimenez, J.; Martinez, M.; de Pablo, J.; Rovira, M.; Duro, L. Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard. Mater. 2007, 141, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.F.H.; Li, C.; Zhang, H.; Chen, J.; Wang, W.; Dong, Y. Arsenic removal from contaminated water using natural adsorbents: A review. Coatings 2021, 11, 1407. [Google Scholar] [CrossRef]
- Cruz, G.J.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gómez, M.M.; Solis, J.L.; Lang, J. Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zerovalent iron encapsulated chitosan nanospheres—A novel adsorbent for the removal of total inorganic Arsenic from aqueous systems. Chemosphere 2012, 86, 150–155. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Martin-Alarcon, D.A.; Gutiérrez, M.; Reynoso-Cuevas, L.; Martín-Domínguez, A.; Olmos-Márquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 134168. [Google Scholar] [CrossRef]
- Morales, I.; Villanueva-Estrada, R.E.; Rodríguez, R.; Armienta, M.A. Geological, hydrogeological and geothermal factors associated to the origin of arsenic, fluoride and groundwater temperature in a volcanic environment “El Bajío Guanajuatense”, Mexico. Environ. Earth Sci. 2015, 74, 5403–5415. [Google Scholar] [CrossRef]
- Del Razo, L.M.; Ledón, J.M.; Velasco, M.N. Hacia el cumplimiento del Derecho Humano al Agua. In Arsénico y Fluoruro en Agua: Riesgos y Perspectivas Desde la Sociedad Civil y la Academia en México, 1st ed.; UNAM-Instituto de Geofísica: Ciudad de México, México, 2021; p. 200. ISBN 978-607-30-4773-9. [Google Scholar]
- Cebrian, M.E.; Albores, A.; Aguilar, M.; Blakely, E. Chronic arsenic poisoning in the north of Mexico. Hum. Toxicol. 1983, 2, 121–133. [Google Scholar] [CrossRef]
- Del Razo, L.M.; García-Vargas, G.G.; Valenzuela, O.L.; Castellanos, E.H.; Sánchez-Peña, L.C.; Currier, J.M.; Drobná, Z.; Loomis, D.; Stýblo, M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ. Health 2011, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Armienta, M.A.; Villaseñor, G.; Rodriguez, R.; Ongley, L.K.; Mango, H. The role of arsenic-bearing rocks in groundwater pollution at Zimapán Valley, México. Environ. Geol. 2001, 40, 571–581. [Google Scholar] [CrossRef]
- Davis, A.D.; Webb, C.J.; Sorensen, J.L.; Dixon, D.J. Thermodynamic constraints on limestone-based arsenic removal from water. Environ. Earth Sci. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Shan, H.; Ma, T.; Wang, Y.; Zhao, J.; Han, H.; Deng, Y.; He, X.; Dong, Y. A cost-effective system for in-situ geological arsenic adsorption from groundwater. J. Contam. Hydrol. 2013, 154, 33. [Google Scholar] [CrossRef]
- Devi, R.R.; Umlong, I.M.; Das, B.; Borah, K.; Thakur, A.J.; Raul, P.K.; Banerjee, S.; Singh, L. Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Appl. Water Sci. 2014, 4, 175–182. [Google Scholar] [CrossRef]
- Cederkvist, K.; Holm, P.E.; Jensen, M.B. Full-scale removal of arsenate and chromate from water using a limestone and ochreous sludge mixture as a low-cost sorbent material. Water Environ. Res. 2010, 82, 401–408. [Google Scholar] [CrossRef]
- NOM-127-SSA1-2021, Agua Para Uso y Consumo Humano. Límites Permisibles de la Calidad del Agua. DOF 02/05/2022. Available online: https://www.dof.gob.mx/nota_detalle_popup.php?codigo=5650705 (accessed on 2 May 2024).
- Ongley, L.K.; Armienta, M.A.; Heggeman, K.; Lathrop, A.S.; Mango, H.; Miller, W.; Pickelner, S. Arsenic removal from contaminated water by the Soyatal Formation, Zimapán Mining District, Mexico—A potential low-cost low-tech remediation system. Geochem. Explor. Environ. Anal. 2001, 1, 23–31. [Google Scholar] [CrossRef]
- Romero, F.M.; Armienta, M.A.; Carrillo-Chavez, A. Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapán, México. Arch. Environ. Contam. Toxicol. 2004, 47, 1–13. [Google Scholar] [CrossRef]
- Micete-Flores, S. Diseño de una planta piloto basada en adsorción en rocas calizas para el tratamiento del agua contaminada con arsénico del pozo Zimapán V en el municipio de Zimapán, Hidalgo. Master’s Thesis, Universidad Autónoma Metropolitana: Azcapotzalco, Mexico City, México, 31 March 2005. [Google Scholar]
- Manzo-Garrido, M. Remoción de Arsénico y Fluoruro de Aguas Subterráneas Naturalmente Contaminadas en Zimapán, Hidalgo Mediante Rocas Calizas. Bachelor’s Thesis, Facultad de Ingeniería, UNAM, Mexico City, México, 2 September 2019. [Google Scholar]
- Juárez-Aparicio, F.; Morales-Arredondo, J.I.; Armienta-Hernández, M.A. Simultaneous removal of fluoride and arsenic from drinking groundwater using limestones from Bajío Guanajuatense, Mexico. Arab. J. Geosci. 2024, 17, 109. [Google Scholar] [CrossRef]
- Solórzano-Ramos, D.E. Influencia de la Matriz Acuosa en la Remoción de Arsénico y Fluoruro. Bachelor’s Thesis, Facultad de Ciencias, UNAM, Mexico City, México, 17 November 2023. [Google Scholar]
- Sosa, A.; Armienta, M.A.; Aguayo, A.; Cruz, O. Evaluation of the influence of main groundwater ions on arsenic removal by limestones through column experiments. Sci. Total Environ. 2020, 727, 138459. [Google Scholar] [CrossRef]
- APHA; AWWA; WWF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Association Water Environment Federation: Washington, DC, USA, 2025. [Google Scholar]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- Fakhreddine, S.; Fendorf, S. The effect of porewater ionic composition on arsenate adsorption to clay minerals. Sci. Total Environ. 2021, 785, 147096. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.; Isgor, O.B.; Weiss, W.J. Predicting sorption Isotherms from thermodynamic calculations. Cement 2025, 19, 100131. [Google Scholar] [CrossRef]
- Sosa-Islas, A.S.; Armienta-Hernández, M.A.; Loredo-Portales, R.; Aguayo, A.; Cruz, O. An identification of arsenic retention mechanisms in column filtration systems packed with limestone. Appl. Geochem. 2023, 156, 105762. [Google Scholar] [CrossRef]
- Alexandratos, V.G.; Elzinga, E.J.; Reeder, R.J. Arsenate Uptake by Calcite: Macroscopic and Spectroscopic Characterization of Adsorption and Incorporation Mechanisms. Geochim. Cosmochim. Acta 2007, 71, 4172–4187. [Google Scholar] [CrossRef]
- Pignatelli, I.; Kumar, A.; Field, K.G.; Wang, B.; Yu, Y.; Pape, Y.L.; Bauchy, M.; Sant, G. Direct Experimental Evidence for Differing Reactivity Alterations of Minerals following Irradiation: The Case of Calcite and Quartz. Sci. Rep. 2016, 6, 20155. [Google Scholar] [CrossRef]
- Chung, H.; Kim, W.; Jeongwon, P.; Cho, J.; Jeong, T.; Pyung-Kyu, P. Application of Langmuir and Freundlich Isotherms to Predict Adsorbate Removal Efficiency or Required Amount of Adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Sosa, A.S. Aplicación de Rocas Calizas Para la Remediación de Acuíferos Contaminados. Master’s Thesis, Universidad Nacional Autónoma de México (UNAM), Mexico City, México, 2019. [Google Scholar]
- Sø, H.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption and Desorption of Arsenate and Arsenite on Calcite. Geochim. Cosmochim. Acta 2008, 72, 5871–5884. [Google Scholar] [CrossRef]
- Yolcubal, I.; Akyol, N.H. Adsorption and Transport of Arsenate in Carbonate-Rich Soils: Coupled Effects of Nonlinear and Rate-Limited Sorption. Chemosphere 2008, 73, 1300–1307. [Google Scholar] [CrossRef]
- Juárez-Aparicio, F. Evaluación de Rocas Calizas del Bajío Guanajuatense en la Remoción de arsénico y Fluoruro en el Agua Subterránea. Master’s Thesis, Posgrado en Ciencias de la Tierra, UNAM, Mexico City, México, 2019. [Google Scholar]
- Goldberg, S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 2002, 66, 413–421. [Google Scholar] [CrossRef]
- Labastida, I.; Armienta, M.A.; Lara, R.H.; Briones, R.; González, I.; Romero, F. Kinetic approach for the appropriate selection of indigenous limestones for acid mine drainage treatment with passive systems. Sci. Total Environ. 2019, 677, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Aguayo, A.D. Filtros Basados en Roca Caliza Proveniente de la región del Bajío, Gto., para la Remoción de Elementos Contaminantes como arsénico y Fluoruro en el Agua. Bachelor’s Thesis, Faculty of Chemistry, UNAM, Mexico City, México, 2024. [Google Scholar]
- Moreno-Rojo, L.R. Escalamiento Teórico de Filtros Basados en Roca Caliza para Arsénico a Planta Piloto. Bachelor’s Thesis, Faculty of Chemistry, UNAM, Mexico City, México, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).