Microalgae as Bioindicators of Changes in Permafrost Catchments: A Reference Area of the Olyokma Nature Reserve, Yakutia
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling
2.3. Water Chemistry Analysis
2.4. Algological Analysis
3. Results
3.1. Physico-Chemical Parameters of Waters
3.2. Taxonomical and Floristic Analysis of Microscopic Algae
3.3. Bioindicators
3.4. Species–Environmental Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JASP | Jeffreys’s Amazing Statistics Program |
| WESI | Water Ecosystem State Index |
| TDS | Total dissolved solids |
| COD | Chemical oxygen demand |
Appendix A
| Variable | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 | St 10 | St 11 | St 12 | St 13 | St 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T °C | 18.3 | 20.9 | 27.0 | 23.0 | 23.3 | 24.0 | 18.1 | 21.5 | 25.0 | 19.2 | 22.3 | 3.1 | 15.9 | 23.0 |
| pH | 6.89 | 6.85 | 7.20 | 6.68 | 7.15 | 6.72 | 8.26 | 7.52 | 4.65 | 6.46 | 7.62 | 8.10 | 8.20 | 8.02 |
| Color, Pt/Co° | 19 | 47 | 185 | 72 | 113 | 377 | 13 | 97 | 27 | 92 | 101 | 8 | 42 | 39 |
| COD, mgO2 L−1 | 7.46 | 20.00 | 81.75 | 55.50 | 50.45 | 80.20 | 5.85 | 58.15 | 30.95 | 81.35 | 50.15 | 5.25 | 11.15 | 6.70 |
| TDS, mg L−1 | 54 | 49 | 74 | 44 | 57 | 104 | 359 | 148 | 3405 | 95 | 151 | 745 | 495 | 643 |
| Hardness, mg L−1 | 0.7 | 0.6 | 0.9 | 0.5 | 0.7 | 1.3 | 4.5 | 2.0 | 38.0 | 1.2 | 1.9 | 9.8 | 6.6 | 8.2 |
| Ca, mg L−1 | 4.41 | 4.81 | 10.42 | 4.81 | 8.02 | 12.42 | 45.29 | 15.23 | 585.17 | 6.41 | 20.44 | 141.88 | 76.15 | 86.57 |
| Mg, mg L−1 | 5.35 | 4.37 | 4.62 | 3.64 | 3.64 | 8.26 | 27.22 | 15.07 | 106.92 | 10.69 | 11.18 | 33.54 | 33.54 | 47.63 |
| Na, mg L−1 | 1.70 | 1.27 | 1.23 | 0.73 | 0.92 | 1.34 | 5.17 | 0.78 | 186.40 | 1.47 | 0.82 | 2.84 | 1.42 | 2.93 |
| K, mg L−1 | 0.54 | 0.64 | 0.73 | 0.34 | 0.34 | 0.63 | 0.89 | 0.21 | 10.00 | 0.46 | 0.13 | 1.27 | 0.65 | 1.10 |
| HCO3, mg L−1 | 30.0 | 28.0 | 51.0 | 31.5 | 41.0 | 76.0 | 257.5 | 90.0 | 1846.0 | 70.0 | 110.0 | 410.0 | 308.0 | 485.0 |
| Cl, mg L−1 | 1.30 | 2.30 | 2.50 | 1.20 | 1.50 | 2.23 | 7.00 | 1.20 | 291.08 | 1.40 | 1.10 | 4.46 | 2.80 | 3.66 |
| SO4, mg L−1 | 10.50 | 7.10 | 3.20 | 1.41 | 1.60 | 3.20 | 15.90 | 25.50 | 379.46 | 4.20 | 7.00 | 151.40 | 72.70 | 16.55 |
| N-NH4, mg L−1 | 0.22 | 0.48 | 1.48 | 0.93 | 1.06 | 2.25 | 0.20 | 1.02 | 2.42 | 1.43 | 0.96 | 0.46 | 0.50 | 0.80 |
| N-NO2, mg L−1 | 0.005 | 0.006 | 0.012 | 0.010 | 0.010 | 0.022 | 0.004 | 0.007 | 0.005 | 0.008 | 0.007 | 0.004 | 0.004 | 0.006 |
| N-NO3, mg L−1 | 0.03 | 0.06 | 0.26 | 0.13 | 0.15 | 0.51 | 0.02 | 0.14 | 0.35 | 0.24 | 0.15 | 0.19 | 0.10 | 0.07 |
| Ptot, mg L−1 | <0.04 | <0.04 | 0.05 | <0.04 | <0.04 | 0.10 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 |
| P-PO4, mg L−1 | <0.016 | <0.016 | <0.016 | <0.016 | <0.016 | 0.017 | <0.016 | <0.016 | <0.016 | 0.017 | <0.016 | <0.016 | 0.017 | <0.016 |
| Si, mg L−1 | 2.76 | 1.18 | 4.57 | 1.92 | 2.99 | 8.84 | 2.41 | 2.54 | 5.92 | 5.28 | 2.32 | 3.88 | 7.68 | 4.12 |
| Fetot, mg L−1 | 0.10 | 0.34 | 0.66 | 0.27 | 0.64 | 2.35 | 0.05 | 0.67 | 0.25 | 0.60 | 0.70 | 0.05 | 0.20 | 0.11 |
| Taxa | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 | St 10 | St 11 | St 12 | St 13 | St 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cercozoa Cavalier-Smith | ||||||||||||||
| Spongomonas splendida (F.Stein) M.Hartmann & Chagas | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Charophyta Migula | ||||||||||||||
| Actinotaenium capax var. minus (Schmidle) Teiling ex Ruzicka & Pouzar | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bambusina borreri (Ralfs) Cleve | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Closterium abruptum West | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Closterium closterioides var. intermedium (J.Roy & Bisset) Ruzicka | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium dianae var. arcuatum (Brebisson ex Ralfs) Rabenhorst | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Closterium gracile Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium intermedium Ralfs | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium jenneri Ralfs | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium juncidum Ralfs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium kuetzingii Brébisson | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Closterium leibleinii Kützing ex Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Closterium lineatum Ehrenberg ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Closterium littorale F.Gay | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium moniliferum Ehrenberg ex Ralfs | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Closterium navicula (Brébisson) Lütkemüller | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Closterium setaceum Ehrenberg ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Closterium venus Kützing ex Ralfs | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cosmarium baileyi Wolle | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium bioculatum Brébisson ex Ralfs | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cosmarium blyttii var. novaesylvae West & G.S.West | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium botrytis Meneghini ex Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium brebissonii Meneghini ex Ralfs | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cosmarium circulare Reinsch | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium contractum O.Kirchner | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cosmarium crenulatum Nägeli | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium formosulum Hoff | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Cosmarium granatum Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium hammeri Reinsch | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cosmarium humile Nordstedt ex De Toni | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cosmarium impressulum Elfving | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium meneghinii Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium obsoletum (Hantzsch) Reinsch | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium ochthodes Nordstedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cosmarium pachydermum P.Lundell | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium pachydermum var. minus Nordstedt | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium porteanum W.Archer | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium pseudoexiguum Raciborski | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium pseudopyramidatum P.Lundell | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium punctulatum Brébisson | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cosmarium regnellii Wille | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium regnesi Reinsch | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium reniforme (Ralfs) W.Archer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cosmarium sexnotatum var. tristriatum (Lütkemuller) Schmidle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cosmarium subprotumidum Nordstedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium subtumidum Nordstedt | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium subundulatum Wille | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium trilobulatum var. depressum Printz | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium ungerianum var. subtriplicatum West & G.S.West | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Desmidium aptogonum Brébisson ex Kützing | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Desmidium aptogonum var. ehrenbergii Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Desmidium baileyi (Ralfs) Nordstedt | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Desmidium coarctatum Nordstedt | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Desmidium graciliceps (Nordstedt) Lagerheim | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Desmidium swartzii C.Agardh ex Ralfs | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Elakatothrix parvula (W.Archer) Hindák | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum ansatum Ehrenberg ex Ralfs | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum bidentatum Nägeli | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum binale Ehrenberg ex Ralfs | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum binale var. minus (West) Willi Krieger | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum crassicolle P.Lundell | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum didelta Ralfs | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum elegans Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum gemmatum Ralfs | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euastrum neosinuosum O.V.Anissimova & Guiry | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Euastrum pulchellum Brébisson | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gonatozygon aculeatum W.N.Hastings | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gonatozygon brebissonii De Bary | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gonatozygon monotaenium De Bary | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Groenbladia neglecta (Raciborski) Teiling | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Groenbladia undulata (Nordstedt) Kurt Förster | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haplotaenium rectum (Delponte) Bando | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hyalotheca dissiliens Brébisson ex Ralfs | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyalotheca indica W.B.Turner | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyalotheca mucosa Ralfs | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Micrasterias crux-melitensis Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Micrasterias pinnatifida Ralfs | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Micrasterias truncata Brébisson ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mougeotia laetevirens (A.Braun) Wittrock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Octacanthium bifidum var. latidivergens (West) Petlovany | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penium margaritaceum Brébisson ex Ralfs | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotaenium clavatum (Ralfs) De Bary | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotaenium coronatum (Brébisson) Rabenhorst | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotaenium ehrenbergii (Ralfs) De Bary | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotaenium elongatum (West) Coesel & Meesters | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotaenium trabecula Nägeli | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Sphaerozosma filiforme Ralfs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sphaerozosma laeve (Nordstedt) Thomasson | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spirogyra sp. ster. | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spondylosium ellipticum West & G.S.West | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spondylosium lundellii O.Borge | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spondylosium planum (Wolle) West & G.S.West | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurastrum boreale West & G.S.West | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Staurastrum brevispina Brébisson | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Staurastrum dilatatum Ehrenberg ex Ralfs | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum furcatum Brébisson | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum furcigerum (Brébisson) W.Archer | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum gemelliparum Nordstedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum gracile Ralfs ex Ralfs | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurastrum granulosum Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Staurastrum longispinum (Bailey) W.Archer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum manfeldtii Delponte | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum muticum Brébisson ex Ralfs | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum paradoxum Meyen ex Ralfs | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum punctulatum Brébisson | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Staurastrum spiniferum West | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Staurastrum subarmigerum J.Roy & Bisset | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum submonticulosum J.Roy & Bisset | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum teliferum var. gladiosum (W.B.Turner) Coesel & Meesters | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Staurastrum tetracerum Ralfs ex Ralfs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurastrum vestitum Ralfs | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurodesmus convergens (Ehrenberg ex Ralfs) S.Lillieroth | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurodesmus cuspidatus (Brébisson) Teiling | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurodesmus dejectus (Brébisson) Teiling | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurodesmus glaber (Ralfs) Teiling | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurodesmus incus (Hassal ex Ralfs) Teiling | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurodesmus leptodermus (P.Lundell) Teiling | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Teilingia granulata (J.Roy & Bisset) Bourrelly | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xanthidium antilopaeum Kützing | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Xanthidium cristatum Brébisson ex Ralfs | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlorophyta Reichenbach | ||||||||||||||
| Actinastrum hantzschii var. subtile Wołoszyńska | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acutodesmus acutiformis var. costatus (Huber-Pestalozzi) P.M.Tsarenko & D.M.John | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus arcuatus Korshikov | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus falcatus (Corda) Ralfs | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Ankistrodesmus fusiformis Corda | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aphanochaete repens A.Braun | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Botryococcus braunii Kützing | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Coelastrum astroideum De Notaris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Comasiella arcuata (Lemmermann) E.Hegewald, M.Wolf, Al.Keller, Friedl & Krienitz | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium angulosum var. concinnum (Rabenhorst) West & G.S.West | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Crucigenia fenestrata (Schmidle) Schmidle | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Desmodesmus armatus (Chodat) E.H.Hegewald | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Desmodesmus denticulatus var. linearis (Hansgirg) Hegewald | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Desmodesmus microspina (Chodat) P.M.Tsarenko | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Desmodesmus spinosus (Chodat) E.Hegewald | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dictyosphaerium ehrenbergianum Nägeli | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dimorphococcus lunatus A.Braun | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoraphidium contortum (Thuret) Komárková-Legnerová | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoraphidium komarkovae Nygaard | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Nephrocytium lunatum West | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedogonium undulatum A.Braun ex Hirn | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oocystis lacustris Chodat | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oocystis parva West & G.S.West | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oocystis rhomboidea Fott | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pandorina morum (O.F.Müller) Bory | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Pseudopediastrum boryanum (Turpin) E.Hegewald | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Quadrigula korsikovii Komárek | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scenedesmus ellipticus Corda | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Scenedesmus obtusus f. disciformis (Chodat) Compère | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scenedesmus obtusus Meyen | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Selenastrum bibraianum Reinsch | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Sorastrum spinulosum Nägeli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Stauridium tetras (Ehrenberg) E.Hegewald | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tetradesmus obliquus (Turpin) M.J.Wynne | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Tetraëdron minimum (A.Braun) Hansgirg | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Ulothrix zonata (F.Weber & Mohr) Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Verrucodesmus verrucosus (Y.V.Roll) E.Hegewald | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Volvox aureus Ehrenberg | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Volvox polychlamys Korshikov | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Westella botryoides (West) De Wildeman | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Willea irregularis (Wille) Schmidle | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyanobacteria Stanier ex Cavalier-Smith | ||||||||||||||
| Anabaena augstumalis Schmidle | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anabaena oscillarioides Bory ex Bornet & Flahault | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anagnostidinema amphibium (Gomont) Strunecký, Bohunická, J.R.Johansen & Komárek | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Anathece clathrata (West & G.S.West) Komárek, Kaštovský & Jezberová | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aphanocapsa conferta (West & G.S.West) Komárková-Legnerová & Cronberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aphanocapsa delicatissima West & G.S.West | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aphanocapsa holsatica (Lemmermann) G.Cronberg & Komárek | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aphanocapsa incerta (Lemmermann) G.Cronberg & Komárek | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Chroococcus turgidus (Kützing) Nägeli | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Coelosphaerium kuetzingianum Nägeli | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lyngbya cincinnata (Itzigsohn) Compère | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Merismopedia glauca (Ehrenberg) Kützing | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Merismopedia tranquilla (Ehrenberg) Trevisan | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Microcystis aeruginosa (Kützing) Kützing | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nostoc commune Vaucher ex Bornet & Flahault | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nostoc kihlmanii Lemmermann | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Oscillatoria limosa C.Agardh ex Gomont | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Oscillatoria princeps Vaucher ex Gomont | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Oscillatoria proboscidea Gomont | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oscillatoria rupicola (Hansgirg) Hansgirg ex Forti | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oscillatoria tenuis C.Agardh ex Gomont | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phormidium ambiguum Gomont | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Phormidium breve (Kützing ex Gomont) Anagnostidis & Komárek | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Rivularia biasolettiana Meneghini ex Bornet & Flahault | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scytonema coactile Montagne ex Bornet & Flahault | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Snowella lacustris (Chodat) Komárek & Hindák | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spirulina abbreviata Lemmermann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Woronichinia naegeliana (Unger) Elenkin | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinoflagellata Fensome & al. | ||||||||||||||
| Ceratium cornutum (Ehrenberg) Claparède & J.Lachmann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ceratium hirundinella (O.F.Müller) Dujardin | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peridinium bipes F.Stein | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peridinium cinctum (O.F.Müller) Ehrenberg | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Peridinium willei Huitfeldt-Kaas | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euglenophyta Cavalier-Smith | ||||||||||||||
| Colacium vesiculosum Ehrenberg | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Lepocinclis acus (O.F.Müller) B.Marin & Melkonian | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Phacus limnophilus (Lemmermann) E.W.Linton & Karnkowska | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Phacus longicauda (Ehrenberg) Dujardin | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Phacus orbicularis Hübner | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Phacus pleuronectes (O.F.Müller) Nitzsch ex Dujardin | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trachelomonas acanthostoma var. minor Dreżepolski | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas armata (Ehrenberg) F.Stein | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trachelomonas armata var. steinii Lemmermann | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas australica var. granulata (Playfair) Deflandre | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas crenulatocollis Maskell | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Trachelomonas dubia Svirenko | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas dybowskii Dreżepolski | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas granulosa Playfair | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas hispida (Perty) F.Stein | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas lacustris Dreżepolski | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas oblonga Lemmermann | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas superba Svirenko | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas woycickii Koczwara | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Heterokontophyta Moestrup, R.A.Andersen & Guiry | ||||||||||||||
| Asterionella tekelili D.M.Williams, T.M.Schuster, E.Cesar & Jüttner | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dinobryon bavaricum Imhof | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinobryon cylindricum O.E.Imhof | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dinobryon divergens O.E.Imhof | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinobryon divergens var. schauinslandii (Lemmermann) Brunnthaler | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinobryon sertularia Ehrenberg | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Encyonema silesiacum (Bleisch) D.G.Mann | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Entomoneis ornata (Bailey) Reimer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithemia adnata (Kützing) Brébisson | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Epithemia gibba (Ehrenberg) Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Epithemia turgida (Ehrenberg) Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gomphonema sp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema acuminatum Ehrenberg | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema capitatum Ehrenberg | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Meridion circulare (Greville) C.Agardh | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Nitzschia acicularis (Kützing) W.Smith | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pinnularia nobilis (Ehrenberg) Ehrenberg | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stenopterobia anceps (F.W.Lewis) Brébisson ex Van Heurck | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella librile (Ehrenberg) Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tabellaria fenestrata (Lyngbye) Kützing | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Tabellaria flocculosa (Roth) Kützing | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Tribonema vulgare Pascher | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ulnaria ulna (Nitzsch) Compère | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Urosolenia eriensis (H.L.Smith) Round & R.M.Crawford | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taxa | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 |
|---|---|---|---|---|---|---|---|---|---|
| Charophyta Migula | |||||||||
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | 0.11 | ||||||||
| Bambusina borreri (Ralfs) Cleve | 0.11 | 0.70 | 0.70 | ||||||
| Closterium dianae var. arcuatum (Brebisson ex Ralfs) Rabenhorst | 0.70 | ||||||||
| Closterium gracile Brébisson ex Ralfs | 0.11 | ||||||||
| Closterium intermedium Ralfs | 0.11 | 0.70 | |||||||
| Closterium jenneri Ralfs | 0.11 | ||||||||
| Closterium juncidum Ralfs | 0.11 | ||||||||
| Closterium kuetzingii Brébisson | 0.70 | 0.15 | |||||||
| Closterium leibleinii Kützing ex Ralfs | 0.15 | ||||||||
| Closterium lineatum Ehrenberg ex Ralfs | 0.15 | ||||||||
| Closterium moniliferum Ehrenberg ex Ralfs | 0.11 | 0.15 | |||||||
| Cosmarium bioculatum Brébisson ex Ralfs | 0.11 | 0.23 | 0.70 | 0.70 | 0.70 | ||||
| Cosmarium blyttii var. novaesylvae West & G.S.West | 0.11 | ||||||||
| Cosmarium botrytis Meneghini ex Ralfs | 0.70 | ||||||||
| Cosmarium brebissonii Meneghini ex Ralfs | 0.70 | ||||||||
| Cosmarium circulare Reinsch | 0.70 | 0.70 | |||||||
| Cosmarium contractum O.Kirchner | 0.70 | ||||||||
| Cosmarium crenulatum Nägeli | 0.11 | ||||||||
| Cosmarium formosulum Hoff | 0.15 | 0.70 | 0.70 | ||||||
| Cosmarium humile Nordstedt ex De Toni | 0.11 | 0.15 | 0.70 | ||||||
| Cosmarium obsoletum (Hantzsch) Reinsch | 0.11 | 0.70 | |||||||
| Cosmarium pachydermum P.Lundell | 0.70 | ||||||||
| Cosmarium pseudopyramidatum P.Lundell | 0.11 | ||||||||
| Cosmarium punctulatum Brébisson | 0.11 | 0.70 | 0.23 | ||||||
| Cosmarium reniforme (Ralfs) W.Archer | 0.70 | ||||||||
| Cosmarium subtumidum Nordstedt | 0.70 | 0.70 | |||||||
| Cosmarium subundulatum Wille | 0.70 | ||||||||
| Cosmarium trilobulatum var. depressum Printz | 0.11 | ||||||||
| Cosmarium ungerianum var. subtriplicatum West & G.S.West | 0.70 | ||||||||
| Desmidium aptogonum Brébisson ex Kützing | 0.68 | 0.70 | |||||||
| Desmidium aptogonum var. ehrenbergii Kützing | 0.70 | ||||||||
| Desmidium baileyi (Ralfs) Nordstedt | 0.70 | 0.70 | 6.80 | ||||||
| Desmidium graciliceps (Nordstedt) Lagerheim | 0.70 | ||||||||
| Desmidium swartzii C.Agardh ex Ralfs | 0.11 | 0.70 | 0.70 | 0.70 | 0.70 | ||||
| Euastrum binale Ehrenberg ex Ralfs | 0.70 | ||||||||
| Euastrum binale var. minus (West) Willi Krieger | 0.11 | ||||||||
| Euastrum gemmatum Ralfs | 0.34 | ||||||||
| Gonatozygon aculeatum W.N.Hastings | 0.70 | ||||||||
| Gonatozygon brebissonii De Bary | 0.15 | ||||||||
| Gonatozygon monotaenium De Bary | 0.11 | 1.20 | 0.70 | 0.70 | 0.70 | ||||
| Groenbladia neglecta (Raciborski) Teiling | 0.70 | ||||||||
| Hyalotheca dissiliens Brébisson ex Ralfs | 0.70 | ||||||||
| Hyalotheca indica W.B.Turner | 0.70 | ||||||||
| Hyalotheca mucosa Ralfs | 0.70 | 0.70 | 0.70 | ||||||
| Micrasterias crux-melitensis Ralfs | 0.70 | ||||||||
| Micrasterias pinnatifida Ralfs | 0.70 | 0.70 | |||||||
| Micrasterias truncata Brébisson ex Ralfs | 0.70 | ||||||||
| Pleurotaenium coronatum (Brébisson) Rabenhorst | 0.11 | 0.70 | |||||||
| Pleurotaenium elongatum (West) Coesel & Meesters | 0.70 | ||||||||
| Pleurotaenium trabecula Nägeli | 0.11 | 0.70 | 0.70 | 0.70 | |||||
| Sphaerozosma filiforme Ralfs | 0.11 | ||||||||
| Sphaerozosma laeve (Nordstedt) Thomasson | 0.11 | ||||||||
| Spondylosium ellipticum West & G.S.West | 0.70 | ||||||||
| Spondylosium lundellii O.Borge | 0.70 | 0.70 | |||||||
| Spondylosium planum (Wolle) West & G.S.West | 0.70 | 0.70 | |||||||
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | 0.11 | 0.70 | 0.70 | 0.70 | 0.70 | ||||
| Staurastrum boreale West & G.S.West | 0.11 | 0.23 | 0.70 | 0.15 | 0.70 | 0.70 | |||
| Staurastrum furcatum Brébisson | 0.70 | ||||||||
| Staurastrum furcigerum (Brébisson) W.Archer | 0.70 | ||||||||
| Staurastrum gracile Ralfs ex Ralfs | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | ||||
| Staurastrum longispinum (Bailey) W.Archer | 0.70 | ||||||||
| Staurastrum manfeldtii Delponte | 0.11 | 0.70 | 0.70 | ||||||
| Staurastrum muticum Brébisson ex Ralfs | 0.70 | 0.70 | |||||||
| Staurastrum paradoxum Meyen ex Ralfs | 0.11 | 0.70 | |||||||
| Staurastrum punctulatum Brébisson | 0.70 | ||||||||
| Staurastrum subarmigerum J.Roy & Bisset | 0.70 | 0.70 | |||||||
| Staurastrum submonticulosum J.Roy & Bisset | 0.70 | ||||||||
| Staurastrum teliferum var. gladiosum (W.B.Turner) Coesel & Meesters | 0.70 | ||||||||
| Staurastrum tetracerum Ralfs ex Ralfs | 0.70 | 0.70 | |||||||
| Staurodesmus convergens (Ehrenberg ex Ralfs) S.Lillieroth | 0.70 | ||||||||
| Staurodesmus cuspidatus (Brébisson) Teiling | 0.70 | 0.70 | 0.70 | 0.70 | |||||
| Staurodesmus dejectus (Brébisson) Teiling | 0.70 | ||||||||
| Staurodesmus glaber (Ralfs) Teiling | 0.70 | 0.70 | 0.70 | 0.70 | |||||
| Staurodesmus incus (Hassal ex Ralfs) Teiling | 0.70 | 0.70 | |||||||
| Staurodesmus leptodermus (P.Lundell) Teiling | 0.23 | ||||||||
| Teilingia granulata (J.Roy & Bisset) Bourrelly | 0.70 | 0.45 | 0.70 | 0.70 | |||||
| Xanthidium antilopaeum Kützing | 0.70 | 0.70 | 0.70 | ||||||
| Xanthidium cristatum Brébisson ex Ralfs | 0.70 | ||||||||
| Chlorophyta Reichenbach | |||||||||
| Ankistrodesmus arcuatus Korshikov | 0.11 | 0.11 | |||||||
| Ankistrodesmus falcatus (Corda) Ralfs | 0.70 | 0.70 | 0.70 | ||||||
| Ankistrodesmus fusiformis Corda | 0.68 | 2.72 | 0.15 | 0.70 | |||||
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | 0.70 | ||||||||
| Botryococcus braunii Kützing | 3.40 | 0.11 | 3.15 | 0.70 | 1.81 | 0.70 | 0.70 | ||
| Coelastrum astroideum De Notaris | 0.15 | ||||||||
| Comasiella arcuata (Lemmermann) E.Hegewald, M.Wolf, Al.Keller, Friedl & Krienitz | 0.11 | ||||||||
| Desmodesmus armatus (Chodat) E.H.Hegewald | 0.15 | ||||||||
| Desmodesmus microspina (Chodat) P.M.Tsarenko | 0.70 | 0.70 | 0.70 | 0.15 | |||||
| Desmodesmus spinosus (Chodat) E.Hegewald | 0.70 | ||||||||
| Dimorphococcus lunatus A.Braun | 0.70 | 0.70 | |||||||
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 0.11 | 0.11 | |||||||
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | 1.36 | ||||||||
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | 0.15 | ||||||||
| Nephrocytium lunatum West | 0.70 | ||||||||
| Oocystis lacustris Chodat | 0.70 | 0.70 | |||||||
| Pandorina morum (O.F.Müller) Bory | 2.72 | 0.11 | 0.70 | 0.70 | 0.15 | 0.70 | 0.70 | ||
| Pseudopediastrum boryanum (Turpin) E.Hegewald | 0.11 | 0.70 | 0.70 | 0.70 | 0.15 | ||||
| Quadrigula korsikovii Komárek | 0.11 | 0.70 | 0.70 | 0.70 | |||||
| Scenedesmus ellipticus Corda | 0.11 | 0.70 | 0.70 | ||||||
| Selenastrum bibraianum Reinsch | 0.11 | 0.70 | 0.70 | ||||||
| Sorastrum spinulosum Nägeli | 0.15 | ||||||||
| Stauridium tetras (Ehrenberg) E.Hegewald | 0.11 | 0.15 | |||||||
| Tetradesmus obliquus (Turpin) M.J.Wynne | 0.15 | ||||||||
| Tetraëdron minimum (A.Braun) Hansgirg | 0.15 | ||||||||
| Volvox polychlamys Korshikov | 34.00 | 0.70 | |||||||
| Willea irregularis (Wille) Schmidle | 36.95 | 0.70 | 0.70 | ||||||
| Cyanobacteria Stanier ex Cavalier-Smith | |||||||||
| Anabaena augstumalis Schmidle | 4.80 | ||||||||
| Chroococcus turgidus (Kützing) Nägeli | 0.15 | 0.70 | |||||||
| Coelosphaerium kuetzingianum Nägeli | 11.33 | 0.70 | |||||||
| Merismopedia glauca (Ehrenberg) Kützing | 0.11 | ||||||||
| Nostoc kihlmanii Lemmermann | 34.00 | 0.70 | 0.70 | 0.70 | |||||
| Oscillatoria limosa C.Agardh ex Gomont | 0.11 | 0.70 | 0.15 | 0.70 | |||||
| Oscillatoria princeps Vaucher ex Gomont | 0.11 | 0.70 | |||||||
| Oscillatoria tenuis C.Agardh ex Gomont | 0.70 | ||||||||
| Spirulina abbreviata Lemmermann | 0.15 | ||||||||
| Woronichinia naegeliana (Unger) Elenkin | 17.00 | ||||||||
| Dinoflagellata Fensome & al. | |||||||||
| Ceratium cornutum (Ehrenberg) Claparède & J.Lachmann | 0.70 | ||||||||
| Ceratium hirundinella (O.F.Müller) Dujardin | 0.34 | 0.70 | 0.70 | 3.17 | 0.70 | ||||
| Peridinium bipes F.Stein | 0.11 | 0.70 | 0.45 | 0.70 | |||||
| Peridinium cinctum (O.F.Müller) Ehrenberg | 0.11 | 0.11 | 0.70 | 0.70 | 0.70 | 0.70 | |||
| Peridinium willei Huitfeldt-Kaas | 0.70 | ||||||||
| Euglenophyta Cavalier-Smith | |||||||||
| Colacium vesiculosum Ehrenberg | 9.70 | 0.70 | 0.70 | ||||||
| Lepocinclis acus (O.F.Müller) B.Marin & Melkonian | 0.11 | 0.15 | |||||||
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 0.11 | 0.11 | 0.45 | ||||||
| Phacus limnophilus (Lemmermann) E.W.Linton & Karnkowska | 0.15 | ||||||||
| Phacus longicauda (Ehrenberg) Dujardin | 0.11 | 0.11 | 0.70 | ||||||
| Phacus orbicularis Hübner | 0.11 | 0.15 | |||||||
| Phacus pleuronectes (O.F.Müller) Nitzsch ex Dujardin | 0.11 | 0.70 | |||||||
| Trachelomonas acanthostoma var. minor Dreżepolski | 0.11 | ||||||||
| Trachelomonas armata (Ehrenberg) F.Stein | 0.70 | ||||||||
| Trachelomonas armata var. steinii Lemmermann | 0.11 | ||||||||
| Trachelomonas australica var. granulata (Playfair) Deflandre | 0.11 | ||||||||
| Trachelomonas crenulatocollis Maskell | 0.70 | ||||||||
| Trachelomonas dubia Svirenko | 0.11 | ||||||||
| Trachelomonas dybowskii Dreżepolski | 0.11 | ||||||||
| Trachelomonas granulosa Playfair | 0.70 | ||||||||
| Trachelomonas hispida (Perty) F.Stein | 0.11 | 0.11 | 0.23 | ||||||
| Trachelomonas superba Svirenko | 0.11 | ||||||||
| Heterokontophyta Moestrup, R.A.Andersen & Guiry | |||||||||
| Asterionella tekelili D.M.Williams, T.M.Schuster, E.Cesar & Jüttner | 2.72 | 0.91 | |||||||
| Aulacoseira sp. | 3.23 | ||||||||
| Dinobryon bavaricum Imhof | 0.34 | 0.34 | 0.70 | 0.70 | 3.63 | 0.70 | |||
| Dinobryon cylindricum O.E.Imhof | 0.70 | ||||||||
| Dinobryon divergens O.E.Imhof | 8.84 | 0.70 | |||||||
| Dinobryon divergens var. schauinslandii (Lemmermann) Brunnthaler | 0.70 | ||||||||
| Dinobryon sertularia Ehrenberg | 1.20 | 0.15 | 0.70 | 1.13 | 22.67 | ||||
| Encyonema silesiacum (Bleisch) D.G.Mann | 0.34 | ||||||||
| Entomoneis ornata (Bailey) Reimer | 0.70 | ||||||||
| Epithemia adnata (Kützing) Brébisson | 0.15 | ||||||||
| Epithemia gibba (Ehrenberg) Kützing | 0.70 | 1.36 | |||||||
| Epithemia turgida (Ehrenberg) Kützing | 0.15 | ||||||||
| Gomphonema sp. | 0.23 | 0.70 | |||||||
| Gomphonema acuminatum Ehrenberg | 0.11 | 0.70 | |||||||
| Gomphonema capitatum Ehrenberg | 0.34 | ||||||||
| Pinnularia nobilis (Ehrenberg) Ehrenberg | 0.70 | ||||||||
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 0.11 | ||||||||
| Stenopterobia anceps (F.W.Lewis) Brébisson ex Van Heurck | 0.11 | 0.11 | 0.23 | ||||||
| Tabellaria fenestrata (Lyngbye) Kützing | 0.11 | 2.38 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | ||
| Tabellaria flocculosa (Roth) Kützing | 4.76 | 2.40 | 0.70 | 0.70 | 0.70 | 0.70 | 0.45 | ||
| Ulnaria ulna (Nitzsch) Compère | 1.36 | 0.70 | 0.15 | ||||||
| Urosolenia eriensis (H.L.Smith) Round & R.M.Crawford | 0.34 | 0.70 |
| Taxa | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 |
|---|---|---|---|---|---|---|---|---|---|
| Charophyta Migula | |||||||||
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | 2.22 | ||||||||
| Bambusina borreri (Ralfs) Cleve | 0.85 | 0.55 | 0.57 | ||||||
| Closterium dianae var. arcuatum (Brebisson ex Ralfs) Rabenhorst | 0.68 | ||||||||
| Closterium gracile Brébisson ex Ralfs | 0.90 | ||||||||
| Closterium intermedium Ralfs | 11.11 | 1.10 | |||||||
| Closterium jenneri Ralfs | 0.13 | ||||||||
| Closterium juncidum Ralfs | 0.46 | ||||||||
| Closterium kuetzingii Brébisson | 1.56 | 6.93 | |||||||
| Closterium leibleinii Kützing ex Ralfs | 1.00 | ||||||||
| Closterium lineatum Ehrenberg ex Ralfs | 6.86 | ||||||||
| Closterium moniliferum Ehrenberg ex Ralfs | 34.30 | 1.34 | |||||||
| Cosmarium bioculatum Brébisson ex Ralfs | 0.20 | 0.58 | 0.13 | 0.13 | 0.13 | ||||
| Cosmarium blyttii var. novaesylvae West & G.S.West | 0.29 | ||||||||
| Cosmarium botrytis Meneghini ex Ralfs | 5.36 | ||||||||
| Cosmarium brebissonii Meneghini ex Ralfs | 2.15 | ||||||||
| Cosmarium circulare Reinsch | 1.82 | 21.58 | |||||||
| Cosmarium contractum O.Kirchner | 0.83 | ||||||||
| Cosmarium crenulatum Nägeli | 0.61 | ||||||||
| Cosmarium formosulum Hoff | 4.57 | 2.28 | 2.90 | ||||||
| Cosmarium humile Nordstedt ex De Toni | 0.10 | 0.32 | 0.14 | ||||||
| Cosmarium obsoletum (Hantzsch) Reinsch | 4.96 | 3.30 | |||||||
| Cosmarium pachydermum P.Lundell | 5.11 | ||||||||
| Cosmarium pseudopyramidatum P.Lundell | 3.10 | ||||||||
| Cosmarium punctulatum Brébisson | 0.47 | 1.28 | 0.95 | ||||||
| Cosmarium reniforme (Ralfs) W.Archer | 5.87 | ||||||||
| Cosmarium subtumidum Nordstedt | 2.41 | 3.30 | |||||||
| Cosmarium subundulatum Wille | 3.34 | ||||||||
| Cosmarium trilobulatum var. depressum Printz | 0.32 | ||||||||
| Cosmarium ungerianum var. subtriplicatum West & G.S.West | 2.60 | ||||||||
| Desmidium aptogonum Brébisson ex Kützing | 3.40 | 0.18 | |||||||
| Desmidium aptogonum var. ehrenbergii Kützing | 0.25 | ||||||||
| Desmidium baileyi (Ralfs) Nordstedt | 0.30 | 0.30 | 43.80 | ||||||
| Desmidium graciliceps (Nordstedt) Lagerheim | 0.33 | ||||||||
| Desmidium swartzii C.Agardh ex Ralfs | 1.40 | 0.78 | 0.40 | 0.69 | 0.69 | ||||
| Euastrum binale Ehrenberg ex Ralfs | 0.17 | ||||||||
| Euastrum binale var. minus (West) Willi Krieger | 0.90 | ||||||||
| Euastrum gemmatum Ralfs | 16.94 | ||||||||
| Gonatozygon aculeatum W.N.Hastings | 2.30 | ||||||||
| Gonatozygon brebissonii De Bary | 0.71 | ||||||||
| Gonatozygon monotaenium De Bary | 1.10 | 9.99 | 0.73 | 0.73 | 0.73 | ||||
| Groenbladia neglecta (Raciborski) Teiling | 0.12 | ||||||||
| Hyalotheca dissiliens Brébisson ex Ralfs | 0.19 | ||||||||
| Hyalotheca indica W.B.Turner | 0.14 | ||||||||
| Hyalotheca mucosa Ralfs | 0.60 | 0.30 | 0.29 | ||||||
| Micrasterias crux-melitensis Ralfs | 16.70 | ||||||||
| Micrasterias pinnatifida Ralfs | 2.40 | 6.87 | |||||||
| Micrasterias truncata Brébisson ex Ralfs | 9.90 | ||||||||
| Pleurotaenium coronatum (Brébisson) Rabenhorst | 57.33 | 34.16 | |||||||
| Pleurotaenium elongatum (West) Coesel & Meesters | 28.72 | ||||||||
| Pleurotaenium trabecula Nägeli | 13.47 | 16.26 | 8.81 | 8.98 | |||||
| Sphaerozosma filiforme Ralfs | 0.90 | ||||||||
| Sphaerozosma laeve (Nordstedt) Thomasson | 0.27 | ||||||||
| Spondylosium ellipticum West & G.S.West | 0.90 | ||||||||
| Spondylosium lundellii O.Borge | 0.68 | 0.68 | |||||||
| Spondylosium planum (Wolle) West & G.S.West | 0.20 | 0.10 | |||||||
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | 2.96 | 0.17 | 1.97 | 1.97 | 1.97 | ||||
| Staurastrum boreale West & G.S.West | 1.50 | 2.13 | 0.70 | 1.40 | 0.70 | 0.70 | |||
| Staurastrum furcatum Brébisson | 0.35 | ||||||||
| Staurastrum furcigerum (Brébisson) W.Archer | 3.00 | ||||||||
| Staurastrum gracile Ralfs ex Ralfs | 0.41 | 2.80 | 2.80 | 2.80 | 2.80 | ||||
| Staurastrum longispinum (Bailey) W.Archer | 0.42 | ||||||||
| Staurastrum manfeldtii Delponte | 4.78 | 1.55 | 1.70 | ||||||
| Staurastrum muticum Brébisson ex Ralfs | 0.32 | 0.19 | |||||||
| Staurastrum paradoxum Meyen ex Ralfs | 0.22 | 0.15 | |||||||
| Staurastrum punctulatum Brébisson | 1.41 | ||||||||
| Staurastrum subarmigerum J.Roy & Bisset | 0.17 | 0.90 | |||||||
| Staurastrum submonticulosum J.Roy & Bisset | 1.32 | ||||||||
| Staurastrum teliferum var. gladiosum (W.B.Turner) Coesel & Meesters | 0.29 | ||||||||
| Staurastrum tetracerum Ralfs ex Ralfs | 0.45 | 0.20 | |||||||
| Staurodesmus convergens (Ehrenberg ex Ralfs) S.Lillieroth | 1.28 | ||||||||
| Staurodesmus cuspidatus (Brébisson) Teiling | 0.70 | 0.70 | 0.70 | 0.70 | |||||
| Staurodesmus dejectus (Brébisson) Teiling | 0.60 | ||||||||
| Staurodesmus glaber (Ralfs) Teiling | 0.10 | 0.80 | 0.10 | 0.10 | |||||
| Staurodesmus incus (Hassal ex Ralfs) Teiling | 0.40 | 0.13 | |||||||
| Staurodesmus leptodermus (P.Lundell) Teiling | 1.40 | ||||||||
| Teilingia granulata (J.Roy & Bisset) Bourrelly | 0.20 | 0.80 | 0.20 | 0.20 | |||||
| Xanthidium antilopaeum Kützing | 4.90 | 4.50 | 4.90 | ||||||
| Xanthidium cristatum Brébisson ex Ralfs | 1.99 | ||||||||
| Chlorophyta Reichenbach | |||||||||
| Ankistrodesmus arcuatus Korshikov | 0.10 | ||||||||
| Ankistrodesmus falcatus (Corda) Ralfs | 0.10 | 0.10 | 0.10 | ||||||
| Ankistrodesmus fusiformis Corda | 0.60 | 0.25 | 0.10 | 0.10 | |||||
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | 0.10 | ||||||||
| Botryococcus braunii Kützing | 0.28 | 0.10 | 2.53 | 0.10 | 0.15 | 0.10 | 0.10 | ||
| Coelastrum astroideum De Notaris | 0.20 | ||||||||
| Comasiella arcuata (Lemmermann) E.Hegewald, M.Wolf, Al.Keller, Friedl & Krienitz | 0.20 | ||||||||
| Desmodesmus armatus (Chodat) E.H.Hegewald | 0.10 | ||||||||
| Desmodesmus microspina (Chodat) P.M.Tsarenko | 0.10 | 0.10 | 0.10 | 0.20 | |||||
| Desmodesmus spinosus (Chodat) E.Hegewald | 0.10 | ||||||||
| Dimorphococcus lunatus A.Braun | 0.50 | 0.50 | |||||||
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 0.10 | 0.10 | |||||||
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | 0.90 | ||||||||
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | 0.16 | ||||||||
| Nephrocytium lunatum West | 0.10 | ||||||||
| Oocystis lacustris Chodat | 0.20 | 0.40 | |||||||
| Pandorina morum (O.F.Müller) Bory | 0.72 | 0.30 | 0.20 | 0.20 | 0.40 | 0.20 | 0.20 | ||
| Pseudopediastrum boryanum (Turpin) E.Hegewald | 0.20 | 0.40 | 0.20 | 0.20 | 0.30 | ||||
| Quadrigula korsikovii Komárek | 0.10 | ||||||||
| Scenedesmus ellipticus Corda | 0.10 | 0.10 | 0.20 | ||||||
| Selenastrum bibraianum Reinsch | 0.10 | 0.10 | |||||||
| Sorastrum spinulosum Nägeli | 0.40 | ||||||||
| Stauridium tetras (Ehrenberg) E.Hegewald | 0.10 | 0.20 | |||||||
| Tetradesmus obliquus (Turpin) M.J.Wynne | 0.20 | ||||||||
| Tetraëdron minimum (A.Braun) Hansgirg | 0.30 | ||||||||
| Volvox polychlamys Korshikov | 3.82 | 0.10 | |||||||
| Willea irregularis (Wille) Schmidle | 2.48 | 0.10 | 0.10 | ||||||
| Cyanobacteria Stanier ex Cavalier-Smith | |||||||||
| Anabaena augstumalis Schmidle | 0.21 | ||||||||
| Chroococcus turgidus (Kützing) Nägeli | 2.80 | 1.40 | |||||||
| Coelosphaerium kuetzingianum Nägeli | 0.50 | ||||||||
| Merismopedia glauca (Ehrenberg) Kützing | 0.10 | ||||||||
| Nostoc kihlmanii Lemmermann | 1.13 | 0.10 | 0.10 | ||||||
| Oscillatoria limosa C.Agardh ex Gomont | 0.30 | 0.20 | 0.40 | 0.20 | |||||
| Oscillatoria princeps Vaucher ex Gomont | 0.36 | 0.24 | |||||||
| Oscillatoria tenuis C.Agardh ex Gomont | 0.10 | ||||||||
| Spirulina abbreviata Lemmermann | 0.10 | ||||||||
| Woronichinia naegeliana (Unger) Elenkin | 0.57 | ||||||||
| Dinoflagellata Fensome & al. | |||||||||
| Ceratium cornutum (Ehrenberg) Claparède & J.Lachmann | 8.68 | ||||||||
| Ceratium hirundinella (O.F.Müller) Dujardin | 27.16 | 5.97 | 5.97 | 253.46 | 5.97 | ||||
| Peridinium bipes F.Stein | 1.66 | 7.11 | 43.80 | 7.11 | |||||
| Peridinium cinctum (O.F.Müller) Ehrenberg | 5.72 | 5.72 | 3.81 | 3.81 | 3.81 | 3.81 | |||
| Peridinium willei Huitfeldt-Kaas | 3.62 | ||||||||
| Euglenophyta Cavalier-Smith | |||||||||
| Colacium vesiculosum Ehrenberg | 11.87 | 0.10 | 0.10 | ||||||
| Lepocinclis acus (O.F.Müller) B.Marin & Melkonian | 0.17 | 0.23 | |||||||
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 0.49 | 0.49 | 2.00 | ||||||
| Phacus limnophilus (Lemmermann) E.W.Linton & Karnkowska | 0.39 | ||||||||
| Phacus longicauda (Ehrenberg) Dujardin | 1.69 | 1.69 | 1.13 | ||||||
| Phacus orbicularis Hübner | 1.55 | 2.13 | |||||||
| Phacus pleuronectes (O.F.Müller) Nitzsch ex Dujardin | 0.61 | 0.21 | |||||||
| Trachelomonas acanthostoma var. minor Dreżepolski | 0.37 | ||||||||
| Trachelomonas armata (Ehrenberg) F.Stein | 0.83 | ||||||||
| Trachelomonas armata var. steinii Lemmermann | 2.47 | ||||||||
| Trachelomonas australica var. granulata (Playfair) Deflandre | 0.62 | ||||||||
| Trachelomonas crenulatocollis Maskell | 1.16 | ||||||||
| Trachelomonas dubia Svirenko | 1.35 | ||||||||
| Trachelomonas dybowskii Dreżepolski | 0.84 | ||||||||
| Trachelomonas granulosa Playfair | 0.25 | ||||||||
| Trachelomonas hispida (Perty) F.Stein | 0.74 | 1.29 | 0.34 | ||||||
| Trachelomonas superba Svirenko | 1.90 | ||||||||
| Heterokontophyta Moestrup, R.A.Andersen & Guiry | |||||||||
| Asterionella tekelili D.M.Williams, T.M.Schuster, E.Cesar & Jüttner | 1.80 | 0.36 | |||||||
| Aulacoseira sp. | 2.40 | ||||||||
| Dinobryon bavaricum Imhof | 0.90 | 0.90 | 0.20 | 0.20 | 0.94 | 0.20 | |||
| Dinobryon cylindricum O.E.Imhof | 0.40 | ||||||||
| Dinobryon divergens O.E.Imhof | 4.50 | 0.30 | |||||||
| Dinobryon divergens var. schauinslandii (Lemmermann) Brunnthaler | 0.50 | ||||||||
| Dinobryon sertularia Ehrenberg | 0.31 | 0.60 | 0.20 | 0.35 | 6.93 | ||||
| Encyonema silesiacum (Bleisch) D.G.Mann | 1.47 | ||||||||
| Entomoneis ornata (Bailey) Reimer | 2.70 | ||||||||
| Epithemia adnata (Kützing) Brébisson | 0.30 | ||||||||
| Epithemia gibba (Ehrenberg) Kützing | 2.58 | 47.00 | |||||||
| Epithemia turgida (Ehrenberg) Kützing | 1.64 | ||||||||
| Gomphonema sp. | 1.15 | 0.27 | |||||||
| Gomphonema acuminatum Ehrenberg | 0.17 | 0.13 | |||||||
| Gomphonema capitatum Ehrenberg | 2.24 | ||||||||
| Pinnularia nobilis (Ehrenberg) Ehrenberg | 6.42 | ||||||||
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 5.39 | ||||||||
| Stenopterobia anceps (F.W.Lewis) Brébisson ex Van Heurck | 0.45 | 0.45 | 0.90 | ||||||
| Tabellaria fenestrata (Lyngbye) Kützing | 0.45 | 9.52 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | ||
| Tabellaria flocculosa (Roth) Kützing | 34.27 | 14.69 | 0.54 | 0.54 | 0.54 | 0.54 | 3.26 | ||
| Ulnaria ulna (Nitzsch) Compère | 4.57 | 0.50 | 0.50 | ||||||
| Urosolenia eriensis (H.L.Smith) Round & R.M.Crawford | 0.31 | 0.70 |
| Taxa | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cercozoa Cavalier-Smith | |||||||||||||||
| Spongomonas splendida (F.Stein) M.Hartmann & Chagas | 1 | ||||||||||||||
| Charophyta Migula | |||||||||||||||
| Actinotaenium capax var. minus (Schmidle) Teiling ex Ruzicka & Pouzar | 1 | 1 | |||||||||||||
| Actinotaenium cucurbita (Brébisson ex Ralfs) Teiling | 1 | 1 | 2 | ||||||||||||
| Bambusina borreri (Ralfs) Cleve | 1 | ||||||||||||||
| Closterium abruptum West | 1 | ||||||||||||||
| Closterium closterioides var. intermedium (J.Roy & Bisset) Ruzicka | 1 | ||||||||||||||
| Closterium dianae var. arcuatum (Brebisson ex Ralfs) Rabenhorst | 1 | 1 | |||||||||||||
| Closterium jenneri Ralfs | 5 | ||||||||||||||
| Closterium leibleinii Kützing ex Ralfs | 1 | 1 | 2 | ||||||||||||
| Closterium littorale F.Gay | 1 | ||||||||||||||
| Closterium navicula (Brébisson) Lütkemüller | 1 | ||||||||||||||
| Closterium setaceum Ehrenberg ex Ralfs | 2 | ||||||||||||||
| Closterium venus Kützing ex Ralfs | 1 | 1 | 1 | ||||||||||||
| Cosmarium baileyi Wolle | 1 | ||||||||||||||
| Cosmarium bioculatum Brébisson ex Ralfs | 2 | 2 | 1 | ||||||||||||
| Cosmarium blyttii var. novaesylvae West & G.S.West | 1 | 1 | |||||||||||||
| Cosmarium botrytis Meneghini ex Ralfs | 1 | 1 | 1 | ||||||||||||
| Cosmarium brebissonii Meneghini ex Ralfs | 1 | ||||||||||||||
| Cosmarium circulare Reinsch | 1 | 1 | |||||||||||||
| Cosmarium contractum O.Kirchner | 1 | ||||||||||||||
| Cosmarium formosulum Hoff | 1 | 2 | 1 | 1 | 1 | 1 | |||||||||
| Cosmarium granatum Brébisson ex Ralfs | 3 | ||||||||||||||
| Cosmarium hammeri Reinsch | 1 | ||||||||||||||
| Cosmarium humile Nordstedt ex De Toni | 1 | ||||||||||||||
| Cosmarium impressulum Elfving | 2 | 1 | |||||||||||||
| Cosmarium meneghinii Brébisson ex Ralfs | 2 | ||||||||||||||
| Cosmarium obsoletum (Hantzsch) Reinsch | 2 | 2 | 1 | 1 | |||||||||||
| Cosmarium ochthodes Nordstedt | 3 | ||||||||||||||
| Cosmarium pachydermum P.Lundell | 1 | ||||||||||||||
| Cosmarium pachydermum var. minus Nordstedt | 1 | ||||||||||||||
| Cosmarium porteanum W.Archer | 2 | 1 | |||||||||||||
| Cosmarium pseudoexiguum Raciborski | 1 | ||||||||||||||
| Cosmarium pseudopyramidatum P.Lundell | 1 | ||||||||||||||
| Cosmarium punctulatum Brébisson | 2 | 1 | 1 | 2 | |||||||||||
| Cosmarium regnellii Wille | 1 | 1 | 1 | 2 | 1 | ||||||||||
| Cosmarium regnesi Reinsch | 3 | 1 | |||||||||||||
| Cosmarium sexnotatum var. tristriatum (Lütkemuller) Schmidle | 1 | 1 | |||||||||||||
| Cosmarium subprotumidum Nordstedt | 2 | ||||||||||||||
| Cosmarium subundulatum Wille | 1 | ||||||||||||||
| Cosmarium trilobulatum var. depressum Printz | 1 | 1 | |||||||||||||
| Cosmarium ungerianum var. subtriplicatum West & G.S.West | 1 | 1 | |||||||||||||
| Desmidium aptogonum Brébisson ex Kützing | 2 | ||||||||||||||
| Desmidium baileyi (Ralfs) Nordstedt | 2 | 2 | 3 | 2 | |||||||||||
| Desmidium coarctatum Nordstedt | 1 | ||||||||||||||
| Desmidium graciliceps (Nordstedt) Lagerheim | 1 | ||||||||||||||
| Desmidium swartzii C.Agardh ex Ralfs | 2 | 1 | 2 | ||||||||||||
| Elakatothrix parvula (W.Archer) Hindák | 1 | ||||||||||||||
| Euastrum ansatum Ehrenberg ex Ralfs | 3 | 1 | |||||||||||||
| Euastrum bidentatum Nägeli | 1 | ||||||||||||||
| Euastrum binale var. minus (West) Willi Krieger | 2 | ||||||||||||||
| Euastrum crassicolle P.Lundell | 1 | ||||||||||||||
| Euastrum didelta Ralfs | 1 | ||||||||||||||
| Euastrum elegans Ralfs | 1 | ||||||||||||||
| Euastrum gemmatum Ralfs | 1 | ||||||||||||||
| Euastrum neosinuosum O.V.Anissimova & Guiry | 3 | ||||||||||||||
| Euastrum pulchellum Brébisson | 2 | 1 | |||||||||||||
| Gonatozygon aculeatum W.N.Hastings | 1 | ||||||||||||||
| Gonatozygon brebissonii De Bary | 1 | ||||||||||||||
| Gonatozygon monotaenium De Bary | 1 | ||||||||||||||
| Groenbladia undulata (Nordstedt) Kurt Förster | 2 | ||||||||||||||
| Haplotaenium rectum (Delponte) Bando | 1 | 1 | |||||||||||||
| Hyalotheca mucosa Ralfs | 1 | ||||||||||||||
| Micrasterias crux-melitensis Ralfs | 1 | 1 | |||||||||||||
| Micrasterias pinnatifida Ralfs | 1 | 1 | |||||||||||||
| Mougeotia laetevirens (A.Braun) Wittrock | 6 | ||||||||||||||
| Octacanthium bifidum var. latidivergens (West) Petlovany | 1 | ||||||||||||||
| Penium margaritaceum Brébisson ex Ralfs | 1 | ||||||||||||||
| Pleurotaenium clavatum (Ralfs) De Bary | 1 | ||||||||||||||
| Pleurotaenium ehrenbergii (Ralfs) De Bary | 1 | ||||||||||||||
| Spirogyra sp. st. | 6 | 6 | |||||||||||||
| Spondylosium lundellii O.Borge | 2 | 3 | |||||||||||||
| Staurastrum avicula var. lunatum (Ralfs) Coesel & Meesters | 1 | ||||||||||||||
| Staurastrum boreale West & G.S.West | 1 | 1 | 1 | ||||||||||||
| Staurastrum brevispina Brébisson | 2 | 1 | |||||||||||||
| Staurastrum dilatatum Ehrenberg ex Ralfs | 1 | ||||||||||||||
| Staurastrum gemelliparum Nordstedt | 1 | ||||||||||||||
| Staurastrum gracile Ralfs ex Ralfs | 1 | 1 | |||||||||||||
| Staurastrum granulosum Ralfs | 2 | ||||||||||||||
| Staurastrum manfeldtii Delponte | 1 | ||||||||||||||
| Staurastrum muticum Brébisson ex Ralfs | 1 | 1 | |||||||||||||
| Staurastrum paradoxum Meyen ex Ralfs | 1 | 1 | |||||||||||||
| Staurastrum punctulatum Brébisson | 2 | 2 | 1 | 2 | |||||||||||
| Staurastrum spiniferum West | 1 | ||||||||||||||
| Staurastrum teliferum var. gladiosum (W.B.Turner) Coesel & Meesters | 1 | 1 | |||||||||||||
| Staurastrum vestitum Ralfs | 1 | ||||||||||||||
| Staurodesmus convergens (Ehrenberg ex Ralfs) S.Lillieroth | 2 | 1 | |||||||||||||
| Staurodesmus cuspidatus (Brébisson) Teiling | 1 | ||||||||||||||
| Staurodesmus dejectus (Brébisson) Teiling | 1 | ||||||||||||||
| Staurodesmus glaber (Ralfs) Teiling | 2 | ||||||||||||||
| Teilingia granulata (J.Roy & Bisset) Bourrelly | 2 | 3 | 1 | ||||||||||||
| Xanthidium antilopaeum Kützing | 1 | ||||||||||||||
| Chlorophyta Reichenbach | |||||||||||||||
| Acutodesmus acutiformis var. costatus (Huber-Pestalozzi) P.M.Tsarenko & D.M.John | 2 | ||||||||||||||
| Ankistrodesmus falcatus (Corda) Ralfs | 1 | 3 | 1 | 2 | |||||||||||
| Ankistrodesmus fusiformis Corda | 2 | 1 | 1 | 1 | |||||||||||
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann | 2 | 1 | |||||||||||||
| Aphanochaete repens A.Braun | 1 | ||||||||||||||
| Botryococcus braunii Kützing | 1 | 1 | |||||||||||||
| Cosmarium angulosum var. concinnum (Rabenhorst) West & G.S.West | 1 | 3 | |||||||||||||
| Desmodesmus armatus (Chodat) E.H.Hegewald | 2 | 1 | 1 | 1 | |||||||||||
| Desmodesmus denticulatus var. linearis (Hansgirg) Hegewald | 2 | ||||||||||||||
| Desmodesmus microspina (Chodat) P.M.Tsarenko | 2 | ||||||||||||||
| Dimorphococcus lunatus A.Braun | 1 | 1 | |||||||||||||
| Monoraphidium contortum (Thuret) Komárková-Legnerová | 1 | 3 | |||||||||||||
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová | 3 | 3 | |||||||||||||
| Monoraphidium komarkovae Nygaard | 1 | ||||||||||||||
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz | 2 | ||||||||||||||
| Neglectella solitaria (Wittrock) Stenclová & Kaštovský | 1 | 1 | |||||||||||||
| Oedogonium undulatum A.Braun ex Hirn | 2 | 1 | |||||||||||||
| Oocystis parva West & G.S.West | 1 | ||||||||||||||
| Oocystis rhomboidea Fott | 1 | ||||||||||||||
| Pandorina morum (O.F.Müller) Bory | 1 | 2 | 2 | ||||||||||||
| Pseudopediastrum boryanum (Turpin) E.Hegewald | 1 | 3 | 1 | ||||||||||||
| Quadrigula korsikovii Komárek | 2 | 1 | |||||||||||||
| Scenedesmus ellipticus Corda | 1 | 3 | 1 | 2 | 2 | 1 | |||||||||
| Scenedesmus obtusus f. disciformis (Chodat) Compère | 1 | ||||||||||||||
| Stauridium tetras (Ehrenberg) E.Hegewald | 1 | 2 | 2 | ||||||||||||
| Tetradesmus obliquus (Turpin) M.J.Wynne | 2 | 3 | 2 | ||||||||||||
| Tetraëdron minimum (A.Braun) Hansgirg | 2 | 2 | |||||||||||||
| Ulothrix zonata (F.Weber & Mohr) Kützing | 6 | ||||||||||||||
| Verrucodesmus verrucosus (Y.V.Roll) E.Hegewald | 1 | ||||||||||||||
| Westella botryoides (West) De Wildeman | 1 | ||||||||||||||
| Willea irregularis (Wille) Schmidle | 1 | 1 | |||||||||||||
| Cyanobacteria Stanier ex Cavalier-Smith | |||||||||||||||
| Anabaena oscillarioides Bory ex Bornet & Flahault | 1 | ||||||||||||||
| Anagnostidinema amphibium (Gomont) Strunecký, Bohunická, J.R.Johansen & Komárek | 2 | ||||||||||||||
| Anathece clathrata (West & G.S.West) Komárek, Kaštovský & Jezberová | 2 | ||||||||||||||
| Aphanocapsa conferta (West & G.S.West) Komárková-Legnerová & Cronberg | 3 | ||||||||||||||
| Aphanocapsa incerta (Lemmermann) G.Cronberg & Komárek | 2 | 2 | |||||||||||||
| Chroococcus turgidus (Kützing) Nägeli | 1 | 1 | |||||||||||||
| Lyngbya cincinnata (Itzigsohn) Compère | 1 | ||||||||||||||
| Merismopedia glauca (Ehrenberg) Kützing | 1 | 1 | 1 | ||||||||||||
| Merismopedia tranquilla (Ehrenberg) Trevisan | 1 | 1 | 2 | ||||||||||||
| Nostoc commune Vaucher ex Bornet & Flahault | 6 | ||||||||||||||
| Nostoc kihlmanii Lemmermann | 2 | 1 | 1 | ||||||||||||
| Oscillatoria limosa C.Agardh ex Gomont | 1 | 2 | 6 | 1 | 1 | ||||||||||
| Oscillatoria princeps Vaucher ex Gomont | 6 | 1 | |||||||||||||
| Oscillatoria proboscidea Gomont | 6 | ||||||||||||||
| Oscillatoria rupicola (Hansgirg) Hansgirg ex Forti | 1 | ||||||||||||||
| Oscillatoria tenuis C.Agardh ex Gomont | 1 | 2 | 1 | ||||||||||||
| Phormidium ambiguum Gomont | 2 | ||||||||||||||
| Phormidium breve (Kützing ex Gomont) Anagnostidis & Komárek | 6 | 1 | |||||||||||||
| Rivularia biasolettiana Meneghini ex Bornet & Flahault | 1 | ||||||||||||||
| Scytonema coactile Montagne ex Bornet & Flahault | 6 | 2 | 6 | ||||||||||||
| Snowella lacustris (Chodat) Komárek & Hindák | 1 | 1 | |||||||||||||
| Spirulina abbreviata Lemmermann | 1 | ||||||||||||||
| Dinoflagellata Fensome & al. | |||||||||||||||
| Ceratium hirundinella (O.F.Müller) Dujardin | 1 | ||||||||||||||
| Peridinium bipes F.Stein | 1 | ||||||||||||||
| Euglenophyta Cavalier-Smith | |||||||||||||||
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian | 1 | ||||||||||||||
| Monomorphina pyrum (Ehrenberg) Mereschkowsky | 1 | ||||||||||||||
| Phacus orbicularis Hübner | 1 | 2 | 2 | 1 | |||||||||||
| Trachelomonas armata (Ehrenberg) F.Stein | 1 | ||||||||||||||
| Trachelomonas crenulatocollis Maskell | 1 | ||||||||||||||
| Trachelomonas dybowskii Dreżepolski | 1 | 2 | |||||||||||||
| Trachelomonas granulosa Playfair | 1 | ||||||||||||||
| Trachelomonas lacustris Dreżepolski | 1 | ||||||||||||||
| Trachelomonas oblonga Lemmermann | 1 | ||||||||||||||
| Trachelomonas superba Svirenko | 1 | ||||||||||||||
| Trachelomonas woycickii Koczwara | 1 | ||||||||||||||
| Heterokontophyta Moestrup, R.A.Andersen & Guiry | |||||||||||||||
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 4 | ||||||||||||||
| Dinobryon sertularia Ehrenberg | 1 | 1 | 2 | ||||||||||||
| Entomoneis ornata (Bailey) Reimer | 1 | ||||||||||||||
| Epithemia gibba (Ehrenberg) Kützing | 1 | 1 | |||||||||||||
| Meridion circulare (Greville) C.Agardh | 2 | 2 | 1 | ||||||||||||
| Nitzschia acicularis (Kützing) W.Smith | 2 | ||||||||||||||
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot | 3 | ||||||||||||||
| Stenopterobia anceps (F.W.Lewis) Brébisson ex Van Heurck | 1 | 1 | 1 | ||||||||||||
| Surirella librile (Ehrenberg) Ehrenberg | 1 | ||||||||||||||
| Tabellaria fenestrata (Lyngbye) Kützing | 1 | 2 | 3 | 2 | 3 | 3 | 2 | 3 | |||||||
| Tabellaria flocculosa (Roth) Kützing | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 1 | |||||||
| Tribonema vulgare Pascher | 2 | 2 | |||||||||||||
| Ulnaria ulna (Nitzsch) Compère | 2 |
| Indicator | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 8 | St 11 | St 14 |
|---|---|---|---|---|---|---|---|---|---|
| Habitat | |||||||||
| B | 598.4 | 299.2 | 1579.3 | 224.4 | 675.5 | 1356.6 | 598.4 | 598.4 | 598.4 |
| P-B | 2101.2 | 4676.1 | 14,708.4 | 10,866.4 | 68,890.8 | 23,772.8 | 972.4 | 3832.9 | 4655.7 |
| P | 23,115.5 | 5134.0 | 55,528.8 | 374.0 | 11,931.7 | 37,903.2 | 224.4 | 448.8 | 1196.8 |
| Temperature | |||||||||
| temp | 74.8 | 0 | 2152.2 | 0 | 0 | 112.2 | 0 | 149.6 | 1808.8 |
| eterm | 528.1 | 301.5 | 4984.4 | 74.8 | 74.8 | 2488.8 | 0 | 149.6 | 752.5 |
| warm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 149.6 |
| Oxygen | |||||||||
| aer | 0 | 0 | 0 | 0 | 0 | 224.4 | 74.8 | 0 | 149.6 |
| str | 0 | 0 | 340.0 | 0 | 74.8 | 0 | 0 | 0 | 0 |
| st-str | 1276.1 | 4152.5 | 10,859.6 | 9739.9 | 37,996.1 | 19,138.6 | 523.6 | 1346.4 | 4057.3 |
| st | 149.6 | 299.2 | 23,684.4 | 224.4 | 30,445.9 | 785.4 | 299.2 | 2112.5 | 598.4 |
| Salinity | |||||||||
| hb | 600.7 | 149.6 | 34,224.4 | 299.2 | 525.9 | 1356.6 | 74.8 | 374.0 | 149.6 |
| i | 24,391.6 | 9510.9 | 18,681.3 | 1122.0 | 79,698.3 | 52,907.4 | 972.4 | 3309.3 | 3903.2 |
| hl | 0 | 0 | 564.4 | 149.6 | 224.4 | 0 | 149.6 | 74.8 | 299.2 |
| mh | 0 | 0 | 112.2 | 0 | 0 | 112.2 | 0 | 0 | 453.3 |
| Water pH | |||||||||
| acf | 8076.1 | 600.7 | 5888.8 | 374.0 | 673.2 | 4637.6 | 374.0 | 822.8 | 149.6 |
| ind | 1348.7 | 748.0 | 6670.8 | 10,342.8 | 68,519.1 | 12,903.0 | 748.0 | 2935.3 | 2547.7 |
| alf | 0 | 0 | 1812.2 | 0 | 0 | 0 | 224.4 | 299.2 | 2108.0 |
| alb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 149.6 |
| Autotrophy-Heterotrophy | |||||||||
| ats | 74.8 | 0 | 340.0 | 0 | 74.8 | 0 | 0 | 149.6 | |
| ate | 0 | 0 | 1360.0 | 0 | 0 | 0 | 0 | 149.6 | 1509.6 |
| hne | 0 | 0 | 0 | 0 | 0 | 0 | 74.8 | 0 | 0 |
| Trophic state | |||||||||
| ot | 374.0 | 149.6 | 0 | 528.1 | 149.6 | 224.4 | 0 | 224.4 | 149.6 |
| om | 224.4 | 74.8 | 904.4 | 224.4 | 374.0 | 673.2 | 224.4 | 448.8 | 1659.2 |
| m | 7849.5 | 598.4 | 3508.8 | 523.6 | 12,385.1 | 785.4 | 374.0 | 598.4 | 897.6 |
| me | 149.6 | 0 | 336.6 | 149.6 | 0 | 1020.0 | 149.6 | 149.6 | 299.2 |
| e | 299.2 | 3322.9 | 19,828.8 | 374.0 | 37,619.9 | 3393.2 | 224.4 | 374.0 | 1496.0 |
| Class of Water Quality | |||||||||
| Class 1 | 224.4 | 0 | 792.2 | 74.8 | 301.5 | 0 | 74.8 | 224.4 | 0 |
| Class 2 | 23,641.3 | 8611.1 | 5776.6 | 976.9 | 30,894.7 | 10,414.2 | 598.4 | 2486.5 | 2706.4 |
| Class 3 | 448.8 | 451.1 | 22,195.2 | 9440.7 | 12,006.5 | 11,094.2 | 299.2 | 822.8 | 2094.4 |
| Class 4 | 528.1 | 149.6 | 4872.2 | 74.8 | 149.6 | 2264.4 | 0.0 | 224.4 | 149.6 |
References
- Vincent, W.F.; Laurion, I.; Pienitz, R.; Walter Anthony, K.M. Climate Impacts on Arctic Lake Ecosystems. In Climatic Change and Global Warming of Inland Waters; Goldman, C.R., Kumagai, M., Robarts, R.D., Eds.; Wiley-Blackwel: Chichester, UK, 2012; pp. 27–42. [Google Scholar]
- Pokrovsky, O.S.; Manasypov, R.M.; Kopysov, S.G.; Krickov, I.V.; Shirokova, L.S.; Loiko, S.V.; Lim, A.G.; Kolesnichenko, L.G.; Vorobyev, S.N.; Kirpotin, S.N. Impact of Permafrost Thaw and Climate Warming on Riverine Export Fluxes of Carbon, Nutrients and Metals in Western Siberia. Water 2020, 12, 1817. [Google Scholar] [CrossRef]
- Thompson, M.S.; Wrona, F.J.; Prowse, T.D. Shifts in Plankton, Nutrient and Light Relationships in Small Tundra Lakes Caused by Localized Permafrost Thaw. Arctic 2012, 65, 367–510. [Google Scholar] [CrossRef]
- Wauthy, M.; Rautio, M. Permafrost thaw stimulates primary producers but has a moderate effect on primary consumers in subarctic ponds. Ecosphere 2020, 11, e03099. [Google Scholar] [CrossRef]
- Ayala-Borda, P.; Lovejoy, C.; Power, M.; Rautio, M. Evidence of eutrophication in Arctic lakes. Arct. Sci. 2021, 7, 859–871. [Google Scholar] [CrossRef]
- Jasinski, B.L.; Hewitt, R.E.; Mauritz, M.; Miller, S.N.; Schuur, E.A.; Taylor, M.A.; Walker, X.J.; Mack, M.C. Plant foliar nutrient response to active layer and water table depth in warming permafrost soils. J. Ecol. 2022, 110, 1201–1216. [Google Scholar] [CrossRef]
- Saros, J.E.; Arp, C.D.; Bouchard, F.; Comte, J.; Couture, R.-M.; Dean, J.F.; Lafrenière, M.; MacIntyre, S.; McGowan, S.; Rautio, M.; et al. Sentinel responses of Arctic freshwater systems to climate: Linkages, evidence, and a roadmap for future research. Arct. Sci. 2023, 9, 356–392. [Google Scholar] [CrossRef]
- Yun, H.; Zhu, Q.; Tang, J.; Zhang, W.; Chen, D.; Ciais, P.; Wu, Q.; Elberling, B. Warming, permafrost thaw and increased nitrogen availability as drivers for plant composition and growth across the Tibetan Plateau. Soil Biol. Biochem. 2023, 182, 109041. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Pozzan, R.; de Carvalho, J.C.; Cavali, M.; Mariano, A.B.; Vargas, J.V.C.; Ordonez, J.; Severo, I.A.; Soccol, C.R. The Role of Microalgae as Bioindicators of Aquatic Contamination. In Algae as a Natural Solution for Challenges in Water-Food-Energy Nexus. Environmental Science and Engineering; Kurniawan, T.A., Anouzla, A., Eds.; Springer: Singapore, 2024; pp. 323–347. [Google Scholar]
- Wiłkomirski, B. History of bioindication (Historia bioindykacji). Monit. Sr. Przyr. 2013, 14, 137–142. [Google Scholar]
- Burger, J. Bioindicators: Types, Development, and Use in Ecological Assessment and Research. Environ. Bioindic. 2006, 1, 22–39. [Google Scholar] [CrossRef]
- Vasilyeva, I.I.; Rozhkov, Y.F.; Rozhkova, O.Y. Features of the development of phytoplankton communities of water bodies in the south of Yakutia (using the example of watercourses of Olyokma Nature Reserve). In Biological and Ecological Studies in the Republic of Sakha (Yakutia); Publishing House of the Yakut State University: Yakutsk, Russia, 1996; pp. 150–154. (In Russian) [Google Scholar]
- Vasilyeva, I.I.; Rozhkov, Y.F.; Rozhkova, O.Y. Features of the seasonal dynamics of the development of phyto- and bacterioplankton in watercourses of the Olyokma Nature Reserve (Republic of Sakha (Yakutia). Algologia 1997, 7, 166–170. (In Russian) [Google Scholar]
- Vasilyeva, I.I.; Pshennikova, E.V.; Rozhkova, O.Y. Algae of some rivers of the Olyokma Nature Reserve. In Issues of Ecology and Environmental Education in Yakutia; Publishing House of the Yakut State University: Yakutsk, Russia, 1998; pp. 36–41. (In Russian) [Google Scholar]
- Rozhkova, O. Seasonal dynamics of phytoplankton development. In Strict Nature Reserves (Zapovedniki) of Russia; Sabashnikov publishers: Moscow, Russia, 1996; pp. 155–156. [Google Scholar]
- Rozhkova, O.Y. Research of the algal flora of the Olyokma Nature Reserve. In Flora and Fauna of Specially Protected Natural Areas of the Republican System Ytyk Kere Sirder; Kuduk: Yakutsk, Russia, 2001; pp. 53–72. (In Russian) [Google Scholar]
- Gabyshev, V.A.; Gabysheva, O.I. Spatial structure of the phytoplankton of Olyokma River (Eastern Siberia) in summer and its habitat conditions. Izv. Komi Nauchnogo Cent. UB RAS 2013, 1, 25–31. (In Russian) [Google Scholar]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; p. 535. [Google Scholar]
- Desyatkin, R.V.; Okoneshnikova, M.V.; Desyatkin, A.R. Soils of Yakutia; Bichik Publishing House: Yakutsk, Russia, 2009; p. 64. (In Russian) [Google Scholar]
- Izyumenko, S.A. (Ed.) Climate of the Yakut Autonomous Soviet Socialist Republic (Atlas); Gidrometeoizdat: Leningrad, Russia, 1968; 33p. (In Russian) [Google Scholar]
- Weather and Climate. Available online: http://www.pogodaiklimat.ru/history/30089.htm (accessed on 9 May 2025).
- Semenov, A.D. Guidance on the Chemical Analysis of Surface Waters of the Land; Gidrometeoizdat: Leningrad, Russia, 1977; p. 541. (In Russian) [Google Scholar]
- Water Analysis. In A Practical Guide to Physico-Chemical, Chemical, and Microbiological Water Examination and Quality Assurance; Fresenius, W., Quentin, K.E., Schneider, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; p. 830. [Google Scholar]
- Starmach, K. Chrysophyceae und Haptophyceae; VEB Gustav Fischer: Jena, Germany, 1985; 515p. [Google Scholar]
- Vasilyeva, I.I. Freshwater euglenids and yellow-green algae of water bodies of Yakutia; Nauka: Leningrad, Russia, 1987; 265p. (In Russian) [Google Scholar]
- Palamar-Mordvintseva, G.M. Green algae. Class Conjugates. In Key to Freshwater Algae of the USSR; Nauka: Leningrad, Russia, 1982; Issue 11 (2); 483p. (In Russian) [Google Scholar]
- Popovský, J.; Pfiester, L.A. Dinophyceae (Dinoflagellida); Gustav Fischer: Stuttgart, Germany; Jena, Germany, 1990; 272 s. [Google Scholar]
- Tsarenko, P.M. Brief Guide to Chlorococcal Algae of the Ukrainian SSR; Naukova Dumka: Kyiv, Ukraine, 1990; 208p. (In Russian) [Google Scholar]
- Komárek, J. Heterocytous Genera. Cyanoprokaryota. T. 3, P. 3.; Springer Spektrum: Berlin, Germany, 2013; 1130p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. T. 2. Oscillatoriales; Elsevier: München, Germany, 2005; 759p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. T. 1. Chroococcales; Gustav Fischer: Jena, Germany, 1998; 548p. [Google Scholar]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; 804p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, University of Galway. 2024. Available online: https://www.algaebase.org (accessed on 3 December 2024).
- Hillebrand, H.; Durselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; p. 498. (In Russian) [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949; p. 117. [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; Jointly developed by the Scottish Association for Marine Science and the Natural History Museum: London, UK, 1997; Available online: https://www.sams.ac.uk/science/outputs/ (accessed on 15 January 2025).
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Wessa, P. Person Correlation (v1.0.13) in Free Statistics Software (v1.2.1). Office for Research Development and Education. 2017. Available online: https://www.wessa.net/rwasp_correlation.wasp/ (accessed on 23 December 2024).
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Kyiv, Israel, 2019; p. 367. (In Russian) [Google Scholar]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555588. [Google Scholar] [CrossRef]
- Hustedt, F. Die Diatomeen flora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abh. Des Naturwissenschaftlichen Ver. Zu Brem. 1957, 34, 181–440. [Google Scholar]
- Hustedt, F. Systematische und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra. Arch. Für Hydrobiol. Suppl. 1938–1939, 15–16, 131–177, 393–506, 638–790, 1–155, 274–394. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochim. Et Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Elovskaya, L.G. Classification and Diagnostics of Frozen Soils in Yakutia; Publishing House of the Yakut Branch of the Siberian Division of the USSR Academy of Sciences: Yakutsk, Russia, 1987; p. 172. (In Russian) [Google Scholar]
- Reyes, F.R.; Lougheed, V.L. Rapid Nutrient Release from Permafrost Thaw in Arctic Aquatic Ecosystems. Arct. Antarct. Alp. Res. 2015, 47, 35–48. [Google Scholar] [CrossRef]
- Fouché, J.; Christiansen, C.T.; Lafrenière, M.J.; Grogan, P.; Lamoureux, S.F. Canadian permafrost stores large pools of ammonium and optically distinct dissolved organic matter. Nat. Commun. 2020, 11, 4500. [Google Scholar] [CrossRef]
- Menzel, D.W.; Spaeth, J.P. Occurrence of ammonia in Sargasso Sea waters and in rain water at Bermuda. Limnol. Oceanogr. 1962, 7, 159–162. [Google Scholar] [CrossRef]
- Perelman, A.I. Geochemistry of Natural Waters; Nauka: Moscow, Russia, 1982; p. 154. (In Russian) [Google Scholar]
- Matveev, A.N.; Samusenok, V.P.; Rozhkova, N.A.; Bondarenko, N.A.; Kravtsova, L.S.; Sheveleva, N.G.; Slugina, Z.V.; Yuryev, A.L. Biota of the Vitim Nature Reserve: Structure of the Biota of Aquatic Ecosystems; Academic Publishing House “Geo”: Novosibirsk, Russia, 2006; p. 256. (In Russian) [Google Scholar]
- Biskaborn, B.K.; Nazarova, L.; Kröger, T.; Pestryakova, L.A.; Syrykh, L.; Pfalz, G.; Herzschuh, U.; Diekmann, B. Late quaternary climate reconstruction and lead-lag relationships of biotic and sediment-geochemical indicators at Lake Bolshoe Toko, Siberia. Front. Earth Sci. 2021, 9, 737353. [Google Scholar] [CrossRef]
- Genkal, S.; Gabyshev, V.; Kulikovskiy, M.; Kuznetsova, I. Pliocaenicus bolshetokoensis—A new species from Lake Bolshoe Toko (Yakutia, Eastern Siberia, Russia). Diatom Res. 2018, 33, 145–153. [Google Scholar] [CrossRef]
- Genkal, S.I.; Gabyshev, V.A. New records of centric diatoms from Yakutia (Bolshoe Toko Lake): SEM morphology, ecology and distribution. Nov. Sist. Nizshikh Rastenii 2018, 52, 245–252. [Google Scholar] [CrossRef]
- Genkal, S.I.; Gabyshev, V.A. Diatoms (Bacillariophyta, Fragilariophyceae, and Bacillariophyceae) of Lake Bolshoye Toko (South Yakutia). Inland Water Biol. 2020, 13, 122–130. [Google Scholar] [CrossRef]
- Gusev, E.S.; Guseva, E.E.; Gabyshev, V.A. Taxonomic composition of silica-scaled chrysophytes in rivers and lakes of Yakutia and Magadanskaya oblast (Russia). Nova Hedwig. Beih. 2018, 147, 105–117. [Google Scholar] [CrossRef]
- Stoof-Leichsenring, K.R.; Dulias, K.; Biskaborn, B.K.; Pestryakova, L.A.; Herzschuh, U. Lake-depth related pattern of genetic and morphological diatom diversity in boreal Lake Bolshoe Toko, Eastern Siberia. PLoS ONE 2020, 15, e0230284. [Google Scholar] [CrossRef]
- Genkal, S.I.; Bondarenko, N.A.; Popovskaya, G.I. New representative of the genus Discostella Houk et Klee (Bacillariophyta) from the Eastern Baikal area. Int. J. Algae 2007, 9, 359–364. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of Sediment and Internal Loading of Phosphorus in Shallow Lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Soininen, J.; Luoto, M. Is catchment productivity a useful predictor of taxa richness in lake plankton communities? Ecol. Appl. 2012, 22, 624–633. [Google Scholar] [CrossRef]
- Toporowska, M.; Ferencz, B.; Dawidek, J. Impact of lake-catchment processes on phytoplankton community structure in temperate shallow lakes. Ecohydrology 2018, 11, e2017. [Google Scholar] [CrossRef]
- Ongun Sevindik, O.T.; Çetin, T.; Tekbaba, A.; Güzel, U. Impacts of Lake Surface Area on Phytoplankton Community Dynamics and Ecological Status Across 70 Lentic Systems in Türkiye. Ecohydrology 2025, 18, e70031. [Google Scholar] [CrossRef]
- Lara, M.J.; Lin, D.H.; Andresen, C.; Lougheed, V.L.; Tweedie, C.E. Nutrient release from permafrost thaw enhances CH4 emissions from Arctic tundra wetlands. J. Geophys. Res. Biogeosci. 2019, 124, 1560–1573. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Pavlova, O.A.; Shirokova, L.S.; Vorobyev, S.N. Carbon, nutrient and metal controls on phytoplankton concentration and biodiversity in thermokarst lakes of latitudinal gradient from isolated to continuous permafrost. Sci. Total Environ. 2022, 806, 151250. [Google Scholar] [CrossRef]
- Munawar, M.; Fitzpatrick, M.A.J. Eutrophication in three Canadian Areas of Concern: Phytoplankton and major nutrient interactions. Aquat. Ecosyst. Health Manag. 2018, 21, 421–437. [Google Scholar] [CrossRef]
- Maberly, S.C.; Van de Waal, D.B.; Raven, J.A. Phytoplankton Growth and Nutrients. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Oxford, UK, 2022; pp. 130–138. [Google Scholar]
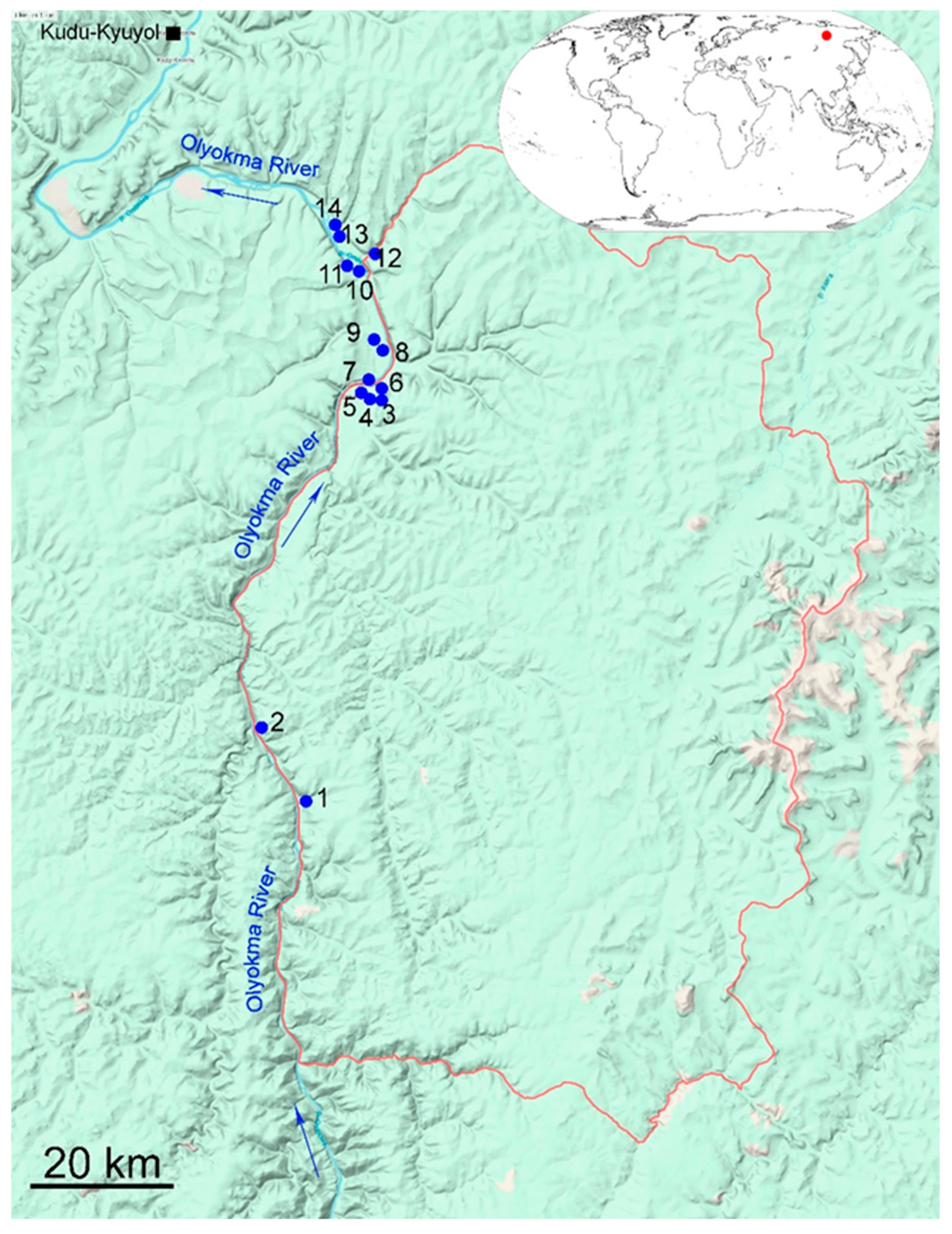

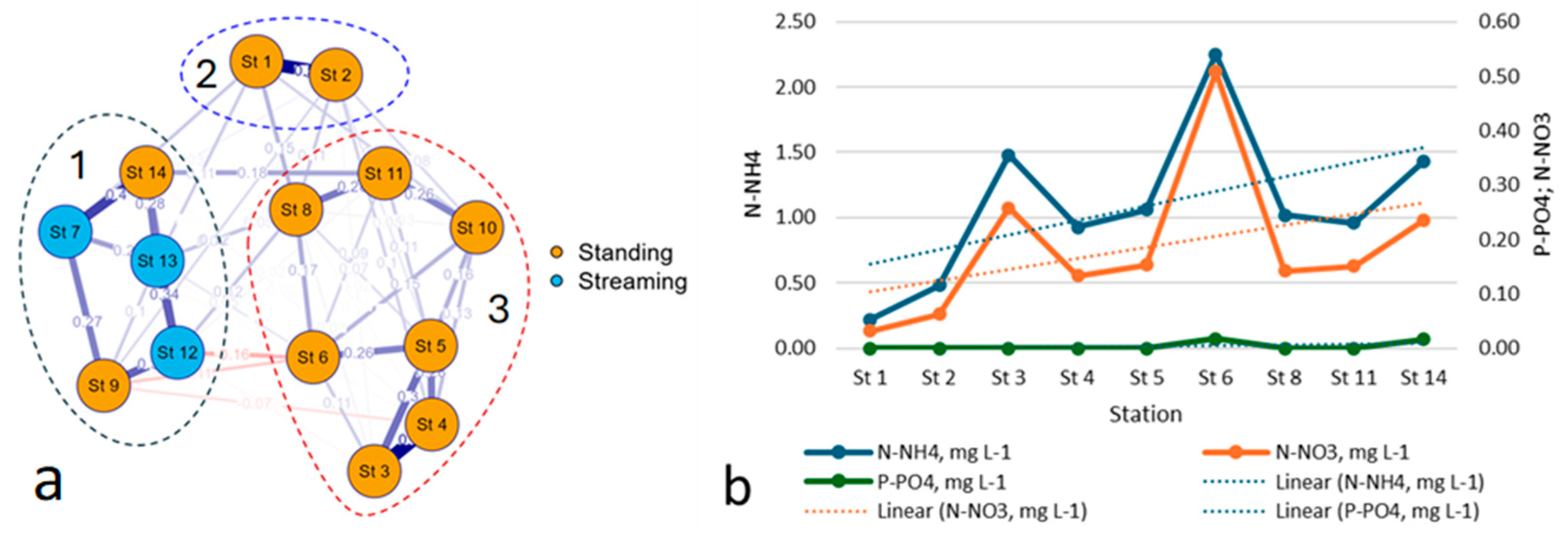
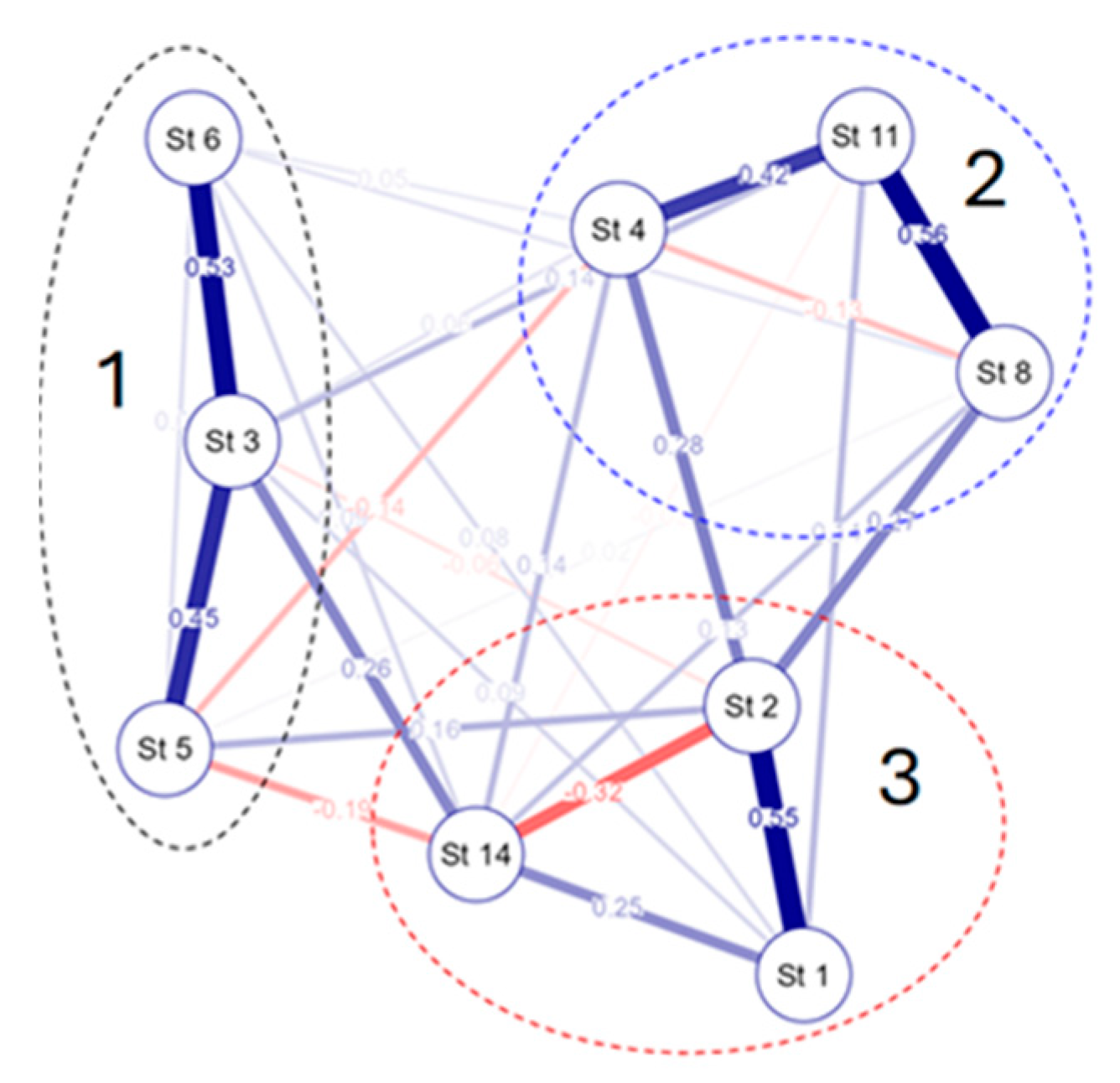
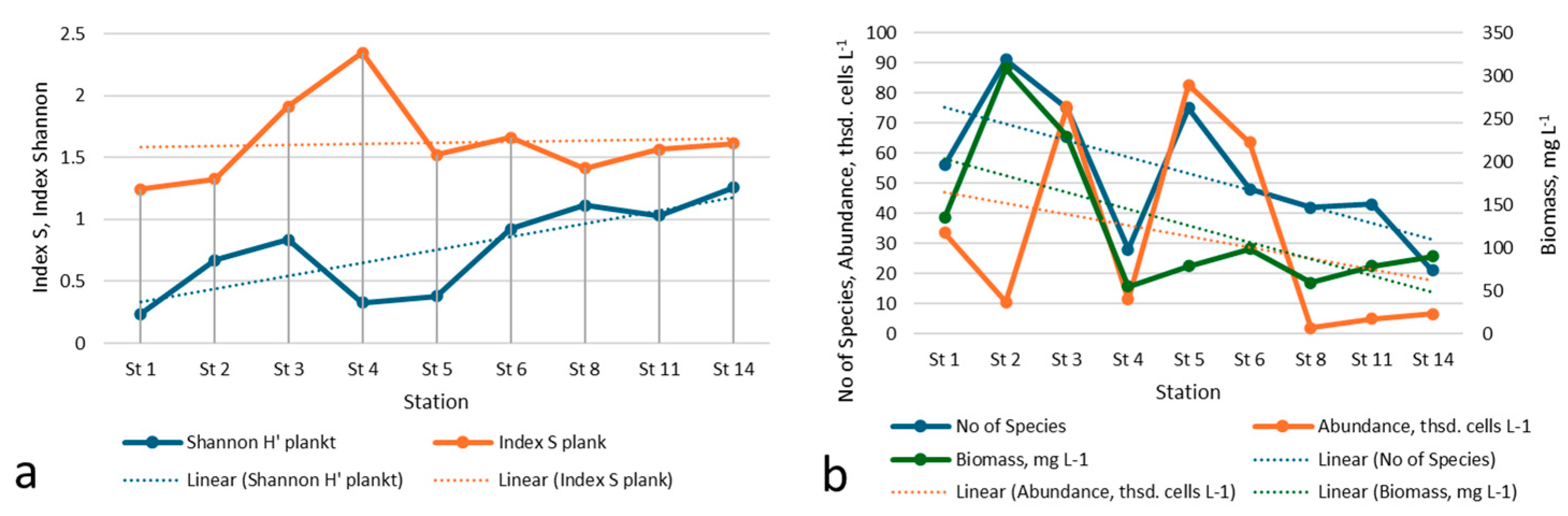
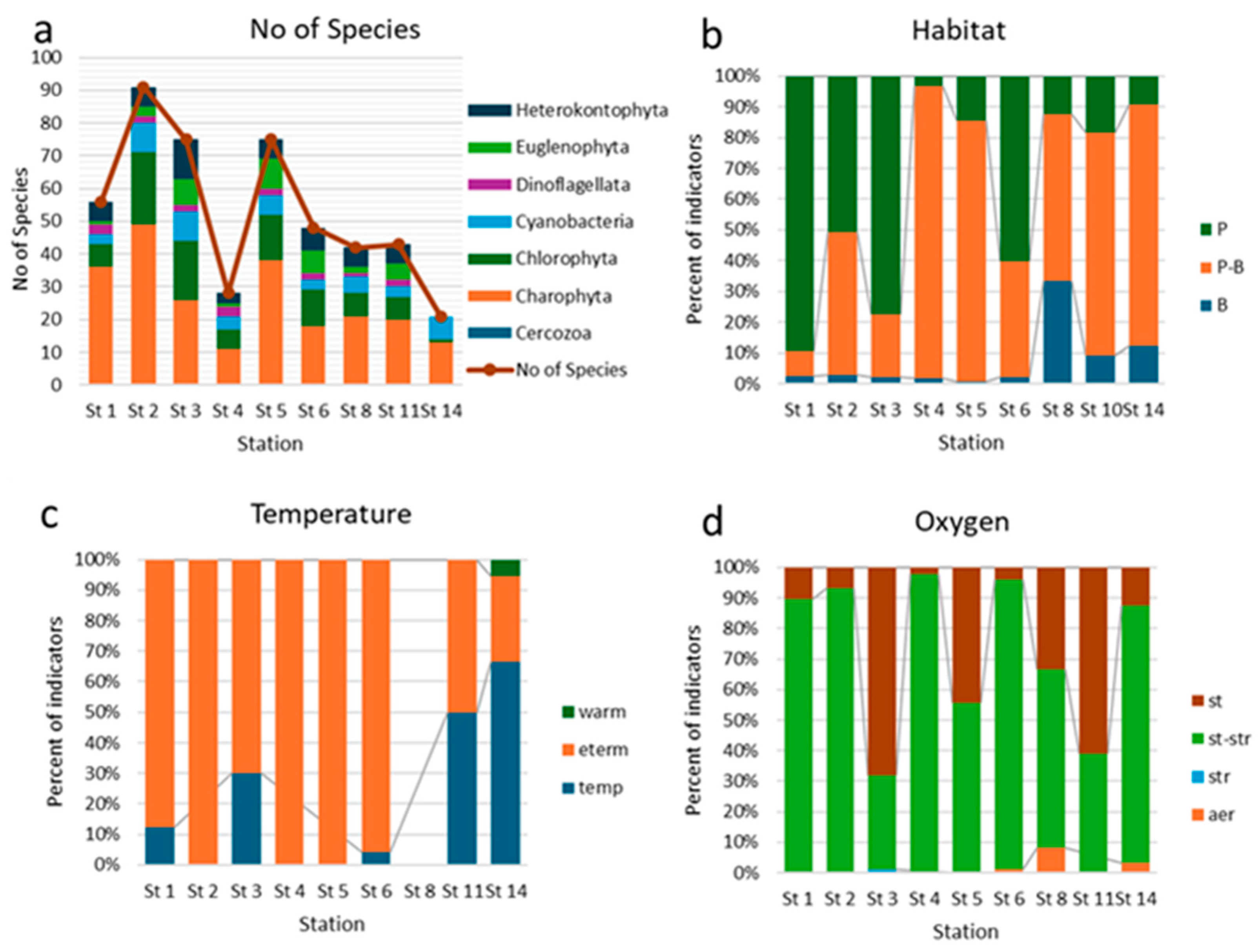
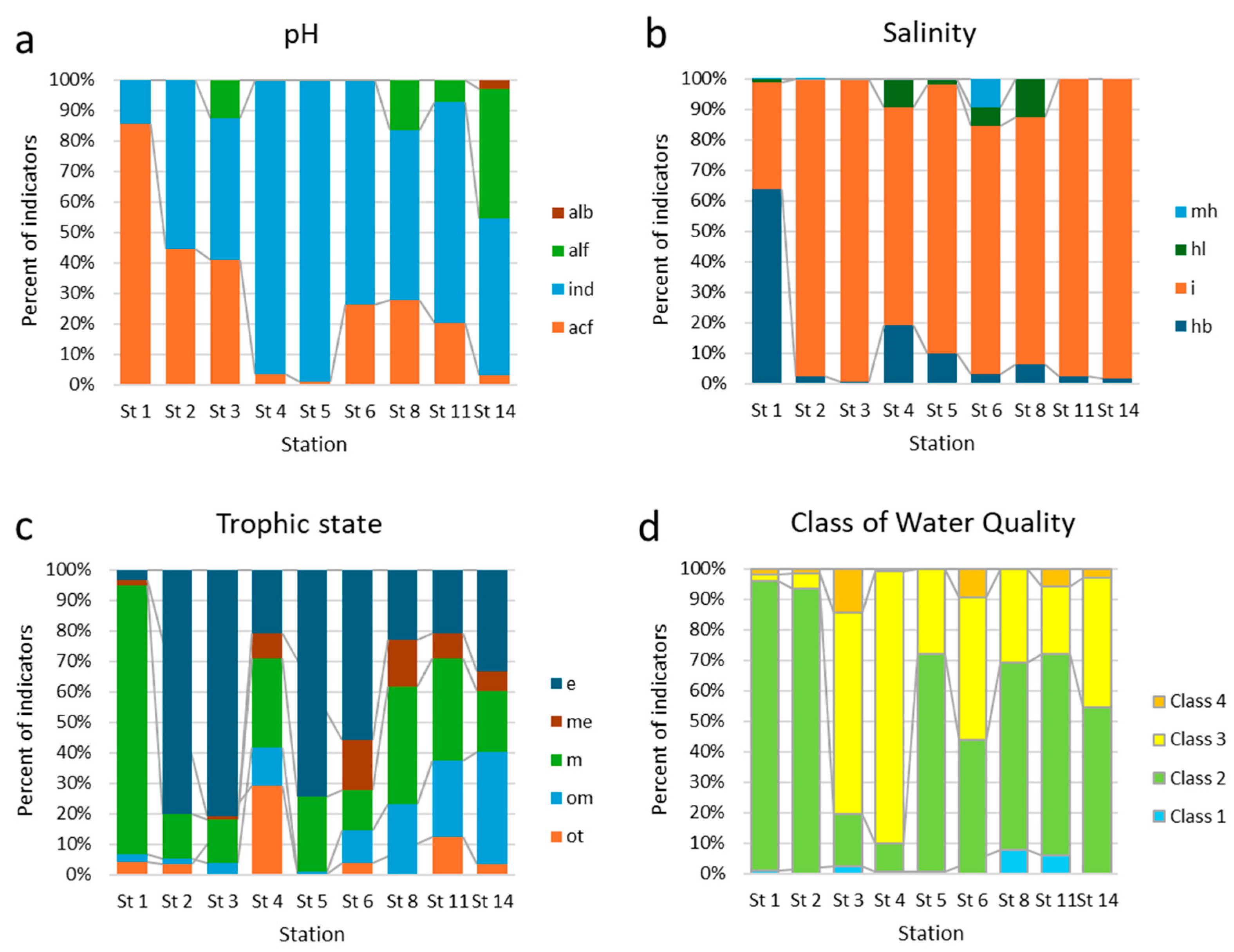
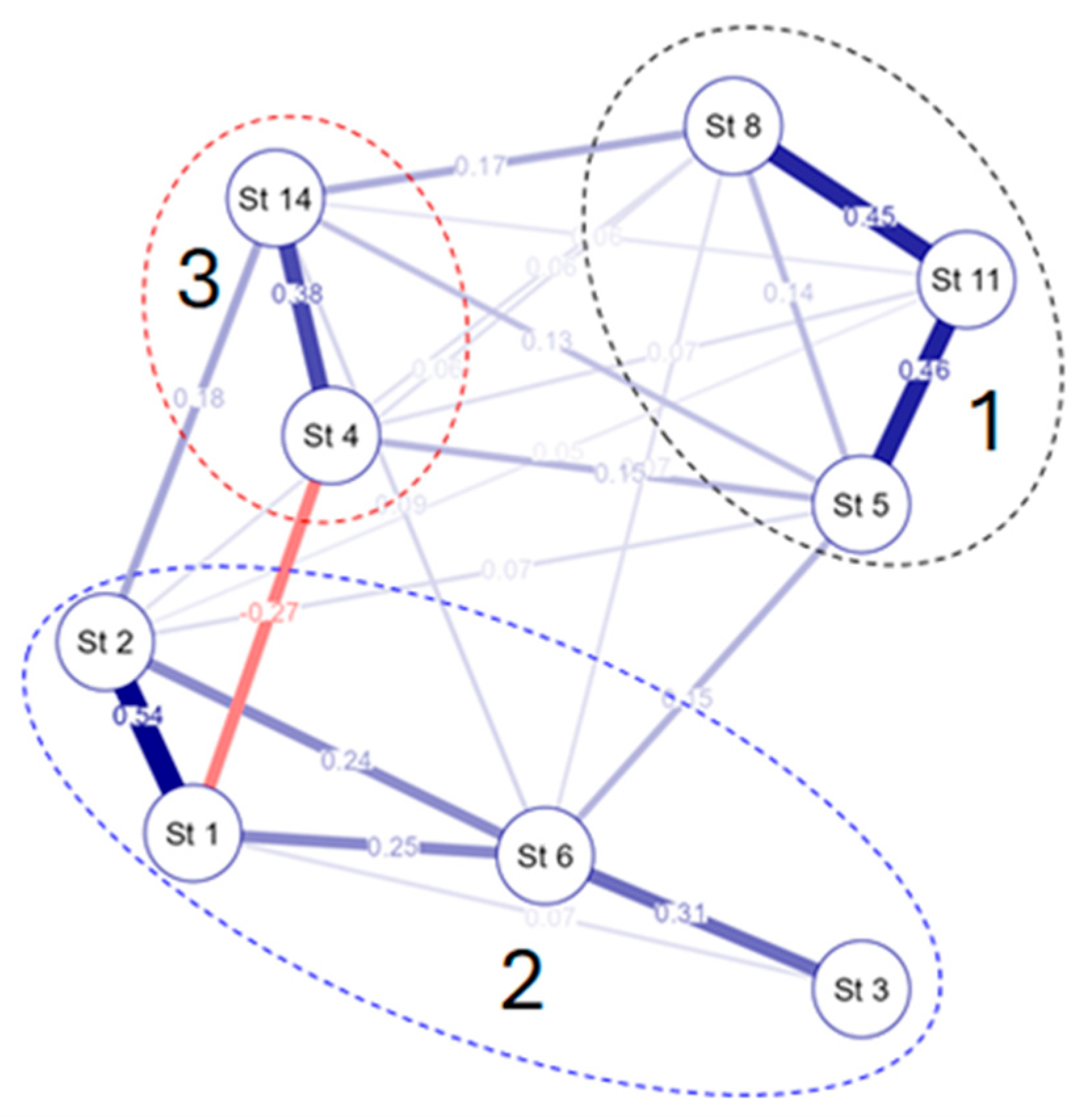
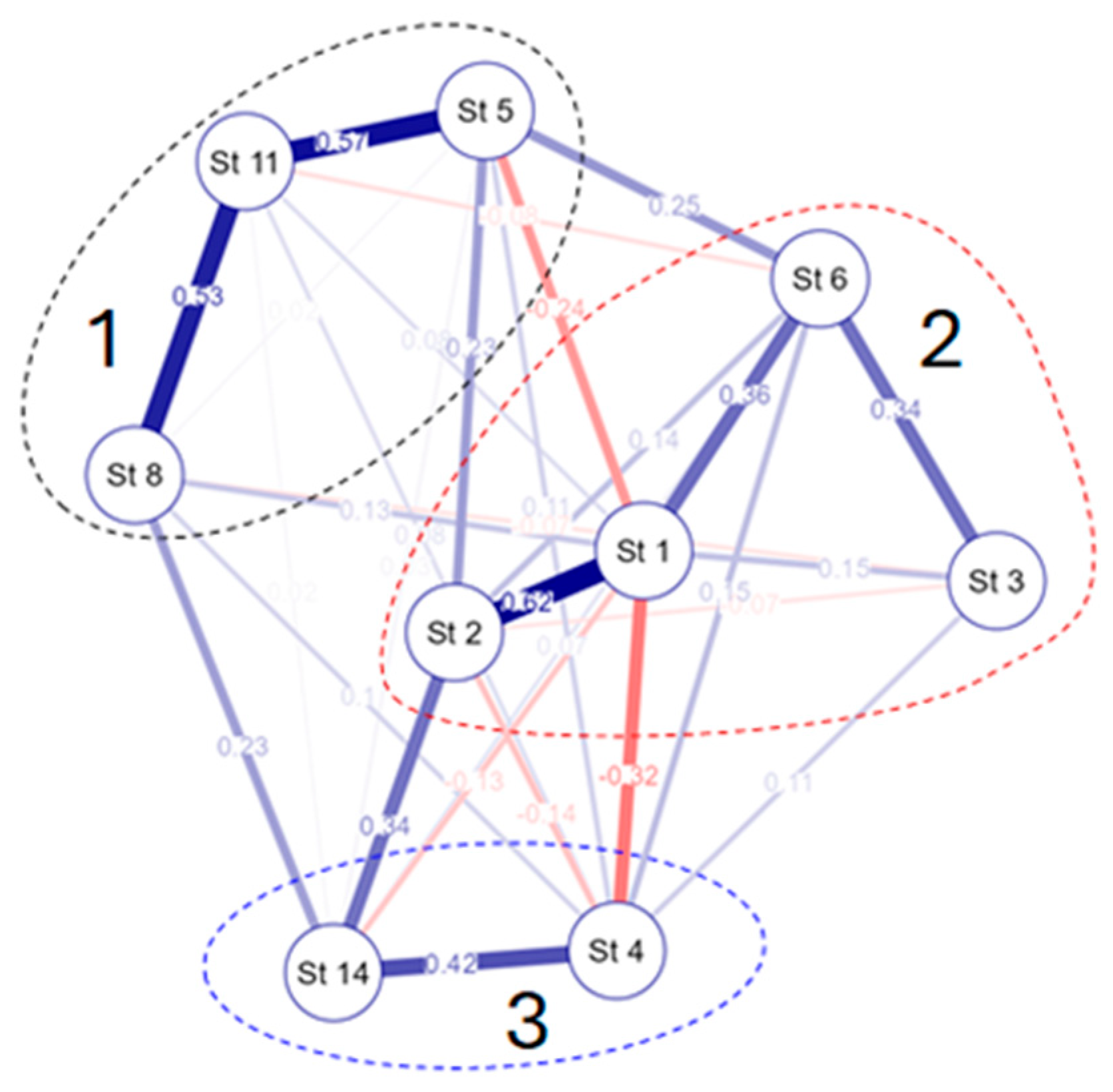
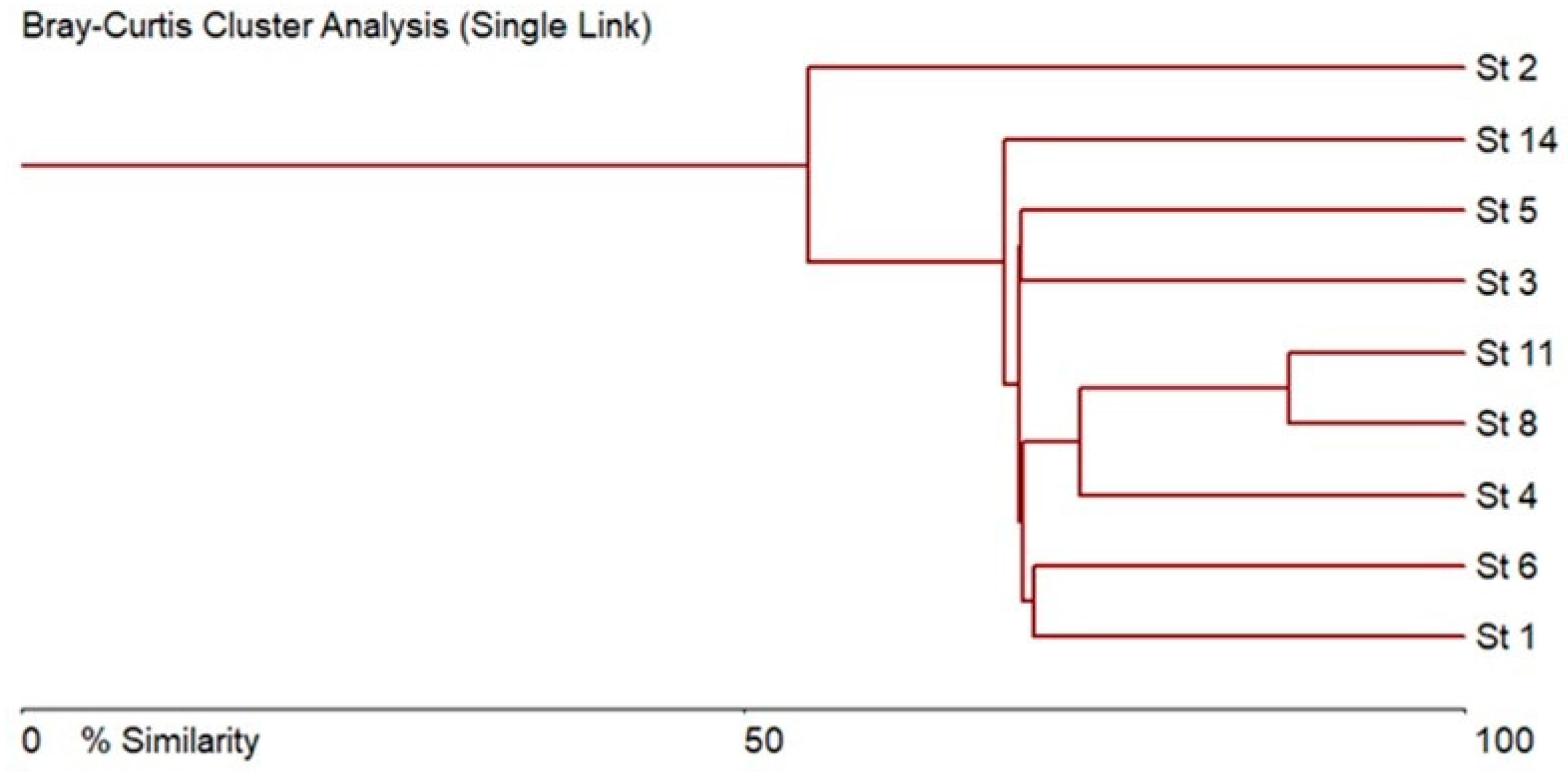
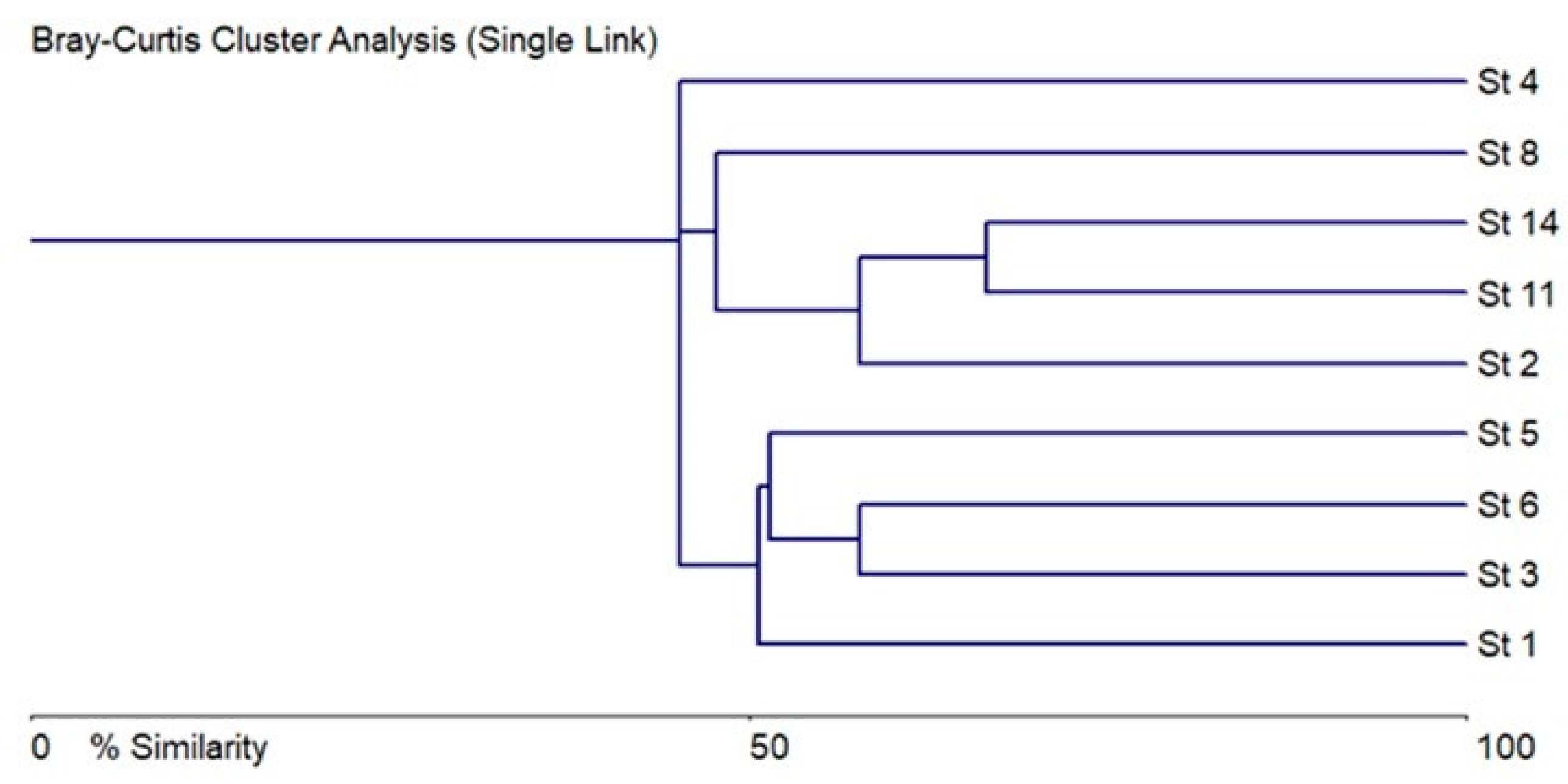
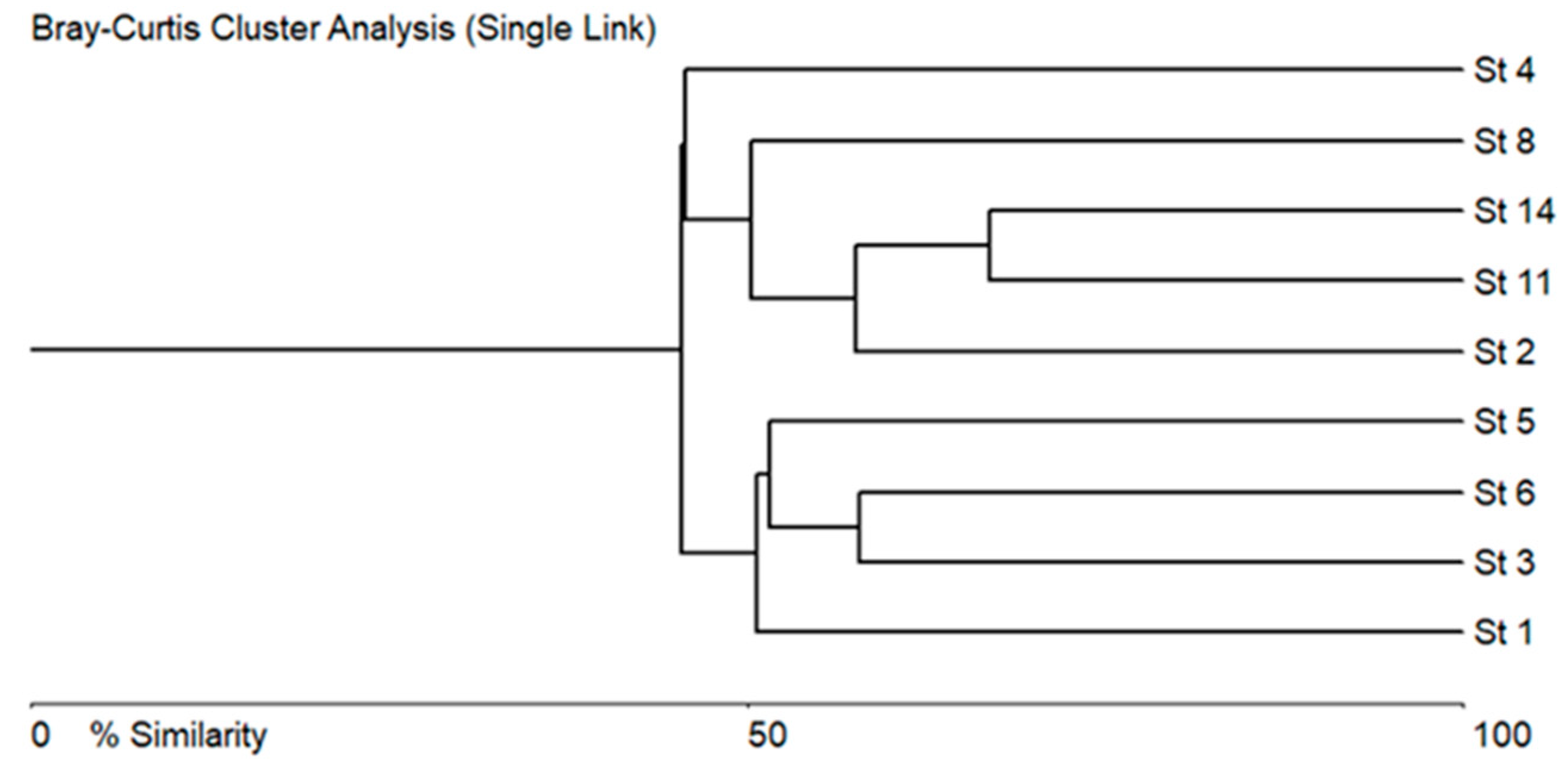
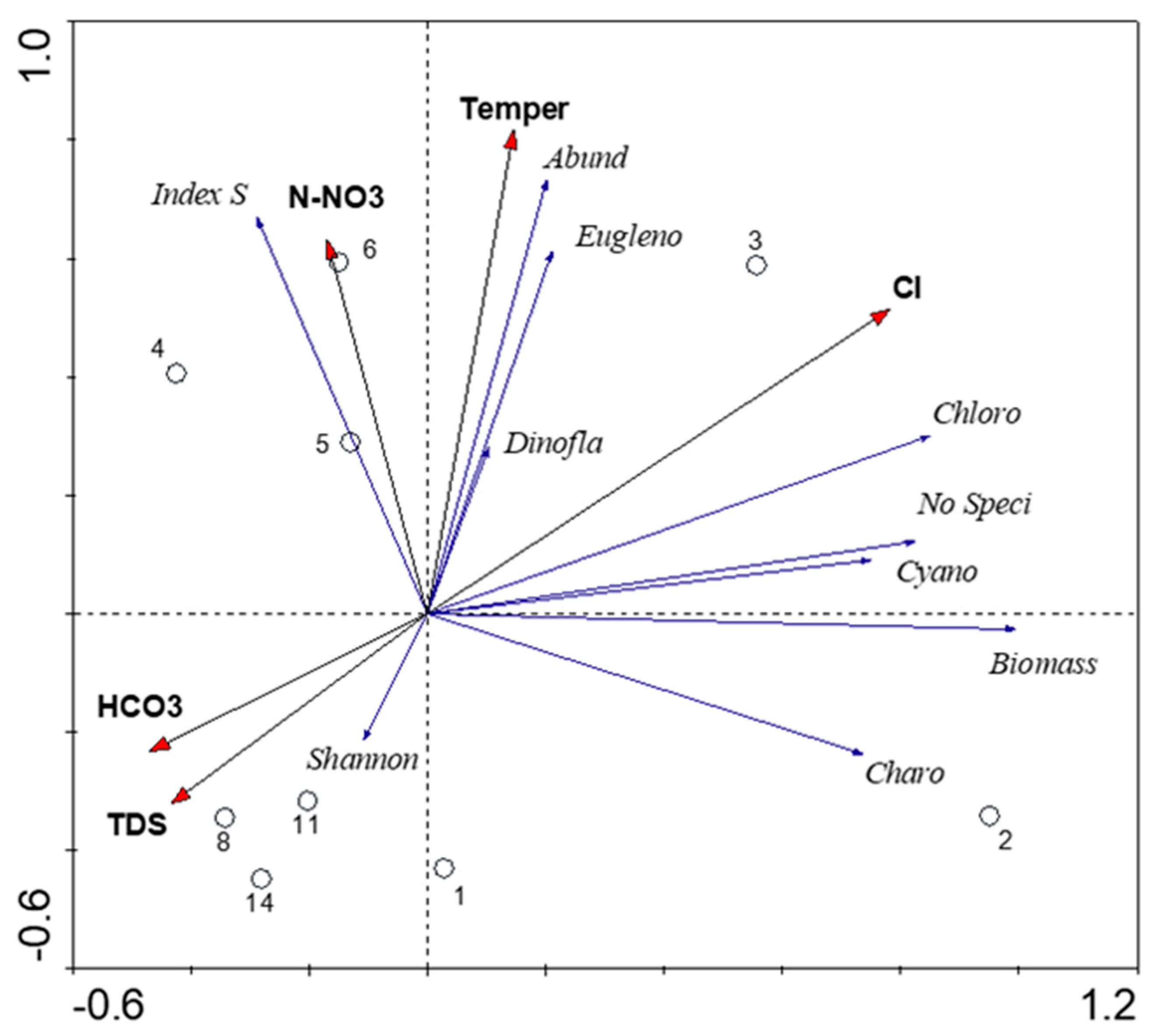
| Station No | Name of Water body | Altitude, m a.s.l. | Sample Type and Its Number | Geographical Position | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Latitude | Longitude | |||
| 1 | Lake 7 | 189 | 9a, 9b | 20 | Ch13 | 58°30′7″ | 121°37′12″ | |||||
| 2 | Podskalnoye Lake | 184 | 8a, 8b | 14 | 16 | 18 | 21 | Ch12 | 58°35′31″ | 121°30′22″ | ||
| 3 | Lake 1 | 171 | 1a, 1b | 23 | Ch1 | 59°2′2″ | 121°46′48″ | |||||
| 4 | Lake 4 | 171 | 4a, 4b | Ch4 | 59°1′48″ | 121°45′40″ | ||||||
| 5 | Lake 3 | 170 | 3a, 3b | 24 | Ch3 | 59°1′55″ | 121°45′25″ | |||||
| 6 | Lake 2 | 170 | 2a, 2b | Ch2 | 59°2′6″ | 121°47′6″ | ||||||
| 7 | Olyokma River | 162 | 17 | Ch14 | 59°2′13″ | 121°46′19″ | ||||||
| 8 | Bolshoy Sordonokh Lake | 173 | 7a, 7b | 13 | 19 | Ch11 | 59°4′26″ | 121°49′5″ | ||||
| 9 | Pool 1 | 170 | 22 | Ch10 | 59°4′41″ | 121°48′29″ | ||||||
| 10 | Swamp 1 | 167 | 10 | Ch6 | 59°11′6″ | 121°45′43″ | ||||||
| 11 | Lake 5 | 162 | 5a, 5b | Ch5 | 59°11′6″ | 121°45′32″ | ||||||
| 12 | Stream 1 | 180 | 11 | 12 | Ch7 | 59°11′53″ | 121°46′59″ | |||||
| 13 | Stream 2 | 161 | 15 | Ch8 | 59°13′8″ | 121°41′46″ | ||||||
| 14 | Mundunda Lake | 163 | 6a, 6b | Ch9 | 59°13′8″ | 121°42′7″ | ||||||
| Phylum | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 | St 10 | St 11 | St 12 | St 13 | St 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cercozoa | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Charophyta | 36 | 49 | 26 | 11 | 38 | 18 | 10 | 21 | 6 | 13 | 20 | 1 | 4 | 8 |
| Chlorophyta | 7 | 22 | 18 | 6 | 14 | 11 | 13 | 7 | 2 | 1 | 7 | 1 | 5 | 12 |
| Cyanobacteria | 3 | 9 | 9 | 4 | 6 | 3 | 2 | 5 | 3 | 7 | 3 | 0 | 2 | 4 |
| Dinoflagellata | 3 | 2 | 2 | 3 | 2 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Euglenophyta | 1 | 3 | 8 | 1 | 9 | 7 | 0 | 2 | 2 | 0 | 5 | 0 | 0 | 4 |
| Heterokontophyta | 6 | 6 | 12 | 3 | 6 | 7 | 5 | 6 | 0 | 0 | 6 | 1 | 8 | 5 |
| Total | 56 | 91 | 75 | 28 | 75 | 48 | 31 | 42 | 13 | 21 | 43 | 3 | 19 | 33 |
| Phylum | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 8 | St 11 | St 14 |
|---|---|---|---|---|---|---|---|---|---|
| Charophyta | 9.2 | 1.1 | 1.5 | 1.2 | 2.0 | 3.5 | 1.1 | 1.5 | 1.2 |
| Chlorophyta | 0.4 | 0.5 | 43.2 | 0.5 | 67.9 | 3.8 | 0.2 | 2.3 | 1.8 |
| Cyanobacteria | 0.1 | 0.2 | 17.6 | 0.3 | 11.5 | 38.2 | 0.2 | 0.2 | 0.6 |
| Dinoflagellata | 0.2 | 3.6 | 0.5 | 0.2 | 0.6 | 0.2 | 0.1 | 0.2 | 0 |
| Euglenophyta | 0.1 | 0.2 | 0.9 | 9.1 | 0 | 0.8 | 0.1 | 0.4 | 0.9 |
| Heterokontophyta | 23.6 | 4.9 | 11.8 | 0.2 | 1.2 | 17.1 | 0.4 | 0.5 | 2.0 |
| Total | 33.5 | 10.5 | 75.3 | 11.5 | 82.7 | 63.6 | 1.9 | 5.0 | 6.5 |
| Phylum | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 8 | St 11 | St 14 |
|---|---|---|---|---|---|---|---|---|---|
| Charophyta | 105.9 | 9.5 | 133.4 | 22.2 | 62.1 | 38.2 | 47.5 | 58.0 | 32.1 |
| Chlorophyta | 0.1 | 0.1 | 5.0 | 0.1 | 5.1 | 0.4 | 0.02 | 0.2 | 0.4 |
| Cyanobacteria | 0 | 0.01 | 1.0 | 0 | 0.1 | 1.4 | 1.4 | 0.3 | 2.93 |
| Dinoflagellata | 16.9 | 296.5 | 32.9 | 13.4 | 9.8 | 16.4 | 7.1 | 12.5 | 0 |
| Euglenophyta | 0.3 | 0.3 | 10.6 | 11.9 | 0 | 5.7 | 0.1 | 3.4 | 4.8 |
| Heterokontophyta | 11.6 | 2.1 | 46.6 | 7.3 | 1.3 | 36.6 | 2.8 | 4.2 | 49.5 |
| Total | 134.7 | 308.7 | 229.4 | 54.8 | 78.3 | 98.6 | 59.0 | 78.7 | 89.7 |
| Variable | St 1 | St 2 | St 3 | St 4 | St 5 | St 6 | St 7 | St 8 | St 9 | St 10 | St 11 | St 12 | St 13 | St 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Species | 56 | 91 | 75 | 28 | 75 | 48 | 31 | 42 | 13 | 21 | 43 | 3 | 19 | 33 |
| Abundance, thou. cells L−1 | 34 | 11 | 75 | 12 | 83 | 64 | n/a | 2 | n/a | 7 | 5 | n/a | n/a | n/a |
| Biomass, mg L−1 | 135 | 309 | 230 | 55 | 78 | 99 | n/a | 59 | n/a | 90 | 79 | n/a | n/a | n/a |
| Shannon H′ plankton | 0.238 | 0.671 | 0.838 | 0.329 | 0.379 | 0.924 | n/a | 1.114 | n/a | 1.256 | 1.033 | n/a | n/a | n/a |
| Index S plankton | 1.25 | 1.32 | 1.91 | 2.35 | 1.52 | 1.66 | n/a | 1.42 | n/a | 1.61 | 1.57 | n/a | n/a | n/a |
| Index S phytoperiphyton—average | 1.59 | 1.93 | 1.77 | 2.35 | 1.87 | 1.66 | 1.87 | 2.37 | 2.02 | 1.45 | 1.57 | 1.50 | 2.06 | 1.61 |
| Index S average | 1.42 | 1.63 | 1.84 | 2.35 | 1.7 | 1.66 | 1.87 | 1.89 | 2.02 | 1.45 | 1.57 | 1.50 | 2.06 | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barinova, S.; Gabyshev, V.A.; Gabysheva, O.I.; Gabyshev, E.M. Microalgae as Bioindicators of Changes in Permafrost Catchments: A Reference Area of the Olyokma Nature Reserve, Yakutia. Water 2025, 17, 1686. https://doi.org/10.3390/w17111686
Barinova S, Gabyshev VA, Gabysheva OI, Gabyshev EM. Microalgae as Bioindicators of Changes in Permafrost Catchments: A Reference Area of the Olyokma Nature Reserve, Yakutia. Water. 2025; 17(11):1686. https://doi.org/10.3390/w17111686
Chicago/Turabian StyleBarinova, Sophia, Viktor A. Gabyshev, Olga I. Gabysheva, and Eduard M. Gabyshev. 2025. "Microalgae as Bioindicators of Changes in Permafrost Catchments: A Reference Area of the Olyokma Nature Reserve, Yakutia" Water 17, no. 11: 1686. https://doi.org/10.3390/w17111686
APA StyleBarinova, S., Gabyshev, V. A., Gabysheva, O. I., & Gabyshev, E. M. (2025). Microalgae as Bioindicators of Changes in Permafrost Catchments: A Reference Area of the Olyokma Nature Reserve, Yakutia. Water, 17(11), 1686. https://doi.org/10.3390/w17111686








