Abstract
The bioaccumulation of trace elements and heavy metals in aquatic organisms is a critical environmental concern due to its potential impact on ecosystem health and human safety. This study investigated the level of trace elements and heavy metals bioaccumulation in Labeo rohita and Mystus seenghala from the River Jhelum in the district Khushab, Punjab, Pakistan. The concentration of calcium, magnesium, iron, nickel, copper, arsenic, cadmium, zinc, chromium, manganese, cobalt, and lead in the gills, liver, and muscle tissues of these fish was measured using inductively coupled plasma-mass spectrometry. Then, the extent of contamination and its possible health risks were assayed. Our findings indicate significant variations in the elemental and metal concentrations among different organs and between species, reflecting their diverse feeding habits and habitats. The health risk assessment based on the estimated daily intake, estimated weekly intake, maximum permissible intake, target hazard quotient, hazard index or total target hazard quotient, health risk index, and target cancer risk revealed potential risks to human consumers of these fish. This study emphasizes the need for continuous monitoring, as new data and insights are crucial for understanding and mitigating these risks. Strict regulatory measures are also necessary to safeguard public health and preserve the ecosystem of Jhelum River.
Keywords:
heavy elements; bioaccumulation; fish; Labeo rohita; Mystus seenghala; human health; risk assessment 1. Introduction
Heavy metals have become an increasing global concern due to their persistence in the environment, impact on biogeochemical cycles, and associated ecological risks [1,2,3,4,5,6,7,8]. In aquatic ecosystems, heavy metals can originate from several sources including industrial discharges, mining activities, agricultural practices, urban runoff, inadequately treated sewage, atmospheric deposition, and the improper disposal of metal-containing products [9]. Once introduced into the aquatic environment, these metals can persist for long periods, leading to long-term ecological consequences [10,11,12,13,14,15]. These metals are typically classified into two categories: potentially toxic metals such as cadmium (Cd), lead (Pb), and nickel (Ni), and essential metals such as copper (Cu), zinc (Zn), iron (Fe), and manganese [16]. Potentially toxic metals pose significant health risks even at low concentrations when ingested over extended periods, potentially leading to serious health conditions including neurological disorders, kidney damage, and various forms of cancer. On the other hand, while essential metals are required for various biological functions, excessive intake can result in toxic effects, disrupting metabolic processes and leading to health issues such as gastrointestinal distress, liver damage, and other systemic effects [16,17,18].
Heavy metal pollution negatively impacts environmental health and biodiversity [13]. Moreover, heavy metals, such as mercury and lead, can accumulate in seafood, posing serious health risks like neurological disorders and cancer. Therefore, bioaccumulation and health risk assessments of heavy metals in finfish and shellfish are crucial to protect human health and environmental quality [12]. Prabakaran et al. [19] found that although zinc (Zn) was the most common metal in fish from the Gulf of Thailand, high levels of mercury (Hg) in some species posed potential health risks to consumers. In Ghana, Blankson et al. [20] observed higher metal accumulation in the fish liver and gills compared to the muscles; however, the health risk indices remained below critical thresholds, indicating no immediate health concerns. Ali et al. [21] reported that arsenic (As) was the most concentrated metal in fish from Bangladesh, with risk assessments indicating minimal health risks despite seasonal variations. Rani et al. [22] found that metal concentrations in brackish water fish from India were generally safe, but continuous monitoring was recommended due to potential long-term health impacts. Hashim et al. [23] showed that fish from the Zhob River had safe metal concentrations, although high levels in the water and soil pointed to potential risks. Köse [24] reported that while metal levels of fish in Türkiye’s Meriç Delta were within the permissible values, the high phosphorus content in water warranted further monitoring. Ray and Vashishth [25] emphasized the global concern of heavy metal bioaccumulation in fish and called for more research on its long-term health effects. Moreover, Habib et al. [26] found that wild fish in Pakistan had higher metal concentrations than farmed fish, although health risks were low, with target hazard quotient (THQ) values below 1. Therefore, assessing these metals helps ensure that seafood remains safe for consumption and informs regulations to maintain food safety standards. Furthermore, high levels of heavy metals in aquatic organisms can indicate broader environmental pollution, which affects entire ecosystems and wildlife [3,6,7,27,28,29].
The issue of heavy metals and trace elements in Pakistan’s water ecosystem is a significant environmental and public health concern. Previous studies have documented a significant deterioration in the quality of aquatic environments in Pakistan due to the direct discharge of toxic chemicals into rivers [30,31]. This pollution can turn these water bodies into death traps for aquatic organisms including fish. Fish are particularly vulnerable to exposure of various toxic elements through bioaccumulation processes within the food chain, potentially becoming hazardous to human health upon consumption [32,33].
The Jhelum River, flowing through Punjab, Pakistan, is a crucial waterway that supports a diverse aquatic ecosystem and is a significant resource for local communities. However, increasing pollution from various anthropogenic activities has compromised the health of this river [34]. It has been observed that the waste from the markets across the district of Khushab is dumped into the river. These markets are located in Jauharabad, Quaidabad, Khushab, and Noshehra. The most notable sources of pollution in the Jhelum river in district Khushab are wastes from markets, agricultural runoff, and domestic waste. Recent studies on the physicochemical characterization of the Jhelum River have indicated varying ranges of various physical and chemical parameters at different sites. However, most of these studies have focused on seasonal fluctuations and water suitability indices at different locations including Azad Jammu and Kashmir, the Kashmir Valley, Kashmir Himalayas, and northern Punjab [35,36,37]. Javed et al. [38,39,40] observed a high contamination degree and moderate risks of potentially toxic elements in the Jhelum River in the district of Jhang. However, studies concerning trace elements or heavy metals in the Jhelum River basin at Khushab are still scarce. The current study is the first of its nature on the Jhelum River in the district of Khushab.
In this study, we focused on two economically and ecologically significant fish species, Labeo rohita (rohu) and Mystus seenghala (singhari), which are commonly found in the Jhelum River and are vital to local fisheries. These species are also a key part of the diet for many local residents. By analyzing the concentrations of heavy elements in different organs of these fish species, specifically the liver, muscles, and gills, this research aims to provide a comprehensive assessment of the contamination levels and potential health risks.
Due to their physiological roles, the liver, muscles, and gills are important organs for metal bioaccumulation [3,6,41,42,43,44]. The liver often acts as a detoxification and storage site for metals [45], while the muscles and gills are involved in nutrient absorption and respiration, respectively [46]. Understanding the distribution of heavy metals in these organs can offer insights into the extent of contamination and the potential health risks posed to humans who consume these fish [47].
Studies concerning the bioaccumulation of heavy metals in fish tissues have been regularly conducted on different fish species across the globe as well as Pakistan. However, there is not a single study on the Jhelum River in the district of Khushab. Therefore, this study was aimed to determine the concentrations of trace elements and heavy metals (Ca, Mg, Fe, Ni, Cu, As, Cd, Zn, Cr, Mn, Co, and Pb) in the dominant fish species of the Jhelum River in the district of Khushab and assess the associated health risks. By comparing these concentrations with the established safety thresholds, the research will evaluate the potential health impacts on consumers and provide recommendations for mitigating pollution and ensuring safe fish consumption.
2. Materials and Methods
2.1. Study Area
The Jhelum River is a significant river within the Indus River system. Originating in the Indian-administered territory of Jammu and Kashmir, it flows through Pakistan’s Punjab province, passing through various ecological zones. The Jhelum River stretches approximately 725 km and has a catchment area that includes parts of both India and Pakistan. The region experiences a subtropical climate, with hot summers and mild winters. Monsoon rains from July to September significantly impact the river’s flow, leading to seasonal water levels and quality variations.
The Jhelum River basin is home to a rich biodiversity including numerous fish species, aquatic plants, and invertebrates. This diverse ecosystem highlights the river’s ecological importance and its role in supporting local livelihoods and environmental health.
2.2. Sampling of Fish
For this study, fish were sampled from different sites in district Khushab along the Jhelum River in Punjab, Pakistan. This site was strategically chosen because it is situated downstream from several industrial zones and agricultural fields, making it an essential location for evaluating the impact of pollutants on aquatic life. The area is influenced by a combination of industrial effluent, agricultural runoff, and domestic waste, which contribute to various contaminants in the water. Two fish species, Labeo rohita (Rohu) and Mystus seenghala (Singhara), were selected for sampling due to their ecological significance and higher consumption rates among the local population. Fishes (nine specimens of each fish species were collected from three different sites such as upstream, mid-stream, and downstream and a total of 27 specimens were collected for each species; n = 27; N = 54) were collected between November 2022 and January 2023 with the help of local professional fishermen, who used cast nets for fish collection. After collection, the dead fish samples were placed in clean, ice-filled containers. The specimens were placed in ice from all sides to avoid contact of the fish specimens with the walls of the container. These specimens were then transported to the “Fisheries and Aquaculture Research Laboratory (Headed by Dr. Sana Ullah)” at the University of Education (Jauharabad Campus) for subsequent analysis.
2.3. Preparation of Samples
The collected fish specimens were rinsed with distilled water to remove surface contaminants. Scales were removed using a stainless steel knife, and tissues such as the muscles, gills, and liver were dissected out. These samples were then weighed, packed, and stored at −20 °C until further analysis. The aqua regia method was employed for digestion, which involves a mixture of concentrated nitric and hydrochloric acids handled with extreme care. The defrosted tissues were washed, blotted, and transferred to digestion flasks. About 2 g of each sample was weighed and digested in a 3:1 solution of HCl (Sigma-Aldrich) and HNO3 (Sigma-Aldrich). The mixture was heated until reduced to 5 mL, cooled to room temperature, and filtered (through 0.45 µm membrane filters—nylon) to remove the undigested material. The filtrate was diluted to 50 mL with distilled water [48].
2.4. Heavy Metal Analysis
The samples were tested for essential elements [calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn)], trace element [nickel (Ni)], and heavy metals [arsenic (As), cadmium (Cd), lead (Pb), and chromium (Cr)] at the Hi-Tech Laboratory of Government College University, Faisalabad, using inductively coupled plasma-mass spectrometry (ICP-MS). The regulatory guidelines of ASTM [49] were followed. To determine the accuracy of the heavy metal results, the following points were considered: (1) Quality control measures including calibration verification (analyzing standards before and after sample analysis), instrument performance check (monitoring sensitivity, resolution, and mass axis stability), and method validation (verification of method detection limits, precision, and accuracy); (2) Interference checks including spectral interferences (monitor potential overlaps) and matrix effects (evaluation of sample matrix impact on analyte signals); (3) Contamination control include blank samples (analysis of procedural blanks to detect contamination), duplicates (running duplicate samples for assessing precision), and spikes (spiked samples were analyzed to evaluate recovery); and (4) Data evaluation including signal-to-noise ratio (S/N), isotopic ration, and result validation. The common interference in ICP-MS used was spectral (argon-based polyatomic ions such as ArO+ or ArN+, and/or molecular ions such as NO+). For the mitigation strategy, matrix-matching standards (for improved accuracy, reduced matrix interference, and enhanced robustness of the method) were employed.
2.5. Human Health Risk Assessment
Evaluating the health risks associated with fish consumption was a vital component of this research. This assessment employed various indices such as the estimated daily intake (EDI) [1], estimated weekly intake [50], target hazard quotient (THQ), total target hazard quotient (TTHQ) or hazard index (HI) [32,41,45,46,47], health risk index (HRI) [48,50,51], target cancer risk (TR) [52,53,54], and maximum permissible intake (MPI) [39,55]. These indices provide a comprehensive understanding of the potential exposure to contaminants and were calculated using data derived from the muscle samples.
2.5.1. Consumption Data
Due to the lack of existing data on daily fish consumption in the study area, a survey was conducted to gather this information. The survey found that a total of 50,000 kg of fish was sold daily within the district (unpublished data). By dividing this quantity by the district’s total population, it was estimated that an average of 39.03 g of fish was consumed per person per day in the district Khushab.
2.5.2. Estimated Daily Intake of Heavy Metals
The estimated daily intake (EDI) of trace elements and heavy metals through fish consumption was calculated using the metal concentrations in the edible part (muscle tissues—flesh—because of its consumption), daily consumption rates, and body weight. The following equation was used to calculate the EDI [51,54].
where FIR is the food ingestion rate (39.03 g), C is the metal concentration in the samples (mg/g), and BW is the body weight of the consumer (70 kg) [52,53].
EDI = (FIR × C)/BW
The estimated weekly intake was calculated using the following equation [50]
EWI = EDI × 7
The estimated daily intake (EDI) can be compared to the provisional tolerable daily Intake (PTDI). In contrast, the EWI can be compared to the provisional tolerable weekly intake (PTWI) to assess the potential human health risks.
2.5.3. Target Hazard Quotient (THQ)
The THQ can be calculated by using the following equation [50]
where C represents the contaminant concentration in fish (mg/g), IR is the ingestion rate of fish (g/day), EF denotes the exposure frequency (365 days/year), ED is the exposure duration (67 years), BW refers to the body weight of the consumer (70 kg), RfD is the reference dose (mg/kg/day) [39,40], and AT is the averaging time (EF × ED) [55]. A THQ value less than 1 indicates a negligible risk, while a THQ value greater than 1 indicates a significant health risk [56,57].
THQ = (C × IR × EF × ED)/(BW × RfD × AT)
2.5.4. Hazard Index (HI)
The hazard index (HI) was calculated by summing the target hazard quotient (THQ) values for each contaminant. The formula for HI is provided in the equation below [58].
Hazard Index HI = ∑THQ
If the HI is less than 1, the risk is considered low. If it exceeds 1, there may be a significant health risk [59].
2.5.5. Daily Metal Intake/Health Risk Index (HRI)
The health risk index (HRI) measures the potential health risk of consuming contaminants and is calculated using the daily metal intake (DIM) from food and the oral reference dose (RfD).
HRI = DIM/RfD
If the HRI is below 1, the risk is minimal. If the HRI is above 1, there is a significant health hazard [57,60].
2.5.6. Target Carcinogenic Risk (TR)
The target carcinogenic risk (TR) factor represents the increased likelihood of an individual developing cancer over their lifetime due to exposure to a potential carcinogen. The TR was calculated using the formula:
TR = (C × IR × CPSo × EF × ED)/(BW × AT) × 10−3
In this formula, C is the concentration of the carcinogenic contaminant in the fish (mg/g), IR is the ingestion rate of fish (g/day), EF is the exposure frequency (days/year), ED is the exposure duration (years), BW represents the body weight of the consumer (in kg), AT stands for the time period over which the average exposure is calculated (in days), and CPSo is the carcinogenic potency slope via oral exposure (mg/kg/BW/day) [61,62].
If the TCR value is below 10−6, the hazard is considered negligible. A TCR value between 10−6 and 10−4 indicates a low hazard, while a TCR value above 10−4 is classified as a high risk [63,64].
2.5.7. Metal Pollution Index (MPI)
The metal pollution index (MPI) is a measure used to estimate the overall bioaccumulation of trace elements and heavy metals in different fish tissues. It is calculated using the formula:
where M1, M2, M3, …, Mn represent the concentrations of various metals in the tissues, and (n) is the number of metals analyzed [65,66].
MPI = M1 × M2 × M3 … Mn1/n
2.6. Statistical Analysis
Analyses were carried out to statistically compare the results observed to determine whether there were significant differences in the elemental and metal concentrations across the liver, gills, and muscles of the studied fish species. Analysis of variance (ANOVA) was performed followed by least significant difference (LSD) to test the homogeneity of variance (multiple variance analysis) [67] at a significance level (p value) of less than 0.05. Furthermore, the two-sample t-test was performed to compare the mean concentration of the studied elements and metals between Labeo rohita and Mystus seenghala at significance levels (p value) less than 0.001, 0.01, and 0.05. Pearson correlation was carried out to study the correlation of the studied heavy metals across the sampled tissues in both fish species. The statistical analysis was carried out using MS Excel (2016) and Statistix (V. 9).
3. Results
3.1. Heavy Metal Concentration
The average concentrations of the studied trace elements and heavy metals in the gills, liver, and muscles of Labeo rohita and Mystus seenghala are provided in Table S1. In the gill tissue of Mystus seenghala, the bioaccumulation of metals followed the order: Ca, Mg, Fe, Ni, Cu, Cr, Mn, Co, As, Cd, Zn, and Pb. In the liver tissue, the order was Mg, Ca, Fe, Cr, Cu, Ni, As, Co, Mn, Cd, Zn, and Pb. For the muscle tissue, the sequence was Mg, Ca, Ni, Cr, Cu, Fe, As, Mn, Co, Cd, Zn, and Pb, while for Labeo rohita, the sequence of metal bioaccumulation in the gill tissue was Mg, Ca, Fe, Mn, Ni, As, Cu, Cd, Cr, Co, Zn, and Pb. In the liver tissue, the order was Mg, Ca, Fe, Mn, Ni, Co, Cd, As, Cu, Zn, Cr, and Pb, and in the muscle tissue, the metals bioaccumulated in the following sequence: Mg, Ca, Fe, Mn, Ni, Cr, Cu, Co, As, Cd, Zn, and Pb.
The tissue-wise comparisons of the accumulation patterns of the studied element and heavy metals within the same species of L. rohita and M. seenghala are illustrated in Figure 1 and Figure 2, respectively. Table 1 and Table 2 show the correlation coefficient matrix of the studied elements and heavy metals in Labeo rohita and Mystus seenghala, respectively. Tables S2 and S3 show the correlation coefficient matrix of the studied elements and heavy metals in liver and gills of Labeo rohita, respectively. Tables S4 and S5 show the correlation coefficient matrix of the studied elements and heavy metals in the liver and gills of Mystus seenghala, respectively.
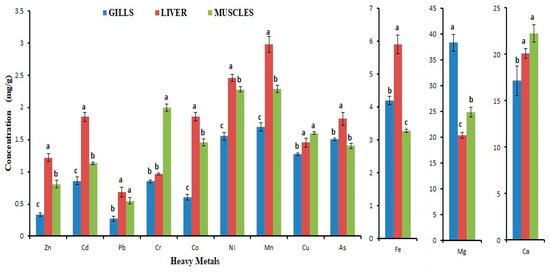
Figure 1.
Level of trace elements and heavy metals in various tissues of Labeo rohita. Data presented as mean ± SE (n = 9). The mean with different letters is significantly different (p < 0.05) (ANOVA followed by LSD test).
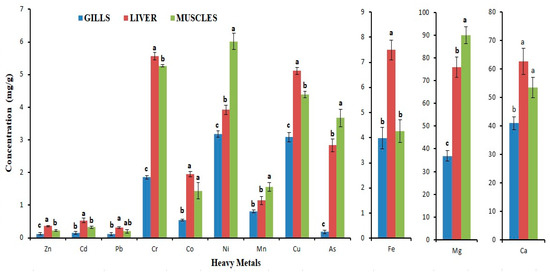
Figure 2.
Level of trace elements and heavy metals in various tissues of M. seenghala. Data presented as mean ± SE (n = 9). The mean with different letters is significantly different (p < 0.05) (ANOVA followed by LSD test).

Table 1.
Correlation coefficient matrix of the studied trace elements and heavy metals in the muscle tissues of Labeo rohita.

Table 2.
Correlation coefficient matrix of the studied trace elements and heavy metals in the muscle tissues of M. seenghala.
Figure 3, Figure 4 and Figure 5 illustrate the tissue-wise comparison of the bioaccumulation patterns of the studied trace elements and heavy metals across species of L. rohita and M. seenghala.
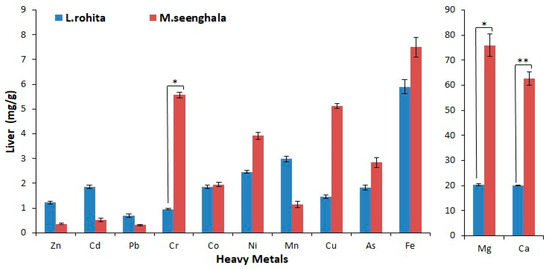
Figure 3.
Concentration of trace elements and heavy metals (mg/g) in the liver tissue of L. rohita and M. seenghala. Asterisks represent the level of significance based on a two-sample t-test assuming equal variance (** = p < 0.01, * = p < 0.05).
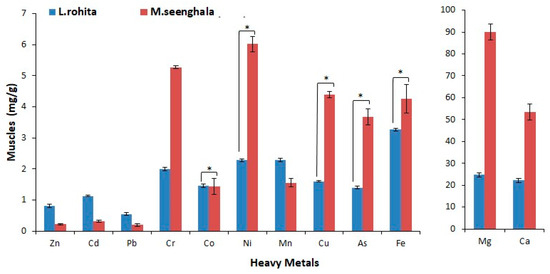
Figure 4.
Concentration of trace elements and heavy metals (mg/g) in the muscle tissue of L. rohita and M. seenghala. Asterisks represent the level of significance based on a two-sample t-test assuming equal variance (* = p < 0.05).
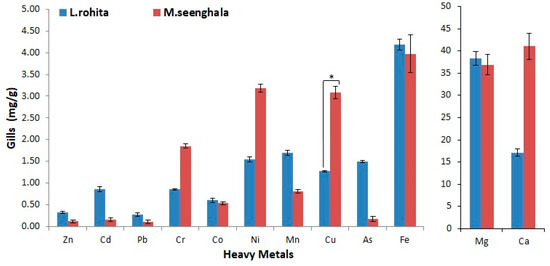
Figure 5.
Concentration of trace elements and heavy metals (mg/g) in the gill tissue of L. rohita and M. seenghala. Asterisks represent the level of significance based on a two-sample t-test assuming equal variance (* = p < 0.05).
3.2. Comparison of the Health Risks Associated with the Consumption of Labeo rohita and Mystus seenghala
The EDI and EWI values for Labeo rohita and Mystus seenghala were determined and contrasted with the PTDI and PTWI values, as shown in Table 3. Table 4 provides a comparison of the TR values for L. rohita and M. seenghala. Additionally, the THQ, TTHQ, HRI, and MPI values for both species are compared in Table 5.

Table 3.
EDI and EWI values of L. rohita and M. seenghala.

Table 4.
Comparison of the TR values of L. rohita and M. seenghala.

Table 5.
Comparison of the THQ, TTHQ, HRI and MPI values of L. rohita and M. seenghala.
4. Discussion
Trace and heavy metals in aquatic environments pose significant health risks to both aquatic organisms and humans [2]. These metals can accumulate in fish tissues through various pathways such as water, sediment, and diet [68]. This bioaccumulation is influenced by factors like water quality, seasonal changes, fish species, and their life stages [69]. Trace elements and heavy metals are persistent in the environment and can magnify through the food chain, leading to elevated concentrations in top predators and consumers including humans [1].
Fish is a major source of easily digestible protein, supplying essential amino acids, fats, macro and trace elements, fat-soluble vitamins, and long-chain polyunsaturated omega-3 fatty acids. Omega-3 fatty acids like eicosapentaenoic acid (EPA, C20:5 n-3), docosahexaenoic acid (DHA, C22:6 n-3), and docosapentaenoic acid (DPA, C22:5 n-3) are crucial for reducing the risk of cancer, cardiovascular diseases, and neurological disorders [70]. With growing awareness of its nutritional and therapeutic benefits, global fish consumption has increased rapidly in recent years [16]. The American Heart Association recommends eating fish at least twice a week to meet the daily intake of omega-3 fatty acids [71]. However, the presence of toxic levels of heavy elements in fish can diminish these beneficial effects [33].
Studies have shown that the levels of heavy metals in fish can vary significantly based on geographical location, industrial activities, and agricultural practices [72]. In this study, we examined the concentrations of trace elements and heavy metals in the gills, liver, and muscle tissues of L. rohita and M. seenghala. One-way ANOVA tests revealed significant differences in the elemental and metal concentrations across the different tissues of the fish species, with a significance level of p < 0.05. A two-sample t-test demonstrated that the concentrations of Cr and Mg in the liver, Co, Cu, Ni, As, and Fe in the muscles, and Cu in the gills varied significantly across the species at a significance level of p < 0.05. Additionally, the concentration of Ca in the liver showed a significant difference at a significance level of p < 0.01, assuming equal variance. The levels of all the elements and metals analyzed in L. rohita and M. seenghala exceeded the international limits listed in Table 6.

Table 6.
Concentrations of trace elements and heavy metals in Labeo rohita and Mystus seenghala compared with international standards.
In this study, we found that the liver tissues had a high bioaccumulation of trace elements and heavy metals, consistent with findings from previous research [12,78]. Among all of the studied elements, Ca, Mg, and Fe were found to be highly bioaccumulated in both fish species because of their crucial roles in bone formation, muscle function, and oxygen transport [79]. These essential biological processes require increased uptake and storage of these metals, and this necessity leads to their significant presence in the tissues. Zn, Cd, and Pb were found to be less bioaccumulated in both fish species compared to other metals. In the liver, their concentrations were 1.220, 1.854, and 0.687 mg/g, respectively, in L. rohita, and 0.361, 0.530, and 0.312 mg/g, respectively, in M. seenghala. In the muscle tissues, L. rohita had concentrations of 0.808, 1.133, and 0.549 mg/g, while M. seenghala had lower levels of 0.221, 0.325, and 0.200 mg/g. In the gills, L. rohita showed concentrations of 0.332, 0.857, and 0.271 mg/g, whereas M. seenghala had even lower levels of 0.124, 0.156, and 0.116 mg/g. The results showed that Zn, Cd, and Pb were found to be higher in L. rohita compared to M. seenghala in all of the studied tissues. This difference might be due to variations in their metabolic rates and physiological mechanisms, leading L. rohita to have a greater capacity for the uptake and retention of these metals [65,66,80,81,82]. In M. seenghala, Ni, Cr, and Cu were found to be highly accumulated in the muscle tissues, with concentrations of 6.019 mg/g, 5.272 mg/g, and 4.389 mg/g, respectively. In the liver, their concentrations were 3.924 mg/g, 5.568 mg/g, and 5.118 mg/g, while in the gills, these were 3.184 mg/g, 1.855 mg/g, and 3.086 mg/g, respectively. These metals are essential for various biological functions in animals and humans, while their excessive intake can lead to adverse health effects. High levels of these metals can cause toxicity and disrupt physiological processes, potentially leading to health issues such as organ damage and metabolic disturbances [52]. The bioaccumulation of heavy elements in L. rohita investigated in the present study was found to be higher than that reported by scientists [83,84,85,86], while the concentration of heavy metals (Fe, Cu, Zn, Mn, Cd, Co, Ni, Pb, and Cr) in M. seenghala was found to be higher than that reported by Sofia and Teresa [87]. The levels of Cu, Zn, Cd, and Pb found in M. seenghala in this study were higher than those reported by Kumar et al. [84,88]. This study indicates that M. seenghala has higher concentrations of trace elements compared to L. rohita. The primary reasons for this difference are the distinct dietary habits and ecological roles of the two species. M. seenghala is a carnivorous fish, consuming other aquatic organisms that may already contain heavy elements, leading to bioaccumulation [89]. Moreover, as a bottom feeder, M. seenghala ingests sediments from the river or lakebed, where heavy metals often settle [90]. Therefore, M. seenghala may be more vulnerable to heavy metal bioaccumulation because of its slow metabolism and growth rate [91].
For the health risk assessment, we used various health risk indices to estimate the level of risk posed to human health by consuming these fish species. We compared the estimated daily intake (EDI) with the provisional tolerable daily intake (PTDI) values and the estimated weekly intake [50] with the provisional tolerable weekly intake (PTWI) values for both fish species (Table 3). Based on the comparisons, it was evident that both fish species, L. rohita and M. seenghala, contained heavy metals in concentrations that generally exceeded the provisional tolerable intake limits for most of the analyzed metals [46,47,48,50,51,52,53,54,66]. In general, M. seenghala had higher EDI and EWI values for Cr, Co, Ni, Fe, Cu, Mg, and Ca compared to L. rohita, while some heavy elements like Zn in both fish species and Ca in L. rohita were within the safe limits.
Table 4 shows the TR value, which revealed that cadmium, nickel, and arsenic were associated with high carcinogenic risks in both fish species, with values significantly above 10−4, while lead showed a low hazard.
Table 5 shows the THQ, TTHQ, HRI, and MPI values of both fish species. The total target hazard quotient, indicating a considerable cumulative health risk, was significantly greater than 1 [46,47], with values 3.800 for L. rohita and 8.28 for M. seenghala. L. rohita showed an MPI value of 1796.59, reflecting a high level of metal pollution, whereas M. seenghala presented a value of 8856.41, indicating an even higher level of metal pollution. Both fish species had HRI values for several metals (Cd, Pb, Cr, Co, Ni, Cu, and As) that far exceeded the standard value of 1 [51,66], which shows a significant risk. Arsenic was the only heavy metal with a THQ value above 1, which was 2.6500 and 0.6828 in L. rohita and M. seenghala, respectively. M. seenghala generally exhibited higher HI, THQ, HRI, and MPI values compared to L. rohita, indicating a greater health risk from its consumption.
5. Conclusions
The investigation into the bioaccumulation of trace elements and heavy metals in Labeo rohita and Mystus seenghala from the Jhelum River has revealed critical insights into the extent of contamination and the associated health risks. The study identified significant trace elements and heavy metal bioaccumulation in the gills, liver, and muscle tissues of both fish species. The concentrations of all of the studied elements and metals were found to be higher than the international standards. The health risk assessment demonstrates potential hazards for humans consuming these fish, highlighting the need for comprehensive and continuous environmental monitoring, effective pollution control strategies, and increased public awareness. Collaborative efforts between governmental bodies, environmental organizations, and local communities are essential to address and mitigate the impacts of heavy metal pollution. Future research should aim at developing sustainable solutions and innovative approaches to ensure the long-term health and safety of both aquatic life and human populations relying on these ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16202994/s1, Table S1: Concentrations of heavy metals (mg/g) in the muscles, liver, and gills of L. rohita and M. seenghala; Tables S2–S5: Correlation coefficient matrix of the studied heavy metals in the liver and gills of L. rohita and M. seenghala, respectively.
Author Contributions
Conceptualization, A.E. and S.U.; Methodology, A.E., S.U. and S.I.; Software, S.U., S.I., A.E. and M.B. (Muhammad Bilal); Validation, A.E., S.U., C.F. and M.B. (Mahdi Banaee); Formal analysis, A.E., S.I., M.B. (Muhammad Bilal) and S.U.; Investigation, A.E., S.U. and M.B. (Muhammad Bilal); Resources, S.U.; Data curation, A.E., S.I. and M.B. (Muhammad Bilal); Writing—original draft preparation, A.E. and S.U.; Writing—review and editing, M.B. (Mahdi Banaee), C.M. and C.F.; Visualization, M.B. (Muhammad Bilal) and M.B. (Mahdi Banaee); Supervision, S.U.; Project administration, S.U.; Funding acquisition, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data associated with this study are provided in this article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Ali, M.M.; Hossain, D.; Al-Imran, A.; Khan, M.; Begum, M.; Osman, M. Environmental pollution with heavy metals: A public health concern. In Heavy Metals-Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021; pp. 771–783. [Google Scholar]
- Banaee, M.; Mohammadipour, S.; Madhani, S. Effects of sublethal concentrations of permethrin on bioaccumulation of cadmium in zebra cichlid (Cichlasoma nigrofasciatum). Toxicol. Environ. Chem. 2015, 97, 200–207. [Google Scholar] [CrossRef]
- Gholamhosseini, A.; Banaee, M.; Sinha, R.; Zeidi, A.; Faggio, C. Bio-concentration of heavy metals in marine crustaceans’ hemolymph: Insights from Oman Sea, Iran. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P. Heavy metal contamination in water and its possible sources. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–189. [Google Scholar]
- Banaee, M.; Di Paola, D.; Cuzzocrea, S.; Cordaro, M.; Faggio, C. Biomarkers in Aquatic Ecotoxicology: Understanding the Effects of Xenobiotics on the Health of Aquatic Organisms. In Biochemical and Physiological Response During Oxidative Stress—From Invertebrates to Vertebrates; Marika, C., Roberta, F., Rosanna, D.P., Eds.; IntechOpen: London, UK, 2024; pp. 1–24. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Mikušková, N.; Faggio, C. Assessing metal toxicity on crustaceans in aquatic ecosystems: A comprehensive review. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Impellitteri, F.; Curpăn, A.-S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A useful tool for assessing the toxicity of microplastics, heavy metals, and pesticides on aquatic invertebrates. Int. J. Environ. Res. Public Health 2022, 19, 16830. [Google Scholar] [CrossRef] [PubMed]
- Hedayatzadeh, F.; Ildoromi, A.; Hassanzadeh, N.; Bahramifar, N.; Banaee, M. Comprehensive monitoring of contamination and ecological-health risk assessment of potentially harmful elements in surface water of Maroon–Jarahi sub-basin of the Persian Gulf, Iran. Environ. Geochem. Health 2024, 46, 411. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Khalil, M.S.; Ghorab, M.A. Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc. J. Toxicol 2018, 3, 555603. [Google Scholar]
- Baby, J.; Raj, J.S.; Biby, E.T.; Sankarganesh, P.; Jeevitha, M.; Ajisha, S.; Rajan, S.S. Toxic effect of heavy metals on aquatic environment. Int. J. Biol. Chem. Sci. 2010, 4, 939–952. [Google Scholar] [CrossRef]
- Gholamhosseini, A.; Shiry, N.; Soltanian, S.; Banaee, M. Bioaccumulation of metals in marine fish species captured from the northern shores of the Gulf of Oman, Iran. Reg. Stud. Mar. Sci. 2021, 41, 101599. [Google Scholar] [CrossRef]
- Merola, C.; Bisegna, A.; Angelozzi, G.; Conte, A.; Abete, M.C.; Stella, C.; Pederiva, S.; Faggio, C.; Riganelli, N.; Perugini, M. Study of heavy metals pollution and vitellogenin levels in brown trout (Salmo trutta trutta) wild fish populations. Appl. Sci. 2021, 11, 4965. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Shiry, N.; Derakhshesh, N.; Gholamhosseini, A.; Pouladi, M.; Faggio, C. Heavy metal concentrations in Cynoglossus arel (Bloch & Schneider, 1801) and sediment in the Chabahar Bay, Iran. Int. J. Environ. Res. 2021, 15, 773–784. [Google Scholar]
- Obiero, K.; Meulenbroek, P.; Drexler, S.; Dagne, A.; Akoll, P.; Odong, R.; Kaunda-Arara, B.; Waidbacher, H. The contribution of fish to food and nutrition security in Eastern Africa: Emerging trends and future outlooks. Sustainability 2019, 11, 1636. [Google Scholar] [CrossRef]
- Kwaansa-Ansah, E.E.; Nti, S.O.; Opoku, F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci. Biotechnol. 2019, 28, 569–579. [Google Scholar] [CrossRef]
- Tuzen, M. Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food Chem. Toxicol. 2009, 47, 1785–1790. [Google Scholar] [CrossRef]
- Prabakaran, K.; Sompongchaiyakul, P.; Bureekul, S.; Wang, X.; Charoenpong, C. Heavy metal bioaccumulation and risk assessment in fishery resources from the Gulf of Thailand. Mar. Pollut. Bull. 2024, 198, 115864. [Google Scholar] [CrossRef] [PubMed]
- Blankson, E.R.; Ohene-Obeng, N.K.; Awuah, B.A.; Oduro, D.; Ewool, J.; Gbogbo, F. Heavy metal bioaccumulation in highly consumed pelagic and benthic fish and associated health risk. Biol. Trace Elem. Res. 2024, 202, 3781–3788. [Google Scholar] [CrossRef]
- Ali, M.M.; Kubra, K.; Alam, E.; Mondol, A.H.; Akhtar, S.; Islam, M.S.; Karim, E.; Ahmed, A.S.; Siddique, M.A.B.; Malafaia, G. Bioaccumulation and sources of metal (loid) s in fish species from a subtropical river in Bangladesh: A public health concern. Environ. Sci. Pollut. Res. 2024, 31, 2343–2359. [Google Scholar] [CrossRef]
- Rani, V.; Vilvest, J.; Yagoo, A. Bioaccumulation of heavy metals in commercial fishes from Adyar estuary (Mugil cephalus and Megalops cyprinoides). Reg. Stud. Mar. Sci. 2024, 74, 103512. [Google Scholar] [CrossRef]
- Hashim, T.; Masood, Z.; Alvi, S.; Gul, J.; Khan, W.; Ahmed, D.; Jamil, J.; Ali, W.; Swelum, A.A. Assessment and bioaccumulation of heavy metal contaminants in Golden Mahseer (Tor putitora Hamilton, 1822). Sci. Total Environ. 2024, 951, 175719. [Google Scholar] [CrossRef]
- Köse, E. The Bioaccumulation of Heavy Metals in the Water and Tissues of Invasive Fish Carassius gibelio (Bloch, 1782) and Non-carcinogenic Health Risk Assessment from Meriç Delta Wetland, Türkiye. Biological Trace Element Research 2024. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Vashishth, R. From Water to Plate: Reviewing the Bioaccumulation of Heavy Metals in Fish and Unraveling Human Health Risks in the Food Chain. Emerg. Contam. 2024, 10, 100358. [Google Scholar] [CrossRef]
- Habib, S.S.; Naz, S.; Fazio, F.; Cravana, C.; Ullah, M.; Rind, K.H.; Attaullah, S.; Filiciotto, F.; Khayyam, K. Assessment and bioaccumulation of heavy metals in water, fish (wild and farmed) and associated human health risk. Biol. Trace Elem. Res. 2024, 202, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Gholamhosseini, A.; Hoseinifar, S.H.; Banaee, M. Investigation of the effects of heavy metals (copper, cobalt, manganese, selenium, and zinc) on fish immune systems: An overview. Ann. Anim. Sci. 2024. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108583. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L.). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108942. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Assessment of potentially toxic heavy metals and health risk in water, sediments, and different fish species of River Kabul, Pakistan. Hum. Ecol. Risk Assess. 2018, 24, 2101–2118. [Google Scholar] [CrossRef]
- Shafi, J.; Mirza, Z.S.; Kosour, N.; Zafarullah, M. Assessment of water quality and heavy metals contamination of River Ravi in Pakistan. Pak. J. Anal. Environ. Chem. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Garai, P.; Banerjee, P.; Mondal, P.; Saha, N. Effect of heavy metals on fishes: Toxicity and bioaccumulation. J. Clin. Toxicol. 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Isangedighi, I.A.; David, G.S. Heavy metals contamination in fish: Effects on human health. J. Aquat. Sci. Mar. Biol. Res. 2019, 2, 7–12. [Google Scholar] [CrossRef]
- Inayat, I.; Batool, A.I.; Rehman, M.F.U.; Ahmad, K.R.; Kanwal, M.A.; Ali, R.; Khalid, R.; Habib, S.S. Seasonal variation and association of heavy metals in the vital organs of edible fishes from the River Jhelum in Punjab, Pakistan. Biol. Trace Elem. Res. 2024, 202, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Muhammad, S.; Umar, M.; Azhar, M.U.; Ahmed, A.; Ahmad, A.; Ullah, R. Spatial distribution of physicochemical parameters and drinking and irrigation water quality indices in the Jhelum River. Health Environ. Geochem. 2024, 46, 263. [Google Scholar] [CrossRef] [PubMed]
- Gull, S.; Shah, S.R.; Dar, A.M. Assessment and interpretation of surface water quality in Jhelum River and its tributaries using multivariate statistical methods. Environ. Monit. Assess. 2023, 195, 746. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, U.; Islam, S.T.; Sabha, I.; Bhat, S.U.; Dar, S.A. Coliform pollution mapping in major watersheds along Jhelum River Basin of Kashmir Himalaya. Environ. Sci. Pollut. Res. 2023, 30, 7930–7941. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Assessment of heavy metals (Cu, Ni, Fe, Co, Mn, Cr, Zn) in rivulet water, their accumulations and alterations in hematology of fish Channa punctatus. Afr. J. Biotechnol. 2014, 13, 492. [Google Scholar]
- Javed, M.; Usmani, N. Accumulation of heavy metals in fishes: A human health concern. Int. J. Environ. Sci. 2011, 2, 659–670. [Google Scholar]
- Javed, M.; Usmani, N. Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. SpringerPlus 2016, 5, 776. [Google Scholar] [CrossRef]
- Banaee, M.; Beitsayah, A.; Prokić, M.D.; Petrović, T.G.; Zeidi, A.; Faggio, C. Effects of cadmium chloride and biofertilizer (Bacilar) on biochemical parameters of freshwater fish, Alburnus mossulensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 268, 109614. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; Piccione, G.; Faggio, C. Dietary arthrospira platensis in rainbow trout (Oncorhynchus mykiss): A means to reduce threats caused by CdCl2 exposure? Toxics 2022, 10, 731. [Google Scholar] [CrossRef]
- Squadrone, S.; Prearo, M.; Brizio, P.; Gavinelli, S.; Pellegrino, M.; Scanzio, T.; Guarise, S.; Benedetto, A.; Abete, M. Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 2013, 90, 358–365. [Google Scholar] [CrossRef]
- Torre, A.; Trischitta, F.; Faggio, C. Effect of CdCl2 on regulatory volume decrease (RVD) in Mytilus galloprovincialis digestive cells. Toxicology 2013, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Omar, W.A.; Zaghloul, K.H.; Abdel-Khalek, A.A.; Abo-Hegab, S. Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch. Environ. Contam. Toxicol. 2013, 65, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Agbugui, M.; Abe, G. Heavy metals in fish: Bioaccumulation and health. Br. J. Earth Sci. Res. 2022, 10, 47–66. [Google Scholar]
- Selvam, S.; Venkatramanan, S.; Hossain, M.; Chung, S.; Khatibi, R.; Nadiri, A. A study of health risk from accumulation of metals in commercial edible fish species at Tuticorin coasts of southern India. Estuar. Coast. Shelf Sci. 2020, 245, 106929. [Google Scholar] [CrossRef]
- Idera, F.; Omotola, O.; Adedayo, A.; Paul, U.J. Comparison of acid mixtures using conventional wet digestion methods for determination of heavy metals in fish tissues. J. Sci. Res. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- ASTM D1976-20; Standard Test Method for Elements in Water by Inductively-Coupled Plasma Atomic Emission Spectroscopy. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- Pokorska-Niewiada, K.; Witczak, A.; Protasowicki, M.; Cybulski, J. Estimation of target hazard quotients and potential health risks for toxic metals and other trace elements by consumption of female fish gonads and testicles. Int. J. Environ. Res. Public Health 2022, 19, 2762. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Raknuzzaman, M.; Ali, M.M.; Eaton, D.W. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environ. Syst. Decis. 2016, 36, 253–265. [Google Scholar] [CrossRef]
- Iqbal, A.; Tabinda, A.B.; Ahmad, F.; Yasar, A.; Siddique, S. Temporal Metal Bioaccumulation in Tissues of Labeo rohita and Cyprinus carpio from Indus River, Pakistan. Asian J. Chem. 2016, 28, 1069. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, M.H. Study of seasonal variations and health risk assessment of heavy metals in Cyprinus carpio from Rawal Lake, Pakistan. Environ. Monit. Assess. 2014, 186, 2025–2037. [Google Scholar] [CrossRef]
- Ullah, A.A.; Maksud, M.; Khan, S.; Lutfa, L.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- Bahreini Esfahani, N.; Jafari, M.; Moravejolahkami, A.R. Heavy metals concentration and target hazard quotients assessment through the consumption of fish muscle Ctenopharyngodon idella (Cyprinidae) from markets in Ahvaz province, Iran. Nutr. Food Sci. 2020, 50, 529–537. [Google Scholar] [CrossRef]
- Fathabad, A.E.; Tajik, H.; Najafi, M.L.; Jafari, K.; Khaneghah, A.M.; Fakhri, Y.; Conti, G.O.; Miri, M. The concentration of the potentially toxic elements (PTEs) in the muscle of fishes collected from Caspian Sea: A health risk assessment study. Food Chem. Toxicol. 2021, 154, 112349. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, L.; Liu, Q.; Li, S.; Li, J.; Zhang, X. Metal concentrations in fish from nine lakes of Anhui Province and the health risk assessment. Environ. Sci. Pollut. Res. 2020, 27, 20117–20124. [Google Scholar] [CrossRef] [PubMed]
- Resma, N.S.; Meaze, A.M.H.; Hossain, S.; Khandaker, M.U.; Kamal, M.; Deb, N. The presence of toxic metals in popular farmed fish species and estimation of health risks through their consumption. Phys. Open 2020, 5, 100052. [Google Scholar] [CrossRef]
- Rakib, M.R.J.; Jolly, Y.; Enyoh, C.E.; Khandaker, M.U.; Hossain, M.B.; Akther, S.; Alsubaie, A.; Almalki, A.S.; Bradley, D. Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci. Rep. 2021, 11, 14642. [Google Scholar] [CrossRef]
- Ghosh, P.; Ahmed, Z.; Alam, R.; Begum, B.A.; Akter, S.; Jolly, Y.N. Bioaccumulation of metals in selected cultured fish species and human health risk assessment: A study in Mymensingh Sadar Upazila, Bangladesh. Stoch. Environ. Res. Risk Assess. 2021, 35, 2287–2301. [Google Scholar] [CrossRef]
- Yin, X.; Martineau, C.; Demers, I.; Basiliko, N.; Fenton, N.J. The potential environmental risks associated with the development of rare earth element production in Canada. Environ. Rev. 2021, 29, 354–377. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Alam, I.; Khattak, M.N.K.; Mulk, S.; Dawar, F.U.; Shahi, L.; Ihsanullah, I. Heavy metals assessment in water, sediments, algae and two fish species from river swat, Pakistan. Bull. Environ. Contam. Toxicol. 2020, 105, 546–552. [Google Scholar] [CrossRef]
- Alam, M.; Rohani, M.F.; Hossain, M.S. Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerg. Contam. 2023, 9, 100254. [Google Scholar] [CrossRef]
- Bazarsadueva, S.V.; Shiretorova, V.G.; Nikitina, E.P.; Zhigzhitzhapova, S.V.; Taraskin, V.V.; Bazarzhapov, T.Z.; Dong, S.; Radnaeva, L.D. Heavy Metal Content in Fish of the Barguzin River (Eastern Cisbaikalia) and Assessment of Potential Risks to Human Health. Water 2023, 15, 3710. [Google Scholar] [CrossRef]
- Abdel-Kader, H.; Mourad, M. Estimation of tilapia fish quality in Lake Edku through physiological analyses regarding trace element accumulation, antioxidant enzymes, proximate composition, and human health risk assessment as the ultimate consumer. Egypt. J. Aquat. Biol. Fish. 2021, 25, 447–463. [Google Scholar] [CrossRef]
- Varol, M.; Sünbül, M.R. Multiple approaches to assess human health risks from carcinogenic and non-carcinogenic metals via consumption of five fish species from a large reservoir in Turkey. Sci. Total Environ. 2018, 633, 684–694. [Google Scholar] [CrossRef]
- Weber, P.; Behr, E.R.; Knorr, C.D.L.; Vendruscolo, D.S.; Flores, E.M.; Dressler, V.L.; Baldisserotto, B. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J. 2013, 106, 61–66. [Google Scholar] [CrossRef]
- Griboff, J.; Wunderlin, D.A.; Horacek, M.; Monferrán, M.V. Seasonal variations on trace element bioaccumulation and trophic transfer along a freshwater food chain in Argentina. Environ. Sci. Pollut. Res. 2020, 27, 40664–40678. [Google Scholar] [CrossRef]
- Badoni, P.; Nazir, I.; Aier, M.; Maity, P.B.; Samanta, S.; Das, A. Significant Role of Fish Nutrients with Special Emphasis to Essential Fatty Acid in Human Nutrition. Int. J. Curr. Microbiol. Appl. Sci 2021, 10, 2034–2046. [Google Scholar]
- Barry, A.R.; Dixon, D.L. Omega-3 fatty acids for the prevention of atherosclerotic cardiovascular disease. Pharmacotherapy 2021, 41, 1056–1065. [Google Scholar] [CrossRef]
- Ai, L.; Ma, B.; Shao, S.; Zhang, L. Heavy metals in Chinese freshwater fish: Levels, regional distribution, sources and health risk assessment. Sci. Total Environ. 2022, 853, 158455. [Google Scholar] [CrossRef]
- FAO Joint; World Health Organization; WHO Expert Committee on Food Additives. Evaluation of Certain Contaminants in Food: Seventy-Second [72nd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Rahman, M.M.; Haque, T.; Mahmud, A.; Al Amin, M.; Hossain, M.S.; Hasan, M.Y.; Shaibur, M.R.; Hossain, S.; Hossain, M.A.; Bai, L. Drinking water quality assessment based on index values incorporating WHO guidelines and Bangladesh standards. Phys. Chem. Earth Parts A/B/C 2023, 129, 103353. [Google Scholar] [CrossRef]
- Wasana, H.M.; Perera, G.D.; Gunawardena, P.D.S.; Fernando, P.S.; Bandara, J. WHO water quality standards vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, 42516. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, L.; Qu, Z.; Wang, C.; Yang, Z. Effects on heavy metal accumulation in freshwater fishes: Species, tissues, and sizes. Environ. Sci. Pollut. Res. 2017, 24, 9379–9386. [Google Scholar] [CrossRef] [PubMed]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B. Health risk assessment of heavy metals and lipid quality indexes in freshwater fish from lakes of Warmia and Mazury region, Poland. Int. J. Environ. Res. Public Health 2019, 16, 3780. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Pietrzak-Fiećko, R.; Purkiewicz, A.; Łuczyński, M.J. Assessment of fish quality based on the content of heavy metals. Int. J. Environ. Res. Public Health 2022, 19, 2307. [Google Scholar] [CrossRef]
- Zaghloul, G.Y.; Eissa, H.A.; Zaghloul, A.Y.; Kelany, M.S.; Hamed, M.A.; Moselhy, K.M.E. Impact of some heavy metal accumulation in different organs on fish quality from Bardawil Lake and human health risks assessment. Geochem. Trans. 2024, 25, 1. [Google Scholar] [CrossRef]
- Jabeen, G.; Javed, M.; Azmat, H. Assessment of heavy metals in the fish collected from the river Ravi, Pakistan. Pak. Vet. J. 2012, 32, 107–111. [Google Scholar]
- Kumar, A.; Kumar, A.; Jha, S.K. Distribution and bioaccumulation of heavy metal in water, sediment and fish tissue from the River Mahananda in Seemanchal zone, North Bihar, India. Int. J. Aquat. Biol. 2020, 8, 109–125. [Google Scholar]
- Mastan, S. Heavy metals concentration in various tissues of two freshwater fishes, Labeo rohita and Channa striatus. Afr. J. Environ. Sci. Technol. 2014, 8, 166–170. [Google Scholar]
- Maurya, P.K.; Malik, D. Bioaccumulation of heavy metals in tissues of selected fish species from Ganga river, India, and risk assessment for human health. Hum. Ecol. Risk Assess. 2019, 25, 905–923. [Google Scholar] [CrossRef]
- Sofia, S.; Teresa, M. Seasonal variations in the biochemical composition and bio accumulation of metals in selected fishes of Chirackal, Ernakulam district, Kerala. J. Pharmacogn. Phytochem. 2019, 8, 2839–2849. [Google Scholar]
- Kumar, M.; Gupta, N.; Ratn, A.; Awasthi, Y.; Prasad, R.; Trivedi, A.; Trivedi, S.P. Biomonitoring of heavy metals in river ganga water, sediments, plant, and fishes of different trophic levels. Biol. Trace Elem. Res. 2020, 193, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Javed, M.; Khan, H.; Khalil-ur-Rahman, K. Toxicity and bioaccumulation of metals (Al and Co) in three economically important carnivorous fish species of Pakistan. Int. J. Agric. Biol. 2018, 20, 1123–1128. [Google Scholar]
- Ashraf, M.; Zafar, A.; Naeem, M. Comparative Studies on the Seasonal Variations in the Nutritional Values of Three Carnivorous Fish Species. Int. J. Agric. Biol. 2011, 13, 701–706. [Google Scholar]
- Rind, K.H.; Aslam, S.; Memon, N.H.; Raza, A.; Saeed, M.Q.; Mushtaq, A.; Ujan, J.A.; Habib, S.F.; Al-Rejaie, S.S.; Mohany, M. Heavy metal concentrations in water, sediment, and fish species in Chashma Barrage, Indus River: A comprehensive health risk assessment. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).