Abstract
The study aims to contribute valuable insights into the potential applications of the photocatalyst, particularly in the realms of sustainable energy and environmental remediation. Here, Zn-doped NiO nanoparticles with different mole percentages of zinc ingredients are produced and analyzed. Synthesized Zn-doped NiO nanoparticles were evaluated structurally, optically, morphologically, elementally, and photocatalytically. According to X-ray diffraction analysis, cubic NiO and hexagonal Zn-doped cubic NiO nanoparticles were formed, and Fourier transform infrared spectroscopy revealed metal dopants and metal-oxygen stretching, as well as Zn substitution and stabilization. A UV analysis revealed that zinc dopants reduced visible light absorption and bandgap. A decrease in bandgap indicates the importance of zinc incorporation and its interface with NiO. Electron scanning microscopy and transmission electron microscopy confirmed that the nanoparticles exhibited quasi-spherical morphologies and contained Ni, Zn, and O elements. Photocatalytic activity of the synthesized Zn-doped NiO nanoparticles increased with increasing Zn content, achieving a maximum at 8% Zn doping into NiO lattices of 92%. Through XPS analysis, the valencies of Zn, Ni, and O elements are demonstrated, as well as electron movements and bonding between the atoms. The zinc dopants on the metal oxide surface led to charge separation and radical reactions, resulting in enhanced degradation of phorate, salbutamol, and rhoda mine B activities. Hence, Zn-doped NiO nanoparticles are proposed as effective photocatalysts for environmental remediation. The findings are expected to have implications for advancing the field of photocatalysis and addressing challenges related to pollution and energy sustainability.
1. Introduction
There is a significant problem with water scarcity in many regions of the world, and with the growing population, freshwater demand is growing, creating even greater challenges. In order to ensure environmental sustainability and provide safe drinking water, wastewater containing various contaminants must be treated [1,2,3]. The advancements in agriculture, medicine, energy production, and chemical industries have created a significant predicament for the modern world: environmental contamination and water scarcity [4,5]. Pollutants discharged into the atmosphere, water bodies, and soil have pernicious effects on human beings, plants, and animals [6,7]. Rhodamine B, a synthetic dye commonly used in the textile, paper, and printing industries, deteriorates water quality, harms aquatic life, and inhibits light transmission [8,9,10]. Various methods have been explored to eliminate such contaminants, including coagulation, electrochemical oxidation, chemical synthesis, reverse osmosis, adsorption, and photocatalysis [11,12,13]. However, traditional techniques are insufficient due to the vast volume of effluent that requires treatment. There is a wide range of applications for semiconductive metal oxides such as TiO2, ZnO, NiO, and Ag2CrO4, due to their unique properties [14,15,16,17]. Their catalytic activity and stability make them suitable candidates for developing sustainable technologies for various industrial uses. There are several methods for synthesizing NiO nanoparticles, a p-type semiconductor with a wide band gap that has shown promise as a photocatalyst [18,19,20]. The introduction of transition metal ions into metal oxides through doping creates additional energy levels in the band gap, increasing the absorption of visible light and promoting the separation of electron–hole pairs generated by photoexcitation. This leads to an overall enhancement in photocatalytic activity. Researchers are using these innovative techniques to develop sustainable solutions to water scarcity and environmental contamination. The photocatalytic activity of metal oxides can be enhanced by transition metal ion dopants under visible light illumination. The photocatalytic activity of Fe-doped TiO2 and ZnO has been reported to be greater than that of their undoped counterparts [21,22]. Transition metals, such as copper (Cu), chromium (Cr), and vanadium (V), have been studied for their potential to enhance the photocatalytic performance of metal oxides [23,24,25]. A photocatalytic system that harnesses visible light is crucial to producing clean energy and restoring the environment. When transition metal ions are included as dopants, they affect the interfacial charge transfer mechanisms and the recombination of electron–hole pairs, which can influence a material’s photocatalytic properties [26,27]. These dopants may act as electron or hole traps, thereby facilitating charge transfer. The degree of metal dispersion on the particle surface and the uniformity of the chemical composition within each particle can be significantly influenced by the doping techniques used and the temperature during calcination. These elements contribute to a wide array of structural and crystalline characteristics. As a p-type transition metal, zinc (Zn) holds the potential to enhance the photocurrent in dye-sensitized solar cells (DSSC) when combined with titanium dioxide (TiO2). As a result of the combination of a p-type semiconductor (Zn) with an n-type semiconductor (TiO2), the solar cell’s overall functionality is enhanced [28]. It enhances the photocatalytic performance of TiO2 across the visible spectrum by reducing its bandgap. It has been demonstrated that the introduction of zinc ions (Zn) into the anatase structure of TiO2 can raise the conduction band boundary of the photoanode, resulting in a shift in the Fermi level. As a result of this shift, open-circuit voltage (VOC) and overall photovoltaic performance can be improved [28]. Due to their distinct disadvantages in aquatic systems, salbutamol and phorate pose environmental concerns. In aquatic environments, salbutamol accumulates and is prolonged exposure, potentially harmful to aquatic organisms. The bioaccumulation of the substance at higher trophic levels is of particular concern since it persists and may disrupt organisms’ normal behavior, growth, and reproduction. Additionally, salbutamol can form transformation products with potentially different toxicological properties during degradation, necessitating a comprehensive understanding of their environmental fate. However, phorate, which is highly toxic to aquatic life, raises serious ecological concerns. Due to its persistence and bioaccumulation, it gradually moves up the food chain, posing risks to higher trophic levels. Agricultural use of phorate can cause water contamination through runoff, affecting aquatic ecosystems nearby, and its ability to leach into groundwater increases the risk of drinking water contamination for years to come. Salbutamol and phorate emphasize the importance of sustainable practices and careful consideration of their impact on aquatic ecosystems [29,30].
In this study, a simple chemical synthesis technique is used to prepare Zn-doped NiO nanoparticles using a direct solvothermal method. As compared to other synthesis methods, solvothermal stands out for its ease and efficiency in generating nanostructures. Zn2+ substitution modifies NiO structure and bandgap, which can reduce the gap between bonds and increase visible light absorption. Furthermore, divalent metal dopants promote reactive oxygen species (ROS) and radical proliferation. The Zn-NiO nanoparticles are synthesized and characterized for their potential applications in the removal of environmental pollutants, namely rhodamine B (Rh-B) dye, phorate, and salbutamol.
2. Materials and Methods
2.1. Materials
The synthesis process involved employing nickel (II) acetate tetrahydrate (C4H6NiO4·4H2O), 99.9% purity, zinc nitrate hexahydrate (Zn(NO3)2·6H2O), NaOH, ammonia solution, and rhodamine B (C28H31ClN2O3), 99.9% purity. These chemicals were procured from HiMedia in Mumbai, India; phorate and salbutamol chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), and all of them met the standards of analytical reagent (AR) grade. These compounds were used in their original, unaltered states, and double-distilled water was employed for both the solution preparation and reaction processes.
2.2. Synthesis of Zn-Doped NiO NPs
The 0.3 M nickel acetate and 3 mL ammonia were mixed by continuous stirring using a magnetic stirrer at 750 rpm. Subsequently, varying mole percentages of a solution containing zinc nitrate hexahydrate were systematically introduced, including 2%, 5%, and 8% of Zn concentrations. To attain a solution pH of 12, a 0.3 M NaOH solution was slowly added. The resulting solution was then heated at 80 °C while being stirred for 6 h, leading to the formation of a precipitate with a bluish-green hue. This precipitate underwent several washes using distilled water and ethanol to eliminate any residual by-products. The collected nanoparticle solution was dried at 100 °C for 24 h, and their output powder was stored for further processing. The dried powder was subsequently subjected to a calcination process at 400 °C for 2 h. The application of heat induced a transformation and modification in the lattice structure of NiO through Zn substitutions, influencing the crystal lattice and thereby altering the material’s properties. These nanoparticles exhibited a distinct black color, thus yielding a nanopowder with these specific characteristics. The same procedure was duplicated to form the nanostructures of nickel oxide doped with zinc. In this case, zinc nitrate solution was added to the precursor solution at concentrations of 2%, 5%, and 8% of Zn [31,32].
2.3. Characterization of Synthesized NPs
The material phase and crystallinity of the synthesized nanoparticles were examined using the PANalytical X’Pert Pro X-ray diffractometer (Malvern Panalytical, Malvern, UK). To identify chemical interactions and functional groups, FT-IR Perkin Elmer spectroscopy (PerkinElmer Inc., Buckinghamshire, UK) was used. The optical properties of the materials were analyzed with UV-visible spectroscopy, specifically employing the UV-2600 Shimadzu instrument (Shimadzu Corporation, Kyoto, Japan). The surface morphology and elemental composition of the samples were investigated by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) using a Carl Zeiss instrument (Jena, Germany), as well as transmission electron microscopy (FEI-Titan 80-300, TEM, Hillsboro, OR, USA). The valency and binding energy of the synthesized nanoparticles were determined by X-ray photoelectron spectroscopy (XPS) with the PHI 5000 VersaProbe III measurement from the Chanhassen, MN, USA [33,34,35].
2.4. Pollutants Photocatalytic Degradation Experiment
To evaluate the photocatalytic performance of the Zn-doped NiO nanoparticles in the presence of Rh-B dye, phorate, and salbutamol, an experiment was conducted using visible light. An initial Rh-B dye solution (40 mg/L) was mixed with 10 mg of nanoparticles in a 100 mL container. The same condition was repeated for phorate and salbutamol contaminants. The mixture was allowed to reach an adsorption-desorption equilibrium in darkness and was then stirred for 30 min. Afterward, the solution was exposed to visible light at a wavelength above 400 nm, generated by a Xenon lamp. The illuminated nanoparticle solution was subjected to absorbance measurements at regular 10-min intervals using UV spectroscopy, with 3 mL of the solution being withdrawn each time. Before measuring absorbance, the samples were centrifuged at a speed of 10,000 revolutions per minute to facilitate the separation of nanoparticles from the polluted solution. The degradation efficiency of the nanoparticles was calculated using the formula provided in reference [36].
where Co represents the initial pollutant absorbance concentration and Ct represents the active pollutant absorbance concentration at 10-min intervals.
Dye degradation (%) = (Co − Ct)/Co × 100
3. Results and Discussions
3.1. XRD Analysis
The X-ray diffraction (XRD) analysis of 2%, 5%, and 8% of zinc-doped nickel oxide nanoparticles typically shows characteristic peaks that correspond to the crystalline materials (Figure 1a–c). The most common crystal structure of nickel oxide is cubic with a rock salt lattice, while zinc oxide has a hexagonal wurtzite structure. The doping of nickel oxide with zinc ions can lead to changes in the crystal structure and lattice parameters, as well as changes in the position, intensity, and width of the XRD peaks. The pronounced diffraction peaks of Zn-doped NiO nanoparticles at 2 theta values are 43.8, 62.3, and 76.8 degrees, which correspond to planes of (111), (200), and (220), respectively. These diffraction peaks closely matched the standard values of JCPDS: 78-0643 for nickel oxide [37,38], indicating the formation of crystalline zinc-doped nickel oxide nanoparticles. The strength of the diffraction patterns reduced with the addition of zinc dopants, suggesting that the incorporation of zinc ions may have caused some distortion in the crystal structure of nickel oxide, leading to a decrease in its crystallinity. Specifically, the crystallinity percentages were measured as follows: 52% for 2% Zn-NiO, 60% for 5% Zn-NiO, and 72% for 8% Zn-NiO. Nonetheless, the observed diffraction peaks confirmed the successful synthesis of zinc-doped nickel oxide nanoparticles with a crystalline structure. The presence and position of these peaks can be used to fix the crystal structure and lattice parameters of the Zn-NiO nanoparticles [39,40]. The decrease in the intensity of the XRD peaks for NiO after the addition of zinc dopants can be attributed to the incorporation of zinc ions into the crystal lattice of NiO. Zinc ions are known to have a smaller ionic radius than nickel ions, and therefore, the crystal structure of NiO can be modified because of their lattice distortion and orientation of crystal structure [37,38,39,40].
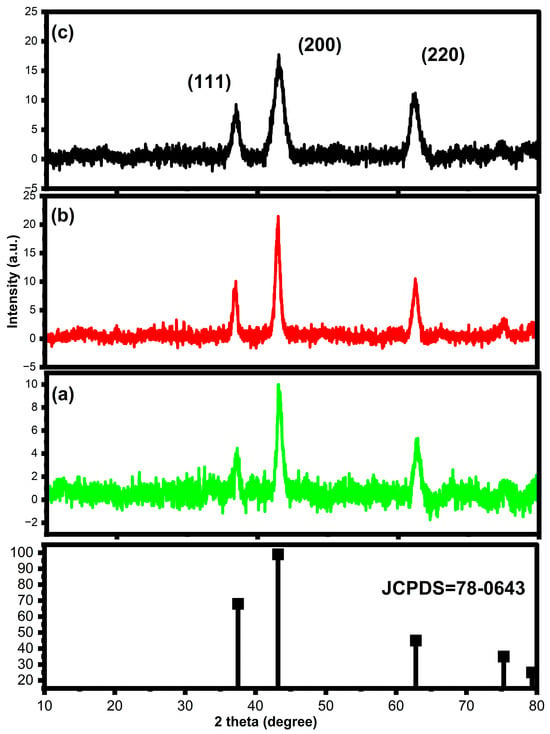
Figure 1.
JCPDS spectra of NiO and different mole percentages of (2%, 5%, and 8%) Zn-doped NiO nanoparticles (a,b) X-ray diffraction patterns.
The Scherrer formula helps to calculate the crystallite size of Zn-NiO nanoparticles, and their resulting values were 17, 16, and 15 nm for 2%, 5%, and 8% concentrations of zinc doping, respectively. The addition of zinc dopants to nickel oxide (NiO) leads to a reduction in the crystallite size of the resulting zinc-doped nickel oxide nanoparticles. The incorporation of zinc ions into the NiO can cause lattice alteration and tension in the crystal structure of the material. This strain can result in the formation of defects and vacancies within the crystal structure, which can inhibit the growth of the crystal and lead to a reduction in the crystallite size [41,42]. The decrease in crystallite size of zinc-doped NiO nanoparticles can have significant impacts on the material properties and performance. Smaller crystallite sizes can increase the surface area of the material, leading to higher reactivity and improved catalytic performance. However, smaller crystallite sizes can also result in decreased stability and mechanical strength of the material. Therefore, controlling the crystallite size of zinc-doped NiO nanoparticles is an important consideration in the design and optimization of these materials for various applications.
3.2. FTIR Analysis
The FTIR spectra of 2%, 5%, and 8% of Zn-NiO NPs (Figure 2a–c) exhibit the various functional groups and vibrational modes present in the nanoparticles. The broad absorption peak at around 2922–3436 cm−1 corresponds to the stretching vibration of hydroxyl (OH) groups, which are commonly found on the surface of metal oxide nanoparticles [43,44]. The absorption peak at about 1625 cm−1 links to the bending vibration of water molecules adsorbed on the nanoparticle surface [45]. The absorption peak at around 1033–1400 cm−1 corresponds to the bending vibration of carbonate (CO32−) groups, which can be present as impurities in the nanoparticles [46,47]. The absorption peak at around 600–775 cm−1 corresponds to the stretching vibration of metal-oxygen (Ni-O) bonds in the nanoparticles [48]. The absorption peak at around 420–600 cm−1 corresponds to the bending vibration of Ni-O-Zn bonds in the nanoparticles, and it can motivate the single phase of the NiO nanostructure [49,50]. FTIR results provide insights into the adsorption and desorption of molecules on the nanoparticle surface. It can be used in corroboration with other techniques, such as XRD and TEM, to understand the material’s properties.
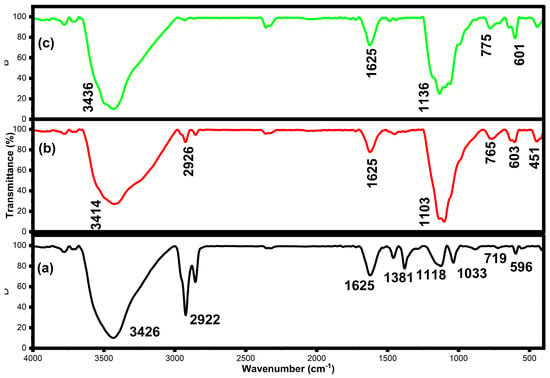
Figure 2.
FTIR spectra of NiO NPs doped with 2% (a), 5% (b), and 8% (c) Zn.
3.3. UV-Visible Analysis
Figure 3a,b displays the UV absorbance and Tauc plot bandgap spectra for the synthesized Zn-NiO NPs, with varying dopant concentrations of 2%, 5%, and 8%. An evident decline in absorbance is observed in these spectra, which can be attributed to the accumulation of electrons within the NiO lattice as a consequence of Zn dopant integration. The shift in absorbance toward the visible spectrum implies that the nanoparticles’ bandgap decreased after Zn doping. Specifically, the calculated bandgap values were determined to be 3.28 eV, 3.05 eV, and 2.86 eV for nanoparticles doped with 2%, 5%, and 8% of zinc, respectively. The reduction in NiO nanoparticles’ bandgap energy due to Zn doping can be ascribed to the adjustments in the band structure induced by doping. This leads to the creation of defect states within the bandgap, resulting in a narrower bandgap [51,52,53,54,55]. Furthermore, the inclusion of Zn dopants into the NiO lattice generates a new impurity energy level near the valence band maximum, reducing the effective bandgap and consequently enhancing the photocatalytic activity of the nanoparticles.
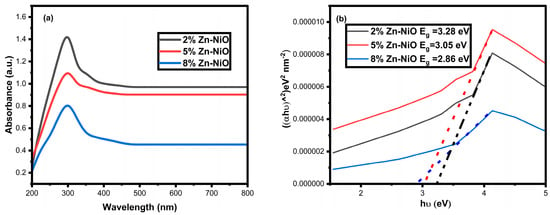
Figure 3.
UV absorbance spectra and Tauc plot bandgap graphs of synthesized NiO NPs doped with 2%, 5%, and 8% Zn.
3.4. SEM with EDX Analysis
Scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) is a powerful method to examine the morphology and elemental structure of the 2%, 5%, and 8% of Zn-NiO NPs, as shown in Figure 4. In the case of Zn-NiO NPs, SEM images revealed that the synthesized nanoparticles have a flower-like, spherical, and aggregated morphology. This morphology is due to the self-assembling behavior of nanoparticles during the synthesis process. The flower-like morphology of Zn-NiO NPs is due to the agglomeration of small nanoparticles into a larger flower-like structure. The aggregated morphology is due to the agglomeration of larger nanoparticles into even larger structures.
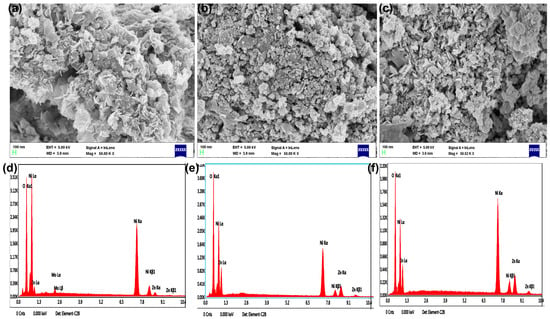
Figure 4.
SEM (a–c) with EDX (d–f) images of synthesized NiO NPs doped with 2%, 5%, and 8% Zn.
The aggregated nanoparticles form clusters of individual nanoparticles that are bound together by relatively weak forces, such as van der Waals forces or electrostatic interactions. Aggregation can occur during the synthesis or handling of nanoparticles and can lead to changes in their properties, such as a decrease in specific surface area and an increase in size distribution [55,56,57,58]. However, aggregation can also have beneficial effects, such as the formation of porous or hierarchical structures, which can enhance catalytic activity or light absorption properties. The EDX analysis of the Zn-NiO NPs confirmed the presence of nickel, zinc, and oxygen elements in the synthesized nanoparticles. The presence of zinc in the EDX analysis indicates the successful doping of NiO NPs with zinc.
The SEM morphology images reveal the prime existence of spherical and semi-spherical Zn-NiO nanoparticles. A substantial portion of these nanoparticles exhibit a flower-like, spherical, and semi-spherical morphology, forming bigger grain-sized particles due to reaction derivatives. The excess reactive compounds create a layer on the NiO surface, facilitating their integration with Zn and the agglomeration of particles. Subsequently, the particles exhibit a variety of shapes, including flowers, semi-spheres, and spheres. The distribution of Zn-NiO nanoparticles was quantified using ImageJ software version 6.0, and the corresponding graphs are presented in Figure 5. Zinc doping in NiO NPs enhances the photocatalytic properties of NiO NPs due to the generation of additional defects and oxygen vacancies that promote electron–hole pair separation and the formation of reactive oxygen species.
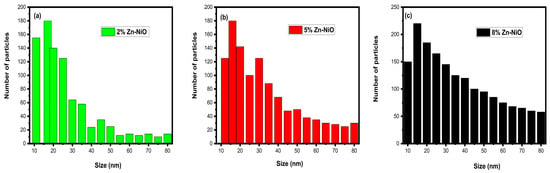
Figure 5.
Histogram (a–c) images of synthesized NiO NPs doped with 2%, 5%, and 8% Zn.
3.5. TEM with SAED Pattern Analysis
Transmission electron microscopy (TEM) analysis is used to provide insights into the dimensions, morphology, and spatial arrangement of a subset comprising 8% of zinc-doped nickel oxide nanoparticles. The observation of a spherical morphology is frequently encountered in various materials, such as nanoparticles of zinc-doped nickel oxide (Figure 6a). Spherical nanoparticles exhibit a notable surface area-to-volume ratio, augmenting their reactivity and rendering them valuable in diverse domains, including catalysis, drug administration, and sensing [59,60]. To achieve a spherical morphology in Zn-NiO NPs, it is feasible to control the synthesis parameters in order to facilitate the aggregation of nanoparticles into spherical clusters. The reaction of NaOH and ammonia can facilitate the modification of nanoparticle growth into a spherical configuration, while the regulation of reaction conditions, such as pH and temperature, can effectively hinder the development of other morphologies. The application of selected area electron diffraction (SAED) patterns serves to derive both the crystal structure and the alignment of the nanoparticles. The SAED patterns (Figure 6b) have the ability to unveil the typical crystal structure of nanoparticles, often associated with a spinel structure. These diffraction patterns may display diffraction spots corresponding to the (111), (200), and (220) crystallographic planes, which are indicative of the spinel structure. The presence and intensity of these diffraction spots can provide valuable insights into the crystal alignment, grain size, and imperfections within the nanoparticles. The use of transmission electron microscopy (TEM) and selected area electron diffraction (SAED) techniques in the study of zinc-doped nickel oxide nanoparticles can offer substantial insights into their morphological attributes, crystal structure, and orientation. The structural information is essential for comprehending the characteristics of Zn-doped NiO nanoparticles and enhancing their effectiveness in various applications.
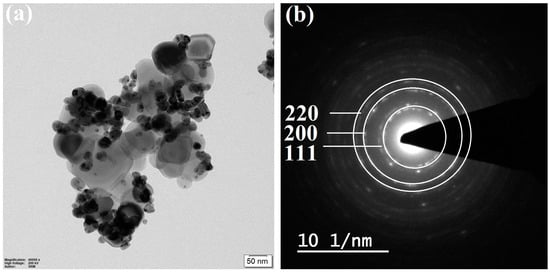
Figure 6.
TEM image (a) and SAED pattern (b) of synthesized 8% of Zn-NiO nanoparticles.
3.6. XPS Analysis
The surface material compositions, valency, and binding energy of the 8% Zn-doped NiO nanoparticles were examined by XPS analysis, as shown in Figure 7. The wide spectrum (Figure 7a) confirmed the synthesized Zn-doped NiO nanoparticles with the existence of Zn, Ni, and O elements. The Ni region exhibited peaks at 854.07 eV and 872.71 eV, which indicate the chemical states of Ni-2p3/2 and Ni-2p1/2, respectively. The remaining peaks of the Ni-2p region are attributed to the formation of Ni2+/Ni3+ and Ni(OH)2. Moreover, additional peaks in the Ni-2p state represent Ni vacancies [61]. The Zn-2p state showed peaks at 1020.84 eV (Zn-2p3/2) and 1043.92 eV (Zn-2p1/2). The spin-orbit coupling of Zn-2p is 23.08 eV, which promotes the bonding between the oxygen [62,63]. In addition, the Zn-2p state modified the electronic structure of the Ni-2p state and ionization energy. The p-type Ni2+/Ni3+ vacancies are altered by Zn dopants due to their p-type Ni material quasi-localized holes, increasing their density by the substitution of Zn-2p state materials [61]. The O-1s spectrum shows peaks at 529.81 eV, which denote the absorbed oxygen associated with Zn and Ni elements [62,63]. The primary formation of Ni-O bonds was confirmed by the peaks at 531.20 eV. The Ni2+ ions were neutralized by O-1s, and Ni and Zn elements shared their localized space with oxygen and established the bond of Zn-Ni-O, which provides the construction of Zn-doped NiO nanoparticles.
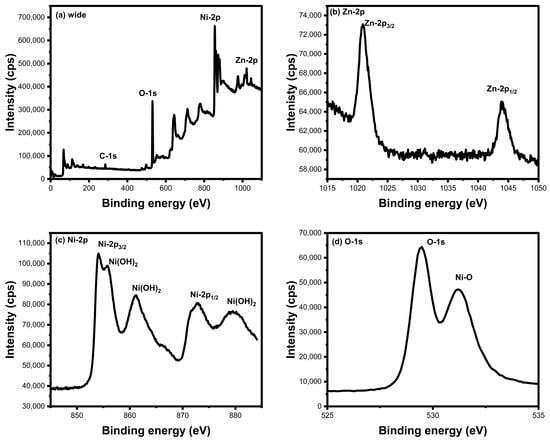
Figure 7.
X-ray photoelectron spectroscopy of synthesized 8% Zn-NiO NPs (a) wide, (b) Zn, (c) Ni, and (d) O spectrum.
3.7. Pollutants Photocatalytic Degradation Analysis
The visible light photocatalytic activity of synthesized 2%, 5%, and 8% Zn-NiO NPs on Rh-B dye, phorate, and salbutamol degradation was evaluated in this work. During this process, the toxic molecules undergo a series of chemical reactions and changes, leading to a shift in their absorption wavelength and a decrease in their absorbance. The shift in the absorption wavelength is due to the formation of intermediate products during the degradation process, which have different chemical structures and optical properties than the parent dye molecule (Figure 8a–c). These intermediates may absorb light at different wavelengths than the original dye, leading to a shift in the absorption spectrum. The decrease in absorbance is a result of the degradation of the dye molecule, which leads to the formation of smaller molecules with lower molar extinction coefficients [64,65]. As the concentration of the dye decreases during the degradation process, the absorbance of the dye solution also decreases.
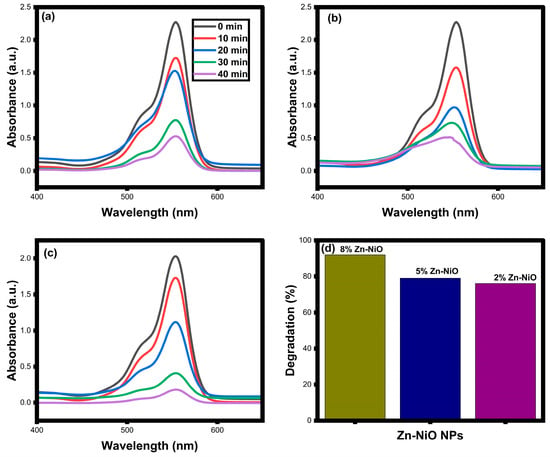
Figure 8.
Photocatalytic dye degradation absorbance spectra (a–c) and degradation efficiency (d) of synthesized 2%, 5%, and 8% Zn-NiO NPs.
The results showed that the addition of zinc to the NiO nanoparticles enhanced their photocatalytic activity, with 8% Zn-NiO nanoparticles exhibiting the highest degradation efficiency of 92% after 40 min (Figure 8d). When zinc was used as a dopant for NiO, an enhancement of radical generation and an increase in surface area were obtained. This, in turn, led to a higher degree of oxidation and reduction during the process involving the Rh-B dye and also reduced the occurrence of fast recombination. As a result of this process, a substantial amount of OH radicals and superoxide ions were generated. These highly reactive species effectively attacked the dye molecules, breaking them down into smaller, harmless molecules such as CO2 and H2O. Both the small molecules and the original dye molecules are considered environmentally safe compounds.
The degradation mechanism is a multi-step process that involves the generation of charge carriers (electrons and holes) when Zn-NiO is exposed to light. The holes on the valence band (VB) produced hydroxyl radicals (OH*) upon interaction with OH-, while the electrons on the conduction band (CB) reacted with O2 to form O2−. The O2− then reacted with H2O to produce HO2* and OH−. The HO2* and OH* radicals produced by these reactions reacted with Rh-B to form supplementary products, CO2 and H2O [66,67,68,69,70]. The pseudo-first-order kinetics and degradation mechanism are presented in Figure 9. The resulting degradation pathway can be summarized as follows:
Zn-NiO + hν → h+VB + e−CB
OH− + h+VB → OH*
O2 + e−CB− → O2−*
O2−* + H2O → HO2* + OH−
HO2* + OH− + h+VB → OH*
Rh-B + OH*/O* → Rh-B* (additional products) + H2O + CO2
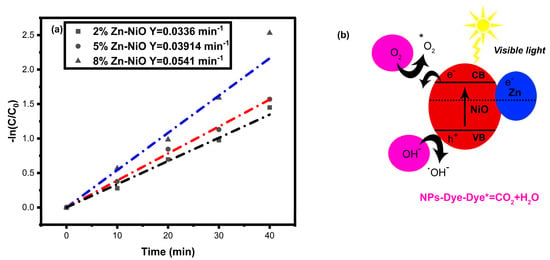
Figure 9.
Photocatalytic dye degradation kinetic graph (a) and mechanism (b) of synthesized Zn-NiO NPs.
The photocatalytic performance of Zn-NiO nanoparticles was assessed in comparison to a diverse array of metal oxide and metal-doped metal oxide nanoparticles reported in the literature (Table 1). The approach of enhancing the photocatalytic capabilities of metal oxide nanoparticles through targeted metal incorporation represented a significant breakthrough. This method effectively surmounted the inherent limitations of metal oxide nanoparticles, resulting in enhanced catalytic performance. An innovative approach involved the introduction of metal doping or decoration into metal oxide nanoparticles. This alteration led to a simultaneous reduction in bandgap energy and particle size, augmenting the nanoparticles’ ability to absorb visible light [71,72,73,74,75,76]. The transformative impacts of noble metal decoration, doping, or coating were pivotal. These adjustments were instrumental in significantly increasing the nanoparticles’ surface area, giving rise to the generation of “hot electrons” driven by plasmonic resonance. This intriguing phenomenon, involving the plasmonic resonance-induced excitation of surface electrons, markedly improved catalytic efficiency. Importantly, this phenomenon outperformed other methods of metal decoration, ushering in an era of heightened effectiveness. The research, in particular, spotlighted the exceptional catalytic activity exhibited by Zn-NiO NPs. When compared to both conventional and noble metal embellishments, these nanoparticles demonstrated superior performance when applied to NiO surfaces.

Table 1.
Photocatalytic properties of undoped and metal-doped metal oxides nanoparticles.
The research discoveries expose a positive correlation between the concentration of Zn doping in NiO nanoparticles and the efficiency of degrading both phorate and salbutamol, as shown in Figure 10. Phorate: increasing Zn doping from 2% to 8% results in a steady enhancement of degradation efficiency from 44% to a peak of 72%. This improvement suggests that higher Zn concentrations positively impact catalytic activity, surface area, or electron transfer properties in NiO nanoparticles. Similarly, for salbutamol, the degradation efficiency follows a similar trend, with 2%, 5%, and 8% Zn doping leading to efficiencies of 55%, 62%, and 70%, respectively. The study underscores the tunability of degradation efficiency in Zn-doped NiO nanoparticles by adjusting Zn doping concentrations, offering promising prospects for designing efficient catalysts in environmental remediation and water treatment applications [77,78,79,80]. Further exploration into the specific mechanisms and roles of Zn in these processes is warranted for a comprehensive understanding and targeted development of catalysts for pollutant removal, addressing concerns related to both agricultural and pharmaceutical contaminants in water systems. This remarkable finding underscores the substantial potential of Zn-NiO nanoparticles as a leading photocatalytic material, especially in the degradation of Rhodamine B dye under visible light. Compared to Rh-B, phorate, and palbutamol contaminants, RhB dye exhibited better degradation potential than phorate and salbutamol compounds. The significance of these Zn-doped NiO nanoparticles is underscored by emphasizing the broader implications of these discoveries in the realms of environmental preservation, sustainable energy, and wastewater treatment.
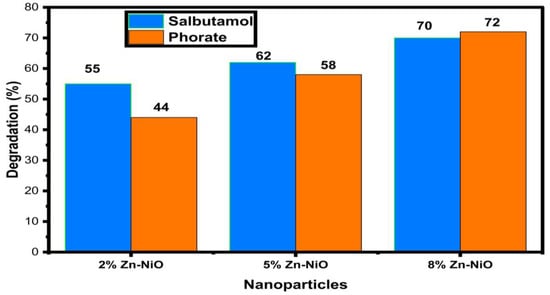
Figure 10.
Photocatalytic degradation of synthesized Zn-NiO NPs on Salbutamol and Phorate.
4. Conclusions
The chemically synthesized zinc-doped nickel oxide nanoparticles showed promising results for the production of efficient photocatalysts. Zinc oxide and replaced nickel oxide produced cubic-phase nanoparticles with reduced bandgaps and altered multivalency of nickel lattices. The spherical morphology of the nanoparticles contributed to their high surface area and photocatalytic activity. Under visible light irradiation, 8% Zn-doped NiO nanoparticles demonstrated a remarkable degradation efficiency of 92% in 40 min and enhanced degradation potential for phorate and salbutamol. A thorough examination of metal-oxygen stretching and substitution of zinc for nickel oxide stretching confirmed the successful synthesis of nanoparticles. Chemical synthesis using conventional methods provided a viable route to synthesize nanoparticles. Nickel oxide nanoparticles doped with zinc have enhanced photocatalytic efficiency, making them potentially useful as environmental remediation agents. Based on the results of this research, zinc-doped nickel oxide nanoparticles have the potential to function as efficient photocatalysts for the removal of organic pollutants and toxins.
Author Contributions
Conceptualization, S.M. (S. Minisha) and A.A.A.; methodology, S.M. (Saikh Mohammad) and W.-C.L.; software, S.M. (S. Minisha), J.K.G. and W.-C.L.; validation, S.A. and W.-C.L.; formal analysis, J.J., A.A.A., J.K.G., A.A.A. and W.-C.L.; investigation, S.M. (S. Minisha), J.K.G. and S.A.; resources, J.J., S.A., A.A.A. and W.-C.L.; data curation, S.M. (Saikh Mohammad) and J.K.G.; writing—original draft, S.M. (S. Minisha); writing—review & editing, S.M. (S. Minisha) and J.J.; visualization, J.J. and S.M. (S. Minisha); supervision, J.J. and W.-C.L.; project administration, S.M. (S. Minisha), J.K.G., S.A. and A.A.A.; funding acquisition, S.M. (S. Minisha), J.K.G., S.A. and W.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Researchers Supporting Project Number (RSP2024R243), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
All research data associated with the manuscript.
Acknowledgments
The authors are grateful to the Researchers Supporting Project Number (RSP2024R243), King Saud University, Riyadh, Saudi Arabia. Minisha drew the thanks to the Department of Physics, Annai Velankani College, Tholayavattam–629157, and Manonmaniam Sundaranar University, Abishekapatti, Tirunelveli-627012, Tamil Nadu, India.
Conflicts of Interest
The author has no conflicts of interest.
References
- Sun, S.; Tang, Q.; Konar, M.; Fang, C.; Liu, H.; Liu, X.; Fu, G. Water transfer infrastructure buffers water scarcity risks to supply chains. Water Res. 2023, 229, 119442. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Z.; Liu, W. Impacts of precipitation variations on agricultural water scarcity under historical and future climate change. J. Hydrol. 2023, 617, 128999. [Google Scholar] [CrossRef]
- Cai, B.; Jiang, L.; Liu, Y.; Zhang, W.; Zhang, Z. The impact of interregional trade on both quantity-and quality-related water scarcity of Beijing-Tianjin-Hebei urban agglomeration in China. Sustain. Cities Soc. 2023, 88, 104270. [Google Scholar] [CrossRef]
- Raj, R.S.; Krishnan, K.A. A comprehensive review on the impact of emerging organophosphorous pesticides and their remedial measures: Special focus on acephate. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100813. [Google Scholar]
- Singh, H.; Pant, G. Phytoremediation: Low input-based ecological approach for sustainable environment. Appl. Water Sci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Daripa, A.; Malav, L.C.; Yadav, D.K.; Chattaraj, S. Metal contamination in water resources due to various anthropogenic activities. In Metals in Water; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–127. [Google Scholar]
- Liu, P.; Dai, J.; Huang, K.; Yang, Z.; Zhang, Z.; Guo, X. Sources of micro (nano) plastics and interaction with co-existing pollutants in wastewater treatment plants. Crit. Rev. Environ. Sci. Technol. 2023, 53, 865–885. [Google Scholar] [CrossRef]
- Xu, M.; Deng, Y.; Li, S.; Zheng, J.; Liu, J.; Tremblay, P.L.; Zhang, T. Bacterial cellulose flakes loaded with Bi2MoO6 nanoparticles and quantum dots for the photodegradation of antibiotic and dye pollutants. Chemosphere 2023, 312, 137249. [Google Scholar] [CrossRef]
- Rajesh, G.; Kumar, P.S.; Akilandeswari, S.; Rangasamy, G.; Lohita, S.; Shankar, V.U.; Ramya, M.; Thirumalai, K. Preparation and characterization of a novel cobalt-substitution cadmium aluminate spinel for the photodegradation of azo dye pollutants. Chemosphere 2023, 323, 138232. [Google Scholar] [CrossRef]
- Reddy, C.V.; Kakarla, R.R.; Cheolho, B.; Shim, J.; Rezakazemi, M.; Aminabhavi, T.M. Highly efficient photodegradation of toxic organic pollutants using Cu-doped V2O5 nanosheets under visible light. Chemosphere 2023, 311, 137015. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Klemeš, J.J.; Bokhari, A.; Wang, C.; Sillanpaa, M.; Amesho, K.T.T.; Vithanage, M. Various advanced wastewater treatment methods to remove microplastics and prevent transmission of SARS-CoV-2 to airborne microplastics. Int. J. Environ. Sci. Technol. 2023, 20, 2229–2246. [Google Scholar] [CrossRef]
- Guerrero-Gualan, D.; Valdez-Castillo, E.; Crisanto-Perrazo, T.; Toulkeridis, T. Methods of Removal of Hormones in Wastewater. Water 2023, 15, 353. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A review on revolutionary technique for phosphate removal in wastewater using green coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Shakil, M.; Inayat, U.; Tanveer, M.; Nabi, G.; Gillani, S.S.A.; Rafique, M.; Tariq, N.H.; Shah, A.; Mahmood, A. NiO and Ag–Cd co-doped NiO nanoparticles: Study of photocatalytic degradation of rhodamine B dye for wastewater treatment. Int. J. Environ. Sci. Technol. 2023, 20, 2021–2036. [Google Scholar] [CrossRef]
- Gatou, M.A.; Lagopati, N.; Vagena, I.A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Chandekar, K.V.; Palanivel, B.; Alkallas, F.H.; Trabelsi, A.B.G.; Khan, A.; Ashraf, I.M.; AlFaify, S.; Shkir, M. Photocatalytic activities of Mg doped NiO NPs for degradation of Methylene blue dye for harmful contaminants: A kinetics, mechanism and recyclability. J. Phys. Chem. Solids 2023, 178, 111345. [Google Scholar] [CrossRef]
- Sudhaik, A.; Hasija, V.; Selvasembian, R.; Ahamad, T.; Singh, A.; Khan, A.A.P.; Raizada, P.; Singh, P. Applications of graphitic carbon nitride-based S-scheme heterojunctions for environmental remediation and energy conversion. Nanofabrication 2023, 8, 1–36. [Google Scholar] [CrossRef]
- Jagan, K.S.G.; Surendhiran, S.; Savitha, S.; Balu, K.S.; Karthick, M.; Vidaarth, T.N.; Karthik, A.; Kalpana, B.; Senthilmurugan, R. Influence of different alkaline actuators in synthesis of NiO NPs: A comparative green approach on photocatalytic and in vitro biological activity. Inorg. Chem. Commun. 2023, 151, 110618. [Google Scholar] [CrossRef]
- Fatimah, I.; Citradewi, P.W.; Purwiandono, G.; Hidayat, H.; Sagadevan, S. Nickel oxide decorated reduced graphene oxide synthesized using single bioreductor of Pometia pinnata leaves extract as photocatalyst in tetracycline photooxidation and antibacterial agent. Inorg. Chem. Commun. 2023, 148, 110287. [Google Scholar] [CrossRef]
- Majeed, K.; Ambreen, J.; Khan, S.A.; Muhammad, S.; Shah, A.A.; Bhatti, M.A.; Batool, S.S.; Farooq, M.; Shah Bukhari, S.N.U.; Chandio, A.D.; et al. Effective Removal of Methylene Blue by Mn3O4/NiO Nanocomposite under Visible Light. Separations 2023, 10, 200. [Google Scholar] [CrossRef]
- Mancuso, A.; Blangetti, N.; Sacco, O.; Freyria, F.S.; Bonelli, B.; Esposito, S.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Crystal Violet Dye under Visible Light by Fe-Doped TiO2 Prepared by Reverse-Micelle Sol–Gel Method. Nanomaterials 2023, 13, 270. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Mottola, S.; Pragliola, S.; Moretta, A.; Vaiano, V.; De Marco, I. Synthesis of Fe-doped ZnO by supercritical antisolvent precipitation for the degradation of azo dyes under visible light. Inorganica Chim. Acta 2023, 549, 121407. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.Y.; Peng, J.F.; Wang, L.H.; Peng, D.Y.; Liu, F.Y.; Xu, S.; Yang, Z.L. Fabrication of Cu2O/ZnTi-LDH pn heterostructure by grafting Cu2O NPs onto the LDH host layers from Cu-doped ZnTi-LDH and insight into the photocatalytic mechanism. Compos. Part B Eng. 2023, 250, 110447. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, M.M.; Akhtar, S. Studies on Synthesis, Characterization, and Photocatalytic Activity of TiO2 and Cr-Doped TiO2 for the Degradation of p-Chlorophenol. ACS Omega 2023, 8, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Singh, A.; Poddar, D.; Jain, P. Fabrication and photocatalytic evaluation of functionalized chitosan decorated vanadium pentoxide nano-adsorbents for water remediation. Ceram. Int. 2023, 49, 8871–8885. [Google Scholar] [CrossRef]
- Perumal, K.; Shanavas, S.; Ahamad, T.; Karthigeyan, A.; Murugakoothan, P. Construction of Ag2CO3/BiOBr/CdS ternary composite photocatalyst with improved visible-light photocatalytic activity on tetracycline molecule degradation. J. Environ. Sci. 2023, 125, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Saadati, A.; Sheibani, S. Modification of a Z-scheme ZnO-CuO nanocomposite by Ag loading as a highly efficient visible light photocatalyst. Mater. Res. Bull. 2023, 158, 112048. [Google Scholar] [CrossRef]
- Saad, S.K.M.; Umar, A.A.; Rahman, M.Y.A.; Salleh, M.M. Porous Zn-doped TiO2 nanowall photoanode: Effect of Zn2+ concentration on the dye-sensitized solar cell performance. Appl. Surf. Sci. 2015, 353, 835–842. [Google Scholar] [CrossRef]
- Bano, N.; Musarrat, J. Isolation and characterization of phorate degrading soil bacteria of environmental and agronomic significance. Lett. Appl. Microbiol. 2003, 36, 349–353. [Google Scholar] [CrossRef]
- Alberti, S.; Rucco, M.; Di Carro, M.; Magi, E.; Ferretti, M.; Benedetti, B. A multidisciplinary approach to the environmental problem of emerging pollution: Synthesis and application of a novel composite photocatalyst and the case study of salbutamol degradation. J. Environ. Chem. Eng. 2023, 11, 110262. [Google Scholar] [CrossRef]
- Wojnarovits, L.; Tóth, T.; Takacs, E. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review. Sci. Total Environ. 2020, 717, 137219. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, D.J.; Jo, A.; Yun, S.H.; Eun, J.B.; Im, M.H.; Shim, J.H.; Abd El-Aty, A.M. Onsite/on-field analysis of pesticide and veterinary drug residues by a state-of-art technology: A review. J. Sep. Sci. 2021, 44, 2310–2327. [Google Scholar] [CrossRef] [PubMed]
- Vazhayail, A.; Thomas, J.; Thomas, N. Synergistic modulation of active site of NiO via cobalt doping by solution combustion for improving oxygen evolution reaction. Mater. Chem. Phys. 2023, 300, 127540. [Google Scholar] [CrossRef]
- Menon, P.S.; Kunjumon, J.; Bansal, M.; Nair, S.S.; Beryl, C.; Vinitha, G.; Maity, T.; Abraham, P.M.; Sajan, D.; Philip, R. Role of surface defects in the third order nonlinear optical properties of pristine NiO and Cr doped NiO nanostructures. Ceram. Int. 2023, 49, 5815–5827. [Google Scholar] [CrossRef]
- Sharmila, M.; Mani, R.J.; Parvathiraja, C.; Kader, S.M.A.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Photocatalytic Dye Degradation and Bio-Insights of Honey-Produced α-Fe2O3 Nanoparticles. Water 2022, 14, 2301. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Chavan, A.U.; Jadhav, L.D.; Jamale, A.P.; Patil, S.P.; Bhosale, C.H.; Bharadwaj, S.R.; Patil, P.S. Effect of variation of NiO on properties of NiO/GDC (gadolinium doped ceria) nano-composites. Ceram. Int. 2012, 38, 3191–3196. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Xia, X.; Qi, M.; Yin, J.; Chen, Q. Self-supported Ni decorated NiO nanoflake arrays as promising cathode materials of hybrid batteries. Mater. Res. Bull. 2016, 76, 113–117. [Google Scholar] [CrossRef]
- Mao, H.K.; Bassett, W.A.; Takahashi, T. Effect of pressure on crystal structure and lattice parameters of iron up to 300 kbar. J. Appl. Phys. 1967, 38, 272–276. [Google Scholar] [CrossRef]
- Spedding, F.H.; Daane, A.H.; Herrmann, K.W. The crystal structures and lattice parameters of high-purity scandium, yttrium and the rare earth metals. Acta Crystallogr. 1956, 9, 559–563. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Temperature dependent Raman modes of reduced graphene oxide: Effect of anharmonicity, crystallite size and defects. Carbon 2021, 184, 437–444. [Google Scholar] [CrossRef]
- Lehmann, V.; Jobst, B.; Muschik, T.; Kux, A.; Petrova-Koch, V. Correlation between optical properties and crystallite size in porous silicon. Jpn. J. Appl. Phys. 1993, 32, 2095. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, T.; Pinna, N.; Kumari, K.P.; Perumal, K.; Jayaprakash, R. Microwave-assisted synthesis and characterization of tin oxide nanoparticles. Mater. Lett. 2008, 62, 3437–3440. [Google Scholar] [CrossRef]
- Morsi, R.E.; Elsalamony, R.A. Superabsorbent enhanced-catalytic core/shell nanocomposites hydrogels for efficient water decolorization. New J. Chem. 2016, 40, 2927–2934. [Google Scholar] [CrossRef]
- Kumar, P.R.; Maharajan, T.M.; Chinnasamy, M.; Pitchiah, A.; Prabu, J.; Kumar, K.S. Hydroxyl radical scavenging activity of La2O3 nanoparticles. Pharma Innov. 2019, 8, 759–763. [Google Scholar]
- Pershina, A.G.; Sazonov, A.E.; Ogorodova, L.M. Investigation of the interaction between DNA and cobalt ferrite nanoparticles by FTIR spectroscopy. Russ. J. Bioorganic Chem. 2009, 35, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, K.S.; Srinivas, C.; Prajapat, C.L.; Meena, S.S.; Mehar, M.V.K.; Potukuchi, D.M.; Sastry, D.L. Structural and magnetic investigations: Study of magnetocrystalline anisotropy and magnetic behavior of 0.1% Cu2+ substituted Ni–Zn ferrite nanoparticles. Ceram. Int. 2018, 44, 1193–1200. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Eskandarzadeh, N.; Jalili, H.; Varzaneh, A.G.; Kameli, P.; Orue, I.; Chernenko, V.; Hajalilou, A.; Ferreira, L.P.; Cruz, M.M. Magnetic hyperthermia properties of CoFe2O4 nanoparticles: Effect of polymer coating and interparticle interactions. Ceram. Int. 2022, 48, 27995–28005. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Haq, S.; Dildar, S.; Ali, M.B.; Mezni, A.; Hedfi, A.; Shahzad, M.I.; Shahzad, N.; Shah, A. Antimicrobial and antioxidant properties of biosynthesized of NiO nanoparticles using Raphanus sativus (R. sativus) extract. Mater. Res. Express 2021, 8, 055006. [Google Scholar] [CrossRef]
- Rao, B.P.; Rao, P.S.; Rao, K.H. X-ray and magnetic studies of scandium substituted Ni-Zn ferrites. IEEE Trans. Magn. 1997, 33, 4454–4458. [Google Scholar]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Kostishyn, V.G.; Trukhanov, S.V.; Trukhanov, A.V.; Baykal, A. Impact of Eu3+ ion substitution on structural, magnetic and microwave traits of Ni–Cu–Zn spinel ferrites. Ceram. Int. 2020, 46, 11124–11131. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Al-Shihri, A.S.; Kalam, A.; Du, G.; Ahmad, T. Microwave synthesis, optical properties and surface area studies of NiO nanoparticles. J. Mol. Struct. 2014, 1058, 56–61. [Google Scholar] [CrossRef]
- Wani, I.A.; Ganguly, A.; Ahmed, J.; Ahmad, T. Silver nanoparticles: Ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater. Lett. 2011, 65, 520–522. [Google Scholar] [CrossRef]
- Agostini, G.; Pellegrini, R.; Leofanti, G.; Bertinetti, L.; Bertarione, S.; Groppo, E.; Zecchina, A.; Lamberti, C. Determination of the particle size, available surface area, and nature of exposed sites for silica−alumina-supported Pd nanoparticles: A multitechnical approach. J. Phys. Chem. C 2009, 113, 10485–10492. [Google Scholar] [CrossRef]
- Hohl, L.; Röhl, S.; Stehl, D.; von Klitzing, R.; Kraume, M. Influence of nanoparticles and drop size distributions on the rheology of w/o Pickering emulsions. Chem. Ing. Tech. 2016, 88, 1815–1826. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Abd Hamid, S.B. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M. Visible-light photocatalysts: Prospects and challenges. Apl Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Sen, T.; Biswas, A.; Rout, T.K.; Thangavel, R.; Nair, U.G. Comparative study of morphological, optical and conductive properties between low and heavily zinc doped nickel oxide thin films as hole transporting material. J. Alloys Compd. 2021, 889, 161613. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Z.; Deng, Q.; Zhang, F.; You, F.; Yao, C. Zinc-Doped Nickel Oxide Hollow Microspheres–Preparation Hydrothermal Synthesis and Electrochemical Properties. Eur. J. Inorg. Chem. 2018, 2018, 4345–4348. [Google Scholar] [CrossRef]
- Thiruchelvan, P.S.; Lai, C.C.; Tsai, C.H. Combustion processed nickel oxide and zinc doped nickel oxide thin films as a hole transport layer for perovskite solar cells. Coatings 2021, 11, 627. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible light assisted photocatalysis of Methylene Blue and Rose Bengal dyes by iron doped NiO nanoparticles prepared via chemical co-precipitation. Phys. B Condens. Matter 2020, 579, 411905. [Google Scholar] [CrossRef]
- Thambidurai, S.; Gowthaman, P.; Venkatachalam, M.; Suresh, S. Enhanced bactericidal performance of nickel oxide-zinc oxide nanocomposites synthesized by facile chemical co-precipitation method. J. Alloys Compd. 2020, 830, 154642. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Kuo, D.H. Phase transformation of bimetal zinc nickel oxide to oxysulfide photocatalyst with its exceptional performance to evolve hydrogen. Appl. Catal. B Environ. 2020, 272, 118985. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous nickel doped titanium dioxide nanoparticles with improved visible light photocatalytic activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Zhang, X.; Kennedy, L.J. Green synthesis of nickel oxide nanoparticles using Solanum trilobatum extract for cytotoxicity, antibacterial and photocatalytic studies. Surf. Interfaces 2020, 20, 100553. [Google Scholar] [CrossRef]
- Malathy, P.; Vignesh, K.; Rajarajan, M.; Suganthi, A. Enhanced photocatalytic performance of transition metal doped Bi2O3 nanoparticles under visible light irradiation. Ceram. Int. 2014, 40, 101–107. [Google Scholar] [CrossRef]
- George, A.; Raj, D.M.A.; Venci, X.; Raj, A.D.; Irudayaraj, A.A.; Josephine, R.; Sundaram, S.J.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Chen, T.-W.; et al. Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res. 2022, 203, 111880. [Google Scholar] [CrossRef]
- Ahmad, I. Catharanthus roseus leaf extract mediated Ag-MgO nanocatalyst for Photocatalytic degradation of congo red dye and their antibacterial activity. J. Mol. Struct. 2022, 1262, 133005. [Google Scholar]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Amer, M.S.; Ahamed, M. Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials 2021, 11, 2915. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.Y.; Burhan, J.; Jaladi, F.; Lim, C.M.; Usman, A.; Kumara, N.; Tsang, S.C.E.; Mahadi, A.H. Effect of Cr doping in CeO2 nanostructures on photocatalysis and H2O2 assisted methylene blue dye degradation. Catal. Today 2021, 375, 506–513. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, W.; Khan, M.N.; Alhazaa, A.N. Enhanced visible light-driven photocatalytic performance of Zr doped CeO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 8291–8300. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, V.; Tanwar, A. Structural, morphological, optical and photocatalytic properties of Ag-doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 2166–2173. [Google Scholar] [CrossRef]
- Shahid, M.; Farrukh, M.A.; Umar, A.A.; Khaleeq-ur-Rahman, M. Solvent controlled synthesis of CaO-MgO nanocomposites and their application in the photodegradation of organic pollutants of industrial waste. Russ. J. Phys. Chem. A 2014, 88, 836–844. [Google Scholar] [CrossRef]
- Wu, R.J.; Chen, C.C.; Lu, C.S.; Hsu, P.Y.; Chen, M.H. Phorate degradation by TiO2 photocatalysis: Parameter and reaction pathway investigations. Desalination 2010, 250, 869–875. [Google Scholar] [CrossRef]
- Shifu, C.; Gengyu, C. Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2·SiO2/beads by sunlight. Sol. Energy 2005, 79, 1–9. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Calza, P.; Medana, C.; Villioti, A.E.; Baiocchi, C.; Pelizzetti, E.; Albanis, T. Heterogeneous photocatalytic degradation of the pharmaceutical agent salbutamol in aqueous titanium dioxide suspensions. Appl. Catal. B Environ. 2007, 77, 135–144. [Google Scholar] [CrossRef]
- Mingmongkol, Y.; Polnok, A.; Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Photocatalytic Degradation Mechanism of the Pharmaceutical Agent Salbutamol Using the Mn-Doped TiO2 Nanoparticles Under Visible Light Irradiation. ACS Omega 2023, 8, 17254–17263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).