Abstract
The electrochlorination (E-Cl) process has attracted much attention as it is a highly efficient method for treating organic compounds in hypersaline wastewater. In this study, the E-Cl process was utilized for the removal of antibiotics. The optimal experimental conditions were determined to be a NaCl concentration of 100 mM, a current density of 1.5 mA/cm2, a pH of 7.0, and a plate spacing of 1 cm, with a levofloxacin (LEV) degradation efficiency reaching as high as 99% using this setup. The effects of the presence of other ions and humic acid on the E-Cl process were investigated, and it was found that the degradation of LEV was not significantly affected by the presence of coexisting substances. In addition, free chlorine was identified as the primary active species for the degradation of LEV by means of a quenching experiment. It was demonstrated by 3D EEM and TOC that LEV was not completely mineralized and that intermediate products may be present. In order to reveal the degradation pathways of LEV, its degradation products were also analyzed via LC-MS, and some possible pathways of LEV degradation in this system were proposed. The successful degradation of LEV demonstrated that the E-Cl process is an efficient and promising technique for the treatment of organic pollutants in high-salinity wastewater.
1. Introduction
High-salinity wastewater (>1% salt), such as that generated by mining, oil production, and chemical manufacturing, poses significant challenges for the treatment and application of wastewater []. The volume of high-salinity wastewater produced has dramatically increased []. Pharmaceutical wastewater contains a high concentration of recalcitrant organic pollutants, a large amount of inorganic salts and different kinds of pharmaceutical residues. Therefore, the development of the pharmaceutical industry and the production of high-salinity wastewater are closely related. Jiang et al. [] used collected pharmaceutical wastewater at a salinity of 2.5% from a pharmaceutical manufacturing plant in Singapore. People are becoming more dependent on the use of antibiotics, and the application of antibiotics in various fields, such as medical care and animal husbandry, among others, has become increasingly extensive. Fluoroquinolones (FQs), including levofloxacin (LEV), moxifloxacin, norfloxacin and ciprofloxacin [], are highly concentrated in the environment [] and pose a high risk to both ecosystems and public health []. Among them, levofloxacin (LEV) is widely overused as an anti-inflammatory agent and is discharged into the environment mainly through the effluent of wastewater treatment plants []. Hanna et al. [] investigated the presence of antibiotic residues in twelve villages in Shandong Province, China. Their results showed that levofloxacin was detected at concentrations ranging between 0.3 and 3.9 ng/L in river water, 1.3 and 12.5 ng/L in wastewater, 0.5 and 21.4 ng/L in drinking water and 0.5 and 2.5 μg/kg in soil. The long-term existence and accumulation of LEV in water bodies pose a significant threat to human beings, aquatic organisms and the ecosystem []. Therefore, it is hoped that an efficient, convenient, environmentally friendly, and safe wastewater treatment method can be found to reduce the environmental and health hazards posed by LEV [].
Many technologies have been developed to treat high-salinity organic wastewater, such as advanced oxidation processes [], membrane separation [], and osmosis technologies []. Among these technologies, advanced oxidation processes (AOPs) represent a promising method for the removal of emerging organic pollutants from water []. These processes involve the generation of highly reactive hydroxyl radicals (e.g., •OH, Cl•, and SO4•−) through the use of various oxidizing agents, such as ozone [], UV radiation [], Fenton reactions [], and electrochemical oxidation []. Wang et al. [], using mesoporous MnO@MnOx microspheres with peroxymonosulfate (PMS), successfully achieved the degradation of LEV. In particular, the MnO@MnOx catalyst achieved 98.1% degradation and 81.4% mineralization of LEV after being irradiated for 30 min. In addition, the stability of the catalyst, its reaction kinetics and the degradation mechanism were also systematically studied. Li et al. [] developed a novel catalyst (MS-N3H) prepared directly from electrolytic manganese slag, and MS-N3H could be applied for the efficient degradation of LEV with a broad pH range from 2.0 to 10. In the MS-N3H/PMS system, after PMS (0.4 g/L) had been added, the concentration of LEV decreased obviously within 60 min with a degradation efficiency of 82.6%. It was demonstrated that while non-radical 1O2 was the dominant contributor, the MS-N3H/PMS was stable for the degradation of LEV in different water matrixes and maintained high recyclability even after four recycling processes. In addition, unlike membrane filtration technologies, AOPs can decompose hazardous substances directly rather than simply transferring the pollutants []. However, conventional AOPs may not be efficient at removing organic pollutants from high-salinity wastewater. Due to the presence of high concentrations of chlorides, sulfates, nitrates, phosphates, bicarbonates, carbonates, and so on in wastewater, the free radicals are largely scavenged [].
The electrochemical oxidation (EO) process is able to degrade organic pollutants through the electrogenesis of reactive radicals and direct electron transfer []. In high-salinity wastewater, when a voltage is applied, the presence of large amounts of Cl− can lead to the in situ generation of reactive chlorine species (RCS), which act as reactive oxidants to degrade organic pollutants []. Moreover, the in situ generation of RCS and free chlorine is less materially restricted and is more cost-effective than the generation of •OH []. Kuang et al. [] proposed a new bipolar-membrane-integrated electrochlorination (BPM-EC) process for removing biorefractory organic pollutants in landfill leachate. This BPM-EC process is efficient for the generation of ClO•, which significantly accelerates the oxidation of ammonium in landfill leachate and its selective transformation into nitrogen. Using this method, it was found that 100% of the NH4+-N present was removed within 3 h, demonstrating the superiority of this method in promoting ammonium oxidation. Huo et al. [] coupled electroporation with electrochlorination to establish an efficient method of water disinfection. Electroporation is sub-lethal to microorganisms, resulting in limited efficiency of microbial inactivation. However, the electrogenerated active chlorine at the anode causes more lethal damage. This process completely disinfected water (>6.0-log) under a high water flux of 2.4 × 104 L/(m2·h) and an applied voltage of 2.0 V, removing bacteria and viruses.
In this study, the electrochlorination (E-Cl) process was applied to treat high-salinity wastewater containing LEV. In the E-Cl system, a Ti/RuIr electrode was first selected as the anode, and a Ti/Pt electrode was selected as the cathode. Next, the effects of several experimental parameters, such as the NaCl concentration, current density, pH, and plate spacing between the electrodes, were investigated. Additionally, the effects of the presence of other substances on the system were explored, and the reactive chlorine species in the system were identified through quenching experiments. Lastly, the possible pathways and intermediates of LEV degradation were hypothesized. The aim of this study was to investigate the optimal degradation conditions and the degradation effect of the E-Cl process on antibiotic LEV and to analyze the degradation mechanism and degradation pathway. Another type of wastewater, high-salinity wastewater, was efficiently utilized to establish an efficient method for the removal of antibiotics from water, providing a new perspective on the treatment of organic pollutants in high-salinity wastewater.
2. Materials and Methods
2.1. Chemicals
Levofloxacin, sodium chloride, methanol, tert-butyl alcohol, sodium thiosulfate, nitrobenzene, sodium sulfate anhydrous, sodium bicarbonate, sodium nitrate, sodium phosphate dibasic (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), humic acid, triethylamine, and acetonitrile (Titan Scientific Co., Ltd., Shanghai, China). Ti/RuIr and Ti/Pt electrodes were purchased from Suzhou Schulte Industrial Technology Co., Ltd., Suzhou, China.
2.2. Electrochlorination Experiments
Experimental setup: A Ti/RuIr electrode was used as the anode and a Pt electrode was used as the cathode, both of which were 50 × 100 mm in size and 1 mm in thickness. A total of 300 mL of synthetic LEV wastewater (10 mg/L) was treated, and the required constant current density was provided using an IT6302 DC power supply (ITECH, Shanghai, China).
Experimental method: The experiment was carried out for 60 min, and samples were collected every 10 min. Magnetic stirring was performed at 300 rpm, and the pH of the solution was adjusted using HCl or NaOH.
2.3. Experimental Instrument
The concentration of LEV was measured using high-performance liquid chromatography (HPLC; Shimadzu, Kyoto, Japan). Total organic carbon (TOC) was used to detect the degree of mineralization of LEV, which was analyzed using a TOC-L analyzer (Shimadzu, Japan). The excitation–emission matrix (EEM) fluorescence spectra were measured using a fluorescence spectrophotometer (Hitachi, Japan). Liquid-phase chromatography/mass spectrometry (LC-MS; Agilent 1290 Infinity, Shanghai, China) was used to detect the intermediate products during the degradation of levofloxacin.
2.4. Analytical Methods
High-performance liquid chromatography (HPLC) with a symmetrical C18 column (150 mm × 4.6 mm i.d., 5 μm) was used to determine the concentration of LEV. The pH of 1% triethylamine was adjusted to 3.0 using phosphoric acid. A mixture of 1% triethylamine and acetonitrile at a volume ratio (86:14, v/v) was used as the mobile phase. The separation was set up at a column temperature of 40 °C. The detection wave lengths for LEV were set at 294 nm. The flow rate was set to 1.0 mL/min. The excitation–emission matrix (EEM) fluorescence spectra was used to investigate the degradation process. The LEV solution displayed fluorescence peaks in the Ex/Em = 200–300/400–550 nm range and the Ex/Em = 300–350/400–500 nm range. The width of the excitation and emission slits was fixed at 5 nm and the scan speed was 12,000 nm/min. The experimental conditions for LC-MS were as follows: a mixture of 0.1% formic acid and acetonitrile at a volume ratio of 2:3 was used as the mobile phase, and the column was a Waters BEH C18 (2.1 × 100 mm i.d., 1.7 μm). A volume of 5 μL of the reaction sample was extracted. The flow rate was set to 1.0 mL/min.
3. Results and Discussion
3.1. Effects of Key Operational Parameters on LEV Degradation
3.1.1. Effects of Cl− Concentration
Cl− is a common anion in water bodies, and the magnitude of its concentration has a direct effect on the generation of RCS in the E-Cl process. As shown in Figure 1, without the addition of NaCl, the degradation efficiency of LEV was only 62.92% after 60 min. With the addition of NaCl, the degradation efficiency increased significantly as the NaCl concentration increased. In the first 10 min, when the concentration of NaCl increased from 25 mM to 200 mM, the degradation efficiency of LEV increased from 41.96% to 87.53%, respectively. As the reaction time increased, the degradation efficiency of LEV further increased; when the reaction time reached 60 min, the degradation efficiency tended to be around 99% if the NaCl concentration was over 50 mM, which is approximately 36% higher compared to the degradation efficiency when no NaCl was added. The experimental results proved that the concentration of chloride is important for pollutant degradation in the E-Cl process; when the chloride concentration reached 100 mM, nearly complete LEV degradation was achieved.
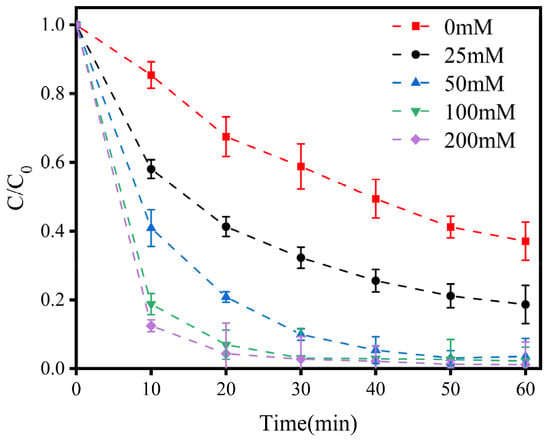
Figure 1.
Variations in LEV degradation at different concentrations of NaCl. Experimental conditions: current density, 1.5 mA/cm2; solution pH, 7.0; plate spacing, 1 cm. C/C0 represents the degradation efficiency of LEV.
3.1.2. Effects of Current Density
In the E-Cl process, generally, as the current density increases, more reactive chlorine species will be produced, which can accelerate the reaction and improve the degradation efficiency []. Therefore, it is of great significance to study the effect of the current density on the degradation of LEV in the E-Cl process.
As depicted in Figure 2, generally, the LEV degradation efficiency increased with the increase in current density. In the first 10 min, the difference in the LEV degradation efficiency was obvious with the variation in current density. The LEV degradation efficiency was only 17.26% when the current density was 0.5 mA/cm2; when the current density increased from 1 mA/cm2 to 2.5 mA/cm2, the LEV degradation efficiency increased from 34.76% to 92.95%, respectively. When the reaction time reached 20 min, the LEV degradation efficiency was close to 94% as the current density increased above 1.5 mA/cm2. Therefore, considering the cost-effectiveness of LEV degradation, a current density of 1.5 mA/cm2 was selected for further research.
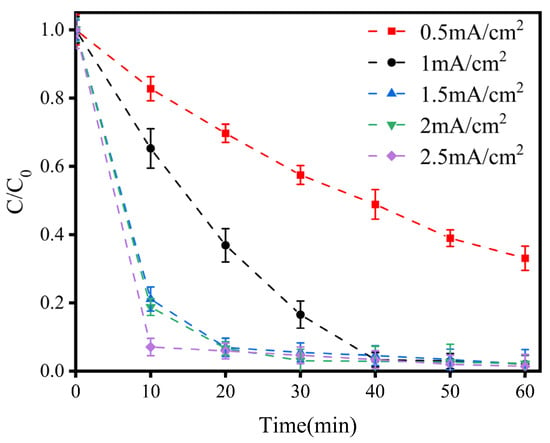
Figure 2.
Variations in LEV degradation at different current densities. Experimental conditions: concentration of NaCl = 100 mM; solution pH = 7.0; plate spacing = 1 cm. C/C0 represents the degradation efficiency of LEV.
3.1.3. Effects of pH
The pH level is also important to consider. It has been reported that pH determines the form of reactive chlorine species—Cl2 (acidic), HOCl (neutral), or OCl− (alkaline) []. Therefore, the effect of pH on the degradation of LEV via the E-Cl process was studied.
As depicted in Figure 3, within 10 min, as the pH value decreased, the efficiency of LEV degradation increased. However, the efficiency of LEV degradation at pH levels ranging from three to nine tended to be consistent when the reaction time reached 20 min, and the degradation efficiency was only obviously suppressed when the pH was eleven. The LEV degradation efficiency also decreased to 78.33% at a reaction time of 60 min and a pH of eleven. Although there was an obvious degradation effect, the degradation rate became slower. Therefore, a pH of seven was chosen as the optimal condition in this experiment. Meanwhile, the experiment showed that the E-Cl process, which has a wide range of applicability in acidic and alkali conditions, had an inhibitory effect on the efficiency and rate of LEV degradation under a strong alkali pH of 11 or more, demonstrating good application prospects.
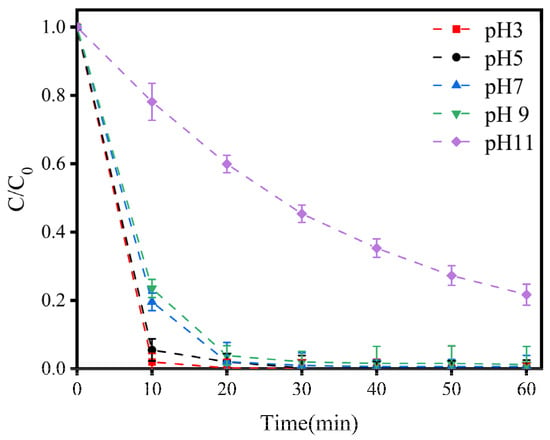
Figure 3.
Variations in LEV degradation at different pH values. Experimental conditions: concentration of NaCl = 100 mM; current density = 1.5 mA/cm2; plate spacing = 1 cm. C/C0 represents the degradation efficiency of LEV.
3.1.4. Effects of Plate Spacing
Plate spacing is also an important conditioning factor in this type of study, and it exhibits an effect on the degradation of LEV in this system; thus, we investigated it in the present work.
As shown in Figure 4, as the electrode plate spacing increased, the efficiency of LEV degradation significantly decreased. The smaller the electrode plate spacing was, the more efficient the LEV degradation was. The efficiency of LEV degradation within 60 min increased from 80.14% to 96.64% when the electrode plate spacing decreased from 3 cm to 1 cm. Considering that the best degradation results were obtained at a plate spacing of 1 cm, this spacing was chosen as the optimal condition.
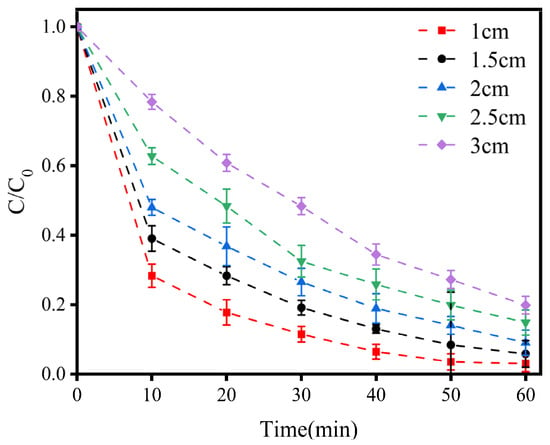
Figure 4.
Variations in LEV degradation with different polar plate spacings. Experimental conditions: concentration of NaCl = 100 mM; current density = 1.5 mA/cm2; solution pH = 7.0. C/C0 represents the degradation efficiency of LEV.
3.1.5. Effects of Coexisting Substances
In AOPs, the presence of other substances affects the degradation efficiency. These coexisting substances, which are mainly inorganic anions (including SO42−, PO43−, HCO3−, and NO3−) and natural organic matter (NOM), may compete with active species and decrease their degradation efficiency. In high-salinity wastewater, there may be several coexisting substances that compete with active chlorine species and decrease the efficiency of LEV degradation. For this reason, the effects that coexisting substances had on the system were investigated. In order to reveal the specific effects of coexisting substances on E-Cl, their effects on the degradation of the pollutant were investigated. Figure 5a shows the effect of SO42− on the E-Cl process. When the reaction time reached 60 min, the efficiency of the degradation of LEV from the solution reached 97%, regardless of whether SO42− had been added or not, proving that sulfate had little effect on the E-Cl process. The same phenomenon can be observed in Figure 5b; when the reaction time reached 60 min, the efficiency of LEV degradation reached 98%, regardless of whether NO3− had been added or not, indicating that NO3− had virtually no effect on the process. As shown in Figure 5c, in the first 10 min, the presence of HCO3− had an inhibitory effect on the degradation efficiency of LEV. The degradation efficiency decreased from 82.6% to 60.47% when the concentration of HCO3− increased from 0 mg/L to 400 mg/L. After 60 min, the efficiency of LEV degradation reached 95% regardless of the HCO3− concentration. As shown in Figure 5d, in comparison with the abovementioned coexisting ions, PO43− exerted a more significant effect within 20 min, especially in the first 10 min, during which the degradation efficiency was hindered and decreased from 82.6% to 50.74% as the PO43− concentration increased from 0 mg/L to 50 mg/L. After 60 min, the degradation efficiency reached 96%. A likely reason for this is that PO43− causes a change in the pH of the solution, thus altering the equilibrium of HClO and ClO− conversion and inhibiting the degradation efficiency in the first 20 min.
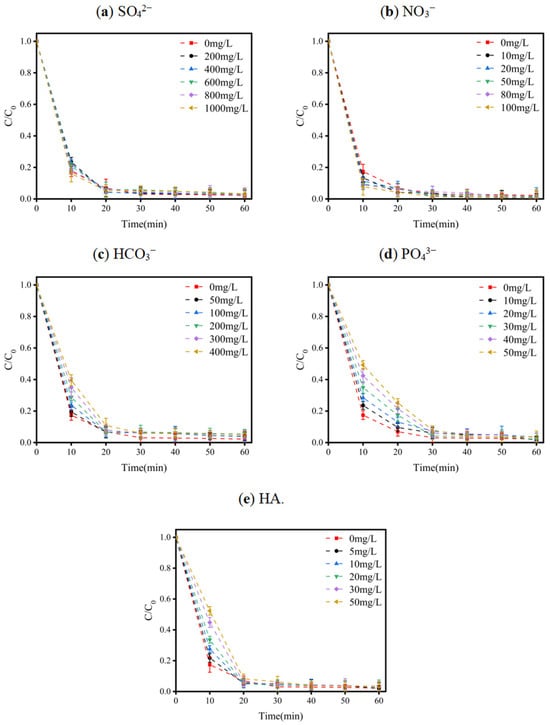
Figure 5.
Variations in LEV degradation when background matter of different types and concentrations was added to the system: (a) SO42−; (b) NO3−; (c) HCO3−; (d) PO43−; (e) HA. Experimental conditions: concentration of NaCl = 100 mM; current density = 1.5 mA/cm2; solution pH = 7.0; plate spacing = 1 cm. C/C0 represents the degradation efficiency of LEV.
In conventional AOPs, HA scavenges the free radicals that compete with organic pollutants and reduces the degradation efficiency. A similar phenomenon exists in the E-Cl process. As shown in Figure 5e, the degradation efficiency was inhibited during the first 10 min, and as the concentration of HA increased from 0 mg/L to 50 mg/L, the efficiency of LEV degradation decreased from 82.6% to 47.7%. The reason for this phenomenon was that HA consumed the RCS and •OH in the system and interacted with the active chlorine species to inhibit the degradation of LEV. However, as the reaction time reached 20 min, regardless of the HA concentration, the LEV degradation efficiency reached 93%, and eventually reached 96% after 60 min.
Considering the above results, the degradation of LEV via the E-Cl process was not significantly affected by the presence of coexisting substances, indicating that the E-Cl process is more robust to variations in water properties compared with conventional AOP processes, making it more applicable in actual practice.
3.2. Identification of Reactive Species
In EO wastewater treatment, anodic oxidation can produce active species, such as superoxide anions (O2•−), hydroxyl radicals (•OH), sulfate (SO4•−), chlorine (Cl•), and oxychloride (ClO•) [].
When wastewater contains a high concentration of salt, a large amount of Cl− is converted into reactive chlorine species. The identification of reactive chlorine species is essential in order to reveal the mechanism of LVE degradation in the E-Cl process [].
Firstly, Na2S2O3 has been reported to be capable of quenching free radicals such as •OH, Cl•, Cl2•−, ClO•, and free chlorine []. As shown in Figure 6, the efficiency of LEV degradation was reduced by 75% in comparison with that when Na2S2O3 was absent, proving that the degradation of LEV did not occur due to electrochemical oxidation alone, but required free radicals and free chlorine.
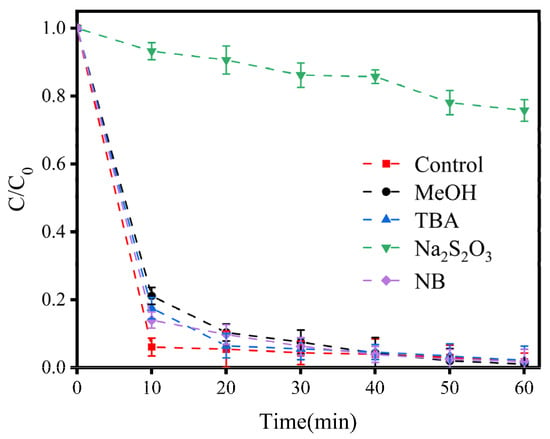
Figure 6.
Variations in LEV degradation in the presence of different scavengers ([Na2S2O3] = [MeOH] = [TBA] = [NB] = 50 mM). Experimental conditions: concentration of NaCl = 100 mM; current density = 1.5 mA/cm2; solution pH = 7.0; plate spacing = 1 cm. C/C0 represents the degradation efficiency of LEV.
MeOH is widely applied to quench •OH and Cl2•− []. In the first 10 min following the addition of 50 mM MeOH, the efficiency of LEV degradation decreased by 15.08%. However, after 60 min of the reaction, the efficiency of LEV degradation reached 99%. This result demonstrated that the scavenging of •OH and Cl2•− delayed LEV degradation but did not affect it in the long run. TBA is generally used to quench •OH, ClO•, and Cl• []. In the first 10 min after the addition of TBA, the LEV degradation efficiency reduced by 11.46%, but when the reaction time reached 60 min, the efficiency of LEV degradation reached 97%. Moreover, NB has been shown to quench •OH []. Although the addition of NB reduced LEV’s degradation efficiency by 7.95% in the first 10 min after its addition, the efficiency of LEV degradation ultimately reached 97%, indicating that •OH was not the prominent active substance in the system. The above results suggest that free chlorine may be the RCS contributing to the degradation of LEV in the E-Cl process.
3.3. Possible Pathways of LEV Degradation
Three-dimensional EEM can be used to characterize and analyze organic compound species and observe their changes []. To better understand the degradation of LEV, 3D EEM fluorescence spectra of the LEV samples were observed before and after the reaction. As shown in Figure 7a, the initial LEV solution displayed fluorescence peaks at Ex/Em = 200–300/400–550 nm and Ex/Em = 300–350/400–500 nm []. As shown in Figure 7b, after 60 min, no fluorescence signals could be detected, indicating that the functional group of LEV was destroyed [] and that LEV had been degraded via the E-Cl process.
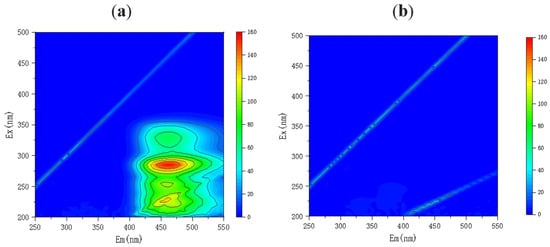
Figure 7.
Three-dimensional EEM of the levofloxacin solution after 0 min (a) and 60 min (b) of degradation via the E-Cl process.
However, the degradation efficiencies of LEV when studied using TOC and HPLC are quite different. As shown in Figure 8, the degradation efficiency of LEV determined using HPLC was 99%, but when detected using TOC, the degradation efficiency of LEV dramatically dropped to 57.17%, Similarly, Wang et al. [] used the E-Cl process to treat atrazine (ATZ) in pesticide wastewater. Although the degradation efficiency of ATZ reached 98.66% after 60 min, the chemical oxygen demand (COD) removal efficiency was 86.43% and an obvious gap was observed between the results obtained from different analysis methods. It can be deduced that the mineralization of organic pollutant by E-Cl was incomplete and the products of LEV’s degradation must be urgently studied.
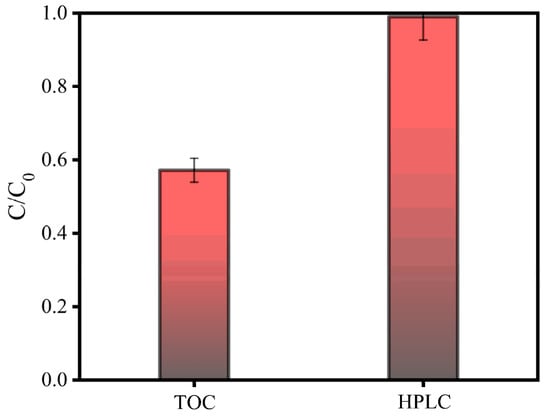
Figure 8.
Efficiencies of LEV degradation, as detected using TOC and HPLC.
To reveal the degradation pathway of LEV, LC-MS was also applied to analyze the degradation products. Some possible pathways of LEV degradation in this system are proposed.
As shown in Figure 9, in pathway I, the piperazine in LEV is attacked by •OH, opening the piperazine ring to form P1 (m/z = 336); then, P1 (m/z = 336) is converted into P2 (m/z = 279) through deamination and dealkylation reactions. P3 (m/z = 239) is generated from P2 (m/z = 279) by opening the cycloxazine ring and via dealkylation reactions [,]. In pathway II, LEV is converted into the intermediate product P4 (m/z = 318) via a decarboxylation reaction, and P4 (m/z = 318) is oxidized into P5 (m/z = 338); P5 (m/z = 338) is converted into P6 (m/z = 262) through defluorination, decarboxylation, and demethylation reactions [,]. In pathway III, LEV is converted into P8 (m/z = 348) via the demethylation of the piperazine ring, and P8 (m/z = 348) is degraded into P9 (m/z = 364) through dehydroxylation reactions. The byproducts generated in AOP-based water treatment are attracting increasing attention. Wang et al. [] developed the E-Cl process and treated high-salinity wastewater containing atrazine (ATZ). To compare the variations in the acute toxicity of wastewater before and after treatment, a method was employed using a bio-toxicity assay with bioluminescent bacteria. The results showed that the ATZ showed a 37.53% inhibitory effect on bioluminescence at high concentrations, and the bioluminescence was restored to 77.49% with the decomposition of ATZ by the E-Cl process. He et al. [] prepared a ZnnCoOx anode for electrocatalytic generation of RCS to remove 4-chlorophenol (4-cp) from wastewater containing Cl−. The potential ecotoxicity of 4-cp and its degradation intermediates was assessed with the ECOSER procedure. The results showed that the acute and chronic toxicity of the treated wastewater containing 4-cp decreased in all four pathways of degradation after treatment. When AOPs were applied to LEV degradation, the byproducts of P3, P6, and P9 could often be detected, and these byproducts were able to further convert into small molecules [,,]. Although LEV degradation by the E-Cl process generates a number of intermediate products, they are expected to be further degraded with a longer reaction time; therefore, the environmental risks of the byproducts can be limited.
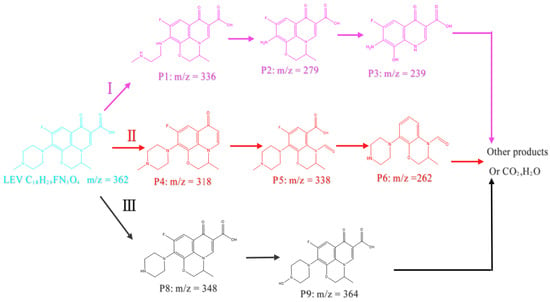
Figure 9.
Possible pathways of LEV degradation in the E-Cl system.
4. Conclusions
The E-Cl process was successfully performed for the treatment of LEV, and efficient degradation was achieved. The optimal conditions for E-Cl were determined by selecting a NaCl concentration of 100 mM, a current density of 1.5 mA/cm2, a pH of 7, and a polar plate spacing of 1 cm. The degradation efficiency of LEV via the E-Cl process is not significantly limited by the presence of other substances in the water matrix, demonstrating that the E-Cl process possesses a potentially higher applicability than conventional AOP processes. When wastewater contains high concentrations of Cl−, this Cl− can be converted into reactive chlorine species; among these, free chlorine was identified as the dominant reactive species. Despite a nearly complete degradation of LEV being achieved using the E-Cl process, a number of intermediate products were detected using LC-MS. Therefore, the mineralization efficiency of LEV should be further increased. In conclusion, the E-Cl process provides a new solution to treating organic pollutants in high-salinity wastewater, which is especially advantageous over traditional AOP processes due to its resistance to the presence of coexisting substances in the water matrix.
Author Contributions
Investigation, J.L. and B.J.; Writing—original draft, M.W.; Writing—review & editing, X.L. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Shandong Provincial Natural Science Foundation (ZR2021QE223).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tawabini, B.S.; Plakas, K.V.; Fraim, M.; Safi, E.; Oyehan, T.; Karabelas, A.J. Assessing the efficiency of a pilot-scale GDE/BDD electrochemical system in removing phenol from high salinity waters. Chemosphere 2020, 239, 124714. [Google Scholar] [CrossRef]
- Cao, W.R.; Hu, C.; Zhang, P.; Qiu, T.; Wang, S.G.; Huang, G.H.; Lyu, L. Salinity-mediated water self-purification via bond network distorting of H2O molecules on DRC-surface. Proc. Natl. Acad. Sci. USA 2023, 120, e2311920120. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Ng, H.Y. Aerobic granular sludge systems for treating hypersaline pharmaceutical wastewater: Start-up, long-term performances and metabolic function. J. Hazard. Mater. 2021, 412, 125229. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500, 250–269. [Google Scholar] [CrossRef]
- Lv, H.; Han, P.; Li, X.; Mu, Z.; Zuo, Y.; Wang, X.; Tan, Y.; He, G.; Jin, H.; Sun, C.; et al. Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater. Materials 2021, 14, 6814. [Google Scholar] [CrossRef]
- John, Y.; David, V.E., Jr.; Mmereki, D. A Comparative Study on Removal of Hazardous Anions from Water by Adsorption: A Review. Int. J. Chem. Eng. 2018, 2018, 3975948. [Google Scholar] [CrossRef]
- Gonzalez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganes, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernandez-Pinas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.W.; Yang, X.W.; Ji, X.; Zou, H.Y.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Patil, D.V.; Jang, M.; Paeng, K.-J.; Jeon, B.-H. Biodegradation and metabolic fate of levofloxacin via a freshwater green alga, Scenedesmus obliquus in synthetic saline wastewater. Algal Res.-Biomass Biofuels Bioprod. 2017, 25, 54–61. [Google Scholar] [CrossRef]
- Yang, K.D.; Han, P.W.; Liu, Y.N.; Lv, H.X.; Chen, X.F.; Lei, Y.H.; Yu, L.; Ma, L.; Duan, P.Z. Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes. Molecules 2024, 29, 662. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef]
- Fonseca Pierangeli, G.M.; Ragio, R.A.; Benassi, R.F.; Gregoracci, G.B.; Subtil, E.L. Pollutant removal, electricity generation and microbial community in an electrochemical membrane bioreactor during co-treatment of sewage and landfill leachate. J. Environ. Chem. Eng. 2021, 9, 106205. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Ma, L.; Huang, Y.; Wang, H. Characteristics and mechanisms of catalytic ozonation with Fe-shaving-based catalyst in industrial wastewater advanced treatment. J. Clean. Prod. 2019, 222, 174–181. [Google Scholar] [CrossRef]
- Yang, F.; Sheng, B.; Wang, Z.; Xue, Y.; Liu, J.; Ma, T.; Bush, R.; Kusic, H.; Zhou, Y. Performance of UV/acetylacetone process for saline dye wastewater treatment: Kinetics and mechanism. J. Hazard. Mater. 2021, 406, 124774. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Hong, M.; Peng, B.; Bao, C.; Xu, X.; Li, D.; Chen, J.; Wang, B.; Zhang, Q. Ferrocene-based resin as heterogeneous fenton-like catalyst for efficient treatment of high salinity wastewater at acidic, neutral, and basic pH. Chem. Eng. J. 2023, 464, 142450. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Wang, A.Q.; Chen, Z.; Zheng, Z.K.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2020, 379, 122340. [Google Scholar] [CrossRef]
- Li, M.K.; Huang, F.L.; Hu, L.; Sun, W.; Li, E.P.; Xiong, D.L.; Zhong, H.; He, Z.G. Efficient activation of peroxymonosulfate by a novel catalyst prepared directly from electrolytic manganese slag for degradation of recalcitrant organic pollutes. Chem. Eng. J. 2020, 401, 126085. [Google Scholar] [CrossRef]
- Nabgan, W.; Saeed, M.; Jalil, A.A.; Nabgan, B.; Gambo, Y.; Ali, M.W.; Ikram, M.; Fauzi, A.A.; Owgi, A.H.K.; Hussain, I.; et al. A state of the art review on electrochemical technique for the remediation of pharmaceuticals containing wastewater. Environ. Res. 2022, 210, 112975. [Google Scholar] [CrossRef]
- Nejumal, K.K.; Manoj, P.R.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of a pharmaceutical waste, atenolol, in aqueous medium. Environ. Sci. Pollut. Res. 2014, 21, 4297–4308. [Google Scholar] [CrossRef]
- Hai, H.; Xing, X.; Li, S.; Xia, S.; Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 2020, 738, 139909. [Google Scholar] [CrossRef]
- Sheng, B.; Huang, Y.; Wang, Z.; Yang, F.; Ai, L.; Liu, J. On peroxymonosulfate-based treatment of saline wastewater: When phosphate and chloride co-exist. RSC Adv. 2018, 8, 13865–13870. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Urea Degradation by Electrochemically Generated Reactive Chlorine Species: Products and Reaction Pathways. Environ. Sci. Technol. 2014, 48, 11504–11511. [Google Scholar] [CrossRef]
- Kuang, W.J.; Yan, Z.; Chen, J.X.; Ling, X.T.; Zheng, W.X.; Huang, W.J.; Feng, C.H. A Bipolar Membrane-Integrated Electrochlorination Process for Highly Efficient Ammonium Removal in Mature Landfill Leachate: The Importance of ClO• Generation. Environ. Sci. Technol. 2022, 57, 18538–18549. [Google Scholar] [CrossRef]
- Huo, Z.Y.; Winter, L.R.; Wang, X.X.; Du, Y.; Wu, Y.H.; Hubner, U.; Hu, H.Y.; Elimelech, M. Synergistic Nanowire-Enhanced Electroporation and Electrochlorination for Highly Efficient Water Disinfection. Environ. Sci. Technol. 2022, 56, 10925–10934. [Google Scholar] [CrossRef]
- Khelifa, A.; Aoudj, S.; Moulay, S.; Hecini, M.; De Petris-Wery, M. Degradation of EDTA by in-situ electrogenerated active chlorine in an electroflotation cell. Desalination Water Treat. 2009, 7, 119–123. [Google Scholar] [CrossRef]
- Bonola, B.; Aguilar, D.; Fuentes-Camargo, I.; Sosa-Rodríguez, F.S.; Altamirano, R.H.; Luna-Sánchez, R.; Vazquez-Arenas, J. Implications to enhance sulfamethoxazole degradation using statistical optimization of either active chlorine concentration or ORP in an electrochemical reactor. J. Environ. Chem. Eng. 2020, 8, 104179. [Google Scholar] [CrossRef]
- Xie, J.Z.; Zhang, C.Y.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Wojnarovits, L.; Takacs, E. Rate constants of dichloride radical anion reactions with molecules of environmental interest in aqueous solution: A review. Environ. Sci. Pollut. Res. 2021, 28, 41552–41575. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.; Guo, J.; Yang, C.; Yan, L.; Feng, W.; Zhang, Y.; Wang, J. Elimination of pesticide from high salinity wastewater by electrochlorination process: Active chlorine species and scale-up performance. Sep. Purif. Technol. 2023, 306, 122572. [Google Scholar] [CrossRef]
- Song, T.H.; Gao, Y.J.; Pang, S.Y.; Hu, R.H.; Li, H.B. Study of Fe3O4/PS System in Degrading BPA in Aqueous Solution. J. Braz. Chem. Soc. 2021, 32, 2264–2271. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Wang, P.F.; Wang, Y.C.; Wei, W.; Zhu, S.G. A novel solar-activated chlorine dioxide process for atrazine degradation in drinking water. Water Res. 2023, 239, 120056. [Google Scholar] [CrossRef]
- Xiang, Y.Y.; Fang, J.Y.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef]
- Lu, X.; Wu, L.; Liang, L.; Liu, D.; Chen, Y.; Zeng, Y.; Zhong, M.; Jia, B. Levofloxacin degradation by porous Cox/CN activated peroxymonosulfate: Investigation of efficiency, mechanism, and degradation pathways. J. Water Process Eng. 2023, 56, 104427. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.-M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Zhang, M.X.; Xing, M.Y.; Dong, B.; Sun, X.J.; Zhang, H.X.; Wang, C.L.; Zhu, H.X. Preparation of BiVO4/CO32−-Bi2O2CO3 heterojunctions for enhanced photocatalytic activity in the degradation of levofloxacin under visible light. J. Alloys Compd. 2023, 965, 171471. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Lu, S.Y.; Wang, Z.; Wang, Y.Q.; Zhang, G.D.; Guo, X.C.; Guo, W.; Zhang, T.T.; Xi, B.D. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.B.; Owens, G.; Chen, Z.L. Toward green nano adsorbents and catalysts: Highly active Fe/Mn nanoparticles for enhanced oxidation of oxytetracycline and levofloxacin. J. Colloid Interface Sci. 2023, 632, 299–310. [Google Scholar] [CrossRef]
- Fang, C.; Shao, C.; Wang, S.; Wu, Y.; Liu, C.; Huang, Q. Simultaneous removal of levofloxacin and sulfadiazine in water by dielectric barrier discharge (DBD) plasma: Enhanced performance and degradation mechanism. Process Saf. Environ. Prot. 2023, 171, 459–469. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.Y.; Zhang, Z.Y.; Chen, X.Z.; Jiang, Y.L.; Guo, H.; Yuan, J.P.; Wang, J.H. Removal of levofloxacin by an oleaginous microalgae Chromochloris zofingiensis in the heterotrophic mode of cultivation: Removal performance and mechanism. J. Hazard. Mater. 2022, 425, 128036. [Google Scholar] [CrossRef]
- He, L.; Ma, Y.Y.; Lei, X.; Zhou, H.J.; Yuan, Y.; Du, W.T.; Liu, Z.L.; Miao, R.R.; Guan, Q.Q. Degradation of 4-chlorophenol through in situ generation of reactive chlorine species in a Zn2CoOx electrocatalytic system. J. Environ. Chem. Eng. 2024, 12, 111911. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic Considerations for the Advanced Oxidation Treatment of Fluoroquinolone Pharmaceutical Compounds using TiO2 Heterogeneous Catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef]
- Mahjoore, M.; Honarmand, M.; Aryafar, A. Plant-based green fabrication of CuO-CdO-bentonite S-scheme heterojunction with enhanced photocatalytic performance for the degradation of levofloxacin. Environ. Sci. Pollut. Res. 2023, 30, 44439–44456. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Zhang, Y.Q.; Li, X.; Chen, T.Y.; Adéoye, A.B.E.; Peter, P.O.; Sun, Q. Microalgal mediated antibiotic co-metabolism: Kinetics, transformation products and pathways. Chemosphere 2022, 292, 133438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).