Abstract
Chile has numerous areas that lack sewage collection, including in the capital city. Sanitation in these cases is managed through individual solutions like septic tanks or small wastewater treatment plants (WWTPs) that use biological treatment, usually activated sludge with extended aeration. In general, the design of these systems adheres to the quality standards mandated by regulations for discharge, infiltration, or irrigation. In this scenario, traditional methods like increasing dissolved oxygen (DO) or hydraulic retention time (HRT) were unable to effectively reduce excessive nutrients. Therefore, literature related to nutrient excess and denitrification systems is consulted and reviewed to compile different solutions suitable for the presented issue. Potential solutions were modeled and verified using the free simulation software WRc STOAT. The software accurately predicted the unsatisfactory results of the current setup and provided parameters for the proposed modifications. Experience, precise user definition, influential characteristics, and modeling are essential in the design of WWTPs.
1. Introduction
In Chile, the collection and treatment of sewage are handled by concessioned sanitation companies. These companies have the important task of capturing, conveying, and treating the effluent from each individual land plot or building. When the project is situated outside the concession area, and the property does not have access to a public sewerage network, it is necessary for the property to have its own system for collecting, conveying, treating, and disposing of domestic sewage. Regarding treatment, it is worth noting that as of 2020, the coverage of treated sewage collected stands at 99.98% [1].
For water to be utilized for irrigation purposes, it is necessary to meet the requirements set by the Chilean Standard Nch 1.333/78, which establishes the Quality Standard for Water Resources. Compliance with Decree-Law No. 46/2002 [2] and Decree-Law No. 90/2000 [3] is necessary based on the body of water used for infiltration or recharge of the water table. These laws establish emission standards for discharges of liquid waste into marine and surface continental waters. The design parameters commonly used for domestic wastewater are based on the regulations outlined in Decree-Law No. 609/1998 [4]. These regulations specify the characteristics of Chilean wastewater for a population of 100 people, taking into account a provision of 200 [L/per/day] and a recovery factor of 0.8. According to Díaz M.A. et al. [5], this recovery factor can potentially reach 0.9. The pollutant load shown in the table for BOD5 (biochemical oxygen demand incubated for 5 days) falls within the typical range mentioned by the Environmental Protection Agency (EPA) in its manual for small communities [6]. The manual states that the general population usually has values between 35 and 60 g/day, while educational institutions have an average of 30 g/day of BOD5. According to Table 1, the value ranges from 40 to 50 g/day. BOD is an indicator that measures the level of pollution in wastewater. A high value indicates water that is heavily contaminated with organic matter [7].

Table 1.
Characterization of domestic wastewater corresponding to 100 inhabitants DS N°609/1998.
The primary option for individual systems is biological treatment, which involves the use of microorganisms to degrade organic matter [6,7,8,9,10]. A wide variety of microorganisms are responsible for carrying out the removal of BOD, the coagulation of colloidal solids, and the stabilization of organic matter [11,12,13]. Based on the literature, the concentration of total nitrogen (TN) varies between 35 to 100 mg/L, and total phosphorus (TP) varies between 18 to 29 mg/L for domestic wastewater [14]. Tomczak and Grita [15] classify BOD5 as low if it is less than 200 mg/L.
Discharges containing high nutrient concentrations result in a notable reduction in the levels of dissolved oxygen (DO) in the water [14,16,17,18,19]. This has negative effects on aquatic environments, the overall environment, and human beings [15,20,21]. Phosphates play a crucial role in the growth of algae and plankton [22], further exacerbating the situation. These are the primary factors contributing to eutrophication [23,24,25,26], with domestic and industrial wastewater being the primary sources [13]. The threshold levels for TN and TP to cause eutrophication vary within specific ranges [27]. Cui et al. [28] highlights the rising international standards. For instance, the European Union has enhanced discharge standards for TN, reducing them from 10 to 15 mg/L to 6 mg/L. Similarly, certain regions in the United States and China now demand even lower values, below 5 mg/L.
The results presented in Table 2 are obtained from a WWTP in full operation at a higher education institution. It can be observed that the concentrations of effluent surpass the standards set by regulations, which could potentially pose a threat to both health and the environment. Therefore, it is essential to model treatment systems in order to assess the efficacy of the proposed treatment type and design; this provides the opportunity for pre-emptive corrective measures to be taken if required. Cost and energy considerations are disregarded in this study, as the main focus is to comply with water quality.

Table 2.
The result of the chemical analysis of wastewater in wastewater treatment plants (WWTPs).
The relevance of this study lies in demonstrating to designers of small-scale compact plants that a design solely based on BOD parameters and manual calculations, with estimated contaminants and their values, is insufficient during the design stage. It is a common practice to assume that all wastewater has the same quality and that effluents will meet regulatory requirements. Therefore, this study aims to introduce the use of software in modeling, emphasizing its importance in the design process.
2. Materials and Methods
For the study, we thoroughly reviewed relevant literature, research, journals, and books on wastewater treatment and nutrient excess. We utilized resources like Google Scholar and the university’s electronic library database to conduct searches using specific keywords related to the topic, such as “nutrient excess”, “water treatment”, “simulation”, “Stoat”, etc. This approach sought to make the optimal decision regarding the solution to implement in the current system, aiming to utilize existing resources with minimal changes and expenses. In addition, WRc STOAT version 5.0, a popular free modeling program for WWTPs, was used to conduct software modeling. This program is widely used for predicting and enhancing wastewater performance. Simulating plants is ideal [29,30,31].
WRc STOAT has been used to study the biological and physical processes of secondary treatment in WWTPs, with a focus on treating high-nutrient content [32]. The dynamic simulation will be based on the BOD and TSS profiles, utilizing various available models [33], with an operation period of 2 months and approximately three sludge ages.
The design in this work was executed using STOAT for three distinct scenarios. The first scenario involves simulating the existing process units without any modification (model ASAL1). The second and third scenarios involve modifying and simulating a WWTP, which includes offering different models (ASAL1A and ASAL5A) while keeping the existing layout in the field. In general terms, the main components in wastewater are organic matter (OM), nutrients, microorganisms, and metals [15,21]. All these components can be present in dissolved or suspended form and can be assessed through the concentration of material in suspension or dissolved [11,13,24].
It has been estimated that the levels of nutrients are higher among users who typically belong to higher income categories, such as the upper-class (AB) and upper-middle-class (C1a) [34]. They do not utilize toilets for excreta disposal, instead opting to discard urine periodically, possibly because of their close proximity to the study site. It is widely recognized that human urine contains a significantly higher concentration of nutrients compared to feces. In fact, it is responsible for nearly 80% of the nitrogen load and 56% of phosphorus in treatment plants [19,35,36].
This alters the properties of the influent and does not align with the projected daily flow rates for each user, resulting in a decrease in the amount of organic matter and flow. This describes a situation in wastewater where there is an imbalance between nitrogen compounds and carbonaceous compounds, leading to a low C/N ratio [22,37,38].
Conventional wastewater treatment includes physical, chemical, and biological procedures to remove solids and organic/inorganic materials from wastewater [15,31]. The activated sludge process is a biological process where microbial biomass oxidizes and mineralizes the organic matter present in wastewater and also serves for phosphorus and nitrogen removal [6,12,35,39,40].
These microbiological reactions can occur under aerobic conditions (presence of dissolved oxygen, DO), anoxic conditions (absence of DO, presence of nitrates), or anaerobic conditions (absence of DO and nitrates) [41]. In the aerobic zone, microorganisms are kept in suspension in the water by aeration [13].
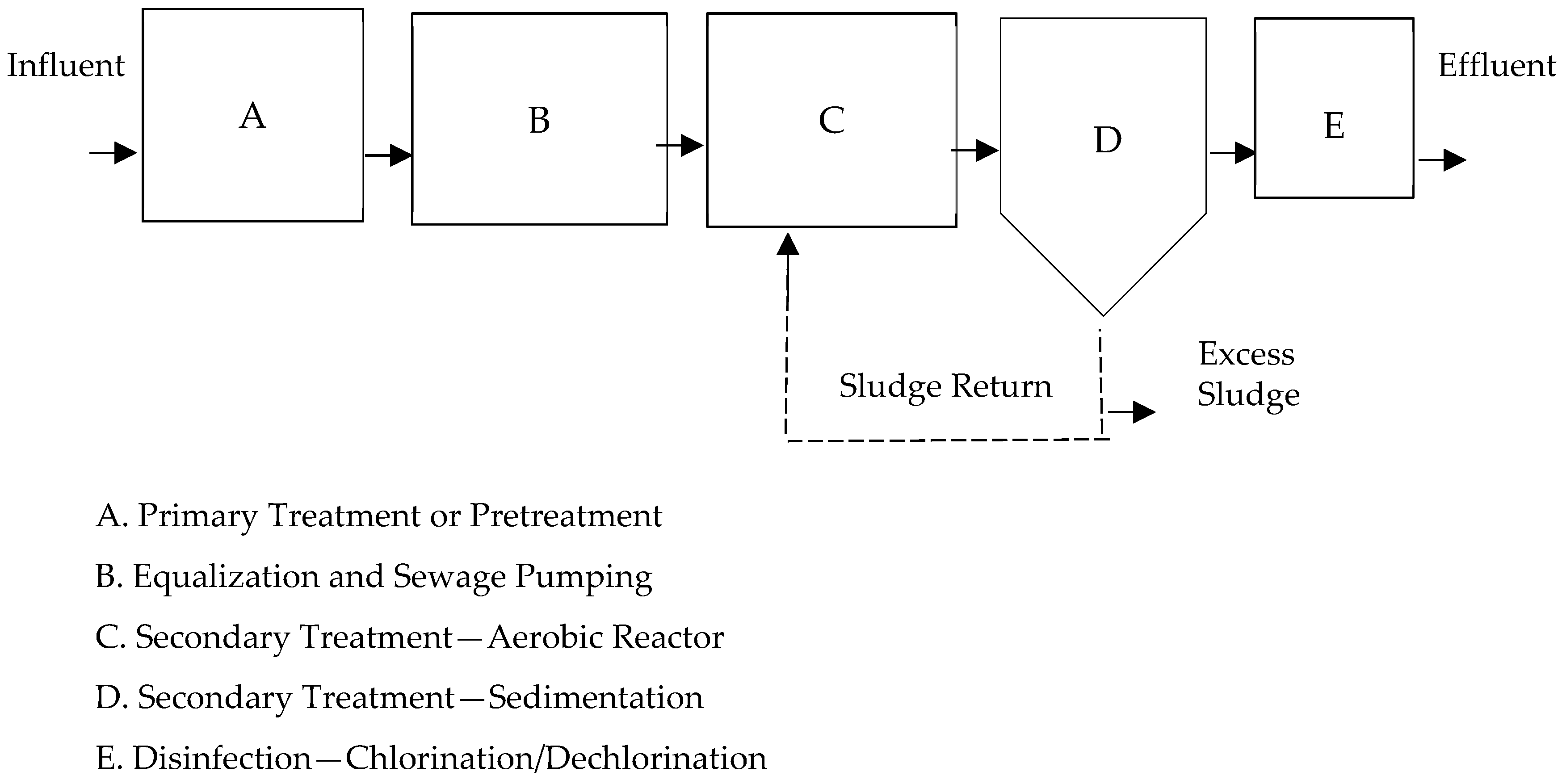
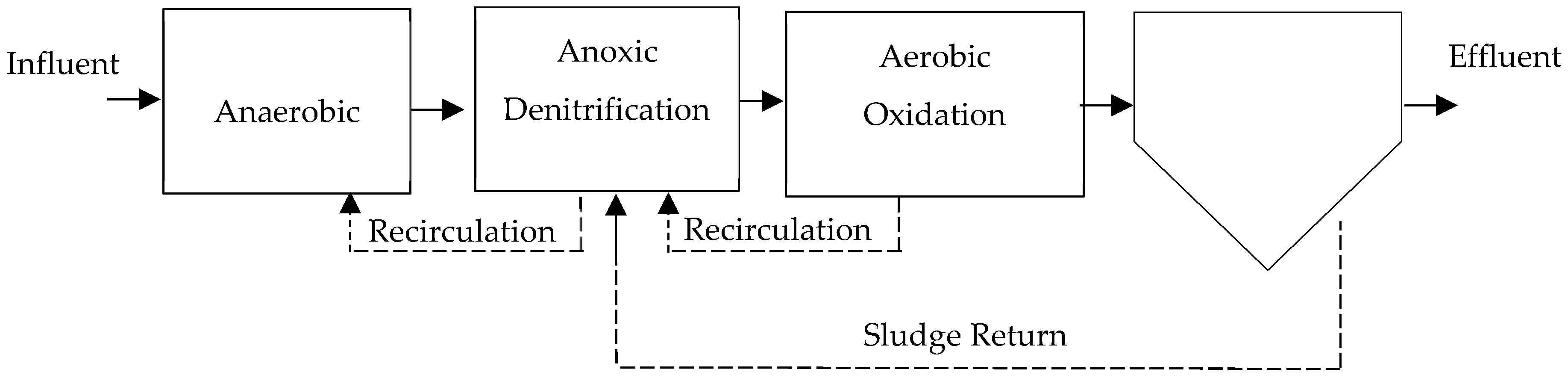
The extended aeration process corresponds to a variant of the conventional process, where the solids retention times (SRT) in the system are usually longer [42], typically between 20 and 30 days, an example in Figure 1. It operates in the endogenous phase of the growth curve, which requires a reduced organic load and prolonged aeration periods [9,25,43].
Figure 1.
Typical extended aeration wastewater treatment plant (WWTP) layout; adapted from [44].
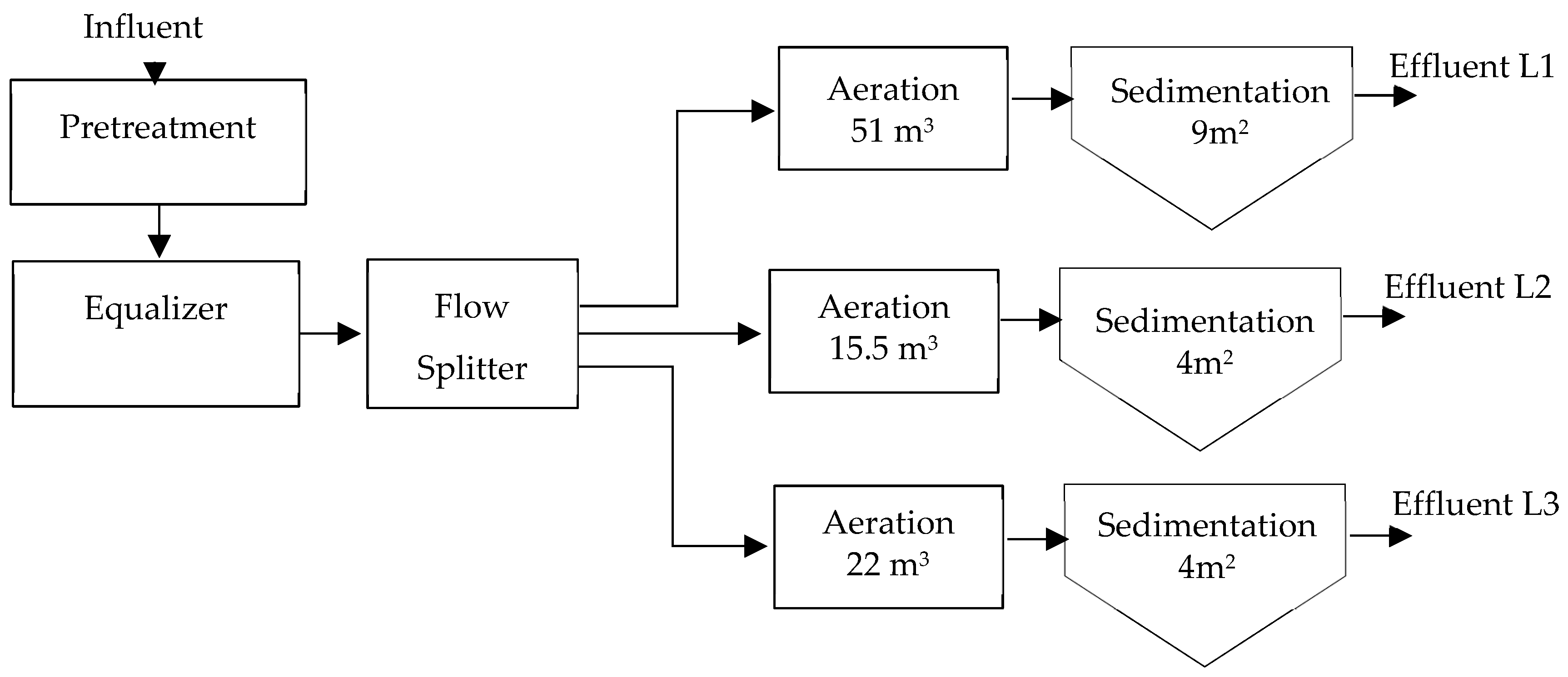
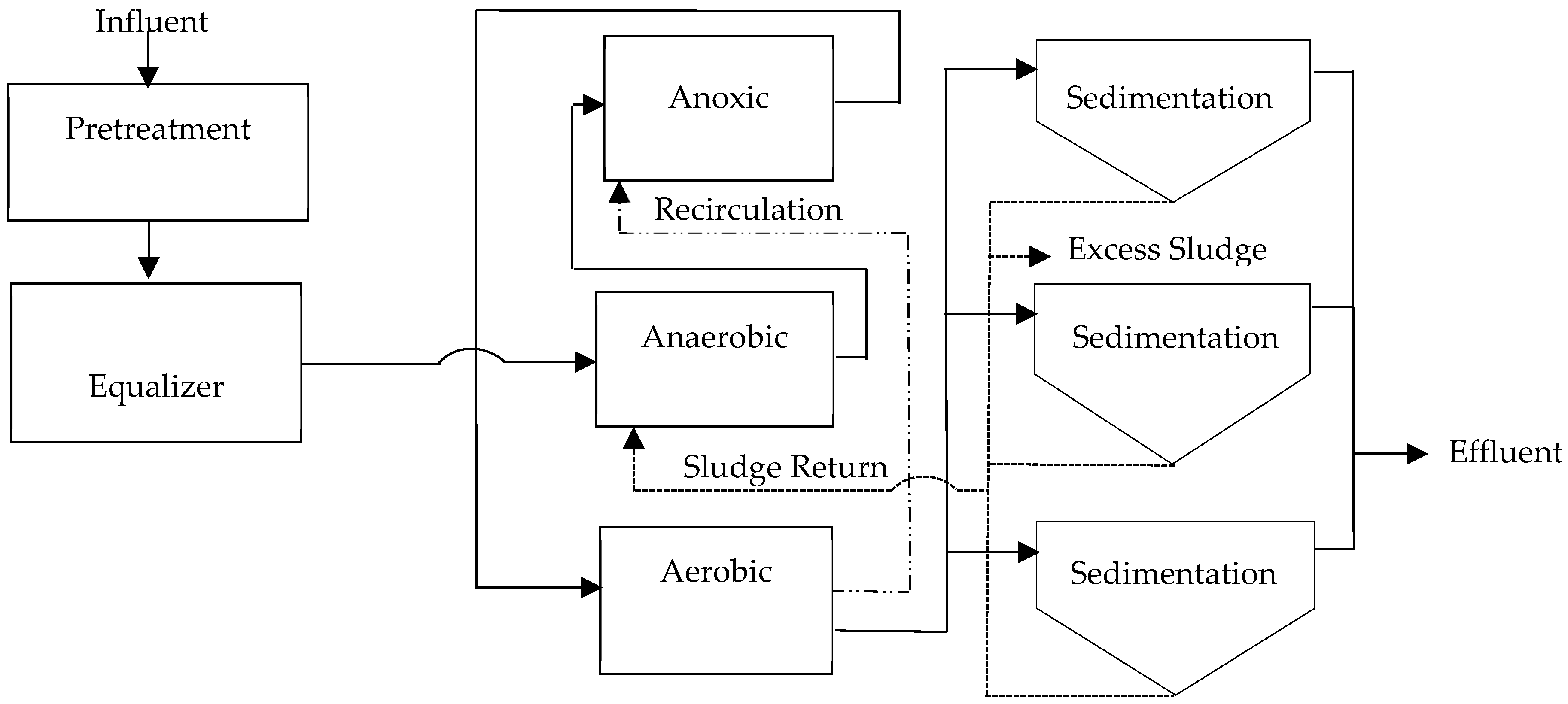
The flows in the existing plant vary throughout the day and during the week due to the class schedule regime. The system has an interceptor chamber for oils and fats as pretreatment. As for ambient temperature, on a normal day, a delta of 15 °C is easily reached between day and night. The secondary treatment process is carried out by three lines in parallel, distributing the flow proportionally to the volume, see diagram in Figure 2.
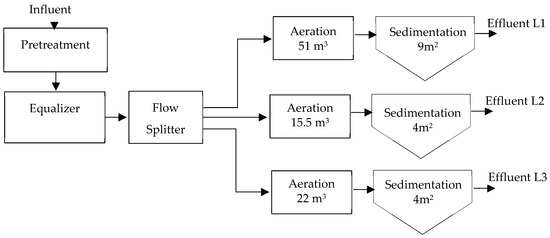
Figure 2.
Existing diagram installed in parallel of extended aeration wastewater treatment plants (WWTPs); self-made.
This configuration was determined because lines 1 and 2 were existing from an initial stage, and a third line was added. Each of the secondary stages receives the flow from a flow splitter proportionally. As additional data in the design, a 100% sludge recirculation is considered, a BOD5 of 250 mg/L (a higher value than what is present in the field), NTK less than 50 mg/L, and TSS of 4200 mg/L in the reactor, which differs substantially from reality.
James Barnard’s work in the early 1970s led to significant advancements in processes for biological nitrogen and phosphorus removal using BOD in the influent [29,45]. He initially created a process configuration with internal recycling that utilized the BOD of the influent for denitrification. This configuration eventually became the widely accepted nitrogen removal process, now referred to as the modified Ludzack-Ettinger (MLE) process [16,20,46,47]. This process demonstrates varying levels of efficiency, ranging from 48% to 84% [48].
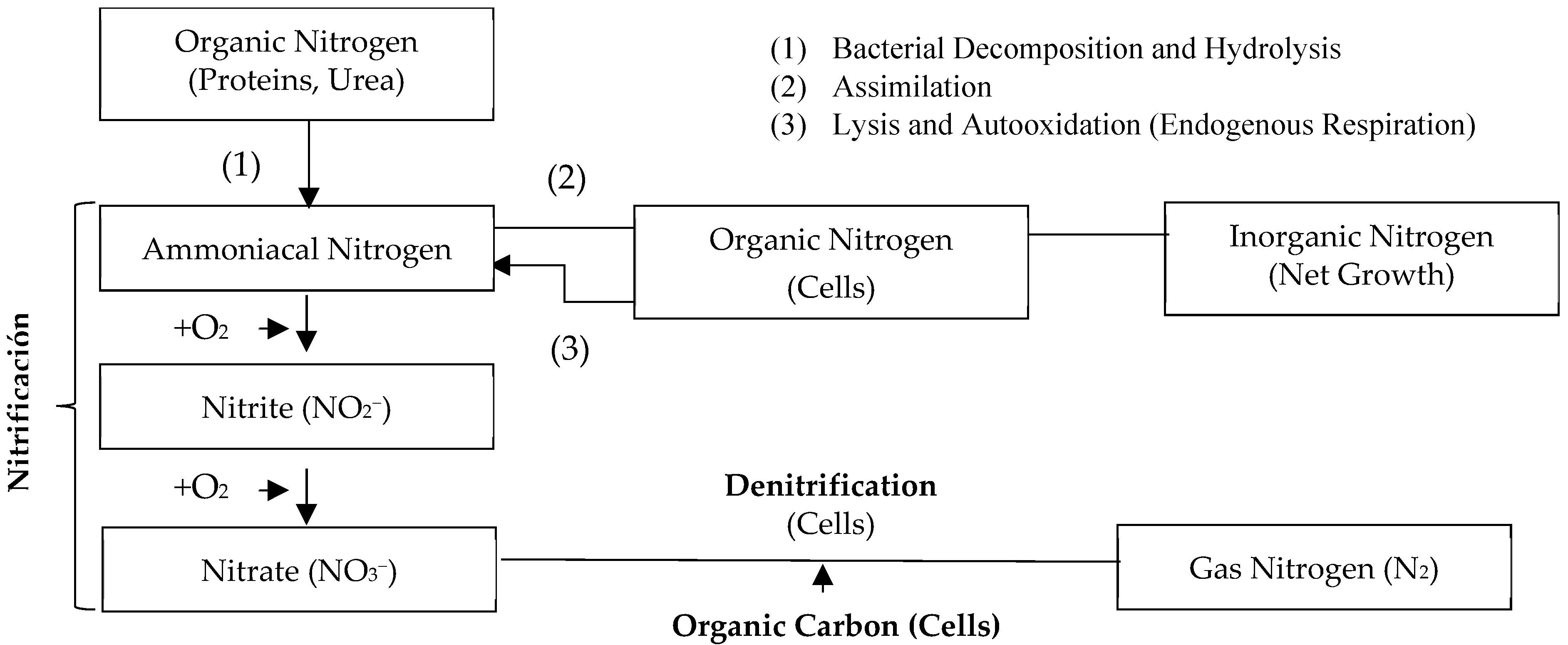
Biological nutrient removal (BNR) is an economically feasible process [9,17,45]. It removes total nitrogen and phosphorus from wastewater using microorganisms under different environmental conditions [14,46]. The availability of carbon affects the removal capacity of N and P [18,37]; for this reason, the delicate balance between organic carbon, N, and P levels has a significant impact on the removal of the latter in these treatment systems [49]. The nitrogen transformations are schematized below in Figure 3.
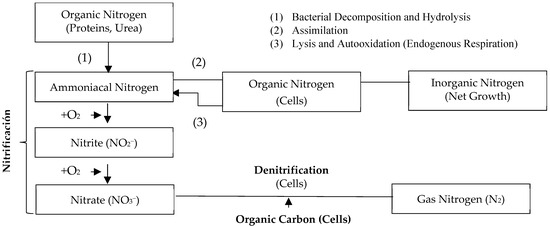
Figure 3.
Nitrogen transformations in biological treatment. Adapted from [50].
Despite the above, there is no consensus regarding the efficiency of phosphorus removal. In a system designed for simultaneous nitrification and denitrification, this may not always be efficient. Meanwhile, other studies have hypothesized that the microbial population responsible for phosphorus removal has a group capable of using oxygen or nitrate as an electron acceptor, a process carried out by a unique group of bacteria called Anammox [51,52,53]. These bacteria would allow simultaneous denitrification and phosphorus removal using the same substrate source [24] with almost 90% efficiency [54]. This is important considering that the organic matter content is often limited for simultaneous processes [13].
Under anoxic conditions, biomass releases phosphorus into the liquor in the form of soluble orthophosphates. Under aerobic conditions (high aeration rate), the phosphorus-poor biomass absorbs this compound [22].
Some researchers highlight the potential of aerobic granular sludge (AGS) technology as a viable alternative to conventional activated sludge systems for simultaneous nitrification and denitrification [55]. Another option is micro-aerobic-activated sludge (MAS), a single-stage process that operates with 0.5 to 1.0 mgO2/L [56].
2.1. Existing Situation and Case Analysis
In this section, we provide an overview of the commonly used systems for treating excess nutrients. We discuss their main characteristics, configurations, and processes in accordance with the available literature. In addition, the current state is being modeled, which involves incorporating influent data into WRc STOAT.
In Table 3, it is evident that the values of nitrogen and phosphorus in the effluent are high due to the conditions of low organic load or little substrate, as described earlier, which were already predictable through modeling, being very similar to the effluent results in Table 1, with a value of 69.9 mg/L.

Table 3.
Modeling results with WRc STOAT Software. Current situation for each treatment line.
Table 4 compares the removal capacities of TN and TP of common BNR configurations in general. It should be noted that each location dictates the performance of each process.

Table 4.
Comparison of common Biological Nutrient Removal (BNR) configurations.
2.2. Biological Nitrogen Removal
The nitrogen that exists in urban wastewater is primarily in the form of ammoniacal nitrogen and organic nitrogen, with proportions of 60% and 40%, respectively. The presence of nitric and nitrous forms is minimal, accounting for less than 1%. WWTPs that are designed to facilitate nitrification and denitrification processes have the ability to eliminate a significant portion of inorganic nitrogen, typically ranging from 80% to 95%. However, the removal of organic nitrogen is generally not as effective [16].
2.2.1. Factors Controlling Biological Nitrogen Removal
Most aerobic wastewater treatment systems contain microorganisms capable of nitrification (belonging to the following genera: Nitrosomonas, Nitrosococcus, Nitrobacter) [25,38,57] and denitrification (belonging to the following genera: Alcaligenes, Paracoccus, Pseudomonas, Thiobacillus, Rhizobium, Thiosphaera), provided that the conditions of cell retention time (CRT) and temperatures are appropriate [12,24,38,57,58]. Below, Venegas [24] and CONAGUA [25] describe some important factors in nitrogen removal.
- Temperature: Temperature significantly affects the growth rate (µmax) of nitrifying organisms. Nitrification occurs within a temperature range of 5 to 50 °C, with optimal performance at temperatures of 25 to 36 °C. Experimental studies report that the denitrification rate increases up to a certain point (35–50 °C) and then decreases.
- Dissolved Oxygen Concentration: Dissolved oxygen (DO) is a limiting factor, considering that Nitrosomonas are aerobic bacteria. The reference value in the aerobic zone for nitrogen removal is between 0.5 and 2.5 mgO2/L. Some studies on nitrification with high ammoniacal nitrogen content indicate an optimal DO of 1.7 mg/L. In winter, 2 to 3 mgO2/L is suggested.
- pH: Optimal nitrification rates are 7.0 < pH < 8.5, with a sharp decrease outside of this range. Metcalf and Eddy [43] reported a pH range between 6.0 and 9.0.
All biological nitrogen removal processes include an aerobic zone where nitrification takes place. This is the two-step biological process where free and saline ammonium (NH4+-N) is oxidized to nitrite (NO2-) and nitrite is further oxidized to nitrate (NO3-) by autotrophic aerobic bacteria [9,12,42,58,59], thus completing the denitrification to nitrogen [60].
2.2.2. Simultaneous Nitrification and Denitrification (SND)
In accordance with the traditional understanding of nitrogen removal, it has been believed that achieving simultaneous nitrification and denitrification is not possible. This is because nitrification happens in the presence of oxygen, while denitrification requires the absence of molecular oxygen [12,25,38,50,58]. In addition, the rate of nitrification is slow and expensive; autotrophs are easily affected by high levels of organic matter, and denitrifiers are sensitive to oxygen [14].
Denitrification is the biological reduction of nitrate to nitric oxide, nitrous oxide, and nitrogen gas [11,16,25,55]. This process is carried out by heterotrophic and autotrophic biocenosis [58,61]. Most of these microorganisms are facultative aerobes, meaning they can use oxygen and nitrate or nitrite as electron acceptors, and in some cases, they can even carry out fermentation in the absence of nitrate or oxygen [41,43]. To carry out denitrification, microorganisms require a source of organic carbon to oxidize [11,58,62].
Wastewater must contain sufficient organic matter in both quantity and quality for biological nutrient removal processes to occur. Heterotrophic microorganisms responsible for phosphorus removal also require sources of carbon/energy; thus, a higher C/N ratio is needed to achieve the simultaneous removal of nitrogen and phosphorus [37,63,64].
If the carbon is provided by the wastewater itself, oxygen consumption in aerobic phases will decrease, as much of the organic matter will have oxidized during the denitrification process. However, if the organic matter is depleted beforehand, an external carbon source will be necessary [18,27,37,41]. The carbon source is an important cost factor to consider in operation [28].
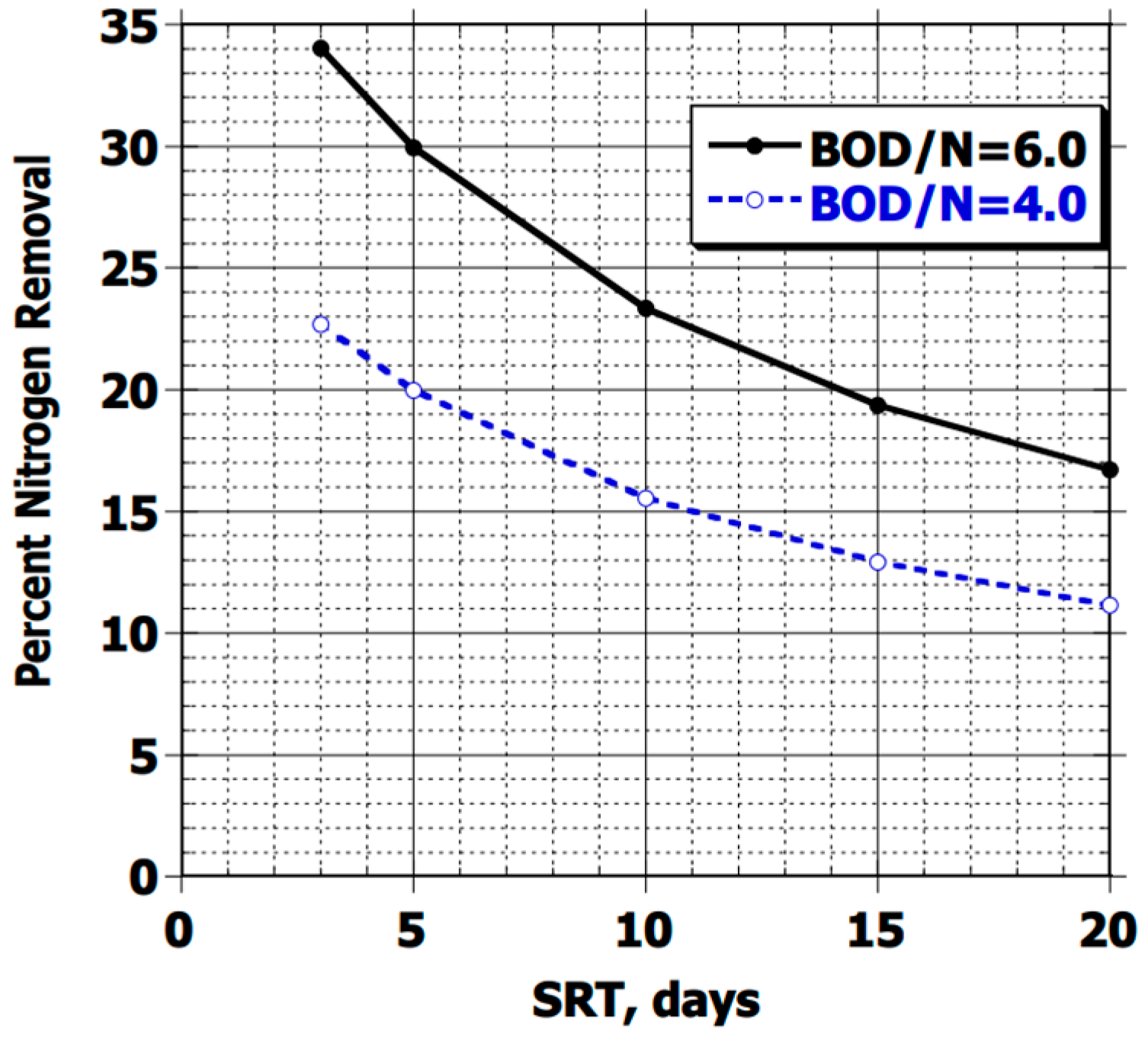
In the literature, various external sources of organic matter are proposed, including methanol [13,24,25,28,58,63,65], which yields better results than methane, acetic acid, ethanol, and sugar (sucrose) [16,42,49,61]. Depending on the source used, different denitrification rates can be achieved, but at an additional operating cost [18,63]. A general rule is that 4 g of BOD from the influent is needed per gram of NO3− to be removed through biological treatment according to the USEPA [16]. The latter also indicates that the efficiency of nitrogen removal through biomass synthesis depends on the biological process of the influent, that is, on the ratio of DBO/NT and the biomass solid retention time (SRT), as shown in Figure 4. This coincides with what Cao et al. [62] mentioned, with a ratio for BNR of 3 and 4.3, and 5 for industrial water, according to Qi et al. [63].
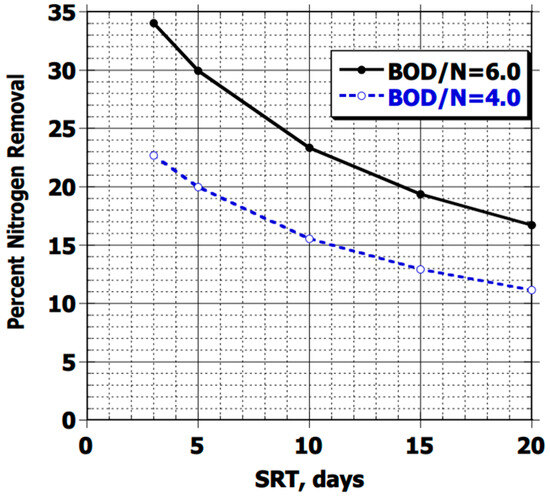
Figure 4.
Percentage of nitrogen removal due to biomass synthesis as a function of SRT and influent DBO/N ratio, obtained from [16].
- Bardenpho System: This system is widely recognized and consists of four stages that alternate between anoxic and aerobic zones (anoxic/aerobic/anoxic/aerobic) [16,29]. The system observed in Figure 5, utilizes the carbon found in the raw water and the carbon produced from the breakdown of biomass for biological denitrification.To accomplish this, distinct stages are employed for each process: carbon oxidation, nitrification, and denitrification [43,50]. Therefore, the water flows into a zone of anoxic denitrification. In this zone, mixed liquor from the oxidation and nitrification tank, which is located downstream of the first anoxic zone, is recirculated [22,24,25,58].
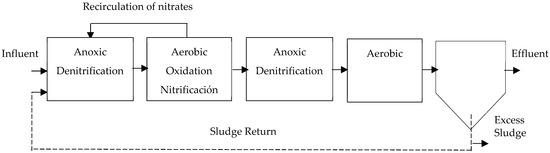
Figure 5.
Bardenpho 4-stage process, adapted from [13].
Figure 5.
Bardenpho 4-stage process, adapted from [13].
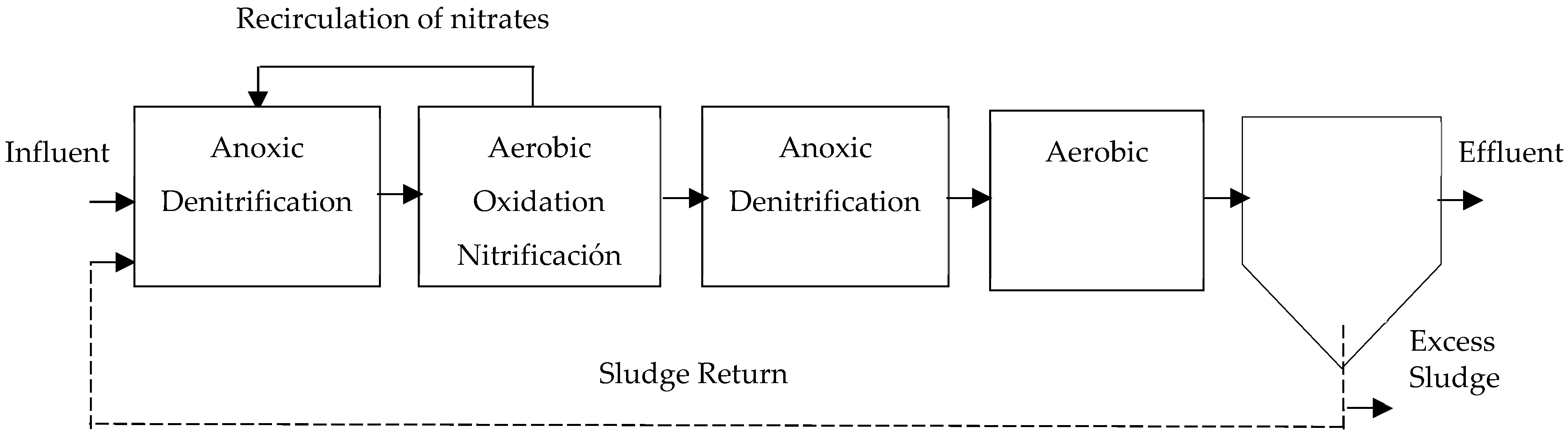
The removal efficiency in the Bardenpho system reaches 90%, but it requires large-sized reactors [25]. Complete nitrogen removal is achieved by using two denitrification zones, resulting in very high hydraulic retention times [41]. The addition of an external carbon substrate (methanol) can achieve highly efficient nitrogen removal [26,29].
2.3. Biological Phosphorus Removal
Organic phosphorus can exist in different forms, such as soluble, colloidal, or particle. Typically, particulate and colloidal phosphorus settle and are eliminated with the sludge [14,66]. Phosphorus is present in domestic wastewater in various forms, including orthophosphates, polyphosphates, and organic phosphorus. The main sources of phosphorus in wastewater are human excreta, detergents, and wastewater from chemical treatments [22,24,41,66]. As per the research conducted by Metcalf and Eddy [43], the concentration of total phosphorus (TP) in urban wastewater typically ranges from 4 to 15 mgP/L, with the majority of it being soluble inorganic phosphorus.
In anaerobic zones, certain bacteria are capable of accumulating excessive amounts of phosphorus in the form of polyphosphates; these microorganisms are called phosphorus-accumulating organisms (PAO) [10,25,46]. They convert available organic matter (for example, volatile fatty acids) into carbon compounds called polyhydroxyalkanoates (PHA) [12,17,46]. In the aerobic zone, there is molecular growth based on the absorption of soluble BOD and the oxidation of orthophosphates, resulting in energy production. Orthophosphate is accumulated in the cellular material as polyphosphate [41]. As the organic matter (OM) increases, there is a proportional increase in volatile fatty acids (VFA) that promotes the growth of PAO [48]. Some PAOs use nitrate instead of free oxygen to oxidize stored PHA and absorb phosphorus. These denitrifying PAO remove phosphorus in the anoxic zone instead of the aerobic zone [17,46].
2.3.1. Factors Controlling Biological Phosphorus Removal
The removal process of phosphorus can also be affected by environmental factors such as pH, type, and availability of organic matter, excessive aeration, cell retention time, temperature, and the presence of nitrates and nitrites, among others. Venegas [24] and Curtin et al. [57] indicate the following factors, which coincide with those described by Wang [17] and CONAGUA [25].
- Availability of volatile fatty acids (VFA): The availability of easily biodegradable substrate, mainly in the form of VFA, is of great importance for the development and performance of the biological phosphorus removal process.
- Nitrate concentration: Where nitrification is required to meet the effluent concentration, the presence of nitrates affects the efficiency of a treatment system aimed at biological phosphorus removal.
- Dissolved oxygen concentration: This parameter is necessary for the aerobic phase to carry out the assimilation of orthophosphates, and it has been indicated that a concentration of 1.5–3.0 mg O2/L may be optimal without affecting the anoxic or anaerobic states of a combined process.
- pH: The pH (hydrogen potential) is a crucial element in the biological phosphorus elimination process and should be controlled within the range of 6.5–8.0.
- Temperature: Microbial populations are impacted by temperature fluctuations ranging from 20 °C to 35 °C. Maintaining a temperature below 30 °C offers specific benefits to the PAO in terms of metabolism. Lower temperatures need longer retention durations.
2.3.2. Proposed Schemes for Phosphorus Removal
Biological phosphorus removal procedures encompass several types that involve the combination of aerobic and anaerobic conditions. An uncomplicated procedure involves the use of two reactors arranged in a sequential manner, with the initial reactor being anaerobic and the subsequent one being aerobic [24,41,43,58]. The three-stage Phoredox or A2/O process, which was introduced by Barnard, is referred to as such [24,25,45].
- A2/O System: This process is based on the A/O (anaerobic/aerobic) system, which was developed as the Phoredox system in South Africa and later patented in the United States as A/O [16,57]. It was designed specifically for phosphorus removal, incorporating an anoxic phase in the middle of the flow line [37,41,67]. Therefore, the arrangement consists of two sets of tanks, one for anoxic conditions and the other for aerobic conditions, placed consecutively, as can be appreciated in Figure 6. The first tank is in an anaerobic environment [33].Sludge recirculation to the anaerobic tank occurs at varying rates between 100 and 400% [24,25,43]. Hu et al. [37] states that efficiencies in TN removal above 50% and TP removal above 40% were achieved without the need for an external carbon source. Authors have noted significant efficiencies ranging from 67 to 89% [48].
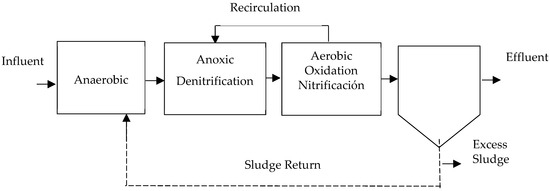 Figure 6. A2/O process, adapted from [41].Figure 6. A2/O process, adapted from [41].
Figure 6. A2/O process, adapted from [41].Figure 6. A2/O process, adapted from [41].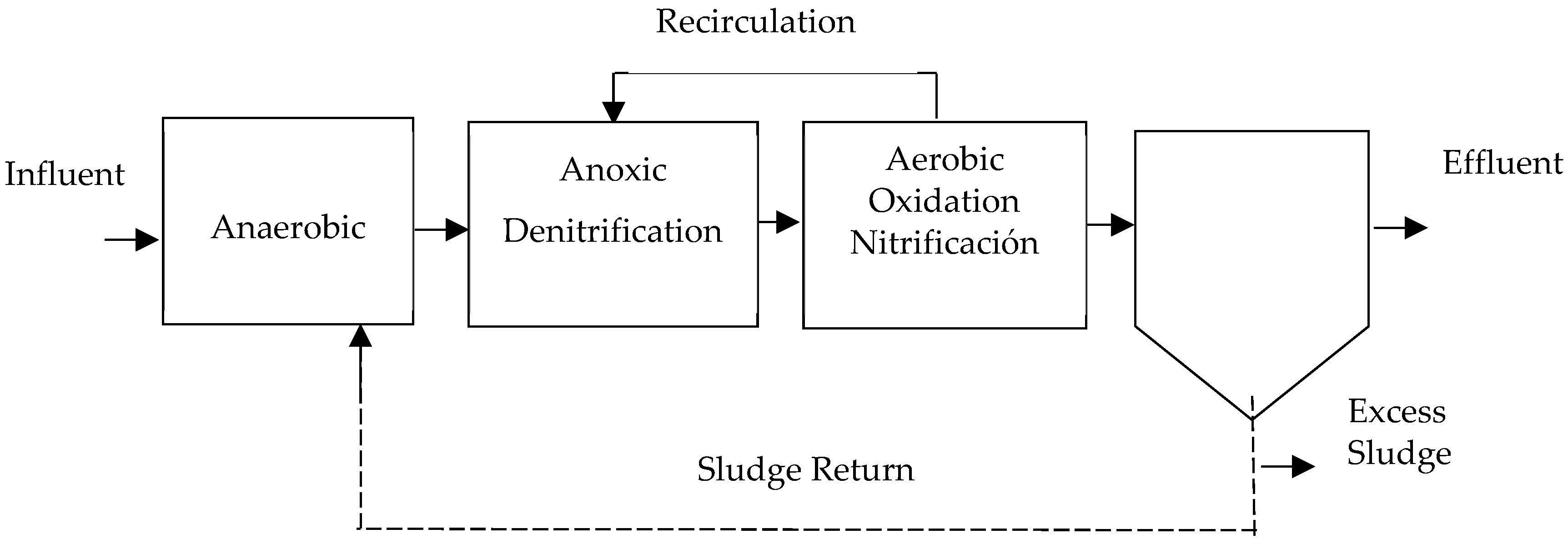
- Modified Bardenpho System: It is an addition to the earlier four-stage process that removes phosphorus by adding an anaerobic step at the beginning of the line, as shown in the configuration in Figure 7, as explained by Escaler and Mujeriego [41] and Curtin et al. [57]. It is also known as the five-stage Bardenpho or Phoredox [12]. They also describe it as a system for the simultaneous removal of N and P, which aligns with what CONAGUA [25] describes. Authors report efficiencies between 67 and 89% for this system [48].
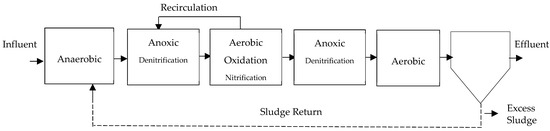 Figure 7. Modified 5-stage Bardenpho process, adapted from [43].Figure 7. Modified 5-stage Bardenpho process, adapted from [43].
Figure 7. Modified 5-stage Bardenpho process, adapted from [43].Figure 7. Modified 5-stage Bardenpho process, adapted from [43].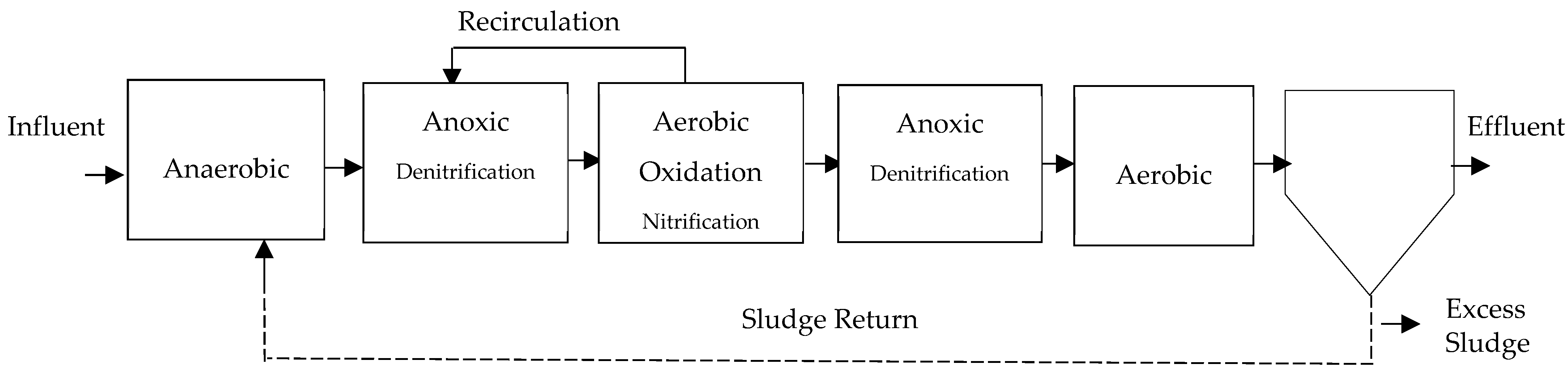
Venegas [24] states that the use of an external carbon supply can result in nitrogen removal rates up to 90%. Additionally, around 70% of the nitrate created in the system is removed during the first anoxic phase. During the aerobic phase, ammonium is oxidized, phosphorus is stored, and organic matter is removed. In the following stage, under oxygen-depleted circumstances, an extended period is upheld to allow microorganisms to undergo denitrification by their own respiration process. During this process, nitrates are utilized as electron acceptors instead of oxygen. According to Curtin et al. [57], the ideal dissolved oxygen (DO) levels in the anaerobic stage should be 0 mgO2/L; in the anoxic stage, it should be between 0 and 0.1 mgO2/L; and in the aerobic zone, it should be between 1.5 and 2.0 mgO2/L. Research indicates a reduction in the ultimate levels of biodegradable chemical oxygen demand (COD), ammonia, and nitrate when compared to the conventional Bardenpho system [29].
- University of Cape Town (UCT): The UCT process was designed to reduce nitrates in the anaerobic zone when high nitrate removal in the effluent is not required, similar to the A2/O [43,67]. Like other nitrogen and phosphorus removal technologies, it consists of three stages: an anaerobic stage, an anoxic stage, and an aerobic stage [12], as seen in Figure 8. The return-activated sludge returns from the clarifier to the anoxic zone instead of the anaerobic zone to allow for denitrification. The modified process divides the anoxic zone into two stages. Nitrate-rich recycle from the aerobic zone is recycled to the head of the second anoxic stage [16,67,68].
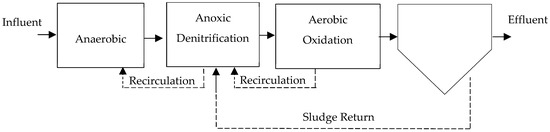 Figure 8. UCT configuration for combined biological removal, adapted from [43].
Figure 8. UCT configuration for combined biological removal, adapted from [43].
3. Results
The findings of Table 5 provide a concise overview of the outcomes from many simulations conducted through the utilization of the WRc STOAT program. In the Bardenpho procedures, sedimentation tanks were utilized as anoxic tanks to optimize the utilization of all available elements and volumes on site.

Table 5.
Comparison of configurations using WRc STOAT software.
Now, there is only one treatment line (arranged in series) that will not affect the system since the flows, according to the records and in situ measurements, are much lower than those projected in the design stage.
After analyzing the relevant literature and simulations, the shortcomings of the project were identified, and the following general plant layout was proposed to obtain better results in water analyses. The configuration with the best results in the modeling is the A2O system. Existing module and system locations will be utilized, with modifications only to recirculation and feed lines, according to the proposed scheme below in Figure 9.
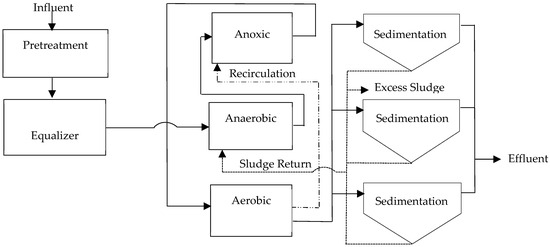
Figure 9.
Proposal for modification of wastewater treatment plant (WWTP) for nutrient removal.
4. Discussion
Research has shown that a higher anoxic/aerobic ratio results in improved efficiency in the removal of biological nitrogen [69]. Here, the ratio is around 2, which accounts for the enhanced nitrogen removal effectiveness when there is a large volume in the anoxic stage. Shen et al. [18] suggests that stepped feeding may be used to improve the distribution of carbon sources.
The absence of substrate or organic matter (human excreta) has a direct impact on the functioning of the WWTP in terms of nutrient removal. Hence, it is advisable to incorporate an additional carbon source [65], as several simulations have demonstrated that nitrogen removal alone is inadequate. Another option to consider is the utilization of magnetic pyrite as a medium for the formation of biofilms. This can create a conducive environment for denitrification, as suggested by Zhang et al. [70]. Additionally, artificial wetlands have been proven effective in removing nutrients, as demonstrated by Díaz et al. [5] and Bai et al. [38].
The study does not take into account the cost/energy impact when solving the system—the primary objective was to identify a viable strategy to decrease the surplus nutrients that exceeded the discharge limitations. Conversely, confirming that the flow to be processed is less than the originally planned flow requires making adjustments and decreasing the airflow or pumping duration in the aerobic reactor, leading to a decrease in energy consumption.
In the future, it may be worth considering the separation of urine, as suggested by Jimenez et al. [35], Pradhan et al. [71], and Saetta et al. [72]. This may be achieved by the use of pee-diverting toilets, as proposed by Flanagan and Randall [36], to recover nutrients that can be reused in soil, for instance [73]. Another option is the diversion of nitrite, which is a method of partially controlling nitrification. This method limits the conversion of ammonia to nitrite and requires 40% less carbon compared to typical nitrification–denitrification, as stated by Hodgson [48].
Moreover, the literature shows that food waste can be utilized as a carbon source for nutrient removal in wastewater treatment [74]. It can also be used to recover volatile fatty acids (VFAs) for the denitrification stage of the nitrification/denitrification process, serving as an alternative method for nitrogen removal, as suggested by Qi et al. [63,75].
Finally, another valid option could be chemical treatments, such as advanced oxidation processes (AOPs), which exhibit a notable capacity to efficiently remove nitrogen from wastewater [27], with ozonation being one of them.
5. Conclusions
It was found that simulation is highly necessary in the design stage. It is common for traditional designs of compact plants (small-scale) to primarily focus on BOD removal while overlooking other contaminants. However, this narrow focus can lead to inadequate treatment of other pollutants, such as nutrients (nitrogen and phosphorus), pathogens, and emerging contaminants. This is where the importance of being able to conduct a simple simulation of compact systems lies. By utilizing simulation tools, designers and engineers can assess the performance of compact treatment systems beyond BOD removal.
The systems were oversized, which allowed for the necessary combinations to be made to find the best arrangement and solution for nutrient reduction. The literature review was fundamental for this purpose. It is also worth noting that the modeling results, regarding nutrient removal, show percentages very similar to those expressed by Demir et al. [67] in their study.
It is highly recommended to conduct an analysis of the population to be served, including their habits, activities, socioeconomic group, influent characteristics, etc., in order to more accurately determine the type of treatment system to be implemented, which aligns with the findings of [21]. Additionally, whenever possible, taking samples and analyzing the influent can better characterize the water to be treated and make decisions based on the results of such tests.
Relying solely on calculations based on empirical results of projects carried out repeatedly does not guarantee that design conditions or criteria will be met, as previously demonstrated. Technological advancements in simulation should be utilized to improve designs, helping to predict and address events or issues that could potentially harm the environment and human health.
In summary, compact treatment systems represent a universe that is no smaller in relation to large plants. Each compact system, individually, can contribute to environmental damage and excess pollution due to lower regulatory oversight. Simple and free software solutions can serve as a useful tool for verifying manual calculations in these types of plants.
Author Contributions
Conceptualization, M.A.D. and D.B.; methodology, M.A.D.; validation, R.C.-J. and D.B.; formal analysis, M.A.D. and R.C.-J.; investigation, M.A.D.; software, M.A.D. and D.L.C.; resources, M.A.D.; writing—original draft preparation, M.A.D.; writing—review and editing, M.A.D. and A.D.; visualization, D.L.C. and A.D.; supervision, M.A.D. and M.B.A.-C.; project administration, D.B. and P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SISS. Superintendencia de Servicios Sanitarios, Informe de Coberturas Sanitarias. 2020. Available online: https://www.siss.gob.cl/586/articles-19543_recurso_1.pdf (accessed on 29 April 2024).
- Chile—Ministerio Secretaría General de la Presidencia (MINSEGPRESa). Decreto Supremo N°46, Establece Norma de Emisión de Residuos Líquidos a Aguas Subterraneas. 2003. Available online: https://bcn.cl/2f864 (accessed on 29 April 2024).
- Chile—Ministerio Secretaría General de la Presidencia (MINSEGPRESb). Decreto Supremo N°90, Establece Norma de Emisión Para la Regulación de Contaminantes Asociados a las Descargas de Residuos Líquidos a Aguas Marinas y Continentales Superficiales. 2000. Available online: https://bcn.cl/2f7i3 (accessed on 29 April 2024).
- Chile—Ministerio Secretaría General de la Presidencia (MINSEGPRESc). Decreto Supremo N°609, Establece Norma de Emisión Para la Regulación de Contaminantes Asociados a las Descargas de Residuos Industriales Líquidos a Sistemas de Alcantarillado. 1998. Available online: https://bcn.cl/2f8hn (accessed on 29 April 2024).
- Díaz, M.A.; Decinti, A.; Blanco, D.; Vasquez, K. Metodología para la reutilización de aguas grises en viviendas ubicadas en áreas de estrés hídrico y estrés hídrico extremo—Caracterización, calidad y opciones de tratamiento para su reuso en Chile. Inf. Construcción 2021, 73, e408. [Google Scholar] [CrossRef]
- Ireland. Environmental Protection Agency (EPA). Wastewater Treatment Manuals: Treatment Systems for Small Communities; Business, Leisure Centres and Hotels; Environmental Protection Agency: Johnstown Castl Estate, Co.: Wexford, Ireland, 1999; Available online: https://www.epa.ie/publications/compliance--enforcement/waste-water/EPA_water_treatment_manual_-small-comm_business.pdf (accessed on 29 April 2024).
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the Domestic Wastewater Treatment (DWWT) Regimes: A Review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Barañao, P.; Tapia, L. Tratamiento de las Aguas Servidas: Situación en Chile. Rev. Cienc. Trab. Número 2004, 13, 111–117. Available online: https://research.csiro.au/gestionrapel/wp-content/uploads/sites/79/2016/11/Tratamiento-de-las-aguas-servidas-Situaci%C3%B3n-en-Chile-2005.pdf (accessed on 29 April 2024).
- Pérez Oddershede, A. Selección de Sistema de Tratamiento de Aguas Residuales para Localidad de Santa Bárbara Usando Metodología de Decisión Multicriterio, A.H.P; Tesis para Ingeniería Civil, Universidad de Chile: Santiago, Chile, 2010; p. 110. Available online: https://repositorio.uchile.cl/handle/2250/103989 (accessed on 29 April 2024).
- Jing, W.; Sajnani, S.; Zhou, M.; Zhu, H.; Xu, Y. Evaluación del impacto ecológico de las descargas de aguas residuales en la dinámica microbiana y de contaminantes en los ríos. Agua 2024, 16, 377. [Google Scholar] [CrossRef]
- Choi, I.S.; Dombrowski, E.M.; Wiesmann, U. Fundamentals of Biological Wastewater Treatment; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 978-3-527-31219-1. [Google Scholar]
- Henze, M.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008; ISBN 9781780401867. [Google Scholar] [CrossRef]
- Claros Bedoya, J.A. Estudio del Proceso de Nitrificación y Desnitrificación Vía Nitrito para el Tratamiento Biológico de Corrientes de Agua Residual con Alta Carga de Nitrógeno Amoniacal. Ph.D. Thesis, Departamento de Ingeniería Hidráulica y Medio Ambiente, Universitat Politècnica de València, Valencia, Spain, 2012. [Google Scholar] [CrossRef]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient removal from domestic wastewater: A comprehensive review on conventional and advanced technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review. Energies 2022, 15, 4981. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Treating for Nutrients in Wastewater. Office of Research and Development/National Risk Management Research Laboratory. 2010. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1008KTD.txt (accessed on 29 April 2024).
- Wang, Q.; Chen, Q. Simultaneous denitrification and denitrifying phosphorus removal in a full-scale anoxic–oxic process without internal recycle treating low strength wastewater. J. Environ. Sci. 2016, 39, 175–183. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, D.; Wu, Y.; Zhang, H.; Zhang, X. Operation mode of a step-feed anoxic/oxic process with distribution of carbon source from anaerobic zone on nutrient removal and microbial properties. Sci. Rep. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Potencial global y regional de las aguas residuales como fuente de agua, nutrientes y energía. Nat. Recurso. Foro. 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Slavov, A. Performance of Complete-Mix And Plug-Flow Systems During Treatment of Low Loaded Nitrogen Deficient Waste Water—Simulation with Asal1 Model. Food Environ. Saf. J. 2017, 15, 1–7. Available online: http://fens.usv.ro/index.php/FENS/article/view/211 (accessed on 29 April 2024).
- Wang, Y.; Cheng, Y.; Liu, H.; Guo, Q.; Dai, C.; Zhao, M.; Liu, D. A Review on Applications of Artificial Intelligence in Wastewater Treatment. Sustainability 2023, 15, 13557. [Google Scholar] [CrossRef]
- Cotoruelo, L.; Dolores Marqués, M. Eliminación de nutrientes en aguas residuales (I) Eliminación del fósforo. Ing. Química 1999, 365, 161–171. [Google Scholar]
- Winkler, M.; Coats, E.R.; Brinkman, C.K. Advancing post-anoxic denitrification for biological nutrient removal. Water Res. 2011, 45, 6119–6130. [Google Scholar] [CrossRef]
- Venegas, C.B. Eliminación Biológica de Nutrientes en Aguas Residuales con Alto Contenido de Nitrógeno Amoniacal Utilizando un Reactor Biológico Secuencial. Ph.D. Thesis, Escuela Técnica Superior de Ingenieros de Caminos, Canales y Puertos. Universidad De Cantabria, Cantabria, Spaña, 2016; p. 172. Available online: http://hdl.handle.net/10902/8451 (accessed on 29 April 2024).
- Comisión Nacional del Agua (CONAGUA). Libro 35. Manual de Agua Potable, Alcantarillado y Saneamiento, Diseño de Plantas de Tratamiento de Aguas Residuales Municipales: Procesos Avanzados con Fines de Reúso 2019; Secretaria de Medio Ambiente y Recursos Naturales: Tlalpan, México; ISBN 978-607-626-032-6. Available online: https://files.conagua.gob.mx/conagua/mapas/SGAPDS-1-15-Libro35.pdf (accessed on 29 April 2024).
- Zhang, H.; Li, R.; Shi, Y.; Pan, F. Effect of Aeration and External Carbon Source on Nitrogen Removal and Distribution Patterns of Related-Microorganisms in Horizontal Subsurface Flow Constructed Wetlands. Water 2024, 16, 632. [Google Scholar] [CrossRef]
- Omar, A.; Almomani, F.; Qiblawey, H.; Rasool, K. Advances in Nitrogen-Rich Wastewater Treatment: A Comprehensive Review of Modern Technologies. Sustainability 2024, 16, 2112. [Google Scholar] [CrossRef]
- Cui, X.; You, J.; Liao, K.; Ding, L.; Hu, H.; Ren, H. Carbon Source in Tertiary Denitrification Regulates Dissolved Organic Nitrogen in Wastewater Effluent. Environ. Sci. Technol. 2024, 58, 4648–4661. [Google Scholar] [CrossRef]
- Sarkar, U.; Dasgupta, D.; Bhattacharya, T.; Pal, S.; Chakroborty, T. Dynamic simulation of activated sludge based wastewater treatment processes: Case studies with Titagarh Sewage Treatment Plant, India. Desalination 2010, 252, 120–126. [Google Scholar] [CrossRef]
- Wang, W.; Shi, C.; Yang, J.; Zeng, M.; Dai, Z.; Zhang, Z. Modelling performance of oxidation ditch in wastewater treatment plant by STOAT software. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 032065. [Google Scholar] [CrossRef]
- Abbasi, N.; Ahmadi, M.; Naseri, M. Quality and cost analysis of a wastewater treatment plant using GPS-X and CapdetWorks simulation programs. J. Environ. Manag. 2021, 284, 111993. [Google Scholar] [CrossRef]
- Issa, H.M. Optimization of Wastewater Treatment Plant Design using Process Dynamic Simulation: A Case Study from Kurdistan, Iraq. Aro-Sci. J. Koya Univ. 2019, 7, 59–66. [Google Scholar] [CrossRef]
- Gao, Y.N.; Liu, X.Z.; Zhang, R.X.; Shan, J.J.; Sun, M.Q.; Zong, Z.X.; Li, C.W.; Wang, X.Z. Operation and management of Liaoning waste water treatment plants by STOAT Simulation. MATEC Web Conf. 2016, 63, 04019. [Google Scholar] [CrossRef]
- ANDA. Asociación Nacional de Avisadores A.G. Anda Chile. Estilos de Vida de los Nuevos Grupos Socioeconómicos en Chile. 2019. Available online: https://www.anda.cl/wp-content/uploads/2019/05/GfK_GSE_190502_FINAL.pdf (accessed on 29 April 2024).
- Jimenez, J.; Bott, C.; Love, N.; Bratby, J. Source Separation of Urine as an Alternative Solution to Nutrient Management in Biological Nutrient Removal Treatment Plants. Water Environ. Res. 2015, 87, 2120–2129. [Google Scholar] [CrossRef]
- Flanagan, C.P.; Randall, D.G. Development of a novel nutrient recovery urinal for on-site fertilizer production. J. Environ. Chem. Eng. 2018, 6, 6344–6350. [Google Scholar] [CrossRef]
- Hu, X.; Xie, L.; Shim, H.; Zhang, S.; Yang, D. Biological Nutrient Removal in a Full Scale Anoxic/Anaerobic/Aerobic/Pre-anoxic-MBR Plant for Low C/N Ratio Municipal Wastewater Treatment. Chin. J. Chem. Eng. 2014, 22, 447–454. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Chang, S. Effects of Different Carbon and Nitrogen Ratios on Nitrogen Removal Efficiency and Microbial Communities in Constructed Wetlands. Water 2023, 15, 4272. [Google Scholar] [CrossRef]
- Ya, L.; Gu, J.; Yu, L. Energy self-sufficient biological municipal wastewater reclamation: Present status, challenges and solutions forward. Bioresour. Technol. 2018, 269, 513–519. [Google Scholar] [CrossRef]
- Tiar, S.M.; Bessedik, M.; Abdelbaki, C.; ElSayed, N.B.; Badraoui, A.; Slimani, A.; Kumar, N. Steady-State and Dynamic Simulation for Wastewater Treatment Plant Management: Case Study of Maghnia City, North-West Algeria. Water 2024, 16, 269. [Google Scholar] [CrossRef]
- Escaler, M.; Mujeriego, R. Eliminación biológica de nutrientes (nitrógeno y fósforo) mediante un proceso discontinuo de fangos activados. Ingeniería Del Agua 2001, 8, 67–77. [Google Scholar] [CrossRef][Green Version]
- Cisneros-Ortiz, M.; Noyola, A. Metano Como Fuente de Carbono y Energía Para la Desnitrificación Biológica de Aguas Residuales. Rev. Aidis Ing. Cienc. Ambient. Investig. Desarro. Práctica 2011, 3, 74–85. [Google Scholar]
- Metcalf & Eddy, Inc. Treatment and Reuse, 4th ed.; Wastewater Engineering: New York, NY, USA; Mc Graw Hill: New York, NY, USA, 2003. [Google Scholar]
- Dunner, I. Evaluación Integral de PTAS y Alternativas de Tratamiento en Localidades Rurales Concentradas. Ph.D. Thesis, Programa de Magíster en Ciencias de la Ingeniería Mención Recursos y Medio Ambiente Hídrico, Universidad de Chile, Santiago, Chile, 2004; p. 172. Available online: https://repositorio.uchile.cl/handle/2250/102800 (accessed on 29 April 2024).
- Oleyiblo, J.; Jia-Shun, C.; Xiao-Guang, L. Troubleshooting a Full-scale Wastewater Treatment Plant for Biological Nutrient Removal. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 745–753. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Biological Nutrient Removal Processes and Costs, National Service Center for Environmental Publications (NSCEP). 2007. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/60000G2U.PDF?Dockey=60000G2U.PDF (accessed on 29 April 2024).
- Choi, D.; Shin, H.; Jung, J. Control parameters in three-stage deammonification process for a mature leachate treatment based on a traditional Modified Ludzack-Ettinger process. J. Water Process Eng. 2022, 48, 102863. [Google Scholar] [CrossRef]
- Hodgson, B.; Sharvelle, S. Development of generalized empirical models for comparing effectiveness of wastewater nutrient removal technologies. Environ. Sci. Pollut. Res. 2019, 26, 27915–27929. [Google Scholar] [CrossRef]
- Guerrero, J.; Guisasola, A.; Baeza, J. The Nature of the Carbon Source Rules the Competition between PAO and Denitrifiers in Systems for Simultaneous Biological Nitrogen and Phosphorus Removal. Water Res. 2011, 45, 4793–4802. [Google Scholar] [CrossRef]
- Rodríguez, A.; Letón, P.; Rosal, R.; Dorado, M.; Villar, S.; García, J. Tecnologías Convencionales. In Tratamientos Avanzados de Aguas Residuales Industriales; Tratamientos biológicos, Universidad de Alcála del Círculo de Innovación en Tecnologías Medioambientales y Energía (CITME): Madrid, España, 2006; Available online: https://www.madrid.org/bvirtual/BVCM001696.pdf (accessed on 29 April 2024).
- Persson, F.; Suarez, C.; Hermansson, M.; Plaza, E.; Sultana, R.; Wilén, B. Community structure of partial nitritation-anammox biofilms at decreasing substrate concentrations and low temperature. Microb. Biotechnol. 2017, 10, 761–772. [Google Scholar] [CrossRef]
- McCarty, P.L. What is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef]
- Ikem, J.; Chen, H.; Delatolla, R. Design strategy and mechanism of nitrite oxidation suppression of elevated loading rate partial nitritation system. Front. Microbiol. 2023, 14, 1142570. [Google Scholar] [CrossRef]
- Bianco, F.; Ali Saeed Al-Gheethi, A.; Race, M. Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions. Processes 2023, 11, 458. [Google Scholar] [CrossRef]
- Campo, R.; Sguanci, S.; Caffaz, S.; Mazzoli, L.; Ramazzotti, M.; Lubello, C.; Lotti, T. Efficient carbon, nitrogen and phosphorus removal from low C/N real domestic wastewater with aerobic granular sludge. Bioresour. Technol. 2020, 305, 122961. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Zheng, S.; Duan, S. Impact of dissolved oxygen and loading rate on NH3 oxidation and N2 production mechanisms in activated sludge treatment of sewage. Microb. Biotechnol. 2021, 14, 419–429. [Google Scholar] [CrossRef]
- Curtin, K.; Duerre, S.; Fitzpatrick, B.; Meyer, P. Biological Nutrient Removal. Minnesota Pollution Control Agency, US. 2011. Available online: https://www.pca.state.mn.us/sites/default/files/wq-wwtp8-21.pdf (accessed on 29 April 2024).
- Ramalho, R.S. Tratamiento Terciario de Aguas Residuales; Jimenez, D., Lora, F., Ramalho, R., Eds.; Tratamiento de aguas residuales; Editorial Reverté: Barcelona, España, 2009; pp. 642–646. [Google Scholar]
- Ma, T.-F.; Ma, H.-X.; Wu, J.; Yu, Y.-C.; Chen, T.-T.; Yao, Y.; Liao, W.-L.; Feng, L. The Inhibition of Engineered Nano-ZnO in the Biological Nitrogen Removal Process: A Review. Water 2024, 16, 17. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, L.M.; Han, W.; Wang, X.; Guo, Z.B.; Peng, F.Q.; Yang, F.; Kong, M.; Gao, Y.X.; Chao, J.Y.; et al. Nitrate removal, spatiotemporal communities of denitrifiers and the importance of their genetic potential for denitrification in novel denitrifying bioreactors. Bioresour. Technol. 2017, 241, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Rovira, L. Eliminación Catalítica de Nitratos y Bromatos en Aguas. Master’s Thesis, Instituto Universitario Mixto de Tecnología Química (UPV-CSIC), Universitat Politècnica de València, Valencia, Spaña, 2012; p. 55. Available online: https://riunet.upv.es/bitstream/handle/10251/30008/PFM%20Laura%20Rovira%20Gonz%E1lez.pdf?sequence=1 (accessed on 29 April 2024).
- Cao, J.; Oleyiblo, O.J.; Xue, Z.; Otache, Y.M.; Feng, Q. Achieving low effluent, N.O.3.-N.; TN concentrations in low influent chemical oxygen demand (COD) to total Kjeldahl nitrogen (TKN) ratio without using external carbon source. Chin. J. Ocean. Limnol. 2015, 33, 1039–1052. [Google Scholar] [CrossRef]
- Qi, S.; Lin, J.; Wang, Y.; Yuan, S.; Wang, W.; Xiao, L.; Zhan, X.; Hu, Z. Fermentation liquid production of food wastes as carbon source for denitrification: Laboratory and full-scale investigation. Chemosphere 2021, 270, 129460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Y.; Wang, B.; Zhang, H.; Ding, J. Effects of Stepwise Adjustment of C/N during the Start-Up of Submerged Membrane Bioreactors (SMBRs) on the Aerobic Denitrification of Wastewater. Water 2021, 13, 3251. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Wastewater Treatment Fact Sheet: External Carbon Sources for Nitrogen Removal; US EPA, Office of Wastewater Management: Washington, DC, USA, 2013. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100IL8F.txt (accessed on 29 April 2024).
- Nadeem, K.; Alliet, M.; Plana, Q.; Bernier, J.; Azimi, S.; Rocher, V.; Albasi, C. Modeling, simulation and control of biological and chemical P-removal processes for membrane bioreactors (MBRs) from lab to full-scale applications: State of the art. Sci. Total Environ. 2022, 809, 151109. [Google Scholar] [CrossRef] [PubMed]
- Demir, S. Comparison of performances of biological nutrient removal systems for municipal wastewater treatment. Sigma J. Eng. Nat. Sci. 2021, 38, 1235–1248. [Google Scholar]
- Rahman, S.; Eckelman, M.; Onnis-Hayden, A.; Gu, A. Life-Cycle Assessment of Advanced Nutrient Removal Technologies for Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, L.; Lv, J. Influence of anoxic to aerobic volume ratio on sludge settleability and bacterial community structure in a denitrifying–nitrifying activated sludge system. Desalination Water Treat. 2015, 56, 1863–1876. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, C.; Liu, T.; Wang, X. Removal of Nitrate Nitrogen from Municipal Wastewater Using Autotrophic Denitrification Based on Magnetic Pyrite. Water 2023, 15, 4292. [Google Scholar] [CrossRef]
- Pradhan, S.; Mikola, A.; Vahala, R. Nitrogen and phosphorus harvesting from human urine using a stripping, absorption, and precipitation process. Environ. Sci. Technol. 2017, 51, 5165–5171. [Google Scholar] [CrossRef]
- Saetta, D.; Zheng, C.; Leyva, C.; Boyer, T. Impact of acetic acid addition on nitrogen speciation and bacterial communities during urine collection and storage. Sci. Total Environ. 2020, 745, 141010. [Google Scholar] [CrossRef] [PubMed]
- Dereszewska, A.; Cytawa, S. Circular Economy in Wastewater Treatment Plants—Potential Opportunities for Biogenic Elements Recovery. Water 2023, 15, 3857. [Google Scholar] [CrossRef]
- Kim, M.; Nakhla, G.; Keleman, M. Modeling the impact of food wastes on wastewater treatment plants. J. Environ. Manag. 2019, 237, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Plaza, E.; Elginöz, N.; Atasoy, M.; Khatami, K.; Perez-Zabaleta, M.; Cabrera-Rodríguez, C.; Yesil, H.; A Tugtas, A.; Calli, B.; et al. Conceptual system for sustainable and next-generation wastewater resource recovery facilities. Sci. Total Environ. 2023, 885, 163758. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).