Abstract
Water contaminated with arsenic is a worldwide problem. This review presents the arsenic contamination in groundwater, its sources, and possible health risk to humans. Groundwater pollution is the most common route of inorganic arsenic exposure in humans. Arsenic concentrations in different countries were analyzed and projected on a map. Because arsenic is widely spread throughout the Earth’s crust, it is present in trace amounts in practically all waterways. Harmful levels of this toxin have been identified in drinking water in some regions. For drinking purposes, the majority of people use groundwater; excess arsenic levels in groundwater have been linked to a variety of negative health impacts on people. Arsenic exposure is the world’s leading environmental cause of cancer. The main aim of this review is to summarize the effective technologies to remove arsenic from drinking water, such as ion exchange, coagulation/flocculation, and membrane technologies like ultra-filtration and electrodialysis, helping to deal with the adverse effects caused by arsenic exposure. All these technologies present different advantages and disadvantages. Electrocoagulation, adsorption, and phytoremediation are the most efficient and cost-effective technologies. The removal efficiencies of arsenic using these technologies and prospects were also included.
1. Introduction
Water is a critical resource for human life, and the issue of water security directly impacts society and residents’ quality of life [1,2,3,4]. The rising population, industrial development, and modern lifestyles have increased the use of water [5,6,7]. Chemicals and microbial contamination frequently challenge water safety [8,9,10]. Arsenic is a metalloid that is highly toxic and carcinogenic in nature, and it is the 20th most abundant element [11]. Arsenic exists in two forms: arsenite As(III) and arsenate As(V). As(III) is uncharged in the reducing domain, while As(V) is a single or double charge in the oxidizing domain [12,13,14]. Due to industrial developments, there is a serious increase in arsenic concentrations in water that vary between 0.5 and 5000 μg/L [15]. Arsenic contamination of ground and drinking water is an outcome of both natural and man-made activities [16,17] including farming activities [11], urbanization, industrialization [18], mining [19], volcanic ash/ eruption, weathering processes, and agricultural pesticides [3,20]. The majority of people are exposed to arsenic through food and drinking water. Long-term arsenic poisoning occurs because of eating food grown in arsenic-rich groundwater; this water has been revealed to be used in the cultivation of agricultural products, vegetables, and rice that are used for human consumption [21]. Arsenic has been found in almost all rice products; however, the levels vary significantly [22,23]. Arsenic concentration higher than the permissible limit has affected more than 300 million people around the world [24], causing severe health problems in humans like cancer [25], cardiovascular diseases [26], and dermatologic, reproductive, developmental, neurological, and respiratory effects [27].
According to the World Health Organization (WHO), the permissible limit for arsenic is 10 μg/L, but the review of the literature showed arsenic concentrations higher than the permissible limit in many countries including Bangladesh [28], Iran [29], Pakistan [15,30], Mexico [31,32], Saudi Arabia [33], China (Yangtze River basin, Han River) [34], Latin America [35], the USA [36], and Ethiopia [37]. Asia is at the highest risk of drinking arsenic-contaminated water [38]. To reduce the treatment costs of diseases caused by arsenic exposure, Dutch water companies aim to reduce arsenic concentration up to <1 μg/L, a far lower level compared with the WHO’s permissible limit [39]. Groundwater in Bangladesh contains high arsenic concentrations, far higher than the permissible limit, which is 50 μg/L [28]. In Bam, southeastern Iran, arsenic concentration in groundwater ranges from 9.26 μg/L to 14.65 μg/L, while exposure to arsenic through ingestion is causing more diseases than the dermal route [29]. According to [31], 45% of the water samples in five zones of the metropolitan area of San Luis Potosí, Mexico showed an arsenic concentration above the WHO guidelines. A study carried out by [32] in which 44 groundwater samples were taken from two areas of the northeastern part of the province of La Pampa, Argentina showed arsenic concentrations ranging from 5.9 to 535.1 μg/L and from 17.5 to 248.4 μg/L for both sites. Podgorski and Berg reviewed the global threat of arsenic in groundwater and concluded that globally 13 regions are highly contaminated with groundwater arsenic [40]. Arsenic concentrations were projected on a map in the range of <10 μg/L, 10–50 μg/L, and >50 μg/L. Arsenic concentrations in different countries and their sources are presented in Table 1.

Table 1.
Documented groundwater arsenic concentration in different countries.
In recent years, the number of publications on arsenic water treatment has shown a significant increase, as shown in Figure 1. This study is evidence that the treatment of arsenic-contaminated groundwater is of major importance because it is the major source of drinking water for many countries. For this purpose, different treatment technologies are used to combat polluted water. These technologies include electrocoagulation [12], magnetic biochar [47], oxidation, ion exchange, membrane filtration, coagulation and electrocoagulation, lime softening, capacitive deionization (CDI), adsorption [46], stabilization/encapsulation [47], phytoremediation [49], and bioremediation [50]. The review also outlines the future perspectives related to arsenic-contaminated water treatment.
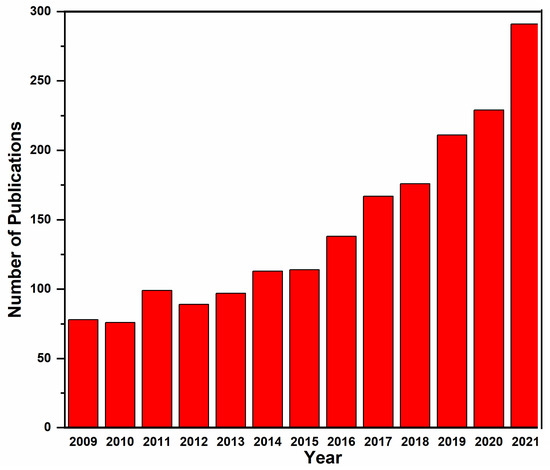
Figure 1.
Number of articles published in recent years, Source: Scopus “Arsenic in water” as on 1 July 2021.
2. Toxicity of Arsenic and Its Health Effects
Studies show that arsenic has several health effects on human health, which might be acute or chronic, as shown in Figure 2 [33,51]. Different diseases caused by arsenic exposure that are documented in the literature are presented in Table 2. Arsenic can cause cancerogenic and non-cancerogenic effects. WHO announced arsenic as a Class-1 carcinogen [12]. Severe and toxic health effects caused by arsenic are mostly due to water contamination because every human consumes water without understanding its safety and uses it for various purposes [15,52]. A significant percentage of arsenic, over the WHO guideline, is present in the drinking water used by millions of people worldwide [47]. People in Southeast Asia are most adversely affected by exposure to arsenic [30]. The cancer rate is higher in areas where rice consumption is higher because it contains inorganic arsenic, and millions of people are affected by inorganic arsenic exposure [53,54]. Arsenic can cause cancer when consumed in high doses, but this does not imply that it is harmless when consumed in low doses; prolonged exposure to arsenic can result in a variety of health issues [47]. Arsenic ingestion comes from a variety of sources, including food chains, skin contact, and inhalation, in addition to water. If taken orally, arsenic is extremely deadly [38]. Arsenic in chronic form causes keratosis [30]. Long-term exposure to arsenic can result in neurological issues, cardiovascular ailments, cancer, and skin blemishes [45,55]. High diabetes rates in Pakistan are also linked to arsenic consumption [15]. The initial chronic effect of arsenic is on the skin including hyperkeratosis and hypo- and hyperpigmentation. Changes in the skin are known as arsenicosis or arseniasis [36]. Arsenic intake by food can cause cancer, nerve damage, kidney damage, eventually failure, diabetes, and hepatic and renal syndromes [51]. A study shows that if a mother is taking As from whatever the source is, the impact must also be on the infant, and not only this, different types of problems can occur during pregnancy such as low birth weight, stillbirths, infant mortality, and infant infectious morbidity [56]. According to a study, exposure to arsenic does not yet clearly cause breast cancer, but when combined with Cd, it acts to do so [57]. Arsenic pollution mainly occurs in rural areas of poor nations. No particular techniques exist in rural areas to purify tainted water [58].
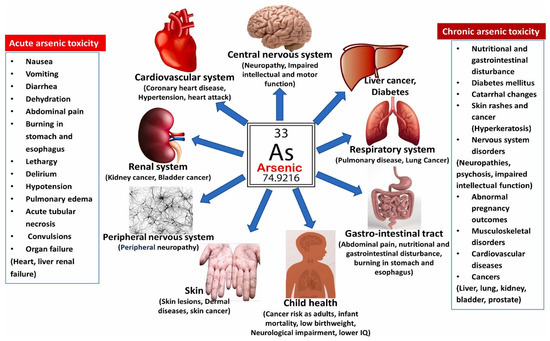
Figure 2.
Health effects of arsenic contamination [59].

Table 2.
Common diseases caused by exposure to arsenic.
3. Arsenic Removal Technologies
The technologies for purifying water that has been contaminated with arsenic are the main subject of this study. The different known arsenic removal techniques are discussed in this review paper (Figure 3), including ion exchange, coagulation/flocculation, phytoremediation, oxidation, adsorption, bioremediation, and membrane techniques including electrodialysis and ultra-filtration.
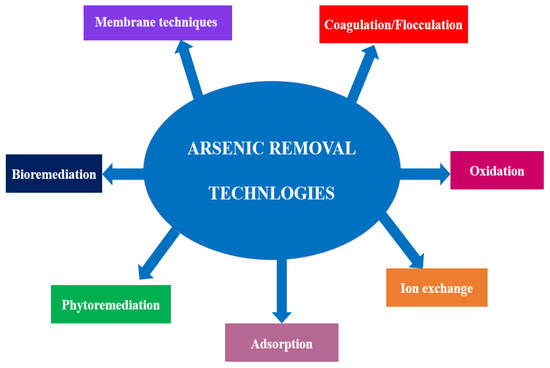
Figure 3.
Various arsenic removal technologies.
3.1. Ion-Exchange
Ion exchange is a method in which the same signs are swapped reversibly between solid and liquid in a highly insoluble solution. Ion exchange chromatography is an ion exchange technique that is popular because of its high capacity and resolving power. The ion exchange method is used for water purification (especially converting hard water into soft water) and also for other purifications. It can detect inorganic salts. Cationic exchangers and ionic exchangers are two types of ion exchangers. Two techniques are used for ion exchange: a batch method and a column method [64].
For ages, the ion exchange method has been used to purify water from arsenic. The ion exchange method works for arsenic with few total dissolved solids (TDS) and little sulfate. For the arsenic ion exchange method, effectiveness is increased by different factors such as TDS and competing ions [58]. The study explains the tailored anion exchanger used to decrease the As concentration. Simulated ion exchange resins are effective for As(V) adsorption. Chloride ions are easily exchanged with As(III) or As (V). In water, the arsenic concentration, competing ions, and ion exchange resins are important elements for arsenic [37]. Graphene oxide and composites of graphite oxide iron-modified clinoptilolite are good composites used for arsenic removal using the ion exchange or adsorption method [65]. A study defines its goal to remove As(V) from a solution by using hybrid ion exchange/electrodialysis. Some other technologies can be used for arsenic removal, such as nanofiltration or reverse osmosis, but these have some drawbacks like regenerate ion exchange or membrane cleaning, so here, ion-exchange electrodialysis is more convenient, which overcomes the drawbacks of other technologies [66]. In the ion-exchange method, no pH acclimations are needed and do not rely on influent concentration. In the ion-exchange method, the removal of As(III) is low, and some precipitates block the process [67].
3.2. Coagulation/Flocculation
Arsenic contamination water is treated using coagulation, which is then followed by the neutralization and filtration processes. Various arsenic species are transformed into flocs for further filtration using various coagulants. The coagulation process is also preferable because of its simplicity and removal efficiency [52]. This process is applicable on small scales and large scales, but pH adjustments are obligatory [67]. A study briefly describes ferric coagulation. If the dosage of coagulant increases, there is more removal of arsenic [68]. Sludge is produced during the coagulation process; however, this process can remove very high amounts of arsenic concentrations [69].
According to studies, choosing a certain type of arsenic removal procedure is primarily influenced by the nature of the water. Numerous modifications to coagulation were being made, such as electrocoagulation as a substitute for aqueous arsenic removal [37]. Coagulation techniques, particularly electrocoagulation processes, are used to treat the various health problems brought on by consuming arsenic-contaminated water [70]. Although electrocoagulation has been used to cleanse water for human consumption, its use is not widespread because of the need for energy. Solids are easily removed; however, they cannot be disposed of directly because of the arsenic residue produced. The process of coagulation is economically viable [37]. Iron and aluminum are popular electrode materials for the electrocoagulation process. For the removal of arsenic from water, the researchers employed iron anodes rather than aluminum anodes. More than 27 researchers utilized iron or stainless-steel anodes to electrolytically generate iron hydroxides, whereas more than 15 employed aluminum anodes to electrolytically generate aluminum hydroxides. The major reasons for this electrode preference are its inexpensive cost, convenient availability, and improved efficiency [71,72]. The electrocoagulation method is capable of converting arsenite to arsenate, which is essential for the effective removal of aresenite. The efficacy of aluminum and iron for the removal of arsenite from an aqueous medium was investigated, and equal removal efficiencies were reported for both electrodes for arsenite concentrations ranging from 75 ppm to 500 ppm. Aluminum, on the other hand, has a lower effectiveness for arsenic removal than iron. The removal efficiency of the coagulation–flocculation technique is shown in Table 3.

Table 3.
The removal efficiency of coagulation–flocculation technique.
3.3. Phytoremediation
Phytoremediation is a cost-effective biological technique that can easily safeguard human health and the environment from the poisonous effects caused by arsenic [49]. It is a procedure in which plants accumulate pollutants from the soil.
Plants used for remediation and their accumulation of arsenic are mentioned in Table 4. Different species of plants have been found to be remediation options for removing heavy metals from the soil. Lemna minor, also called common duckweed [74,75], also presented 70% removal efficiency of L minor in 15 days. The removal efficiency of Colocasia esculenta L. Schott increases from 30 to 90 days and decreases after 122 days [76]. Plant species Pistia stratiotes decreased with an increase in initial concentration when exposed to different arsenic concentrations and showed high removal efficiency at a low concentration, that is, 10 µg/L [77]. Among other plants, Pteris vittata L. can endure high arsenic concentrations [78] up to 5000 mg/kg; it is a genetically engineered plant having high potential for phytoremediation of arsenic-polluted soil, but its geographic and environmental dispersion restricts the scope of its application because its natural growth is in warm climate [79].

Table 4.
Arsenic accumulation by different plant species.
To determine the phytoremediation capability of Pistia stratiotes L. to accumulate arsenite [60], an experiment was conducted with different factors in which, among four treatments, 10 µM As(III) showed a high accumulation of arsenite from the medium. A study conducted by Moreira, V., et al., to remove arsenic from the aqueous media under controlled conditions in which plant species Lemna Valdiviana showed arsenic reduction up to 82%, accumulating 1190 mg/kg of As from water [61]. Plant samples were collected from different areas of Enugu State, southeastern Nigeria. Arsenic in leaves, roots, and soil is monitored separately. The phytoaccumulation capacity of plants was measured by different factors (bioconcentration, translocation, and accumulation). Pteridium Aquilinum with 622.0 mg kg−1 accumulation capacity is the most efficient plant species for the phytoremediation of arsenic from water, followed by Lasimorpha Senegalesis and Sacciolepis Cymbiandra [62]. Phytoremediation studies were led to assess the arsenic take-up capability of Salix atrocinerea in 30 days, in which plant roots showed higher intake of arsenic than leaves [63].
3.4. Oxidation
The arsenite As(III) form of arsenic is highly versatile and needs conversion into a less mobile form, As(V), because most of the treatment procedures viably eliminate just As(V); that is why pre-oxidation of As(III) to As(V) is needed and, for this purpose, different oxidation processes like Fenton’s reagent, hypochlorite, and permanganate [13] and oxidizing agents like ozone, chlorine, bleaching powder, and hydrogen peroxide [13] are used.
Manganese-oxidizing aerobic granular sludge (Mn-AGS) is very effective in removing arsenic from organic wastewater, especially with the addition of Fe (II) attributed to the Fenton reactions [13]. pH dependence appears to be cons of Fenton reaction. Depending on the circumstances, As(III) removal from contaminated water can be effectively remedied using the CuFe2O4 reaction with peroxymonosulfate [84]. Commercial activated carbon has been showed promising results toward As(III) oxidation under different parameters including residence time, pH, dissolved oxygen, and initial As(III) concentration [85]. Photocatalytic oxidation is an efficient method because of its low cost [86]. Comparing the present study of photocatalytic oxidation with the previous studies to access its benefits, this study showed improved photocatalytic performance compared with different reports in view of comparative materials. Reference [87] studied the self-floating copper loading catalyst as an oxidizer of As(III) that can be used for the treatment of arsenic-contaminated water. Reference [88] reported that different lead extracts like eucalyptus (Eucalyptus globulus), mango (Mangifera indica), jamun (Syzygium cumini), and guava (Psidium guajava) also play an important role in arsenic oxidation. Zero-valent iron nanoparticles obtained from these leaf extracts are used as oxidants. Results showed 70% arsenic (III) oxidation at a period of 10 min and, among all the leaf extracts, guava leaves were able to oxidize arsenic at all pH values (3, 7, and 9).
3.5. Adsorption
Adsorption widely relies upon the porosity and movement of the adsorbent in disposing of or bringing down the grouping of a wide scope of toxins (natural, inorganic, and organic) from the arrangement [89]. Adsorption is an ex situ technique used to remove heavy metals, e.g., arsenic [90]. The different adsorbents used for the removal of As(III) and As (IV) include ferric hydroxide and activated alumina (SFAA) [91], chitosan–magnetic graphene oxide (CMGO) nanocomposite [92], magnetic gelatin-modified biochar [93], iron-modified activated carbons [94], iron-ore sludge [95], and magnetite nanoparticles [96].
By electrochemical adsorption with birnessite, Liu, L., et al. demonstrated a decrease in total arsenic (As T) and As(III) from 3808.7 to 73.7 µg/L and 682.8 to 21.4 µg/L, respectively [97]. With an adsorption capacity of 166.94 mg/g via a Langmuir isotherm model, Mn-doped MgAl-LDHs is the effective absorbent for removing As(V) from the aqueous medium [98]. Xu, F., et al. indicated that starch-stabilized ferromanganese binary oxide (starch-FMBO) generated with Fe/Mn at a 1:2 ratio has a greater adsorption influence on As (III), demonstrating substantial adsorption capacity [99]. Some binary oxides are widely employed for arsenic removal because they are affordable and environmentally friendly of their ecofriendly nature and cost-effectiveness [100]. Arsenic removal from water is greatly improved by microbial conversion of arsenite in combination with various adsorption approaches [101]. The combination of two techniques, adsorption (ADS) and dielectrophoresis (DEP) appear to be cost-effective and efficient; the adsorbent fly ash showed high adsorption capacity removing 91.4% As(V) from industrial wastewater [102]. Langmuir and Freundlich’s experiments were performed to evaluate As(III) and As(V) removal efficiencies by Fe-FeS2 prepared with mechanical ball milling at a pH ranging from 3–10 [103,104], and used Fe/olivine composite for arsenic removal that showed great adsorption capacity at a minimal expense. Another adsorbent, iron-coated S. Muticum, can be used as an alternative treatment for the expulsion of arsenic that also has an ecological benefit over other techniques [19].
Many studies demonstrated that adsorption is a beneficial technique for the treatment of contaminated water [105]. During adsorption, As(III) oxidizes to As(V) to improve removal efficiency as As(III) is not easy to remove [106]. Ferric salts and iron oxides are most convenient when they are used to purify water from As contamination [105,106]. Iron oxide is cost effective and has a higher charismatic character. Initially, adsorption performance is low but activated carbon enhances its effectiveness. After the result, activated carbon can be removed with the help of a magnetic process [107]. Activated carbon is a venerable adsorbent. There are several types of adsorptions; some can regenerate after use and become impregnated [108]. It is expected in the next 10 years that some advanced adsorbents will be made that will be very effective to clean the environment [108].
Adsorption is a worthy technique that can be used at home. It is an effective technique and increases the quality of things after removing contamination [90]. This process can be processed with the water having multiple pollutants and there is less waste production at the end of processing [107]. This technique gets attention because of its low cost, ease, simplicity of operation, and high efficiency standard [109].
Many adsorbents are neither practically nor financially viable for usage in underdeveloped nations. As a result, locally accessible natural adsorptive materials may provide viable and affordable alternatives for removing As pollution in low-income nations. These organic substances include hydroxylapatite and struvite, zeolites, clays, rocks, soils, and sorbents [0]. Only the right pH is suitable for this technique. Sometimes the performance is affected by the minerals or nutrients in the water and soil [90]. Due to its weight, unstructured nature, and flocculant properties, the iron-based adsorbent is relatively limited in removing arsenic [107]. Sometimes there is no revival of adsorbents, which become secondary pollutants [108].
Adsorption kinetics and adsorption isotherm models are different adsorption models used for the efficient removal of arsenic from groundwater, as shown in Table 5.

Table 5.
Different adsorbents used for arsenic remediation.
3.6. Bioremediation
Arsenic is hazardous to health—not only to human health, but it is also dangerous for marine life when it is present in water [112]. Now it extends around the globe, so bioremediation is a technique used to remove arsenic [50]. Bioremediation is a helpful technique to remove arsenic from water, soil, and mine tailings [113,114]. Bioremediation is of two types, in situ and ex situ, which are further classified, too [115]. Though we had some old technologies to remove arsenic, they consume a lot of money and are complicated. The use of microorganisms is a little tricky but removes the As contamination more in a less expensive way [116]. Some bioremediation techniques used for As removal are Prangos ferulacea (Pf) and Teucrium polium [117], microbial fuel cells [50], field-pilot bioreactor [114], biogenic pyrite [113], etc. In some bioremediation processes, arsenic mobility increases so it can be removed easily [113]. As removal capability also depends upon its bonding with other metals, which makes its removal easy or tough in the bioremediation process [114]. Studies show that different elements are present with arsenic, so the removal of all, including arsenic microorganisms, is used. Sometimes for As, the tolerance level is unpredictable, and bacterial activity is done on it, which then clarifies its tolerance concentration. Arsenic-resistant bacteria are used for arsenic removal from soil because the soil contaminates food. A study had been done for the removal of As in which for 193.7 nm As the line is segregated for processing so, after all, the processing result shows that greater than 90% As(III) and As(V) had been removed [118].
Bioremediation techniques are inexpensive and eco-friendly [113,119]. The study illustrates that there are two stages for arsenic removal: arsenite is oxidized to arsenate and, in the second step, arsenate is removed successively and the bioremediation technique holds this two-step processing [117]. Microbes used in bioremediation have high forbearance and defiance powers toward metal and metalloids like arsenic. Microbial activity helps convert heavy metals’ insolubility to solubility [115]. The mobility of arsenic can be controlled by the bioremediation technique [120]. One more advantage of this technique is the use of natural processes for cleaning the environment [121]. The removal of arsenic by microbial agents is shown in Table 6.

Table 6.
Microbial agents used for arsenic removal.
There are some drawbacks to the bioremediation process. Bioremediation needs sometimes aerobic and sometimes anaerobic conditions to run a process; otherwise, fluctuations may occur. The nutrient requirement is very important in this technique. Sometimes the process is slow and faces difficulties when it works for inorganic things. The most severe drawback is that sometimes it converts into a more highly toxic material after processing than the original [123]. Suitable soil, climate, and other suitable conditions are required for good performance [90].
3.7. Membrane Technology
As the name suggests, it is a membrane structure that serves as a barrier, forcing contamination to stop as molecules flow through it. A porous membrane serves as the material for the barrier. Nano-filtration, micro-filtration, ultra-filtration, and electrodialysis are the four basic forms of membrane technology [124]. Electrodialysis is a technique used to remove salts and chemicals from water. It works when an electric current is applied to it and ions pass through the selective membrane [125]. Nanofiltration needs pretreatment, removes arsenic and bacteria and viruses, and makes water drinkable. It can remove 93.8% of arsenic at pH 8. With microfiltration, with the help of electrocoagulation and micro-coagulation, As is removed at a minimum cost [13]. In ultrafiltration, pressure is applied to the semipermeable membrane to split the contamination. Its pore size is large [24]. In the separation technique, pressure is applied to a harmful substance to produce a contaminant-free product [126].
The study illustrated that by modifying an adsorptive membrane, seven tons of water can be purified to drinking level by using a 1 m2 adsorptive membrane and giving a high level of water security [127]. A study has been done that shows reverse osmosis can clean water up to 70–90% from As(III) and As(V) [128]. Another study encapsulates that under reverse osmosis and nanofiltration using their different membranes, up to 91% arsenic was removed, which are very efficient results [129]. The study has been undertaken to determine membrane technology’s cost- and energy-efficiency benefits. The results showed that for 20,000 residents, water costs USD 1041 per day, producing drinking water costs USD 0.52 per cubic meter, and electricity costs just 35 percent of the total cost [126]. Nanofiltration is a well-founded technique proved by an experiment in which contaminated groundwater was treated, decreasing its contamination level from 435 µg/L to 10 µg/L [130].
This technology not only helps to remove arsenic but also removes the total dissolved solids, salt, turbidity, and other unsuitable material. This method eliminates significant waste because it has a high filtration potential [90,131]. There is no sludge production in the membrane technique as in adsorption and chemical precipitation [132]. In addition, less energy is used in this method [128]. The main advantage distinguishing membrane technology from other methods is that there is no use of chemicals here [124,128]. Being easy to scale added a plus point to the membrane technique [67].
Membrane filtration required a very good price to process, so it burdens the economy [133]. A heavy amount is required for its maintenance, not only for processing. Substantial waste production is less but water contamination production is high. Linear scaling is not very feasible [90]. Temperature maintenance is also required [63], as is high expenditure and utilization of electricity [67]. A high amount of water could not filter here [124]. The defilement of the membrane can disrupt the whole process [67]. It is not preferable to change As(III) oxidation to As(V) because there is a chance of membrane damage [124].
3.7.1. Electrodialysis
The increasing population has elevated the requirement for water resources [134]. On the other hand, electrodialysis is a technique used to remove water contamination [135]. It is an electrochemical process [136]. Electrodialysis is a type of membrane technology in which anion and cation are migrated through the membrane by electric force. Initially, this technique was not used for As removal but for salt removal [136,137]. With the help of an electric field, ions are separated, shifted, and concentrated [138]. Electrodialysis is a very useful technology to enhance water quality and alter salt concentration [136]. The study was performed to remove arsenic with the help of electrodialysis, deep eutectic solvent enhancement, by which 82% As was removed successfully [139]. A study shows that As(V) removal is faster than As(III) under the mass transfer coefficient. It is increased in the case of As(V) because it is negatively charged, but not for As(III) as it has had no charge and the pH is not affected [140]. Another experiment has been done to show the efficiency of this technology. The result was electrodialysis removal efficiency of 80% at pH 10 [139].
Electrodialysis is an environmentally friendly technique. Compared to reverse osmosis, electrodialysis recovery rate is high. It can perform two things at once: it can remove salinity and contamination from water at the same time [135]. Defilement can be reduced more easily as compared to reverse osmosis [136]. Electrodialysis membranes are long-lasting, as compared to nanofiltration, and easy to start and reboot [141]. This technology helps to remove harmful secondary substances and decrease their risk to people. Moreover, they also help to improve the economy and environment by removing organic and inorganic contamination [142]. Its membrane can tolerate high pH and chlorine levels [141]. The arsenic removal from water by the electrodialysis technique is tabulated in Table 7.

Table 7.
Arsenic removal from water by electrodialysis technique.
Electrodialysis has a disadvantage in that it requires a labor force. Defilement can affect performance. Prior treatments and restoring minerals are required in this technique [135]. Another technical problem is that ED has less storage capacity. There would be chances of energy losses and blocking [144].
3.7.2. Ultra-Filtration
In the present era when there is an economic race everywhere, developing countries are unable to reach water purification technologies that are expensive. Simply, they cannot afford them, so in this case, ultrafiltration is a viable technique to remove contamination [145]. It is a low-pressure membrane technique [146]. Hydrogen peroxide is helpful to oxidize As(III) into As(V) at the membrane [147]. Micellar-enhanced ultrafiltration (MEUF) is a modification to ultrafiltration where a little decrease in the surface tension of the heavy metal is made to improve removal efficiency [148].
A study demonstrated the reinforced conventional treatment is 96.9% successful by ultrafiltration [149]. An experiment has shown that there is efficient arsenic removal with the help of ultrafiltration in collaboration with cetylpyridinium chloride, by which 91% and 84% of contaminated water were obtained [150]. The study elaborated that a negatively charged membrane efficiently removes particles at neutral pH. It has been studied that there is more arsenic removal caused by the increase in UF membrane by increasing negative charge and increasing pH [24]. The arsenic removal from water by the ultrafiltration technique is tabulated in Table 8.

Table 8.
Arsenic removal from water by ultrafiltration technique.
Ultrafiltration membrane technology helps to increase the validity and solidity of the process. In this process, unwanted contaminants are removed and color and turbidity are improved, bringing the water to drinking level. Even then, if its membrane life-span is short, it is feasible because of its suitable price [149]. This technique helps to decrease the fouling potential [153]. The flux rate is high and requires less energy, and is economically feasible [13].
Some enhancements are required in ultrafiltration because, alone, it will not give an effective result. Modifications are required to increase its rejection rate. The modification can be on the membrane or the whole system. The large pore size of ultrafiltration affects the purity and helps small particles pass out [154]. Some essential ions like Ca and Mg are not filtered here [13].
4. Future Prospects
Arsenic concentration in groundwater is mostly higher in developing countries. Backward places, such as those in India, Bangladesh, West Africa, Nepal, etc., have more vulnerable populations and greater rates of arsenic exposure [38]. Apart from the higher contamination risk rate, the poor economy does not support coping with this problem easily and day by day people are getting severely affected [155]. So, these problems are demanding some techniques for arsenic removal that are easy to use and handle. Awareness programs with some financial aid are also required. Acid mine drainage is helpful to remove contamination from mine wastewater. It is a biological method that uses potato peels and compost batches and helps to remove 73–87% of the arsenic. This percentage took 14 days to complete. Biological methods are easy to use in developing countries and very reliable for developed countries. Arsenic removal efficiency is better, up to 99%, if there is less concentration of heavy metals at low sulfide concentration. This reactor worked for approximately 500 days [156].
The management of arsenic toxicity is extremely important (Figure 4), which includes proper separation of sewerage water and groundwater, effectively saving drinkable water. Sometimes mine or tillage raw materials get into contact with safe water, causing them to produce impurities in water. The separate system to dump the trash or the remainder will be helpful to get rid of contamination and save many lives. These practices help to save the economy, labor force, and advanced working technology. The current study suggests conducting future investigations considering a larger scale to recommend efficient management strategies, urbanization planning, and ensuring safe irrigation and drinking water to prevent groundwater pollution.

Figure 4.
Arsenic toxicity management.
In the case of soil, agricultural land is also getting highly affected. Acid mine drainage sludge (AMDS), cement, and sand fly ash or Ca(OH)2 are some agents that are useful for the removal of contamination; their efficiency is stated as greater than 80%, which is a valuable amount [157]. The most favorable technique that is available for less-developed countries is bioremediation. It is easy to use and easy to apply. Biotic components were easily available compared to the chemicals that are not easily available and not affordable. The microorganisms that are used for removing contamination can be native or can be implanted from some outsourcing [121]. Bioremediation can accumulate approximately 98–99% of the arsenic contamination, which is a valuable quantity and useful for those who cannot easily afford any expensive technology and labor effort [120].
5. Conclusions
Water tainted with arsenic is a concern on a global scale. The WHO’s 10 μg/l standard for arsenic are exceeded by millions of people all over the world who are exposed to excessive concentrations of the toxin. It is particularly harmful in its inorganic form, both in the food chain and the environment. The “air we breathe, the water we drink, and the food we eat” all contain small amounts of arsenic. Another way to be exposed to arsenic is through man-made items. Most arsenic compounds have no taste or smell and are quickly dissolved in water, which make them a serious health risk. If the issue is not effectively addressed, it will have negative effects on both human and environmental systems. Different organs can develop cancer when arsenic levels are high, but this does not suggest that exposure to low arsenic concentrations is safe for human health. A lot of research has been done on the toxic effect of arsenic and its remedial technologies. The present review tried to summarize the different treatment technologies used for arsenic contamination. Membrane technologies are widely used for arsenic treatment. Although it is a simple and efficient technique, it cannot be used for small and medium industries because of its high operation costs, high energy requirements, and maintenance costs. Electrocoagulation, adsorption, and phytoremediation are effective treatment technologies for arsenic removal from water. The adsorption technique presents high performance, easy operation, and cost effectiveness. Oxidation also plays an important role in the conversion of As (III) to As (IV), which is helpful because most technologies effectively remove As(V). The phytoremediation process is in its research stage; this technique has low installation and maintenance costs, and it is environmentally feasible because it helps to clean the soil from arsenic contamination.
Any technology can be considered cost effective if it is environmentally friendly and fulfills the needs of both developed and underdeveloped countries. Future research should focus on the technologies that are economically viable and provide solutions that are also sustainable in terms of the environment.
Author Contributions
Writing—original draft; F.D. and J.I.; conceptualization; M.Y.J.B. and M.M.; methodology; F.D., J.I. and M.M.; software; formal analysis; M.M.A., M.S., A.M., S.S. and I.S.; review and editing; M.M.A., K.H.H.A., I.M., A.M. and I.S.; visualization; supervision; M.Y.J.B., M.M.A., I.M., I.S. and K.H.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data will be provided on request to the corresponding author.
Acknowledgments
The authors are thankful to the “COMSATS University Islamabad, Abbottabad Campus, Pakistan, and Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan”, who gave us a platform and all possible availabilities for this review paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iqbal, J.; Su, C.; Rashid, A.; Yang, N.; Baloch, M.Y.J.; Talpur, S.A.; Ullah, Z.; Rahman, G.; Rahman, N.U.; Earjh, E.; et al. Hydrogeochemical Assessment of Groundwater and Suitability Analysis for Domestic and Agricultural Utility in Southern Punjab, Pakistan. Water 2021, 13, 3589. [Google Scholar] [CrossRef]
- Talpur, S.A.; Noonari, T.M.; Rashid, A.; Ahmed, A.; Jat Baloch, M.Y.; Talpur, H.A.; Soomro, M.H. Hydrogeochemical signatures and suitability assessment of groundwater with elevated fluoride in uncon-fined aquifers Badin district, Sindh, Pakistan. SN Appl. Sci. 2020, 2, 1038. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Chai, J.; Li, S.; Alqurashi, M.; Rehman, G.; Tariq, A.; Talpur, S.A.; Iqbal, J.; Munir, M.; et al. Shallow Groundwater Quality Assessment and Its Suitability Analysis for Drinking and Irrigation Purposes. Water 2021, 13, 3361. [Google Scholar] [CrossRef]
- Baloch MY, J.; Mangi, S.H. Treatment of synthetic greywater by using banana, orange and sapodilla peels as a low cost activated carbon. J. Mater. Environ. Sci. 2019, 10, 966–986. [Google Scholar]
- Baloch, M.Y.J.; Talpur, S.A.; Talpur, H.A.; Iqbal, J.; Mangi, S.H.; Memon, S. Effects of Arsenic Toxicity on the Environment and Its Remediation Techniques: A Review. J. Water Environ. Technol. 2020, 18, 275–289. [Google Scholar] [CrossRef]
- Arifullah; Changsheng, H.; Akram, W.; Rashid, A.; Ullah, Z.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M. Quality Assessment of Groundwater Based on Geochemical Modelling and Water Quality Index (WQI). Water 2022, 14, 3888. [Google Scholar] [CrossRef]
- Tariq, A.; Mumtaz, F.; Zeng, X.; Baloch, M.Y.J.; Moazzam, M.F.U. Spatio-temporal variation of seasonal heat islands mapping of Pakistan during 2000–2019, using day-time and night-time land surface temperatures MODIS and meteorological stations data. Remote. Sens. Appl. Soc. Environ. 2022, 27, 100779. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, J.; Li, S.; Wang, X.; Wu, S.; Liang, Z.; Baloch, M.Y.J.; Silva, L.F.; Zhang, D. Physiological characteristics, geochemical properties and hydrological variables influencing pathogen mi-gration in subsurface system: What we know or not? Geosci. Front. 2022, 13, 101346. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Gu, R.; Liang, Z.; Xu, W.; Baloch, M.Y.J. Health Risk Assessment during In Situ Remediation of Cr(VI)-Contaminated Groundwater by Permeable Reactive Barriers: A Field-Scale Study. Int. J. Environ. Res. Public Health 2022, 19, 3079. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Al Shoumik, B.A.; Nigar, A.; Elhassan, A.A.M.; Elshekh, A.E.A.; Bashir, M.O.; Ebrahim, A.F.M.S.; Mohamed, K.A.A.; Iqbal, J. Hydrogeochemical Mechanism Associated with Land Use Land Cover Indices Using Geospatial, Remote Sensing Techniques, and Health Risks Model. Sustainability 2022, 14, 6768. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Zia, Z.; Abbas, S.; Hammad, H.M.; Shah, G.M.; Khalid, S.; Shahid, N.; Sajjad, M.; Fahad, S. Factors controlling arsenic contamination and potential remediation measures in soil-plant systems. Groundw. Sustain. Dev. 2019, 9, 100263. [Google Scholar] [CrossRef]
- Alidokht, L.; Anastopoulos, I.; Ntarlagiannis, D.; Soupios, P.; Tawabini, B.; Kalderis, D.; Khataee, A. Recent advances in the application of nanomaterials for the remediation of arsenic-contaminated water and soil. J. Environ. Chem. Eng. 2021, 9, 105533. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A.; Singh, R.; Bhadouria, R.; Singh, V.K.; Devi, P. A critical review on the research trends and emerging technologies for arsenic decontamination from water. Groundw. Sustain. Dev. 2021, 14, 100607. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef]
- Nath, S.G.; Debsarkar, A.; Dutta, A. Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2018, 13, 277–303. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Zhang, D.; Al Shoumik, B.A.; Iqbal, J.; Li, S.; Chai, J.; Farooq, M.A.; Parkash, A. Evolution Mechanism of Arsenic Enrichment in Groundwater and Associated Health Risks in Southern Punjab, Pakistan. Int. J. Environ. Res. Public Health 2022, 19, 3325. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Mi, W.; Xie, S.; Ji, L. Land-use change caused by anthropogenic activities increase fluoride and arsenic pollution in groundwater and human health risk. J. Hazard. Mater. 2020, 406, 124337. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.R.; Pintor, A.M.; Boaventura, R.A.; Botelho, C.M.; Santos, S.C. Arsenic removal from water using iron-coated seaweeds. J. Environ. Manag. 2017, 192, 224–233. [Google Scholar] [CrossRef]
- Monteiro De Oliveira, E.C.; Caixeta, E.S.; Santos VS, V.; Pereira, B.B. Arsenic exposure from groundwater: Environmental contamination, human health effects, and sustainable solutions. J. Toxicol. Environ. Health Part B 2021, 24, 119–135. [Google Scholar] [CrossRef]
- Clair-Caliot, G.; Marks, S.J.; Hug, S.J.; Bretzler, A.; N’Guessan, N.D.; Tihe, S.F.K.; Lalanne, F. Uptake of Arsenic by Irrigated Vegetables and Cooked Food Products in Burkina Faso. Front. Water 2021, 3, 667308. [Google Scholar] [CrossRef]
- Karagas, M.R.; Punshon, T.; Davis, M.; Bulka, C.M.; Slaughter, F.; Karalis, D.; Argos, M.; Ahsan, H. Rice Intake and Emerging Concerns on Arsenic in Rice: A Review of the Human Evidence and Methodologic Challenges. Curr. Environ. Health Rep. 2019, 6, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Baloch MY, J.; Su, C.; Talpur, S.A.; Iqbal, J.; Bajwa, K. Arsenic Removal from Groundwater using Iron Pyrite: Influences Factors and Removal Mechanism. J. Earth Sci. 2022, in press. [Google Scholar]
- Kundu, S.; Naskar, M.K. Perspective of Membrane Processes for the Removal of Arsenic from Water: An Overview. Trans. Indian Ceram. Soc. 2021, 80, 28–40. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ. Technol. Innov. 2019, 16, 100462. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sastry, S. Problem Definition and Community Participation in Environmental Health Interventions: An Exploratory Study of Groundwater Arsenic Remediation. Health Commun. 2020, 37, 717–725. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Udensi, U.K.; Pacurari, M.; Stevens, J.J.; Patlolla, A.K.; Noubissi, F.; Kumar, S. State of the science review of the health effects of inorganic arsenic: Perspectives for future research. Environ. Toxicol. 2018, 34, 188–202. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Mukherjee, S.C.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S.; et al. Status of groundwater arsenic contamination in Bangladesh: A 14-year study report. Water Res. 2010, 44, 5789–5802. [Google Scholar] [CrossRef]
- Toolabi, A.; Bonyadi, Z.; Paydar, M.; Najafpoor, A.A.; Ramavandi, B. Spatial distribution, occurrence, and health risk assessment of nitrate, fluoride, and arsenic in Bam groundwater resource, Iran. Groundw. Sustain. Dev. 2020, 12, 100543. [Google Scholar] [CrossRef]
- Qurat-ul-Ain Farooqi, A.; Sultana, J.; Masood, N. Arsenic and fluoride co-contamination in shallow aquifers from agricultural suburbs and an industrial area of Punjab, Pakistan: Spatial trends, sources and human health implications. Toxicol. Ind. Health 2017, 33, 655–672. [Google Scholar] [CrossRef]
- Fernández-Macias, J.C.; Ochoa-Martínez, C.; Orta-García, S.T.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Probabilistic human health risk assessment associated with fluoride and arsenic co-occurrence in drinking water from the metropolitan area of San Luis Potosí, Mexico. Environ. Monit. Assess. 2020, 192, 712. [Google Scholar] [CrossRef] [PubMed]
- Alcaine, A.A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.-P.; Mörth, C.-M.; Sracek, O.; Ahmad, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rao, T.S.; Isloor, A.M.; Ibrahim, G.S.; Inamuddin; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.V.; Guo, X.; Gan, Y.; Benner, S.G.; Griffin, A.M.; Gorski, C.A.; Wang, Y.; Fendorf, S. Redox controls on arsenic enrichment and release from aquifer sediments in central Yangtze River Basin. Geochim. Cosmochim. Acta 2017, 204, 104–119. [Google Scholar] [CrossRef]
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Quezada, V.D.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New findings on source, mobilization and mobility in human environments in 20 countries based on decadal research 2010–2020. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1727–1865. [Google Scholar] [CrossRef]
- 3Tsuji, J.S.; Chang, E.T.; Gentry, P.R.; Clewell, H.J.; Boffetta, P.; Cohen, S.M. Dose-response for assessing the cancer risk of inorganic arsenic in drinking water: The scientific basis for use of a threshold approach. Crit. Rev. Toxicol. 2019, 49, 36–84. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Singh, R.; Gayen, A.; Kumar, S.; Dewangan, R. Geo-spatial distribution of arsenic contamination of groundwater resources in intricate crystalline aquifer system of Central India: Arsenic toxicity manifestation and health risk assessment. Hum. Ecol. Risk Assessment: Int. J. 2021, 27, 1588–1612. [Google Scholar] [CrossRef]
- Ahmad, A.; van der Wens, P.; Baken, K.; de Waal, L.; Bhattacharya, P.; Stuyfzand, P. Arsenic reduction to <1 µg/L in Dutch drinking water. Environ. Int. 2019, 134, 105253. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Mushtaq, N.; Masood, N.; Khattak, J.A.; Hussain, I.; Khan, Q.; Farooqi, A. Health risk assessment and source identification of groundwater arsenic contamination using agglomerative hierarchical cluster analysis in selected sites from upper Eastern parts of Punjab province, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2020, 27, 999–1018. [Google Scholar] [CrossRef]
- Bandara UG, C.; Diyabalanage, S.; Hanke, C.; van Geldern, R.; Barth, J.A.; Chandrajith, R. Arsenic-rich shallow groundwater in sandy aquifer systems buffered by rising carbonate waters: A geo-chemical case study from Mannar Island, Sri Lanka. Sci. Total Environ. 2018, 633, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Astatkie, H.; Ambelu, A.; Beyene, E.M. Sources and level of heavy metal contamination in the water of Awetu watershed streams, southwestern Ethiopia. Heliyon 2021, 7, e06385. [Google Scholar] [CrossRef]
- Chen, J.; Qian, H.; Gao, Y.; Li, X. Human Health Risk Assessment of Contaminants in Drinking Water Based on Triangular Fuzzy Numbers Approach in Yinchuan City, Northwest China. Expo. Health 2017, 10, 155–166. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef]
- Maity, J.P.; Ho, P.-R.; Huang, Y.-H.; Sun, A.-C.; Chen, C.-C. The removal of arsenic from arsenic-bearing groundwater in In-situ and Ex-situ environment using novel natural magnetic rock material and synthesized magnetic material as adsorbent: A comparative assessment. Environ. Pollut. 2019, 253, 768–778. [Google Scholar] [CrossRef]
- Ruj, B.; Chakrabortty, S.; Nayak, J.; Chatterjee, R. Treatment of arsenic sludge generated from groundwater treatment plant: A review towards a sustainable solution. S. Afr. J. Chem. Eng. 2021, 37, 214–226. [Google Scholar] [CrossRef]
- Da’ana, D.A.; Zouari, N.; Ashfaq, M.Y.; Abu-Dieyeh, M.; Khraisheh, M.; Hijji, Y.M.; Al-Ghouti, M.A. Removal of toxic elements and microbial contaminants from groundwater using low-cost treatment options. Curr. Pollut. Rep. 2021, 7, 300–324. [Google Scholar] [CrossRef]
- Mateo, C.; Navarro, M.; Navarro, C.; Leyva, A. Arsenic Phytoremediation: Finally a Feasible Approach in the Near Future. In Environmental Chemistry and Recent Pollution Control Approaches; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Leiva, E.; Leiva-Aravena, E.; Rodríguez, C.; Serrano, J.; Vargas, I. Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci. Total Environ. 2018, 645, 471–481. [Google Scholar] [CrossRef]
- Hussain, S.; Habib-Ur-Rehman, M.; Khanam, T.; Sheer, A.; Kebin, Z.; Jianjun, Y. Health Risk Assessment of Different Heavy Metals Dissolved in Drinking Water. Int. J. Environ. Res. Public Health 2019, 16, 1737. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Davis, M.A.; Signes-Pastor, A.J.; Argos, M.; Slaughter, F.; Pendergrast, C.; Punshon, T.; Gossai, A.; Ahsan, H.; Karagas, M.R. Assessment of human dietary exposure to arsenic through rice. Sci. Total Environ. 2017, 586, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.; Bundschuh, J. Arsenic in cooked rice foods: Assessing health risks and mitigation options. Environ. Int. 2019, 127, 584–591. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2449–2484. [Google Scholar] [CrossRef]
- Rahman, A.; Granberg, C.; Persson L, Å. Early life arsenic exposure, infant and child growth, and morbidity: A systematic review. Arch. Toxicol. 2017, 91, 3459–3467. [Google Scholar] [CrossRef]
- Jablonska, E.; Socha, K.; Reszka, E.; Wieczorek, E.; Skokowski, J.; Kalinowski, L.; Fendler, W.; Seroczynska, B.; Wozniak, M.; Borawska, M.; et al. Cadmium, arsenic, selenium and iron–implications for tumor progression in breast cancer. Environ. Toxicol. Pharmacol. 2017, 53, 151–157. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, D.; Li, B.; Dong, L.; Sun, G. Development of Arsenic Removal Technology from Drinking Water in Developing Countries. In Arsenic Contamination in Asia; Springer: Singapore, 2018; pp. 163–179. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S.J.E.P. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E. Stabilization and encapsulation of arsenic-/antimony-bearing mine waste: Overview and outlook of existing techniques. Crit. Rev. Environ. Sci. Technol. 2021, 52, 1–33. [Google Scholar] [CrossRef]
- Sarkar, M.; Pal, S.C. Human health hazard assessment for high groundwater arsenic and fluoride intact in Malda district, Eastern India. Groundw. Sustain. Dev. 2021, 13, 100565. [Google Scholar] [CrossRef]
- Poonia, T.; Singh, N.; Garg, M.C. Contamination of Arsenic, Chromium and Fluoride in the Indian groundwater: A review, meta-analysis and cancer risk assessment. Int. J. Environ. Sci. Technol. 2021, 18, 2891–2902. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron YA, R.; Santos LV, S.; de Paula, E.C.; Amaral, M.C.S. Arsenic contamination, effects and remediation techniques: A special look onto membrane separation processes. Process Saf. Environ. Prot. 2021, 148, 604–623. [Google Scholar] [CrossRef]
- Mohammed, J.S. A brief review on Ion Exchange Chromatography. PharmaTutor 2017, 5, 30–38. [Google Scholar]
- Baskan, M.B.; Hadimlioglu, S. Removal of arsenate using graphene oxide-iron modified clinoptilolite-based composites: Adsorption kinetic and column study. J. Anal. Sci. Technol. 2021, 12, 22. [Google Scholar] [CrossRef]
- Ortega, A.; Oliva, I.; Contreras, K.E.; González, I.; Cruz-Díaz, M.R.; Rivero, E.P. Arsenic removal from water by hybrid electro-regenerated anion exchange resin/electrodialysis process. Sep. Purif. Technol. 2017, 184, 319–326. [Google Scholar] [CrossRef]
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Torres, E.M.F.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Technologies for arsenic removal from groundwater sources, investment costs and waste management practices. Sci. Total Environ. 2019, 690, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zheng, T.; Dai, Q.; Chen, J. Sulfide and arsenic compounds removal from liquid digestate by ferric coagulation and toxicity evaluation. Water Environ. Res. 2019, 91, 1613–1623. [Google Scholar] [CrossRef]
- Robledo-Peralta, A.; López-Guzmán, M.; Morales-Amaya, C.; Reynoso-Cuevas, L. Arsenic and Fluoride in Groundwater, Prevalence and Alternative Removal Approach. Processes 2021, 9, 1191. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Thiam, A.; Salazar, R. Arsenic and fluoride removal by electrocoagulation process: A general review. Sci. Total Environ. 2020, 753, 142108. [Google Scholar] [CrossRef]
- Nidheesh, P.; Singh, T.A. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.Z.; Zoromba, M.S.; Bassyouni, M.; Sedahmed, G.H. Removal of nitrates from water by electrocoagulation using a cell with horizontally oriented Al serpentine tube anode. J. Ind. Eng. Chem. 2020, 82, 105–112. [Google Scholar] [CrossRef]
- Kumar, I.; Quaff, A.R. Comparative study on the effectiveness of natural coagulant aids and commercial coagulant: Removal of arsenic from water. Int. J. Environ. Sci. Technol. 2018, 16, 5989–5994. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Gałczyńska, M.; Mańkowska, N.; Milke, J.; Buśko, M. Possibilities and limitations of using Lemna minor, Hydrocharis morsus-ranae and Ceratophyllum demersum in removing metals with contaminated water. J. Water Land Dev. 2019. Available online: https://www.jwld.pl/files/Galczynska-et-al.pdf (accessed on 13 December 2022). [CrossRef]
- Thathong, V.; Tantamsapya, N.; Yossapol, C.; Liao, C.-H.; Wirojanagud, W.; Padungthon, S. Role of Colocasia esculenta L. schott in arsenic removal by a pilot-scale constructed wetland filled with laterite soil. Heliyon 2019, 5, e01233. [Google Scholar] [CrossRef]
- Paul, M.; Goswami, C.; Mukherjee, M.; Roychowdhury, T. Phyto-remedial detoxification of arsenic by Pistia stratiotes and assessment of its anti-oxidative enzymatic changes. Bioremediation J. 2019, 23, 175–184. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef]
- Yan, H.; Gao, Y.; Wu, L.; Wang, L.; Zhang, T.; Dai, C.; Xu, W.; Feng, L.; Ma, M.; Zhu, Y.-G.; et al. Potential use of the Pteris vittata arsenic hyperaccumulation-regulation network for phytoremediation. J. Hazard. Mater. 2019, 368, 386–396. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.D.; Borges, A.C.; Braga, A.F.; Veloso, R.W.; de Matos, A. T Phytoremediation of arsenic-contaminated water by Lemna Valdiviana: An optimization study. Chemosphere 2019, 234, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Onyia, P.C.; Ozoko, D.C.; Ifediegwu, S.I. Phytoremediation of arsenic-contaminated soils by arsenic hyperaccumulating plants in selected areas of Enugu State, Southeastern, Nigeria. Geol. Ecol. Landscapes 2020, 5, 308–319. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Tsang, D.C.; Song, L.; Zhang, C.; Yin, M.; Liu, J.; Xiao, T.; Zhang, G.; Wang, J. Hyperaccumulation and transport mechanism of thallium and arsenic in brake ferns (Pteris vittata L.): A case study from mining area. J. Hazard. Mater. 2019, 388, 121756. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mathur, J. Phytoremediation efficiency of Helianthus annuus L. for reclamation of heavy metals-contaminated industrial soil. Environ. Sci. Pollut. Res. 2020, 27, 29954–29966. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, H.; Liu, C.; Luo, S.; Liu, Y.; Yu, X.; Ma, J.; Yin, K.; Feng, H. Fast and efficient removal of As(III) from water by CuFe2O4 with peroxymonosulfate: Effects of oxidation and adsorption. Water Res. 2018, 150, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mahandra, H.; Ghahreman, A. Novel Continuous Column Process for As(III) Oxidation from Concentrated Acidic Solutions with Activated Carbon Catalysis. Ind. Eng. Chem. Res. 2020, 59, 9882–9889. [Google Scholar] [CrossRef]
- Navarrete-Magaña, M.; Estrella-González, A.; May-Ix, L.; Cipagauta-Díaz, S.; Gómez, R. Improved photocatalytic oxidation of arsenic (III) with WO3/TiO2 nanomaterials synthesized by the solgel method. J. Environ. Manag. 2021, 282, 111602. [Google Scholar] [CrossRef]
- An, Y.; Han, M.; Zheng, H.; Ding, W.; Sun, Q.; Hu, C.; Zheng, L. Hollow structured copper-loaded self-floating catalyst in sulfite-induced oxidation of arsenic(III) at neutral pH: Kinetics and mechanisms investigation. Chem. Eng. J. 2020, 407, 127193. [Google Scholar] [CrossRef]
- Rana, A.; Kumari, N.; Tyagi, M.; Jagadevan, S. Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics. Chem. Eng. J. 2018, 347, 91–100. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A comprehensive review on removal of arsenic using activated carbon prepared from easily available waste materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef]
- Luong, V.T.; Kurz, E.E.C.; Hellriegel, U.; Luu, T.L.; Hoinkis, J.; Bundschuh, J. Iron-based subsurface arsenic removal technologies by aeration: A review of the current state and future prospects. Water Res. 2018, 133, 110–122. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Sahu, O. Arsenate and fluoride removal from groundwater by sawdust impregnated ferric hydroxide and activated alumina (SFAA). Groundw. Sustain. Dev. 2020, 12, 100490. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.-G.; Liu, S.-B.; Liu, H.-Y.; Zeng, G.-M.; Tan, X.-F.; Yang, C.-P.; Ding, Y.; Yan, Z.-L.; Cai, X.-X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Gutiérrez-Martínez, J.; Rangel-Méndez, J.R. Modified activated carbon with interconnected fibrils of iron-oxyhydroxides using Mn2+ as morphology regulator, for a superior arsenic removal from water. J. Environ. Sci. 2019, 76, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.M.; Nguyen, B.Q.; Nguyen, H.T.; Nguyen, H.T. Adsorption of Arsenic and Heavy Metals from Solutions by Unmodified Iron-Ore Sludge. Appl. Sci. 2019, 9, 619. [Google Scholar] [CrossRef]
- Lung, I.; Stan, M.; Opris, O.; Soran, M.L.; Senila, M.; Stefan, M. Removal of lead (II), cadmium (II), and arsenic (III) from aqueous solution using magnetite nanoparticles pre-pared by green synthesis with Box–Behnken design. Anal. Lett. 2018, 51, 2519–2531. [Google Scholar] [CrossRef]
- Liu, L.; Tan, W.; Suib, S.L.; Qiu, G.; Zheng, L.; Su, S. Enhanced adsorption removal of arsenic from mining wastewater using birnessite under electrochemical redox reactions. Chem. Eng. J. 2019, 375, 122051. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Xiao, M.; Zhang, T.; Yao, S.; Zang, S.; Fan, H.; Shen, Y.; Zhang, Z. Enhanced arsenate removal from aqueous solution by Mn-doped MgAl-layered double hydroxides. Environ. Sci. Pollut. Res. 2019, 26, 12014–12024. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.; Dai, Y.; Wu, S.; Tang, X. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J. Environ. Manag. 2019, 245, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As (III) adsorption on Fe-Mn binary oxides: Are Fe and Mn oxides synergistic or antagonistic for arsenic removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Biswas, R.; Sarkar, A. A two-step approach for arsenic removal by exploiting an autochthonous Delftia sp. BAs29 and neutralized red mud. Environ. Sci. Pollut. Res. 2020, 28, 40665–40677. [Google Scholar] [CrossRef]
- Jin, Q.H.; Cui, C.Y.; Chen, H.Y.; Wang, Y.; Geng, J.; Wu, Y.H. Efficient removal of arsenic from water by dielectrophoresis-assisted adsorption. Water Supply 2018, 19, 1066–1072. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Kattil, K.V.; Yadav, M.K.; Gupta, A.K. Adsorptive removal of arsenic by novel iron/olivine composite: Insights into preparation and adsorption process by response surface methodology and artificial neural network. J. Environ. Manag. 2018, 209, 176–187. [Google Scholar] [CrossRef]
- Zhu, N.; Qiao, J.; Ye, Y.; Yan, T. Synthesis of mesoporous bismuth-impregnated aluminum oxide for arsenic removal: Adsorption mechanism study and application to a lab-scale column. J. Environ. Manag. 2018, 211, 73–82. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Sasani, M.; Marghzari, S. Removal of Dyes from the Environment by Adsorption Process. Chem. Mater. Eng. 2018, 6, 31–35. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, P.; Du, P.; Deng, L.; Wei, Y.; Liu, D.; Zhong, X.; Zhou, J. A novel halloysite–CeOx nanohybrid for efficient arsenic removal. Appl. Clay Sci. 2020, 186, 105450. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance Evaluation of Small Sized Powdered Ferric Hydroxide as Arsenic Adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Raichur, A.M. Enhanced adsorption capacity of activated alumina by impregnation with alum for removal of As(V) from water. Chem. Eng. J. 2008, 138, 179–186. [Google Scholar] [CrossRef]
- Diaz-Vanegas, C.; Casiot, C.; Liming, L.; de Windt, L.; Djibrine, A.; Malcles, A.; Hery, M.; Desoeuvre, A.; Bruneel, O.; Battaglia-Brunet, F. Performances of a semi-passive field-pilot for bioremediation of As-rich Acid Mine Drainage at the Carnoulès mine (France). In Proceedings of the 14th International Mine Water Association Congress, Christchurch, New Zealand, 9–30 November 2020. [Google Scholar]
- Saunders, J.A.; Lee, M.-K.; Dhakal, P.; Ghandehari, S.S.; Wilson, T.; Billor, M.Z.; Uddin, A. Bioremediation of arsenic-contaminated groundwater by sequestration of arsenic in biogenic pyrite. Appl. Geochem. 2018, 96, 233–243. [Google Scholar] [CrossRef]
- Fernandez-Rojo, L.; Casiot, C.; Laroche, E.; Tardy, V.; Bruneel, O.; Delpoux, S.; Desoeuvre, A.; Grapin, G.; Savignac, J.; Boisson, J.; et al. A field-pilot for passive bioremediation of As-rich acid mine drainage. J. Environ. Manag. 2019, 232, 910–918. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.-T.; Bahari, Z.M.; Manan, F.A. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602. [Google Scholar] [CrossRef]
- Karimi, P.; Javanshir, S.; Sayadi, M.H.; Arabyarmohammadi, H. Arsenic Removal from Mining Effluents Using Plant-Mediated, Green-Synthesized Iron Nanoparticles. Processes 2019, 7, 759. [Google Scholar] [CrossRef]
- Rodríguez-Castrejón, U.E.; Serafin-Muñoz, A.H.; Alvarez-Vargas, A.; Cruz-Jímenez, G.; Noriega-Luna, B. Isolation and molecular identification of native As-resistant bacteria: As(III) and As(V) removal capacity and possible mechanism of detoxification. Arch. Microbiol. 2022, 204, 191. [Google Scholar] [CrossRef]
- Chitara, M.K.; Chauhan, S.; Singh, R.P. Bioremediation of Polluted Soil by Using Plant Growth–Promoting Rhizobacteria. In Microbial Rejuvenation of Polluted Environment; Springer: Singapore, 2021; pp. 203–226. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Mohkami, A.; Habibi-Pirkoohi, M. Arsenic Removal from Aqueous Media using Scenedesmus obliquus: The Promoting Impact of Microalgae-Bacteria Consortium. J. Phycol. Res. 2019, 3, 301–311. [Google Scholar] [CrossRef]
- Sayqal, A.; Ahmed, O.B. Advances in Heavy Metal Bioremediation: An Overview. Appl. Bionics Biomech. 2021, 2021, 1609149. [Google Scholar] [CrossRef] [PubMed]
- Zakhar, R.; Derco, J.; Čacho, F. An overview of main arsenic removal technologies. Acta Chim. Slovaca 2018, 11, 107–113. [Google Scholar] [CrossRef]
- Mir, N.; Bicer, Y. Integration of electrodialysis with renewable energy sources for sustainable freshwater production: A review. J. Environ. Manag. 2021, 289, 112496. [Google Scholar] [CrossRef]
- Abejón, A.; Garea, A.; Irabien, A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interface Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, A.; Figoli, A. Pressure-driven and thermally-driven membrane operations for the treatment of arsenic-contaminated waters: A comparison. J. Hazard. Mater. 2019, 370, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jarma, Y.A.; Karaoğlu, A.; Tekin, Ö.; Baba, A.; Ökten, H.E.; Tomaszewska, B.; Bostancı, K.; Arda, M.; Kabay, N. Assessment of different nanofiltration and reverse osmosis membranes for simultaneous removal of arsenic and boron from spent geothermal water. J. Hazard. Mater. 2021, 405, 124129. [Google Scholar] [CrossRef]
- Figoli, A.; Fuoco, I.; Apollaro, C.; Chabane, M.; Mancuso, R.; Gabriele, B.; De Rosa, R.; Vespasiano, G.; Barca, D.; Criscuoli, A. Arsenic-contaminated groundwaters remediation by nanofiltration. Sep. Purif. Technol. 2019, 238, 116461. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2020, 278, 123805. [Google Scholar] [CrossRef]
- Rajendran, M.; Thangavelu, D. Removal of As(V) from water using galvanically coupled sacrificial metals. J. Hazard. Mater. 2020, 409, 124564. [Google Scholar] [CrossRef]
- Kepel, B.; Bodhi, W.; Tallei, T.E. Isolation and Identification of Arsenic-resistant Bacteria for Possible Application in Arsenic Bioremediation. Pak. J. Biol. Sci. 2019, 23, 63–67. [Google Scholar] [CrossRef]
- Mendoza, R.M.O.; Dalida, M.L.P.; Kan, C.-C.; Wan, M.-W. Groundwater treatment by electrodialysis: Gearing up towards green technology. Desalination Water Treat. 2018, 127, 178–183. [Google Scholar] [CrossRef]
- Aliaskari, M.; Schäfer, A.I. Nitrate, arsenic and fluoride removal by electrodialysis from brackish groundwater. Water Res. 2020, 190, 116683. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of polyethyleneimine coating on membrane permselectivity and desalination performance during pilot-scale electrodialysis of reverse osmosis concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar] [CrossRef]
- Tuan Minh, P.; Nishihama, S.; Yoshizuka, K. Removal of Arsenic from Water Environment by Electrodialysis. Available online: https://www.academia.edu/59977609/Removal_of_Arsenic_from_Water_by_Electrocoagulation_and_Electrodialysis_Techniques (accessed on 13 December 2022).
- Graça, N.S.; Rodrigues, A.E.J.C.T. The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technol. 2022, 4, 63. Available online: https://www.mdpi.com/2571-8797/4/4/63 (accessed on 13 December 2022). [CrossRef]
- Almeida, J.; Craveiro, R.; Faria, P.; Silva, A.; Mateus, E.; Barreiros, S.; Paiva, A.; Ribeiro, A. Electrodialytic removal of tungsten and arsenic from secondary mine resources—Deep eutectic solvents enhancement. Sci. Total Environ. 2019, 710, 136364. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Khan, T.A.; Asim, M.J.S.; reviews, p. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Environ. Technol. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Cohen, B.; Lazarovitch, N.; Gilron, J. Upgrading groundwater for irrigation using monovalent selective electrodialysis. Desalination 2018, 431, 126–139. [Google Scholar] [CrossRef]
- Almeida, J.S.R.d. Recovery of Mining Residues for Eco-Efficient Mortar Production. PhD Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2021. [Google Scholar]
- Almeida, J.; Magro, C.; Rosário, A.R.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic treatment of secondary mining resources for raw materials extraction: Reactor design assessment. Sci. Total Environ. 2021, 752, 141822. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gokcek, O.B.; Uzal, N. Arsenic removal by the micellar-enhanced ultrafiltration using response surface methodology. Water Supply 2019, 20, 574–585. [Google Scholar] [CrossRef]
- Rosário, A.R.A. Application of the Electrodialytic Process for Tungsten Recovery and Arsenic Removal from Mine Tailings. PhD Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2018. [Google Scholar]
- Ma, S.; Yang, F.; Chen, X.; Khor, C.M.; Jung, B.; Iddya, A.; Sant, G.; Jassby, D. Removal of As(III) by Electrically Conducting Ultrafiltration Membranes. Water Res. 2021, 204, 117592. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H. Heavy metals removal from aqueous solution through micellar enhanced ultrafiltration: A review. Environ. Eng. Res. 2018, 24, 363–375. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.D.S.; Amaral, M.C.S. Dead-end ultrafiltration as a cost-effective strategy for improving arsenic removal from high turbidity waters in conventional drinking water facilities. Chem. Eng. J. 2020, 417, 128132. [Google Scholar] [CrossRef]
- Chen, M.; Shafer-Peltier, K.; Randtke, S.J.; Peltier, E. Modeling arsenic (V) removal from water by micellar enhanced ultrafiltration in the presence of competing anions. Chemosphere 2018, 213, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, P.; Maleki, A.; Rezaee, R.; Khamforoush, M.; Yetilmezsoy, K.; Athar, S.D.; Gharibi, F. Simultaneous removal of arsenate and nitrate from aqueous solutions using micellar-enhanced ultrafiltration process. J. Water Process. Eng. 2018, 27, 24–31. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T.; Wu, Y.; Cao, X.; Hankins, N.P. Inorganic anion removal using micellar enhanced ultrafiltration (MEUF), modeling anion distribution and suggested improvements of MEUF: A review. Chem. Eng. J. 2020, 398, 125413. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; Isloor, A.M.; Asiri, A.M.; Ismail, A.F.; Kumar, R.; Ahamed, M.I. Performance intensification of the polysulfone ultrafiltration membrane by blending with copolymer encompassing novel derivative of poly (styrene-co-maleic anhydride) for heavy metal removal from wastewater. Chem. Eng. J. 2018, 353, 425–435. [Google Scholar] [CrossRef]
- Hamid, M.F.; Yusof, N.; Ismail, N.M.; Azali, M.A. Role of Membrane Surface Charge and Complexation-Ultrafiltration for Heavy Metals Removal: A Mini Review. J. Appl. Membr. Sci. Technol. 2020, 24, 39–49. [Google Scholar] [CrossRef]
- Kabir, F.; Chowdhury, S. Arsenic removal methods for drinking water in the developing countries: Technological developments and research needs. Environ. Sci. Pollut. Res. 2017, 24, 24102–24120. [Google Scholar] [CrossRef]
- Alam, R.; McPhedran, K. Applications of biological sulfate reduction for remediation of arsenic—A review. Chemosphere 2019, 222, 932–944. [Google Scholar] [CrossRef]
- Yun, H.-S.; Jang, M.; Shin, W.-S.; Choi, J. Remediation of arsenic-contaminated soils via waste-reclaimed treatment agents: Batch and field studies. Miner. Eng. 2018, 127, 90–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).