The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters
Abstract
:1. Introduction
2. Overview of Electrocoagulation Process
2.1. Chemical Coagulation vs. Electrocoagulation
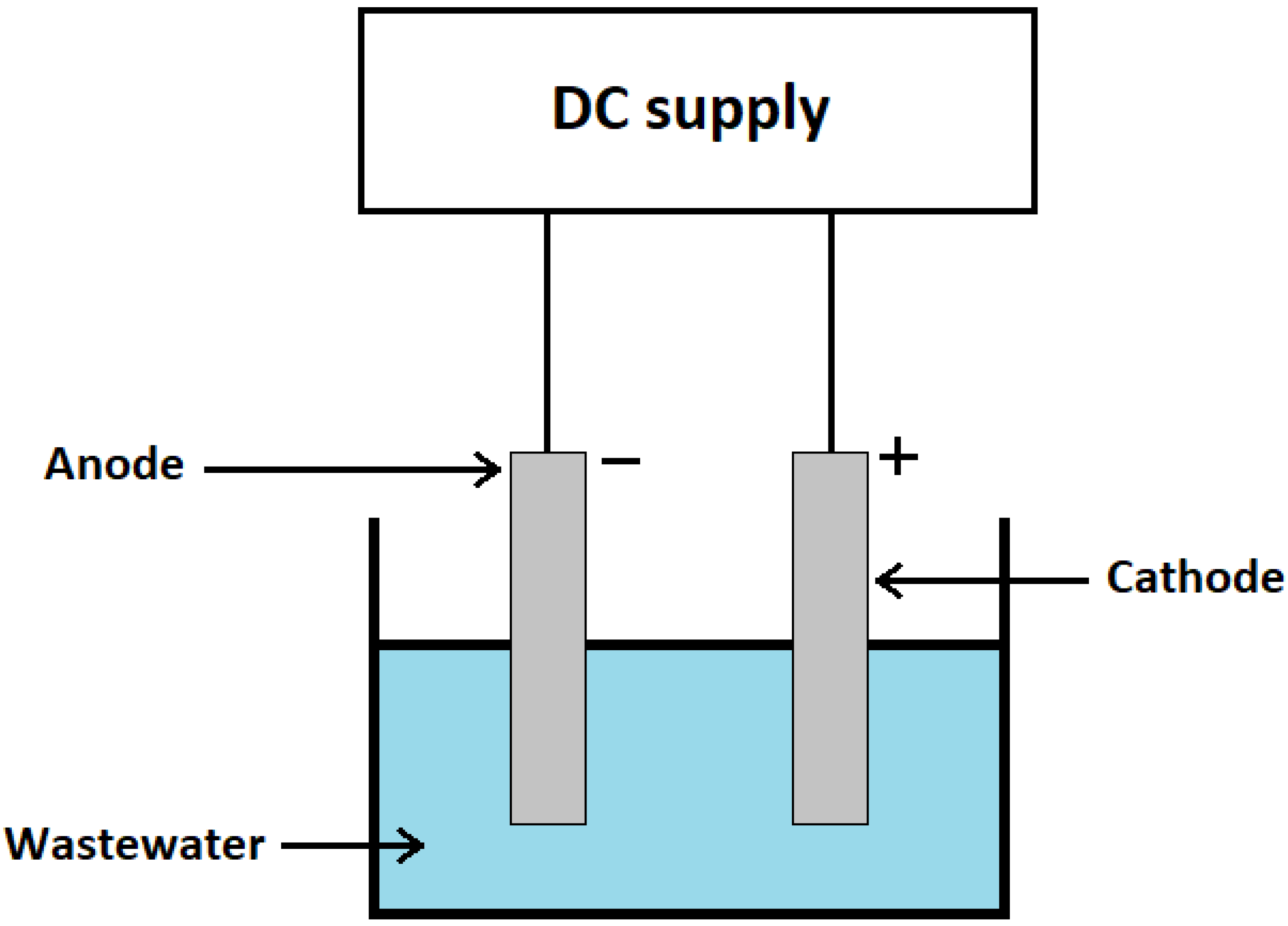
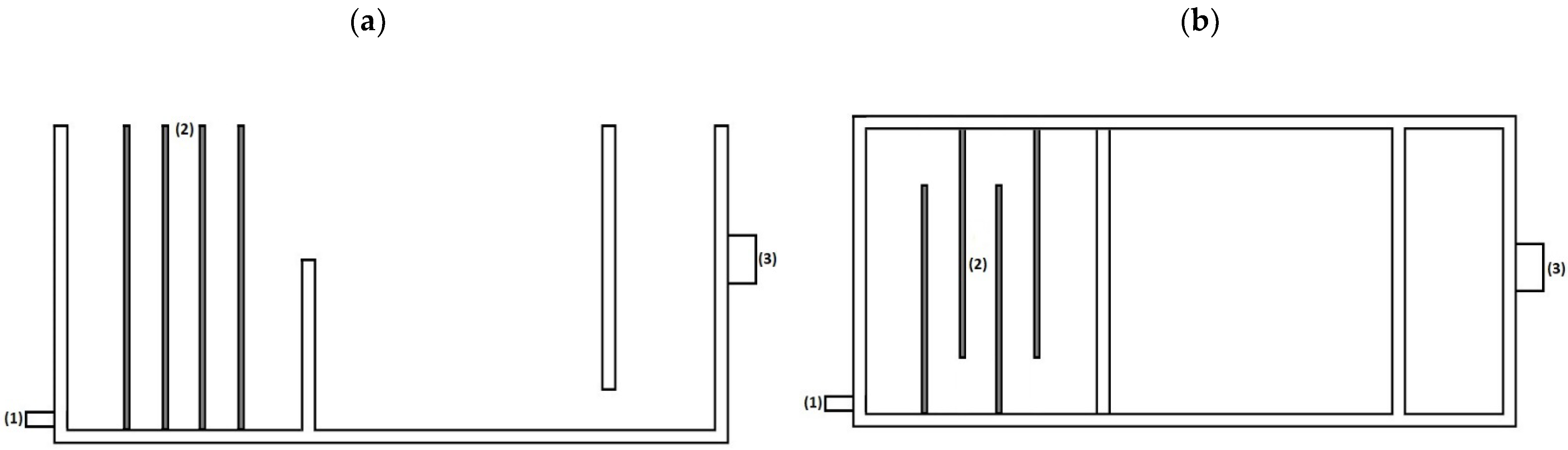
2.2. Electrocoagulation Process Set-Up
2.3. Factors Affecting Electrocoagulation
2.3.1. Current Intensity
2.3.2. Initial pH
2.3.3. Inter-Electrode Distance
2.3.4. Electrode Arrangement
2.3.5. Electrode Material
2.3.6. Other Factors
2.4. Application of the EC Process on Wastewater Treatment
| Wastewater | Initial Concentration of Pollutants | Electrodes | Removal Efficiency | Reference |
|---|---|---|---|---|
| Industrial wastewater | 873 mg/L (COD) | 91.7% | [100] | |
| Municipal wastewater | 143 mg/L (TSS) 68 mg/L (particulate BOD) | 95.4% (TSS) 99% (particulate BOD) | [101] | |
| Textile wastewater | 1400 mg/L (Acid Red 336) | 99% (turbidity) 95% (color) | [102] | |
| Textile wastewater | 278 ADMI (color) 339 mg/L (COD) 610 mg/L (silica) | 88.1% (color) 70.5% (COD) 100% (silica) | [103] | |
| Cold meat industry Wastewater | 3482 mg/L (COD) | 92.9% | [104] | |
| Palm oil mill effluent | 25,500 mg/L (COD) 15,600 mg/L (BOD) 12,300 mg/L (TSS) | steel wool | 95% (COD) 94% (BOD) 96% (TSS) | [105] |
| Carpet cleaning wastewater | 20.4 mg/L (methylene blue substances) 674 mg/L (COD) 122 NTU (turbidity) | 85.5% (methylene blue substances) 84.4% (COD) 90.5% (turbidity) | [106] | |
| Hospital wastewater | 32.5 mg/L (ciprofloxacin) | 90.4% | [107] | |
| Simulated phenolic wastewater | 327 mg/L (total phenolic content) 1118 mg/L (COD) | 84.2% (total phenolic content) 40.3% (COD) | [108] | |
| Olive mill effluent | 57,800 mg/L (COD) 2420 mg/L (polyphenol) | 76% (COD) 91% (polyphenol) 95% (dark color) | [109] | |
| Landfill leachate | 11,000 mg/L (COD) | 65.9% | [110] | |
| Wastewater from an industrial park | 2300 mg/L (COD) 7450 mg/L (color (Pt-Co)) 550 NTU (turbidity) 1080 mg/L (TOC) | 89% (COD) 97% (color) 91% (turbidity) 48% (TOC) | [111] | |
| Pulp and paper industry bleaching effluent | 255 CU (color) 620 mg/L (COD) 210 mg/L (BOD) | 94% (color) 90% (COD) 87% (BOD) | [112] | |
| Cork boiling wastewater | 1594 mg/L (COD) 880 mg/L (TOC) 38.6 mg/L (TN) 271 mg/L (TSS) | 93.5% (COD) 82.5% (TOC) 88.9% (TN) 99% (TSS) | [113] | |
| Tannery wastewater | 14,001 mg/L (COD) 6000 mg/L (TDS) | carbon-steel | 23% (COD) 76% (TDS) | [114] |
3. Overview of the Adsorption Process
- 1-
- Bulk diffusion: transport of the adsorbate molecules in the solution phase.
- 2-
- Film diffusion: transport of the adsorbate molecules from the bulk solution to the adsorbent surface through a hydrodynamic boundary layer (film).
- 3-
- Intra-particle diffusion: transport of the adsorbate molecules from the adsorbent external surface through the adsorbent pores.
- 4-
- Adsorption: uptake of the adsorbates on the adsorbent.
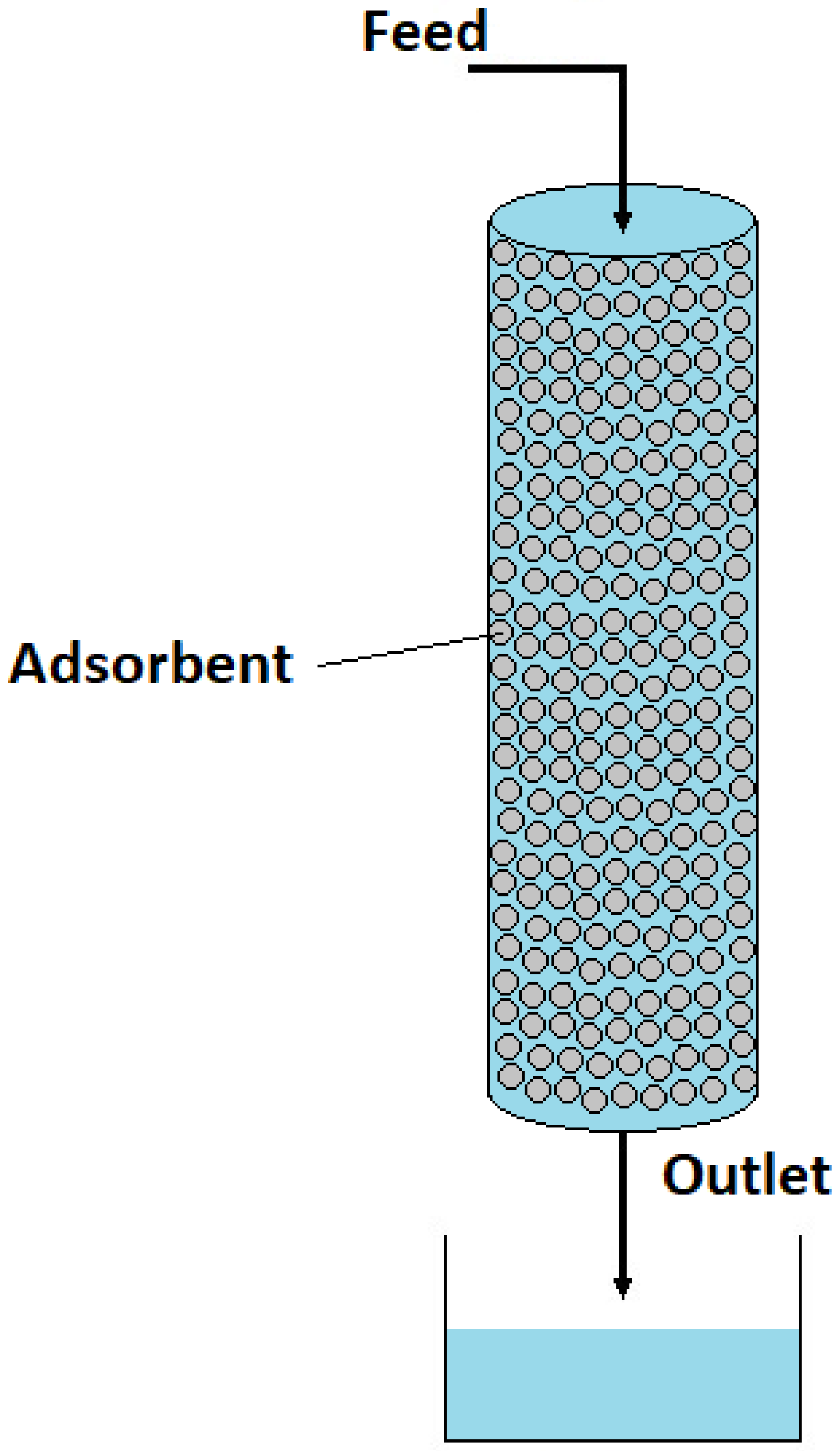
3.1. Adsorption Process Set-Up
3.2. Factors Affecting Adsorption
3.2.1. Adsorbent Material
3.2.2. Adsorbent Dosage
3.2.3. Concentration of Pollutants
3.2.4. Temperature
3.2.5. Solution pH
3.3. Applications of Low-Cost Adsorbents
4. Combined Electrocoagulation and Adsorption Processes
4.1. Applications of Combined Electrocoagulation and Adsorption Processes
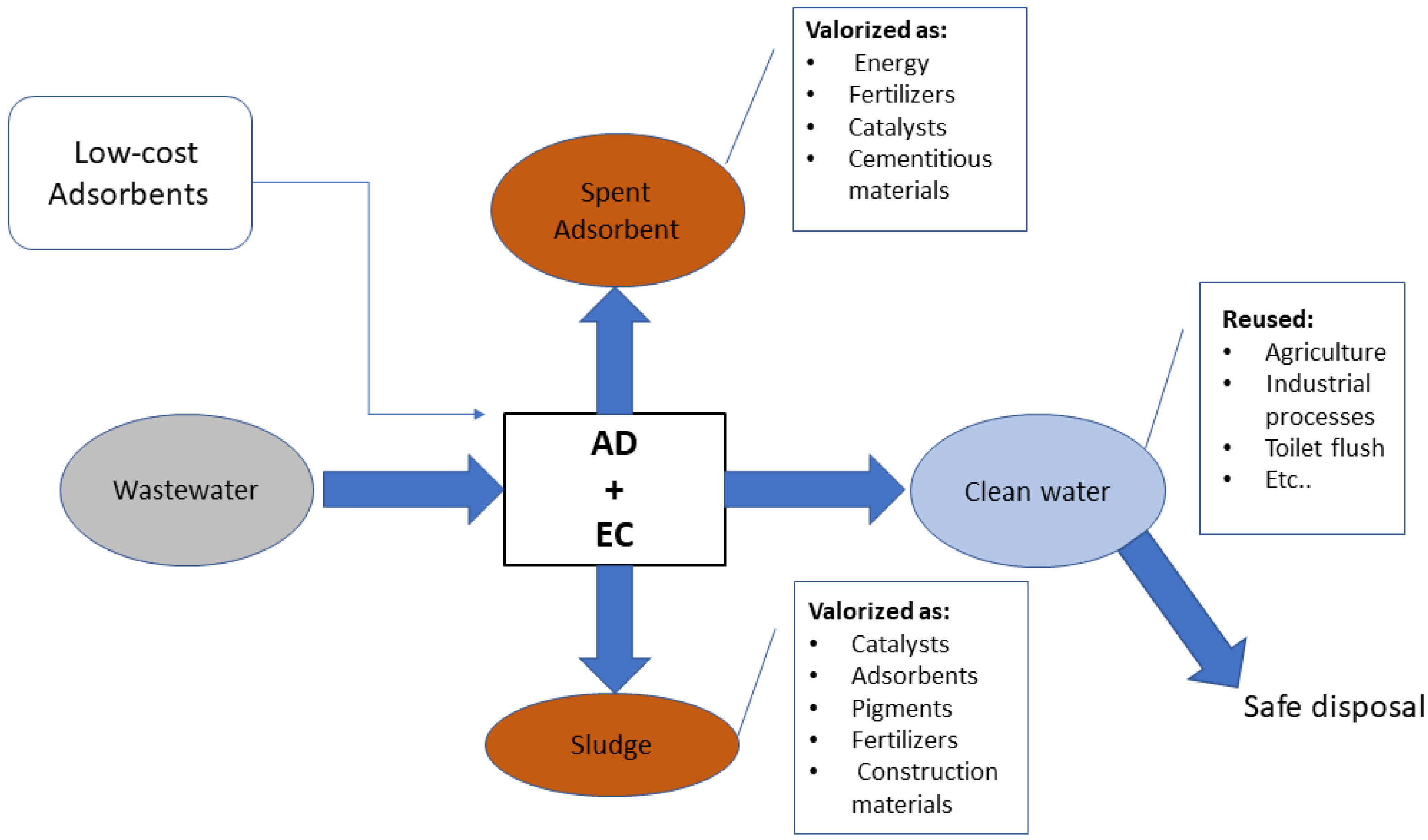
4.2. The Combined Process from the Perspective of Circular Economy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- The United Nations. World Water Development Report 2019: Leaving no one behind WWDR 2019; UNESCO: Paris, France, 2019. [Google Scholar]
- The United Nations. World Water Development Report 2016: Water and Jobs WWDR 2016; UNESCO: Paris, France, 2016. [Google Scholar]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wei, S.; Liu, Y.; Zhang, X.; Jiang, Z.; Tao, Y.; Zhang, G.; Zhang, B.; Wang, L.; Zhang, Y. Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria. J. Hazard. Mater. 2021, 423, 127043. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Otero, M.; Rocha, L.S.; Gil, M.V.; Ferreira, P.; Esteves, V.I.; Calisto, V. Multivariable optimization of activated carbon production from microwave pyrolysis of brewery wastes—Application in the removal of antibiotics from water. J. Hazard. Mater. 2022, 431, 128556. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sadhanala, H.K.; Mastai, Y.; Porat, Z.; Gedanken, A. Sonochemically Prepared BSA Microspheres as Adsorbents for the Removal of Organic Pollutants from Water. Langmuir 2021, 37, 9927–9938. [Google Scholar] [CrossRef]
- The United Nations. World Water Development Report 2009: Water in a Changing World WWDR 2009; UNESCO: Paris, France, 2009. [Google Scholar]
- United Nations. SDG 6 Synthesis Report 2018 on Water and Sanitation; United Nations: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2015, 149, 222–235. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Hu, C.; Zeng, G.; Cheng, M.; Xu, P.; Gong, X.; Wang, R.; Xue, W. Combination of Fenton processes and biotreatment for wastewater treatment and soil remediation. Sci. Total Environ. 2016, 574, 1599–1610. [Google Scholar] [CrossRef]
- Bhuptawat, H.; Folkard, G.; Chaudhari, S. Innovative physico-chemical treatment of wastewater incorporating Moringa oleifera seed coagulant. J. Hazard. Mater. 2007, 142, 477–482. [Google Scholar] [CrossRef]
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Álvarez, J.; Otero, L.; Lema, J.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Lopez-Vizcaíno, R.; Sáez, C.; Cañizares, P.; Rodrigo, M. Electrocoagulation of the effluents from surfactant-aided soil-remediation processes. Sep. Purif. Technol. 2012, 98, 88–93. [Google Scholar] [CrossRef]
- Jara, C.C.; Fino, D.; Specchia, V.; Saracco, G.; Spinelli, P. Electrochemical removal of antibiotics from wastewaters. Appl. Catal. B Environ. 2007, 70, 479–487. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachta, M. Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chem. Eng. Process. Process Intensif. 2019, 143, 107619. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martinez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-H.; Wang, K.-S.; Liang, H.-H.; Chen, H.-Y.; Li, H.-C.; Peng, T.-H.; Su, Y.-C.; Chang, C.-Y. Treatment of Reactive Black 5 by combined electrocoagulation–granular activated carbon adsorption–microwave regeneration process. J. Hazard. Mater. 2010, 175, 850–857. [Google Scholar] [CrossRef]
- Dindaş, G.B.; Çalışkan, Y.; Çelebi, E.E.; Tekbaş, M.; Bektaş, N.; Yatmaz, H.C. Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-fenton and photocatalytic oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103777. [Google Scholar] [CrossRef]
- Apshankar, K.R.; Goel, S. Review and analysis of defluoridation of drinking water by electrocoagulation. J. Water Supply Res. Technol. 2018, 67, 297–316. [Google Scholar] [CrossRef]
- Titchou, F.E.; Afanga, H.; Zazou, H.; Akbour, R.A.; Hamdani, M. Batch elimination of cationic dye from aqueous solution by electrocoagulation process. Mediterr. J. Chem. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2017, 33, 263–292. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K. Intermolecular force. Interface Sci. Technol. 2011, 18, 1–57. [Google Scholar]
- Alexander, J.T.; Hai, F.I.; Al-Aboud, T.M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manag. 2012, 111, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2016. [Google Scholar]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Y.; Morkovsky, P.; Gomes, J.A.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, D. Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological; CRC Press: London, UK, 2016. [Google Scholar]
- Rodriguez, J.; Stopić, S.; Krause, G.; Friedrich, B. Feasibility assessment of electrocoagulation towards a new sustainable wastewater treatment. Environ. Sci. Pollut. Res. 2007, 14, 477–482. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Eyvaz, M.; Kobya, M. Treatment of the textile wastewater by electrocoagulation: Economical evaluation. Chem. Eng. J. 2007, 128, 155–161. [Google Scholar] [CrossRef]
- Brahmi, K.; Bouguerra, W.; Hamrouni, B.; Elaloui, E.; Loungou, M.; Tlili, Z. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar] [CrossRef]
- Graça, N.S.; Ribeiro, A.M.; Rodrigues, A.E. Removal of Fluoride from Water by a Continuous Electrocoagulation Process. Ind. Eng. Chem. Res. 2019, 58, 5314–5321. [Google Scholar] [CrossRef]
- Nuñez, P.; Hansen, H.K.; Aguirre, S.; Maureira, C. Electrocoagulation of arsenic using iron nanoparticles to treat copper mineral processing wastewater. Sep. Purif. Technol. 2011, 79, 285–290. [Google Scholar] [CrossRef]
- Zewail, T.; Yousef, N. Chromium ions (Cr6+ & Cr3+) removal from synthetic wastewater by electrocoagulation using vertical expanded Fe anode. J. Electroanal. Chem. 2014, 735, 123–128. [Google Scholar] [CrossRef]
- Un, U.T.; Koparal, A.S.; Ogutveren, U.B. Fluoride removal from water and wastewater with a bach cylindrical electrode using electrocoagulation. Chem. Eng. J. 2013, 223, 110–115. [Google Scholar] [CrossRef]
- Lacasa, E.; Cañizares, P.; Saez, C.; Fernández, F.J.; Rodrigo, M.A. Electrochemical phosphates removal using iron and aluminium electrodes. Chem. Eng. J. 2011, 172, 137–143. [Google Scholar] [CrossRef]
- Eiband, M.M.; Trindade, K.C.D.A.; Gama, K.; de Melo, J.V.; Martínez-Huitle, C.A.; Ferro, S. Elimination of Pb2+ through electrocoagulation: Applicability of adsorptive stripping voltammetry for monitoring the lead concentration during its elimination. J. Electroanal. Chem. 2014, 717–718, 213–218. [Google Scholar] [CrossRef]
- Un, U.T.; Kandemir, A.; Erginel, N.; Ocal, S.E. Continuous electrocoagulation of cheese whey wastewater: An application of Response Surface Methodology. J. Environ. Manag. 2014, 146, 245–250. [Google Scholar] [CrossRef]
- Hamdan, S.S.; El-Naas, M.H. Characterization of the removal of Chromium(VI) from groundwater by electrocoagulation. J. Ind. Eng. Chem. 2014, 20, 2775–2781. [Google Scholar] [CrossRef]
- Graça, N.S.; Ribeiro, A.M.; Rodrigues, A.E. Modeling the electrocoagulation process for the treatment of contaminated water. Chem. Eng. Sci. 2019, 197, 379–385. [Google Scholar] [CrossRef]
- Picard, T.; Cathalifaud-Feuillade, G.; Mazet, M.; Vandensteendam, C. Cathodic dissolution in the electrocoagulation process using aluminium electrodes. J. Environ. Monit. 2000, 2, 77–80. [Google Scholar] [CrossRef]
- Jiménez, C.; Sáez, C.; Martínez, F.; Cañizares, P.; Rodrigo, M.A. Electrochemical dosing of iron and aluminum in continuous processes: A key step to explain electro-coagulation processes. Sep. Purif. Technol. 2012, 98, 102–108. [Google Scholar] [CrossRef]
- Mechelhoff, M.; Kelsall, G.H.; Graham, N.J. Super-faradaic charge yields for aluminium dissolution in neutral aqueous solutions. Chem. Eng. Sci. 2013, 95, 353–359. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Graça, N.S.; Ribeiro, A.M.; Rodrigues, A.E. Electrocoagulation process for the removal of co-existent fluoride, arsenic and iron from contaminated drinking water. Sep. Purif. Technol. 2018, 197, 237–243. [Google Scholar] [CrossRef]
- Dimoglo, A.; Sevim-Elibol, P.; Dinç, Ö.; Gökmen, K.; Erdoğan, H. Electrocoagulation/electroflotation as a combined process for the laundry wastewater purification and reuse. J. Water Process Eng. 2019, 31, 100877. [Google Scholar] [CrossRef]
- Bashir, M.J.; Lim, J.H.; Abu Amr, S.S.; Wong, L.P.; Sim, Y.L. Post treatment of palm oil mill effluent using electro-coagulation-peroxidation (ECP) technique. J. Clean. Prod. 2018, 208, 716–727. [Google Scholar] [CrossRef]
- Changmai, M.; Pasawan, M.; Purkait, M. Treatment of oily wastewater from drilling site using electrocoagulation followed by microfiltration. Sep. Purif. Technol. 2018, 210, 463–472. [Google Scholar] [CrossRef]
- Reilly, M.; Cooley, A.P.; Tito, D.; Tassou, S.A.; Theodorou, M.K. Electrocoagulation treatment of dairy processing and slaughterhouse wastewaters. Energy Procedia 2019, 161, 343–351. [Google Scholar] [CrossRef]
- Deveci, E.; Akarsu, C.; Gönen, Ç.; Özay, Y. Enhancing treatability of tannery wastewater by integrated process of electrocoagulation and fungal via using RSM in an economic perspective. Process Biochem. 2019, 84, 124–133. [Google Scholar] [CrossRef]
- Nawarkar, C.; Salkar, V. Solar powered Electrocoagulation system for municipal wastewater treatment. Fuel 2018, 237, 222–226. [Google Scholar] [CrossRef]
- Verma, A.K. Treatment of textile wastewaters by electrocoagulation employing Fe-Al composite electrode. J. Water Process Eng. 2017, 20, 168–172. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M. Operating cost and treatment of metalworking fluid wastewater by chemical coagulation and electrocoagulation processes. Process Saf. Environ. Prot. 2017, 105, 79–90. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, B.; Lee, C. Effects of low temperature on aluminum(III) hydrolysis: Theoretical and experimental studies. J. Environ. Sci. 2008, 20, 907–914. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, H.A.; Kasiri, M.B. Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, W.; Barhoumi, A.; Ibrahim, N.; Brahmi, K.; Aloui, L.; Hamrouni, B. Optimization of the electrocoagulation process for the removal of lead from water using aluminium as electrode material. Desalination Water Treat. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and Fe–Al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Ghosh, D.; Medhi, C.; Purkait, M. Treatment of fluoride containing drinking water by electrocoagulation using monopolar and bipolar electrode connections. Chemosphere 2008, 73, 1393–1400. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2013, 21, 2397–2413. [Google Scholar] [CrossRef]
- Mechelhoff, M.; Kelsall, G.H.; Graham, N.J. Electrochemical behaviour of aluminium in electrocoagulation processes. Chem. Eng. Sci. 2013, 95, 301–312. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Flores, O.J.; Nava, J.L.; Carreño, G. Arsenic removal from groundwater by electrocoagulation process in a filter-press-type FM01-LC reactor. Int. J. Electrochem. Sci. 2014, 9, 6658–6667. [Google Scholar]
- Moreno, C.H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E.; Garcia, C. Electrochemical Reactions for Electrocoagulation Using Iron Electrodes. Ind. Eng. Chem. Res. 2009, 48, 2275–2282. [Google Scholar] [CrossRef]
- Singh, M.M.; Szafran, Z.; Ibanez, J.G. Laboratory Experiments on the Electrochemical Remediation of Environment. Part 4: Color Removal of Simulated Wastewater by Electrocoagulation-Electroflotation. J. Chem. Educ. 1998, 75, 1040. [Google Scholar] [CrossRef]
- Safwat, S.M.; Hamed, A.; Rozaik, E. Electrocoagulation/electroflotation of real printing wastewater using copper electrodes: A comparative study with aluminum electrodes. Sep. Sci. Technol. 2018, 54, 183–194. [Google Scholar] [CrossRef]
- Danial, R.; Abdullah, L.C.; Sobri, S. Potential of Copper Electrodes in Electrocoagulation Process for Glyphosate Herbicide Removal. MATEC Web Conf. 2017, 103, 06019. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, A.K.; Chaudhari, P.K.; Pal, D.; Chandrakar, A.; Choudhary, R. Electrocoagulation treatment of rice grain based distillery effluent using copper electrode. J. Water Process Eng. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Kamaraj, R.; Vasudevan, S. Evaluation of electrocoagulation process for the removal of strontium and cesium from aqueous solution. Chem. Eng. Res. Des. 2015, 93, 522–530. [Google Scholar] [CrossRef]
- Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod. 2017, 147, 206–216. [Google Scholar] [CrossRef]
- Çırak, M. High-temperature electrocoagulation of colloidal calcareo-argillaceous suspension. Powder Technol. 2018, 328, 13–25. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Perdicakis, B.; Sadrzadeh, M. Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Sci. Total Environ. 2018, 645, 560–572. [Google Scholar] [CrossRef]
- Song, S.; Yao, J.; He, Z.; Qiu, J.; Chen, J. Effect of operational parameters on the decolorization of C.I. Reactive Blue 19 in aqueous solution by ozone-enhanced electrocoagulation. J. Hazard. Mater. 2008, 152, 204–210. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Wu, M.; Hu, Y.; Liu, R.; Lin, S.; Sun, W.; Lu, H. Electrocoagulation method for treatment and reuse of sulphide mineral processing wastewater: Characterization and kinetics. Sci. Total Environ. 2019, 696, 134063. [Google Scholar] [CrossRef]
- Keshmirizadeh, E.; Yousefi, S.; Rofouei, M.K. An investigation on the new operational parameter effective in Cr(VI) removal efficiency: A study on electrocoagulation by alternating pulse current. J. Hazard. Mater. 2011, 190, 119–124. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. J. Hazard. Mater. 2011, 192, 26–34. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Koyuncu, S.; Arıman, S. Domestic wastewater treatment by real-scale electrocoagulation process. Water Sci. Technol. 2020, 81, 656–667. [Google Scholar] [CrossRef]
- Gasmi, A.; Ibrahimi, S.; Elboughdiri, N.; Tekaya, M.A.; Ghernaout, D.; Hannachi, A.; Mesloub, A.; Ayadi, B.; Kolsi, L. Comparative Study of Chemical Coagulation and Electrocoagulation for the Treatment of Real Textile Wastewater: Optimization and Operating Cost Estimation. ACS Omega 2022, 7, 22456–22476. [Google Scholar] [CrossRef]
- Ge, J.; Qu, J.; Lei, P.; Liu, H. New bipolar electrocoagulation-electroflotation process for the treatment of laundry wastewater. Sep. Purif. Technol. 2004, 36, 33–39. [Google Scholar] [CrossRef]
- Elnakar, H.; Buchanan, I. Soluble chemical oxygen demand removal from bypass wastewater using iron electrocoagulation. Sci. Total Environ. 2019, 706, 136076. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zampeta, C.; Mastrantonaki, M.; Katsaouni, N.; Frontistis, Z.; Koutsoukos, P.; Vayenas, D.V. Treatment of printing ink wastewater using a continuous flow electrocoagulation reactor. J. Environ. Manag. 2022, 314, 115033. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A. Removal of water turbidity by the electrocoagulation method. J. Res. Health Sci. 2008, 8, 18–24. [Google Scholar] [PubMed]
- Un, U.T.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and cod from real textile wastewater. J. Environ. Manag. 2013, 123, 113–119. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, T.; Kim, T.-K.; Joo, S.-W.; Zoh, K.-D. Degradation mechanism of perfluorooctanoic acid (PFOA) during electrocoagulation using Fe electrode. Sep. Purif. Technol. 2020, 247, 116911. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Y.; Ning, K.; Niu, X.; Tan, S.; Su, P. Remediation of Perfluoroalkyl Substances in Landfill Leachates by Electrocoagulation. CLEAN–Soil Air Water 2013, 42, 1740–1743. [Google Scholar] [CrossRef]
- Mao, X.; Ciblak, A.; Baek, K.; Amiri, M.; Loch-Caruso, R.; Alshawabkeh, A.N. Optimization of electrochemical dechlorination of trichloroethylene in reducing electrolytes. Water Res. 2012, 46, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Keyikoglu, R.; Can, O.T. The role of dye molecular weight on the decolorization performance of the electrocoagulation. Environ. Dev. Sustain. 2020, 23, 3917–3928. [Google Scholar] [CrossRef]
- Sankar, R.; Sivasubramanian, V. Application of statistical design to optimize the electrocoagulation of synthetic Congo red dye solution and predicting the mechanism. Int. J. Environ. Sci. Technol. 2020, 17, 1373–1386. [Google Scholar] [CrossRef]
- Balarak, D.; Ganji, F.; Choi, S.S.; Lee, S.S.; Shim, M.J. Effects of operational parameters on the removal of acid blue 25 dye from aqueous solutions by electrocoagulation. Appl. Chem. Eng. 2019, 30, 742–748. [Google Scholar]
- Bracher, G.H.; Carissimi, E.; Wolff, D.B.; Graepin, C.; Hubner, A.P. Optimization of an electrocoagulation-flotation system for domestic wastewater treatment and reuse. Environ. Technol. 2020, 17, 2669–2679. [Google Scholar] [CrossRef]
- Omwene, P.; Kobya, M. Treatment of domestic wastewater phosphate by electrocoagulation using Fe and Al electrodes: A comparative study. Process Saf. Environ. Prot. 2018, 116, 34–51. [Google Scholar] [CrossRef]
- Elazzouzi, M.; Haboubi, K.; Elyoubi, M. Enhancement of electrocoagulation-flotation process for urban wastewater treatment using Al and Fe electrodes: Techno-economic study. Mater. Today Proc. 2019, 13, 549–555. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C., Jr. Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J. Hazard. Mater. 2019, 361, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Y.; Ögütveren, Ü.B. Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. J. Environ. Manag. 2017, 207, 151–158. [Google Scholar] [CrossRef]
- Bukhari, A.A. Investigation of the electro-coagulation treatment process for the removal of total suspended solids and turbidity from municipal wastewater. Bioresour. Technol. 2008, 99, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Khemila, B.; Merzouk, B.; Chouder, A.; Zidelkhir, R.; Leclerc, J.-P.; Lapicque, F. Removal of a textile dye using photovoltaic electrocoagulation. Sustain. Chem. Pharm. 2018, 7, 27–35. [Google Scholar] [CrossRef]
- Amri, N.; Ismail, S.; Azha, S.F.; Abdullah, A.Z. Behaviors and Mechanism of Color, COD, and Silica Removals in the Electrocoagulation of Batik Wastewater Using Waste Aluminum Electrodes. Int. J. Environ. Res. 2021, 15, 509–525. [Google Scholar] [CrossRef]
- Morales-Rivera, J.; Sulbarán-Rangel, B.; Gurubel-Tun, K.; Del Real-Olvera, J.; Zúñiga-Grajeda, V. Modeling and Optimization of COD Removal from Cold Meat Industry Wastewater by Electrocoagulation Using Computational Techniques. Processes 2020, 8, 1139. [Google Scholar] [CrossRef]
- Nasrullah, M.; Zularisam, A.; Krishnan, S.; Sakinah, M.; Singh, L.; Fen, Y.W. High performance electrocoagulation process in treating palm oil mill effluent using high current intensity application. Chin. J. Chem. Eng. 2018, 27, 208–217. [Google Scholar] [CrossRef]
- Shakeri, E.; Mousazadeh, M.; Ahmadpari, H.; Kabdasli, I.; Jamali, H.A.; Graça, N.S.; Emamjomeh, M.M. Electrocoagulation-flotation treatment followed by sedimentation of carpet cleaning wastewater: Optimization of key operating parameters via RSM-CCD. Desalination Water Treat. 2021, 227, 163–176. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Rodrigues, R.F.; Martins, R.C.; Castro, L.M.; Quinta-Ferreira, R.M. Phenolic wastewaters treatment by electrocoagulation process using Zn anode. Chem. Eng. J. 2015, 275, 331–341. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. Process Intensif. 2004, 43, 1281–1287. [Google Scholar] [CrossRef]
- Orkun, M.O.; Kuleyin, A. Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro-coagulation and electro-fenton technique. Environ. Prog. Sustain. Energy 2010, 31, 59–67. [Google Scholar] [CrossRef]
- García-García, A.; Martínez-Miranda, V.; Martínez-Cienfuegos, I.G.; Almazán-Sánchez, P.T.; Castañeda-Juárez, M.; Linares-Hernández, I. Industrial wastewater treatment by electrocoagulation–electrooxidation processes powered by solar cells. Fuel 2015, 149, 46–54. [Google Scholar] [CrossRef]
- Sridhar, R.; Sivakumar, V.; Immanuel, V.P.; Maran, J.P. Treatment of pulp and paper industry bleaching effluent by electrocoagulant process. J. Hazard. Mater. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Silva, J.R.; Carvalho, F.; Vicente, C.; Santos, A.D.; Quinta-Ferreira, R.M.; Castro, L.M. Electrocoagulation treatment of cork boiling wastewater. J. Environ. Chem. Eng. 2022, 10. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Kadier, A.; Singh, R.; Navarro-Mendoza, R.; Bandala, E.; Peralta-Hernández, J.M. Post-tanning wastewater treatment using electrocoagulation: Optimization, kinetics, and settlement analysis. Process Saf. Environ. Prot. 2022, 165, 872–886. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Cooney, D.O. Adsorption Design for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Sharma, S.K. Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Anfar, Z.; Ahsaine, H.A.; Zbair, M.; Amedlous, A.; El Fakir, A.A.; Jada, A.; El Alem, N. Recent trends on numerical investigations of response surface methodology for pollutants adsorption onto activated carbon materials: A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1043–1084. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of Persistent Organic Pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw. Sustain. Dev. 2021, 13, 100575. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar]
- Tempkin, M.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327. [Google Scholar]
- Dubinin, M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR. 1947, 55, 327–329. [Google Scholar]
- Crittenden, B.; Thomas, W.J. Adsorption Technology and Design; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Wu, Z.; Liu, Y.; Li, R.; Wang, D.; Wang, S.; Wei, S.; Zhang, J.; Tao, Y.; Jiang, Z.; et al. Ball milling potassium ferrate activated biochar for efficient chromium and tetracycline decontamination: Insights into activation and adsorption mechanisms. Bioresour. Technol. 2022, 360, 127407. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Salman, J.; Njoku, V.; Hameed, B.H. Adsorption of pesticides from aqueous solution onto banana stalk activated carbon. Chem. Eng. J. 2011, 174, 41–48. [Google Scholar] [CrossRef]
- Gupta, S.S.; Sreeprasad, T.S.; Maliyekkal, S.M.; Das, S.K.; Pradeep, T. Graphene from Sugar and its Application in Water Purification. ACS Appl. Mater. Interfaces 2012, 4, 4156–4163. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Campos, M.; Rodrigues, C.A. Adsorption of textile dye Reactive Red 120 by the chitosan–Fe(III)-crosslinked: Batch and fixed-bed studies. J. Environ. Chem. Eng. 2013, 1, 1350–1358. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Peng, Y.; Ye, G.; Shang, X.; Wang, S.; Zhou, J. Adsorption of organic matter from papermaking wastewater by CoFe2O4-coated sand in batch and fixed-bed systems. BioResources 2021, 16, 5806–5820. [Google Scholar] [CrossRef]
- El Mouhri, G.; Merzouki, M.; Belhassan, H.; Miyah, Y.; Amakdouf, H.; Elmountassir, R.; Lahrichi, A. Continuous Adsorption Modeling and Fixed Bed Column Studies: Adsorption of Tannery Wastewater Pollutants Using Beach Sand. J. Chem. 2020, 2020, 7613484. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.; Weatherley, L.R. Textile Wastewater Treatment Using Granular Activated Carbon Adsorption in Fixed Beds. Sep. Sci. Technol. 2000, 35, 1329–1341. [Google Scholar] [CrossRef]
- DiChiara, A.B.; Weinstein, S.J.; Rogers, R.E. On the Choice of Batch or Fixed Bed Adsorption Processes for Wastewater Treatment. Ind. Eng. Chem. Res. 2015, 54, 8579–8586. [Google Scholar] [CrossRef]
- Graça, N.S.; Ribeiro, A.M.; Ferreira, A.; Rodrigues, A.E. Application of Adsorption Processes for the Treatment of Diluted Industrial Effluents. In Porous Materials; Springer: Cham, Switzerland, 2021; pp. 175–195. [Google Scholar] [CrossRef]
- Costa, C.A.V.; Rodrigues, A.E.; Grevillot, G.; Tondeur, D. Purification of phenolic wastewater by parametric pumping: Nonmixed dead volume equilibrium model. AIChE J. 1982, 28, 73–85. [Google Scholar] [CrossRef]
- Chen, H.T.; Reiss, E.H.; Stokes, J.D.; Hill, F.B. Separations via semicontinuous parametric pumping. AIChE J. 1973, 19, 589–595. [Google Scholar] [CrossRef]
- Sweed, N. Parametric Pumping. Prog. Sep. Purif. 1971, 4, 171–240. [Google Scholar]
- Rice, R.G.; Mackenzie, M. A Curious Anomaly in Parametric Pumping. Ind. Eng. Chem. Fundam. 1973, 12, 486–487. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Rodrigues, A.E. Phenolic wastewaters purification by thermal parametric pumping: Modeling and pilot-scale experiments. Water Res. 2005, 39, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Browne, T.E.; Cohen, Y. Aqueous-phase adsorption of trichloroethene and chloroform onto polymeric resins and activated carbon. Ind. Eng. Chem. Res. 1990, 29, 1338–1345. [Google Scholar] [CrossRef]
- Aumeier, B.M.; Dang, A.H.Q.; Ohs, B.; Yüce, S.; Wessling, M. Aqueous-Phase Temperature Swing Adsorption for Pesticide Removal. Environ. Sci. Technol. 2018, 53, 919–927. [Google Scholar] [CrossRef]
- Kunin, R. Polymeric adsorbents for treatment of waste effluents. Polym. Eng. Sci. 1977, 17, 58–62. [Google Scholar] [CrossRef]
- Brião, G.V.; Jahn, S.L.; Foletto, E.L.; Dotto, G.L. Highly efficient and reusable mesoporous zeolite synthetized from a biopolymer for cationic dyes adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Khalek, M.A.; Rahman, M.A.; Francis, A. Exploring the adsorption behavior of cationic and anionic dyes on industrial waste shells of egg. J. Environ. Chem. Eng. 2017, 5, 319–327. [Google Scholar] [CrossRef]
- Titchou, F.E.; Akbour, R.A.; Assabbane, A.; Hamdani, M. Removal of cationic dye from aqueous solution using Moroccan pozzolana as adsorbent: Isotherms, kinetic studies, and application on real textile wastewater treatment. Groundw. Sustain. Dev. 2020, 11, 100405. [Google Scholar] [CrossRef]
- Nsami, J.N.; Mbadcam, J.K. The Adsorption Efficiency of Chemically Prepared Activated Carbon from Cola Nut Shells by ZnCl 2 on Methylene Blue. J. Chem. 2013, 469170. [Google Scholar] [CrossRef]
- Baral, S.; Das, N.; Ramulu, T.; Sahoo, S.; Das, S.; Chaudhury, G.R. Removal of Cr(VI) by thermally activated weed Salvinia cucullata in a fixed-bed column. J. Hazard. Mater. 2009, 161, 1427–1435. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M.; Pouya, E.S. Aqueous phase adsorption of cephalexin by walnut shell-based activated carbon: A fixed-bed column study. Appl. Surf. Sci. 2016, 375, 144–153. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef]
- Garg, V. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dye. Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Zhang, X.; Sun, M.; Tao, Y.; Zhang, X.; Zhang, G.; Ge, C.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yaswanthraj, M. Modeling and analysis of a packed-bed column for the effective removal of zinc from aqueous solution using dual surface-modified biomass. Part. Sci. Technol. 2017, 36, 934–944. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; García, J. Removal of Atenolol and Isoproturon in Aqueous Solutions by Adsorption in a Fixed-Bed Column. Ind. Eng. Chem. Res. 2012, 51, 5045–5055. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Namasivayam, C.; Yamuna, R. Adsorption of chromium (VI) by a low-cost adsorbent: Biogas residual slurry. Chemosphere 1995, 30, 561–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, J.; Yuan, Y.; Song, H.; Liu, Y.; Wang, S.; Tao, Y.; Zhao, Y.; Li, Z. Simultaneous scavenging of Cd(II) and Pb(II) from water by sulfide-modified magnetic pinecone-derived hydrochar. J. Clean. Prod. 2022, 341, 130758. [Google Scholar] [CrossRef]
- Ahmed, M.; Okoye, P.; Hummadi, E.; Hameed, B. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef]
- Dash, S.; Chaudhuri, H.; Gupta, R.; Nair, U.G. Adsorption study of modified coal fly ash with sulfonic acid as a potential adsorbent for the removal of toxic reactive dyes from aqueous solution: Kinetics and thermodynamics. J. Environ. Chem. Eng. 2018, 6, 5897–5905. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Saha, P. Biosorption of Basic Green 4 from aqueous solution by Ananas comosus (pineapple) leaf powder. Colloids Surf. B Biointerfaces 2011, 84, 520–527. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Lofrano, G. Emerging Compounds Removal from Wastewater: Natural and Solar Based Treatments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Weng, C.-H.; Lin, Y.-T.; Tzeng, T.-W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Zou, W.H.; Zhao, L.; Zhu, L. Efficient uranium(VI) biosorption on grapefruit peel: Kinetic study and thermodynamic parameters. J. Radioanal. Nucl. Chem. Artic. 2012, 292, 1303–1315. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.; Kavitha, D.; Selvakumar, R.; Jayabalan, R.; Choi, J.; Yun, S. Adsorption potential of maize cob carbon for 2,4-dichlorophenol removal from aqueous solutions: Equilibrium, kinetics and thermodynamics modeling. Chem. Eng. J. 2009, 147, 265–271. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by Sugarcane Bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Runping, H.; Pan, H.A.N.; Zhaohui, C.A.I.; Zhao, Z.; Tang, M. Kinetics and isotherms of neutral red adsorption on peanut husk. J. Environ. Sci. 2008, 20, 1035–1041. [Google Scholar]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper(II) from waste water/industrial effluent. Colloids Surf. B Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption Using Modified Orange Peel as Adsorbent. Adv. Mater. Res. 2011, 236–238, 237–240. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Zhu, Z.; Li, L.; Yao, X.; Rudolph, V.; Haghseresht, F. Phosphate removal from wastewater using red mud. J. Hazard. Mater. 2008, 158, 35–42. [Google Scholar] [CrossRef]
- Ding, Y.; Jing, D.; Gong, H.; Zhou, L.; Yang, X. Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour. Technol. 2012, 114, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xiao, D. Adsorption of cadmium ion from aqueous solution by ground wheat stems. J. Hazard. Mater. 2009, 164, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, F.-S.; Xiu, F.-R. Arsenic (V) removal from aqueous system using adsorbent developed from a high iron-containing fly ash. Sci. Total Environ. 2009, 407, 5780–5786. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Shin, M.-C.; Choi, H.-D.; Seo, C.-I.; Baek, K. Removal mechanisms of copper using steel-making slag: Adsorption and precipitation. Desalination 2008, 223, 283–289. [Google Scholar] [CrossRef]
- Otero, M.; Rozada, F.; Calvo, L.; García, A.; Morán, A. Elimination of organic water pollutants using adsorbents obtained from sewage sludge. Dye. Pigment. 2003, 57, 55–65. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kametani, T.; Maruyama, T. Removal of heavy metals from aqueous solution by nonliving Ulva seaweed as biosorbent. Water Res. 2005, 39, 1803–1808. [Google Scholar] [CrossRef]
- Rengaraj, S.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Adsorption kinetics of o-cresol on activated carbon from palm seed coat. Indian J. Chem. Technol. 2000, 7, 127–131. [Google Scholar]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Liquid-phase adsorption of dyes and phenols using pinewood-based activated carbons. Carbon 2003, 41, 487–495. [Google Scholar] [CrossRef]
- Oliveira, D.Q.; Gonçalves, M.; Oliveira, L.C.; Guilherme, L.R. Removal of As(V) and Cr(VI) from aqueous solutions using solid waste from leather industry. J. Hazard. Mater. 2008, 151, 280–284. [Google Scholar] [CrossRef]
- McKay, G.; Porter, J.F.; Prasad, G.R. The Removal of Dye Colours from Aqueous Solutions by Adsorption on Low-cost Materials. Water Air Soil Pollut. 1999, 114, 423–438. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A. Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 2010, 173, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, K.S.; Ramesh, S.T. Removal of dyes using agricultural waste as low-cost adsorbents: A review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.A.; Daud, W.W.; Aroua, M.K. Adsorption kinetics of various gases in carbon molecular sieves (CMS) produced from palm shell. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 131–135. [Google Scholar] [CrossRef]
- Aygun, A.; Yenisoy-Karakas, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Juang, R.-S.; Wu, F.-C.; Tseng, R.-L. Characterization and use of activated carbons prepared from bagasses for liquid-phase adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2002, 201, 191–199. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, L.; Zhu, L. Adsorption of uranium(VI) by grapefruit peel in a fixed-bed column: Experiments and prediction of breakthrough curves. J. Radioanal. Nucl. Chem. Artic. 2012, 295, 717–727. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass-Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.A.; Cunha, F.A.; Ruotolo, L.A.M. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J. Clean. Prod. 2019, 229, 956–963. [Google Scholar] [CrossRef]
- Singh, M.; Rano, S.; Roy, S.; Mukherjee, P.; Dalui, S.; Gupta, G.K.; Kumar, S.; Mondal, M.K. Characterization of organophosphate pesticide sorption of potato peel biochar as low cost adsorbent for chlorpyrifos removal. Chemosphere 2022, 297, 134112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, Y.; Zhang, M.; Ming, Z.; Yang, S.; Arkin, A.; Fang, P. Functionalized agricultural biomass as a low-cost adsorbent: Utilization of rice straw incorporated with amine groups for the adsorption of Cr(VI) and Ni(II) from single and binary systems. Biochem. Eng. J. 2016, 105, 27–35. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange peel as low-cost adsorbent in the elimination of Cd (II) ion: Kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Jalali, A.; Mirnezami, F.; Lotfi, M.; Shafiee, M.; Mohammadi, A.H. Biosorption of lead ion from aqueous environment using wheat stem biomass. Desalination Water Treat. 2021, 233, 98–105. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Yuan, Y.; Zhang, X.; Wang, L.; Tao, Y.; Jiang, Z.; Yu, H.; Dong, M.; Zhang, Y. Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. J. Hazard. Mater. 2021, 425, 127876. [Google Scholar] [CrossRef]
- Attari, M.; Bukhari, S.S.; Kazemian, H.; Rohani, S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J. Environ. Chem. Eng. 2017, 5, 391–399. [Google Scholar] [CrossRef]
- Bhatnagar, A. Removal of bromophenols from water using industrial wastes as low cost adsorbents. J. Hazard. Mater. 2007, 139, 93–102. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef] [Green Version]
- Aris, A.Z.; Hir, Z.A.M.; Razak, M.R. Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: A review. Appl. Mater. Today 2020, 21, 100796. [Google Scholar] [CrossRef]
- Tahiri, S.; DeLaGuardia, M. Treatment and valorization of leather industry solid wastes: A review. J. Am. Leather Chem. Assoc. 2009, 104, 52–67. [Google Scholar]
- Méndez, A.; Barriga, S.; Fidalgo, J.; Gascó, G. Adsorbent materials from paper industry waste materials and their use in Cu(II) removal from water. J. Hazard. Mater. 2009, 165, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Clarified sludge (basic oxygen furnace sludge)—An adsorbent for removal of Pb (II) from aqueous solutions–kinetics, thermodynamics and desorption studies. J. Hazard. Mater. 2009, 170, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Fan, B. Characteristics of PAHs adsorption on inorganic particles and activated sludge in domestic wastewater treatment. Bioresour. Technol. 2011, 102, 5305–5311. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Utilisation of sewage sludge derived adsorbents for the removal of recalcitrant compounds from wastewater: Mechanistic aspects, isotherms, kinetics and thermodynamics. Bioresour. Technol. 2015, 194, 214–224. [Google Scholar] [CrossRef]
- Li, Y.-S.; Liu, C.-C.; Chiou, C.-S. Adsorption of Cr(III) from wastewater by wine processing waste sludge. J. Colloid Interface Sci. 2004, 273, 95–101. [Google Scholar] [CrossRef]
- Nielsen, L.; Bandosz, T.J. Analysis of sulfamethoxazole and trimethoprim adsorption on sewage sludge and fish waste derived adsorbents. Microporous Mesoporous Mater. 2016, 220, 58–72. [Google Scholar] [CrossRef]
- Yoshida, H.; Fukuda, S.; Okamoto, A.; Kataoka, T. Recovery of Direct Dye and Acid Dye by Adsorption on Chitosan Fiber–Equilibria. Water Sci. Technol. 1991, 23, 1667–1676. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef]
- Holan, Z.R.; Volesky, B.; Prasetyo, I. Biosorption of cadmium by biomass of marine algae. Biotechnol. Bioeng. 1993, 41, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.L.; Cruz, C.C.; Luna, A.S.; Henriques, C.A. Sorption and desorption of Pb2+ ions by dead Sargassum sp. biomass. Biochem. Eng. J. 2006, 27, 310–314. [Google Scholar] [CrossRef]
- Kibe, K.; Takahashi, M.; Kameya, T.; Urano, K. Adsorption equilibriums of principal herbicides on paddy soils in Japan. Sci. Total Environ. 2000, 263, 115–125. [Google Scholar] [CrossRef]
- Gao, P.; Feng, Y.; Zhang, Z.; Liu, J.; Ren, N. Comparison of competitive and synergetic adsorption of three phenolic compounds on river sediment. Environ. Pollut. 2011, 159, 2876–2881. [Google Scholar] [CrossRef]
- Bouyarmane, H.H.; Asri, S.; Rami, A.; Roux, C.; Mahly, M.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Pyridine and phenol removal using natural and synthetic apatites as low cost sorbents: Influence of porosity and surface interactions. J. Hazard. Mater. 2010, 181, 736–741. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, X.; Liu, S.; Li, X.; Wang, S.; Feng, Z.; Wu, Z.; Wang, L.; Jiang, Z.; Zhang, Y. One-step preparation of Fe/N co-doped porous biochar for chromium(VI) and bisphenol a decontamination in water: Insights to co-activation and adsorption mechanisms. Bioresour. Technol. 2022, 361, 127718. [Google Scholar] [CrossRef]
- Hernández, I.L.; Barrera-Díaz, C.; Roa, G.; Bilyeu, B.; Ureña-Núñez, F. A combined electrocoagulation–sorption process applied to mixed industrial wastewater. J. Hazard. Mater. 2007, 144, 240–248. [Google Scholar] [CrossRef]
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization of continuous electrocoagulation-adsorption combined process for the treatment of a textile effluent. Chin. J. Chem. Eng. 2021, 44, 310–320. [Google Scholar] [CrossRef]
- Muryanto, M.; Marlina, E.; Sari, A.A.; Harimawan, A.; Sudarno, S. Treatment of beverage industry wastewater using a combination of electrocoagulation and adsorption processes. AIP Conf. Proc. 2018, 2024, 020004. [Google Scholar] [CrossRef]
- Jalil, S.N.A.; Amri, N.; Ajien, A.A.; Ismail, N.F.; Ballinger, B. A hybrid electrocoagulation-adsorption process for fluoride removal from semiconductor wastewater. J. Physics Conf. Ser. 2019, 1349, 012056. [Google Scholar] [CrossRef]
- Anugrah, P.; Said, M.; Bahrin, D. Produced Water Treatment using Electrocoagulation Combination Method with Aluminum (Al) and Iron (Fe) Electrodes and Activated Carbon Adsorption Treatment. Int. J. Adv. Sci. Eng. Inf. Technol. 2022, 12, 703–711. [Google Scholar] [CrossRef]
- Ziouvelou, A.; Tekerlekopoulou, A.G.; Vayenas, D.V. A hybrid system for groundwater denitrification using electrocoagulation and adsorption. J. Environ. Manag. 2019, 249, 109355. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C. Unification electrocoagulation-adsorption treatment for removal of COD and surfactant from automobile wastewater. Int. J. Chem. React. Eng. 2021, 19, 961–968. [Google Scholar] [CrossRef]
- Elabbas, S.; Adjeroud, N.; Mandi, L.; Berrekhis, F.; Pons, M.N.; Leclerc, J.P.; Ouazzani, N. Eggshell adsorption process coupled with electrocoagulation for improvement of chromium removal from tanning wastewater. Int. J. Environ. Anal. Chem. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Pizutti, J.T.; Santos, R.D.C.D.; Hemkemeier, M.; Piccin, J.S. Electrocoagulation coupled adsorption for anaerobic wastewater post-treatment and reuse purposes. Desalination Water Treat. 2019, 160, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Bellebia, S.; Kacha, S.; Bouyakoub, A.Z.; Derriche, Z. Experimental investigation of chemical oxygen demand and turbidity removal from cardboard paper mill effluents using combined electrocoagulation and adsorption processes. Environ. Prog. Sustain. Energy 2012, 31, 361–370. [Google Scholar] [CrossRef]
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Removal of Cr(VI) from Model Solutions by a Combined Electrocoagulation Sorption Process. Chem. Eng. Technol. 2012, 36, 147–155. [Google Scholar] [CrossRef]
- Barhoumi, A.; Ncib, S.; Chibani, A.; Brahmi, K.; Bouguerra, W.; Elaloui, E. High-rate humic acid removal from cellulose and paper industry wastewater by combining electrocoagulation process with adsorption onto granular activated carbon. Ind. Crop. Prod. 2019, 140, 111715. [Google Scholar] [CrossRef]
- De Carvalho, H.P.; Huang, J.; Zhao, M.; Liu, G.; Dong, L.; Liu, X. Improvement of Methylene Blue removal by electrocoagulation/banana peel adsorption coupling in a batch system. Alex. Eng. J. 2015, 54, 777–786. [Google Scholar] [CrossRef]
- Zhu, M.; Yin, X.; Chen, W.; Yi, Z.; Tian, H. Removal of sulphate from mine waters by electrocoagulation/rice straw activated carbon adsorption coupling in a batch system: Optimization of process via response surface methodology. J. Water Reuse Desalination 2018, 9, 163–172. [Google Scholar] [CrossRef]
- Cherifi, M.; Guenfoud, S.; Bendaia, M.; Hazourli, S.; Laefer, D.F.; Leclerc, J.P.; Mecibah, W. Comparative study between electrocoagulation used separately and coupled with adsorption for dairy wastewater treatment using response surface methodology design. Desalination Water Treat. 2021, 223, 235–245. [Google Scholar] [CrossRef]
- Wang, X.; Ni, J.; Pang, S.; Li, Y. Removal of malachite green from aqueous solutions by electrocoagulation/peanut shell adsorption coupling in a batch system. Water Sci. Technol. 2017, 75, 1830–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.C.C.; Tang, P.-L.; Yen, C.-H. Removal of micropollutants from municipal wastewater by graphene adsorption and simultaneous electrocoagulation/electrofiltration process. Water Sci. Technol. 2017, 75, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.V.; Ganesan, M. Use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. J. Hazard. Mater. 2009, 161, 575–580. [Google Scholar] [CrossRef]
- Castañeda-Díaz, J.; Pavón-Silva, T.; Gutiérrez-Segura, E.E.; Colín-Cruz, A. Electrocoagulation-Adsorption to Remove Anionic and Cationic Dyes from Aqueous Solution by PV-Energy. J. Chem. 2017, 2017, 5184590. [Google Scholar] [CrossRef] [Green Version]
- Rubí-Juárez, H.; Barrera-Díaz, C.; Ureña-Nuñez, F. Adsorption-assisted electrocoagulation of real car wash wastewater with equilibrium and kinetic studies. Pollut. Res. 2017, 36, 175–184. [Google Scholar]
- Claude, N.J.; Shanshan, L.; Khan, J.; Yifeng, W.; Dongxu, H.; Xiangru, L. Waste tea residue adsorption coupled with electrocoagulation for improvement of copper and nickel ions removal from simulated wastewater. Sci. Rep. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalination Water Treat. 2015, 57, 9203–9215. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant—Challenges and Barriers. Proceedings 2018, 2, 614. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2022:34:FIN (accessed on 13 October 2022).
- Widiastuti, N.; Wu, H.; Ang, M.; Zhang, D.-K. The potential application of natural zeolite for greywater treatment. Desalination 2008, 218, 271–280. [Google Scholar] [CrossRef]
- Verstraete, W.; Vlaeminck, S.E. ZeroWasteWater: Short-cycling of wastewater resources for sustainable cities of the future. Int. J. Sustain. Dev. World Ecol. 2011, 18, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Molinos-Senante, M.; Hernandez-Sancho, F.; Sala-Garrido, R. Tariffs and Cost Recovery in Water Reuse. Water Resour. Manag. 2012, 27, 1797–1808. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Hu, T.; Nurmesniemi, E.-T.; Tuomikoski, S.; Lassi, U. Phosphate and Ammonium Removal from Water through Electrochemical and Chemical Precipitation of Struvite. Processes 2021, 9, 150. [Google Scholar] [CrossRef]
- Un, U.T.; Onpeker, S.E.; Ozel, E. The treatment of chromium containing wastewater using electrocoagulation and the production of ceramic pigments from the resulting sludge. J. Environ. Manag. 2017, 200, 196–203. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, H. Utilization of electrocoagulation-treated spent wash sludge in making building blocks. Int. J. Environ. Sci. Technol. 2015, 13, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Golder, A.; Samanta, A.; Ray, S. Anionic reactive dye removal from aqueous solution using a new adsorbent—Sludge generated in removal of heavy metal by electrocoagulation. Chem. Eng. J. 2006, 122, 107–115. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Olfati, D.; Gohari, F.; Hassani, A. TiO2 nanoparticles removal by electrocoagulation using iron electrodes: Catalytic activity of electrochemical sludge for the degradation of emerging pollutant. J. Mol. Liq. 2020, 310, 113217. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2018, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Phalakornkule, C.; Sukkasem, P.; Mutchimsattha, C. Hydrogen recovery from the electrocoagulation treatment of dye-containing wastewater. Int. J. Hydrogen Energy 2010, 35, 10934–10943. [Google Scholar] [CrossRef]
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste Material Adsorbents for Zinc Removal from Wastewater: A Comprehensive Review. Int. J. Chem. Eng. 2014, 2014, 347912. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, H.; Wang, H.; Zhang, L.; Liu, P.; Feng, L. Fast adsorption of nickel ions by porous graphene oxide/sawdust composite and reuse for phenol degradation from aqueous solutions. J. Colloid Interface Sci. 2014, 436, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.C.; Mbewe, P.B.; Kong, S.Y.; Šavija, B. Agricultural Solid Waste as Source of Supplementary Cementitious Materials in Developing Countries. Materials 2019, 12, 1112. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, G.; Martín-Lara, M.A.; Dionisio-Ruiz, E.; Tenorio, G.; Calero, M. Copper biosorption by pine cone shell and thermal decomposition study of the exhausted biosorbent. J. Ind. Eng. Chem. 2012, 18. [Google Scholar] [CrossRef]



| Model | Equation | Description |
|---|---|---|
| Langmuir [121] | Assumes monolayer adsorption and a homogenous surface energy distribution. (Qmax is the maximum adsorption capacity (mg g−1), kL is the Langmuir constant (L mg−1)) | |
| Freundlich [122] | Assumes a heterogeneous surface with a non-uniform distribution of heat of adsorption over the surface. (kF is the Freundlich constant (mg g−1) (L mg−1) −n, n is the intensity of adsorption constant) | |
| Sips [123] | This model results from the combination of both the Langmuir and Freundlich models. It has the ability to take into account the heterogeneity of the adsorbent surface while overcoming the limitations associated with the increased adsorbate concentrations of the Freundlich model. (KS is the Sips model constant (L mg−1), aS is the Sips constant (L mg−1), nS is the Sips model exponent) | |
| Redlich–Peterson [124] | It is a hybrid model of the Langmuir and Freundlich isotherms. It can be used to describe adsorption on both homogeneous and heterogeneous surfaces. (KRP is the Redlich–Peterson constant (L mg−1), aRP is the Redlich–Peterson constant (L mg−1), and β is the Redlich–Peterson exponent) | |
| Temkin [125] | Assumes uniform binding energy distribution and a linear decrease in the heat of adsorption with the surface coverage. (AT is the Temkin isotherm equilibrium binding constant (L mg−1), and BT is the Temkin isotherm constant) | |
| Dubinin–Radushkevich [126] | Assumes a Gaussian distribution of energy onto a heterogeneous surface. (Qs is the theoretical isotherm saturation capacity (mg g−1), kDR is the Dubinin–Radushkevich isotherm constant (mol2 J−2), and ε is the Polanyi potential) |
| Material | Adsorbate | Adsorption Capacity | Reference |
|---|---|---|---|
| Pineapple leaf powder | Methylene blue | 9.28 × 10−4 mol/g | [172] |
| Grapefruit peel | U(VI) | 140.79 mg/g | [173] |
| Banana peel | Methyl orange Methylene blue Rhodamine B Congo red Methyl violet Amido black 10B | 17.2 mg/g 15.9 mg/g 13.2 mg/g 11.2 mg/g 7.9 mg/g 7.9 mg/g | [174] |
| Olive stone waste | Pb(II) Ni(II) Cu(II) Cd(II) | 4.47 × 10−5 mol/g 3.63 × 10−5 mol/g 3.19 × 10−5 mol/g 6.88 × 10−5 mol/g | [175] |
| Maize cob | 2,4-Dichlorophenol | 17.94 mg/g | [176] |
| Sugarcane bagasse | Hg(I) | 35.71 mg/g | [177] |
| Peanut husk | Neutral red | 37.5 mg/g | [178] |
| Potato peel charcoal | Cu(II) | 0.3877 mg/g | [179] |
| Orange peel | Cu(II) Cd(II) Pb(II) Zn(II) Ni(II) | 59.77 mg/g 125.63 mg/g 141.84 mg/g 45.29 mg/g 49.14 mg/g | [180] |
| Red mud | Phosphate | 0.23–0.58 mg/g | [181] |
| Rice straw | Cd(II) | 13.9 mg/g | [182] |
| Ground wheat stems | Cd(II) | 0.1032 mmol/g | [183] |
| Iron-containing fly ash | As(VI) | 19.46 mg/g | [184] |
| Steel-making slag | Cu(II) | 6.2–17.4 mg/g | [185] |
| Sewage sludge | Crystal violet Indigo carmine Phenol | 184.68–270.88 mg/g 30.82–60.04 mg/g 5.56–42.04 mg/g | [186] |
| Ulva seaweed | Cd(II) Zn(II) Cu(II) | 90.7 mg/g 74.6 mg/g 57.3 mg/g | [187] |
| Palm seed coat | o-Cresol | 19.58 mg/g | [188] |
| Pinewood | Basic Blue 9 | 556 mg/g | [189] |
| Leather industry waste | Cr(VI) As(V) | 133 mg/g 26 mg/g | [190] |
| Rice husk | Safranine | 838 mg/g | [191] |
| EC Limitations | AD Limitations |
|---|---|
|
|
| Wastewater | Adsorbent | Electrodes | Removal Efficiency | Reference |
|---|---|---|---|---|
| Industrial wastewater | Ectodermis of Opuntia | 84% (COD) 78% (BOD) 97% (color) 98% (turbidity) 99% (fecal coliforms) | [228] | |
| Aqueous solution | Granular activated carbon | 99.88% (Pb(II)) | [19] | |
| Textile wastewater | Crude Tunisian clay | 96.87% (color) 89.77% (COD) 84.46% (TSS) | [229] | |
| Beverage industry wastewater | Activated carbon | 98.66% (COD) 92.15% (TSS) 90.12% (color) | [230] | |
| Semiconductor wastewater | Activated carbon | 67.25% (fluoride) | [231] | |
| Produced water | Coconut shell activated carbon | 98.39% (COD) 93.54% (TDS) 75.16% (ammonia) 97.56% (oil content) 92.5% (phenol) | [232] | |
| Nitrate-contaminated ground water | Zeolite | 96% (nitrates) | [233] | |
| Automobile wastewater | Activated carbon | 71.58% (COD) 77.91% (surfactant) | [234] | |
| Tanning wastewater | Eggshell | 99% (Cr(VI)) | [235] | |
| Anaerobic wastewater | Granular activated carbon | 100% (COD) 100% (BOD) 96.5% (turbidity) 97.5% (phosphorus) | [236] | |
| Paper mill effluent | Granular activated carbon | 98.97% (COD) | [237] | |
| Model solution | Red onion skin | 97% (Cr(VI)) | [238] | |
| Cellulose and paper industry wastewater | Granular activated carbon | 93% (humic acid) | [239] | |
| Dye solution | Banana peel | 99% (methylene blue) | [240] | |
| Mine waters | Rice straw activated carbon | 95.2% (sulphate) | [241] | |
| Dairy wastewater | Granular activated carbon | 99.39% (turbidity) 87.12 (COD) | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, N.S.; Rodrigues, A.E. The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technol. 2022, 4, 1020-1053. https://doi.org/10.3390/cleantechnol4040063
Graça NS, Rodrigues AE. The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technologies. 2022; 4(4):1020-1053. https://doi.org/10.3390/cleantechnol4040063
Chicago/Turabian StyleGraça, Nuno S., and Alírio E. Rodrigues. 2022. "The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters" Clean Technologies 4, no. 4: 1020-1053. https://doi.org/10.3390/cleantechnol4040063
APA StyleGraça, N. S., & Rodrigues, A. E. (2022). The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technologies, 4(4), 1020-1053. https://doi.org/10.3390/cleantechnol4040063







