Abstract
A manganese dioxide-modified red mud (Mn-RM) was developed as an adsorbent for the effective removal of lead ions (Pb2+) from wastewater. Various methods were used to characterize the prepared Mn-RM, analyze its adsorption performance, and evaluate the associated environmental risks post-adsorption. The results revealed that Mn-RM has a large surface area (38.91 m2/g) and a developed porous structure (0.02 cm3/g). The adsorption process exhibited good agreement with the Langmuir isotherm and pseudo-second-order kinetic models, showcasing a theoretical maximum saturation adsorption capacity of 721.35 mg/g. The adsorption mechanism primarily involves electrostatic attraction, ion exchange, and chemical precipitation. The optimal treatment conditions were determined by utilizing a response surface model, resulting in a maximum Pb2+ removal efficiency of 87.45% at pH 5.21, a dosage of 0.83 g/L, and an initial concentration of 301.04 mg/L. The risk assessment code (RAC) for each heavy metal in Mn-RM was less than 1%, indicating low environmental risk. Furthermore, the synthetic toxicity index (STI) values showed a significant decrease post-treatment. This study introduces the concept of “controlling waste with waste”, offering a cost-effective approach to both utilizing red mud and removing aqueous Pb2+ while ensuring environmental safety and minimal ecological impact.
1. Introduction
In the field of environmental contamination, heavy metal pollution stands out due to its high toxicity and enduring residual time, posing a significant threat to global water security [1]. Among the myriad of toxic elements, lead is particularly detrimental to human health and is ubiquitous in numerous industrial processes [2]. Lead ions (Pb2+) can readily enter the human body via aquatic and air environments, inflicting harm on the central nervous system and the gastrointestinal tract [3,4]. At present, adsorption, chemical precipitation, ion exchange, membrane filtration, and biological and electrodialysis processes are used to remove Pb2+ [5]. In terms of remediation strategies, adsorption has been proven to be efficient and economical for Pb2+ extraction from aquatic environments, which is attributed to its simplicity, operational ease, cost-effectiveness, and superior adsorption performance [6]. It is primarily based on the porous structure of adsorbent materials and their surface-active sites that enable adsorption to occur, facilitating the removal of heavy metals through a physicochemical interaction with heavy metal ions. Certain adsorbents can be regenerated and reused via suitable desorption processes. Years of dedicated research has led to the development of a diverse array of adsorbent materials that possess robust adsorption capacity for heavy metals. Nonetheless, despite cost and time constraints, most adsorbent materials remain limited in their practical application. Due to this, the most preferred wastewater treatment method remains the development of cost-effective, high-efficiency heavy metal adsorbents [7].
In recent years, the byproduct of alumina production, known as red mud, has garnered considerable interest due to its substantial accumulation and the challenges associated with its utilization as a resource [5]. Red mud exhibits strong alkalinity, high porosity, and extensive dispersion, conferring upon it effective adsorption capacity for metal cations [8]. Concurrently, heavy metal wastewater treatment can benefit both the environment and the economy by using it as an adsorbent [9]. Nevertheless, the inherent adsorption capacity and the structural robustness of red mud are somewhat limited, and its final removal efficiency fails to satisfy the required concentration thresholds. This constraint impedes the broader application of red mud in wastewater treatment. Consequently, alterations such as acid activation, calcination activation, and additive compounding are necessary [10,11,12,13]. However, these modification techniques either incur high expenses, consume excessive energy, pose implementation challenges, or lead to low adsorption efficiency. It is possible to enhance the adsorption abilities of red mud by altering its surface and structure through utilizing metal compounds.
Manganese dioxide (MnO2) is recognized as an eco-friendly metal oxide, characterized by its substantial specific surface area, elevated surface activity, affordability, and accessibility. Of particular note is the stable chemical nature of MnO2 and its distinct redox reactions [14]. The exceptional adsorptive properties of MnO2 can be attributed to the combination of its robust chemical stability (both in alkaline and acidic environments) with its ability to form complexes with heavy metal ions (e.g., Cd2+, Cu2+, Zn2+, and Pb2+) [15,16]. Amorphous manganese dioxide, for example, demonstrates selective removal capabilities for three specific metal ions (Pb2+, Cd2+, and Zn2+) [15]. A study conducted by Lin et al. explained that MnO2 nanoflowers demonstrate a highly effective capability in the removal of Pb2+, with a remarkable peak adsorption capacity of 239.7 mg/g, while also providing a detailed explanation of the removal mechanism which involves a combination of physical and chemical adsorption [17]. Nevertheless, the practical application of MnO2 is constrained by its poor dispersibility and propensity for agglomeration [18,19]. An effective way to enhance MnO2 adsorption and dispersion is to load it onto a large surface area carrier [20,21]. However, most materials with large surface areas are difficult and costly to produce, which limits their practical use and scalability. As a result, it is vitally important to identify cost-effective carriers for MnO2. In this context, loading MnO2 onto red mud could augment the material’s adsorption performance and dispersion capability, while simultaneously achieving the objective of “controlling waste by waste”. Despite the potential of this approach, there is a paucity of research on the modification of red mud using manganese dioxide.
This study proposes using potassium permanganate as a manganese source and red mud as a base material to prepare manganese dioxide-modified red mud (Mn-RM), which can remove Pb2+ from wastewater at a low cost. The optimal treatment parameters are obtained using the response surface method (RSM). Finally, on this basis, isotherm adsorption models, adsorption kinetics, and thermodynamic models are constructed. Combined with multiple characterization methods, the adsorption mechanism is explored, and the environmental risk posed by the material after adsorption is evaluated, providing theoretical and technical support for its use in the remediation of heavy metal-polluted wastewater.
2. Materials and Methods
2.1. Red Mud and Chemicals
The chemical reagents and red mud used in this study are listed in Text S1 (Supplementary Materials).
2.2. Synthesis and Characterization of Mn-RM
In this study, Mn-RM was fabricated as follows: (1) a total of 2.212 g KMnO4 was mixed with 3 g red mud in 30 mL deionized water, followed by stirring for 30 min; (2) a total of 3.194 g (NH4)2S2O8 was introduced into the mixture, followed by stirring for another 30 min; (3) the aforementioned mixed substance was transferred into a hydrothermal reactor and subjected to hydrothermal treatment at 90 °C for 12 h; (4) the obtained material was washed with deionized water 5 times, and then dried in an oven at 65 °C. Finally, the material was ground and sieved to yield manganese-modified red mud (Mn-RM). Text S2 details the methods used to determine the physicochemical characteristics of the synthesized Mn-RM.
2.3. Batch Adsorption Experiments
The batch adsorption experiments included: (1) adsorption kinetics; (2) adsorption isotherms and thermodynamics; (3) multi-factor effects on the adsorption experiment; (4) the effect of co-cation types on Pb2+ adsorption; and (5) adsorption–regeneration experiments. Detailed information can be found in Text S3.
2.4. RSM Design
The initial pH, solid–liquid ratio (w/v), and initial concentration of Pb2+ were optimized using the Box–Behnken design. The ranges and levels of these three factors are listed in Table 1. The response variable was the removal efficiency of Pb2+ (Y1). The experimental design and response results are listed in Table S1.

Table 1.
Range and levels of the factors by Box-Behnken design for Pb2+ adsorption using Mn-RM.
2.5. Environmental Risk Assessment
To examine the environmental risk of the used Mn-RM, the toxicity characteristic leaching procedure (TCLP) and the HJ/T299-2007 [22] leaching test were carried out. The TCLP uses an unbuffered acetic acid solution (pH 2.88 ± 0.05) as a leaching solution at a solid–liquid ratio of 1:20 and a rotation speed of 30 rpm for an 18 h period. The HJ/T299-2007 leaching test procedure was as follows: a total of 4 g of dry RM sample and 40 mL of sulfuric acid–nitric acid (mass ratio = 2:1, pH 3.20 ± 0.05) solution were added into a 50 mL high-density polyethylene (HDPE) centrifuge tube at a ratio of 1 part solid to 10 parts liquid, and the sample was rotated at 30 rpm for 24 h at room temperature. After the leaching period, the suspension was centrifuged (3500 rpm, 15 min) and filtered (0.45 μm); then, the concentrations of the extracted heavy metals were measured.
After the leaching test, the speciation of heavy metals in Mn-RM was investigated by applying Tessier’s sequential extraction procedure [23]. The risk assessment code (RAC) model and synthesis toxicity index (STI) model were adopted for the environmental risk assessment of Mn-RM. The details of this process are provided in Text S4.
3. Results and Discussion
3.1. Characterization of As-Prepared Mn-RM
The scanning electron microscopy (SEM) results for red mud (RM) and Mn-RM are presented in Figure 1. It can be observed that RM exhibits an irregular surface with large agglomerates and flakes and numerous irregular small particles are found within the gaps between them (Figure 1a,b). After modification (Figure 1c,d), Mn-RM is covered with numerous spherical and flocculent particles. It is hypothesized that these particles are amorphous manganese dioxide. As a result of the three-dimensional porous structure formed when Mn is introduced, there are more adsorption sites in the interlayer domain, in the pore channels, and on the surface, which can enhance Mn-RM adsorption performance. Upon comparing the EDS spectra of RM and Mn-RM (Figure S1), it is found that Mn was not detected in the original red mud, and the increase in manganese content indicates that MnO2 may have been successfully loaded on the red mud.
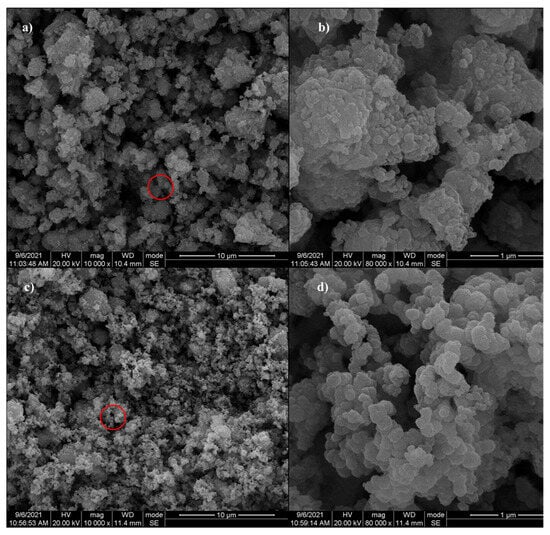
Figure 1.
SEM images of (a,b) RM; (c,d) Mn-RM.
The surface functional groups of RM and Mn-RM were analyzed via Fourier transform infrared spectroscopy (FTIR). As shown in Figure 2a, both RM and Mn-RM exhibit broad peaks at 3444.296 cm−1 and 3438.51 cm−1, respectively, corresponding to the stretching vibration of the hydroxyl group O-H [24,25]. However, the characteristic peak of Mn-RM is more intense, indicating a higher abundance of functional groups. Mn-RM shows an obvious peak at 1637.274 cm−1, which is absent in RM, representing the stretching vibration of O-H in Mn-OH [26]. Additionally, Mn-RM exhibits a broad peak at 522.6227 cm−1 that is not present in RM, which is attributed to the stretching vibration of Mn-O [27]. The appearance of these two characteristic peaks confirms the successful loading of MnO2. Moreover, three characteristic peaks are observed at 684.62 cm−1, 620.98 cm−1, and 458.98 cm−1 in RM, corresponding to the stretching vibrations of Al-O, Si-O-Al, and Fe-O, respectively [28,29,30]. These peaks disappear in Mn-RM, confirming significant alterations in the functional groups after modification. Therefore, it can be concluded that Mn-RM has abundant surface functional groups and possesses strong adsorption capability.
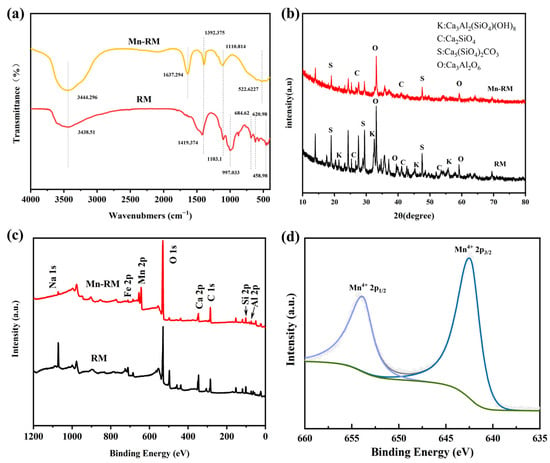
Figure 2.
(a) FTIR spectra of RM and Mn-RM; (b) XRD spectra of RM and Mn-RM; (c) XPS survey of RM and Mn-RM; (d) Mn 2p of Mn-RM.
Figure 2b presents the XRD spectra of RM and Mn-RM. The mineral component of RM is relatively complex, mainly consisting of calcium, silicon, and aluminum. The identified phases include Ca3Al2(SiO4)(OH)8, Ca2SiO4, Ca5(SiO4)2CO3, and Ca3Al2O6. Interestingly, no new characteristic peaks are observed after loading with MnO2, indicating that the amorphous MnO2 on the Mn-RM surface exhibit superior adsorption properties compared with crystallized MnO2 [31]. This may be attributed to the structural change in the MnO2 crystalline phase during the hydrothermal process.
X-ray photoelectron spectroscopy (XPS) was carried out to identify the elemental composition of Mn-RM. In Figure 2c, an increase in Mn 2p peak intensity can be observed at 643.08 eV, indicating the successful introduction of Mn into RM. Mn 2p XPS spectra with peaks at 642.42 eV and 653.95 eV correspond to Mn 2p3/2 and Mn 2p1/2, respectively. There is an energy difference of 11.54 eV between Mn 2p1/2 and Mn 2p3/2, suggesting that Mn on Mn-RM has a valence state of mainly Mn4+, further confirming the presence of MnO2 on its surface [27].
N2 adsorption–desorption isotherms of Mn-RM were detected to analyze its adsorption capability. Figure S2a shows the BET curves for RM and Mn-RM. Mn-RM consistently exhibits a higher adsorption capacity than RM with increasing relative pressure, and their nitrogen adsorption isotherms also increases with relative pressure. Mn-RM has approximately six times the adsorption capacity of RM when the relative pressure is one. It can be observed that both RM and Mn-RM exhibit distinct hysteresis loops, which are attributed to Type IV adsorption isotherms based on the IUPAC classification [32]. This result suggests that both materials are mesoporous. The pore size distributions of RM range from 1.8 nm to 65 nm, while those of Mn-RM range from 1.7 nm to 70 nm, indicating a higher proportion of mesopores and fewer micropores in both materials (Figure S2b). According to Table S2, the specific surface area of Mn-RM is 82.45 m2·g−1 and the pore volume is 0.15 cm3·g−1, both of which are greater than those of RM (specific surface area of 10.22 m2·g−1 and pore volume of 0.02 cm3·g−1). The Mn modification significantly increases the specific surface area and pore volume of RM, but the decrease in average pore size may be due to the successful loading of MnO2 obstructing the micropores of red mud. The increase in pore volume facilitates faster diffusion of pollutants into the internal pores of the adsorbent, while the increase in specific surface area provides more sites for Pb2+ adsorption, enhancing surface activity of the adsorbent. Therefore, Mn-RM is an adsorbent with excellent adsorption capability.
3.2. Adsorption Kinetics
Four kinds of adsorption kinetic models were used to determine the Pb2+ adsorption process [33,34,35]. Figure 3 presents the fitting results of the experimental data, and the fitting parameters are shown in Table 2. Mn-RM adsorption is best described by the pseudo-second-order kinetic model, which has a fitting coefficient (R2) of 0.9879, compared with the pseudo-first-order model, suggesting that the process involves chemical adsorption where the adsorbate engages in electron sharing or electron transfer with the adsorbent [36]. The fitting of the pseudo-first-order kinetic model to the Pb2+ adsorption process of RM is marginally superior to the pseudo-second-order kinetic model, indicating that the removal of Pb2+ using RM is typically associated with a faster adsorption process, in which the substance directly adsorbs from the solution onto the surface of the RM without significant energy barriers [37]. Moreover, within the first 180 min, the Elovich model presents an R2 of 0.8966 for the Pb2+ adsorption, indicating that Mn-RM adsorption is dominated by external diffusion during the initial stage [36]. Furthermore, the closeness of the R2 values of the pseudo-first-order and pseudo-second-order kinetic models suggests that the adsorbing process is often more complex than predicted by theoretical conditions, potentially involving multiple interaction steps and mechanisms.
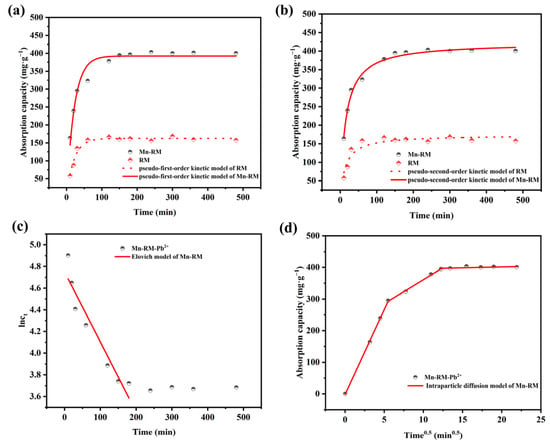
Figure 3.
Adsorption kinetic fitting results of (a) pseudo-first-order; (b) pseudo-second-order; (c) Elovich; and (d) intraparticle diffusion models of Pb2+ on Mn-RM.

Table 2.
Adsorption kinetic parameters for Pb2+ adsorption using Mn-RM.
In addition, an intraparticle diffusion model is employed to analyze the rate-limiting stages in the Pb2+ adsorption process. According to Figure 3d, the relationship between qt and t0.5 is linear, as the line does not pass through the origin. Intraparticle diffusion is not the only step controlling the adsorption rate, and other steps may be involved. There are three linear segments in the fitted curve, each with a rate constant of kp,1 > kp,2 > kp,3. Adsorption is most rapid in the first segment, which represents membrane diffusion. As a result, Pb2+ interacts quickly with the vacant adsorption sites on Mn-RM’s surface during the initial adsorption process. Intraparticle diffusion is represented in the second segment. Adsorption sites on the surface gradually disappear during this process, and adsorption capacity reaches its maximal level. Pb2+ adsorbed on the surfaces of the mesopores is massively resisted by the mesopore exterior due to the reduction of adsorption sites, leading to a constant adsorption rate of kp,3 and allowing adsorption equilibrium to be achieved. This indicates the formation of a Pb-O bond between Pb2+ and Mn-RM during adsorption [38]. In conclusion, it can be inferred that Mn-RM exhibits superior Pb2+ adsorption performance compared to RM. The modification of MnO2 improved both the rate and capacity of Pb2+ adsorption for RM.
3.3. Adsorption Isotherms and Thermodynamics
The adsorption isotherms and fitted parameters are shown in Figure 4 and Table 3, respectively. It can be observed that the Langmuir model [39] has a higher correlation coefficient R2 than the Freundlich model [40], indicating that the adsorption of Pb2+ is monolayer adsorption and the surface active sites on Mn-RM are uniformly distributed [41].
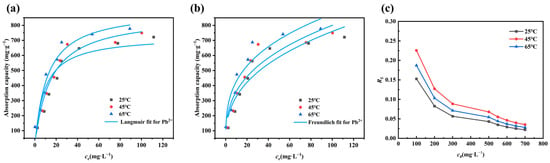
Figure 4.
Adsorption isotherms of (a) Langmuir and (b) Freundlich; (c) RL of Pb2+ on Mn-RM. Reaction conditions: Initial pH = 5, Mn-RM dosage = 1.0 g·L−1, t = 240 min, and T = 25 °C.

Table 3.
Adsorption isotherm parameters for Pb2+ adsorption using Mn-RM.
The dimensionless separation factor RL is expressed as RL = 1/(1 + KLC0), where C0 represents the initial Pb2+ concentration and KL is the Langmuir constant [42]. As shown in Figure 4c, under the same conditions, the RL of Pb2+ decreases with increasing temperature and RL remains between 0 and 1 at different temperatures, indicating that higher temperatures favor the adsorption of Pb2+ using Mn-RM. Additionally, at the same temperature, RL decreases as C0 increases, indicating that the increment of the initial Pb2+ concentration is beneficial for the adsorption process [28]. According to Table 3, the values of 1/n for all three temperatures are less than 1 and greater than 0.1, indicating that Mn-RM favors the adsorption of Pb2+ [43,44].
To study the thermodynamics of adsorption of Pb2+ on Mn-RM, the thermodynamic parameters ΔG, ΔH, and ΔS were calculated and are listed in Figure S3 and Table S3. At different temperatures, ΔH is positive, indicating an endothermic Pb2+ adsorption process using Mn-RM. The ΔG parameter remains negative at different temperatures and concentrations, indicating the favorable and spontaneous removal of Pb2+. A measurement of ΔS > 0 suggests an increase in the degree of freedom at the solid–liquid interface during the adsorption process, possibly related to ion exchange [45]. The positive values of ΔH and ΔS reveal that the adsorption process is both endothermic and entropy-increasing, which aligns with the findings of the Freundlich model.
3.4. Optimization of Reaction Conditions for Pb2+ Adsorption using Mn-RM
As shown in Figure S4a–c, different reaction factors, including the initial pH, solid–liquid ratio (w/v), and initial Pb2+ concentration, were examined. In Figure S4a, as the pH ranges from 2 to 8, both the efficiency in removing Pb2+ and the Mn-RM adsorption capacity increases with increasing pH value. At lower pH values, the Pb2+ removal efficiency is lower, which may be attributed to the protonation of the manganese hydroxyl functional groups on the surface of Mn-RM, resulting in a positive charge as well as an increase in H3O+ ions in the solution. Additionally, the positively charged surface sites compete with H3O+ for the adsorption of Pb2+, compromising the Pb2+ removal efficiency of Mn-RM [45]. As the initial pH increases, the concentration of H3O+ decreases while the concentration of OH- increases, causing deprotonation of the adsorbent surface and the generation of a negative charge on Mn-RM; this leads to enhanced electrostatic attraction between Mn-RM and Pb2+, ultimately improving Pb2+ removal efficiency. When the pH value exceeds 6, there is a continuing improvement in removal efficiency, possibly due to the precipitation of Pb2+ in hydroxide during alkaline conditions.
As the solid–liquid ratio increases, Pb2+ adsorption capacity of Mn-RM gradually decreases, while its removal efficiency continues to increase (Figure S4b). When the solid–liquid ratio exceeds 3 g·L−1, the Pb2+ removal efficiency reaches its maximum, but the adsorption capacity is low. A lower dosage of the adsorbent ensures that most of the adsorption sites are utilized for removing contaminant ions from the solution. However, with an increase in the solid–liquid ratio, more empty adsorption sites are generated alongside the increased number of adsorption sites, leading to a decrease in the adsorption capacity of the adsorbent. Moreover, an excessive amount of adsorbent can cause self-aggregation, further reducing the number of useful adsorption sites. Therefore, taking into account adsorption capacity, removal efficiency, and practicality, 1 g·L−1 was ultimately determined as the optimal solid–liquid ratio.
As depicted in Figure S4c, when the initial Pb2+ concentration is lower than 150 mg·L−1, the Pb2+ adsorption capacity of Mn-RM increases with increasing initial concentration. However, when the concentration exceeds 150 mg·L−1, the adsorption capacity increases slowly and tends to reach equilibrium, while the removal efficiency decreases. This is because the Mn-RM surface has more adsorption sites at lower ion concentrations, but at higher concentrations, adsorption saturation occurs and the adsorption capacity remains relatively constant.
Table S1 shows the experimental and predicted Pb2+ removal efficiencies determined using the Box–Behnken design. An analysis of variance (ANOVA) was conducted to investigate the interactions among the parameters influencing Pb2+ adsorption in the regression model. As shown in Table S4, the F-value of the regression model is 84.37, and the p-value is <0.0001, indicating its considerable significance. The p-value of the lack of fit stands at 0.0714 (>0.05), and the correlation coefficient of the model (R2) is 0.9909, indicating significant fitting performance. Upon comparing the F-values of various factors, the order of their influence on Y1 is as follows: B2 > BC > C2 > A > C > A2 > B > AB. As shown in Figure 5a, Pb2+ removal efficiency exhibits a trend of an initial increase followed by a decrease with increasing solid–liquid ratio and pH value; this may be attributed to the fact that when the solid–liquid ratio is relatively low, Mn-RM may not possess an adequate number of adsorption sites for Pb2+ removal. Consequently, as the solid–liquid ratio of Mn-RM increases, its removal efficiency also rises. However, an excessive solid–liquid ratio of Mn-RM can lead to agglomeration, preventing full contact with Pb2+ and resulting in a decrease in removal performance. Furthermore, the alkali in the red mud itself may be released into the solution, causing an elevated pH level. This, in turn, leads to metal precipitation, hindering the attachment of solid substances to Mn-RM and causing a reduction in its removal efficiency as the solid–liquid ratio increases. While the interaction effect between the initial Pb2+ concentration and the pH is not pronounced (as shown in Figure 5b), based on ANOVA analysis the Pb2+ concentration and solid–liquid ratio have a significant interaction, which is consistent with the results (Figure 5c). This is primarily due to the fact that an increased solid–liquid ratio provides more adsorption sites, and the higher initial concentration of Pb2+ in the solution facilitates a greater interaction with Mn-RM.
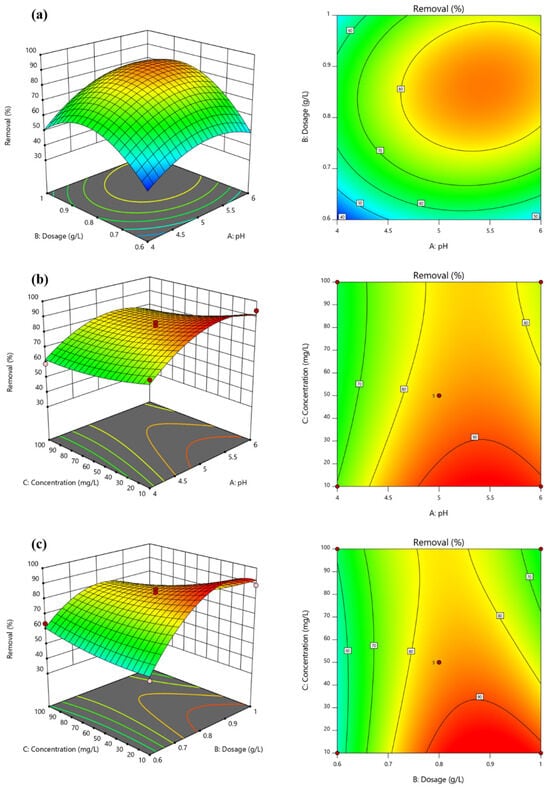
Figure 5.
RSM contours of Pb2+ removal efficiency. (a) Initial pH vs. solid–liquid ratio; (b) initial pH vs. initial concentration; and (c) solid–liquid ratio vs. initial concentration.
The optimal treatment conditions are as follows: initial pH = 5.21; solid–liquid ratio = 0.83 g·L−1; and initial Pb2+ concentration = 301.04 mg·L−1. According to Table S5, the average Pb2+ removal efficiency of the three validations is 87.20%, with an acceptable relative error (0.03%) compared to the predictive results (87.45%), indicating the feasibility of using the response surface method to optimize the operational conditions for Pb2+ removal using Mn-RM.
3.5. Effect of Co-Cation Types on Pb2+ Adsorption
Co-cations might influence the adsorptive capacity of Mn-RM. Na+, K+, Ca2+, and Mg2+ are natural cations commonly found in wastewater, and can easily compete with the target heavy metal ions in adsorption [15]. Therefore, the coexistence of Na+, K+, Ca2+, and Mg2+ ions was used to evaluate the adsorption selectivity of Mn-RM towards Pb2+. As shown in Figure 6, the presence of coexisting ions significantly decreased both the Pb2+ adsorption capacity and the removal efficiency of Mn-RM. This is mainly attributed to the presence of coexisting ions, which has a substantial influence on the interaction between adsorbate and adsorbent at the solid–liquid interface. The presence of coexisting ions weakens the electrostatic interactions between the adsorbate and adsorbent, and competes with the heavy metal ions for negatively charged adsorption sites [7]. Under the same concentration, the impact of coexisting ions on Pb2+ adsorption follows the sequence of Mg2+ > K+ > Ca2+ > Na+, which is closely related to the ion hydration radius and ion charge density. Na+ and K+ have the same electronic distribution in their outer shells but K+ has a smaller hydrated radius, resulting in a higher adsorption affinity than Na+. Therefore, K+ exhibits a stronger inhibition effect on Pb2+ adsorption compared to Na+. Due to the relatively small hydrated ion radius of Ca2+, it has a stronger competitive ability, thus showing a higher impact than Mg2+ [46]. Based on the influence of coexisting ions on adsorption performance, it can be concluded that the adsorption of Pb2+ using Mn-RM involves both ion exchange and electrostatic interactions.
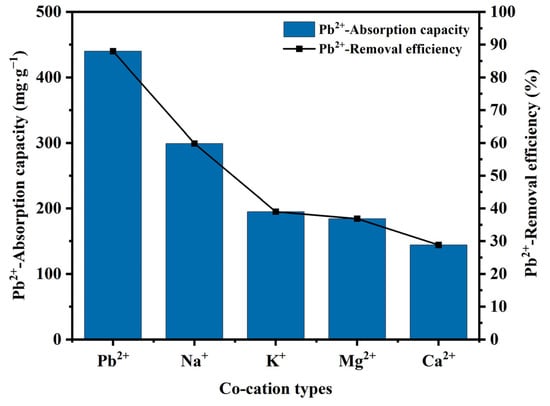
Figure 6.
Effects of Na+, K+, Mg2+, and Ca2+ on Pb2+ adsorption using Mn-RM.
3.6. Adsorption Mechanisms
To investigate the mechanism of Pb2+ removal using Mn-RM, FT-IR and XPS analyses were employed to analyze Mn-RM after Pb2+ adsorption. As depicted in Figure S5a, the FT-IR spectrum of Mn-RM after Pb2+ adsorption does not exhibit any new vibrational peaks, but there are changes in the peak intensity and wave numbers for functional groups. The characteristic peak, originally at 3450.08 cm−1, weakens and shifts to 3446.22 and 3406.37 cm−1, suggesting the participation of hydroxyl groups on Mn-RM in the adsorption process. Additionally, the adsorption peak intensity of Mn-OH noticeably diminishes and shifts due to Pb2+ entering Mn-RM and undergoing ion exchange with protons on the O atoms in the Mn-OH groups [15]. Consequently, Mn-OH groups play a significant role in heavy metal ion adsorption through ion exchange, further verifying the ion exchange capability of Mn-RM [47]. Mn-O groups also exhibit shifts, possibly due to the formation of Pb-O bonds between heavy metal ions and Mn-RM.
As shown in Figure S5b, after Pb2+ adsorption, the XPS survey reveals the appearance of peaks for Pb 4d and Pb 4f, indicating the successful adsorption of Pb2+ using Mn-RM. The peak fitting of Pb 4f in Figure S5c reveals two characteristic binding energies at 142.89 eV and 138.14 eV for Pb 4f5/2 and Pb 4f7/2, respectively. The presence of Pb 4f7/2 suggests the exclusive adsorption of Pb2+ with -O- or -OH functional groups on Mn-RM’s surface, forming Pb-O chemical bonds, which is consistent with the FTIR results. Before Pb2+ adsorption, O 1s exhibits three peaks at 529.28, 531.08, and 531.44 eV, corresponding to metal oxides (M-O), hydroxylated metals (M-OH), and oxygen in adsorbed H2O, respectively (Figure 7a) [27,48]. After Pb2+ adsorption, the percentage of M-OH decreases from 70.17% to 36.85%, indicating an interaction between Pb2+ and M-OH, leading to the formation of hydroxyl complexes or ion exchange (Figure 7b). Meanwhile, the content of M-O increases from 27.93% to 49.89%, suggesting that Pb-O formed on the surface of Mn-RM. In addition, Mn-RM reacts with Pb2+, which consumes M-OH and increases Mn-O, allowing H2O to be formed and thereby elevating its relative level [49].
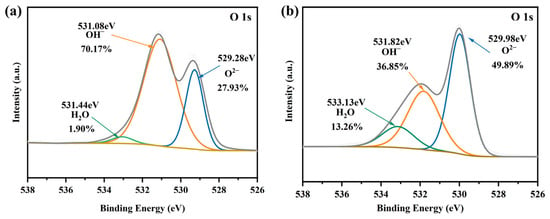
Figure 7.
O 1s XPS spectra of Mn-RM (a) before and (b) after Pb2+ adsorption.
The aforementioned characterizations and adsorption characteristics suggest that electrostatic attraction, ion exchange, and chemical adsorption are possible mechanisms for lead adsorption using Mn-RM.
3.7. Comparison of Mn-RM with Other Adsorbents for Pb2+ Removal
In order to evaluate Mn-RM’s adsorption capacity further, a comparison was made between it and other commonly used heavy metal-adsorbent materials (pH = 5; T = 25 °C; dosage = 1 g·L−1; t = 240 min; and initial Pb2+ concentration = 500 mg∙L−1). According to Table 4, Mn-RM provides greater removal of Pb2+ than other adsorbent materials previously reported. Thus, Mn-RM can be used to remove medium-to-heavy metals from aqueous solutions, and MnO2 is a cheap metal oxide that can be prepared at a low cost since RM is an industrial waste.

Table 4.
Adsorption capacities of different adsorbents for Pb2+ removal.
3.8. Reusability and Regeneration of Mn-RM
Recycling and reusing an adsorbent are key elements of a cost-effective and ideal adsorbent. The number of Pb2+ adsorption–desorption cycles that influence Mn-RM’s Pb2+ adsorption capacity is illustrated in Figure 8. The findings indicate that after undergoing five cycles of Pb2+ adsorption–desorption, the Pb2+ adsorption capacity of Mn-RM samples decreased by approximately 25%. Evidently, Mn-RM exhibits robust stability in Pb2+ adsorption and a noteworthy capacity for regeneration.
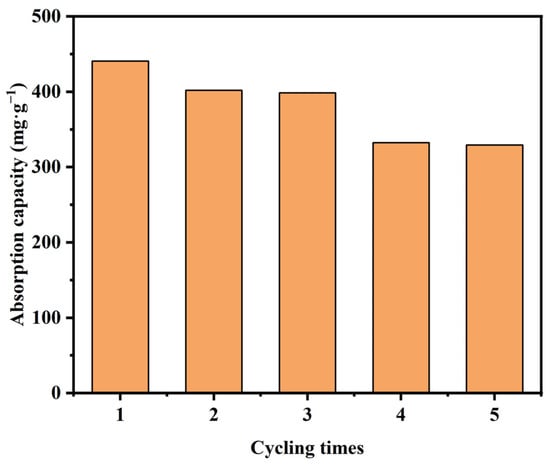
Figure 8.
Reusability and regeneration of Mn-RM under different adsorption–desorption cycles.
3.9. Environmental Risk Assessment
3.9.1. Leaching of Heavy Metals
Toxicity leaching tests were conducted on RM, Mn-RM, and Mn-RM after Pb2+ adsorption (Mn-RM-Pb2+), with the results presented in Table 5. It can be observed that the degree of leaching of heavy metals from red mud itself is relatively low. The degree of leaching from Mn-RM is even lower, with reduced leaching of Cd, Pb, Cr, As, Zn, and Cu. Furthermore, the leaching of heavy metals from Mn-RM after adsorption does not show a significant increase. Upon comparing the leaching results of different leaching methods, it is noted that the degree of leaching of heavy metals is higher when using the HJ/T299-2007 method. This is attributed to the higher solubility of heavy metals under acidic conditions [50]. The concentrations of leached metals from Mn-RM and Mn-RM-Pb2+ are all below the limits specified by the national safety standards (GB5085.3-2007 Leaching Toxicity Identification Standards [51]). Therefore, the employment of Mn-RM as an adsorbent material in aquatic settings is not only feasible, but also secure.

Table 5.
Leaching of heavy metals from RM, Mn-RM, and Mn-RM-Pb2+ (mg∙L−1).
3.9.2. Fractionation of Heavy Metals
Sequential extraction was conducted for RM, Mn-RM, and Mn-RM-Pb2+, and the fractionation of heavy metals within the three samples was analyzed. As shown in Figure S6, Cd primarily exists in exchangeable and residual fractions in RM, while after modification it is mainly distributed in organic-bound and residual fractions in Mn-RM. This indicates that Cd has a lower mobility and better stability in Mn-RM. After the adsorption of Pb2+, Cd in Mn-RM is mainly present in organic-bound and residual fractions, displaying the characteristics of low transformation and migration. Pb in RM mainly exists in carbonate-bound and residual fractions, with the residual fraction accounting for as much as 89% after modification. This suggests that Pb in Mn-RM is less likely to transform and migrate into the environment. After adsorption, the proportions of different fractions of Pb in Mn-RM-Pb2+ show slight alterations, but mainly remain in stable organic-bound and residual fractions.
Before modification, Zn and Cu are primarily present in exchangeable, iron–manganese oxide and carbonate-bound fractions, constituting 83% and 81% of their respective content. This indicates higher potential for the release and migration of these heavy metals, posing greater environmental risk. In Mn-RM, Zn and Cu are mainly present in residual and iron–manganese oxide fractions, and this distribution remains consistent after adsorption. Cr in RM is distributed in exchangeable, organic-bound, and residual fractions, with modification significantly increasing the proportion of the residual fraction, enhancing stability. As in all three samples, As primarily exists in the residual fraction, demonstrating its high stability [52].
In summary, MnO2 modification effectively enhances the stability of red mud itself, making it less prone to transformation and migration. While the stability of Mn-RM decreases slightly after Pb2+ adsorption, it still poses relatively low heavy metal mobility.
3.9.3. Environmental Risk Assessment
An environmental risk assessment was conducted for RM, Mn-RM, and Mn-RM-Pb2+ using the synthesis toxicity index (STI) model and risk assessment code (RAC) model. As shown in Figure 9, a higher STI value indicates greater toxicity, while a lower value suggests lower toxicity. After modification, the STI value for Mn-RM significantly decreases, indicating reduced comprehensive toxicity. After the adsorption of Pb2+, the STI value shows a slight increase, but remains smaller than that of RM. The RAC values for the three samples were calculated. Cd, Pb, Cr, Zn, and Cu all exhibit a certain level of environmental risk in RM, with Cd posing a high risk and Pb, Cr, Zn, and Cu falling into the medium-risk category (Figure 10). Before and after modification, the RAC values for As remain below 1%, indicating that it poses zero environmental risk. After modification, the RAC values for all heavy metals in Mn-RM are below 1%, indicating that MnO2 modification effectively reduces the environmental risk of the original RM. After the adsorption of Pb2+, the corresponding RAC values for Cd and Pb show slight increases but still remain within the low-risk range, posing minimal environmental risk. Consequently, the process of preparing Mn-RM and using it for Pb2+ adsorption carries a relatively low environmental risk.
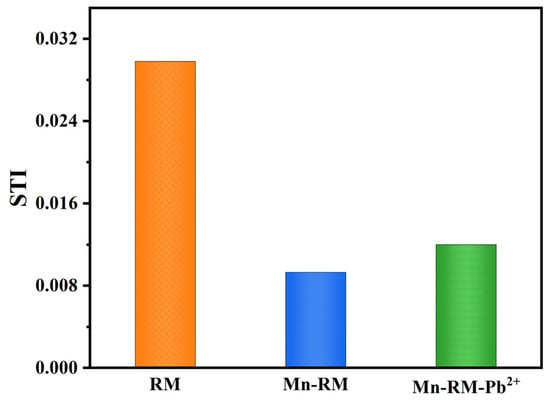
Figure 9.
STI Values of RM, Mn-RM, and Mn-RM-Pb2+.
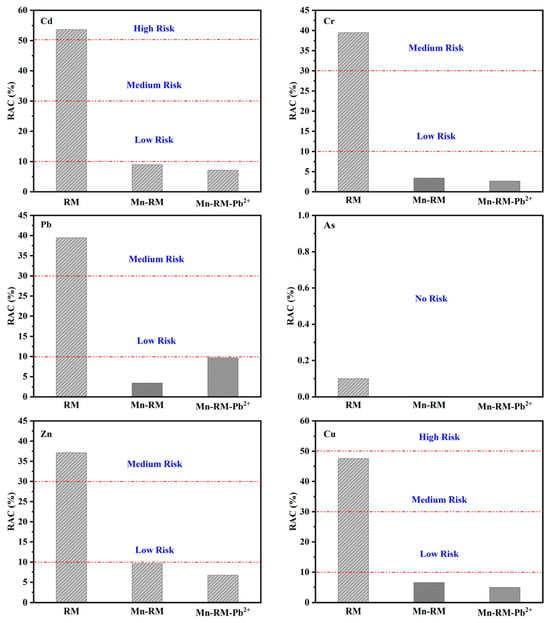
Figure 10.
RAC Values of heavy metals in RM, Mn-RM, and Mn-RM-Pb2+.
The prepared Mn-RM provides an efficient and renewable approach to address heavy metal pollution, particularly in the removal of Pb2+. This holds significant importance for safeguarding water resources and reducing the threat posed by heavy metals to ecosystems and human health. From the perspective of sustainable waste management, this study offers a novel approach of converting discarded red mud into valuable heavy metal adsorbents, providing a new direction for the recycling of industrial solid waste. This could not only aid in reducing the accumulation of red mud and its adverse environmental impacts, but could also accomplish effective resource recovery and utilization, contributing to the establishment of a sustainable environmental management system.
4. Conclusions
This study focuses on the preparation of a heavy metal-adsorbent material, manganese-modified red mud, by loading MnO2 onto the surface of red mud using the hydrothermal synthesis method (Mn-RM). The main findings are as follows:
- (1)
- The characterization results indicate that compared to raw red mud, the surface of Mn-RM is rougher and contains many spherical and flocculent particles. The specific surface area and the pore volume of Mn-RM increases by approximately eight times, and the loaded manganese dioxide exists in an amorphous phase structure. Mn-RM exhibits superior adsorption stability and regeneration ability, with a 25% decrease in Pb2+ adsorption capacity after five adsorption–desorption cycles.
- (2)
- Adsorption isotherms of Pb2+ on Mn-RM are better explained by the Langmuir isotherm model, and adsorption kinetics are better explained by a pseudo-second-order kinetic model. According to the thermodynamic analysis, it can be concluded that the adsorption of Pb2+ using Mn-RM is a process that absorbs heat and leads to an increase in entropy. The theoretically calculated maximum saturation adsorption capacity is 721.35 mg·g−1. Based on FTIR and XPS characterization, the adsorption of Pb2+ using Mn-RM mainly involves electrostatic attraction, ion exchange, and chemical adsorption.
- (3)
- The response surface analysis demonstrates that the removal of Pb2+ is mainly influenced by the interaction between the initial concentration and solid–liquid ratio, and between the solid–liquid ratio and pH value. The response surface model calculates the optimum treatment conditions as pH = 5.21, dosage = 0.83 g·L−1, and initial concentration = 301.04 mg·L−1, resulting in the highest Pb2+ removal efficiency of 87.45%.
- (4)
- The manganese modification of red mud effectively reduces the leaching of heavy metal components, and the leaching contents are within the specified range. Heavy metal speciation analysis reveals that manganese modification transforms heavy metals which were originally present in an unstable form in RM into a relatively stable state. The risk assessment code (RAC) values of each heavy metal in Mn-RM are less than 1%, and the synthesis toxicity index (STI) values decrease significantly. The RAC and STI values of Pb increase slightly after adsorption, but remain within the low-risk range.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15244314/s1, Text S1: Chemicals and materials; Text S2: Characterization methods for Mn-RM; Text S3: Details of batch experiments; Text S4: Methods and Procedures of environmental risk assessment; Table S1: Box-Behnken Design for Pb2+ adsorption by Mn-RM; Table S2: BET surface area, pore volume and average pore diameter of the RM and MRM; Table S3: Adsorption thermodynamic parameters for Mn-RM on Pb2+; Table S4: ANOVA for response surface quadratic model; Table S5: Validation of RSM results; Table S6: Bioavailability coefficient of each chemical speciation of HMs; Figure S1: EDS mapping of (a) RM and (b) Mn-RM; Figure S2: (a) N2 Adsorption-Desorption Isotherms and (b) Pore Size Distribution of RM and Mn-RM; Figure S3: Thermodynamic Curves of Mn-RM for Pb2+ adsorption; Figure S4: Effects of (a) initial pH, (b) solid-liquid ratio (w/v) and (c) initial Pb2+ concentration on Pb2+ adsorption by Mn-RM; Figure S5: (a) FTIR, (b) XPS survey, and (c) Pb 4f spectra of Mn-RM after Pb2+ adsorption; Figure S6: Fractionation of heavy metals in (a) RM, (b) Mn-RM, and (c) Mn-RM-Pb2+. References [36,53,54,55,56,57,58] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.B.; methodology, Y.B. and Y.P.; software, Y.B. and Y.P.; investigation, Z.W.; resources, Z.W. and Z.Z.; data curation, Y.P. and Z.W.; writing—original draft preparation, Y.B. and Y.P.; writing—review and editing, X.L., J.J., H.W. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Sichuan Province, China (2022NSFSC1171), the Key Research and Development Program of Sichuan Province, China (No. 2021YFN0130), and the Open Fund of Sichuan Provincial Engineering Research Center of City Solid Waste Energy and Building Materials Conversion and Utilization Technology (No. GF2022YB004).
Data Availability Statement
The data used in this study are available from the corresponding author upon request.
Acknowledgments
The authors extend their appreciation to the Shiyanjia Lab (www.shiyanjia.com) for their support with the SEM, TEM, XPS, and FTIR tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A Mini-Review on Functional Nucleic Acids-Based Heavy Metal Ion Detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) Ions from Aqueous Solutions by Biochars Derived from Potassium-Rich Biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, T.; Gao, L.; Cui, L.; Hu, L.; Yan, L.; Du, B.; Wei, Q. Magnetic Hydroxypropyl Chitosan Functionalized Graphene Oxide as Adsorbent for the Removal of Lead Ions from Aqueous Solution. Desalin. Water Treat. 2016, 57, 3975–3984. [Google Scholar] [CrossRef]
- Senthil Kumar, P. Adsorption of Lead(II) Ions from Simulated Wastewater Using Natural Waste: A Kinetic, Thermodynamic and Equilibrium Study. Environ. Prog. Sustain. Energy 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Abhinaya, R.V.; Thiruvengadaravi, K.V.; Baskaralingam, P.; Sivanesan, S. Lead(II) Adsorption onto Sulphuric Acid Treated Cashew Nut Shell. Sep. Sci. Technol. 2011, 46, 2436–2449. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced Removal of Heavy Metal Ions from Aqueous Solution Using Manganese Dioxide-Loaded Biochar: Behavior and Mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhou, P.; Wang, J.; Ma, Y.; Wu, J.; Su, C. Groundwater Pollution Model and Diffusion Law in Ordovician Limestone Aquifer Owe to Abandoned Red Mud Tailing Pit. Water. 2022, 14, 1472. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A Review of the Use of Red Mud as Adsorbent for the Removal of Toxic Pollutants from Water and Wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Tsamo, C.; Djomou Djonga, P.N.; Dangwang Dikdim, J.M.; Kamga, R. Kinetic and Equilibrium Studies of Cr(VI), Cu(II), and Pb(II) Removal from Aqueous Solution Using Red Mud, a Low-Cost Adsorbent. Arab. J. Sci. Eng. 2018, 43, 2353–2368. [Google Scholar] [CrossRef]
- Almeida, A.C.M.; do Nascimento, R.A.; Amador, I.C.B.; de Sousa Santos, T.C.; Martelli, M.C.; de Faria, L.J.G.; da Paixão Ribeiro, N.F. Chemically Activated Red Mud: Assessing Structural Modifications and Optimizing Adsorption Properties for Hexavalent Chromium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127325. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd (II) Sorption by Red Mud with Heat Treatment: Performance and Mechanisms of Sorption. J. Environ. Manag. 2020, 255, 109866. [Google Scholar] [CrossRef] [PubMed]
- Grudić, V.V.; Perić, Đ.; Blagojević, N.Z.; Vukašinović-Pešić, V.L.; Brašanac, S.; Mugoša, B. Pb (II) and Cu (II) Sorption from Aqueous Solutions Using Activated Red Mud--Evaluation of Kinetic, Equilibrium, and Thermodynamic Models. Polish J. Environ. Stud. 2013, 22, 377–385. [Google Scholar]
- He, C.; Xie, F. Adsorption Behavior of Manganese Dioxide towards Heavy Metal Ions: Surface Zeta Potential Effect. Water Air Soil Pollut. 2018, 229, 77. [Google Scholar] [CrossRef]
- Su, Q.; Pan, B.; Wan, S.; Zhang, W.; Lv, L. Use of Hydrous Manganese Dioxide as a Potential Sorbent for Selective Removal of Lead, Cadmium, and Zinc Ions from Water. J. Colloid Interface Sci. 2010, 349, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, L.; Zhang, L.; Zheng, S.; Wan, H.; Sun, J.; Zhu, D.; Xu, Z. Removal of Aqueous Pb (II) by Adsorption on Al2O3-Pillared Layered MnO2. Appl. Surf. Sci. 2017, 406, 330–338. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Z. A Facile One-Step Synthesized Epsilon-MnO2 Nanoflowers for Effective Removal of Lead Ions from Wastewater. Chemosphere 2020, 250, 126329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Liu, J.; Hu, P.; Meng, K.; Lv, F.; Tong, W.; Chu, P.K. Dealkalization of Red Mud by Carbide Slag and Flue Gas. CLEAN-Soil Air Water 2018, 46, 1700634. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization Processes of Bauxite Residue: A Comprehensive Review. J. Hazard. Mater. 2021, 403, 123671. [Google Scholar] [CrossRef]
- Fayazi, M.; Afzali, D.; Ghanei-Motlagh, R.; Iraji, A. Synthesis of Novel Sepiolite–Iron Oxide–Manganese Dioxide Nanocomposite and Application for Lead(II) Removal from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 18893–18903. [Google Scholar] [CrossRef]
- Afzali, D.; Fayazi, M. Deposition of MnO2 Nanoparticles on the Magnetic Halloysite Nanotubes by Hydrothermal Method for Lead(II) Removal from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- HJ/T299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. State Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.; Wang, L.; Liu, R.; Sun, W.; He, D. Efficient Removal of Pb (II) Ions from Aqueous Solution by Modified Red Mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Nejadshafiee, V.; Islami, M.R. Intelligent-Activated Carbon Prepared from Pistachio Shells Precursor for Effective Adsorption of Heavy Metals from Industrial Waste of Copper Mine. Environ. Sci. Pollut. Res. 2020, 27, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, F.; Sambenedetto, C.; Furlani, G.; Vegliò, F.; Toro, L. Preparation and Characterisation of Chemical Manganese Dioxide: Effect of the Operating Conditions. J. Power Sources 2007, 166, 567–577. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Q.; Tie, B.; Lei, M.; Yang, J.; Luo, S.; Song, Z. Manganese Dioxide Nanosheet Suspension: A Novel Absorbent for Cadmium (II) Contamination in Waterbody. J. Colloid Interface Sci. 2015, 456, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Narayanan, S.; Venkatesan, G.; Vetha Potheher, I. Equilibrium Studies on Removal of Lead (II) Ions from Aqueous Solution by Adsorption Using Modified Red Mud. Int. J. Environ. Sci. Technol. 2018, 15, 1687–1698. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium Uptake, Isotherm and Kinetic Studies of Cd(II) Adsorption onto Iron Oxide Activated Red Mud from Aqueous Solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Antonović, D. The Influence of Citrate Anion on Ni(II) Removal by Raw Red Mud from Aluminum Industry. Chem. Eng. J. 2013, 214, 327–335. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Huang, B.; Dong, H.; Huang, J.; Yang, Z.; Wei, J.; Hu, L.; Zhang, Q. Phase Transformation of Crystalline Iron Oxides and Their Adsorption Abilities for Pb and Cd. Chem. Eng. J. 2016, 284, 247–259. [Google Scholar] [CrossRef]
- Liu, D.-H.; Guo, Y.; Zhang, L.-H.; Li, W.-C.; Sun, T.; Lu, A.-H. Switchable Transport Strategy to Deposit Active Fe/Fe3C Cores into Hollow Microporous Carbons for Efficient Chromium Removal. Small 2013, 9, 3852–3857. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F.C. Kinetics of Adsorption and Desorption and the Elovich Equation. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1970; Volume 21, pp. 1–49. [Google Scholar]
- Wang, Z.; Su, J.; Hu, X.; Ali, A.; Wu, Z. Isolation of Biosynthetic Crystals by Microbially Induced Calcium Carbonate Precipitation and Their Utilization for Fluoride Removal from Groundwater. J. Hazard. Mater. 2021, 406, 124748. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe--N Modification on the Properties of Biochars and Their Adsorption Behavior on Tetracycline Removal from Aqueous Solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Equilibrium, Kinetics and Thermodynamics Study on the Adsorption of Cr(VI) and As(III) by Diatomite-Modified MnO2. J. Dispers. Sci. Technol. 2020, 43, 859–872. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Ren, Z.; Chen, F.; Wang, B.; Song, Z.; Zhou, Z.; Ren, D. Magnetic Biochar from Alkali-Activated Rice Straw for Removal of Rhodamine B from Aqueous Solution. Environ. Eng. Res. 2020, 25, 536–544. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. Adsorption Characteristics and the Removal Mechanism of Two Novel Fe-Zn Composite Modified Biochar for Cd (II) in Water. Bioresour. Technol. 2021, 333, 125078. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A. Removal of Hexavalent Chromium from Aqueous Solution Using Activated Carbon Prepared from Walnut Shell Biomass through Alkali Impregnation Processes. Clean Technol. Environ. Policy 2014, 16, 361–368. [Google Scholar] [CrossRef]
- Wu, S.; Xie, F.; Chen, S.; Fu, B. The Removal of Pb (II) and Cd (II) with Hydrous Manganese Dioxide: Mechanism on Zeta Potential and Adsorption Behavior. Environ. Technol. 2020, 41, 3219–3232. [Google Scholar] [CrossRef]
- Xiyili, H.; Çetintaş, S.; Bingöl, D. Removal of Some Heavy Metals onto Mechanically Activated Fly Ash: Modeling Approach for Optimization, Isotherms, Kinetics and Thermodynamics. Process Saf. Environ. Prot. 2017, 109, 288–300. [Google Scholar] [CrossRef]
- Huang, X.; Hao, H.; Zhang, G.; Li, J.; Ji, P. Adsorption of Cd2+ from Wastewater by Modified Fly Ash. Chin. J. Appl. Ecol. 2019, 30, 3215–3223. [Google Scholar]
- Sari, A.; Tuzen, M. Cd (II) Adsorption from Aqueous Solution by Raw and Modified Kaolinite. Appl. Clay Sci. 2014, 88, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Villalobos, M.; Tan, W.; Liu, F. Sorption Behavior of Heavy Metals on Birnessite: Relationship with Its Mn Average Oxidation State and Implications for Types of Sorption Sites. Chem. Geol. 2012, 292, 25–34. [Google Scholar] [CrossRef]
- Meng, K.; Wu, X.; Zhang, X.; Su, S.; Huang, Z.; Min, X.; Liu, Y.; Fang, M. Efficient Adsorption of the Cd (II) and As (V) Using Novel Adsorbent Ferrihydrite/Manganese Dioxide Composites. ACS Omega 2019, 4, 18627–18636. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, Q.; Gu, K.; Sun, Y.; Wang, Y.; Zhang, P.; Zheng, Z.; Guo, Y.; Xin, M.; Bian, R. Stability Evaluation of Potentially Toxic Elements in MSWI Fly Ash during Carbonation in View of Two Leaching Scenarios. Sci. Total Environ. 2022, 803, 150135. [Google Scholar] [CrossRef] [PubMed]
- GB5085.3-2007; Hazardous Waste Identification Criteria: Leaching Toxicity Identification Standards. State Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- Qi, X.; Wang, H.; Zhang, L.; Xu, B.; Shi, Q.; Li, F. Removal of Cr (III) from Aqueous Solution by Using Bauxite Residue (Red Mud): Identification of Active Components and Column Tests. Chemosphere 2020, 245, 125560. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.A.; Triwahyono, S.; Yaakob, M.R.; Azmi, Z.Z.A.; Sapawe, N.; Kamarudin, N.H.N.; Setiabudi, H.D.; Jaafar, N.F.; Sidik, S.M.; Adam, S.H.; et al. Utilization of Bivalve Shell-Treated Zea Mays L. (Maize) Husk Leaf as a Low-Cost Biosorbent for Enhanced Adsorption of Malachite Green. Bioresour. Technol. 2012, 120, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhu, F.; Zhao, L.; Jiang, H.; Zhang, Z. Heavy Metal Speciation in Various Types of Fly Ash from Municipal Solid Waste Incinerator. J. Mater. Cycles Waste Manag. 2014, 16, 608–615. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching Behaviour of Elements from Coal Combustion Fly Ash: An Overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Z.; Zhou, J.; Zhao, J.; Ruan, X.; Liu, J.; Qian, G. Chemical Characteristics and Risk Assessment of Typical Municipal Solid Waste Incineration (MSWI) Fly Ash in China. J. Hazard. Mater. 2013, 261, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Huang, Y.; Shimaoka, T.; Wang, H.; Wang, Y.; Ma, L.; Zhang, D. Evaluation of Chemical Speciation and Environmental Risk Levels of Heavy Metals during Varied Acid Corrosion Conditions for Raw and Solidified/Stabilized MSWI Fly Ash. Waste Manag. 2019, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Chai, M.; Li, R. Heavy Metal Migration and Potential Environmental Risk Assessment during the Washing Process of MSW Incineration Fly Ash and Molten Slag. Procedia Environ. Sci. 2016, 31, 351–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).