Abstract
To determine the impact of replacing fish meal (FM) in the diet with various levels of soybean meal (SBM) on the spotted knifejaw Oplegnathus punctatus, a 56 day feeding trial was done. Seven diets were formulated with SBM to replace 0% (SBM0), 30% (SBM30), 40% (SBM40), 50% (SBM50), 60% (SBM60), and 70% (SBM70) of FM protein, and SBM50 + T was developed on the basis of SBM50 with the addition of 1.2% taurine. There were triplicate groups of 18 fish (initial weight: 14.62 ± 0.02 g). The weight gain (WG), specific growth rate (SGR), feed efficiency (FE), and protein efficiency ratio (PER) values of the SBM0, SBM30, and SBM50 + T groups were found to be significantly higher than those of the SBM60 and SBM70 groups (p < 0.05). The daily energy gain (DEG), daily nitrogen gain (DNG), daily lipid gain (DLG), energy retention (ER), nitrogen retention (NR), and lipid retention (LR) values decreased significantly with increasing dietary SBM levels (p < 0.05). The highest retention of most amino acids (except lysine) was observed in the SBM30 group (p < 0.05). The lipid content of the whole body and dorsal muscle decreased significantly as dietary SBM levels increased (p < 0.05). Fish fed the SBM70 diet had the lowest serum triglyceride (TG) concentrations (p < 0.05). The effects of different treatments on total cholesterol (T-CHO) were not significant (p > 0.05). Fish fed the SBM0 and SBM30 diets had the highest amylase (AMS) and lipase (LPS) activities (p < 0.05). The lowest liver superoxide dismutase (SOD) and catalase (CAT) activities were observed in the SBM70 group. The malondialdehyde (MDA) concentration of the SBM50 to SBM70 groups were significantly higher than that of other groups (p < 0.05). The levels of interleukin 8 (il-8) mRNA were highest in fish fed the SBM0, SBM30, and SBM50 + T diets (p < 0.05), while the level of transforming growth factor β1 (tgf-β1) was the opposite (p < 0.05). According to the broken line regression of WG and FE, the highest level of FM substitution by SBM for Oplegnathus punctatus was 24.07–25.31%.
1. Introduction
Fish meal (FM) has been used as a major protein source in aqua-feeds due to its relatively balanced amino acid pattern, high mineral and vitamin contents, and long-chain omega-3 fatty acids [1]. Over the last 20 years, although the global FM production has remained relatively stable, it has not been able to match the rapid growth of the worldwide aquaculture industry. The limited supply of FM has led to a continuous rise of the price of commercial diets [2,3,4]. Therefore, it is critical to seek alternative sources of protein to ensure a steady supply of commercial diets. Numerous researchers who use plant protein sources such as soybean meal, canola meal, pea protein, corn gluten meal, and so on to partially or completely replace FM have reported significant progress in different fish species [5,6].
Compared with other plant proteins, soybean meal (SBM) is regarded as a nutritious feedstuff with a high crude protein content, wide availability, relatively balanced amino acid profile, and a stable supply [7,8,9]. Some studies have demonstrated considerable success in the partial or complete replacement of FM with SBM in the diets of many fish species [10,11]. However, some species have a limited capacity to use SBM. High dietary levels of soybean meal also significantly reduce the protease, amylase, and lipase activities in the digestive tract of Oreochromis niloticus and Myxocyprinus asiaticus [11,12]. Even in some fish, such as some salmonids, high levels of SBM can cause severe enteritis [13]. Therefore, it is worth noting that the ability to utilize SBM as a protein source varies among different species.
As a non-proteinogenic essential amino acid or conditionally essential amino acid, taurine has received special attention from nutritionists [14]. Taurine plays a vital role in regulating the physiological functions of fish, including growth promotion, immune response modulation, feeding stimulation, cellular osmoregulation, antioxidant action, and detoxification [15,16,17,18,19]. Although most animals can synthesize taurine from methionine and cysteine, some species have a limited capacity to synthesize this sulfur-containing amino acid due to the lack of L-cysteine sulphinate decarboxylase [14,20,21]. In addition, plant-based ingredients are severely deficient in cysteine, methionine, and serine and do not contain taurine, compared to animal-based ingredients [15]. Thus, diets containing plant protein must provide sufficient taurine to meet the physiological needs of fish for optimal growth, health, and development. It is reported that taurine supplementation can increase growth performance and feed utilization efficiency in various fish fed diets containing high levels of plant proteins, such as Argyrosomus regius [22], Diplodus sargus [23], Oreochromis niloticus [24], Acanthopagrus schlegelii [25], and Oncorhynchus mykiss [26]. These results suggest the potential for incorporating high levels of SBM into the diets with added taurine.
The spotted knifejaw, Oplegnathus punctatus, is a carnivorous marine fish with high economic value due to its beautiful appearance and good taste [27,28]. In recent years, the aquaculture of Oplegnathus punctatus has increased due to the continuous expansion of the market demand in China. However, studies on the nutritional requirements of this species are limited. For example, the optimal lipid and protein requirements were estimated to be 10.46–12.83% and 42.92–46.44%, respectively [29,30]. The current culture of Oplegnathus punctatus mainly relies on the commercial diets of other species, for example, Pseudosciaena crocea. It is incompatible with the development of the emerging aquaculture culture industry. There are currently no studies on alternative protein sources for this species, especially the impact of SBM on the growth, antioxidant capacity, and immunity of juvenile Oplegnathus punctatus. Therefore, there is an urgent need to develop an effective diet that provides balanced nutrition for Oplegnathus punctatus.
2. Materials and Methods
2.1. Experimental Diets
Seven isonitrogen (43% crude protein) and isoenergy (20.00 kJ g−1) diets were prepared. FM protein was replaced with 0%, 30%, 40%, 50%, 60%, and 70% SBM protein, and 1.2% taurine was added on the basis of 50% SBM (designated as SBM0, SBM30, SBM40, SBM50, SBM60, and SBM50 + T, respectively). The composition and formula of the diets used in the experiment are listed in Table 1. Table 2 shows the composition of amino acids in the diets. All dry ingredients were mixed thoroughly for 15 min using a mixer. Subsequently, oil (soybean oil and fish oil) and water were added to the dry mixture sequentially, which was mixed again for 15 min. Afterward, the diets (size, 1.5 mm) were obtained by a twin-screw extruder, air-dried in a 45 °C oven overnight, and then stored in a refrigerator at −20 °C until use.

Table 1.
The formulation and proximate composition of the experimental diets (% dry matter).

Table 2.
Amino acid composition (% total amino acid) of the test diets.
2.2. Experimental Fish and Feeding Trial
The fish used in the experiments were provided by China Qingdao Mingbo Aquatic Products Co., Ltd., and the feeding experiment was carried out in the Key Laboratory of Mariculture and Improvement, Zhejiang Institute of Marine Fisheries, Zhoushan, China. Before starting the feeding experiments, fish were fed commercial diets for 20 days to adapt to the laboratory environment. After that, 21 circular tanks filled with 300 L of seawater were stocked with 18 fish (average initial body weight, 14.62 ± 0.02 g per). Fish were fed twice (8:30 and 16:30) per day for 8 weeks. During feeding, a root blower was used for uninterrupted aeration to guarantee that dissolved oxygen surpassed 6 mg L−1. The water temperature was maintained at 28.7 ± 1.4 °C, and the ammonia nitrogen concentration did not exceed 0.05 mg L−1. The salinity and pH value of the water were 24 ± 0.8 g L−1 and 7.5 ± 0.1 respectively. Experiments were performed under a natural photoperiod, and each tank’s lighting was kept consistent.
2.3. Sample Collection and Analysis
Before the feeding test, 18 fish were randomly chosen as initial samples and were frozen for the subsequent whole-body composition analysis. At the end of the culture experiment, all fish were starved for 24 h before sampling, and the total number and weight of fish in each tank were subsequently recorded. Three fish were randomly sampled from each tank for whole-body composition and total energy analyses. Blood samples were collected from the caudal vein with a hypodermic syringe and centrifuged at 4000× g (4 °C) for 10 min (CT15RE centrifuge, Hitachi, Japan). The viscera, intraperitoneal fat, liver, and intestine were extracted and weighed. All of these samples were processed using liquid nitrogen and then stored at −80 °C.
According to AOAC (1995), the analysis of the whole body and the tissues and the composition of the feed were determined. Moisture was detected by weight removal using a drying oven (diet, 105 °C) or a lyophilizer (LL1500, Thermo Scientific, Waltham, MA, USA) (fish and tissue, −110 °C). The crude protein of the sample was analyzed by an Auto Kjeldahl System (K355/K437, Buchi, Flawil, Switzerland) according to the Kjeldahl method. The ash content was determined in a muffle furnace at 550 °C for 12 h. Crude lipids were determined by ether extraction using a Soxhlet apparatus (E816, Buchi, Flawil, Switzerland). With the use of a calorimeter (HWR-15E, Shangli, Shanghai, China), the total energy of the diet and that of the whole body were assessed. To test the amino acid composition of the samples, the amino acid samples were sent to a professional laboratory for measurement using an automatic analyzer (L-8900, HITACHI, Tokyo, Japan).
The triglyceride (TG) and total cholesterol (T-CHO) contents in serum were evaluated through the method provided by [31]. The activities of lipase (LPS) and amylase (AMS) in the intestinal tract were determined, as described by [32]. Catalase (CAT) was analyzed using the methods provided by [33]. Superoxide dismutase (SOD) activity was measured according to the method provided by [34]. The concentration of malondialdehyde (MDA) was determined by the method provided by [35]. All parameters above were measured by commercial test kits (Nanjing Jianchen Bioengineering Institute, Nanjing, China) and a microplate reader (Multiskan Go, Thermo Scientific, Waltham, MA, USA).
2.4. Gene Expression
Total RNA from liver was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and quantified using 1.0% agarose electrophoresis. Subsequently, according to the manufacturer’s scheme, 1 μg of RNA was used for cDNA synthesis using a PrimeScriptTM RT Reagent kit (perfect Real Time; Takara, Dalian, China). The relative expressions of interleukin-8 (il-8) and transforming growth factor β1 (tgf-β1) genes were determined by a Real-Time PCR System (QuantStudioTM 6 Flex, Life Technologies, Carlsbad, CA, USA) according to [36]. The β-atin gene was chosen as an internal reference. The specific primers used in this study are shown in Table 3. Relative expression levels were calculated based on the 2−△△CT equation according to [37]. Each treatment was performed in triplicate and was repeated three times per sample.

Table 3.
The sequences of primers used in this study.
2.5. Calculation and Statistical Analysis
- Weight gain (%) = 100 × (final body weight − initial body weight)/initial body weight.
- Specific growth rate = 100 × (Ln (final body weight) − Ln (initial body weight))/days.
- Feed efficiency = wet weight gain/dry feed consumed.
- Protein efficiency ratio = wet weight gain/protein intake.
- Daily feed intake = 100 × feed offered/average total weight/days.
- Viscerosomatic index = 100 × (viscera weight/whole body weight).
- Intraperitoneal fat ratio = 100 × (intraperitoneal fat weight/whole body weight).
- Hepatosomatic index = 100 × (hepatosomatic weight/whole body weight).
- Condition factor = 100 × (live weight/length3).
- Average body weight (ABW) = (initial body weight + final body weight)/2.
- Daily nitrogen intake = feed intake nitrogen/ABW × days.
- Daily nitrogen gain = (final body weight × final body nitrogen −initial body weight × initial body nitrogen)/ABW × days.
- Nitrogen retention = 100 × daily nitrogen gain/daily nitrogen intake.
- Daily energy intake = feed intake energy/ABW × days.
- Daily energy gain = (final body weight × final body energy − initial body weight × initial body energy)/ABW × days.
- Energy retention = 100 × daily nitrogen gain/daily nitrogen intake.
- Daily lipid intake = feed intake lipid/ABW × days.
- Daily lipid gain = (final body weight × final body lipid −initial body weight × initial body lipid)/ABW × days.
- Lipid retention = 100 × daily lipid gain/daily lipid intake.
Data were expressed as an average (n = 3) and analyzed statistically using SPSS 24.0 (IBM, Chicago, IL, USA). After Levene’s test was used to analyze the homogeneity of each treatment, one-way analysis of variance (ANOVA) was used to determine the treatment effect, and Duncan’s multi-range test was used to determine the treatment deviation. Linear and quadratic regression models were constructed to test the correlation between results and dietary SBM substitution FM levels. The statistical significance level was 5% (p < 0.05). Based on WG and FE, a polyline model was applied to determine the maximum substitution level of SBM for FM.
3. Results
3.1. Growth Performance, Feed Utilization, and Biometric Parameters
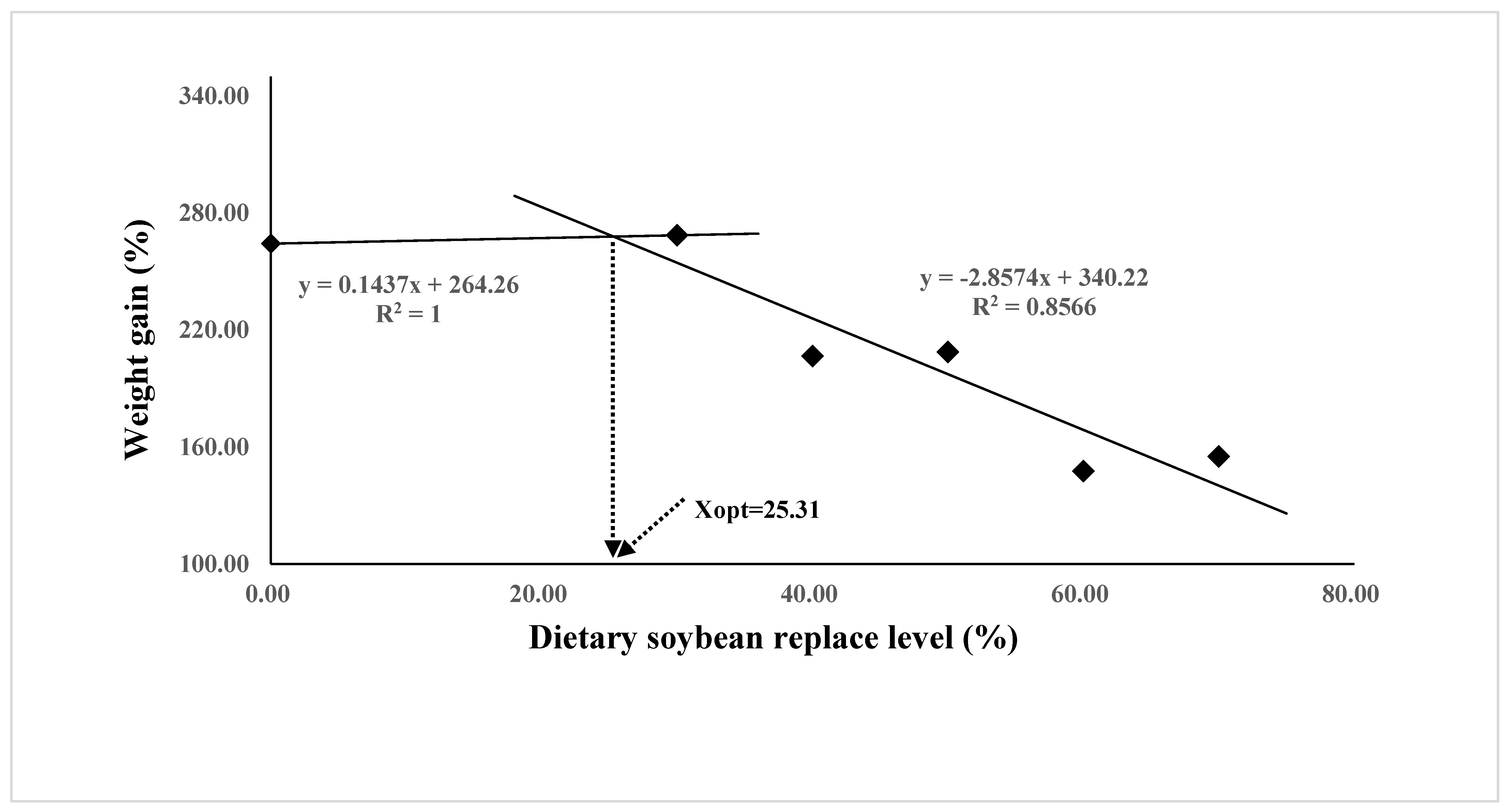
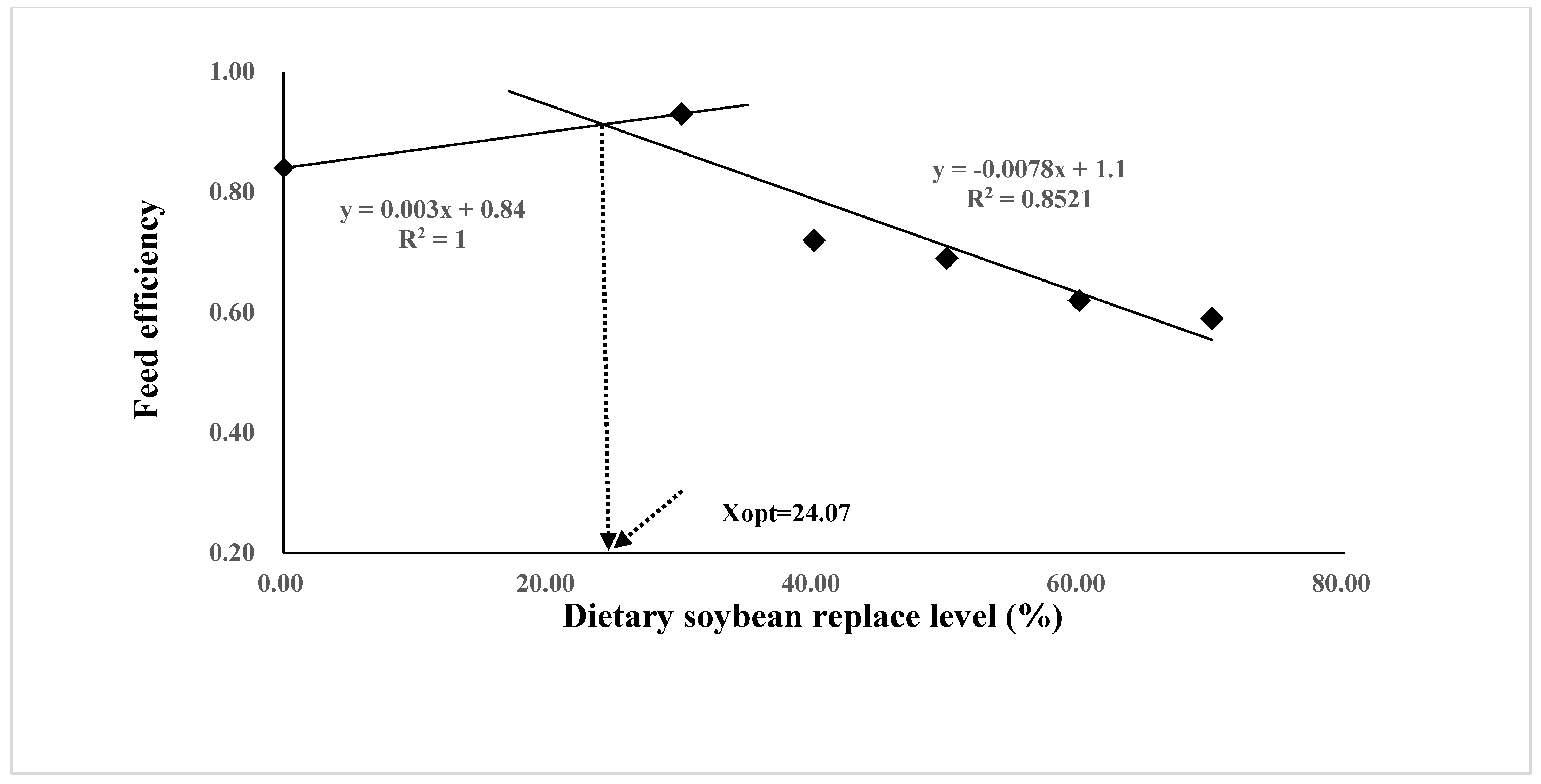
The growth performance and feed utilization of juvenile Oplegnathus punctatus were significantly affected by replacing FM in diets with different levels of SBM (Table 4). Although not statistically different, the weight gain (WG), specific growth rate (SGR), and protein efficiency ratio (PER) values of the SBM0 and SBM30 groups were numerically higher than those of the SBM40 and SBM50 groups (p > 0.05) and significantly higher than those of the SBM60 and SBM70 groups (p < 0.05). The regression analysis of WG and FE in the two-slope broken-line model showed that the highest level of FM substitution in Oplegnathus punctatus by SBM was 24.07–25.31% (Figure 1 and Figure 2). In addition, the content of SBM had no significant influence on the viscerosomatic index (VSI), hepatosomatic index (HSI), and condition factor (CF) (p > 0.05).

Table 4.
Growth, feed utilization, and morphometrical parameters of juvenile Oplegnathus punctatus fed diets containing different levels of soybean meal.
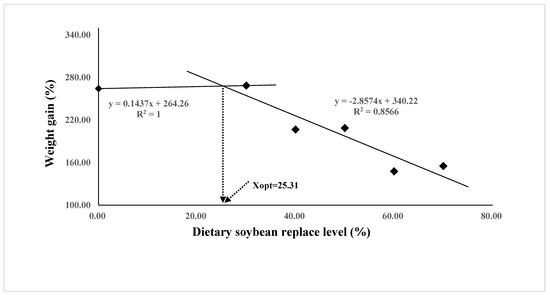
Figure 1.
Relationship between the weight gain (WG, %) and the dietary soybean level based on two slope broken-line regression analysis, where Xopt represents the optimal dietary soybean replacement level for the maximum WG.
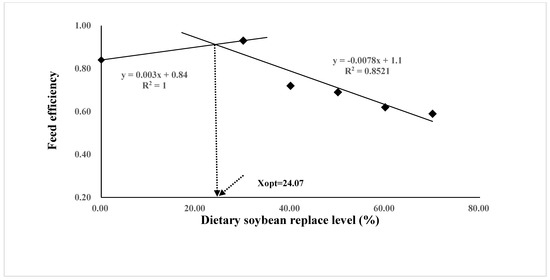
Figure 2.
Relationship between the feed efficiency (FE) and the dietary soybean level based on two slope broken-line regression analysis, where Xopt represents the optimal dietary soybean replacement level for the maximum FE.
3.2. Whole-Body and Tissue Composition
The proximal components of the body and tissues of the fish fed different treatments are listed in Table 5. The lipid content of the whole body and dorsal muscle decreased significantly as SBM dietary levels increased (p < 0.05). However, the effects of different treatments on the protein content were not significant (p > 0.05). In this study, except for proline, the content of most amino acids in the whole body was not affected by the dietary treatment (p > 0.05; Table 6).

Table 5.
Whole-body and tissue proximate composition in juvenile Oplegnathus punctatus fed diets containing different levels of soybean meal (as a % of the wet weight basis).

Table 6.
Amino acid composition (% of total amino acids) of the whole body of juvenile Oplegnathus punctatus fed diets containing different levels of FM replaced with SBM.
3.3. Retention and Deposition of Energy, Nitrogen, and Lipids
In Table 7, the effects of different treatments on the feed intake (FI), daily nitrogen intake (DNI), and daily energy intake (DEI) values were not significant (p > 0.05). However, the daily energy gain (DEG), daily nitrogen gain (DNG), daily lipid gain (DLG), energy retention (ER), nitrogen retention (NR), and lipid retention (LR) decreased significantly with increasing SBM (p < 0.05). The NR values of the SBM50 + T group were significantly higher than those of the SBM50 group (p < 0.05). Moreover, fish fed the SBM30 diet had significantly higher retention of most amino acids than those fed the SBM50, SBM60, and SBM70 diets, except for lysine (p < 0.05; Table 8).

Table 7.
Nitrogen, energy, and lipid utilization in juvenile Oplegnathus punctatus fed diets containing different levels of soybean meal.

Table 8.
Essential amino acid (EAA) retention (%) of juvenile Oplegnathus punctatus fed diets containing difference levels of SBM and FM.
3.4. Hematological Parameters
Hematological parameter data are listed in Table 9. The effects of different treatments on total cholesterol (T-CHO) were not significant (p > 0.05). Serum triglyceride (TG) concentrations were significantly higher in fish fed the SBM0, SBM30, and SBM50 + T diets compared with the SBM70 diet.

Table 9.
Biochemical indices in the serum of juvenile Oplegnathus punctatus fed experimental diets for eight weeks.
3.5. Activity of the Digestive Enzymes in the Intestine
In the present study, fish fed the SBM0 diet had the highest lipase (LPS) activity (p > 0.05; Table 10). The amylase (AMS) activity of the SBM0 group was significantly higher than that of the other treatment groups (p < 0.05).

Table 10.
Digestive enzyme activity in the intestine of juvenile Oplegnathus punctatus fed experimental diets for eight weeks.
3.6. Liver Antioxidant Enzyme and Biochemical Indices
Although there was no significant difference in superoxide dismutase (SOD) under different dietary SBM levels, the lowest SOD value was observed in SBM70 (p > 0.05; Table 11). Catalase (CAT) activity decreased significantly as dietary SBM levels increased (p < 0.05). The concentration of malondialdehyde (MDA) in the SBM0, SBM30, and SBM50 + T groups was significantly lower than that in the SBM50–SBM70 groups (p < 0.05).

Table 11.
Antioxidant parameters in the liver of juvenile Oplegnathus punctatus fed experimental diets for eight weeks.
3.7. The mRNA Expression Levels of il-8 and tgf-β1
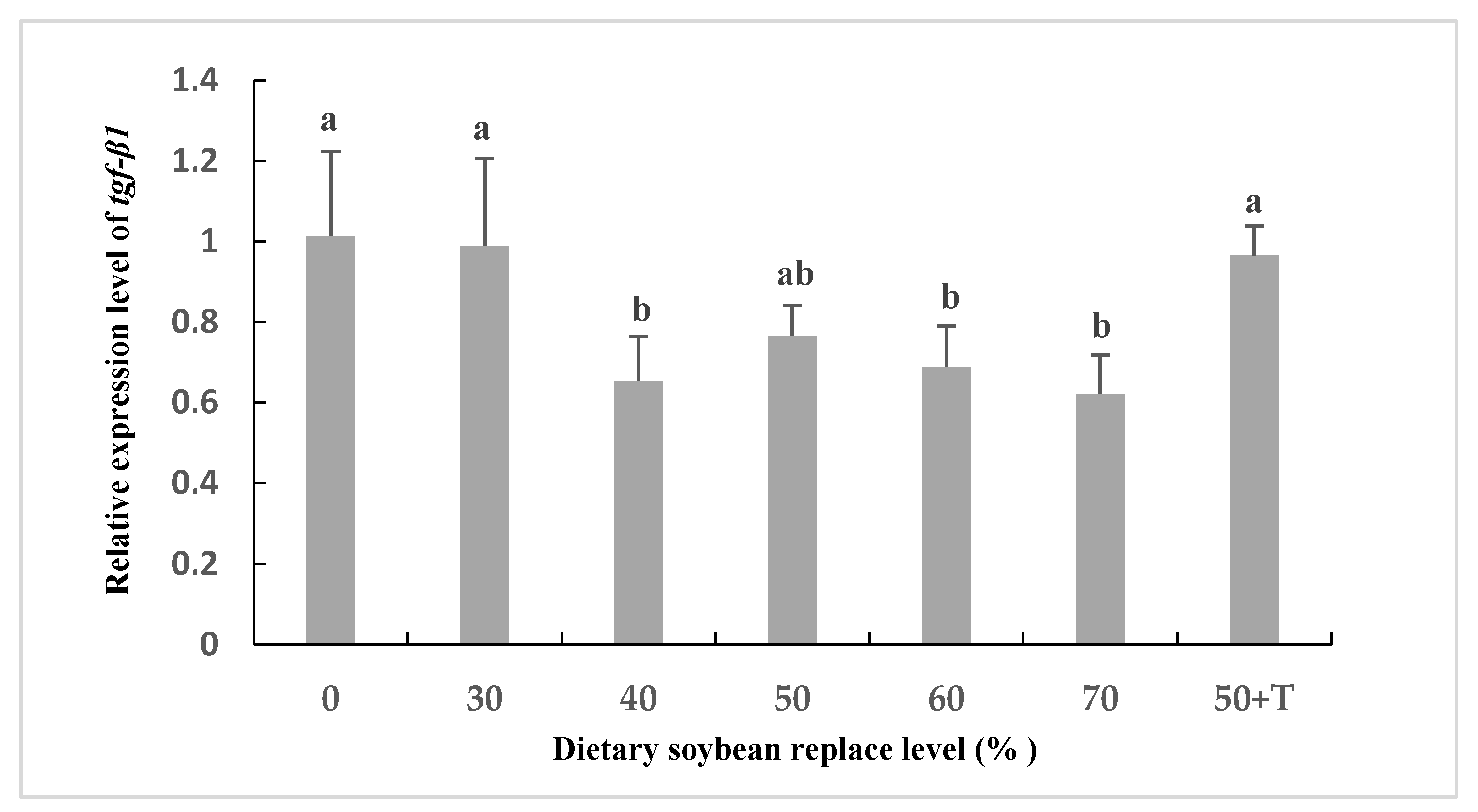
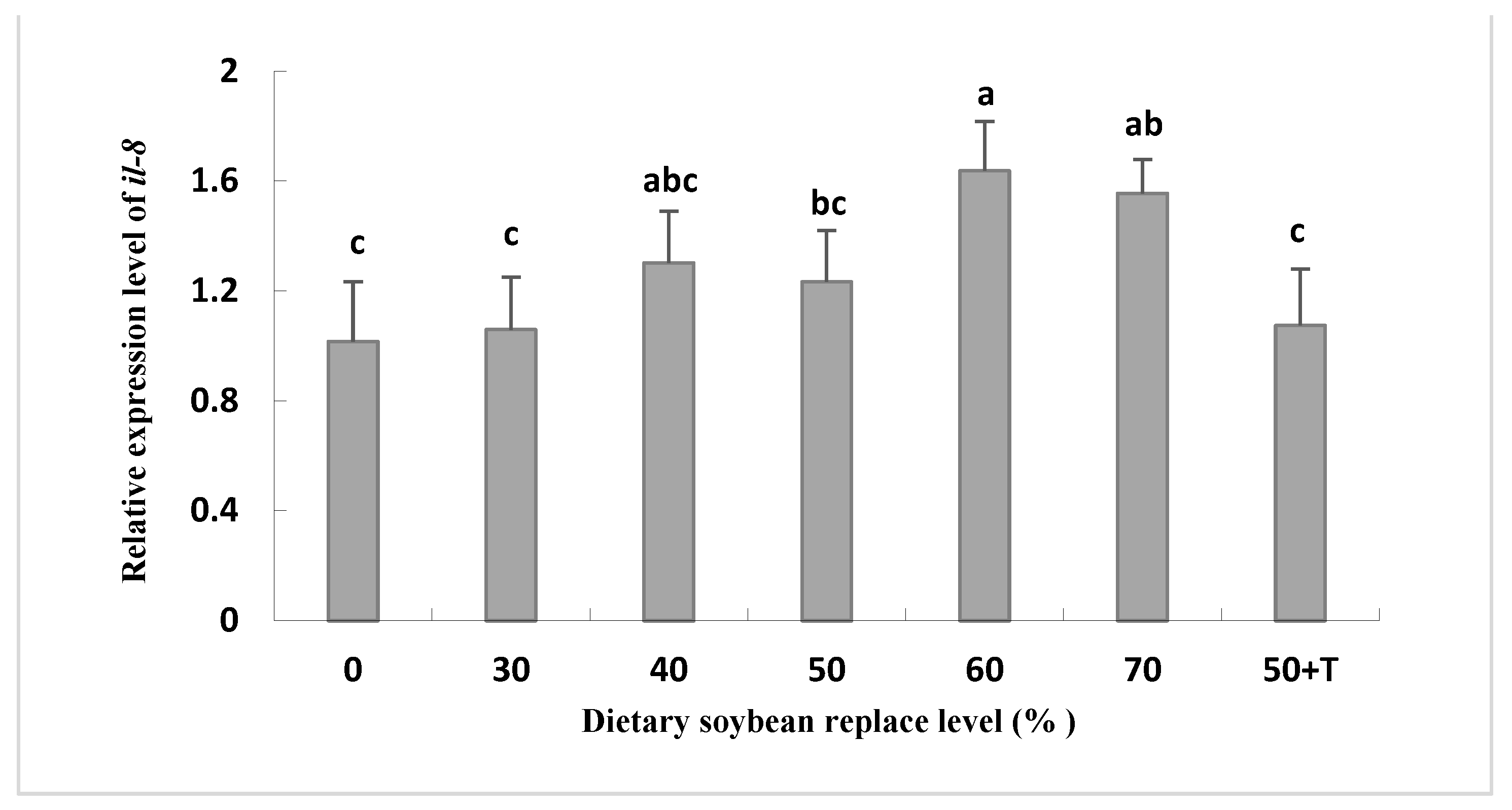
The mRNA levels of il-8 were higher in the livers of the SBM40 to SBM70 groups compared to those in the SBM0 and SBM30 groups. However, the mRNA expression of tgf-β1 was reversed (Figure 3). In addition, the mRNA level of il-8 in the SBM50 + T group was lower than that in the SBM40–SBM70 group, which was equivalent to that in the SBM0 and SBM30 groups (Figure 4).
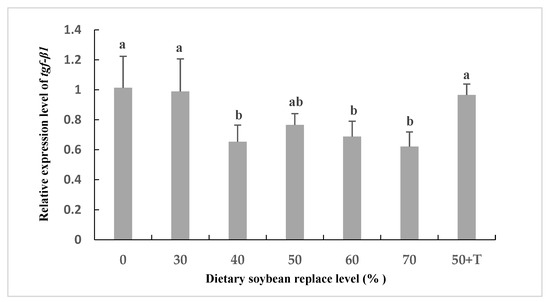
Figure 3.
Relative expression of the mRNA for transforming growth factor β1 (tgf-β1) in the liver of Oplegnathus punctatus fed the seven diets. Means in the same row with different superscripts are significantly different (p < 0.05).
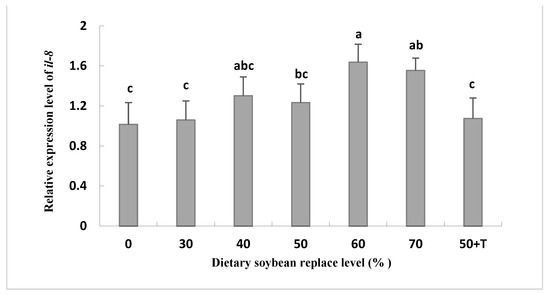
Figure 4.
Relative expression of the mRNA for cytokine interleukin-8 (il-8) in the liver of Oplegnathus punctatus fed the seven diets. Means in the same row with different superscripts are significantly different (p < 0.05).
4. Discussion
After an eight week feeding trial, the growth performance and feed utilization of Oplegnathus punctatus were significantly affected by different dietary treatments. The highest WG values were observed in the SBM0 (264.26 ± 49.45%) and SBM30 (268.57 ± 24.37%) groups. Although not statistically different, the WG values of the SBM0 and SBM30 groups were numerically higher than those of the SBM40 and SBM50 groups and were significantly higher than those of the SBM60 and SBM70 groups. Similar results were also found in other fish species such as Takifugu rubripes [38], Hemibagrus wyckioides [39], and Channa striata [40], with a maximum replacement level of 30%. In addition, the highest FE value occurred in the SBM30 group, which was significantly higher than that in the SBM40 and SBM50 groups. According to the broken line regression analysis of WG and FE, the optimal SBM replacement FM level for spotted knifejaw ranged from 24.07% to 25.31%. These values are similar to those reported for other fish species, such as 24% for juvenile Paralichthys olivaceus [41], 25% for Oreochromis niloticus [42], and 25% for Rhabdosargus sarba [43]. Notably, the SGR and WG values of the SBM50 + T group (with 1.2% taurine added) were significantly higher than those of the corresponding SBM50 group but were not statistically different from those in the SBM30 group. This indicated that FM replacement levels could reach 50% using SBM with 1.2% taurine added to the diet without negatively affecting the growth performance of Oplegnathus punctatus. The beneficial influences of dietary taurine on enhancing the efficacy of plant proteins have also been reported for other carnivorous fish such as Dicentrarchus labrax [44] and Channa striata [45].
When using high levels of SBM instead of FM in the diet, the decrease in feed intake (FI) caused by poor palatability was probably one of the key factors for the decline in the growth performance in some fish species. In this investigation, the FI values of all groups were not significantly different. Consistent with this, there were no significant differences in DNI values (from 1.52 to 1.79) among fish groups treated with increasing dietary SBM. In aquaculture practice, it was observed that the species actively feed on algae attached to the cage. Therefore, it can be concluded that palatability should not be a major factor in the decline of the growth performance of Oplegnathus punctatus or can be ignored. Similar results were also found in studies on N. miichthioides [46] and Lutjanus campechanus [47].
SBM ingredients commonly contain various anti-nutritional factors (ANFS), such as protease inhibitors, phytate, saponins, lectins, and oligosaccharides. In the industrial production of SBM, the activity of some ANFs in soybean can be reduced by heat treatment, such as trypsin inhibitors and other heat-sensitive ANFs [48]. However, this treatment is basically ineffective in destroying NSPs, lectins, saponins, and phytic acid [49,50]. In fact, the main antigenic components existing in SBM, such as glycinin and β-conglycinin, may be mainly responsible for inducing abnormal intestinal structural changes and activating the immune system, leading to harmful inflammatory reactions [51,52,53]. In Atlantic salmon, numerous studies reported that plant feedstuffs can result in changes in intestinal histomorphology and gene expression related to immune responses within four weeks [54,55,56,57]. In the present study, after eight-week culture experiments, no symptoms of intestinal inflammation were observed histologically in all groups (data not provided). However, it was found that the mRNA levels of the pro-inflammatory cytokine interleukin-8 (il-8) were higher in the livers of fish fed high replacement levels of SBM (SBM40 to SBM70) compared with the SBM0 and SBM30 groups, and the expression of the anti-inflammatory cytokine transforming growth factor β1(tgf-β1) was suppressed. il-8 is an important pro-inflammatory cytokine that recruits and activates macrophages and neutrophils to clear cellular debris and invading microorganisms and promotes the regeneration of damaged tissues [58]. Meanwhile, tgf-β acts as an anti-inflammatory cytokine, counteracting the production of pro-inflammatory cytokines and limiting the inflammatory response [59]. Significantly, the mRNA level of the pro-inflammatory cytokine il-8 in the SBM50 + T group was lower than that in the SBM40 to SBM70 groups, which was equivalent to that in the SBM0 and SBM30 groups, whereas the expression of the anti-inflammatory cytokine tgf-β1 was the opposite. Researchers [60] have demonstrated that the primary role of taurine is to protect and maintain the homeostasis of cells involved in acute and chronic inflammation. Therefore, in this study, the supplementation of taurine in the SBM50 + T group may improve the health status of Oplegnathus punctatus. However, [61] found that changes in gene expression associated with immune responses were detected on the third day of a plant-based diet, which was much earlier than the signs of inflammation in histological assessment. Since different species have different levels of susceptibility to ANFs, the effect of supplementing taurine in SBM instead of FM on the digestive tract structure of Oplegnathus punctatus needs long-term evaluation.
In this study, the content of most amino acids in whole body remained unchanged except proline, which is consistent with the fact that the composition of essential amino acids in the whole body is relatively stable and is hardly affected by fish size or dietary composition, as the biosynthesis of body protein is genetically determined [62,63]. It is well known that the essence of amino acid balance is that the ratio of each amino acid constituting a protein should be appropriate. If one amino acid is deficient, it will adversely affect the synthesis of intact protein molecules and will affect the availability of other amino acids [64]. The retention of protein and essential amino acids is considered to be the most sensitive indicator of insufficient amino acid supply [65]. In the current research, the methionine content (1.77% to 1.41%) of the experimental diets gradually decreased with increasing levels of dietary SBM, which significantly affected the retention of essential amino acids. The retention of most amino acids was significantly higher in the SBM30 group than in the SBM50, SBM60, and SBM70 groups, except lysine. This was also confirmed by higher DNG and NR values in the SBM30 group compared to the SBM40 to SBM70 groups. These results suggest that methionine deficiency affected the utilization of other amino acids in the high SBM groups (SBM50 to SBM70). In these groups, due to the lack of methionine, there was a relative surplus of other amino acids, which were used for oxidative breakdown rather than synthesis, ultimately inhibiting growth. Therefore, the imbalance of amino acids in the diet is probably the key factor for the decreased growth performance of Oplegnathus punctatus in the high SBM groups. A similar situation was observed in Pseudobagrus ussuriensis [66], Epinephelus fuscoguttatus [67], and Gadus morhua L. [68] fed soybean meal-based diets. Interestingly, although not statistically different from the SBM50 group, the retention values of most essential amino acids were higher in the SBM50 + T group than in the SBM50 group. Moreover, the NR and SGR values of the SBM50 + T group were significantly higher than those of the SBM50 group. When taurine is insufficient in the diet, part of methionine may be converted into taurine to meet the physiological needs of fish, thus aggravating the lack of methionine and adversely affecting growth. After taurine addition, methionine is saved to a certain extent. More importantly, the saved methionine will be used for growth [15,69,70,71,72].
The inclusion of SBM in the diet may have a negative impact on the digestibility of fish, for example, adding SBM to the diet decreased lipid deposition, digestible protein, and digestible energy in Salmo salar [73]. In this study, although DNI and DEI were not significantly different in all treatment groups, DEG, DNG, DLG, ER, NR, and LR decreased notably with increasing levels of SBM. A similar situation was observed in Liza H. [74]. In addition, the amylase (AMS) activity of the SBM0 and SBM30 groups was significantly higher than that of the SBM40 to SBM70 groups, which may partly be responsible for the decreased dietary digestibility after SBM replaced FM. Triglycerides (TGs) and total cholesterol (T-CHO) are mainly synthesized in the liver, and their changes reflect the lipid metabolism in body to a certain extent. Increased levels of TG and T-CHO indicate that the endogenous fat transport is active [75,76]. Several studies have found that nonstarch polysaccharides (NSP) can reduce plasma and liver T-CHO levels in fish, possibly because they interfere with fat digestion and absorption [49,77]. In this study, the levels of TG and T-CHO in the high SBM groups were lower than those in the low SBM and control groups. Therefore, the significant reduction in the whole body and muscle lipid content in the high replacement groups may be related to NSP. A similar result was seen for redlip mullet Liza haematocheila [74]. Interestingly, the T-CHO and TG values of the SBM50 + T group were higher than those of the SBM50 group. Researchers [78] showed that taurine has a beneficial effect on fat metabolism. However, there were no significant differences in the whole fish and muscle lipid content between the SBM50 + T and SBM50 groups. The specific reason is not clear, so the effect of taurine on lipid metabolism in Oplegnathus punctatus needs further evaluation.
Antioxidant enzymes (such as SOD and CAT) in the antioxidant defense system can effectively remove excess reactive oxygen species and protect cells from oxidative damage [79]. The plant protein in the diet may have a harmful influence on the antioxidant status of fish. For example, when feeding a diet containing high levels of SBM, the antioxidant capacity of Monopterus albus decreased significantly [80]. SOD activity was not significantly different among the groups in the study, but the SBM70 treatment group had the lowest SOD activity. As the SBM content in the diet increased, CAT activity decreased significantly. The results were similar to those of Ctenopharyngodon I. [81]. In addition, malondialdehyde (MDA) produced by endogenous oxidative damage in vivo is one of the final metabolites of lipid peroxidation, which can reflect the extent of lipid peroxidation and the extent of cell injury [82]. Our study found that the supply of high levels of SBM (SBM50 to SBM70) significantly increased the concentration of MDA in the liver of Oplegnathus punctatus, indicating that the health status of Oplegnathus punctatus could be affected by using SBM instead of FM. Many studies showed that taurine can have beneficial effects in diets containing plant proteins. For example, supplementation of taurine in plant protein diets significantly improved the growth performance as well as the feed conversion ratio of Diplodus sargus [23]. In Totoaba macdonaldi, supplementation of 1.2% taurine in a diet that replaced 60% FM with soybean protein concentrate restored the level of lipid peroxidation and increased the activities of catalase and the key enzymes of intermediate metabolism to normal levels [83]. According to current results, taurine supplementation significantly improved the growth and feed efficiency of Oplegnathus punctatus. Compared with the SBM50 group, the SOD and CAT enzyme activities were higher in the SBM50 + T group, while the MDA level was lower. Importantly, taurine has a wide range of biological effects, including membrane stability, neurotransmitter regulation, and antioxidant effects, especially in osmotic pressure regulation and hormone release [15]. The effects of taurine added to a high-level SBM diet on the growth rate, feed efficiency, and antioxidant stress of Oplegnathus punctatus should be studied further. These findings can significantly change the quantities and types of substitute proteins that could be effectively added to the diets of juvenile Oplegnathus punctatus and could reduce the dependency of the industry on FM supplies.
5. Conclusions
The results of this study showed that the use of soybean meal instead of FM in the diet could reach a level of up to 30% without obvious adverse impacts on Oplegnathus punctatus growth and feed utilization. However, a higher SBM content significantly decreased the growth performance, feed utilization, digestive enzyme activity, as well as the antioxidant capacity of fish, which may primarily be related to the existence of ANFS and the lack of essential amino acid in diets including SBM. Through the broken line regression analysis of WG and FE, it is suggested that replacing 24.07–25.31% FM with SBM is appropriate for Oplegnathus punctatus. Moreover, the addition of 1.2% taurine increased the replacement level to 50% without negatively affecting the performance in Oplegnathus punctatus, but longer-term experiments are needed for further validation. Finally, we suggest that future research should explore the feasibility of replacing fishmeal with different plant proteins, which can help save the production cost of feed.
Author Contributions
Methodology, Formal analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, D.W.; Conceptualization, Methodology, Investigation, Data Curation, H.X.; Conceptualization, Methodology, Investigation, Data Curation, Supervision. Y.Y.; Conceptualization, Methodology, Investigation, Data Curation. W.F.; Conceptualization, Methodology, Supervision, Project administration, Funding acquisition. T.H.; J.W.: Conceptualization, Methodology, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Research and Development Program of Zhejiang Province (2021C04016, 2021C02047).
Institutional Review Board Statement
This study was reviewed and approved by Institutional Animal Care and Use Committee of Zhejiang Ocean University. Approval Code: 2022059.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olsen, R.L.; Hasan, M.R. A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Food Sci. Technol. 2012, 27, 120–128. [Google Scholar] [CrossRef]
- Faggio, C.; Piccione, G.; Marafioti, S.; Arfuso, F.; Fazio, F. Metabolic Response to Monthly Variations of Sparus aurata Reared in Mediterranean On-Shore Tanks. Turk. J. Fish. Aquat. Sci. 2014, 14, 567–574. [Google Scholar] [CrossRef]
- Faggio, C.; Piccione, G.; Marafioti, S.; Arfuso, F.; Fazio, F. Monthly variations of haematological parameters of Sparus aurata and Dicentrarchus labrax reared in Mediterranean land off-shore tanks. Cah. De Biol. Mar. 2014, 55, 437–443. [Google Scholar]
- Fazio, F.; Marafioti, S.; Filiciotto, F.; Buscaino, G.; Faggio, C. Blood Hemogram Profiles of Farmed Onshore and Offshore Gilthead Sea Bream (Sparus aurata) from Sicily, Italy. Turk. J. Fish. Aquat. Sci. 2013, 13, 415–422. [Google Scholar] [CrossRef]
- Daniel, N. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018, 6, 164–179. [Google Scholar]
- Shukla, A.; Kumar, M.; Gupta, G.; Pathak, N.; Mishra, V. A review on replacing fish meal in aqua feeds using plant and animal protein sources. Int. J. Chem. Stud. 2019, 7, 4732–4739. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Fish and Shrimp; National Academies Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Storebakken, T. Soy products as fat and protein sources in fish feeds for intensive aquaculture. Soy Anim. Nutr. 2000, 127–170. [Google Scholar]
- Trushenski, J.T.; Kasper, C.S.; Kohler, C.C. Challenges and opportunities in finfish nutrition. N. Am. J. Aquac. 2006, 68, 122–140. [Google Scholar] [CrossRef]
- Mohammadinafchi, F.; Mohammadiazarm, H.; Yavari, V. Evaluation effect of soybean meal and baker’s yeast on resistance to anoxia stress and blood biochemical parameters of fingerlings (Mesopotamichthys sharpeyi Günther, 1874). Int. J. Biosci. 2014, 5, 215–222. [Google Scholar] [CrossRef]
- Pervin, M.; Jahan, H.; Akter, R.; Omri, A.; Hossain, Z. Appraisal of different levels of soybean meal in diets on growth, digestive enzyme activity, antioxidation, and gut histology of tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2020, 46, 1397–1407. [Google Scholar] [CrossRef]
- Yu, D.; Gong, S.; Yuan, Y.; Lin, Y. Effects of replacing fish meal with soybean meal on growth, body composition and digestive enzyme activities of juvenile Chinese sucker, Myxocyprinus asiaticus. Aquac. Nutr. 2013, 19, 84–90. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Park, G.S.; Takeuchi, T.; Seikai, T.; Yokoyama, M. The effects of dietary taurine on growth and taurine levels in whole body of juvenile Japanese flounder Paralichthys olivaceus. Bull. Jpn. Soc. Sci. Fish. 2001, 67, 238–243. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Han, Y.; Koshio, S.; Jiang, Z.; Ren, T.; Ishikawa, M.; Yokoyama, S.; Gao, J. Interactive effects of dietary taurine and glutamine on growth performance, blood parameters and oxidative status of Japanese flounder Paralichthys olivaceus. Aquaculture 2014, 434, 348–354. [Google Scholar] [CrossRef]
- Hano, T.; Ito, M.; Ito, K.; Kono, K.; Ohkubo, N. Dietary taurine supplementation ameliorates the lethal effect of phenanthrene but not the bioaccumulation in a marine teleost, red sea bream, Pagrus major. Ecotoxicol. Environ. Saf. 2017, 137, 272–280. [Google Scholar] [CrossRef]
- Lim, S.-J.; Oh, D.-H.; Khosravi, S.; Cha, J.-H.; Park, S.-H.; Kim, K.-W.; Lee, K.-J. Taurine is an essential nutrient for juvenile parrot fish Oplegnathus fasciatus. Aquaculture 2013, 414, 274–279. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.Y.; Xie, S.Q.; Zhang, P.-Y.; Yang, Z.-C. Effects of taurine supplementation on growth performance and feed utilization in aquatic animals: A meta-analysis. Aquaculture 2022, 551, 737896. [Google Scholar] [CrossRef]
- Jacobsen, J.G.; Smith, L. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Kaneniwa, M.; Sakaguchi, M. Metabolites of L-[35S] cysteine injected into the peritoneal cavity of rainbow trout. Fish. Sci. 1997, 63, 799–801. [Google Scholar] [CrossRef]
- de Moura, L.B.; Diógenes, A.F.; Campelo, D.A.; de Almeida, F.L.; Pousão-Ferreira, P.M.; Furuya, W.M.; Oliva-Teles, A.; Peres, H. Taurine and methionine supplementation as a nutritional strategy for growth promotion of meagre (Argyrosomus regius) fed high plant protein diets. Aquaculture 2018, 497, 389–395. [Google Scholar] [CrossRef]
- Magalhães, R.; Martins, N.; Lopes, T.; Diáz-Rosales, P.; Pousão-Ferreira, P.; Oliva-Teles, A.; Peres, H. Is dietary taurine required for white seabream (Diplodus sargus) juveniles? Aquaculture 2019, 502, 296–302. [Google Scholar] [CrossRef]
- Al-Feky, S.S.A.; El-Sayed, A.F.M.; Ezzat, A.A. Dietary taurine enhances growth and feed utilization in larval Nile tilapia (Oreochromis niloticus) fed soybean meal-based diets. Aquac. Nutr. 2015, 22, 457–464. [Google Scholar] [CrossRef]
- Tong, S.; Wang, L.; Kalhoro, H.; Volatiana, J.A.; Shao, Q. Effects of supplementing taurine in all-plant protein diets on growth performance, serum parameters, and cholesterol 7α-hydroxylase gene expression in black sea bream, Acanthopagrus schlegelii. J. World Aquac. Soc. 2020, 51, 990–1001. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Teague, A.M.; Barrows, F.T. Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2006, 37, 509–517. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Du, X.; Sun, M.; Wang, X.; Li, W.; Zhai, J.; Liu, J.; Yu, H.; Zhang, Q. Spotted knifejaw (Oplegnathus punctatus) MyD88: Intracellular localization, signal transduction function and immune responses to bacterial infection. Fish Shellfish. Immunol. 2019, 89, 719–726. [Google Scholar] [CrossRef]
- Shimada, Y.; Nokubi, K.; Yamamoto, S.; Murata, O.; Kumai, H. Reproduction between Oplegnathus fasciatus and O. punctatus, and fertility of their interspecies. Fish. Sci. 2009, 75, 521–523. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Zheng, P.; Xu, H.; Su, H.; Tao, H.; Yang, Y. Effect of dietary lipid levels on growth performance, body composition, and feed utilization of juvenile spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2021, 21, 100797. [Google Scholar] [CrossRef]
- Wang, J.; Rongxing, H.; Han, T.; Zheng, P.; Xu, H.; Su, H.; Wang, Y. Dietary protein requirement of juvenile spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2021, 21, 100874. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Hacmanjek, M.; Popovic, N.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef]
- Li, J.S.; Li, J.L.; Wu, T.T. Distribution and properties of amylase and lipase in alimentary tract of tilapia Oreochromis niloticus × O. aureus. J. Fish. Sci. China 2004, 5, 473–477. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zheng, P.Q.; Wang, J.T.; Han, T.; Yang, M.; Li, X.Y.; Wang, C.L. Effect of dietary cholesterol levels on growth performance, body composition and gene expression of juvenile mud crab Scylla paramamosain. Aquac. Res. 2018, 49, 3434–3441. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, S.S.; Ko, G.Y.; Song, J.W.; Oh, D.H.; Kim, J.D.; Lee, K.J. Fish meal replacement by soybean meal in diets for Tiger puffer, Takifugu rubripes. Aquaculture 2011, 313, 165–170. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Wang, H.; Lin, B.; Chen, L.; Li, G.; Wang, Q.; Deng, J. Evaluation of soybean meal as alternative to fish meal in diet for juvenile Asian red-tailed catfish (Hemibagrus wyckioides). Aquac. Nutr. 2019, 25, 1036–1049. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Be, T.T.; Lee, C.M.; Bengtson, D.A. Development of formulated diets for snakehead (Channa striata and Channa micropeltes): Can phytase and taurine supplementation increase use of soybean meal to replace fish meal? Aquaculture 2015, 448, 334–340. [Google Scholar] [CrossRef]
- Ye, J.; Liu, X.; Wang, Z.; Wang, K. Effect of partial fish meal replacement by soybean meal on the growth performance and biochemical indices of juvenile Japanese flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Sharda, P.; Sharma, O.; Saini, V. Replacement of fishmeal with soybean meal in Nile tilapia (Oreochromis niloticus) diet. J. Entomol. Zool. Stud. 2017, 5, 845–849. [Google Scholar]
- El-Sayed, A.F.M. Evaluation of soybean meal, spirulina meal and chicken offal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture 1994, 127, 169–176. [Google Scholar] [CrossRef]
- Jose, B.M.; Chatzifotis, S.; Divanach, P.; Takeuchi, T. Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fish. Sci. 2010, 70, 74–79. [Google Scholar] [CrossRef]
- Hongmanee, P.; Wongmaneeprateep, S.; Boonyoung, S.; Yuangsoi, B. The optimal dietary taurine supplementation in zero fish meal diet of juvenile snakehead fish (Channa striata). Aquaculture 2022, 553, 738052. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, L.-J.; Li, C.; Bureau, D.P. Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of cuneate drum (Nibea miichthioides). Aquaculture 2006, 261, 1307–1313. [Google Scholar] [CrossRef]
- Walsh, S.; Davis, R.; Weldon, A.; Reis, J.; Davis, D.A. Effects of fishmeal replacement, attractants, and taurine removal on juvenile and sub-adult Red Snapper (Lutjanus campechanus). Aquaculture 2021, 544, 737054. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mai, K.; Xu, W.; Deng, J.; Cheng, Z. Comparison of high-protein soybean meal and commercial soybean meal partly replacing fish meal on the activities of digestive enzymes and aminotransferases in juvenile Japanese seabass, Lateolabrax japonicus (Cuvier, 1828). Aquac. Res. 2014, 45, 1051–1060. [Google Scholar] [CrossRef]
- Refstie, S.; Svihus, B.; Shearer, K.D.; Storebakken, T. Nutrient digestibility in Atlantic salmon and broiler chickens related to viscosity and non-starch polysaccharide content in different soyabean products. Anim. Feed. Sci. Technol. 1999, 79, 331–345. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.; Liu, Y.; Lu, Y. Effects of Aspergillus oryzae 3.042 fermented soybean meal on growth performance and plasma biochemical parameters in broilers. Anim. Feed. Sci. Technol. 2007, 134, 235–242. [Google Scholar] [CrossRef]
- Hao, Y.; Zhan, Z.; Guo, P.; Piao, X.; Li, D. Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Arch. Anim. Nutr. 2009, 63, 188–202. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Liu, J.; Yang, P.; Zhang, Y.; Ai, Q.; Xu, W.; Zhang, W.; Mai, K. Dietary soya allergen β-conglycinin induces intestinal inflammatory reactions, serum-specific antibody response and growth reduction in a carnivorous fish species, turbot Scophthalmus maximus L. Aquac. Res. 2017, 48, 4022–4037. [Google Scholar] [CrossRef]
- Bakke-McKellep, A.M.; Penn, M.H.; Salas, P.M.; Refstie, S.; Sperstad, S.; Landsverk, T.; Ringø, E.; Krogdahl, Å. Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2007, 97, 699–713. [Google Scholar] [CrossRef]
- Krogdahl, A.; Bakke-McKellep, A.; Roed, K.; Baeverfjord, G. Feeding Atlantic salmon Salmo salar L. soybean products: Effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquac. Nutr. 2000, 6, 77–84. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Skugor, S.; Grisdale-Helland, B.; Refstie, S.; Afanasyev, S.; Vielma, J.; Krasnov, A. Gene expression responses to restricted feeding and extracted soybean meal in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2011, 17, 505–517. [Google Scholar] [CrossRef]
- Yin, G.; Li, W.; Lin, Q.; Lin, X.; Lin, J.; Zhu, Q.; Jiang, H.; Huang, Z. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish Shellfish. Immunol. 2014, 41, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Aranguren, R.; Secombes, C.J.; Castrillo, J.; Novoa, B.; Figueras, A. Molecular characterisation of sea bream (Sparus aurata) transforming growth factor β1. Fish Shellfish. Immunol. 2003, 14, 405–421. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef]
- Sahlmann, C.; Sutherland, B.J.; Kortner, T.M.; Koop, B.F.; Krogdahl, Å.; Bakke, A.M. Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish. Immunol. 2013, 34, 599–609. [Google Scholar] [CrossRef]
- Alam, M.; Yaniharto, D.; Sumule, O.; Ishikawa, M.; Koshio, S. Assessment of reference dietary amino acid pattern for juvenile red sea bream, Pagrus major. Aquac. Int. 2005, 13, 369–379. [Google Scholar] [CrossRef]
- Whiteman, K.W.; Gatlin, D.M., III. Evaluation of crystalline amino acid test diets including pH adjustment with red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops× Morone saxatilis). Aquaculture 2005, 248, 21–25. [Google Scholar] [CrossRef]
- Harper, A.; Benevenga, N.; Wohlhueter, R. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev. 1970, 50, 428–558. [Google Scholar] [CrossRef]
- Bulbul, M.; Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effect of replacing fishmeal with canola meal on growth and nutrient utilization in kuruma shrimp M arsupenaeus japonicus (Bate). Aquac. Res. 2014, 45, 848–858. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Wang, Y.; Che, J.; Yang, Y. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion of juvenile Pseudobagrus ussuriensis. Aquac. Res. 2016, 47, 3145–3155. [Google Scholar] [CrossRef]
- Shapawi, R.; Ebi, I.; Yong, A. Soybean meal as a source of protein in formulated diets for tiger grouper, Epinephelus fuscoguttatus juvenile. Part I: Effects on growth, survival, feed utilization and body compositions. Agric. Sci. 2013, 4, 317–323. [Google Scholar] [CrossRef]
- Hansen, A.C.; Rosenlund, G.; Karlsen, R.; Koppe, W.; Hemre, G.I. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I—Effects on growth and protein retention. Aquaculture 2007, 272, 599–611. [Google Scholar] [CrossRef]
- Goto, T.; Matsumoto, T.; Takagi, S. Distribution of the hepatic cysteamine dioxygenase activities in fish. Fish. Sci. 2001, 67, 1187–1189. [Google Scholar] [CrossRef]
- Goto, T.; Tiba, K.; Sakurada, Y.; Takagi, S. Determination of hepatic cysteinesulfinate decarboxylase activity in fish by means of OPA-prelabeling and reverse-phase high-performance liquid chromatographic separation. Fish. Sci. 2001, 67, 553–555. [Google Scholar] [CrossRef]
- Lunger, A.N.; McLean, E.; Gaylord, T.; Kuhn, D.; Craig, S. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Refstie, S.; Storebakken, T.; Roem, A.J. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture 1998, 162, 301–312. [Google Scholar] [CrossRef]
- Liu, T.; Han, T.; Wang, J.; Liu, T.; Bian, P.; Wang, Y.; Cai, X. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquac. Rep. 2021, 20, 100756. [Google Scholar] [CrossRef]
- Black, D.; Kirkpatrick, S.; Skinner, E. Lipoprotein lipase and salt-resistant lipase activities in the livers of the rainbow trout and cod. Biochem. Soc. Trans. 1983, 11, 708–795. [Google Scholar] [CrossRef]
- IJlst, L.; Mandel, H.; Oostheim, W.; Ruiter, J.; Gutman, A.; Wanders, R. Molecular basis of hepatic carnitine palmitoyltransferase I deficiency. J. Clin. Investig. 1998, 102, 527–531. [Google Scholar] [CrossRef]
- Hossain, M.; Focken, U.; Becker, K. Galactomannan-rich endosperm of Sesbania (Sesbania aculeata) seeds responsible for retardation of growth and feed utilisation in common carp, Cyprinus carpio L. Aquaculture 2001, 203, 121–132. [Google Scholar] [CrossRef]
- Sampath, W.; Rathnayake, R.; Yang, M.; Zhang, W.; Mai, K. Roles of dietary taurine in fish nutrition. Mar. Life Sci. Technol. 2020, 2, 360–375. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Bhavan, P.S.; Seenivasan, C.; Shanthi, R.; Muralisankar, T. Replacement of fishmeal with Spirulina platensis, Chlorella vulgaris and Azolla pinnata on non-enzymatic and enzymatic antioxidant activities of Macrobrachium rosenbergii. J. Basic Appl. Zool. 2014, 67, 25–33. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, L.; Chi, S.; Chu, W.; Liu, Y.; Hu, Y. Sodium butyrate supplementation in high-soybean meal diets for juvenile rice field eel (Monopterus albus): Effects on growth, immune response and intestinal health. Aquaculture 2020, 520, 734952. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.X.; Pan, Q.; Wu, S.L.; Feng, T.; Ye, H. Effects of replacing soybean meal with faba bean meal on growth, feed utilization and antioxidant status of juvenile grass carp, Ctenopharyngodon idella. Aquac. Nutr. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos-Vargas, I.; López, L.M.; Pérez-Jiménez, A.; Peres, H. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba macdonaldi). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 170, 18–25. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).