Abstract
Container crop production has become increasingly popular, but daily water requirements of those crops from transplanting to marketable or harvestable stages are largely unavailable. To address this concern, daily water consumption of two container-grown fern species, Davallia bullata and Nephrolepis exaltata from initial transplanting to marketable size were studied using a canopy closure model. Daily actual evapotranspiration (ETA) of D. bullata ranged from 4.6 mL to 76.5 mL with an average of 29.0 mL per plant per day. The mean cumulative ETA was 13.2 L during 431 days of production spanning from 8 November 2006 to 4 February 2008. Two crops of N. exaltata were produced. Daily ETA per N. exaltata plant produced in crop 1 varied from 19.0 to 241.2 mL with an average of 69.5 mL, and daily ETA of crop 2 differed from 5.7 to 136.8 mL with a mean of 74.0 mL. Both crops had a cumulative ETA of 9.4 L. Such differences in daily ETA and cumulative ETA between the two fern species raised further concern of irrigation practices in commercial foliage plant production as multiple species are commonly produced in one greenhouse and share the same irrigation schedule. Comparing daily ETA and cumulative ETA values of the ferns with the other studied foliage plants indicated that daily ETA and cumulative ETA are species specific. Therefore, to improve irrigation efficiency, daily ETA and cumulative ETA values of major container-grown plants should be established. Implementing the research-based daily ETA and cumulative ETA in container plant production should reduce irrigation water leaching and runoff and conserving freshwater resources.
1. Introduction
Container-grown plants or container plants refers to those produced from seedlings or liners (tissue-cultured plugs or rooted cuttings) in substrate-filled pots or containers to harvestable stages or marketable sizes [1]. A wide range of plants are produced in containers, largely horticultural crops, such as floriculture, fruit, nursery, and vegetable plants. Substrates or growing media used in container plant production are formulated predominately using lightweight peat, vermiculate, perlite, sand, soil, or composted materials in variable proportions. A distinct characteristic of container substrates is low nutrient- and water-holding capacities [2]; thus, water and chemical fertilizers must be applied frequently to ensure that marketable plants are produced on schedule.
Irrigation of container plants, however, has been under increasing scrutiny, which is due to the following reasons: First, freshwater is an irreplaceable natural resource [3], but container plant production is one of the heaviest consumers. Irrigation of container-grown vegetables and nursery crops uses up to 1500 mm [4] and 2900 mm freshwater [5], respectively. Production of 212,055 hectare of container horticultural crops in open field used 776 billion liters of water annually in the United States (U.S.) [6]. Second, such a heavy consumption of freshwater is not completely based on plant requirements, but rather relies on growers’ intuition or experience [7]. To avoid drought stress, growers often over irrigate plants [8]. Over irrigation has been documented to cause 25% to 90% of applied water to be either leached or runoff [9,10]. The production of poinsettia in a highly water-efficient greenhouse used 121 L of water over a 16-week production period [11]. Third, leachate and/or runoff water contains nutrients, largely nitrogen (N) and phosphorus (P) [12,13]. Both N and P movement in waterways may result in the contamination of ground and/or surface water, known as non-point source water pollution [7,14]. A survey of 11 nurseries in southern California suggested that the concentrations of NO3-N in runoff were greater than 10 mg/L in a majority of nurseries [15]. NO3-N in irrigation runoff in a southern Florida foliage nursery varied from 41 to 386 mg/L depending on the methods used for irrigation [16].
Florida is second to California in the production of nursery and greenhouse crops and ranks first in producing ornamental foliage plants [17]. The national wholesale value of foliage plants was $653.08 million in 2018, of which Florida accounted for 63.4% [18]. Plants from over 100 genera are produced as foliage plants [2,19]. However, there has been little information on daily water requirement of those cultivated foliage plants until three recent publications where daily water use of Chamaedorea elegans and Asplenium nidus [20], Guzmania and Vriesea [21], and Calathea and Stromanthe [22] were quantified, which provide important information on revising irrigation practices for foliage plant production.
The quantification of the above six foliage plant species was primarily based on Beeson’s canopy closure model [23] used for estimating actual evapotranspiration (ETA) of container-grown woody ornamental plants. ETA was determined by an autonomous weighing lysimeter system [9]. Reference evapotranspiration (ETO) was calculated using the Campbell Scientific version of the Penmen–Monteith equation [24]. Projected canopy area (PCA) was recorded during the course of plant growth. Based on the relationships of ETA, ETO, and PCA of a given species, a plant factor (PF) [25] was calculated. PF is also known as Water Need Index (WNI) [20,21,22] or crop coefficient (Kc) [26]. Kc is dimensionless. It is the ratio between the ETA of the plant studied to the reference evapotranspiration (ETO) and is a function of the fraction of crop ground cover and crop height [26]. Kc values have been recommended for a wide range of agronomic crops [26], but its values for horticultural crops are limited [25]. PF here is a function of the degree of canopy closure among groups of plants that relate individual plant ETA to plant size, canopy ventilation, and incoming solar radiation [27]. The model was successfully used to estimating daily ETA of Ligustrum japonicum [23], Viburnum odoratissimum [28,29], Rhaphiolepis indica [30], and the six foliage plant species mentioned above. Irrigating container plants based on daily ETA can significantly reduce runoff volume and nutrient load in a nursery setting without affecting plant growth [31,32].
Ferns, a group of primitive plants in the division Pteridophyta, are important ornamental foliage plants [2]. Among them, the Boston fern (Nephrolepis exaltata) is the most popular species as it was the first and almost only plant grown from 1913 to the early 1930s in Florida [33] and now more than 50 cultivars are in production [19]. To gain information on water use of foliage plants, 22 foliage plants, including the Boston fern, along with eight bedding plants were evaluated through overhead irrigation or an ebb-and-flow system (a subirrigation with no irrigation water leaching and runoff) using ground water and captured rainwater and irrigation runoff from a landscape production [12]. A total of 1080 container plants were produced over two and half years. On average, each plant used 10.2 L of water when produced through the subirrigation system, suggesting regardless of species, a foliage plant produced in a 15 cm container generally requires about 10 L of irrigation water from transplanting to attaining a marketable size. However, when produced through overhead irrigation, 35.5 L water was used per plant. Moreover, overhead irrigation resulted in half of applied N leached [14]. Subirrigation could be an ideal irrigation method for minimizing water and nutrient loss, but it has been limited due to the cost and potential disease problems. Overhead irrigation still occurs in foliage plant production, but most foliage plant facilities have switched to drip irrigation.
Here we aimed to determine ETA of the Boston fern along with a rabbit-foot fern (Davallia bullata) from liners to marketable plants in a shaded greenhouse and also to develop models to predict daily ETA values using the canopy closure model [23]. The quantification of daily ETA and cumulative ETA of the two fern species could help growers improve irrigation efficiency in container-fern production through drip irrigation.
2. Materials and Methods
2.1. Experimental Setup, Plant Materials, and Their Growth
We conducted this study in a greenhouse with supplemental shading at the University of Florida’s Mid-Florida Research and Education Center in Apopka. A Florida Automated Weather Network (FAWN; https://fawn.ifas.ufl.edu/) station located about 46 m east of the greenhouse, logged incoming shortwave radiation Rn, air temperatures (Ta, 0.6, 1.8, and 9.1 m heights), soil temperature, wet bulb temperature, relative humidity (RH), and rainfall at 1.8 m, and wind speed (WS) at 10 m every 15 min and also daily evapotranspiration outside the greenhouse. The datalogger calculated hourly and daily local reference evapotranspiration (ETO) from Rn, Ta, RH, and WS inputs as per the ASCE (American Society of Civil Engineers) Penman–Monteith (PM) equation [34].
Tissue cultured plantlets (from 72-cell plug trays) of the Boston fern and Davallia bullata were planted into 15 cm cross section containers with a soilless substrate of 60% Canadian peat, 20% perlite, and 20% vermiculite by volume. The Boston fern study was replicated twice. The first was transplanted on 27 December 2005 and harvested 7 May 2006, and the second planted 22 May 2006 and harvested on 25 September 2006. Each plant was top dressed with 5 g of a controlled released fertilizer (CRF), Osmocote Pro 19–5–9, 8–9 month, Scotts Co., Marysville, Ohio, USA, per container three weeks after potting. Because of its characteristically slow growth habit, the rabbit foot fern was only grown once, transplanted on 8 November 2006 and harvested on 4 February 2008. These plants were fertilized twice with the same CRF three and 30 weeks after transplanting. All study plants were positioned on raised benches in the shaded greenhouse where the maximum photosynthetic active radiation (PAR) was 200 µmol/m2/s. The experimental layout was a completely randomized block design with four replicates (blocks). Each block had 15 plants per species which were spaced 30 cm apart in three rows, with five plants along the bench long side. Center plants were placed in a mini suspension weighing lysimeter [9], and four plants around the lysimeter plant were considered as the interior border plants for subsequent repeated measurements of canopy height and widths. Inside the shaded greenhouse, there was an automated Weatherhawk weather station (Campbell Scientific, Inc., Logan, UT, USA), which was positioned the center at 50 cm above the lysimeter tripods.
The miniature weighing lysimeter system comprised of a CR10X data logger wired to an SDM-AM16-32 multiplexer and SDM-CD16AC relay control module (Campbell Scientific Inc., Logan, UT, USA) attached to control/data collection board. The datalogger was wired to a load cell (SSM-50-AJ, Interface Inc., Scottsdale, AZ, USA) attached to the top underside of a miniature tripod 60 cm tall and 2.5 cm above the wire table. Study plants were suspended from the load cell with a small-linked chain. The load cells were pre-calibrated with a seven-point curve using standardized weights. The data logger program recorded weight change in each lysimeter every half hour. At midnight, the program calculated the previous day’s actual water use ETA for each lysimeter as we observed no transpiration from either species from midnight to 0500 h.
Plants were irrigated with a drip system between 0800 h and 0900 h, which allowed a 10% leachate fraction. Water was delivered through pressure compensated inline drip tubing (Netafilm USA, Fresno, CA, USA) located at 30.5 cm intervals along the tubing. Four emitters were cut along the length of the tubing, which was rolled to form two loops joined by a T-barb. The loop just fit snugly inside the rim of each container. Using equal lengths of 6 mm tubing, the loops were connected to 19 mm polyethylene tubing. The Christensen’s Coefficients of Uniformity were tested with 15 loops of randomly selected plants from each bench. The coefficients ranged from 0.93 to 0.96 with a mean of 0.94. Typical irrigation rates on each bench were 187 mL/min.
2.2. Data Collection
Pairing with the outside weather station, the inside Weatherhawk station recorded greenhouse Rn, Ta, and RH and calculated ETO using the same ASCE PM equation [34]. This interior weather station allowed us to evaluate the value of using the PM equation estimate water demand inside a controlled environment with the understanding these atmospheric inputs are not consistent with boundary layer equilibrium assumptions fundamental to the PM equation.
Beginning a week after transplanting, every three weeks for all studies we measured canopy average height, widest width, width perpendicular to the widest width of the lysimeter study plants, as well as the adjacent four border plants per replication. Multiplication of the two widths provided the two-dimensional PCA. When PCA was multiplied by the average height, i.e., widest width × width perpendicular to the widest width × height, canopy volume or growth index (GI) was estimated [35], which assumed a rectangular form for the three-dimensional canopy. For each study plant we collected total number of leaves that were then run through a LI-3100 Area Meter (LI-Cor, Lincoln, NE, USA) to derive total leaf area per plant. Plants were harvested by separating shoots and roots. Roots were washed to remove substrate and blotted with a paper towel. After recording shoot and root fresh weights, they were dried at 80 °C for 48 h, and dry weights measured. Plant water use efficiency was determined by total dry weight (g) divided by the volume of actual water used, i.e., the (ETA) of the plant [36].
2.3. Modelling of Plant Water Use
Study plant water use was calculated from measured ETA, ETO, and PCA. Briefly, canopy area and percent canopy closure (%CC) were determined based on container size and spacing, i.e., for each measurement, the %CC was calculated by adding half the PCA of each of the four border plants to the PCA of the lysimeter plant and dividing the sum by allocated bench space for each plant (929 cm2 in this study). Because plants were not respaced, canopies overlapped from plant growth over time. As such, overlapping sometimes resulted %CC greater than 100%. Using %CC, we calculated a PF that relates plant water use (corrected for %CC) to ETO, in this case greenhouse ETO (ETOgreenhouse). A PF should be independent from container size and should have no need to apply Fourier curve transformations to account for changes in growth variation due to the season or ETA. For each lysimeter study plant, ETA (cm3) was recorded for seven days and converted to a depth by dividing with its average PCA (cm2). ETA (cm) was normalized by dividing with its corresponding ETO (cm) each day, and then averaged over the seven days to calculate a PF using the equation of (PF = (ETA ÷ PCA) ÷ ETOgreenhouse) for each lysimeter plant at each of the measurement dates. Calculated PF values derived from four lysimeter replicates for each date were plotted against their corresponding %CC values. The plot was fitted to a three-parameter exponential decay curve using SigmaPlot (Version10; SPSS Inc., Chicago, IL, USA). An equation for the non-linear line was derived using a three-level inverse polynomial equation (Version10; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Reference Evapotranspiration
Outdoor ETO values from the FAWN weather station from December 2005 to February 2008 were up to 5.4 mm/day in April due to the more sunlight and May, then declined the rest of the period with the onset of clouds summer rains, and then shorter days, with a mean of 2.5 mm/day. The ETO in the shaded greenhouse was approximately 12% of that calculated from the outdoor FAWN during the same period due to different environmental conditions. Evaporative coolers regulated RH, wind and air temperature within a narrower range than outdoors; yet applying the Penman–Monteith equation in a controlled greenhouse environment can be a useful proxy of plant water because useful solar radiation. In humid conditions like a greenhouse incoming solar radiation is the dominant driver of evapotranspiration even though in this study it was about 30% of full sun outdoors.
3.2. Plant Growth
Canopy widths and height of D. bullata during 431 days of growth increased polynomially (data not shown). Growth index also increased in a polynomial manner (Figure 1A). D. bullata produced 233 leaves with a total leaf area of 4562 cm2 at the time of harvest. Shoot and root fresh weights were 162.7 and 13.0 g; and shoot and root dry weights were 33.1 and 4.5 g, respectively (Table 1).
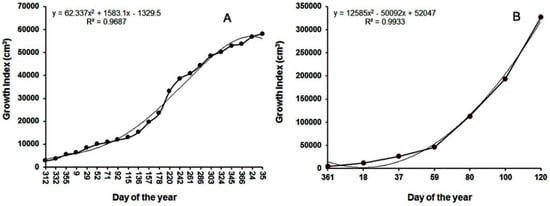
Figure 1.
Growth indices (cm3) of Davallia bullata (A) and Nephrolipis exaltata (B, crop 2) grown in 15 cm plastic containers from liners to marketable sizes. The equations are the best fit line (thin line).

Table 1.
Plant growth parameters measured at harvest by species. They were harvested once their common commercial canopy sizes were attainedz.
Canopy heights and widths of two crops of N. exaltata increased linearly over the respective production time (data not shown), but growth index increased in a polynomial fashion (Figure 1B, data for crop 1 were similar and not shown). At harvest, the mean numbers of leaves of crops 1 and 2 were 110.7 and 127.4, respectively. The corresponding total leaf areas were 7739.2 and 10,344.3 cm2, shoot fresh weights were 119.6 and 173.7 g, and root fresh weights were 31.0 and 47.2 g. Shoot dry weights of crop 1 was 18.8 g and crop 2 was 25.8 g, and root dry weight were 3.4 and 4.8 g, respectively (Table 1).
3.3. Plant ETA
Daily ETA of D. bullata ranged from 4.6 mL to 76.5 mL (Figure 2A) with an overall mean of 29.0 mL per day per plant. Initially, daily average ETA was about 20 mL, decreased to only 4.6 mL after a week of transplanting, and then remained in a range from 5.0 mL to 23 mL for about 100 days from November 2006 to early February 2007. As the weather became warmer, daily ETA increased to the highest (76.5 mL) in May 2007, sustained in an average of 40 mL till October, then declined from October to December 2007. There was a large fluctuation in daily ETA from January to early February 2008 until harvest. Mean cumulative ETA was 13.2 L over 431 days of production period spanning from 8 November 2006 to 4 February 2008.
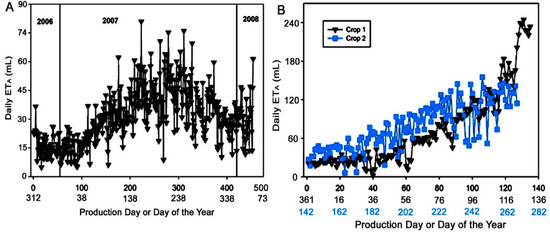
Figure 2.
Mean daily ETA of Davallia bullata (A) and Nephrolipis exaltata (B), this species was produced twice, (i.e., two crops) grown in 15 cm plastic containers during production from liners to marketable sizes. Each point is the mean of four plant replicates.
Daily ETA for crop 1 of the Boston fern produced from late December 2005 to early May 2006 varied from 8.9 to 241.2 mL with an average of 69.5 mL (Figure 2B). The mean cumulative ETA value was 9.4 L per plant over the duration of the entire production period. Average daily ETA was initially about 20 mL, fluctuated in February from lowest of 8.9 mL to 52.0 mL, and then increased thereafter as high as 241.2 mL in May 2006.
Crop 2 of N. exaltata was produced right after the harvest of crop 1 from 22 May 2006 to 25 September 2006. Daily ETA was initially about 40 mL, increased to 136.8 in July. A characteristic of the crop 2, was that the daily ETA values fluctuated highly from 5.7 mL to 58.0 mL in June and from 45.7 to 136.8 mL from July to August. The mean daily ETA of crop 2 was 74.0 mL. Interestingly, the mean cumulative ETA value was also 9.4 L per plant during the four-month growth period.
3.4. Data Analysis and Modeling
The %CC model was used for modelling PF [23]. The best fit models for D. bullata and N. exaltata, are presented in Table 2. The r2 values were 0.80 for D. bullata and 0.86 for two crops of N. exaltata. While the %CC increased from 20% to 80%, the PF of D. bullata rapidly decreased and then slowly declined to below 1 (Figure 3A). For the crop of N. exaltata, the PF quickly decreased to 0.75 when the %CC reached 100%, and then slowly decreased around 0.5 (Figure 3B).

Table 2.
Best fit models for predicting daily ETA values for D. bullata and N. exaltata plants produced from liners to marketable sizes.
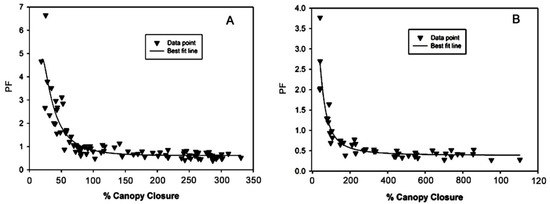
Figure 3.
Inverse polynomial relationship between % Canopy Closure (%CC) and plant factor (PF) or the water need index (WNI) for Davallia bullata (A) and Nephrolipis exaltata (B). Data points are four plant replicates, and equation for the best fit line presents in Table 2.
4. Discussion
This study for the first time documented the daily ETA and cumulative ETA of two popular fern species. The Boston fern is a fast-growing plant with a large canopy, while the rabbit foot fern represents an epiphytic type of fern, which have thick and wax leaves with slow-growth style. Commercially, both are produced in a greenhouse with supplementary shading from tissue-cultured liners to marketable sizes. Daily ETA values of N. exaltata crop 1 varied from 8.9 to 241.2 mL during a 133-day production period and crop 2 differed from 5.7 mL to 136.8 mL during 123 days of production. Thus, one year can produce at least two crops of N. exaltata. The mean daily ETA values of crop 1 and 2 were 69.5 mL and 74.0 mL, respectively. Interestingly, the mean cumulative ETA for both crops was 9.4 L. Additionally, the two crops exhibited a similar water requirement pattern, i.e., initially low in water requirement and then gradually increased with plant growth regardless of seasonal variation (Figure 2B). This pattern is different from D. bullata, Guzmania and Vriesea [21], Asplenium nidus [20], and Calathea [22]. Due to the difference in production time, Boston fern crop 1 took an extra 10 days to reach marketable size with a water use efficiency of 2.4 g/L, lower than crop 2 (3.3 g/L). On the other hand, the daily water requirements of D. bullata ranged from 4.6 mL to 76.5 mL (Figure 2A) with a mean daily ETA of 29.0 mL, substantially lower than those of the Boston fern. The mean cumulative ETA of D. bullata was 13.17 L, which was higher than the Boston fern, attributable to prolonged production time (431 days). The water requirement of D. bullata largely followed the %CC and seasonal variation. Plants were transplanted on 8 November 2006, daily ETA values were low, and plant growth was slow (Figure 1A and Figure 2A). Starting from early March, daily ETA started increasing, reaching the highest level in summer, and decreased from October 2007 (Figure 2A). The pattern of D. bullata in water requirement is similar to both Guzmania and Vriesea [21].
The differences in daily ETA and accumulated ETA between these two fern species raises a serious concern about irrigation management in commercial foliage plant production. It is common that several species are produced in a greenhouse where irrigation schedules are set the same [2]. Even in some specialized nurseries, i.e., producing one type of crop only, for example fern growers producing different fern species in the same greenhouse, irrigation time and volume are usually set based on the species with the greatest water demand. As a result, a slow growing species, such as the rabbit foot fern would be heavily overirrigated, and a large volume of irrigation water could be leached out of the containers. To further analyze this concern, we summarize mean daily ETA and cumulative ETA and some related information from our previous publications in Table 3 and hope to identify some commonalities among the studied foliage plant species. Plants requiring a prolonged production time, such as Guzmania (665 days) have a higher cumulative ETA, but Vriesea requires much shorter production time (224 days) than Guzmania, Vriesea has a rather high cumulative ETA. Neither dry weight accumulation nor total leaf area is particularly associated with the cumulative ETA. The mean daily ETA values among the eight species range from 21.6 mL to 74.0 mL, cumulative ETA varies from 4.8 L to 16.7 L, and water use efficiency differs from 0.7 g/L to 3.3 g/L. No commonalities have been identified among the studied foliage plants. Thus, it is apparent that a plant’s requirement for water relies on the genetic makeup and physiological expression of a given species, and different species have different overall daily ETA and cumulative ETA values. This conclusion could also be true to other container plants. Yearly ETA of Quercus virginiana, Chilopsis linearis, and Prosopis alba grown in 3.8 L containers irrigated with a leaching fraction of 0.25 were 442.2, 781.3, and 540.4 L, respectively [37]. Cumulative ETA for producing R. indica was 38% less than that of V. odoratissimum when both were grown in 11.4 L containers [29]. Average daily ETA of five bamboo species varied from 4 mm to 7 mm [38]. Thus, irrigating multiple plant species in a greenhouse or a production facility with the same volume and frequency could result in leaching and/or runoff of irrigation water and potentially non-point source pollution.

Table 3.
Summary of leaf area, dry weight, day of plant growth, mean daily ETA, cumulative ETA, and water use efficiency of eight foliage plant species produced from liners to marketable sizes in the same location.
In fact, conserving freshwater through improving irrigation efficiency has become increasingly urgent to container plant growers. Regulations or laws have been issued in several states including Florida in restriction of water consumption [39,40]. Relying on personal experience in scheduling irrigation will no longer be enough to meet current state regulations. Various irrigation methods have been adopted for improving irrigation, including time clock based, climate monitoring, soil or substrate monitoring, and phyto-sensing [8]. However, a consensus is that the best irrigation practice is to water plants based on their requirements [8,21,22,40]. Thus, daily ETA of each important species of container plants should be established. The canopy closure model probably represents an easy and convenient way of quantifying daily ETA of plants of interest. This method has been used to quantify daily ETA and cumulative ETA of Acer rubrum from liner to 8 m tall trees over 4.75 years [41], Ilex x ‘Nellie R. Stevens’ from liners to 4 m tall trees for 5.75 years [42], and Quercus virginiana to 7.2 m tall trees in five years [43] as well as this and previous studied container-grown greenhouse plants [20,21,22]. Thus, ETA values for the other container plants should also be able to quantify using this or other reliable methods. The availability of daily ETA and cumulative ETA would allow growers to group plants with similar ETA in the same greenhouse and irrigate them with the same volume and frequency, which should reduce leaching and runoff, minimize N and P loss, improve container plant quality, and also conserve fresh water resources.
5. Conclusions
This study established daily ETA, cumulative ETA, and PF or WNI for D. bullata and N. exaltata. Results suggest the two species markedly differ in mean daily ETA and cumulative ETA as well as production time. D. bullata is a slow-growing plant requiring prolonged production time and a low amount of daily water. However, N. exaltata is a fast-growing plant requiring short production time but a high volume of water. The differences lead to analyzing daily ETA and cumulative ETA of other studied foliage plant species, but no commonalities were identified in water requirements. Our results suggest daily ETA and cumulative ETA is species specific. Traditional irrigation practices based on container size or the same group of plants, not plants’ requirements, could be leading causes of overirrigation. As the best practice is to irrigate plants based on their need, daily ETA, cumulative ETA of major container plants should be established based on %CC and changes in water use with solar radiation over a production cycle, and irrigation methods based on daily ETA should be developed. Application of the developed irrigation practices based on daily ETA will transform conventional container plant production, while conserving freshwater and protecting the environment.
Author Contributions
Conceptualization, R.C.B.J. and J.C.; methodology, R.C.B.J.; resources, R.C.B.J. and J.C.; investigation, R.C.B.J. and J.C.; formal analysis, R.C.B.J.; writing of original draft, J.C.; editing and revision, R.C.B.J., J.C., and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported in part by the Southwest Florida Water Management District, FL, USA.
Acknowledgments
The authors would like to thank Russell Caldwell for assistance in completion of the experiments and Terri Mellich for revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Wei, X. Controlled-Released Fertilizers as A Means to Reduce Nitrogen Leaching and Runoff in Container-Grown Plant Production. In Nitrogen in Agriculture-Updates; Khan, A., Fahad, S., Eds.; InTechOpen Limited: London, UK, 2018; pp. 33–52. [Google Scholar]
- Chen, J.; McConnell, D.B.; Norman, D.L.; Henny, R.J. The foliage plant industry. Hort. Rev. 2005, 31, 47–112. [Google Scholar]
- Berger, M.; Finkbeiner, M. Water footprinting: How to address water use in life cycle assessment? Sustainability 2010, 2, 919–944. [Google Scholar] [CrossRef]
- Bacci, L.; Battista, P.; Cardarelli, M.; Carmassi, G.; Rouphael, Y.; Incrocci, L.; Malorgio, F.; Pardossi, A.; Rapi, B.; Colla, G. Modelling Evapotranspiration of Container Crops for Irrigation Scheduling. In Evapotranspiration—From Measurements to Agricultural and Environmental Applications; Gerosa, G., Ed.; IntechOpen Limited: London, UK, 2011; pp. 263–282. [Google Scholar]
- Fulcher, F.A.; Buxton, J.W.; Geneve, R.L. Developing a physiological-based, on-demand irrigation system for container production. Sci. Hortic. 2012, 138, 221–226. [Google Scholar] [CrossRef]
- Million, J.B.; Yeager, T.H. Periodic versus real-time adjustment of a leaching fraction-based microirrigation schedule for container-grower plants. HortScience 2020, 55, 83–88. [Google Scholar] [CrossRef]
- Mack, R.; Owen, J.S.; Niemiera, A.X.; Latimer, J. Virginia nursery and greenhouse grower survey of best management practices. HortTechnology 2017, 27, 386–392. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Katsoulas, N.; Kittas, C. Irrigation of greenhouse crops. Horticulturae 2019, 5, 7. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Suspension lysimeter systems for quantifying water use and modulating water stress for crops grown in organic substrates. Agric. Water Manag. 2011, 98, 967–976. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Ristvey, A.G.; Lea-Cox, J.D. Water and nutrient management in the production of container-grown ornamentals. Hort. Rev. 2011, 38, 253. [Google Scholar]
- Ingram, D.L.; Hall, C.R.; Knight, J. Modeling container-grown Euphorbia pulcherrima production system components: Impacts on carbon footprint and variable costs using a life cycle assessment. HortScience 2019, 54, 262–266. [Google Scholar] [CrossRef]
- Chen, J.; Beeson, R.C., Jr.; Yeager, T.H.; Stamps, R.H.; Felter, L.A. Evaluation of captured rainwater and irrigation runoff for greenhouse foliage and bedding plant production. HortScience 2003, 38, 228–233. [Google Scholar] [CrossRef]
- Jahromi, N.B.; Fulcher, A.; Walker, F.; Altland, J. Optimizing substrate available water and coir amendment rate in pine bark substrate. Water 2020, 12, 362. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Caldwell, R.D. Best management practices for minimizing nitrate leaching from container-grown nurseries. Sci. World J. 2001, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mangiafico, S.S.; Gan, J.; Wu, L.; Lu, J.; Newman, J.P.; Faber, B.; Merhaut, D.J.; Evans, R. Detention and Recycling Basins for Managing Nutrient and Pesticide Runoff from Nurseries. HortScience 2008, 43, 393–398. [Google Scholar] [CrossRef]
- Wilson, C.; Albano, J.; Mozdzen, M.; Riiska, C. Irrigation water and nitrate-nitrogen loss characterization in Southern Florida nurseries: Cumulative volumes, runoff rates, nitrate-nitrogen concentrations and loadings, and implications for management. HortTechnology 2010, 20, 325–330. [Google Scholar] [CrossRef]
- USDA, USDA National Agricultural Statistics Service. Nursery Crops 2006 Summary; USDA: Washington, DC, USA, 2007.
- USDA, USDA National Agricultural Statistics Service. Floriculture Crop 2018 Summary; USDA: Washington, DC, USA, 2019.
- Henny, R.J.; Chen, J. Cultivar development of ornamental foliage plants. Plant Breed. Rev. 2003, 23, 245–290. [Google Scholar]
- Chen, J.; Beeson, R.C., Jr. Actual evapotranspiration of Asplenium nidus and Chamaedorea elegans during production from liners to marketable plants. Acta Hortic. 2013, 990, 339–344. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr.; Chen, J. Quantification of Daily Water requirements of container grown Calathea and Stromanthe produced in a shaded greenhouse. Water 2018, 10, 1194. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr.; Chen, J. Daily evapotranspiration of Guzmania ‘Irene’ and Vriesea ‘Carly’ bromeliads produced in a shaded greenhouse. HortScience 2018, 53, 1814–1819. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Modeling actual evapotranspiration of Ligustrum japonicum from rooted cuttings to commercially marketable plants in 12-liter black polyethylene containers. Acta Hortic. 2004, 664, 71–77. [Google Scholar] [CrossRef]
- Campbell Scientific. Application Note 4-D; Campbell Scientific Ltd.: Logan, UT, USA, 1991. [Google Scholar]
- Kjelgren, R.; Beeson, R.C., Jr.; Pittenger, D.R.; Montague, D.T. Simplified landscape irrigation demand estimation: Slide rules. Appl. Eng. Agric. 2016, 32, 363–378. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements. Irrigation and Drainage Paper No. 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Beeson, R.C., Jr. Modeling irrigation requirements for landscape ornamentals. HortTechnology 2005, 15, 18–22. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Response of evapotranspiration of Viburnum odoratissimum to canopy closure and the implications for water conservation during production and in landscapes. HortScience 2010, 45, 359–364. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Modeling actual evapotranspiration of Viburnum odoratissimum during production from rooted cuttings to market size plants in 11.4-L containers. HortScience 2010, 45, 1260–1264. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Development of a simple reference evapotranspiration model for irrigation of woody ornamentals. HortScience 2012, 47, 264–268. [Google Scholar] [CrossRef]
- Hagen, E.; Mambuthiri, S.; Fulcher, A.; Geneve, R. Comparing substrate moisture-based daily water use and on-demand irrigation regimes for oakleaf hydrangea grown in two container sizes. Sci. Hortic. 2014, 179, 132–139. [Google Scholar] [CrossRef]
- Pershey, N.A.; Cregg, B.N.; Andresen, J.A.; Fernandez, R.T. Irrigating based on daily water use reduces nursery runoff volume and nutrient load without reducing growth of four conifers. HortScience 2015, 50, 1553–1561. [Google Scholar] [CrossRef]
- Chen, J.; Henny, R.J.; McConnell, D.B. Development of New Foliage Plant Cultivars. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 466–472. [Google Scholar]
- Allen, R.G.; Walter, I.; Elliot, R.; Howell, T. The ASCE Standardized Reference Evapotranspiration Equation; American Society of Civil Engineers: Reston, VA, USA, 2005. [Google Scholar]
- Henny, R.J.; Holm, J.R.; Chen, J.; Scheiber, M. In vitro induction of tetraploids in Dieffenbachia x ‘Star Bright M-1’ by colchicine. HortScience 2009, 44, 646–650. [Google Scholar] [CrossRef]
- Stanhill, G. Water use efficiency. Adv. Agron. 1987, 39, 53–85. [Google Scholar]
- Devitt, D.A.; Morris, R.L.; Neuman, D.S. Evapotranspiration and growth response of three woody ornamental species placed under varying irrigation regimes. J. Am. Soc. Hortic. Sci. 1994, 119, 452–457. [Google Scholar] [CrossRef]
- Piouceau, J.; Panfili, F.; Bois, G.; Anastase, M.; Dufosse, L.; Arfi, V. Actual evapotranspiration and crop coefficients for five species of three-year-old bamboo plants under a tropical climate. Agric. Water Manag. 2014, 137, 15–22. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr.; Arnold, M.A.; Bilderback, T.E.; Bolusky, B.; Chandler, S.; Gramling, H.M.; LeaCox, J.D.; Harris, J.R.; Klinger, P.J.; Mathers, H.M.; et al. Strategic vision of container nursery irrigation in the next ten years. J. Environ. Hort. 2004, 22, 113–115. [Google Scholar]
- Warsaw, A.L.; Fernandez, R.T.; Cregg, B.M.; Andresen, J.A. Water conservation, growth, and water use efficiency of container grown woody ornamentals irrigated based on daily water use. HortScience 2009, 44, 1308–1318. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Evapotranspiration and above ground biomass of Acer rubrum from liners to 8 m tall trees. Am. J. Plant Sci. 2016, 7, 2440–2456. [Google Scholar] [CrossRef][Green Version]
- Beeson, R.C., Jr.; Duong, H.T.T.; Kjelgren, R.J. Developing a simple water use model for Ilex x ‘Nellie R Stevens’ from liners to four-meter tall trees. J. Agric. Stud. 2017, 5, 83–96. [Google Scholar]
- Beeson, R.C., Jr.; Duong, H.T.T.; Kjelgren, R.J. Water use of juvenile live oak (Quercus virginiana) trees over five years in a humid climate. Open J. For. 2018, 8, 1–14. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).