Abstract
Pristine nickel aluminate and the one decorated with graphene quantum dots were prepared via a cost-effective co-precipitation method. Both were fully characterized by thermogravimetry (TGA), differential scanning calorimetry (DSC), attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy, transmission electron microscopy (TEM), and UV–Vis techniques. The photocatalytic activity of nickel aluminate under simulated solar light irradiation was demonstrated towards potential pollutants, including a series of dyes (rhodamine B, quinoline yellow, eriochrome black T, methylene blue), toxic phenol and fungicide (thiram). Further profound enhancement of the photocatalytic activity of nickel aluminate was achieved after its decoration with graphene quantum dots. The mechanism of the photocatalytic degradation in the presence of the NiAl2O4/graphene quantum dots (GQDs) composite was investigated; hydroxyl radicals were found to play the leading role. This work offers new insight into the application of the conjunction of the inorganic spinel and the carbon nanostructure (i.e., GQDs), but also provides a simple and highly efficient route for potential water remediation from common pollutants, including dyes and colorless harmful substances.
1. Introduction
Nanocrystalline spinel aluminates with the general formula of MAl2O4 (M = Ni, Zn, Mn, Co, Mg, etc.) attract research interest due to their versatile properties. Aluminates have high thermal stability, mechanical resistance, hydrophobicity and low surface acidity. Nickel aluminates are one of the most important aluminate materials, and have been studied for their many applications, including electrochemical sensing [1], pigments [2], catalysts [3,4,5,6,7], photocatalysts [8,9,10,11,12], magnetic [13] and refractory materials [14]. NiAl2O4 has also attracted attention as a hydrogen storage material [15,16], oxygen carrier in combustion loop reactors [17] and as a component of supercapacitor electrode materials [18]. Very recently, attention has been focused on its photocatalytic performance [10,11,12]. First reports described the utilization of nickel-aluminum layered hydroxides for carbon dioxide conversion [19,20] and for dye degradation [21,22]. However, to the best of our knowledge the photocatalytic activity of the nickel aluminate of the spinel structure was demonstrated for the first time in 2015 by A. Sobhani-Nasab et al. [8]. The catalyst was synthesized via sol-gel method, to then be applied for methyl orange degradation under visible light irradiation. M. Rahimi-Nasrabadi et al. [9] performed analogous experiments using ultraviolet light. The photocatalytic activity of the nickel aluminate spinel against a series of dyes (i.e., rhodamine B (RhB), methylene blue (MB), methyl orange (MO), methyl red) was demonstrated by T. Tangcharoen et al. [10]. In the past year, the enhanced photocatalytic performance of NiAl2O4 was achieved by its substitution with copper, nickel and magnesium ions [11,12]. These photocatalytic tests were run against MB, MO and Congo red, both using ultraviolet and solar light irradiation.
In our studies we decided to utilize carbon nanostructures, namely graphene quantum dots (GQDs), to decorate spinel nickel aluminate in order to achieve enhanced photocatalytic performance of the composite. Carbon nanoforms, including fullerenes [23,24,25,26], carbon nanotubes [27], graphite [28], graphene [29], graphene oxide [30] and graphene quantum dots [31], have already been used for this purpose. GQDs are nanoscale (diameter < 100 nm) fragments of graphene, revealing size-dependent luminescence. Due to their extended π-electron system, GQDs broaden the spectral range that can be harvested by the composite. Additionally, thanks to discrete electronic levels, they allow for hot electron injection and efficient charge separation. Moreover, GQDs can be prepared from cheap and accessible precursors [31,32]. Therefore, GQDs have already been used to form composites with inorganic semiconductors, which exhibited photocatalytic activity [33,34]. Nevertheless, to the best of our knowledge nickel and aluminum oxides or mixed Ni/Al oxides have not been examined in that context.
On the other hand, the constantly rising production of municipal and industrial wastes and a constant struggle with ineffective methods of water remediation requires us to seek new efficient and economically attractive procedures of wastewater treatment. Heterogenous photocatalysis utilizing solid catalysts that are active under solar light irradiation provides hope that it is possible to meet all mentioned requirements. Therefore, we decided to combine the attractive properties of spinel nickel aluminate and graphene quantum dots by preparing a NiAl2O4/GQDs composite for superior photocatalytic performance. Our report aimed to achieve several goals: (i) reveal the universality of nickel aluminate as a photocatalyst; reveal its capability to degrade pollutants, forming both colorful and colorless aqueous solutions; (ii) enhance its photocatalytic activity by decorating it with graphene quantum dots (GQDs); (iii) assess photocatalyst activity under solar light irradiation; (iv) examine the photodegradation mechanism in the presence of the NiAl2O4/GQDs composite.
2. Materials and Methods
2.1. Materials
Ammonium oxalate, aluminum nitrate nonahydrate, dimethyl sulfoxide, isopropyl alcohol, nickel(II) nitrate hexahydrate, phenol, quinoline yellow, RhB, terephthalic acid and thiram were purchased from Sigma-Aldrich (Warsaw, Poland). MB was supplied by Park Scientific (Northampton, UK). Aqueous ammonia, citric acid monohydrate, eriochrome black and sodium hydroxide were obtained from POCh (Gliwice, Poland).
2.2. Synthetic Procedures
2.2.1. Synthesis of NiAl2O4
Nickel aluminate catalyst was prepared by a coprecipitation method. Nickel and aluminum were coprecipitated from an aqueous nitrate solution (5 mmol·L−1) by adding an aqueous ammonia (1 mol·L−1) solution to produce a pH of about 8. The molar ratio of Al/Ni in the solution was 2.0. The precipitates were collected by filtration, washed with water and dried at 100 °C for 6 h. The as-synthesized nickel aluminate was subjected to calcination in air at 400, 600 and 800 °C for 3 h to obtain the crystalline spinel.
2.2.2. Synthesis of GQDs and NiAl2O4/GQDs
GQDs were prepared according to the procedure described elsewhere [32]. The NiAl2O4/GQDs composite was formed by introducing the crystalline NiAl2O4 spinel during the synthesis of GQDs. Briefly, 0.62 mmol of citric acid monohydrate and 0.85 mmol of the crystalline spinel were heated to 200 °C for 30 min until the transparent liquid changed color through yellow to amber. Subsequently, the heating temperature was reduced to 140 °C, and 10 mL of deionized water were added. The obtained mixture was heated under stirring until the complete evaporation of water.
2.3. Methods
DSC and TGA analyses were performed by Thermal Analyzer TGA/DSC 1 (METTLER TOLEDO, Giessen, Germany) with a heating rate of 15 °C·min−1 under a nitrogen environment with a flow rate of 20 mL·min−1. All runs were carried out from 25 to 1550 °C. The measurements were made in alumina crucibles with lids.
The powder X-ray diffraction data were measured at 293 K using a SuperNova diffractometer (Rigaku, The Woodlands, TX, USA) with a charge-coupled device (CCD) and a Cu-Kα radiation source at a150 mm sample-to-detector distance.
Scanning electron microscopy images were recorded by secondary-electron SEM with the use of an INSPECT S50 scanning electron microscope (FEI, Hillsboro, OR, USA). The accelerating voltage of the electron beam was 15 keV and the working distance was 10 mm. Images were also obtained with a TEM system (FEI Teknai T20 G2 X-TWIN, Hillsboro, OR, USA) operating at 200 kV, equipped with an LaB6 source.
The ATR-FTIR spectra (3200–500 cm−1) were obtained using a Nicolet Model 6700 FT-IR spectrometer with a DTGS detector (Thermo Scientific, Madison, WI, USA). The crystal-diamond spectra were obtained with 4 cm−1 resolution, and 32 scans for each sample spectrum were obtained. Diffuse reflectance UV–Vis spectra (DRS) were recorded on a Jasco V-30 UV–Vis/NIR spectrophotometer (Jasco, De Meern, Netherlands) equipped with an integrating sphere 60 mm in diameter using BaSO4 as a reference.
The UV–Vis spectra were recorded with a HITACHI U-2800A UV–Vis spectrophotometer (Hitachi, Tokyo, Japan) equipped with a double monochromator and a single-beam optical system (190–700 nm). A SUNTEST CPS+ (ATLAS, Mount Prospect, IL, USA) solar simulator apparatus was used to perform photocatalytic degradation experiments. The emission spectra were recorded on a Hitachi F-7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan): excitation bandwidths 5.0 nm; emission bandwidths 5.0 nm; scan speed 1200 nm·min−1.
2.4. Photocatalysis Experiments
The photocatalytic degradation experiments were investigated in a 50-mL glass cell. The reaction mixture consisted of 20 mL of the model pollutant RhB (2 × 10−5 mol·L−1), quinoline yellow (QY, 2 × 10−5 mol·L−1), eriochrome black (EB, 4 × 10−5 mol·L−1), MB (8 × 10−5 mol·L−1), phenol (PH, 10−4 mol·L−1) or thiram (TM, 8 × 10−5 mol·L−1), aqueous solution and a photocatalyst (1.5 g·L−1). Prior to photocatalytic experiments, the catalyst was settled in suspension for 60 min in the dark for the adsorption–desorption equilibrium. All the above-mentioned chemicals were analytical-grade reagents and used without further treatment. All solutions were prepared using deionized water, which was obtained by a Polwater apparatus (Polwater, Cracow, Poland).
2.5. Terephthalic Acid Probe Method
The generation of hydroxyl radicals as a consequence of the irradiation of the aqueous suspension of NiAl2O4 catalyst with the simulated solar light was examined using the terephthalic acid (TPA) probe method [35]. The nickel aluminate particles (2 mg·mL−1) were dispersed in a 3 mmol·L−1 TPA solution prepared in a 10 mmol·L−1 NaOH solution. Afterwards, the obtained suspension was sonicated for 10 min and exposed to sunlight for 2 h while vigorous stirring continued. After a given time the suspension was centrifuged, and the fluorescence emission spectrum was measured at the excitation wavelength of λ = 312 nm.
2.6. Reactive Species Scavenging
The generation of electron holes, hydroxyl radicals and electrons was determined by treating the reaction suspensions with ammonium oxalate (AO), isopropyl alcohol (IPA) and dimethyl sulfoxide (DMSO) as respective scavengers [36].
3. Results and Discussion
3.1. Structural and Morphological Study
3.1.1. NiAl2O4
Thermogravimetric studies were performed to examine the temperature required for the formation of the crystalline form of nickel aluminate. Figure 1 presents DSC, TGA and derivative thermogravimetry (DTG) curves of the as-synthesized NiAl2O4 before annealing. The TGA curve shows distinct mass loss in the temperature range of 230–400 °C, represented by the peak on the DTG curve at 284 °C. In the same temperature window, exothermic peaks on the DSC curve were attributed to the decomposition of the Ni(Ac)2∙4H2O and the following structural ordering of the nickel aluminate spinel phase. Therefore, it was concluded that 400 °C should be the lowest temperature used for the annealing to obtain the stable final inorganic crystalline product. Accordingly, the as-synthesized nickel aluminate was divided into three portions, which were annealed respectively at 400, 600 and 800 °C.
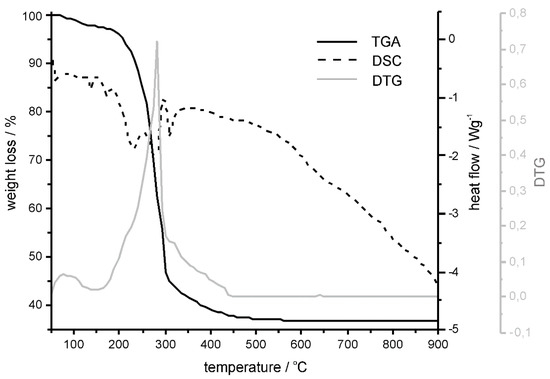
Figure 1.
Differential scanning calorimetric (DSC), thermogravimetric (TGA) and derivative thermogravimetric (DTG) curves of the as-synthesized NiAl2O4 before annealing.
XRD images of the NiAl2O4 annealed at 400, 600 and 800 °C (Figure 2) showed that the contribution of the spinel structure increased with increasing applied temperature. Nickel oxide was observed at lower temperatures (400 and 600 °C), as indicated by diffraction patterns assigned to [200] and [220] lattices (JCPDS No. 47-1049). The pure spinel crystalline form of nickel aluminate (well-matched with JCPDS No. 44-0160), with no remaining cubic NiO, was obtained after annealing at 800 °C. The apparent crystallite diameter (Dc) of NiAl2O4 particles was found to be of 3, 4 and 8 nm for samples annealed at 400, 600 and 800 °C, respectively. The latter numbers were calculated according to the Scherrer equation: Dc = kλ/βcosθ, where β is the full width at the half maximum of the diffraction peak, k is the empirical constant (0.9), θ is the angular position of the diffraction peak, and λ is the wavelength of the X-ray source (here 1.5405 Å). The observed increase of crystallite sizes with the increase of the annealing temperature was in agreement with the findings of others reported for inorganic semiconductors (e.g., nickel oxide) [37].
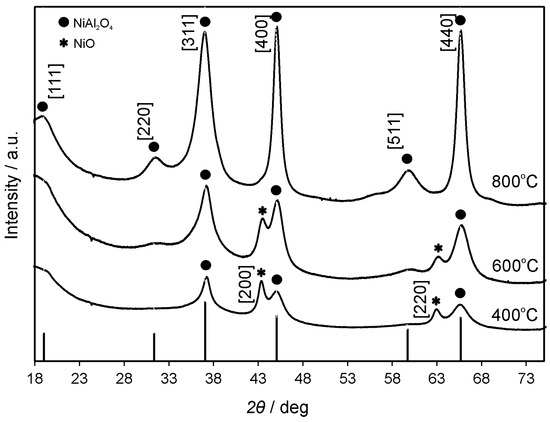
Figure 2.
X-ray diffraction (XRD) patterns of NiAl2O4 annealed at 400, 600 and 800 °C.
ATR-FTIR spectroscopy studies were undertaken to confirm the purity of nickel aluminate nanoparticles and to investigate the presence of the functional groups on their surface. ATR-FTIR spectra of the samples annealed at 400, 600 and 800 °C were registered in the range of 500–3600 cm−1 (Figure 3). The bands observed at low frequencies within 500–700 cm−1 were attributed to the stretching vibrations of Ni–O, Al–O and Ni–O–Al bonds [8,38]. Moreover, the bands observed in the range of 3200–3500 cm−1 indicated the presence of the O–H surface bonds on the catalyst surface. The bands depicted in Figure 3 were observed for the samples annealed at all applied temperatures, from 400 to 800 °C. However, their intensity increased alongside the increase of the applied annealing temperature, indicating well-developed crystalline structures for nickel aluminate annealed at 800 °C.
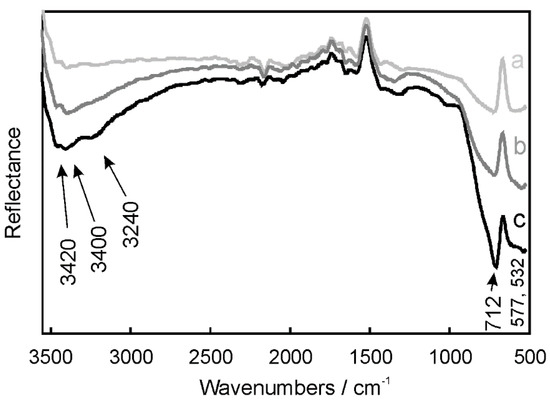
Figure 3.
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra of NiAl2O4 after annealing at (a) 400, (b) 600 and (c) 800 °C.
The morphology of the NiAl2O4 particles was evaluated based on SEM analysis. Figure 4 demonstrates that as-synthesized nickel aluminate formed agglomerates with particle sizes in the range of 50–200 μm. However, after annealing the size of the agglomerates decreased with increasing temperature. The highest homogeneity was observed for the material annealed at 800 °C (Figure 4(4a,b)).
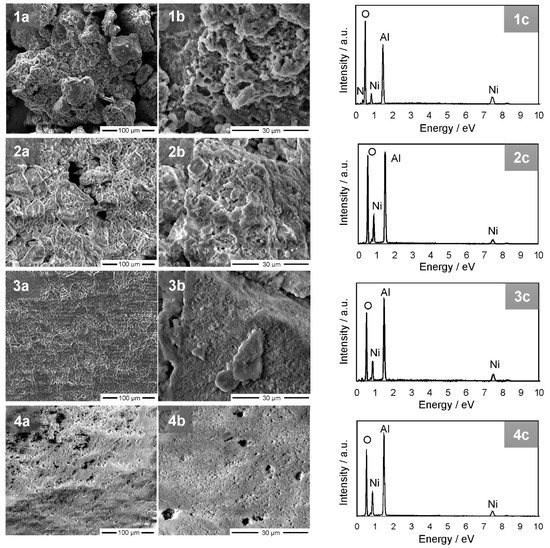
Figure 4.
Scanning electron microscopy (SEM) images and X-ray (EDX) spectra of NiAl2O4 without (1a–c) and after annealing at (2a–c) 400, (3a–c) 600 and (4a–c) 800 °C.
The chemical composition and purity of the synthesized nickel aluminate were evaluated using EDX analysis. As shown in Figure 4(1c–4c), Ni, O and Al were the only observed elements in all of the registered curves. Moreover, the decrease of the intensity of the peak attributed to the O element was noticed after annealing. This was due to the formation of the crystalline form of the spinel structure.
3.1.2. GQDs and NiAl2O4/GQDs Composite
The pristine GQDs exhibited structure of high porosity, as shown in SEM images (Figure 5(1a,b)), as distinguished from the crystalline NiAl2O4 (Figure 4(4a,b)). Therefore, an increase in the porosity of the NiAl2O4/GQDs composite compared to the pristine spinel was observed, as shown in Figure 5(2a,b). TEM images of GQDs particles (Figure 5(1c,d)) demonstrated their uniform sizes ranging from 2 to 7 nm. However, due to profound differences in the size of GQDs and metal oxide particles, carbon nanostructures could not be distinguished in the TEM image of the NiAl2O4/GQDs. Nevertheless, their presence in the composites led to an increase in the dispersity of the spinel nanoparticles (Figure 5(2c)). The TEM image of the pristine NiAl2O4 (Figure 5(3)) shows an agglomerated structure with particles having an average size of 20 nm. On the other hand, the particles of the NiAl2O4/GQDs (Figure 5(2c)) had smaller diameters (between 7–10 nm) and appeared to be separate from each other. The carbon content in GQDs and in the NiAl2O4/GQDs composites was examined by EDX (see Figure 6). This showed that GQDs presented a moderate oxygen content of 16%. The value of the latter is relevant for photocatalytic activity since surface oxygen groups contribute to the photocatalytic activity on defect sites [39].
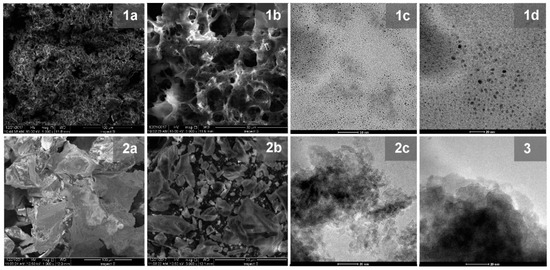
Figure 5.
Images of graphene quantum dots (GQDs). (1a,b) SEM, (1c,d) transmission electron microscopy (TEM); NiAl2O4/GQDs: (2a,b) SEM, (2c) TEM; and NiAl2O4: (3) TEM.
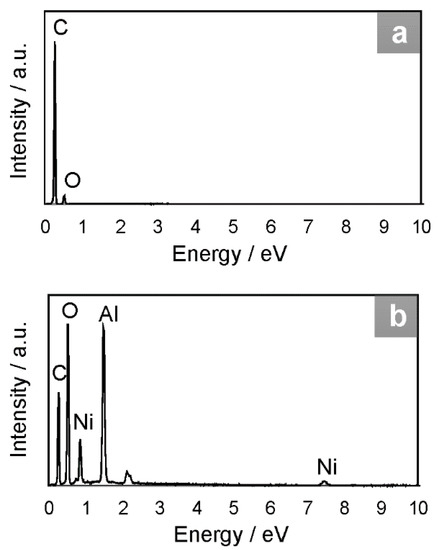
Figure 6.
EDX spectra of (a) GQDs and (b) NiAl2O4/GQDs.
The UV–Vis diffuse reflectance spectrum (Figure 7) of the crystalline NiAl2O4 (after annealing at 800 °C) displayed a significant absorption in the ultraviolet spectrum range. Additionally, absorption in the visible region, due to the d–d transition of Ni(II) and Al(III) was seen. As typical for the normal spinel structure with the tetrahedrally coordinated Ni(II) in the NiAl2O4 lattice absorption, a maximum around 650 nm was found. However, a presence of the inverse spinel structure was also revealed, as indicated by the absorption appearing around 380 and 770 nm. This is known to arise from the octahedral Ni(II) ions [40]. Based on the extrapolation of the linear part of the Kubelka–Munk vs. energy plot, the energy bandgap was calculated to be 2.9 and 2.5 eV for NiAl2O4 and the NiAl2O4/GQDs composite, respectively. The calculated Eg value of the pristine nickel aluminate was close to that reported for spinel (see Table 1). Meanwhile, the synthesized NiAl2O4/GQDs composite showed a significantly narrower band edge, which corresponded to 470 nm. This wavelength was in the solar spectrum range of the highest intensity [41], indicating a significant potential to harvest renewable solar energy.
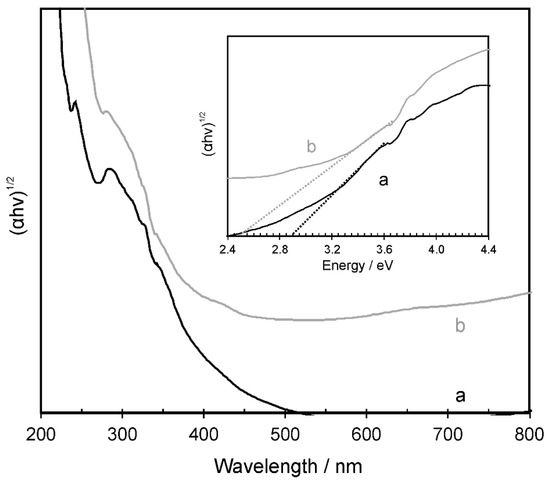
Figure 7.
UV–Vis diffuse reflectance profile of the (a) NiAl2O4 and the (b) NiAl2O4/GQDs composite annealed at 800 °C.

Table 1.
Energy band gap (Eg) of the NiAl2O4/GQDs composite and NiAl2O4 reported in this work and elsewhere.
3.2. Photocatalytic Activity Study
The photocatalytic activity of the NiAl2O4 nanoparticles was tested against a series of potential water pollutants. These included a series of dyes (i.e., RhB, QY, EB and MB), along with PH and the commonly used fungicide TM. The degradation efficiency is illustrated in Figure 8A as a decrease of the residual concentration ratio (Ct/C0) of each compound during the time of irradiation with the simulated solar light. All examined model contaminants were found to decompose under the applied conditions. The degradation of all model pollutants followed pseudo–first-order kinetics. Therefore, based on the plots presented in Figure 8B, the pseudo-first-order rate constants were calculated and are compared in Table 2. The determined k values increased in the following order: RhB < QY < EB < PH < TM < MB. Among dyes, the most resistant turned out to be RhB, while MB decomposed the easiest. The resistance to photocatalytic decomposition of MB was close that of TM. Tetramethylthiuram disulfide, unlike PH—which represents aromatic compounds—underwent photo-oxidation easily.
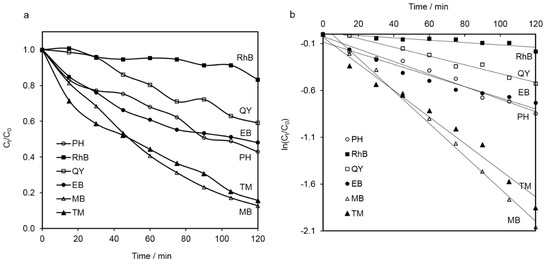
Figure 8.
(a) Residual concentration ratio (Ct/C0) and (b) apparent first-order kinetic lines of rhodamine B (RhB), quinoline yellow (QY), eriochrome black (EB), methylene blue (MB), phenol (PH) and thiram (TM) as a function of time under simulated solar light irradiation in the presence of NiAl2O4.

Table 2.
The apparent first-order rate constants k (min−1) for the degradation of RhB, QY, EB, MB, PH and TM under simulated solar light irradiation in the presence of NiAl2O4.
The photocatalytic activity of the NiAl2O4/GQDs was examined towards RhB as a representative dye and towards PH (representative of toxic compounds forming colorless aqueous solutions). Each of the chosen model pollutants from the two examined groups exhibited the most resistance to degradation. The results of the photocatalytic studies obtained in the presence of the synthesized composite were compared with those performed using pristine spinel (see Figure 9).
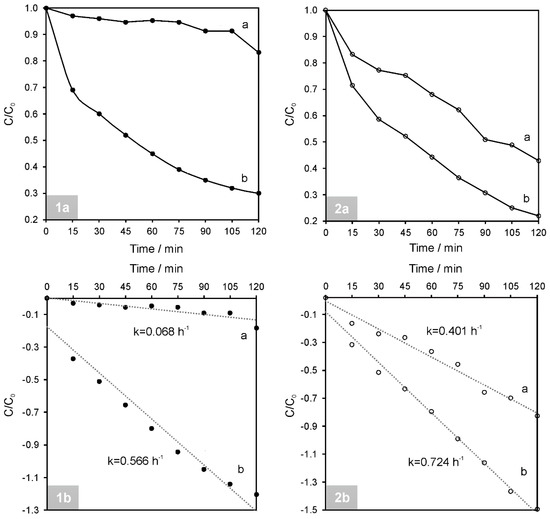
Figure 9.
Residual (1a and 2a) concentration ratio (Ct/C0) and (1b and 2b) kinetic curves as a function of time under the simulated solar light irradiation of (1) RhB and (2) PH aqueous solutions in the presence of (a) NiAl2O4 and (b) NiAl2O4/GQDs.
To examine the mechanism of the photocatalytic activity of NiAl2O4/GQDs, a composite hydroxyl radical generation probe method with TPA was applied. Figure 10 shows fluorescence spectra as observed for the supernatant solution of the NiAl2O4/GQDs catalyst suspension irradiated with terephthalate (TP) for various durations. A strong fluorescence emission peak was observed at λem = 426 nm. This was assigned to the formation of an adduct (hTP) between TP and hydroxyl radical (Scheme 1), indicating the formation of •OH species in the irradiated suspension. The intensity of the observed emission peak increased linearly within the irradiation time, as shown in the inset of Figure 10.
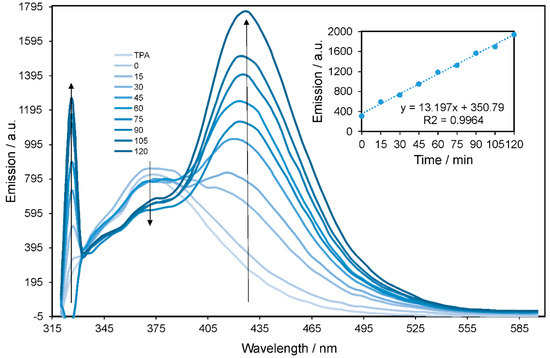
Figure 10.
Fluorescence spectra of the solution of terephthalic acid (TPA) under simulated solar light irradiation in the presence of the NiAl2O4/GQDs catalyst within 2 h. Inset: time dependence of the fluorescence intensity at 426 nm.
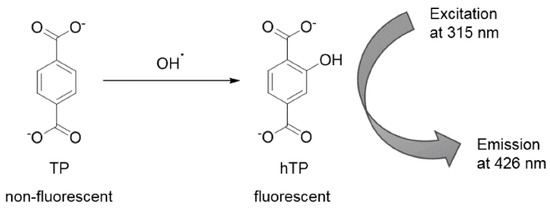
Scheme 1.
Formation of fluorescent 2-hydroxyterephthalate (hTP) via the reaction of hydroxyl radicals with terephthalate (TP).
In order to investigate which other active species were contributing to the photocatalytic activity of NiAl2O4/GQDs, a series of experiments with established scavengers was performed. Ammonium oxalate (AO), isopropyl alcohol (IPA) and dimethylsulfoxide (DMSO) as electron hole, hydroxyl radical and electron scavenger, respectively, were separately mixed with the reactant mixture containing RhB and NiAl2O4. RhB was subjected to photocatalytic degradation under simulated solar light. As shown in Figure 11, the biggest influence on the photocatalytic degradation of RhB was observed in the presence of hydroxyl radicals. However, since AO (being the hole scavenger) also had a significant influence, it indicated that hydroxyl radicals were generated involving both valence band holes and conduction band electrons. The smallest effect was observed in the presence of DMSO, which may point to the instant reaction of the electrons in the conduction band after excitation of the semiconductor. These observations indicated the low electron–hole recombination effect in the synthesized catalyst. The suggested mechanism of the photocatalytic degradation of the organic pollutants in the presence of the NiAl2O4/GQDs composite is presented in Scheme 2. It shows that after GQDs harvest the sunlight, they give rise to the generation of the electron–hole pairs. The same phenomenon occurs in NiAl2O4 since it also absorbs light from the visible spectrum range. Subsequently, the electrons injected in the conduction band of NiAl2O4 may react with oxygen and lead to the generation of hydroxyl radicals, as shown in the Scheme 2. GQDs prolong the recombination rate of the charge carriers. They also contribute to harvesting the sunlight and are responsible for the adsorption of the pollutants, which ultimately decompose.
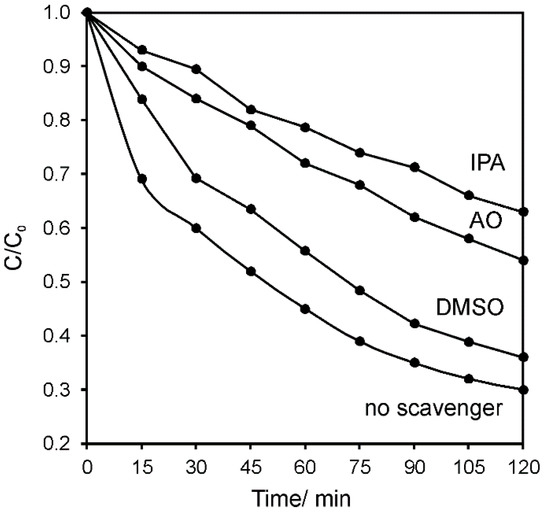
Figure 11.
Photocatalytic degradation of RhB in the presence of the NiAl2O4/GQDs composite under simulated solar light irradiation without and in the presence of scavengers: ammonium oxalate (AO), isopropyl alcohol (IPA) and dimethyl sulfoxide (DMSO) used to capture holes, hydroxyl radicals and electrons, respectively.
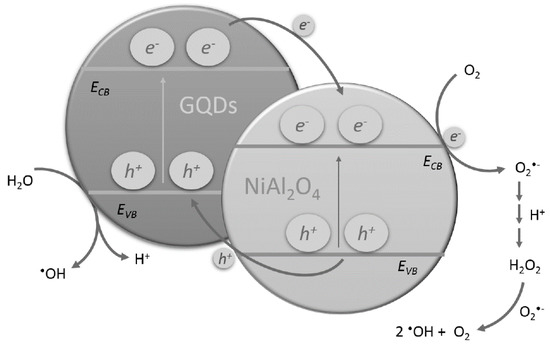
Scheme 2.
The proposed mechanism of photocatalysis using the NiAl2O4/GQDs composite.
The reusability of NiAl2O4/GQDs was studied in four successive recycling experiments for the photocatalytic degradation of RhB. The catalyst was separated from the reaction suspension by centrifugation, washed with ethanol and water (four times each) and dried in the oven at 100 °C. As shown in Figure 12, NiAl2O4/GQDs retained its photocatalytic activity after four successive experimental runs. A slight decrease was observed after the first use. However, in consecutive runs the photocatalytic activity remained unchanged and retained 96% of its original efficiency.
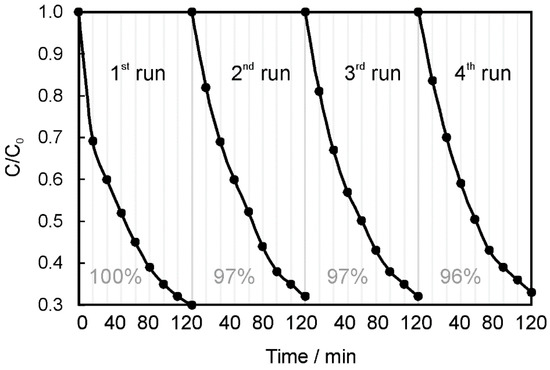
Figure 12.
Reusability of the NiAl2O4/GQDs composite.
4. Conclusions
Highly efficient nickel aluminate nanoparticles with spinel crystal structures were successfully synthesized via a simple and cost-effective co-precipitation method. A comprehensive study of the photocatalytic performance of the degradation of different water pollutants, including a series of dyes (i.e., rhodamine B, quinoline yellow, eriochrome black T, methylene blue), phenol and fungicide (thiram) under simulated solar light irradiation was carried out in this study. Moreover, we succeeded in improving the photocatalytic performance of NiAl2O4 by decorating it with GQDs. We presented the physicochemical characterization of the obtained photocatalyst alongside studies of its photocatalytic activity towards rhodamine B and phenol degradations. The mechanism of the photocatalysis in the presence of the NiAl2O4/GQDs composite was studied using the TPA method and a series of scavengers. Hydroxyl radicals were found to play a leading role in the photocatalytic activity of the investigated composite. This work not only offers new insight into the application of the conjunction of the inorganic spinel and the carbon nanostructure (i.e., GQDs), but also provides a simple and highly efficient route for potential water remediation from common pollutants, including dyes and colorless harmful substances. Moreover, the synthesized composite exhibited multifunctionality, which will be further investigated in an upcoming paper.
Author Contributions
Conceptualization, E.R.; methodology, E.R.; TEM investigation, A.B.; investigation other than TEM analysis, E.R. and J.B.; writing—original draft preparation, E.R.; writing—review and editing, E.R., J.B. and A.B.
Funding
We gratefully acknowledge the financial support from the Polish Ministry of Science and Higher Education under subsidy granted to the Faculty of Biology and Chemistry, University of Bialystok for R&D and related tasks aimed at development of young scientists and PhD students and for maintaining the research potential of the Faculty of Biology and Chemistry, University of Bialystok. Diffractometer, IR spectrometer, SEM and TEM microscopes, DSC and TGA instruments, UV–Vis/NIR spectrophotometer and spectrofluorometer were funded by EU, as part of the Operational Programme Development of Eastern Poland, projects Nr POPW.01.03.00-20-034/09-00 and POPW.01.03.00-20-004/11-00.
Acknowledgments
The authors thank A. Wilczewska (University of Bialystok, Poland) for DSC-TGA measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, G.; Yang, M.; Li, C.; Tan, H.; Deng, L.; Xie, S.; Xu, F.; Wang, L.; Song, Y. Preparation of spinel nickel-cobalt oxide nanowrinkles/reduced graphene oxide hybrid for nonenzymatic glucose detection at physiological level. Electrochim. Acta 2016, 220, 545–553. [Google Scholar] [CrossRef]
- Gaudon, M.; Robertson, L.C.; Lataste, E.; Duttine, M.; Ménétrier, M.; Demourgues, A. Cobalt and nickel aluminate spinels: Blue and cyan pigments. Ceram. Int. 2014, 40, 5201–5207. [Google Scholar] [CrossRef]
- Vitorino, N.M.D.; Kovalevsky, A.V.; Ferro, M.C.; Abrantes, J.C.C.; Frade, J.R. Design of NiAl2O4 cellular monoliths for catalytic applications. Mater. Des. 2017, 117, 332–337. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Yue, B.; He, H. Ni/Al2O3 catalysts derived from spinel NiAl2O4 for low-temperature hydrogenation of maleic anhydride to succinic anhydride. Chin. J. Catal. 2017, 38, 1166–1173. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum and rhodium catalysts. Appl. Catal. Gen. 2012, 437–438, 53–62. [Google Scholar]
- Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J. Synthesis and characterization of several Ni/NiAl2O4 catalysts active for the 1,2,4-trichlorobenzene hydrodechlorination. Appl. Catal. B Environ. 2000, 25, 213–227. [Google Scholar] [CrossRef]
- Farahani, M.D.; Dasireddy, V.D.B.C.; Friedrich, H.B. Oxidative Dehydrogenation of n-Octane over Niobium-Doped NiAl2O4: An Example of Beneficial Coking in Catalysis over Spinel. ChemCatChem 2018, 10, 2059–2069. [Google Scholar] [CrossRef]
- Maddahfar, M.; Ramezani, M.; Sadeghi, M.; Sobhani-Nasab, A. NiAl2O4 nanoparticles: Synthesis and characterization through modify sol–gel method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2015, 26, 7745–7750. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Ahmadi, F.; Eghbali-Arani, M. Different morphologies fabrication of NiAl2O4 nanostructures with the aid of new template and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 2415–2420. [Google Scholar] [CrossRef]
- Tangcharoen, T.; T-Thienprasert, J.; Kongmark, C. Optical properties and versatile photocatalytic degradation ability of MAl2O4 (M = Ni, Cu, Zn) aluminate spinel nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 8995–9006. [Google Scholar] [CrossRef]
- Elakkiya, V.; Agarwal, Y.; Sumathi, S. Photocatalytic activity of divalent ion (copper, zinc and magnesium) doped NiAl2O4. Solid State Sci. 2018, 82, 92–98. [Google Scholar] [CrossRef]
- Akika, F.Z.; Benamira, M.; Lahmar, H.; Tibera, A.; Chabi, R.; Avramova, I.; Suzer, Ş.; Trari, M. Structural and optical properties of Cu-substitution of NiAl2O4 and their photocatalytic activity towards Congo red under solar light irradiation. J. Photochem. Photobiol. Chem. 2018, 364, 542–550. [Google Scholar] [CrossRef]
- Jayasree, S.; Manikandan, A.; Antony, S.A.; Uduman Mohideen, A.M.; Barathiraja, C. Magneto-Optical and Catalytic Properties of Recyclable Spinel NiAl2O4 Nanostructures Using Facile Combustion Methods. J. Supercond. Nov. Magn. 2016, 29, 253–263. [Google Scholar] [CrossRef]
- Deraz, N.M. Synthesis and Characterization of Nano-Sized Nickel Aluminate Spinel Crystals. Int. J. Electrochem. Sci. 2013, 5203–5212. [Google Scholar]
- Gholami, T.; Salavati-Niasari, M.; Varshoy, S. Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int. J. Hydrog. Energy 2017, 42, 5235–5245. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Salehabadi, A.; Amiri, M.; Shabani-Nooshabadi, M.; Rezaie, M. Electrochemical hydrogen storage properties of NiAl2O4/NiO nanostructures using TiO2, SiO2 and graphene by auto-combustion method using green tea extract. Renew. Energy 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, H.; Jiang, Q.; Deng, Y.; Jin, H.; Kang, Q. Development of a chemical-looping combustion reactor having porous honeycomb chamber and experimental validation by using NiO/NiAl2O4. Appl. Energy 2018, 211, 259–268. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Wu, X.; Tang, D.; Cai, K.; Zhang, Q. Carbon quantum dots/Ni–Al layered double hydroxide composite for high-performance supercapacitors. RSC Adv. 2016, 6, 39317–39322. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water using fluorinated layered double hydroxides as photocatalysts. Appl. Catal. Gen. 2016, 521, 160–167. [Google Scholar] [CrossRef]
- Iguchi, S.; Hasegawa, Y.; Teramura, K.; Hosokawa, S.; Tanaka, T. Preparation of transition metal-containing layered double hydroxides and application to the photocatalytic conversion of CO2 in water. J. CO2 Util. 2016, 15, 6–14. [Google Scholar] [CrossRef]
- Khodam, F.; Rezvani, Z.; Amani-Ghadim, A.R. Fabrication of a novel ZnO/MMO/CNT nanohybrid derived from multi-cationic layered double hydroxide for photocatalytic degradation of azo dye under visible light. RSC Adv. 2015, 5, 19675–19685. [Google Scholar] [CrossRef]
- Salehi, G.; Abazari, R.; Mahjoub, A.R. Visible-Light-Induced Graphitic–C3N4@Nickel–Aluminum Layered Double Hydroxide Nanocomposites with Enhanced Photocatalytic Activity for Removal of Dyes in Water. Inorg. Chem. 2018, 57, 8681–8691. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Synthesis of Fullerene-, Carbon Nanotube-, and Graphene-TiO2 Nanocomposite Photocatalysts for Selective Oxidation: A Comparative Study. ACS Appl. Mater. Interfaces 2013, 5, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Regulska, E.; Rivera-Nazario, D.M.; Karpinska, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Enhanced Photocatalytic Performance of Porphyrin/Phthalocyanine and Bis(4-pyridyl)pyrrolidinofullerene modified Titania. ChemistrySelect 2017, 2, 2462–2470. [Google Scholar] [CrossRef]
- Regulska, E.; Karpińska, J. Investigation of novel material for effective photodegradation of bezafibrate in aqueous samples. Environ. Sci. Pollut. Res. 2014, 21, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Regulska, E.; Karpinska, J. Investigation of Photocatalytic Activity of C60/TiO2 Nanocomposites Produced by Evaporation Drying Method. Pol. J. Environ. Stud. 2014, 23, 2175–2182. [Google Scholar]
- Hamadanian, M.; Shamshiri, M.; Jabbari, V. Novel high potential visible-light-active photocatalyst of CNT/Mo, S-codoped TiO2 hetero-nanostructure. Appl. Surf. Sci. 2014, 317, 302–311. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Fu, H.-B.; Zhu, Y.-F. Efficient TiO2 Photocatalysts from Surface Hybridization of TiO2 Particles with Graphite-like Carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, Q.; Zhang, Y.; Xu, Y.-J. Graphene–TiO2 nanocomposite photocatalysts for selective organic synthesis in water under simulated solar light irradiation. RSC Adv. 2014, 4, 15264–15270. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloy Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Chinnusamy, S.; Kaur, R.; Bokare, A.; Erogbogbo, F. Incorporation of graphene quantum dots to enhance photocatalytic properties of anatase TiO2. Mrs Commun. 2018, 8, 137–144. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kedawat, G.; Agrawal, Y.; Kumar, P.; Dwivedi, J.; Dhawan, S.K. A Novel Strategy to Enhance Ultraviolet Light Driven Photocatalysis from Graphene Quantum Dots Infilled TiO2 Nanotube Arrays. RSC Adv. 2015, 5, 10623–10631. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.-X. Graphene Quantum Dots (GQDs) and Its Derivatives for Multifarious Photocatalysis and Photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a Probe for Photochemically Generated Hydroxyl Radical. J. Environ. Monit. 2010, 12, 1658–1665. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, S.; Chen, Z.; Lou, X.; Zhang, D. A Facile Method of Activating Graphitic Carbon Nitride for Enhanced Photocatalytic Activity. Phys. Chem. Chem. Phys. 2015, 17, 27826–27832. [Google Scholar] [CrossRef]
- Maniammal, K.; Madhu, G.; Biju, V. X-ray Diffraction Line Profile Analysis of Nanostructured Nickel Oxide: Shape Factor and Convolution of Crystallite Size and Microstrain Contributions. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 85, 214–222. [Google Scholar] [CrossRef]
- Motahari, F.; Mozdianfard, M.R.; Soofivand, F.; Salavati-Niasari, M. NiO nanostructures: Synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv. 2014, 4, 27654–27660. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, W.; Wang, L.; Zhang, J.Z.; Li, Y.; Liu, X.; Li, Y. Optimizing Oxygen Functional Groups in Graphene Quantum Dots for Improved Antioxidant Mechanism. Phys. Chem. Chem. Phys. 2019, 21, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, W.Y. Partial Oxidation of Methane to Syngas over Calcined Ni–Mg/Al Layered Double Hydroxides. Catal. Lett. 2002, 83, 65–70. [Google Scholar] [CrossRef]
- Serway, R.A.; Beichner, R.J.; Jewett, J.W. Physics for Scientists and Engineers, 5th ed.; Saunders Golden Sunburst Series; Saunders College Publishing: Fort Worth, TX, USA, 2000; ISBN 978-0-03-022654-0. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).