Abstract
A causal analysis was conducted in order to examine the relationship between residents’ inhalation exposure to PCDDs/PCBs and the incidence of cancer. A significant association was identified between chronic inhalation exposure to 2,3,7,8-TCDD and the overall incidence of malignant neoplasms in the study area. This association was stronger among men (p = 0.0020) than among women (p = 0.0027). A significantly higher overall incidence of malignant neoplasms was observed among the inhabitants of villages (CY, GN) exposed to higher levels of PCDD/F mixture in comparison with the reference village (p < 0.001). The present study observed the phenomenon in both male (p = 0.008) and female (p = 0.013). Moreover, a considerably elevated incidence of morbidity was observed in the male population of the CY village (p = 0.034) in comparison with the female population, where atmospheric air pollution with PCDD/Fs has been recorded at its most elevated levels. A higher frequency of cancers was also observed among the inhabitants of the villages GN and CY compared to the inhabitants of the reference village. The observed differences were consistent in cancers of the digestive system (p < 0.001), respiratory system and thoracic organs (p < 0.001), skin (p = 0.023), urinary system (p < 0.001), lymphatic and hematopoietic system (p = 0.032), as well as cancers occurring in women (p = 0.041).
1. Introduction
In recent decades, there has been a notable increase in the global incidence of malignant tumours, including in Poland, with implications for both the quality and length of life. On 1 February 2024, the International Agency for Research on Cancer (IARC) published a report on the global incidence of cancer in 2020. The report estimated that approximately 20 million new cases of cancer were diagnosed worldwide in 2020, with 9.7 million deaths attributable to the disease. The same report predicts a staggering 77% increase in cancer cases by 2050, potentially resulting in 35 million new cases annually [1]. As is the case on the other side of the Atlantic, cancer statistics in Europe remain equally alarming. It is estimated that in 2022, there were nearly 4.5 million new cases and approximately 1.99 million deaths [2]. In 2022, the incidence rate of malignant tumours in European men reached 319.6 cases per 100,000, thus surpassing the rate in women, which was 253.4 cases per 100,000. In the male population, prostate cancer (20.0%), lung cancer (13.4%), and colorectal cancer (12.3%) constituted almost half of all cases. Among women, the most prevalent types were breast cancer (26.4%), colorectal cancer (11.8%), and lung cancer (7.9%) [2]. In Poland, during the same 2022 year, 208,900 new cancer cases and 119,992 cancer-related deaths were recorded. The most prevalent malignant neoplasms were observed to be prostate (20.6%), lung (17.6%), and colorectal (14.3%) cancers in men, and breast (24.5%), colorectal (11.3%), and lung (11.2%) cancers in women [2]. It is noteworthy that the incidence of lung cancer in Polish men and women exceeded the European average by 3.3% and 4.2%, respectively.
Cancer is primarily the consequence of interactions between genetic predispositions, epigenetic and hormonal factors, lifestyle differences, occupational exposures and environmental conditions. Recent research has indicated that the development of nearly all cancers is influenced by both genetic factors (accounting for approximately 10%) and individual predispositions of the affected individual. Conversely, external carcinogenic factors significantly interact with genetic factors and a person’s lifestyle in various combinations, initiating, extending or accelerating the path of the mechanism of cancer development [1]. The most recent data published by the UK Office for National Statistics indicates that the most significant risk factors related to lifestyle are: the consumption of alcohol, tobacco smoking, obesity and excessive exposure to solar radiation. According to British experts, unhealthy lifestyles and unsafe habits have a particularly deleterious effect on increasing the risk of many types of cancer [3]. These are modifiable risk factors for cancer development at the individual level. However, a significant factor that is both modifiable and system-wide, as opposed to being individual-level, is environmental pollution.
It is estimated that environmental factors account for approximately 80% of global cancer cases, with air pollutants being among the most significant contributing factors [4]. In 2013, the IARC classified particulate matter (PM) as a Group I carcinogen, citing its ability to induce oxidative stress and DNA mutations [5,6,7]. In a similar manner, dioxins, particularly 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD), are classified as Group I carcinogens according to the IARC classification system. These compounds are among the most toxic and biologically active in this category [8]. Dioxin-like polychlorinated biphenyls (dl-PCBs), which are classified by the IARC as probable human carcinogens, also pose significant risks [9]. Consequently, prolonged inhalation exposure to air pollutants has been linked to elevated cancer incidence rates [10,11].
It is imperative to comprehend the role of environmental determinants in cancer development in order to formulate effective measures that will contribute to reducing morbidity and mortality on a global scale. Air pollution is a significant factor in the development of cancer, but it is one of many factors that influence the development of cancer. As a result, it is difficult to obtain clear confirmation of the participation of a given factor in causing cancer. It is, therefore, vital to acknowledge the significance of each study in this area, as they contribute novel insights to the field [12]. As demonstrated in the studies conducted by Mostafalou and Abdollahi, even low concentrations of air pollutants have been shown to have a detrimental effect on human health, with adverse outcomes including cancer [12]. Numerous studies have been conducted that demonstrate a correlation between elevated concentrations of Persistent Organic Pollutants (POPs), particularly polychlorinated dioxins and polychlorinated furans (PCDD/Fs), in the adipose tissue of individuals diagnosed with cancer in comparison to those who are cancer-free [13,14,15]. However, there is a paucity of studies that have been able to confirm a higher probability of cancer occurrence in the population living in areas characterised by higher air pollution. It is precisely this kind of research that can be useful in the proper planning of preventive measures and their implementation in the appropriate area. Prior to the implementation of preventive measures, it would be challenging to analyse the content of individual toxins in the tissues of each resident. The present study provides further evidence to support the hypothesis that place of residence exerts a significant influence on exposure levels. The analysis indicates that populations residing in industrialised regions are more susceptible to health loss and premature death due to malignancies.
Poland, and particularly the Silesia Province, serves as a pertinent case study in this regard. The European Environment Agency (EEA) report for 2021–2023 reveals that 23 of the 50 most polluted cities in the European Union are located in Poland, with seven of these cities ranking among the top ten. Of these seven cities, four are located in the Silesia Province [16]. Silesia Province has the distinction of being the most industrialised administrative area in Poland. The region has a long industrial heritage, with a history of significant activity in the extraction of coal, the processing of metals, and the generation of electricity. Notwithstanding this fact, the region remains subject to considerable environmental challenges. Despite such a high concentration of industry in Silesia Province, the major problem at present is the emission of particulate pollutants into the air from residential homes heated with coal, the cheapest and most easily accessible energy source in the region. The problem is more crucial in places where in order to increase the energy efficiency of poorer-quality coal (so-called culm or coal dust), plastic waste often co-burned. Such practices take place in the area covered by this study, where most of the inhabitants are engaged in the production of polyvinyl chloride (PVC) products (i.a. Christmas trees, wreaths, flowers, etc.) and the waste remaining from this production is burned in home furnaces. In 2022, the incidence of cancer in Upper Silesia exceeded the national average, with approximately 475 cases per 100,000 women (compared to 468 per 100,000 in Poland) and 511 cases per 100,000 men (compared to 491 per 100,000 nationwide) [17]. Notwithstanding the region’s industrial legacy, low-level emissions from residential coal heating now represent the primary source of air pollution. The utilisation of coal, compounded by the coincineration of plastic waste, engenders the emission of deleterious pollutants, encompassing particulate matter, sulphur dioxide, dioxins, furans, and PCBs [18,19]. It is particularly evident in communities such as Cynków and Gniazdów, where the tradition of plastic production is deeply entrenched, that the practice of incinerating post-production waste in domestic heating stoves has led to a marked increase in pollution levels. The objective of this study is to analyse the relationship between community exposure to selected POPs and cancer incidence rates.
2. Materials and Methods
2.1. Study Area
The geographical area under consideration comprised two neighbouring villages: the geographical location of Cynków (CY) and Gniazdów (GN) is specified as being within the municipality of Koziegłowy (KZ) (50°35′59.89° N 19°09′46.50° E) in the Silesia Province, situated in the southern part of Poland, in Central Europe. The population of the villages in the years analysed (2005–2022) was comparable. In the CY district, the average population was 1176 individuals, whereas in the GN district it was 918 individuals. The municipality of KZ, encompassing the studied villages, had an average population of 14,117 between 2005 and 2022, with the studied villages constituting less than 15% of the municipality’s population. In the designated study area, the female population constituted slightly less than 52% of the total population, while the male population represented slightly more than 48%.
The reference population was selected in the conducted study at both the village level (Kochcice, KO) and the municipal level (Kochanowice, KC). The village of KO is located approximately 50 km from the municipality of KZ, with an average population of 1835 individuals in the years 2005–2022 [20]. In contrast, the reference municipality of KC had an average population of 6862 residents during the same period (Table 1). The control population exhibited a comparable female-to-male ratio to the study population, with the female gender accounting for just over 51% and the male gender just under 49%. It was determined that neither the reference municipality nor the reference village exhibited any additional sources of exposure to dioxins, furans and PCBs during the study period, with the exception of the standard sources of low-level emissions that are pervasive throughout the country.

Table 1.
The structure of the population living in the study areas, taking into account age groups and gender.
The study area comprised two villages (CY and GN), as well as the KZ commune to which they belong. These locations were selected due to the ongoing, long-term co-incineration of post-industrial waste, which consists of plastic materials and has been occurring since the 1990s. This process constitutes a significant source of exposure to POPs for the local residents. A village (KO) in the commune (KC) was selected as the reference area. This village had a similar socio-economic status of residents to those in the study villages, but it was free from additional sources of environmental exposure to POPs.
2.2. Data Sources
The study was based on data obtained from the National Health Fund (NHF) on the number of new cases of malignant tumours by gender, as determined by provided health services from 2005 to 2022, with diagnoses: According to the International Classification of Diseases and Health Problems, 10th Revision (ICD-10), the range is C00–C97 [21]. The data set under consideration encompassed new cancer cases among the population residing within the designated study area, encompassing the villages of CY and GN and the KZ municipality, in addition to the reference area, which included the village of KO and the KC municipality. In order to undertake comparative analyses of the incidence of specific types of malignant neoplasms between the studied populations, the cancers were categorised according to their anatomical location. This classification has been derived from the categorisation of cancers as outlined by the Polish National Cancer Registry (NCR) [22]. The NCR constitutes a nationwide database of incidences of malignant neoplasms in Poland. It is the only source of epidemiological data that maintains a population-based register in this area according to standards, ensuring comparability with other countries.
Cancers are divided into the following groups:
- cancer of the lip, oral cavity, pharynx—C00–C14,
- cancer of the digestive system—C15–C26,
- cancer of the respiratory system and thoracic organs—C30–C39,
- cancer of the bones and articular cartilage—C40–C41,
- cancer of the skin—C43–C44,
- cancer of connective tissue, soft tissue, mesothelioma—C45–C49,
- cancer neoplasms specific to female—C50–C57,
- cancer specific to male—C60–C63, C50,
- cancer of the urinary tract—C64–C68,
- cancer of the eye, brain, spinal cord and other parts of the nervous system—C69–C72,
- cancer of the thyroid, the adrenal gland and other endocrine glands—C73–C75,
- secondary cancer of various and unspecified locations—C76–C80,
- cancer of the lymphatic and haematopoietic system—C81–C97.
As demonstrated in the results section of the paper, a criterion was employed to exclude groups of malignant tumours for which statistically insignificant results were obtained following statistical analysis. Utilising this criterion, the following groups are presented in the subsequent section of the results of the paper:
- cancer of the digestive system,
- cancer of the respiratory system and thoracic organs,
- cancer of the skin,
- cancer specific to female,
- cancer specific to male,
- cancer of the urinary tract,
- cancer of the lymphatic and haematopoietic system.
The Central Statistical Office (CSO) of Poland and the Local Statistical Offices in the investigated communes and villages obtained data on the number of inhabitants of the respective villages and communes for the years 2005–2022, taking gender into account. The incidence rate (IR) was calculated for the population of the villages (CY, GN, KO) and communes (KZ, KC) in the years 2006–2022, by gender, based on the acquired data. The formula used was as follows:
For the purposes of the present study, data on air pollution in the study villages and municipalities with selected halogenated POP compounds were utilised, employing the results of the studies described in publication [23] (Table 2), which also provides details of the sampling methodology and detection of the concentrations of the studied compounds. The present author conducted the studies over a period of three heating periods [23]:

Table 2.
24 h average concentrations of PCDD/Fs, dl-PCBs in air samples collected in villages: CY, GN, KO and municipalities—KZ, KC of Silesia Province. Source: [23].
- (a)
- heating season 2014/2015,
- (b)
- heating season 2021,
- (c)
- heating season 2022/2023.
In order to ascertain whether the concentration of the analysed POPs is comparable, and therefore whether the exposure of residents is proportional each year, it was decided to carry out the research in several heating seasons. In consideration of the financial constraints associated with the analysis of environmental samples and the limited financial resources available within the scope of the project, the decision was made to conduct the tests during the three heating seasons previously referenced. A comparison of the obtained results indicates that the exposure of residents in the study and reference areas is proportional, with regard to the 2014/2015 and 2022/2023 heating seasons. However, it should be noted that the results of the analyses conducted during the 2021 heating season indicate higher concentrations of PCDD/F and PCB. One potential explanation for this phenomenon could be the impact of the ongoing pandemic, which resulted in individuals spending a significant amount of time at home. This prolonged period of home residence may have led to an increase in emissions from low-intensity sources, as indicated in Table 2.
2.3. Statistical Analysis
As part of the statistical analysis of the study’s results, a comparison was made in the first stage of the incidence rates of malignant tumours between the three villages analysed and between the two municipalities. Initially, the normality of the distribution of incidence rates was verified, and following positive verification, parametric tests were applied: one-way ANOVA—comparison of socio-communities; two sample one-tailed t-test—comparison of communes. The implementation of non-parametric tests was necessitated by the initial negative verification of normality. The subsequent analysis will utilise both the Kruskal–Wallis test and the Mann–Whitney test. In instances where substantial discrepancies in the incidence rate were identified between the analysed hamlets, appropriate post hoc tests (Dunn–Bonferroni and Conover) were employed.
The study also sought to evaluate the impact of concentrations of dioxins, furans and dioxin-like polychlorinated biphenyls (independent variables) on the occurrence of malignant neoplasms (dependent variables) in the studied population of socio-districts and municipalities. Multiple regression analysis was employed in this study to evaluate the significance and direction of the impact of dioxins and furans on the incidence rate of cancer. It was hypothesised that the multiple regression equation would meet two criteria. It is imperative to note that the coefficient of determination, R2, must exhibit a substantial discrepancy from zero, and each coefficient of the equation must demonstrate a significant deviation from zero. The calculations were performed using a commonly accepted significance level value of p = 0.05. Calculations for the incidence rate of malignant neoplasms (IR) were performed separately for the total population, for the female subgroup and for the male subgroup.
The calculations and figures presented herein were performed using Microsoft Office 2013 spreadsheets (MS Word, MS Excel, MS Access). The following levels of statistical significance were contemplated during the formulation of conclusions:
- p > 0.05—no statistical significance
- p < 0.05—statistical significance
- p < 0.01—high statistical significance
- p < 0.001—very high statistical significance
3. Results
3.1. Total Incidence of Cancer in the Study and Reference Population
With regard to the villages, the highest incidence of total cancer was recorded in GN (172.62 cases per 1000 inhabitants), followed by CY (129.26 cases per 1000 inhabitants), and the lowest in KO (104.69 cases per 1000 inhabitants). The incidence of cancer among the female and male population followed a similar pattern. During the study period (2006–2022), the total cancer incidence among the female population of the villages was documented at the following rates: 161.35/1000 in GN, 108.09/1000 in CY, and 93.06/1000 in KO. Conversely, the male population exhibited higher incidence rates, with rates of 183.79/1000 in GN, 150.74/1000 in CY, and 116.98/1000 in KO, respectively. As illustrated in Figure 1, the result of the Kruskal–Wallis test was employed to facilitate a comparison of the female cancer incidence rate in the villages that were the subject of this study. In consideration of the findings from the Dunn–Bonferroni post hoc test, the incidence rate in GN is found to be significantly higher than the results obtained from the other two socio-districts. The p-values for the same comparisons according to Conover’s test exceed the commonly used significance level value of α = 0.05. As demonstrated in Figure 2, the results of the evaluation of the significance of differences in the incidence rate of males from the studied villages are presented. The significance of these differences was demonstrated by the findings of the two post hoc tests, which did not fully align. Fisher’s exact test result indicates a significantly higher incidence rate in the GN village compared to the CY and KO villages. However, the Sheffe test reveals that the GN village has a higher incidence rate only when compared to the KO village.
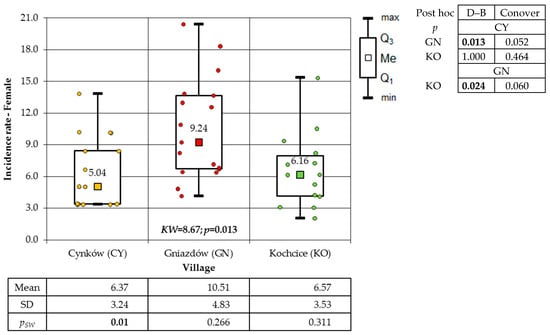
Figure 1.
Incidence rates of female cancer in the study villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni and Conover).
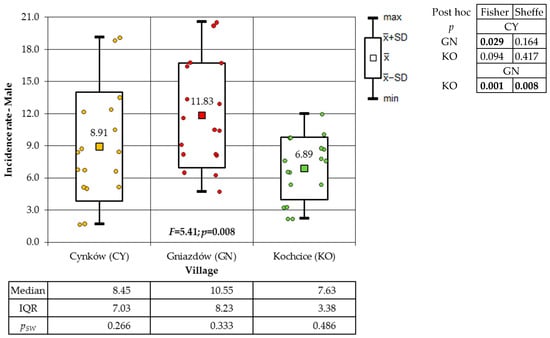
Figure 2.
Incidence rates of male cancer in the study villages (result of one-way ANOVA and results of post hoc tests: LSD Fisher and Sheffe).
A one-way analysis of variance (ANOVA) was employed to analyse the variation in the incidence of cancer among the total population of the villages under study. This analysis revealed the presence of significant differences in incidence. The findings of both post hoc tests demonstrate that the incidence of GN residents was significantly higher in comparison to the other two villages (Figure 3).
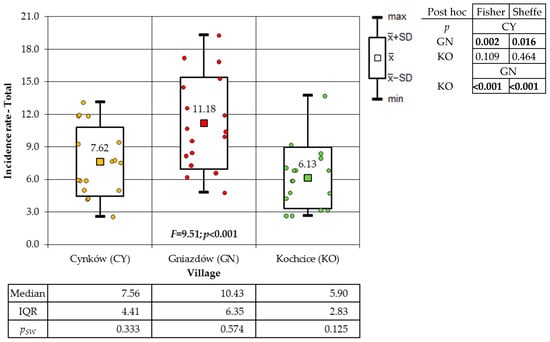
Figure 3.
Incidence rates of cancer of the total population in the study villages (result of one-way ANOVA and results of post hoc tests: LSD Fisher and Sheffe).
In the subsequent phase of the analysis, a comparison was conducted between the incidence of cancer among women, men, and the total population in the municipalities of KZ and KC. In the period between 2006 and 2022, a higher incidence of cancer was observed in the total population of the municipality of KC (86.23 cases per 1000) than in the municipality of KZ, where 57.98 cases of cancer per 1000 inhabitants were recorded during the same period. A higher incidence rate was also observed in the municipality of KC, affecting both the female population (80.46/1000) and the male population (92.30/1000). In the municipality of KZ, the incidence of cancer was found to be 37.63 cases per 1000 women and 79.72 cases per 1000 men during the observed period. The results of the non-parametric Mann–Whitney U test, which was applied because only among the male in the KZ municipality was a normal distribution of the data found, demonstrated that the incidence in the KC municipality was higher than in the KZ municipality. It is noteworthy that no statistical significance was observed in the male population (p = 0.145), a finding that was corroborated by the two-sample one-tailed t-test (p = 0.086) (Figure 4). The Mann–Whitney test was employed to analyse the incidence of total cancer in both female and male residing in the villages, given that the normality of the distribution was not consistently confirmed. A statistically significant disparity was observed in the gender ratio, with a higher proportion of male in comparison to female, a finding that was exclusively observed in CY (Figure 5). The Mann–Whitney test was also applied to municipalities. The calculations demonstrated a substantially reduced incidence of women in comparison to men in the municipality of KZ, whilst no statistically significant difference was identified in the municipality of KC. The latter result was confirmed by the two-means t-test (p = 0.180), which was additionally applied as the result of the Shapiro–Wilk test allowed its use (Figure 6).
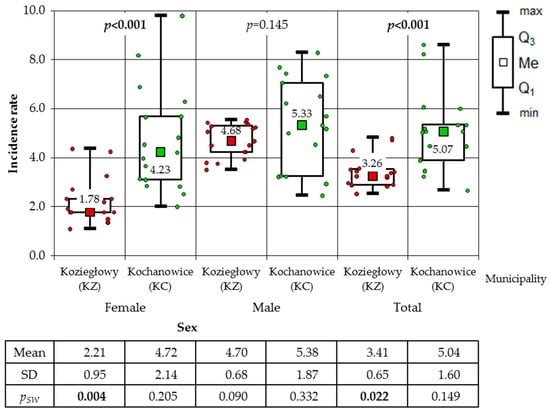
Figure 4.
Incidence rates of cancer for women, men and total population in the municipalities of Koziegłowy and Kochanowice (results of Mann–Whitney test).
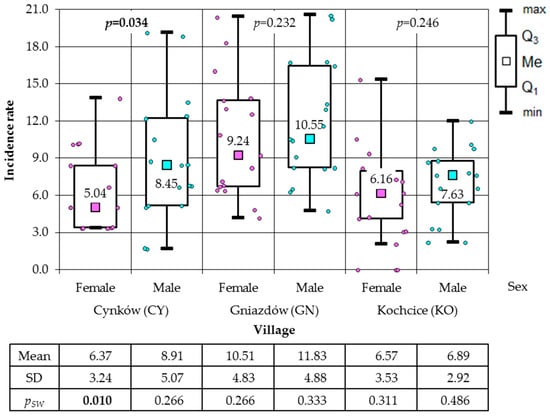
Figure 5.
Incidence rates of female and male total cancer in the study villages (results of Mann–Whitney test).
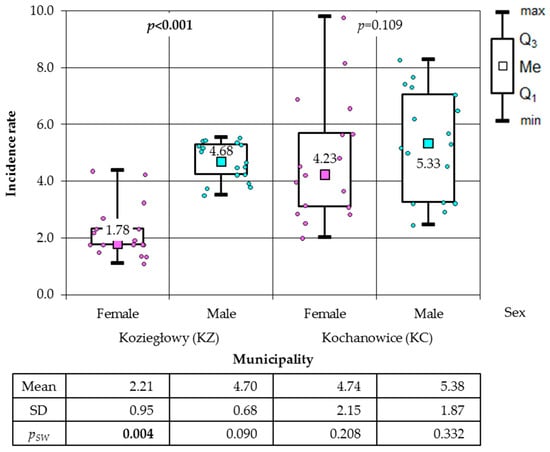
Figure 6.
Incidence rates of cancer among women and men in the municipalities of Koziegłowy (KZ) and Kochanowice (KC) (results of Mann–Whitney test).
3.2. Incidence of Cancer by Group, Taking into Account Their Location, in the Study and Reference Population
This subsection presents the results of the study, which explore the incidence of malignant neoplasms in the study population, categorised according to type. In the case of cancer included in the group of cancer of the digestive system, statistically significant differences were found in the incidence of women (p = 0.002), men (p = 0.002), and total population (p < 0.001) between the villages. The highest incidence was observed in CY, while the lowest was recorded in KO (see Figure 7).
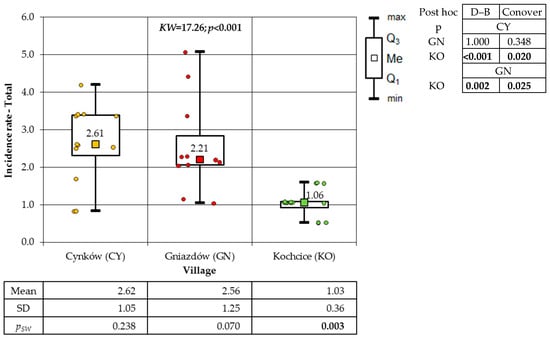
Figure 7.
Incidence rates of cancer of the digestive system of the total population in studies villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover).
Within the group of respiratory and thoracic cancer, statistically significant differences in incidence were identified between the villages for women (p = 0.011), men (p = 0.001), and the total population (p < 0.001). The highest incidence was demonstrated in GN, and the lowest in KO (Figure 8).
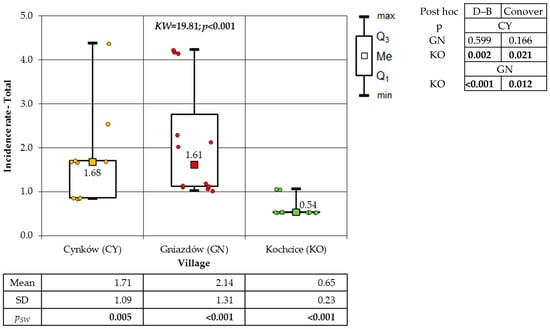
Figure 8.
Incidence rates of respiratory and thoracic cancer of total population in the surveyed villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover).
The incidence of skin cancer exhibited statistical disparities among the inhabitants of the villages, with significant differences observed between the villages for the total population (p = 0.023) and for women (p = 0.005). However, no statistical significance was observed among men (p = 0.288). The incidence of cutaneous malignancies was found to be significantly lower in KO than in GN. However, no significant differences were observed between CY and GN, nor between CY and KO (Figure 9).
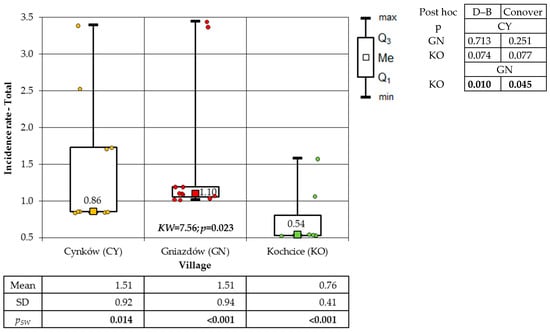
Figure 9.
Incidence rates of total resident skin cancer in the study villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover.
The subsequent group under consideration was that of cancerous developments among women. A statistically significant difference was identified between the villages (p = 0.041). The veracity of this result was corroborated by the Dunn–Bonferroni post hoc test. A higher incidence was observed in GN compared to the other two villages (Figure 10). Conversely, no substantial statistical disparities (p = 0.112) were observed between the villages with respect to the incidence of cancer among male populations.
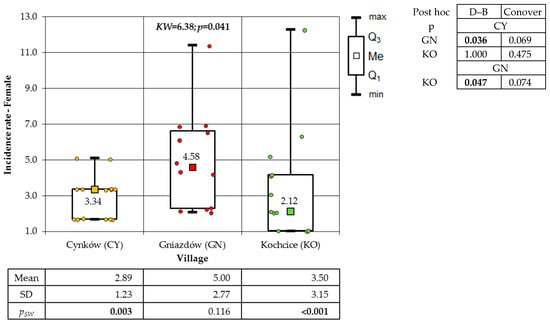
Figure 10.
Incidence rates of cancer occurring in women in the studied villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover).
A significant discrepancy was identified in the incidence of urinary tract cancer among the total population (p < 0.001) and among the male population (p < 0.001) in each village. However, a non-significant difference in incidence was observed among women (p = 0.090). The population of GN exhibited the highest incidence, while the population of KO demonstrated the lowest (Figure 11).
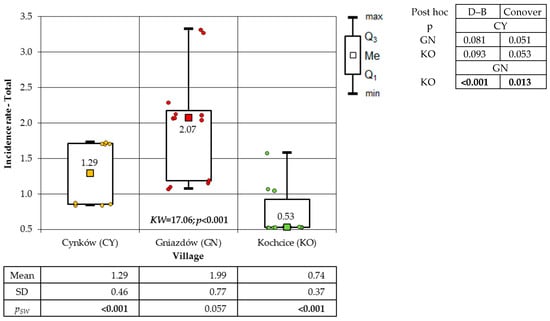
Figure 11.
Incidence rates of urinary tract cancer of residents in the studied villages overall (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover).
The final group under consideration was malignant neoplasms of the lymphatic and haematopoietic systems. A statistically significant discrepancy was identified in the incidence of cancer within the total population of the studied villages (p = 0.032) and the male population (p = 0.028), as determined by comprehensive statistical analysis. No statistically significant differences (p = 0.074) were identified among the female population of the various socio-districts (Figure 12).
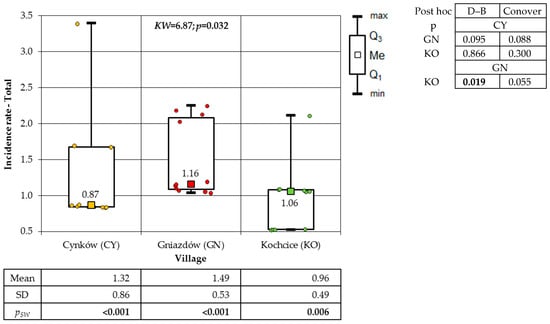
Figure 12.
Incidence rates of cancer of the lymphatic and hematopoietic systems of the total population in the studied villages (result of Kruskal–Wallis test and post hoc tests: Dunn–Bonferroni (D–B) and Conover).
The following section will discuss the variation in incidence of selected cancers among the residents of the studied municipalities of KZ and KC (Table 3). The results indicate that in the KC municipality, the incidence of residents’ cancer of the digestive system was significantly higher than in the KZ municipality, both in the total population (p = 0.007) and among women (p = 0.003) and men (p = 0.037). The Mann–Whitney test revealed a higher incidence of respiratory and thoracic cancer in women (p < 0.001). Utilising the t-test for two means, a higher incidence was observed in the total population (p = 0.042) from the municipality of KC compared to the population from the municipality of KZ. The study also demonstrated that the incidence of skin cancer was significantly higher in women (p < 0.001) and among the total population (p = 0.004) in the municipality of KC compared to the municipality of KZ.

Table 3.
Assessment of the significance of differences in the incidence rates of the total population of the municipalities of Koziegłowy and Kochanowice on malignant neoplasms in the groups.
The incidence of cancer among women was found to be significantly higher (p < 0.001) in the population of women residing in the municipality of KC. Conversely, the incidence of cancer among men did not exhibit a significant difference between the two municipalities under study, a finding corroborated by both the Mann–Whitney test (p = 0.334) and the t-test of two means (p = 0.162). The subsequent group analysed was that of urinary cancer. The study revealed that the incidence of cancer was found to be significantly higher in women from KC (p < 0.001).
Conversely, the incidence of malignant neoplasms of the lymphatic and haematopoietic systems was found to be significantly higher in the municipality of KC among both women (p = 0.001), men (p = 0.020) and the total population (p = 0.004) (Table 3).
3.3. Effect of Long-Term Inhalation Exposure to PCDD/Fs and dl-PCBs on the Incidence of Cancer in the Study Population
The analysis demonstrated that the cancer incidence rate (IR) is contingent on the long-term concentration of the congener 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) in the atmosphere of each locality. The positive slopes of the regression lines indicate a positive correlation between the concentration of 2,3,7,8-TCDD and the incidence of cancer. The observed effect was found to be statistically significant in the male population and the total population living in the socio-communities considered in this study (Figure 13).
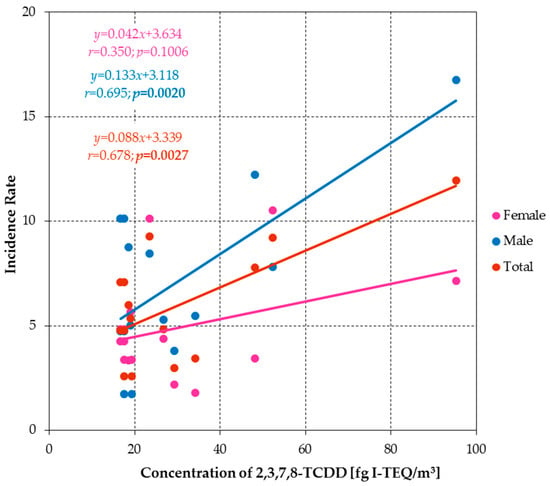
Figure 13.
Regression lines showing the relationship between IR and 2,3,7,8-TCDD concentrations for women, men and total population.
The decision to solely include the 2,3,7,8-TCDD variable in the regression analysis was based on an evaluation of cross-correlations between the explanatory variables. The results of the aforementioned assessment are presented in Table 4.

Table 4.
Results of verification of statistical significance of cross-correlations between the analysed explanatory variables.
The table demonstrates that only 2,3,7,8-TCDD exhibited relatively weak correlations with the other variables. The presence of high intercorrelations among the explanatory variables precludes their simultaneous inclusion in a multiple regression model. An endeavour was made to incorporate a further variable, non-dioxin-like PCB (n-dl-PCB), into the regression analysis. However, the regression coefficient for this variable was found to be statistically non-significant (p > 0.05), and thus it was excluded from the final model (see Table 4).
4. Discussion
The global incidence of cancer is increasing at a steady rate. In high-income countries, cancer is recognised as a chronic disease, while in low- and middle-income countries, it is still considered a fatal disease [1]. The Human Development Index (HDI) is a significant factor influencing variations in cancer incidence across different countries. Among countries with a medium and high HDI, lifestyle-related malignant neoplasms predominate, whereas in countries with a low HDI, the majority of recorded cancer is infection-related [24]. Poland has one of the highest cancer mortality rates in Europe [25]. In consideration of the aforementioned context, it is imperative to ascertain the factors that have contributed to this situation, including the environmental conditions associated with the individual’s place of residence. For a considerable number of years, the region encompassing the Silesia Province area has been distinguished by the most severe levels of air pollution, both within the national context and on a European scale. The problem is especially pronounced during the heating season, which encompasses the autumn and winter months [26,27]. It is imperative to accentuate the substantial disparities in the concentrations of air pollutants, including suspended particulate matter (PM2.5 and PM10), as well as PCDD/Fs and PCBs, that have been documented in various localities within the Silesia Province [28]. In this study, two villages (CY, GN) in the municipality (KZ) were selected, where residents experienced significantly higher inhalation exposure to PCDD/Fs and dl-PCBs than in the reference village (KO), which is located in the municipality of KC. The observed variations in the magnitude of exposure resulted in an increased total cancer incidence in the populations of the villages CY and GN compared to the reference population (KO). Concurrently, a substantial correlation was identified between the aggregate cancer incidence among males and the general population, on the one hand, and long-term inhalation exposure to 2,3,7,8-TCDD among residents in each locality, on the other. The findings of the study demonstrated a positive correlation between the increase in the concentration of 2,3,7,8-TCDD in the atmosphere and the rise in the incidence of cancer in the population. The study also revealed a significantly higher incidence of cancer among men compared to women in the village of CY, which was characterised by the highest concentrations of 2,3,7,8-TCDD in the air. A significant impact of population exposure to this compound and increased risk of cancer-related morbidity and mortality was also demonstrated in a meta-analysis by Xu J. et al. (2016) [29]. A review of studies published up to 2015 revealed a significant association between higher levels of external exposure to 2,3,7,8-TCDD and mortality from total cancer [29].
The process of carcinogenesis is a complex, long-term process influenced by many factors. The role of 2,3,7,8-TCDD in this process is significant [30,31]. A study by Connsoni et al. [32] analysed the long-term effects of the 1976 environmental disaster in Seveso (northern Italy), during which the local environment was heavily contaminated with 2,3,7,8-TCDD. The observations comprised a cohort of residents from 11 municipalities, including six municipalities that were contaminated to varying degrees by 2,3,7,8-TCDD (divided into three zones) and five municipalities that were uncontaminated, constituting the reference zone. The study was conducted over the course of several years: 1976–2013. In the initial ten years following the incident, there was a notable increase in the prevalence of soft tissue sarcoma among the male population residing in the area most distant from the primary epicentre of the accident. In the second decade, an increase in the incidence of multiple myeloma was observed in women from the area moderately distant from the epicentre and men from the area that is farthest from the accident epicentre. Three decades following the incident, an elevated occurrence of non-Hodgkin’s lymphoma was identified among the female population residing within Zone 2. Concurrently, a heightened rate of leukaemia was documented among the male population inhabiting Zone 3. The findings of the long-term observations provide substantiation for the carcinogenic prolonged effects of population exposure to varying concentrations of 2,3,7,8-TCDD [32].
This study presents the results for 17 years of observation of the effects of population exposure to elevated concentrations of PCDD/Fs in the villages of CY and GN. The range of recorded values of the analysed compounds was from 176.19 fg I-TEQ/m3 to 484.13 fg I-TEQ/m3 in CY and GN, in comparison to the reference village KO, where the range was from 169.05 fg I-TEQ/m3 to 238.10 fg I-TEQ/m3. The recorded concentrations of dioxins and furans in the atmosphere of KO were comparable to the levels of air pollution observed in the entire municipality of KC (178.57 fgI-TEQ/m3), which includes KO. In contrast, significantly lower concentrations of the analysed halogenated POPs compounds were detected in the air in the KZ municipality, ranging from 138.10 fg I-TEQ/m3 to 110.90 fg I-TEQ/m3, compared to the villages of CY and GN, which are parts of this municipality. The present study demonstrated that the total incidence of cancer in the analysed municipalities was strongly correlated with air quality. A higher incidence of cancer was observed in the KC municipality (80.46/1000 women; 92.30/1000 men) compared to the KZ municipality (37.63/1000 women; 79.72/1000 men). The variation in inhalation exposure to dioxins and furans among the inhabitants of the KZ municipality resulted in differences in cancer incidence in the population of the villages within this area. In the village of CY, the incidence of cancer was found to be almost three times higher (291%) among women (108.09/1000) and almost twice as high (189%) among men (150.74/1000) compared to the average for the entire KZ municipality.
A study of cancer incidence in the female (161.35/1000) and male (183.79/1000) populations of the GN village revealed an almost 4.5 times (436%) higher incidence in females and a more than twofold increase (232%) in males compared to the average for the entire municipality. Concurrently, in the designated reference village of KO, the incidence of cancer manifested itself at 16% higher among female (92.02 per 1000) and 27% higher among male (116.98 per 1000) in comparison with the respective incidence rates for females and males within the broader KC municipality. The significant variation in cancer incidence observed within villages and municipalities suggests that the impact of elevated levels of dioxins and furans in the atmosphere is limited to specific geographical areas.
An assessment of the relation between blood concentrations of pollutants such as heavy metals (As, Hg, Cd, Pb), PCDD/Fs, PCBs and PBDEs (polybrominated diphenyl ethers) in cancer patients and cancer incidence was undertaken in Forte et al. 2020 [33]. The study was conducted on a group of 95 patients of varying ages with clinically confirmed cancer, residing within the municipalities of the provinces of Naples and Caserta, Italy. The selected study area exhibited characteristics consistent with those previously documented in this study, namely widespread practices of illegal waste dumping and burning. The reference population comprised 27 healthy individuals. The results indicated the presence of halogenated persistent organic pollutants in the blood of individuals from the municipalities of Naples and Caserta Provinces, with concentrations that appeared to be distributed evenly. Concurrently, cancer patients were found to have significantly higher concentrations of heavy metals (Cd, Hg) in their blood compared to healthy individuals [33]. A significant impact of dioxins and furans mixtures on cancer was also reported in the study by Alawi et al. 2018 [13]. The research demonstrated that the concentrations of 17 PCDD/Fs congeners in fresh human adipose tissue samples were three times higher in total cancer patients than in healthy individuals [13].
In this study, an analysis of the incidence of specific types of cancer was also conducted. The results indicated a higher incidence of cancer among residents of GN and CY villages compared to those residing in the reference village, KO. The observed differences were evident in all the examined cancer groups, including cancers of the digestive system, respiratory and thoracic organs, skin, urinary system, lymphatic and haematopoietic systems, as well as female-specific cancers. The only exceptions to this observation were male-specific cancers, for which no statistically significant differences in incidence were observed. It is noteworthy that residents of GN and CY villages were exposed to elevated concentrations of PCDD/Fs in comparison to residents of KO. Analogous correlations were observed in the comparison of cancer incidence between the populations of the two analysed municipalities. A significantly higher incidence of all examined cancer groups was found among residents of KC, who were more heavily exposed to dioxins and furans via inhalation, compared to the less exposed population of the KZ municipality.
The villages constituting the study areas (CY and GN) belong to the KZ commune, while the KO village (reference area) belongs to the KC commune (Table 1). The statistical analysis conducted in this study demonstrated that the aggregate cancer incidence, as well as the incidence of cancer among both female and male members of the study population, was elevated in both villages constituting the study area (GN, CY) in comparison to the reference village (KO). This phenomenon may be attributable to the exposure of the local population to POPs, thereby substantiating the hypothesis proposed by the authors of the manuscript. The study also demonstrated an inverse relationship in the case of the study communes. A considerably elevated overall cancer incidence was observed in the population of the reference commune (KC) in comparison with the study commune (KZ). The inverse relationship observed in the case of the communes, as opposed to the relationships noted for the villages, may be attributable to the fact that the communes CY and GN selected for the study are two of the 26 villages belonging to the KZ commune. It is in these villages that the greatest number of plastic products are manufactured, and consequently, post-production waste is incinerated there. The majority of other villages are not subject to such additional sources of POP emissions; therefore, the results for the KZ commune may be the inverse (“diluted”).
Furthermore, analysis of the incidence of individual cancer groups also showed significantly higher rates of digestive system (Figure 7), respiratory system (Figure 8) and urinary system cancer (Figure 11) in the population living in the studied villages (CY or GN) compared to the reference village (KO). The findings of this study corroborate the hypothesis that exposure to carcinogenic compounds present in the atmosphere may have a bearing on the incidence of the aforementioned cancer groups within the exposed population. In the case of communes, an inverse relationship was observed, similar to the results concerning cancers in general. In the reference commune, the incidence of the analysed cancer groups was higher compared to the studied commune, the reason for which is probably the same as in the case of cancers in general.
The analysis demonstrated that the cancer incidence rate (IR) is contingent on the long-term concentration of the congener 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) in the atmosphere of each locality (Figure 13). This finding serves to substantiate the impact of the carcinogenic compound in question on the incidence of cancer within the population under study.
The correlation between exposure to persistent organic pollutants (POPs) and cancer response was demonstrated in a pilot study by Perrot-Applanat et al. [34]. The study analysed omental adipose tissue (covering the stomach) in patients with gastric cancer, and compared this with a control group without cancer. The study detected significant concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs) in the omental tissue of patients diagnosed with aggressive diffuse-type gastric cancer relative to the control group.
In a separate analysis, Koukoulakis et al. [35] identified an increased cancer risk among residents of Thriasson, Greece’s most industrialised region, associated with elevated environmental emissions. The study confirmed combustion as the primary source of atmospheric POPs. Despite the comparable concentrations of POPs detected in this study to those reported in the existing literature, the estimated cancer risk suggests that approximately three to four individuals per 100,100 may develop cancer due to inhalation of airborne POPs.
A considerable number of researchers have endeavoured to examine the effect of toxic halogenated persistent organic pollutants on the occurrence of particular cancer types [36,37,38,39,40]. Nevertheless, the multifactorial aetiology of cancer hinders the establishment of robust correlations in this domain. It is evident that exposure to polluted air is capable of exerting a carcinogenic effect; however, it is imperative to acknowledge the role of toxic compounds present in other environmental components, such as water and soil. Nevertheless, given the high cost of analysing persistent organic compounds in environmental samples, a comprehensive analysis of the environmental exposure of society to these compounds is often impossible [41]. A potential limitation of this study is its exclusive emphasis on the impact of POPs present in the air on the occurrence of cancer. The budget constraints of the project and the high cost of laboratory analyses related to the determination of individual POPs in samples meant that the authors decided to focus solely on one, but the most significant, route of exposure of the studied community. The significance of the population’s exposure through inhalation is related to the long-term burning of post-industrial waste, which is generated as a result of the production of plastic products in household furnaces. Furthermore, atmospheric air is contaminated with a mixture of various compounds that exhibit carcinogenic properties. In addition to halogenated POPs, such properties have been demonstrated by certain heavy metals and polycyclic aromatic hydrocarbons (PAHs). These substances have been shown to act synergistically to increase the risk of cancer development; however, they were not analysed in this study [42].
The present study was subject to several limitations, the first of which related to the restricted scope of the medical database, which precluded the execution of multivariate analyses that would have taken into account factors of cancer exposure, including age, diet, smoking, and physical activity level. The data employed in the study, pertaining to the number of residents of the analysed villages and communes afflicted with specific types of cancer, was sourced from the National Health Fund and was presented in the form of an anonymised database. The database obtained from the National Health Fund did not contain the number of cancer patients directly; rather, it was created on the basis of financed medical procedures performed during cancer treatment in a given person who resided in the area under analysis. The study fails to consider key factors such as genetic susceptibility and occupational exposure to carcinogenic substances.
It is recommended that the scope of these studies be expanded in the future to encompass the inclusion of any risk factors that may have been omitted. This expansion would facilitate a comprehensive analysis of all or, at the very least, the majority of the factors that have been demonstrated to exert a significant influence on the development of cancer. Furthermore, it would be advantageous to extend the database of analysed pollutants to encompass additional carcinogenic compounds, which are present in both the atmosphere and other components of the environment. A further key consideration is the analysis of the contamination of foodstuffs cultivated in the designated study area, given that the oral route constitutes the primary means of environmental exposure. This aspect will mark the subsequent stage in the present study.
In light of the findings of the search, the necessity for ongoing endeavours to enhance air quality in Poland is reaffirmed. The preponderance of household energy consumption in Poland is characterised by the utilisation of hard coal and firewood, underscoring the need for strategic interventions to promote sustainable energy practices. As indicated by data provided by the Central Statistical Office (CSO), in 2021, the utilisation of solid fuels for space heating accounted for 32.8% of all households, 22.5% for water heating, and 1.7% for cooking. In 2021, Poland’s per capita consumption of hard coal was ten times higher than the EU-27 average. The proportion of this fuel in relation to total energy consumption in Polish households was found to be 21.7%, whereas the average rate for the EU-27 was only 2.1%. A study of energy consumption in the EU revealed that, within the domestic sector, Poland accounted for 89.8% of the total consumption of this specific energy carrier [43]. As indicated by the data of the Central Emission Evidence of Building (CEEB) as of January 2025, the proportion of buildings relying exclusively on solid-fuel heating sources in the rural municipality of KC was 79.14%, of which 39.17% were non-compliant boilers. In contrast, in the rural areas of the KZ municipality, solid-fuel boilers were present in 75.96% of buildings, with 35.36% of these classified as non-compliant. Meanwhile, within the town of KZ itself, the respective figures for these indicators were 61.69% and 31.73% [44].
In certain local communities, initiatives aimed at enhancing air quality may encounter obstacles due to the prioritisation of cost savings over the professional disposal of waste. This approach, frequently propelled by financial imperatives, has the potential to exert a deleterious effect on environmental quality and population health. The preponderance of households in Poland reliant on coal stoves has been identified as a contributing factor to the pervasive practice of uncontrolled waste burning.
It is, therefore, imperative that government programmes replacing heating sources, implementing thermal modernisation and promoting the development of renewable energy sources be accompanied by continuous public education on the negative effects of air pollution exposure. This includes the promotion of awareness regarding the correlation between air pollution and cancer incidence, in addition to accentuating the environmental and health benefits associated with waste sorting and the transition from fossil fuels as an energy source.
Furthermore, the penetration of air pollutants from the exterior into the interior of the rooms can be mitigated by the implementation of highly efficient modern filters, including those capable of capturing particles as small as PM0.3 [45,46,47].
5. Conclusions
- The present study demonstrated a significant association between chronic inhalation exposure to 2,3,7,8-TCDD among men and the general population residing in the case study area, as well as the overall incidence of cancer.
- In the village of CY, which has been identified as a region with the highest levels of pollution, a significantly higher rate of cancer has been observed among the male population compared to the female population.
- The villages constituting the case study area (CY and GN), where residents experienced prolonged exposure to high concentrations of halogenated persistent organic pollutants (POPs) in the air, exhibited a significantly higher cancer incidence rate than the population of the entire KZ municipality, of which they are a part.
- A significantly higher incidence of cancer was observed among the residents of the case study villages when compared with the residents of the reference village. The cancer cases observed included those affecting the digestive system, respiratory system and thoracic organs, skin, urinary system, lymphatic and haematopoietic systems. Female-specific cancer was observed among residents of the case study villages compared to those in the reference village.
- It is imperative that concerted efforts are made to enhance air quality, not only in urban areas but also in rural regions, with a view to reducing the inhalation exposure of residents to harmful pollutants, including halogenated POPs, which have been demonstrated to contribute to increased cancer incidence.
- It is imperative to extend the ambit of research to encompass unanalysed components of the environment, such as water, soil, and foodstuffs. Furthermore, the scope should be broadened to include additional carcinogenic compounds, including heavy metals and PAHs, in addition to other pertinent factors, such as genetics and the lifestyle of the society under study.
Author Contributions
Conceptualisation, A.D. and A.P.; methodology, A.D., A.P. and G.D.; validation, A.P. and G.D.; formal analysis, A.D., A.P. and G.D.; investigation, A.D.; resources, A.D.; data curation, A.D.; writing—original draft preparation, A.D.; writing—review and editing, A.P. and G.D.; visualisation, A.D.; supervision, A.P. and G.D.; project administration, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by Medical University of Silesia in Katowice by the grant Nos. PCN-2-029/N/2/Z, BNW-2-036/K/3/Z, BNW-1-064/N/3/Z.
Institutional Review Board Statement
Not applicable. This study does not involve humans or animals.
Informed Consent Statement
Not applicable. This study does not involve humans.
Data Availability Statement
Data supporting the obtained results will be available upon reasonable request via the corresponding authors’ e-mail contact within 3 years following the publication.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| POPs | Persistent Organic Pollutants |
| PCDD/Fs | Polichlorinated dibenzo-p-dioxins and Polichlorinated dibenzofurans |
| PCBs | Polichlorinated biphenyls |
| IARC | The International Agency for Research on Cancer |
| PM | Particulate Matter |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| ICD-10 | The International Classification of Diseases and Health Problems |
| NCR | The Polish National Cancer Registry |
| CSO | The Central Statistical Office |
| IR | Incidence rate |
| HDI | The Human Development Index |
| PBDEs | Polybrominated diphenyl ethers |
| PAHs | Polycyclic aromatic hydrocarbons |
| CEEB | The Central Emission Evidence of Building |
References
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services; World Health Organization: Lyon, France; Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 20 October 2024).
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/908-europe-fact-sheet.pdf (accessed on 31 October 2024).
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). World Cancer Report: Cancer Research for Cancer Prevention; WHO Press: Geneva, Switzerland, 2020; ISBN 978-92-832-0447-3. [Google Scholar]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working Group IARC. The carcinogenicity of outdoor air pollution. Lancet. Oncol. 2013, 13, 1262–1263. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A.; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef]
- VoPham, T.; Bertrand, K.A.; Jones, R.R.; Deziel, N.C.; DuPre, N.C.; James, P.; Liu, Y.; Vieira, V.; Tamimi, R.M.; Hart, J.E.; et al. Dioxin exposure and breast cancer risk in a prospective cohort study. Environ. Res. 2020, 186, 109516. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Loomis, D.; Baan, R.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Grosse, Y.; Straif, K. Use of mechanistic data in the IARC evaluations of the carcinogenicity of polychlorinated biphenyls and related compounds. Environ. Sci. Pollut. Res. Int. 2016, 23, 2220–2229. [Google Scholar] [CrossRef]
- Bertazzi, P.A.; Zocchetti, C.; Guercilena, S.; Consoni, D.; Tironi, A.; Landi, M.T.; Pesatori, A.C. Dioxin exposure and cancer risk: A 15-year mortality study after the Seveso accident. Epidemiology 1997, 8, 646–652. [Google Scholar] [CrossRef]
- Bertazzi, A.; Pesatori, A.C.; Consonni, D.; Tironi, A.; Landi, M.T.; Zocchetti, C. Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiology 1993, 4, 398–406. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Environmental pollution by mercury and related health concerns: Renotice of a silent threat. Arh. Hig. Rada. Toksikol. 2013, 64, 179–181. [Google Scholar] [CrossRef]
- Alawi, M.; Masaad, M.; Al-Hussaini, M. Comparative study of persistent organic pollutant (POP)(chlorinated pesticides, PCBs, and dioxins/furans) concentrations in cancer-affected human organs with those of healthy organs. Environ. Monit. Assess. 2018, 190, 470. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, A.L.; Waliszewski, S.M.; Ruiz-Ramos, R.; del Carmen Martinez-Valenzuela, M. Time trend tendency (1988–2014 years) of organochlorine pesticide levels in the adipose tissue of Veracruz inhabitants. Environ. Monit. Assess. 2018, 190, 206. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, J.P.; Fernández-Rodríguez, M.; Artacho-Cordón, F.; Garde, C.; Perez-Carrascosa, F.; Linares, I.; Olea, N. Associations of persistent organic pollutants in serum and adipose tissue with breast cancer prognostic markers. Sci. Total Environ. 2016, 566, 41–49. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). European City Air Quality Viewer; European Environment Agency: Copenhagen, Denmark, 2019; Available online: https://www.eea.europa.eu/themes/air/urban-air-quality/european-city-air-quality-viewer (accessed on 23 April 2025).
- National Cancer Registry, Publications. Cancer in Poland in 2022. Available online: https://onkologia.org.pl/sites/default/files/publications/2025-02/0_krn-2024-book-20250212-secured.pdf (accessed on 15 November 2024).
- Dziubanek, G.; Marchwińska-Wyrwał, E.; Hajok, I. The contribution of inhalation exposure to the total exposure to dioxins and dl-PCBs depending on the season summer, winter)—A pilot study in Upper Silesia, Poland. Centr. Eur. J. Public Health 2016, 24, 115–119. [Google Scholar] [CrossRef]
- Dziubanek, G.; Marchwińska-Wyrwał, E.; Ćwieląg-Drabek, M. Preliminary study of possible relationships between exposure to PCDD/Fs and dl-PCBs in ambient air and the length of life of people. Sci. Total Environ. 2017, 598, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Central Statistical Office in Poland (CSO). Local Data Bank. Population Status. Population in Communes Without Cities with County Rights and in Cities with County Rights by Gender (as of 24 May 2023). Available online: https://bdl.stat.gov.pl/bdl/dane/podgrup/wymiary (accessed on 24 May 2023).
- Brämer, G.R. International Statistical Classification of Diseases and Health Problems–10th Revision; World Health Organization: Geneva, Switzerland, 2008; Volume 41. [Google Scholar]
- National Cancer Registry, Knowledge Base. Search for Cancer Type. Available online: https://onkologia.org.pl/pl/wyszukiwarka (accessed on 18 November 2024).
- Duda, A.; Piekut, A.; Dziubanek, G. Life Expectancy and Mortality in the Aspect of Diverse Environmental Exposure to PCDD/Fs and PCBs–Case Study from the Silesia Province. Poland. Environ. Monit. Assess. 2025; under review. [Google Scholar]
- Arnold, M.; Rentería, E.; Conway, D.I.; Bray, F.; Van Ourti, T.; Soerjomataram, I. Inequalities in cancer incidence and mortality across medium to highly developed countries in the twenty-first century. Cancer Causes Control. 2016, 27, 999–1007. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer. 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Air Quality Monitoring in Poland and Europe. Concentration of PM10, PM2,5 as of 2 January 2025. Available online: https://airly.org/map/en/ (accessed on 2 January 2025).
- Air Quality Monitoring in Poland and Europe. Air Pollution Ranking of European Cities–March 2022. Available online: https://airly.org/pl/ranking-zanieczyszczenia-powietrza-europejskich-miast-marzec-2022/ (accessed on 2 January 2025).
- Dziubanek, G.; Spychała, A.; Marchwińska-Wyrwał, E.; Rusin, M.; Hajok, I.; Ćwieląg-Drabek, M.; Piekut, A. Long-term exposure to urban air pollution and the relationship with life expectancy in cohort of 3,5 million people in Silesia. Sci. Total Environ. 2017, 580, 1–8. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Huang, F.; Chen, H.; Wu, H.; Huang, J.; Hu, J.; Xia, D.; Wu, Y. Association between Dioxin and Cancer Incidence and Mortality: A Meta-Analysis. Sci. Rep. 2016, 6, 38012. [Google Scholar] [CrossRef]
- Yoshida, R.; Ogawa, Y. Oxidative Stress Induced by 2, 3, 7, 8-Tetrachlorodibenzo-P-Dioxin: An Application of Oxidative Stress Markers to Cancer Risk Assessment of Dioxins. Ind. Health. 2000, 38, 5–14. [Google Scholar] [CrossRef]
- Chen, Y.J.; Hung, C.M.; Kay, N.; Chen, C.C.; Kao, Y.H.; Yuan, S.S. Progesterone Receptor Is Involved in 2,3,7,8-Tetrachlorodibenzo- p -Dioxin-Stimulated Breast Cancer Cells Proliferation. Cancer Lett. 2012, 319, 223–231. [Google Scholar] [CrossRef]
- Consonni, D.; Rognoni, M.; Cvalieri d’Oro, L.; Pesatori, A.C. Mortality and Cancer Incidence in a Population Exposed to TCDD after the Seveso, Italy, Accident (1976–2013). Occup. Env. Med. 2024, 81, 349–358. [Google Scholar] [CrossRef]
- Forte, I.M.; Indovina, P.; Costa, A.; Iannuzzi, C.A.; Costanzo, L.; Marfella, A.; Montagnaro, S.; Botti, G.; Bucci, E.; Giordano, A. Blood screening for heavy metals and organic pollutants in cancer patients exposed to toxic waste in southern Italy: A pilot study. J. Cell Physiol. 2020, 235, 5213–5222. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Pimpie, C.; Cano-Sancho, G.; Antignac, J.P.; Pocard, M. Detection of persistent organic pollutants in omental adipose tissue from patients with diffuse-gastric cancer: A pilot study. Cancers 2021, 13, 4874. [Google Scholar] [CrossRef]
- Koukoulakis, K.G.; Kanellopoulos, P.G.; Chrysochou, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Bakeas, E. Atmospheric concentrations and health implications of PAHs, PCBs and PCDD/Fs in the vicinity of a heavily industrialized site in Greece. Appl. Sci. 2020, 10, 9023. [Google Scholar] [CrossRef]
- Parada, H., Jr.; Sun, X.; Tse, C.K.; Engel, L.S.; Hoh, E.; Olshan, A.F.; Troester, M.A. Plasma levels of polychlorinated biphenyls (PCBs) and breast cancer mortality: The Carolina Breast Cancer Study. Int. J. Hyg. Environ. Health 2020, 227, 113522. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Zona, A.; Beccaloni, E.; Carere, M.; Comba, P. Incidence of breast, prostate, testicular, and thyroid cancer in Italian contaminated sites with presence of substances with endocrine disrupting properties. Int. J. Environ. Res. Public Health 2017, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Koual, M.; Cano-Sancho, G.; Bats, A.S.; Tomkiewicz, C.; Kaddouch-Amar, Y.; Douay-Hauser, N.; Ngo, C.; Bonsang, H.; Delomenie, M.; Lecuru, F.; et al. Associations between persistent organic pollutants and risk of breast cancer metastasis. Environ. Int. 2019, 132, 105028. [Google Scholar] [CrossRef]
- Praud, D.; Amadou, A.; Coudon, T.; Duboeuf, M.; Mercoeur, B.; Faure, E.; Grassot, L.; Danjou, A.; Aurelie, M.N.; Salizzoni, P.; et al. Association between Chronic Long-Term Exposure to Airborne Dioxins and Breast Cancer. Int. J. Hyg. Environ. Health 2025, 263, 114489. [Google Scholar] [CrossRef]
- Van Gerwen, M.; Vasan, V.; Genden, E.; Saul, S.R. Human 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Exposure and Thyroid Cancer Risk. Toxicology 2023, 488, 153474. [Google Scholar] [CrossRef]
- Avino, P.; Russo, M.V. A comprehensive review of analytical methods for determining persistent organic pollutants in air, soil, water and waste. Curr. Org. Chem. 2018, 22, 939–953. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Erdal, S.; Chen, H.Y.; Gann, P.H.; Argos, M.; Rauscher, G.H. Metallic air pollutants and breast cancer heterogeneity. Environ. Res. 2019, 177, 108639. [Google Scholar] [CrossRef] [PubMed]
- Cierpiał-Wolan, M. Energy Consumption in Households in 2021; Central Statistical Office (CSO): Warsaw, Poland; Rzeszow, Poland, 2023. [Google Scholar]
- Central Office of Building Inspection. Central Evidence of Emissions from Buildings (CEEB). Main Indicators of the Share of Source Types–Municipalities. Report. 2025. Available online: https://bdl.stat.gov.pl/bdl/dane/podgrup/temat (accessed on 24 January 2025).
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-web-inspired PM0. 3 filters based on self-sustained electrostatic nanostructured networks. Adv. Mater. 2020, 32, 2002361. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, T.; Zhang, X.; Zeng, Z.; Tao, R.; Qu, Q.; Huang, C. Multi-hierarchical nanofiber membrane with typical curved-ribbon structure fabricated by green electrospinning for efficient, breathable and sustainable air filtration. J. Membr. Sci. 2022, 660, 120857. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Lu, T.; Liu, K.; Huang, C. High performance, environmentally friendly and sustainable nanofiber membrane filter for removal of particulate matter 1.0. J. Colloid Interface Sci. 2021, 597, 48–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).