Development of a Novel Air–Liquid Interface Culture System to Investigate the Effects of Nanoplastics on Alveolar Epithelium
Abstract
1. Introduction
2. Materials and Methods
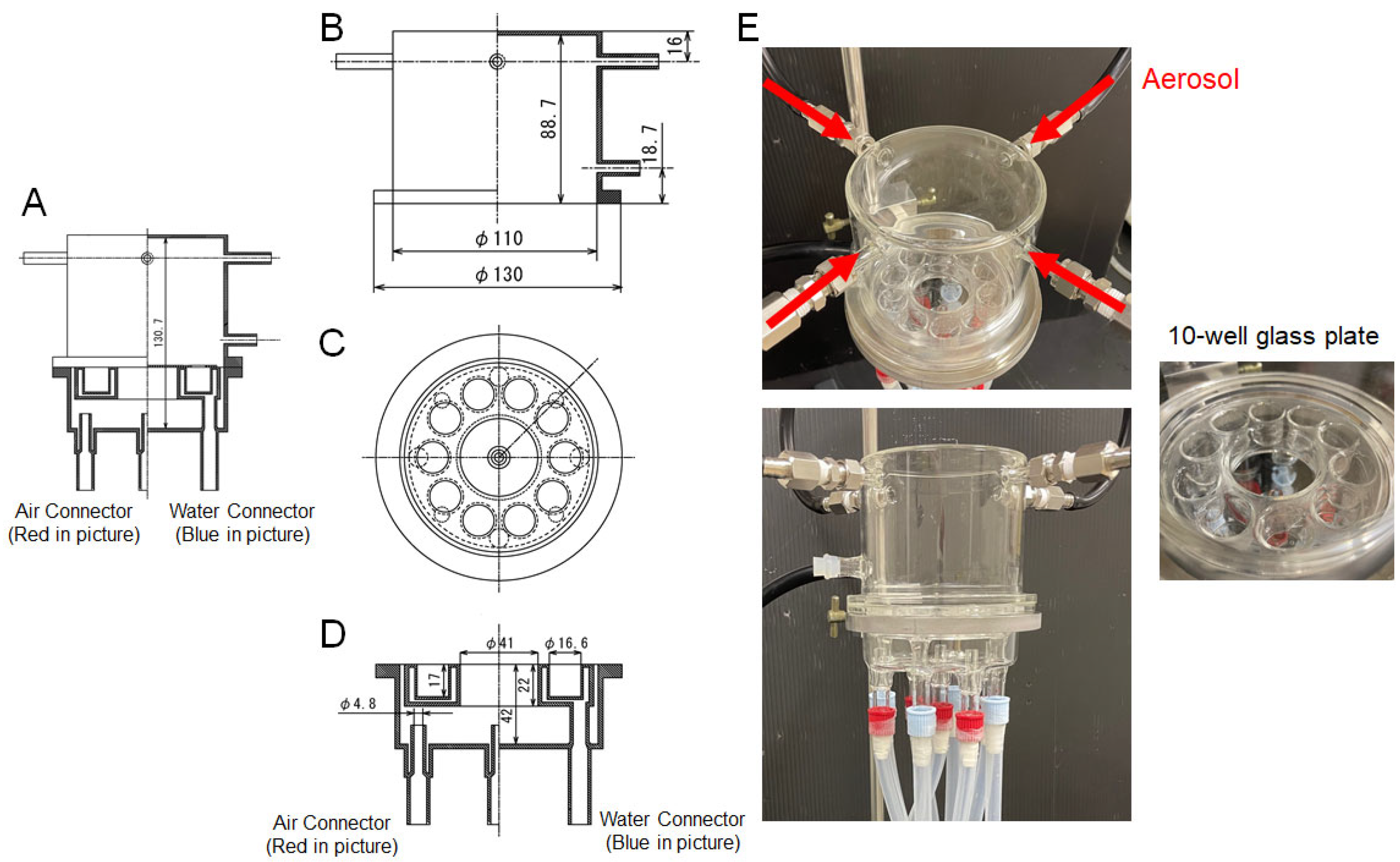
2.1. Air–Liquid Interface (ALI) Exposure System (Figure 1)
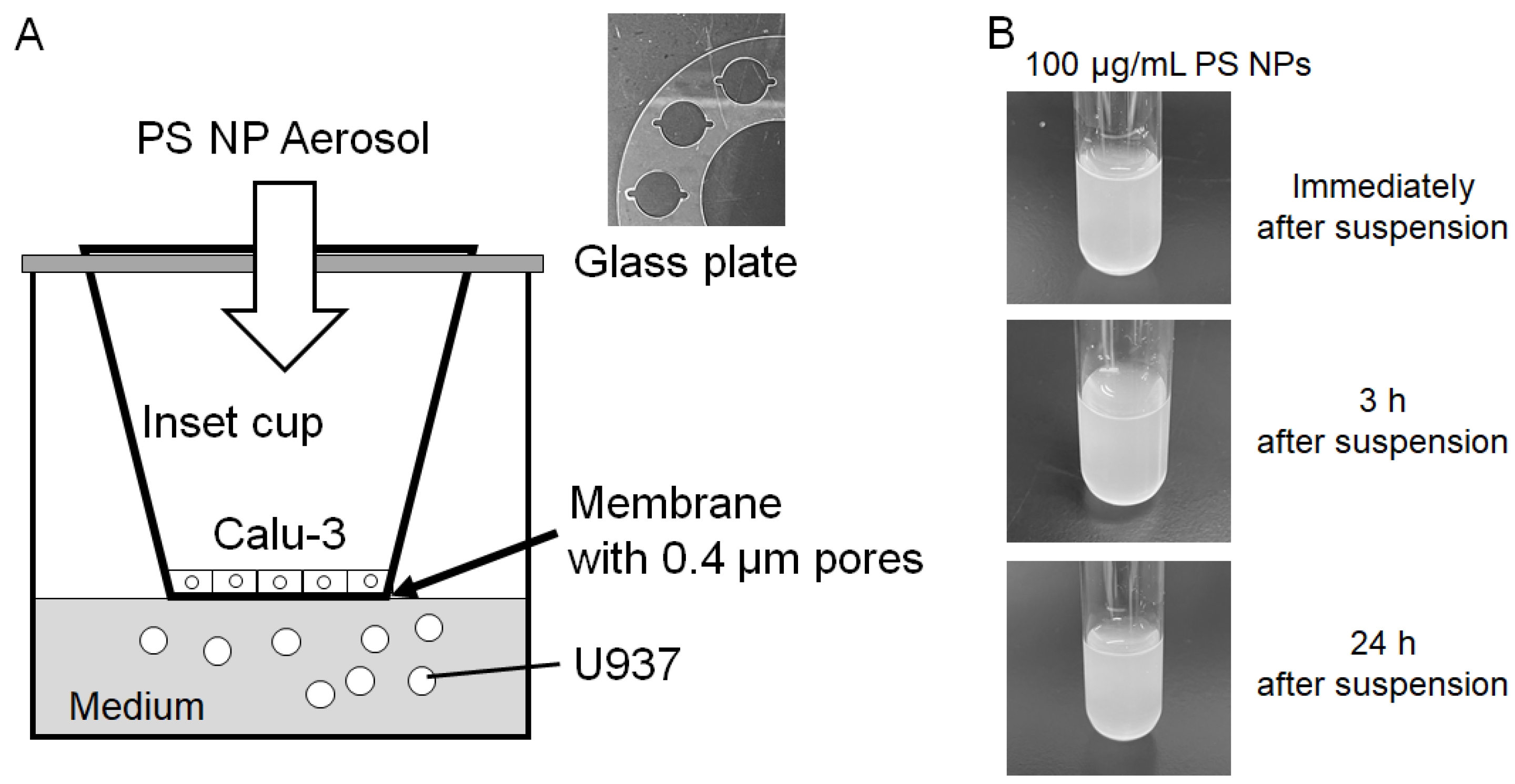
2.2. Calculation of Nano PS Mass Concentration
2.3. Co-Culture of Calu-3 and U937
2.4. Measurement of Trans-Epithelial Electrical Resistance (TEER)
2.5. Cell Viability Assessment
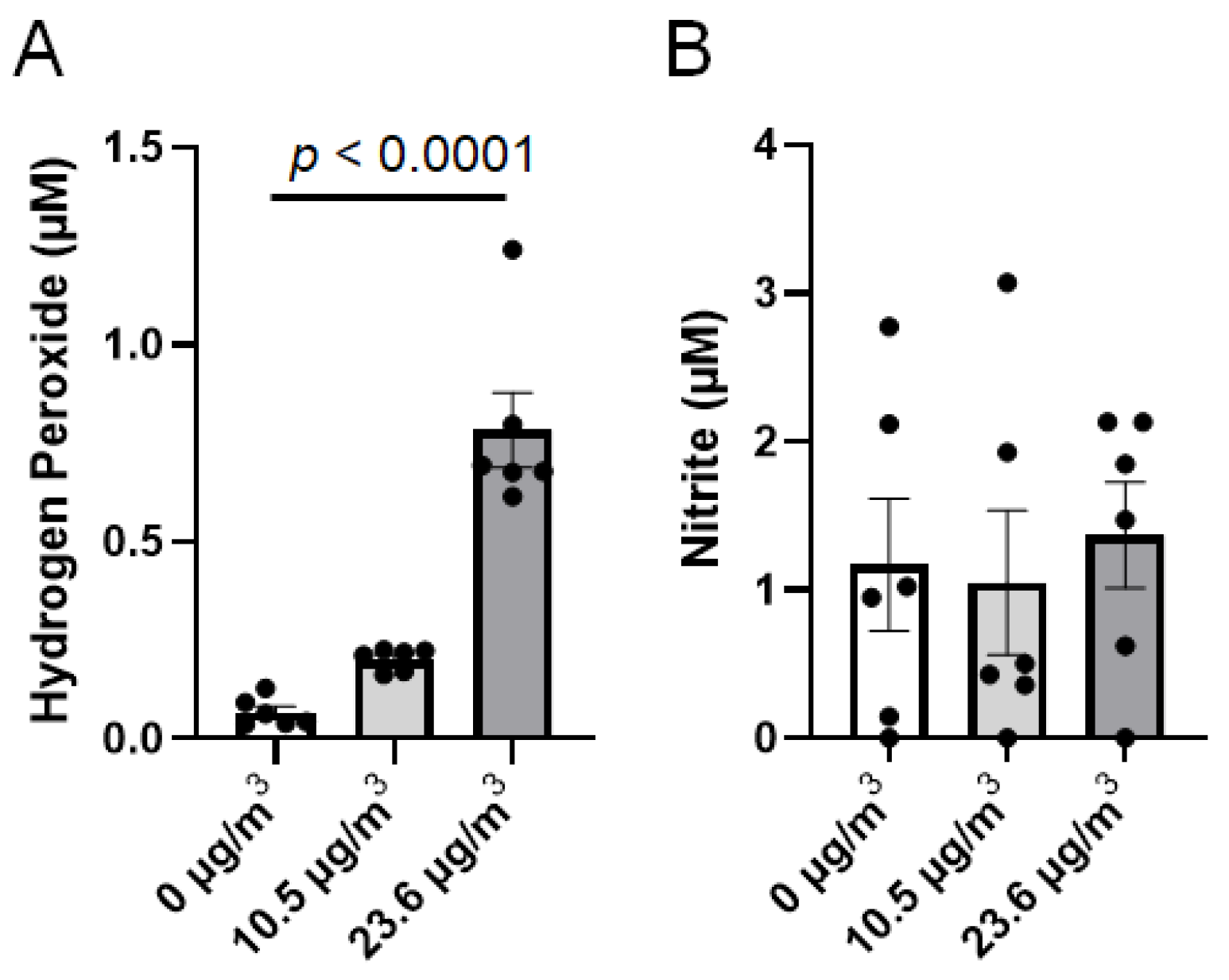
2.6. Determination of Reactive Species in the Medium
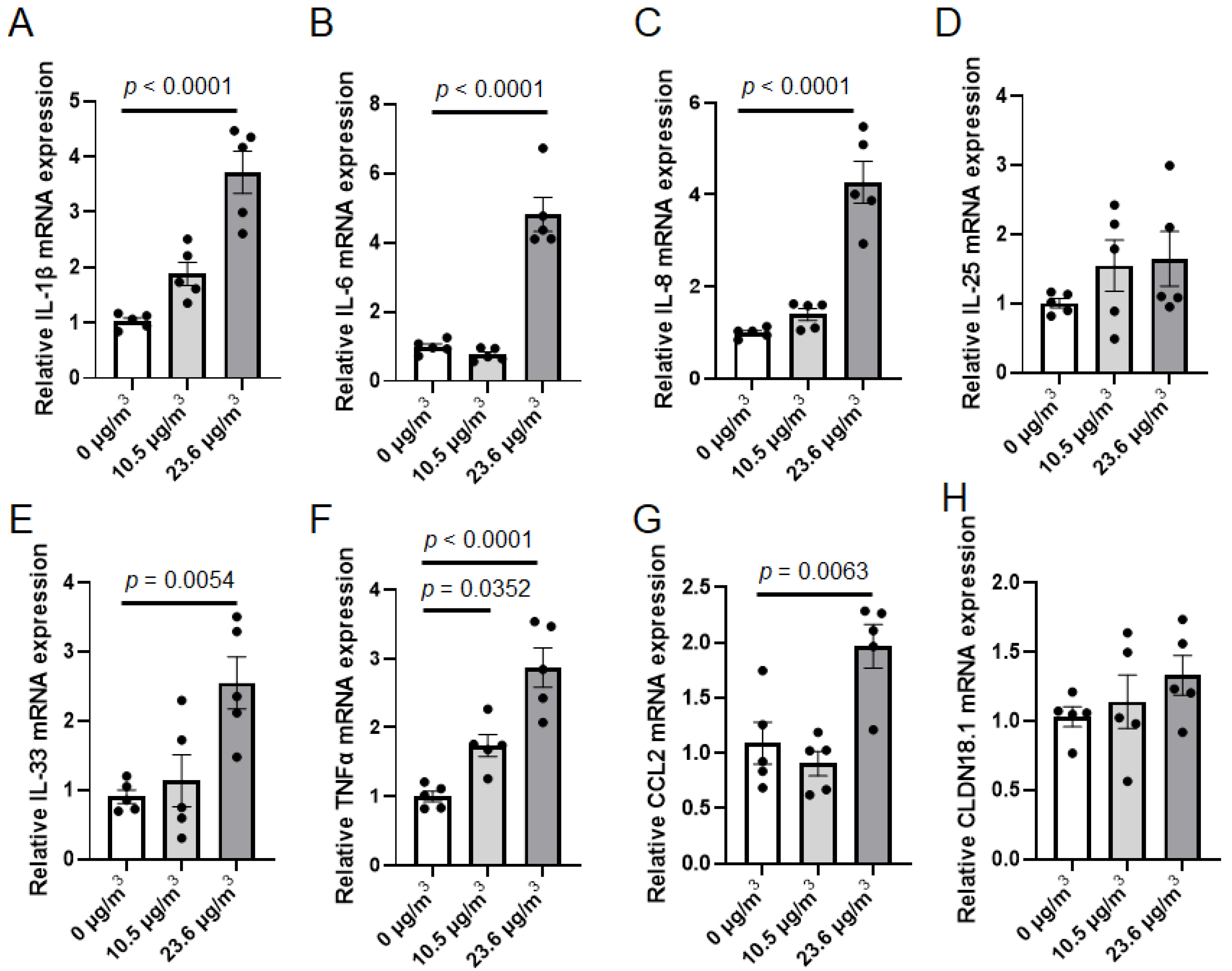
2.7. Total RNA Extraction and Real-Time PCR
2.8. Statistics
3. Results
3.1. Development of the Exposure Camber
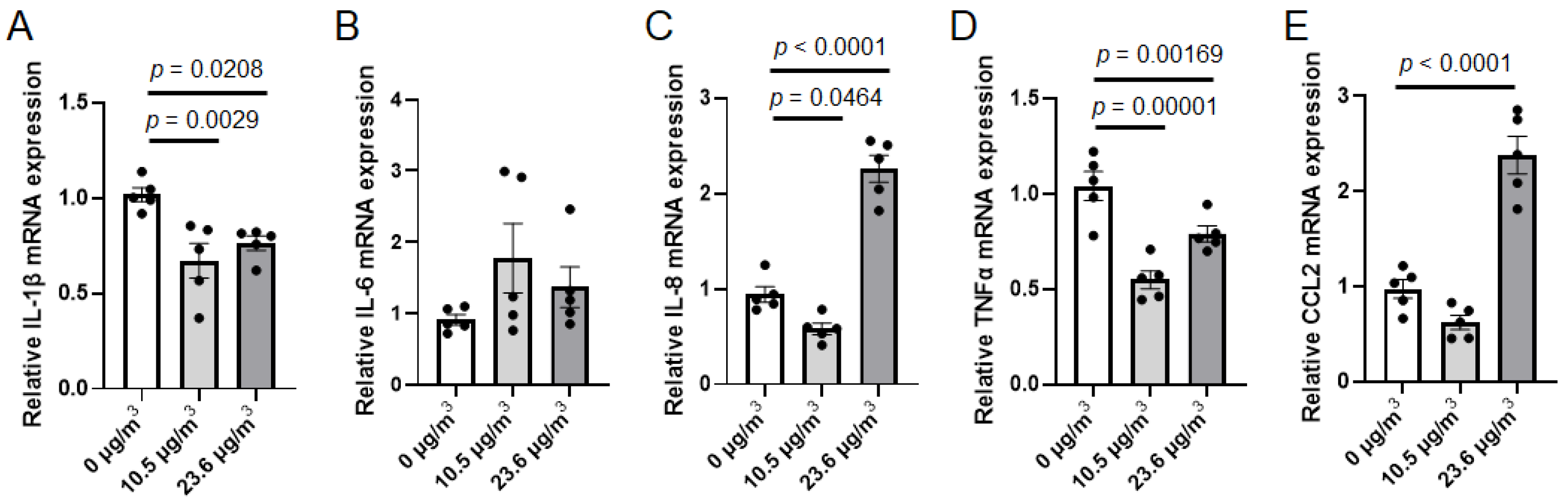
3.2. Effects of PS NP Exposure on Calu-3 Cells
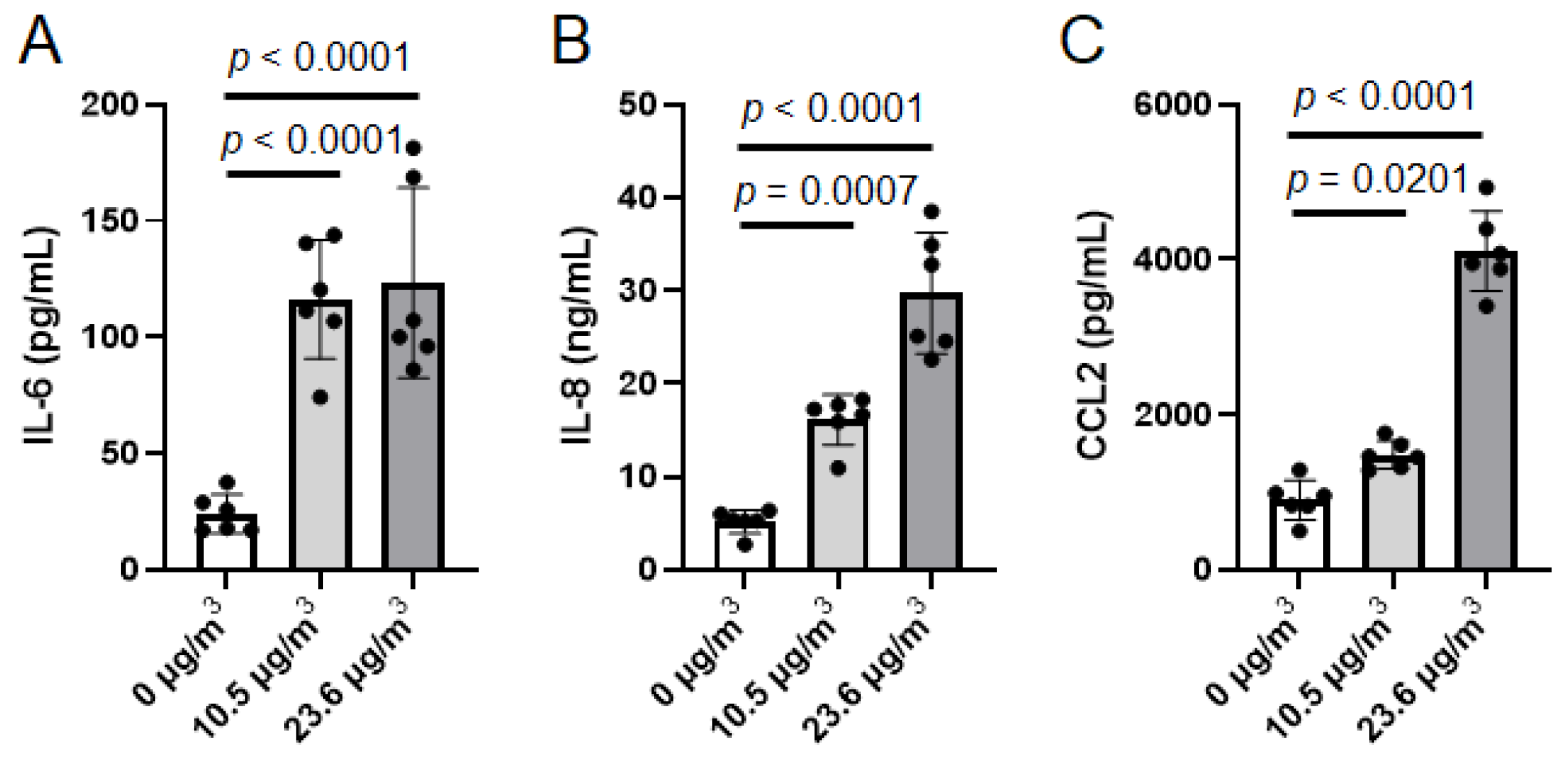
3.3. Effects of PS NP Exposure on U937 Cells Under Cell Culture Insert with Calu-3 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Zarus, G.M.; Muianga, C.; Hunter, C.M.; Pappas, R.S. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021, 756, 144010. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Morioka, T.; Tanaka, S.; Kohama-Inoue, A.; Watanabe, A. The quantification of the airborne plastic particles of 0.43-11 mum: Procedure development and application to atmospheric environment. Chemosphere 2024, 351, 141131. [Google Scholar] [CrossRef]
- Yuan, C.; Li, X.; Lu, C.; Sun, L.; Fan, C.; Fu, M.; Wang, H.; Duan, M.; Xia, S. Micro/nanoplastics in the Shenyang city atmosphere: Distribution and sources. Environ. Pollut. 2025, 372, 126027. [Google Scholar] [CrossRef]
- Luo, D.; Chu, X.; Wu, Y.; Wang, Z.; Liao, Z.; Ji, X.; Ju, J.; Yang, B.; Chen, Z.; Dahlgren, R.; et al. Micro- and nano-plastics in the atmosphere: A review of occurrence, properties and human health risks. J. Hazard. Mater. 2024, 465, 133412. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-kappaB signal in mice. Environ. Pollut. 2023, 320, 121068. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/beta-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kajino, M.; Iwamoto, Y.; Nakane, T.; Nabetani, Y.; Okuda, T.; Kono, M.; Okochi, H. Impact of artificial sunlight aging on the respiratory effects of polyethylene terephthalate microplastics through degradation-mediated terephthalic acid release in male mice. Toxicol. Sci. 2025, 203, 242–252. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Jiang, S.; Shi, R.; Wang, R.; Wu, W. Polystyrene nanoplastics induced lung injury in mice: Insights into lung metabolic disorders. Ecotoxicol. Environ. Saf. 2025, 303, 119060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, W.; Zhou, Y.; Feng, R.; Wang, Y.; Liu, L.; Yuan, Y.; Dai, J.; Liu, Y.; Zhang, X. Polystyrene nanoplastics aggravate house dust mite induced allergic airway inflammation through EGFR/ERK-dependent lung epithelial barrier dysfunction. Ecotoxicol. Environ. Saf. 2025, 298, 118329. [Google Scholar] [CrossRef]
- Jian, X.; Zhang, X.; Chang, S.; Xue, Y.; Shang, P.; Liu, Y.; Chen, H.; Zhou, X.; Wang, W.; Wang, P.; et al. Co-exposure of polystyrene nanoplastics and ozone synergistically induced airway inflammation: Evidence and biomarkers screening. Ecotoxicol. Environ. Saf. 2025, 302, 118643. [Google Scholar] [CrossRef]
- Brouwer, H.; Porbahaie, M.; Boeren, S.; Busch, M.; Bouwmeester, H. The in vitro gastrointestinal digestion-associated protein corona of polystyrene nano- and microplastics increases their uptake by human THP-1-derived macrophages. Part. Fibre Toxicol. 2024, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Bag, S.; Bhowmik, S. Insights into the Binding Interactions between Microplastics and Human alpha-Synuclein Protein by Multispectroscopic Investigations and Amyloidogenic Oligomer Formation. J. Phys. Chem. Lett. 2024, 15, 6560–6567. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Can the impact of micro- and nanoplastics on human health really be assessed using in vitro models? A review of methodological issues. Environ. Int. 2023, 178, 108115. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Takaishi, M.; Okuda, T.; Fujihara, M.; Noguchi, S.; Ishihara, Y. A simple air-liquid interface exposure system for exposing cultured human 3D epidermis and cornea to PM2.5 collected through cyclonic separation. J. Toxicol. Sci. 2024, 49, 61–68. [Google Scholar] [CrossRef]
- Ishihara, Y.; Tsuji, M.; Kawamoto, T.; Yamazaki, T. Involvement of reactive oxygen species derived from mitochondria in neuronal injury elicited by methylmercury. J. Clin. Biochem. Nutr. 2016, 59, 182–190. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kawami, T.; Ishida, A.; Yamazaki, T. Tributyltin induces oxidative stress and neuronal injury by inhibiting glutathione S-transferase in rat organotypic hippocampal slice cultures. Neurochem. Int. 2012, 60, 782–790. [Google Scholar] [CrossRef]
- Huang, Y.; Shang, P.; Li, Y.; Wang, Y. Lung hazards of microplastics and their toxicological mechanisms. Environ. Pollut. 2025, 385, 127149. [Google Scholar] [CrossRef]
- Tanaka, Y.; Wada, Y.; Fujihara, M.; Ishihara, N.; Nakane, T.; Ishihara, Y. Development of a Novel Air-Liquid Interface Exposure System for Particle Exposure in Alveolar Epithelium. Altern. Anim. Test. Exp. 2025, 30, 41–49. [Google Scholar] [CrossRef]
- Buckley, A.; Guo, C.; Laycock, A.; Cui, X.; Belinga-Desaunay-Nault, M.F.; Valsami-Jones, E.; Leonard, M.; Smith, R. Aerosol exposure at air-liquid-interface (AE-ALI) in vitro toxicity system characterisation: Particle deposition and the importance of air control responses. Toxicol. Vitr. 2024, 100, 105889. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Majeed, S.; Kratzer, G.; Vuillaume, G.; Hoeng, J.; Frentzel, S. Characterization of the Vitrocell(R) 24/48 aerosol exposure system for its use in exposures to liquid aerosols. Toxicol. Vitr. 2017, 42, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Leroux, M.M.; Hocquel, R.; Bourge, K.; Kokot, B.; Kokot, H.; Koklic, T.; Strancar, J.; Ding, Y.; Kumar, P.; Schmid, O.; et al. Aerosol-Cell Exposure System Applied to Semi-Adherent Cells for Aerosolization of Lung Surfactant and Nanoparticles Followed by High Quality RNA Extraction. Nanomaterials 2022, 12, 1362. [Google Scholar] [CrossRef]
- Tilly, T.B.; Ward, R.X.; Luthra, J.K.; Robinson, S.; Eiguren-Fernandez, A.; Lewis, G.S.; Salisbury, R.L.; Lednicky, J.A.; Sabo-Attwood, T.L.; Hussain, S.M.; et al. Condensational particle growth device for reliable cell exposure at the air-liquid interface to nanoparticles. Aerosol Sci. Technol. 2019, 53, 1415–1428. [Google Scholar] [CrossRef]
- Kustner, M.J.; Eckstein, D.; Brauer, D.; Mai, P.; Hampl, J.; Weise, F.; Schuhmann, B.; Hause, G.; Glahn, F.; Foth, H.; et al. Modular air-liquid interface aerosol exposure system (MALIES) to study toxicity of nanoparticle aerosols in 3D-cultured A549 cells in vitro. Arch. Toxicol. 2024, 98, 1061–1080. [Google Scholar] [CrossRef]
- Ihalainen, M.; Jalava, P.; Ihantola, T.; Kasurinen, S.; Uski, O.; Sippula, O.; Hartikainen, A.; Tissari, J.; Kuuspalo, K.; Lähde, A.; et al. Design and validation of an air-liquid interface (ALI) exposure device based on thermophoresis. Aerosol Sci. Technol. 2019, 53, 133–145. [Google Scholar] [CrossRef]
- Kapp, N.; Kreyling, W.; Schulz, H.; Im Hof, V.; Gehr, P.; Semmler, M.; Geiser, M. Electron energy loss spectroscopy for analysis of inhaled ultrafine particles in rat lungs. Microsc. Res. Tech. 2004, 63, 298–305. [Google Scholar] [CrossRef]
- Savi, M.; Kalberer, M.; Lang, D.; Ryser, M.; Fierz, M.; Gaschen, A.; Ricka, J.; Geiser, M. A novel exposure system for the efficient and controlled deposition of aerosol particles onto cell cultures. Environ. Sci. Technol. 2008, 42, 5667–5674. [Google Scholar] [CrossRef]
- Petroff, A.; Zhang, L. Development and validation of a size-resolved particle dry deposition scheme for application in aerosol transport models. Geosci. Model. Dev. 2010, 3, 753–769. [Google Scholar] [CrossRef]
- Han, M.; Liang, J.; Wang, K.; Si, Q.; Zhu, C.; Zhao, Y.; Khan, N.A.K.; Abdullah, A.L.B.; Shau-Hwai, A.T.; Li, Y.M.; et al. Integrin A5B1-mediated endocytosis of polystyrene nanoplastics: Implications for human lung disease and therapeutic targets. Sci. Total Environ. 2024, 953, 176017. [Google Scholar] [CrossRef]
- Liu, J.; Xu, F.; Guo, M.; Gao, D.; Song, Y. Nasal instillation of polystyrene nanoplastics induce lung injury via mitochondrial DNA release and activation of the cyclic GMP-AMP synthase-stimulator of interferon genes-signaling cascade. Sci. Total Environ. 2024, 948, 174674. [Google Scholar] [CrossRef]
- Goksel, O.; Sipahi, M.I.; Yanasik, S.; Saglam-Metiner, P.; Benzer, S.; Sabour-Takanlou, L.; Sabour-Takanlou, M.; Biray-Avci, C.; Yesil-Celiktas, O. Comprehensive analysis of resilience of human airway epithelial barrier against short-term PM2.5 inorganic dust exposure using in vitro microfluidic chip and ex vivo human airway models. Allergy 2024, 79, 2953–2965. [Google Scholar] [CrossRef]
- Zimmerman, E.; Sturrock, A.; Reilly, C.A.; Burrell-Gerbers, K.L.; Warren, K.; Mir-Kasimov, M.; Zhang, M.A.; Pierce, M.S.; Helms, M.N.; Paine, R., 3rd. Aryl Hydrocarbon Receptor Activation in Pulmonary Alveolar Epithelial Cells Limits Inflammation and Preserves Lung Epithelial Cell Integrity. J. Immunol. 2024, 213, 600–611. [Google Scholar] [CrossRef]
- Mu, M.; Gao, P.; Yang, Q.; He, J.; Wu, F.; Han, X.; Guo, S.; Qian, Z.; Song, C. Alveolar Epithelial Cells Promote IGF-1 Production by Alveolar Macrophages Through TGF-beta to Suppress Endogenous Inflammatory Signals. Front. Immunol. 2020, 11, 1585. [Google Scholar] [CrossRef]
- Speth, J.M.; Bourdonnay, E.; Penke, L.R.; Mancuso, P.; Moore, B.B.; Weinberg, J.B.; Peters-Golden, M. Alveolar Epithelial Cell-Derived Prostaglandin E2 Serves as a Request Signal for Macrophage Secretion of Suppressor of Cytokine Signaling 3 during Innate Inflammation. J. Immunol. 2016, 196, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Matter, M.; Maye, I.; Im Hof, V.; Gehr, P.; Schurch, S. Influence of airspace geometry and surfactant on the retention of man-made vitreous fibers (MMVF 10a). Environ. Health Perspect. 2003, 111, 895–901. [Google Scholar] [CrossRef][Green Version]
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef]

| Name | Sequence (5′-3′) |
|---|---|
| Human IL-1β Forward | GGGCCTCAAGGAAAAGAATC |
| Human IL-1β Reverse | TTCTGCTTGAGAGGTGCTGA |
| Human IL-6 Forward | GAAGGCAGCAGGCAACAC |
| Human IL-6 Reverse | CAGGAGCCCAGCTATGAACT |
| Human IL-8 Forward | GAAGTTTTTGAAGAGGGCTGAGA |
| Human IL-8 Reverse | TTTGCTTGAAGTTTCACTGGCA |
| Human IL-25 Forward | CCAGGTTGCATTCTTGG |
| Human IL-25 Reverse | TGGCTGTAGGTGTGGGTTCC |
| Human IL-33 Forward | GCCAAAGAAGTTTGCCCCAT |
| Human IL-33 Reverse | TGAAATTCCTGTTTTCAGTGAAGG |
| Human TNFα Forward | CTCTCTCTAATCAGCCCTCTG |
| Human TNFα Reverse | GAGGACCTGGGAGTAGATGAG |
| Human CCL2 Forward | TCAAACTGAAGCTCGCACTCT |
| Human CCL2 Reverse | GGCATTGATTGCATCTGGC |
| Human CLDN18.1 Forward | TCCACCACCACATGCCAAGTG |
| Human CLDN18.1 Reverse | GTGTACATGTTAGCTGTGGAC |
| Human GAPDH Forward | GAGTCAACGGATTTGG |
| Human GAPDH Reverse | TTGATTTTGGAGGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, I.; Wada, Y.; Fujihara, M.; Ishihara, Y. Development of a Novel Air–Liquid Interface Culture System to Investigate the Effects of Nanoplastics on Alveolar Epithelium. Atmosphere 2025, 16, 1343. https://doi.org/10.3390/atmos16121343
Okuda I, Wada Y, Fujihara M, Ishihara Y. Development of a Novel Air–Liquid Interface Culture System to Investigate the Effects of Nanoplastics on Alveolar Epithelium. Atmosphere. 2025; 16(12):1343. https://doi.org/10.3390/atmos16121343
Chicago/Turabian StyleOkuda, Iroha, Yurika Wada, Masashi Fujihara, and Yasuhiro Ishihara. 2025. "Development of a Novel Air–Liquid Interface Culture System to Investigate the Effects of Nanoplastics on Alveolar Epithelium" Atmosphere 16, no. 12: 1343. https://doi.org/10.3390/atmos16121343
APA StyleOkuda, I., Wada, Y., Fujihara, M., & Ishihara, Y. (2025). Development of a Novel Air–Liquid Interface Culture System to Investigate the Effects of Nanoplastics on Alveolar Epithelium. Atmosphere, 16(12), 1343. https://doi.org/10.3390/atmos16121343





