Regional and Whole-Body Dermal Emission Rates of Volatile Sulfur Compounds and Potential Impact on Indoor Air Odour
Abstract
1. Introduction
2. Methods
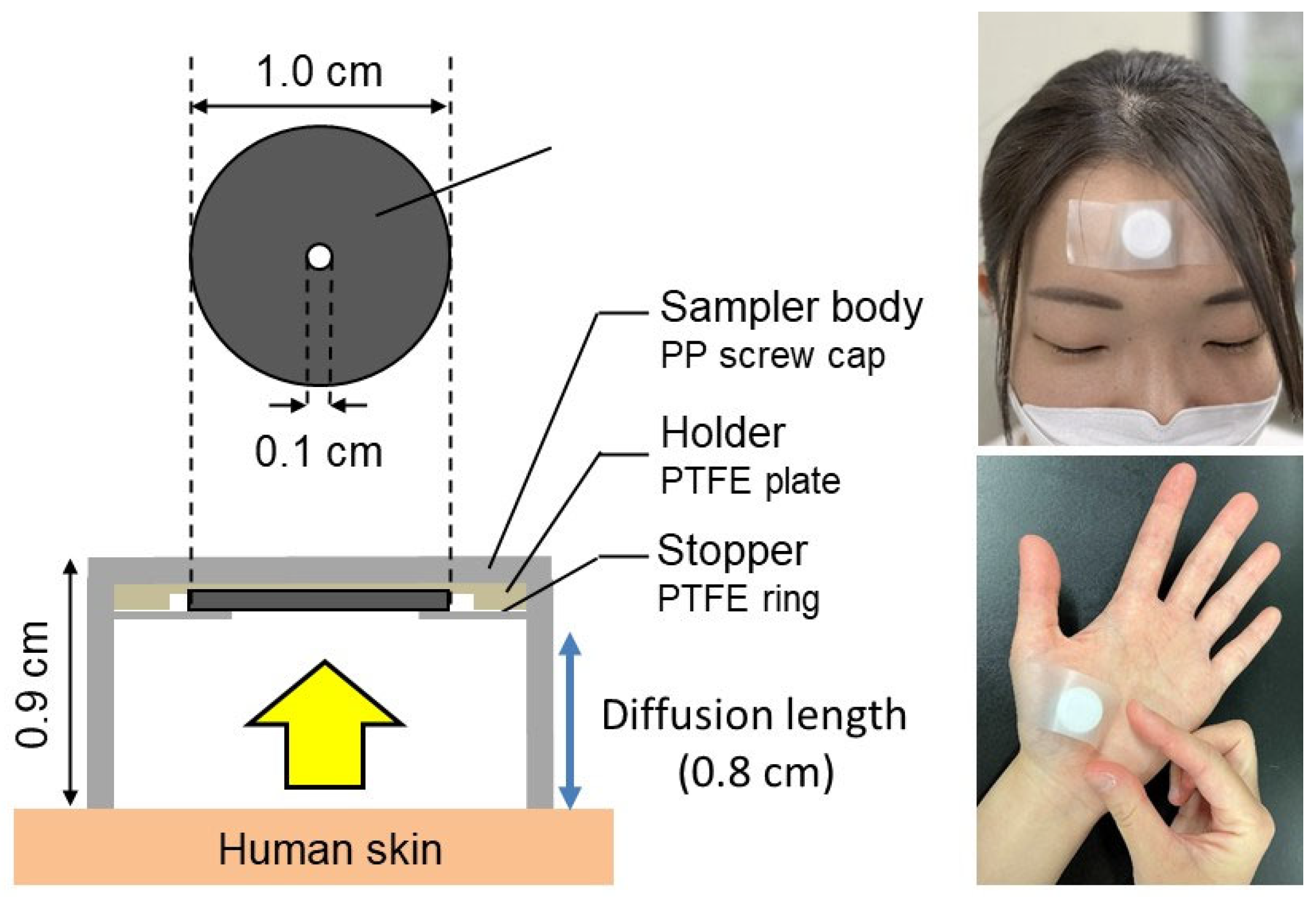
2.1. Subject Test
2.2. Regional and Whole-Body Dermal Emission Rate of VSCs
2.3. Impact of Skin-Derived VSCs on Indoor Air Quality
2.4. Quality Assurance and Quality Control
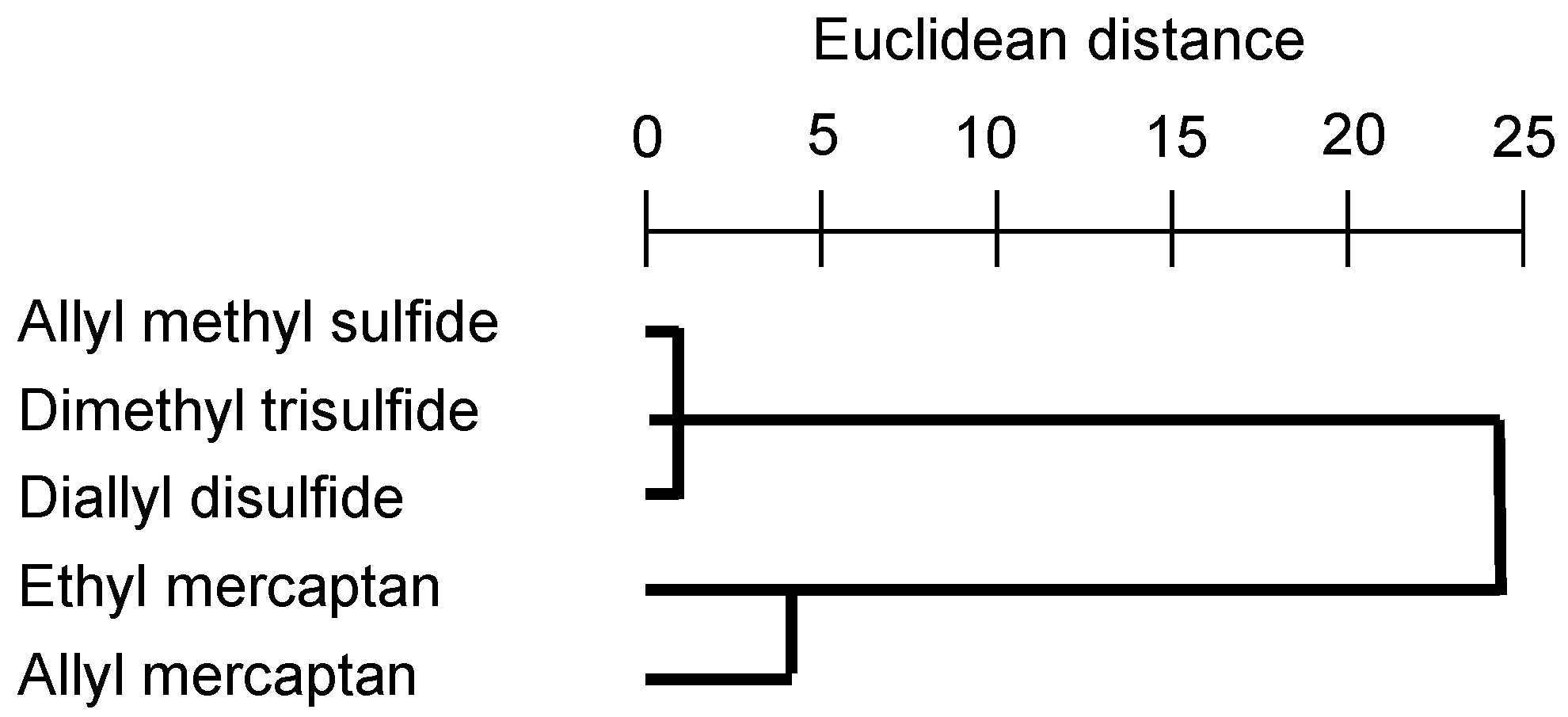
2.5. Statistical Analysis
3. Results and Discussion
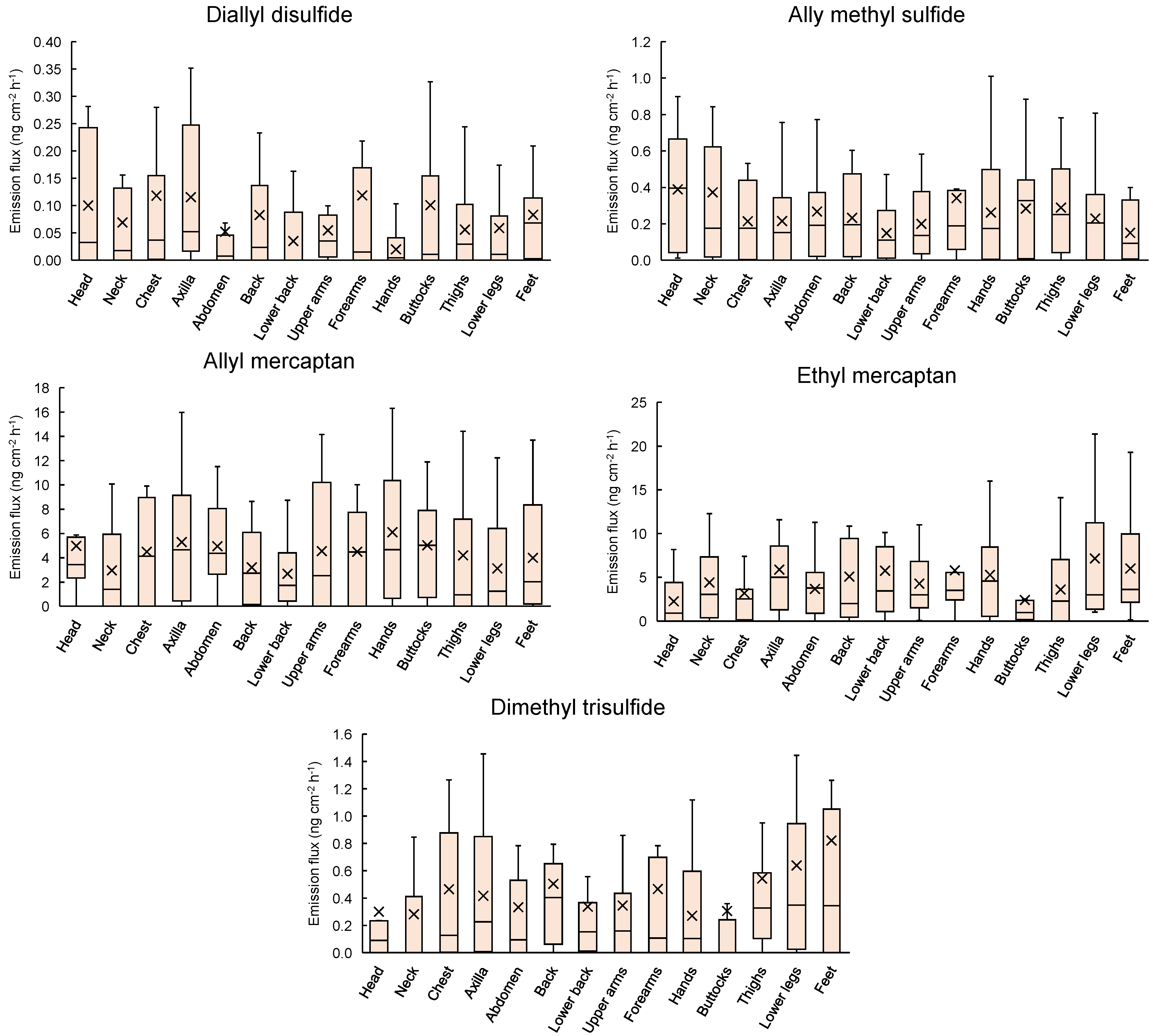
3.1. Dermal Emission Fluxes of VSCs at 14 Sampling Sites
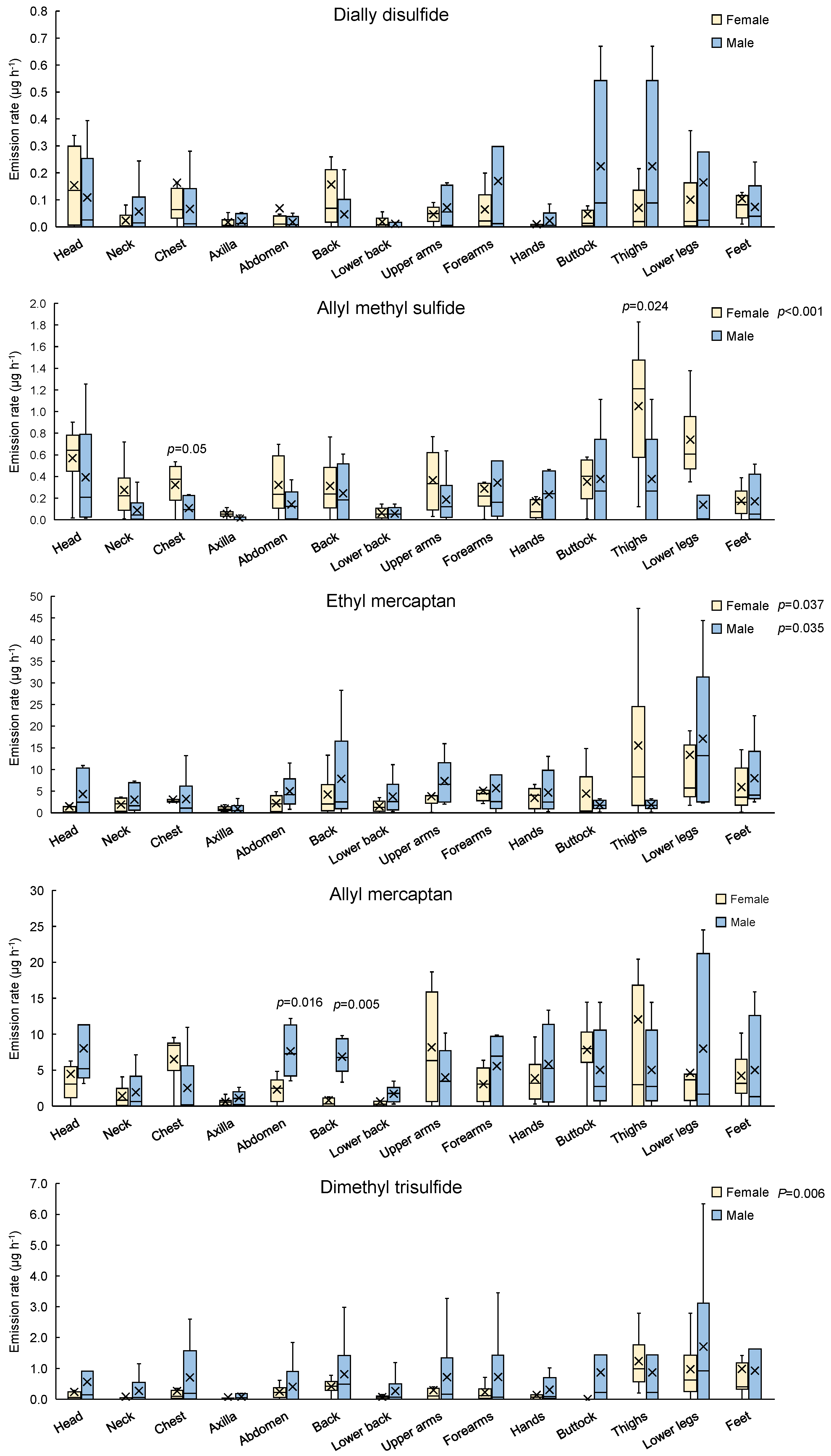
3.2. Regional and Whole-Body Dermal Emission Rate of VSCs
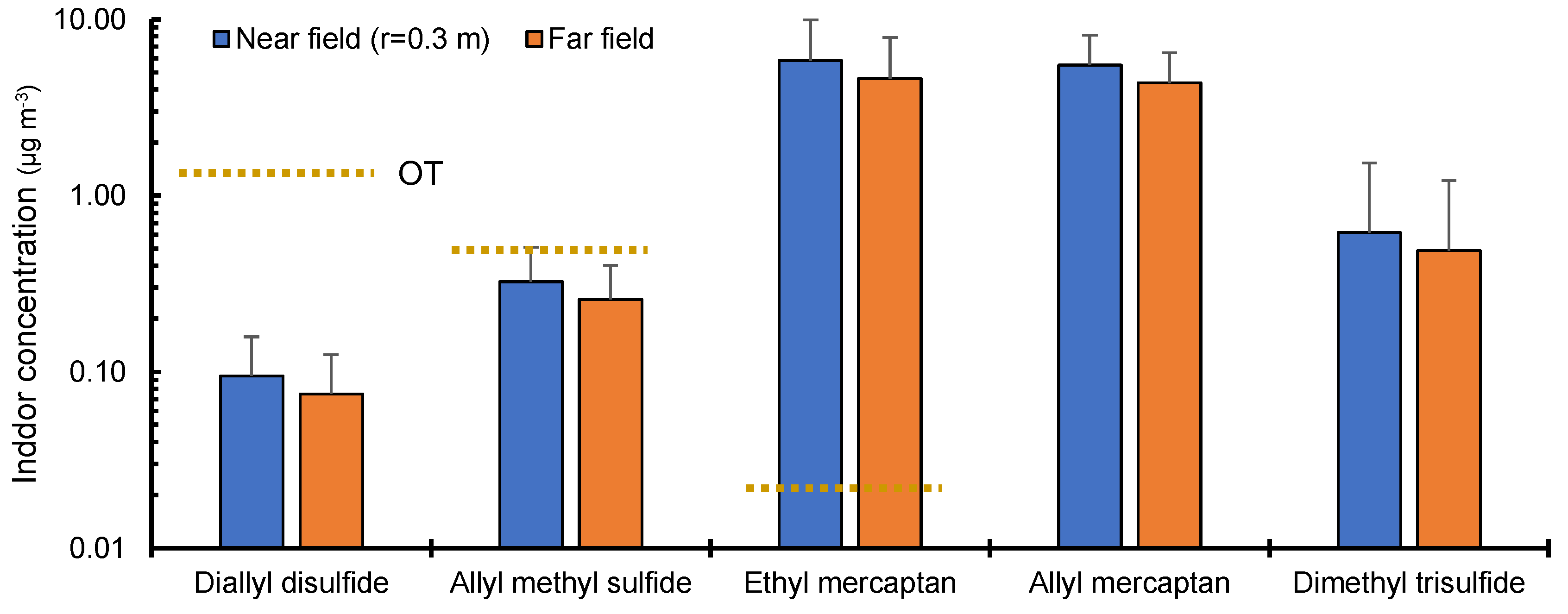
3.3. Impact of Skin-Derived VSCs on Indoor Air Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitko, T.G.; Cowden, S.; Suffet, I.H.M. Evaluation of bioscrubber and biofilter technologies treating wastewater foul air by a new approach of using odor character, odor intensity, and chemical analyses. Water Res. 2022, 15, 118691. [Google Scholar] [CrossRef]
- Mirondo, R.; Barringer, S. Deodorization of garlic breath by foods, and the role of polyphenol oxidase and phenolic compounds. J. Food Sci. 2016, 81, C2425–C2430. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sekine, Y.; Kakumu, Y.; Hiramoto, T. Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic. Sci. Rep. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Haggard, H.W.; Greenberg, L.A. Breath odors from alliaceous substance: Cause and remedy. J. Am. Med. Assoc. 1935, 104, 2160–2163. [Google Scholar] [CrossRef]
- Minami, T.; Boku, T.; Inada, K.; Morita, M.; Okazaki, Y. Odor components of human breath after the ingestion of grated raw garlic. J. Food Sci. 1989, 54, 763–765. [Google Scholar] [CrossRef]
- Taucher, J.; Hansel, A.; Jordan, A.; Lindinger, W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996, 44, 3778–3782. [Google Scholar] [CrossRef]
- Hosomi, K.; Kunisawa, J. Intestinal metabolic environment created with diets and gut microbiota and its impact on our health. J. Jpn. Biochem. Soc. 2023, 95, 450–456. [Google Scholar] [CrossRef]
- Katsuyama, M.; Narita, T.; Nakashima, M.; Kusaba, K.; Ochiai, M.; Kunizawa, N.; Kawaraya, A.; Kuwahara, Y.; Horiuchi, M.; Nakamoto, K. How emotional changes affect skin odor and its impact on others. PLoS ONE 2022, 17, e0270457. [Google Scholar] [CrossRef]
- Kyono, A.; Takei, R.; Murase, M.; Fujioka, Y.; Sekine, Y. Human skin gas components which affect the quality of sleep. In Proceedings of the 37th Annual Conference of the Society for Odor and Flavor Environmental Science, Tokyo, Japan, 29–30 August 2024; p. 4. (In Japanese). [Google Scholar]
- Sekine, Y.; Oikawa, D.; Todaka, M. Human skin gas profile of individuals with the people allergic to me phenomenon. Sci. Rep. 2023, 13, 9471. [Google Scholar] [CrossRef]
- Mitra, A.; Choi, S.; Boshier, P.R.; Razumovskaya-Hough, A.; Belluomo, I.; Spanel, P.; Hanna, G.B. The Human Skin Volatolome: A Systematic Review of Untargeted Mass Spectrometry Analysis. Metabolites 2022, 12, 824. [Google Scholar] [CrossRef]
- Furukawa, S.; Sekine, Y.; Kimura, K.; Umezawa, K.; Asai, S.; Miyachi, H. Simultaneous and multi-point measurement of ammonia emanating from human skin surface for the estimation of whole body dermal emission rate. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1053, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Nikaido, N.; Sato, S.; Todaka, M. Trace biogases emanating from human skin surface—Acetic acid as a possible biomarker for sweating. Frag. J. 2018, 2018, 19–25. (In Japanese) [Google Scholar]
- Wei, Y.; Wang, X.; Ji, M.; Deng, S.; Zhou, M.; Huang, L. Volatile organic compounds in residential indoor environments in winter: A field investigation in Beijing. Air Qual. Atmos. Health 2025, 18, 1115–1126. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, J.K. Indoor concentration distributions of ammonia and sulfur-based odorous substances according to types of laying hen houses in South Korea. Atmosphere 2024, 15, 980. [Google Scholar] [CrossRef]
- You, B.; Zhou, W.; Li, J.; Li, Z.; Sun, Y. A review of indoor Gaseous organic compounds and human chemical Exposure: Insights from Real-time measurements. Environ. Int. 2022, 170, 107611. [Google Scholar] [CrossRef]
- Tsushima, S.; Wargocki, P.; Tanabe, S. Sensory evaluation and chemical analysis of exhaled and dermally emitted bioeffluents. Indoor Air 2018, 28, 146–163. [Google Scholar] [CrossRef]
- Kurazumi, Y.; Horikoshi, T.; Tsuchikawa, T.; Matsubara, N. The body surface area of Japanese. Jpn. J. Biometeorol. 1994, 31, 5–29. (In Japanese) [Google Scholar]
- Ramachandran, G. Occupational Exposure Assessment for Air Contaminants. In Exposure Modelling; CRC Press: Boca Raton, FL, USA, 2005; Chapter 17; pp. 286–289. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, S.Y. A comparative analysis of malodor samples between direct (olfactometry) and indirect (instrumental) methods. Atmos. Environ. 2008, 42, 5061–5070. [Google Scholar] [CrossRef]
- Japan Association on Odor Environment, Odor Threshold Values of Odorous Chemicals. Available online: https://orea.or.jp/gijutsu/kyuukakusokuteihou/odor-threshold-values/ (accessed on 19 February 2023).
- Ultsch, A.; Lötsch, J. Euclidean distance-optimized data transformation for cluster analysis in biomedical data (EDOtrans). BMC Bioinform. 2022, 23, 233. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Q.; Li, H.; Song, X.; Wang, S.; Zhang, W.; Wang, G. The relationship between blood perfusion in the lower extremities and heart rate variability at different positions. Front. Physiol. 2021, 12, 656527. [Google Scholar] [CrossRef]
- Lintzeri, D.A.; Karimian, N.; Blume-Peytavi, U.; Kottner, J. Epidermal thickness in healthy humans: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1191–1200. [Google Scholar] [CrossRef]
- Morioka, D.; Nomura, M.; Lan, L.; Tanaka, R.; Kadomatsu, K. Axillary Osmidrosis: Past, Present, and Future. Ann. Plast. Surg. 2020, 84, 722–728. [Google Scholar] [CrossRef]
- Baumann, T.; Bergmann, S.; Schmidt-Rose, T.; Max, H.; Martin, A.; Enthaler, B.; Terstegen, L.; Schweiger, D.; Kalbacher, H.; Wenck, H.; et al. Glutathione-conjugated sulfanylalkanols are substrates for ABCC11 and γ-glutamyl transferase 1: A potential new pathway for the formation of odorant precursors in the apocrine sweat gland. Exp. Dermatol. 2014, 23, 247–252. [Google Scholar] [CrossRef]

| Body Surface Area (m2) | |||
|---|---|---|---|
| Body Region | Female (n = 6) | Male (n = 6) | All (n = 12) |
| Head | 0.12 ± 0.0092 | 0.13 ± 0.12 | 0.13 ± 0.011 |
| Neck | 0.046 ± 0.0044 | 0.063 ± 0.055 | 0.055 ± 0.0095 |
| Chest | 0.10 ± 0.0074 | 0.11 ± 0.10 | 0.10 ± 0.0084 |
| Axilla | 0.015 ± 0.0012 | 0.017 ± 0.016 | 0.016 ± 0.0015 |
| Abdomen | 0.080 ± 0.0075 | 0.11 ± 0.094 | 0.094 ± 0.016 |
| Back | 0.11 ± 0.0086 | 0.12 ± 0.12 | 0.12 ± 0.010 |
| Lower back | 0.035 ± 0.0037 | 0.053 ± 0.044 | 0.044 ± 0.0094 |
| Upper arms | 0.14 ± 0.011 | 0.15 ± 0.14 | 0.14 ± 0.014 |
| Forearms | 0.087 ± 0.0071 | 0.10 ± 0.094 | 0.094 ± 0.0096 |
| Hands | 0.073 ± 0.0060 | 0.085 ± 0.080 | 0.08 ± 0.0083 |
| Buttocks | 0.13 ± 0.0096 | 0.14 ± 0.13 | 0.13 ± 0.011 |
| Thighs | 0.30 ± 0.020 | 0.29 ± 0.29 | 0.30 ± 0.022 |
| Lower legs | 0.20 ± 0.015 | 0.22 ± 0.21 | 0.21 ± 0.018 |
| Feet | 0.11 ± 0.0085 | 0.12 ± 0.11 | 0.11 ± 0.011 |
| Whole-body | 1.5 ± 0.12 | 1.7 ± 1.6 | 1.6 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaka, T.; Sato, D.; Hosomi, A.; Fukui, M.; Sekine, Y. Regional and Whole-Body Dermal Emission Rates of Volatile Sulfur Compounds and Potential Impact on Indoor Air Odour. Atmosphere 2025, 16, 1331. https://doi.org/10.3390/atmos16121331
Osaka T, Sato D, Hosomi A, Fukui M, Sekine Y. Regional and Whole-Body Dermal Emission Rates of Volatile Sulfur Compounds and Potential Impact on Indoor Air Odour. Atmosphere. 2025; 16(12):1331. https://doi.org/10.3390/atmos16121331
Chicago/Turabian StyleOsaka, Tomomi, Daisuke Sato, Akihiro Hosomi, Mizuki Fukui, and Yoshika Sekine. 2025. "Regional and Whole-Body Dermal Emission Rates of Volatile Sulfur Compounds and Potential Impact on Indoor Air Odour" Atmosphere 16, no. 12: 1331. https://doi.org/10.3390/atmos16121331
APA StyleOsaka, T., Sato, D., Hosomi, A., Fukui, M., & Sekine, Y. (2025). Regional and Whole-Body Dermal Emission Rates of Volatile Sulfur Compounds and Potential Impact on Indoor Air Odour. Atmosphere, 16(12), 1331. https://doi.org/10.3390/atmos16121331






