Abstract
The geological storage of CO2 is a critical technique for reducing emissions, which significantly contributes to the mitigation of the greenhouse effect. Currently, CO2 is often geologically stored in coal seams, hydrocarbon reservoirs, and saline aquifers in order to store CO2 and improve the oil and gas recovery simultaneously. Shale formations, as candidates for CO2 storage, are drawing more attention because of their rich volumes. CO2 storage through shale formations in the Sichuan Basin, China, has tremendous potential because of the readily available CO2 injection equipment, such as abandoned shale gas wells. Therefore, we review the potential of using these wells to store CO2 in this paper. Firstly, we review the status of the geological storage of CO2 and discuss the features and filed applications for the most studied storage techniques. Secondly, we investigate the formation properties, shale gas field development process, and characteristics of the abandoned wells in the Sichuan Basin. Additionally, after carefully studying the mechanism and theoretical storage capacity, we evaluate the potential of using these abandoned wells to store CO2. Lastly, recommendations are proposed based on the current technologies and government policies. We hope this paper may provide some insights into the development of geological CO2 storage using unconventional reservoirs.
1. Introduction
Recently, global warming has attracted more concern because of the increasing consumption of fossil fuels. Therefore, many countries are striving to alleviate global warming by proposing measures to control greenhouse gas emissions. To achieve this goal, a method of carbon neutrality has been proposed [1], the aim of which is to balance anthropogenic emissions via sources and removals via sinks of greenhouse gases in the second half of the 21st century. China has been the world’s largest emitter of greenhouse gases for 14 consecutive years since 2006, according to the statistics from the Global Carbon Atlas [2]. China attaches great importance to this. In September 2020, at the 75th United Nations General Assembly, China solemnly pledged to reach peak carbon dioxide emissions by 2030 and to strive to achieve carbon neutrality by 2060 [3].
A number of emission reduction measures have already been implemented, such as developing wind, solar, hydro, and nuclear energy sources. In particular, the utilization and geological storage of CO2 are also critical matters for emission reductions. Coal seams, saline aquifers, and hydrocarbon reservoirs are the main places for CO2 storage. The Sleipner Project, the world’s first commercial-scale CO2 storage project in Norway, has successfully injected tens of millions of tons of CO2 into the saline formation of Utsira sandstone [4]. The CO2 injection in the Weyburn Project in Canada achieves CO2 storage while enhancing oil recovery [5]. In China, demonstration projects for CO2-enhanced oil and coal belt methane recovery have been implemented in the Jilin Oilfield, Shengli Oilfield, and Qinshui Coalfield [6]. In addition, pilot tests of CO2 sequestration in coal seams have been conducted in Australia and Canada [7]. China’s first demonstration project for geological storage of CO2 in deeply saline aquifers is located in the Ordos Basin of Inner Mongolia. During 2011–2015, CO2 was injected into the saline aquifers at a rate of 100,000 tons/year [8]. The project has largely contributed to the development of CO2 storage technology in China.
Inspired by the successful application of CO2 storage in hydrocarbon reservoirs and saline aquifers, injecting CO2 into abandoned shale gas wells for EOR and CO2 storage has become a potential candidate approach for CO2 storage. The low porosity and low permeability of shale reservoirs require an extensive well network to ensure the efficient extraction of shale gas, resulting in a large number of wells. These wells are abandoned after a certain period of time because of declining production rates or due to engineering factors such as casing damage, aging equipment, and downhole junk. The cost of shutting down and disposing of these abandoned wells is about $50,000, and it would be a huge waste if these abandoned wells are not used wisely [9]. It is critical to employ these abandoned shale gas wells to store CO2 and thereby to further mitigate the greenhouse gas effect, as well as to enhance the recovery factor of shale resources.
China has actively explored the development of relevant theories and technologies and strived to shorten the gap with the international advanced level in terms of unconventional oil and gas recovery, geological storage, and CO2 storage monitoring and early warning systems.
2. Advances in CO2 Geological Storage
Carbon capture and storage (CCS) is one of the most effective ways to mitigate global warming and reduce carbon dioxide emissions [10,11,12]. The most studied techniques involve the storage of CO2 in coal seams, hydrocarbon reservoirs, and saline aquifers [13,14,15]. CO2 storage in shale has drawn growing attention recently. So far, CO2 has been successfully stored in hydrocarbon reservoirs, saline aquifers, and non-profitable reservoirs, which significantly contributes to alleviating the effects of greenhouse gas. Different CO2 storage mechanisms are involved in these geological storage processes, which are thoroughly reviewed below.
2.1. CO2 Storage in Coal Seams
The deeply stored coalbed methane (CBM) formation is the targeted location for CO2 storage. The impermeable overburden rock helps to store CO2 in the pores and fractures of coal seams over the long term. Meanwhile, the injected CO2 can displace the CBM, whose economic gains can lower the cost of the CO2 storage [16].
Figure 1 shows a schematic diagram of the stored CO2 in a coal seam. The compressor at the surface compresses the CO2 into a supercritical state prior to injection. Then, supercritical CO2 is injected into the specified coal seam through the injection wells. The injected CO2 exists mainly in the adsorbed and free states. After about two months of soaking [17], the adsorbed and free gases reach equilibrium in the reservoir, and the injected CO2 is fully adsorbed into the coal seam and displaces the CH4 adsorbed on the inner surface of the coal pores [18]. Because the coal seams have approximately two times the adsorption capacity for CO2 than for CH4 [19,20,21], this implies that the coal seams are capable of adsorbing more CO2. Meanwhile, the injection of CO2 increases the formation energy at the wellhead of the injection well and pushes the gas in the coal seam to flow into the production well. The coalbed methane is then pumped out by deep well pumps and water–gas separation is performed to obtain high-purity coalbed methane.
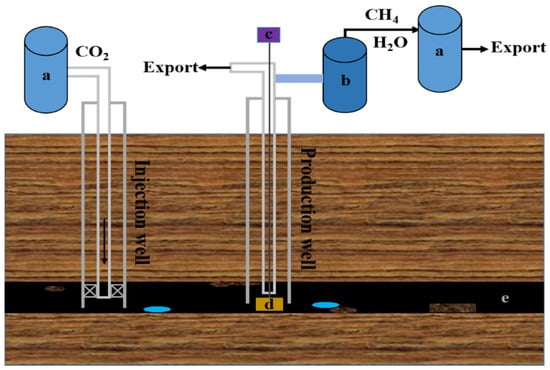
Figure 1.
Schematic diagram of CO2 storage in coal seams: (a) compressor; (b) water–gas separation system; (c) pump; (d) deep well pump; (e) coal seam.
The first tests of geological storage of CO2 in coal seams and improved coal bed methane recovery were applied in the San Juan Basin, USA, in late 1993. Then, a pilot test of injecting pure CO2 was conducted in the Allison coal seam of the San Juan basin; the stimulating effect was remarkable and the recovery factor of the coalbed methane was enhanced by about 18% [22]. Subsequently, pilot tests have been conducted in Alberta in Canada, Ishikari in Japan, and Silesian in Poland, as well as in other coal seams [23,24,25,26]. In 2004, mini-pilot tests involving CO2 huff-puff injection were carried out in the Qin Shui Basin, whose preliminary results confirmed the stimulating effect of CO2 injection and the feasibility of CO2 storage [17]. Parson and Keith [27] estimated that the global CO2 storage capacity of coal seams is about (36.6 − 110) × 1010 t. In fact, these projects have not achieved the desired effect of being able to store large amounts of CO2. The problem of coal swelling due to CO2 injection is still difficult to solve, which greatly limits the large-scale application of this method.
Currently, the storage of CO2 in coal seams is still in the research and development phase, and commercialization and large-scale promotion projects have not yet been implemented. There are still many technical barriers to overcome; for example, the injected CO2 will make the coal swell, reduce the permeability of the coal seams, and contaminate the gas flow path [28].
2.2. CO2 Storage in Hydrocarbon Reservoirs
Mature hydrocarbon reservoirs, which are produced over a long time and exhibit low economic value, are used for CO2 storage. When unexploited, most of the hydrocarbon reserves indicate the integrity of the reservoir when no oil or gas leaks occur for millions of years, which provides a safe location for future CO2 storage. After years of research, this technology is one of the most mature storage technologies.
Existing facilities can be used to store CO2 directly, which significantly increases the efficiency of the CO2 storage. Once the storage depth is deeper than 800 m, the injected CO2 will be in the supercritical state corresponding to the reservoir temperature, resulting in the large-scale and stable storage of CO2 [29]. The majority of the CO2 is stored in the pore space, while part of the CO2 is dissolved in the residual fluid and a small part of the CO2 mineralizes in the underground rock. Meanwhile, both the viscosity and interfacial tension of the crude oil can be lower after the dissolution of CO2 [30]. Furthermore, the injection of CO2 helps maintain the reservoir pressure and displaces hydrocarbon resources, which enhances the oil recovery and extends the producing period of the well [31].
The concept of CO2 flooding was first proposed during the 1920s and matured in the 1980s. In the Weyburn Field, Canada, large-scale CO2 flooding projects have been carried out since 2000; more than 15 billion tons of CO2 have been injected so far [32]. The statistics show that the mass of CO2 that can be stored using globally depleted hydrocarbon reservoirs is about 923 Gt, which is equivalent to 125 years of global CO2 emissions from fossil-fuel-burning power plants [33]. Injecting CO2 into reservoirs has obtained significant results in both EOR and CO2 storage.
2.3. CO2 Storage in Saline Aquifers
Saline aquifers are mostly sandstone, shale, and argillaceous rocks, which are widely distributed in inland and offshore sedimentary basins. Compared with coal seams and hydrocarbon reservoirs, saline aquifers possess better geological conditions for CO2 storage in terms of their storage capacity, duration, technical difficulty, and environmental impact [34]. The CO2 injected into the saline aquifers diffuses and flows into the porous media, which is stored underground after a series of physical and chemical processes.
The main mechanisms for storing CO2 in saline aquifers are summarized in Figure 2, including structural trapping, hydrodynamic trapping, residual trapping, dissolution trapping, and mineralization trapping [7,35]. Structural trapping refers to the immobilization of CO2 under the confinement of a cap rock or structural trap so that the CO2 can be stored. Due to the effect of gas–liquid interfacial tension, part of the CO2 is trapped in the pores, resulting in CO2 storage as the form of residual gas. The hydrodynamic trapping mechanism involves the blockage of CO2 by water, which occurs when the flow pressure of the groundwater is opposite to the buoyancy direction of the CO2 migration and approximately equal to that of the buoyancy force of the CO2. Meanwhile, part of the CO2 can be dissolved in formation water and stored. The key influential factors affecting the solubility of CO2 in water include the temperature, pressure, and salinity of the formation water. As the most stabilized trapping approach, mineralization trapping plays an important role in CO2 storage. The principle of this process is the formation of stable carbonate minerals by the acidized water (H2CO3 (aq), HCO3− and CO32−) after the dissolution of CO2 and mineral ions (Ca2+, Mg2+ and Fe2+) [36].
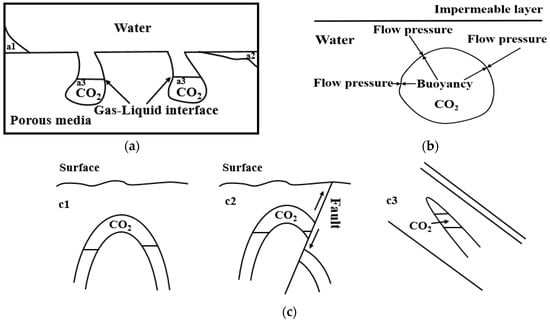
Figure 2.
Schematic diagram of CO2 storage mechanism in saline aquifers: (a) residual trapping ((a1) dissolved trapping; (a2) mineralization trapping; (a3) residual trapping); (b) hydrodynamic trapping; (c) structural trapping ((c1) anticline trapping; (c2) block trapping; (c3) pinch out trapping).
The CO2 can be transformed into a supercritical fluid under supercritical conditions (T > 31.1 °C, p > 7.38 MPa) [37]. The supercritical CO2 exhibits high density and low viscosity, resulting in great solubility and diffusivity in formation water [38]. Injecting CO2 under supercritical conditions can significantly enhance the capacity of the CO2 storage.
Demonstration projects of CO2 storage have been carried out successively in many countries. The Sleipner Project in the North Sea of Norway, started in 1996, was the first demonstration project, with a designed storage capacity of 220 million tons of CO2. In 2004, the United States also started the construction of the Frio Demonstration Project [39]. In China, one of the representative projects of CO2 storage in deep saline aquifers is the CCS Demonstration Project in Shenhua, whose first phase was completed in 2016. A total of 300,000 tons of CO2 was injected into a deep saline aquifer in the Ordos Basin, making it the largest CO2 storage project in the saline aquifer in Asia [40].
From the existing worldwide experience of CO2 injection, the geological storage of CO2 in saline aquifers is feasible from a technical point of view. However, only in certain areas of the world are these saline aquifers available. Additionally, there are many problems to be solved, such as the distribution of storage projects in different subsurface environments around the world, the lack of site characterization information, the need for different monitoring techniques, as well as legal and regulatory issues.
2.4. CO2-ESGR
Using CO2 to enhance the shale gas recovery (CO2-ESGR) is a new method of CO2 geological storage and a shale gas development technique. This method employs CO2 fracturing fluids to replace the conventional fracturing fluids, which can be applied in stimulation, primary recovery, secondary recovery, and CO2 geological storage processes. The CO2 can be massively recycled via this technique, which saves the company money and increases the operational efficiencies. However, this technique is still in the feasibility analysis and pilot testing phase.
Various scholars have demonstrated the great potential of CO2 storage in shale [41,42]. Compared with coal seams, hydrocarbon reservoirs, and saline aquifers, shale is the safest geological site for CO2 storage due to its extremely low permeability. In addition, the shale reservoirs are much more abundant than coal seams or hydrocarbon reservoirs, which further indicates the possibility for massive CO2 storage.
3. Exploitation Status of Shale Gas in the Sichuan Basin
In this section, we review the exploitation status of shale gas in the Sichuan Basin, China. It is estimated that the recoverable reserves of shale gas in China exceed 36 × 1012 m3. The total amount of shale gas resources in the Sichuan Basin is about 41.5 × 1012 m3 [43], which ranks first for shale gas reserves in China. The abundant shale formations and considerable number of wells provide a guarantee for CO2 injection and storage through abandoned shale gas wells. The production status of the shale gas indicates the formation characteristics and provides information for assessing the feasibility of the CO2 storage, which needs to be studied thoroughly.
3.1. Shale Gas and Shale Reservoir Characteristics
Methane is the primary component of shale gas. The main occurrence forms of shale gas are adsorbed and free, and the proportion range of adsorbed gas is about 20–85% [44]. The origins are mainly biological, thermogenic, and a combination of the two [45,46,47].
Shale gas reservoirs are “self-generation and self-storage” gas reservoirs [48,49], with independent oil and gas systems. The reservoirs are characterized by low porosity and ultra-low permeability. The nanoscale pore throat systems are well developed, the porosity is generally less than 10% [50], and the permeability is generally less than 10−4 mD [51]. Shale reservoirs have the characteristics of a large area and wide range. Generally, reservoirs are stored at depths of 200–3500 m and have thicknesses ranging 90–180 m [52].
The shale mainly consists of organic content, quartz, and clay minerals. According to the exploration and development experiences, the threshold values for the average organic carbon content of prospective, favorable, and core areas are 0.5%, 1.5%, and 2.0%, respectively [53]. The total organic content (TOC) values of the shale gas reservoirs in the Wufeng Formation and Longmaxi Formation range from 0.2% to 12% [54], which are at a low level but are greater than the lower limit of shale gas hydrocarbon generation (TOC > 0.5%).
3.2. Exploration and Development Process of Shale Gas
Before 2009, there was a lack of geological understanding and evaluation of shale gas in the Sichuan Basin. After more than a decade of unremitting exploration, the large-scale commercial development of shale gas has occurred successively through formation selection, pilot testing, and demonstration area construction [55].
Since the discovery of the first giant gas field in Puguang, Sichuan Basin, large shale gas fields have been successively explored in the Changning, Weiyuan, and Fuling shale gas fields. The Changning, Weiyuan, and Fuling shale gas blocks are located in the southern, southwestern, and near the eastern boundary of the basin, respectively (Figure 3) [56]. The shale blocks have equivalent vitrinite reflectance (EqVRo, %) values ranging from 2.4% to 3.8%, indicating that they are largely thermally over-mature and in a dry gas generation stage [57,58,59]. Table 1 shows the published production data for these areas. A total of about 13,703 million tons of shale gas has been produced in these areas; the production rate increases year-by-year.
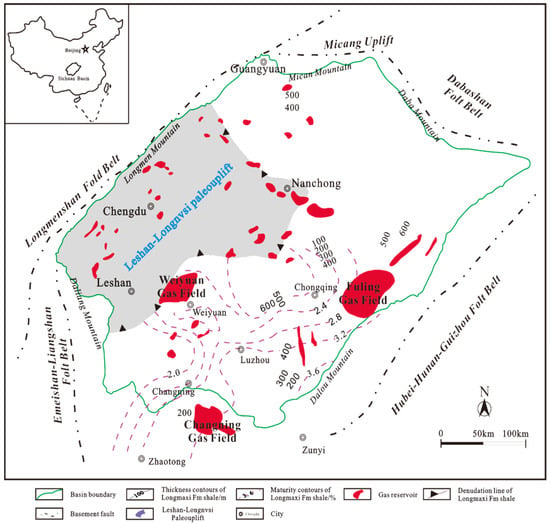
Figure 3.
Geological sketch map of the Sichuan Basin, showing the locations of the major shale gas blocks, isolines of Ro values, and the shale thickness of the Longmaxi Formation (created by Cao et al. [56]).

Table 1.
Production overview of the Changning, Weiyuan, and Fuling shale demonstration blocks [60,61,62,63,64,65,66,67].
Based on the production decline trend in the currently available production data, there is no stable production period for shale gas wells. Even if the production is allocated to less than 1/3 of the open flow rate, the production can only be stable for about 3 years, and the annual decline rate can reach 50% or more. The overall production and development cycle of shale gas wells is estimated to be less than 40 years [68]. To achieve a stabilized or enhanced production rate, we need infill wells that increase the total well number. As the production continues, a large number of wells will be abandoned due to production decline or flawed operations. Taking the Changning, Weiyuan, and Fuling blocks as examples, it is estimated that more than 500 wells will be abandoned shale gas wells by 2030, providing a large number of good injecting channels for storing CO2.
3.3. Characteristics of Abandoned Shale Gas Wells
Due to the ultra-low porosity and permeability of shale reservoirs, fracturing is required to achieve an industrial production rate [69,70]. The stimulating effect of hydraulic fracturing directly affects the fluid flow capacity and CO2 storage potential of the reservoir. Therefore, a better understanding of the fracturing performance is critical to the evaluation of the storage of CO2 in fractured wells. At present, micro-seismic monitoring, post-fracturing evaluation, flow-back data, and production information are used to design and adjust the CO2 storage scheme [71].
The statistics of the hydraulic fracturing operations show that the average lateral length of the stimulated section is about 1500 m and the average fracturing stage is about 21. Various scholars have attempted to understand the fracturing performance and fracture geometry from different perspectives. Some representative results are listed below.
Liu et al. [72] used the microseismic monitoring technique to interpret and analyze the fracture complexity of three fractured wells in Fuling. The results showed that the fracture heights are between 20 to 80 m, and the stimulated reservoir volume (SRV) is greater than 3.56 × 107 m3. Applying the same methodology, the analyses of TYB well in Jiaoshiba showed that the main fracture length range is between 310 m and 1118 m and the SRV is about 3.273 × 107 m3 [73].
Shu et al. [74] proposed a new post-fracturing analysis method using the material balance theory to evaluate the shale gas well productivity. They found that the fracture conductivity of the three tested wells in Jiaoshiba is greater than 673 mD·m. By combining pressure buildup tests and microseismic monitoring, Liu [75] found that the fracture half-length of the Weiyuan Shale Gas I well is about 10 m through curve fitting. The fracture complexity increases and the permeability enhances by several orders of magnitude.
Based on the flowback data, Wang et al. [76] used the aqueous phase seepage mathematical model for gas well production at a constant production rate in the calculation; the estimated fracture width of Longmaxi Well I is about 0.02 m and the fracture pore volume is about 12,572 m3.
The complex fracture network and large SRV provide excellent seepage channels and storage spaces for the injected CO2. The fracturing performances, including the fracture length, number, and conductivity, determine the potential for CO2 storage in fractured shale gas wells. Chen et al. [77] quantitively evaluated the CO2 storage potential through shale gas wells. Table 2 summarizes the recommended indices and major influential factors of this process. Based on the typical fracture scales of hydraulic fracturing operations evaluated via the abovementioned different methods, the shale gas well in Sichuan Basin meets the storage requirement.

Table 2.
Impact of engineering factors on storage [77].
4. Feasibility Assessment of CO2 Storage in Abandoned Shale Reservoirs
4.1. Analysis of Storage Mechanism
The mechanisms of CO2 storage in shale reservoirs are similar to the aforementioned geological storage mechanisms, including structure, hydraulic, residual, dissolved, mineralization, and adsorption trapping mechanisms. Adsorption plays a critical role in CO2 storage [78,79,80], as illustrated in Figure 4. The CO2 storage potential and shale gas recovery significantly increase because of the competitive adsorption between CO2 and CH4 [81].
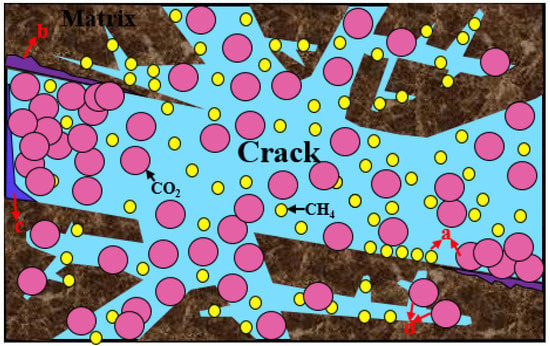
Figure 4.
Schematic figure showing the mechanisms of CO2 storage in shale (a) adsorption trapping; (b) mineralized trapping; (c) dissolved trapping; (d) residual trapping.
Various researchers have thoroughly examined the competitive adsorption mechanisms of CO2 and CH4 to increase the CO2 storage potential in shale. The adsorption characteristics of the organic and inorganic contents are reviewed below.
4.1.1. Competitive Adsorption in Organic Matters of Shale
Effects of temperature and pressure. Sui et al. [82] and Sui et al. [83] used molecular simulation to explore the competitive adsorption behaviors of CH4 and CO2 under different molar ratios and pressures, and analyzed the effect of each atom in kerogen in affecting gas adsorption via a radial distribution function. Coussy [84] and Brochard et al. [85,86] pointed out that the adsorption in the low-pressure stage is the main cause of volumetric strain. Sun et al. [87] simulated the adsorption on a narrow slit kerogen fracture pore in the simulation. The results showed that the coefficient selective adsorption of CO2/CH4 decreased with increasing temperature but first increased and then decreased with rising pressure. Overall, kerogen is strongly affinitive to CO2, which is consistent with the experimental results [88,89,90].
Effects of moisture. Billemont et al. [91] and Huang et al. [92] experimentally and numerically investigated the adsorption behavior of CH4 and CO2 in water-saturated carbon nanopores and found that the average equivalent heat of adsorption for CH4 ranged from 20.42 kJ/mol to 22.10 kJ/mol with respect to the humidity range of 0 to 2.8%. The simulated results were close to the experimental data obtained for activated carbon (29.12 kJ/mol) [93]. At low humidity, the replacement effect of CO2 enhances with increasing humidity content. At high humidity, water molecules tend to form a cage with cluster structures that worsen the effect of the CO2 replacement of CH4 and greatly reduce the amount of adsorbed gas [94]. When the pores are large, gas molecules mainly concentrate in the center of the pore channels [95].
4.1.2. Competitive Adsorption in Inorganic Matters of Shale
Competitive adsorption in quartz. Jing [96] and Xiong et al. [97] constructed pore models for quartz and investigated the effect of moisture on competing CH4/CO2 adsorption. The results showed that the moisture content significantly reduced the total adsorption of the gas mixture, while the adsorption preference of CO2 over CH4 in quartz increased with the increasing moisture content.
Competitive adsorption in montmorillonite. Jin and Firoozabadi [98] conducted a simulation to understand the effect of the moisture content on the competitive adsorption on montmorillonite; the obtained density distribution of CO2/CH4 showed that water inhibited the gas adsorption. In 1 nm pores, water and CO2/CH4 adsorb in the same layer, while in pores whose diameter is large than 2 nm, water molecules adsorb on the first layer and the CO2/CH4 forms a second layer. At relatively low pressure and a moderate water level, montmorillonite exhibits a more pronounced preference for CO2 adsorption [99]. By examining the adsorption equilibrium configuration and calculating the adsorption heat, Yang et al. [100] examined the competitive adsorption mechanism of the mixed gas and found that the charged sodium montmorillonite resulted in stronger adsorption of CO2.
Competitive adsorption in illite. Lu et al. [101] conducted isothermal sorption experiments and found that illite was favorable to increasing the amount of adsorbed gas in shale. Zhang et al. [102] used the GCMC method to examine the effects of the temperature, pressure, and CO2 injection ratio affecting the CO2/CH4 adsorption and desorption in 1 M of illite. When the CO2/CH4 injection ratio was larger than 2 and the formation depth exceeded 2 km, the CO2 adsorption gradually increased with increasing depth and then stabilized at a depth of 5 km. Additionally, their experimental results echoed well with the simulation results shown by Ou [103] and Wang [104].
Competitive adsorption in kaolinite. Heller and Zoback [105] studied competitive adsorption in different types of clay minerals and showed that the adsorption on illite was greater than that of kaolinite. Song et al. [106] constructed a kaolinite supercell model using the GCMC method; most of the CH4 and CO2 molecules were adsorbed in the micropores. Additionally, the adsorbed CH4 and CO2 amounts at different temperatures conformed with those calculated by the Langmuir model.
4.2. Evaluation of Storage Potential
Various models have been proposed to estimate the CO2 storage potential in shale reservoirs [107,108]. Tao et al. [109] developed an algorithm to estimate the CO2 storage capacity of the Marcellus Basin based on historical production data, the CH4/CO2 sorption equilibrium, and kinetic models. They also predicted that 1.04~1.84 billion tons of CO2 would be stored in the region by 2030. Godec et al. [110] predicted that the same region had a CO2 storage capacity of about 0.92 Mt/km2. Sun et al. [111] proposed a dual pore transfer model to investigate the CO2 storage capacity. The results showed that the storage capacity of CO2 enhances with increasing injecting pressure. Zhou et al. [112] estimated the storage of the target shale reservoir using a self-developed CO2 theoretical storage potential calculation formula, and the results showed that the shale has good prospects for CO2 storage, which is larger than the shale gas reserves in the target reservoir. Busch et al. [42], Lu et al. [113], and Tang et al. [114] experimentally demonstrated the considerable storage capacity of shale for CO2.
In this study, the equation we used to estimate CO2 storage in shale was adapted from the method proposed by Zhou et al. [112]. This method was developed based on the mechanism of CO2 storage, of which the competing adsorption mechanism is particularly important, which is where it differs from the mechanism of storing CO2 in conventional oil and gas reservoirs. Adsorption increases the amount of CO2 stored in the shale, which was described in detail in the previous section. We assumed that CO2 is stored in the shale reservoir only in the adsorbed and free states. It was also assumed that the injected CO2 can completely replace the free CH4 in the pore space, ignoring the reservoir mineralization of CO2 and the effect of moisture on the gas. This method requires fewer geological parameters and assumptions than traditional methods for estimating the geological storage of CO2, such as the area method and the volume method [115,116,117,118], and can be calculated quickly when applied in the field as a guide for an initial estimation of a reservoir’s CO2 storage potential. The method can be applied to various shale blocks in the Sichuan Basin, and we have carried out detailed calculations and analyses using the Changning shale gas block as an example. The model formula is as follows:
where X is the percentage of adsorbed CH4 in the reservoir; and are the adsorption capacity of CO2 and CH4 in shale, respectively; and is the shale gas storage volume, m3.
Shale reservoirs in different regions are located in different geological environments, with different formation temperatures and pressures, and have different rock compositions. All of these factors can affect the adsorption of gas by shale. By review, the percentage of absorbance state (X) range in shale is about 20% to 85% [43], and the amounts of CO2 adsorbed in different regions of shale range from 2.68 to 19.41 times the amount of adsorbed CH4, i.e., ranges between 2.68 and 19.41 [112]. This means that to replace 1 mole of adsorbed CH4 gas, 2.68–19.41 moles of CO2 are required. The shale gas reserves in the Changning block are 6 × 1011 m3 (from Table 1), and the potential for storing CO2 in the block can be calculated by substituting the data into Equation (1).
Using these parameters, we calculate the CO2 storage potential of the Changning shale gas block, as shown in Figure 5. When X is 20% and is 2.68, the minimum CO2 storage capacity of the Changning block is 8.016 × 1011 m3 according to Equation (1); when X is 85% and is 19.41, the maximum CO2 storage capacity of the Changning block is 9.821 × 1012 m3 by calculation. Both of these are greater than the shale gas reserves of the block itself (6 × 1011 m3).
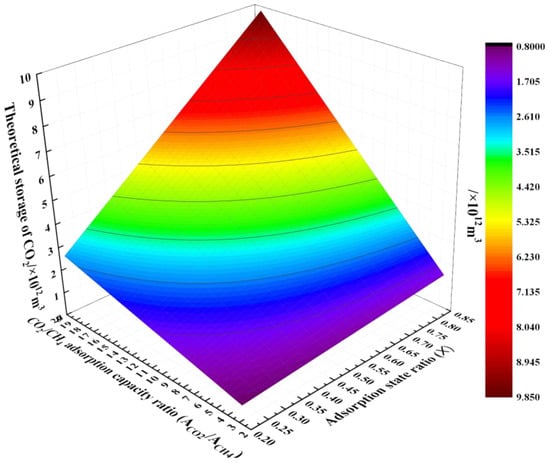
Figure 5.
Theoretical storage of CO2 in the Changning shale block.
Most of the reservoir storage depths in this block range from 2000 to 4000 m [69,119], and the average depth of storage is set at 3000 m. The formation pressure gradient in the Sichuan basin is 0.01 MPa/m, and the average geothermal gradient is 0.024 °C/m [120]. Assuming an average surface temperature of 20 °C and a storage depth of 3000 m, the formation pressure is 30 MPa and the temperature is 92 °C at this time. Thus, the corresponding CO2 density can be derived as 695 kg/m3. After a simple transformation, the minimum CO2 storage and the maximum CO2 storage of the Changning block are about 5.57 × 105 million tons and 6.83 × 106 million tons, respectively. This shows that the Changning shale block has high potential for storing CO2. The huge storage potential could help drive CO2 shale-storage-related projects.
5. Conclusions and Prospects
This paper briefly reviewed CO2 storage projects around the world, focusing on the applications and storage mechanisms related to CO2 storage in coal seams, hydrocarbon reservoirs, and saline aquifers. Inspired by these CO2 storage projects, we evaluated the potential for CO2 storage through abandoned shale gas wells. The abundant shale gas resources and abandoned shale wells in the Sichuan Basin will provide sufficient and effective sites for the storage of CO2, which was adopted here as the research topic.
In a review of the competitive adsorption of CO2/CH4 in shale, both the experimental and simulation results showed that the adsorption of CO2 is significantly greater than that of CH4 under different conditions of temperature and pressure, and the adsorption is more stable, which can help increase the storage of CO2. We calculated the CO2 storage capacity of the Changning shale block in the Sichuan Basin using a mechanistic approach model that considers only the adsorbed and free states of CO2, and the results were greater than for its own shale gas reserves, proving that shale has great potential for CO2 storage. The method is also applicable to other shale blocks in the Sichuan Basin. This provides guidance for initial assessments of reservoir CO2 storage potential. However, the need for improved models that take into consideration the multiple storage states of CO2 in shale is also more urgent to ensure the accuracy of the calculation results. In general, the potential for using abandoned shale gas wells to store CO2 is high. This method is feasible and can effectively reduce carbon emissions and protect the environment.
Based on a review of available CO2 storage techniques and an analysis of the CO2 storage capacity of shale blocks in the Sichuan Basin, China, both technical and policy recommendations are made.
5.1. Technical Recommendations
- Due to the limitations related to the experimental setup and capability of numerical simulations, most studies are conducted in simplified reservoir conditions or assumed unrealistic ones. This will lead to errors in the results and in real life. We should further develop the technical means to ensure the maximum restoration of real situations.
- Storage analyses are conducted by assuming the wells are in relatively good condition. The differences between CO2 storage in abandoned wells and integrity wells are not fully understood. The CO2 injection and storage processes while considering the characteristics of abandoned wells require further investigation. Additionally, the optimization of the operation parameters (i.e., injection volume, time, and pressure) is required to achieve optimal storage.
5.2. Policy Recommendations
- Strengthen state and local government support measures, increase the amount of loans, and lower the interest rates for investment in CO2 shale-storage-related projects, and increase the fiscal and tax incentives and subsidies. Support the implementation of demonstration projects and encourage research centers and key laboratories.
- The government should take the initiative to establish a data management and service system that allows information sharing, to establish a technology and experience exchange platform, and to provide services for enterprise development. The approval and licensing of projects related to CO2 storage in shale optimized, and a safety and environmental protection emergency resource system should be provided. Meanwhile, an effective information disclosure and exchange platform should be established, so that enterprises can fully communicate with society.
Author Contributions
Conceptualization, X.C.; investigation. X.L. and Y.W.; data curation, D.D.; writing—original draft preparation, J.D.; writing—review and editing, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the anonymous reviewers and editors for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.N.; Shen, R.R.; Zhang, X.P.; Kang, J.J.; Yuan, J.H. Summary of the connotation and realization path of China’s carbon neutrality goal. Res. Prog. Clim. Chang. 2022, 18, 240–252. (In Chinese) [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.; Andrew, R.; Hauck, J.; Olsen, A.; Peters, G.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Liu, M.P. The challenges and policy recommendations to achieve the goal of carbon neutrality in China. Contemp. Pet. Petrochem. 2021, 29, 1–9. (In Chinese) [Google Scholar]
- Torp, T.; Gale, J. Demonstrating Storage of CO2 in Geological Reservoirs: The Sleipner and SACS Projects. Energy 2004, 29, 1361–1369. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Mayer, B.; Gunter, W.; Perkins, E. Monitoring of fluid-rock interaction and CO2 storage through produced fluid sampling at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Appl. Geochem. 2005, 20, 1131–1157. [Google Scholar] [CrossRef]
- Yang, Z.J. Study on Reservoir Pressure Control and Well Placement Plan Optimization in CO2-Enhanced Brackish Water Extraction Project. Ph.D. Thesis, Jilin University, Changchun, China, 2019. [Google Scholar]
- White, C.M. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Liu, S.N.; Zhang, L.W.; Su, X.B.; Wang, Y.; Zhao, L.X.; Gan, M.G.; Fu, X.J.; Li, X.C. A review of modeling and parameterization of mineral dissolution and precipitation chemistry for CO2 sequestration in brackish aquifers. Water Resour. Hydropower Technol. 2020, 51, 13–22. (In Chinese) [Google Scholar]
- Texas Railroad Commission Applications and Permits. Available online: http://www.rrc.state.tx.us/oil-gas/applications-and-permits/ (accessed on 22 June 2017).
- Gislason, S.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.; Matter, J.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. Int. J. Greenh. Gas Control 2010, 4, 537–545. [Google Scholar] [CrossRef]
- Celia, M.; Nordbotten, J. Practical Modeling Approaches for Geological Storage of Carbon Dioxide. Ground Water 2009, 47, 627–638. [Google Scholar] [CrossRef]
- Zwaan, B.; Smekens, K. CO2 Capture and Storage with Leakage in an Energy-Climate Model. Environ. Model. Assess. 2007, 14, 135–148. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.; Bardow, A.; Anthony, E.; Boston, A.; Brown, S.; Fennell, P.; Fuss, S.; Galindo, A.; Hackett, L.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Aminu, M.; Nabavi, S.A.; Rochelle, C.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Leung, D.; Caramanna, G.; Maroto-Valer, M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Ye, J.P.; Feng, S.L.; Fan, J.Q.; Wang, G.Q.; William, D.G.; Sam, W.; John, R.R. Micro-pilot test for enhanced coalbed methane recovery by injecting carbon dioxide in south part of Qinshui Basin. Acta Pet. Sin. 2007, 4, 77–80. (In Chinese) [Google Scholar]
- Cai, B.X. Basic Physical Chemistry (Upper Volume), 1st ed.; Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Jiang, W.P.; Cui, Y.J.; Zhang, Q.; Li, Y.H. Quantum chemical study on the coal surface interacting with CH4 and CO2. Meitan Xuebao/J. China Coal Soc. 2006, 31, 237–240. [Google Scholar]
- Zhang, H.T.; Wen, D.G.; Li, Y.L.; Zhang, J.Q.; Lu, J.C. Conditions for CO2 geological sequestration in China and some suggestions. Geol. Bull. China 2005, 24, 1107–1110. [Google Scholar]
- Gentzis, T. Subsurface sequestration of carbon dioxide—An overview from an Alberta (Canada) perspective. Int. J. Coal Geol. 2000, 43, 287–305. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Mavor, M.; Gunter, W.; Robinson, J. Alberta Multiwell Micro-Pilot Testing for CBM Properties, Enhanced Methane Recovery and CO2 Storage Potential. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- Fujioka, M.; Yamaguchi, S.; Nako, M. CO2-ECBM field tests in the Ishikari coal basin of Japan. Int. J. Coal Geol. 2010, 82, 287–298. [Google Scholar] [CrossRef]
- Zhang, C.J.; Shen, J.; Qin, Y.; Ye, J.P.; Zhang, B. CO2 injection to improve coalbed methane recovery rate and CO2 sequestration technology. Coal Sci. Technol. 2016, 44, 205–210. (In Chinese) [Google Scholar]
- Hamelinck, C.; Faaij, A.P.C.; Turkenburg, W.C.; Bergen, F.; Pagnier, H.; Barzandji, O.H.M.; Wolf, K.-H.; Ruijg, G.J. CO2 enhanced coalbed methane production in The Netherlands. Energy 2002, 27, 647–674. [Google Scholar] [CrossRef]
- Parson, E.; Keith, D. Fossil Fuels without CO2 Emissions: Progress, Prospects, and Policy Implications. Science 1998, 282, 1053–1054. [Google Scholar] [CrossRef]
- Goodman, A.; Hakala, A.; Bromhal, G.; Deel, D.; Rodosta, T.; Frailey, S.; Small, M.; Allen, D.; Romanov, V.; Fazio, J.; et al. DOE methodology for the development of geologic storage potential for carbon dioxide at the national and regional scale. Int. J. Greenh. Gas Control 2011, 5, 952–965. [Google Scholar] [CrossRef]
- Li, H.J.; Huang, S.C. Analysis of the Development Prospects of CO2 Capture and Storage (CCS) in China. In Proceedings of the 2010 China Coal Chemical Industry Technology, Market and Information Exchange Conference and Development Strategy Workshop, Chongqing, China, 13–16 April 2010. (In Chinese). [Google Scholar]
- Holloway, S.; Savage, D. The potential for aquifer disposal of carbon dioxide in the UK. Energy Convers. Manag. 1993, 34, 925–932. [Google Scholar] [CrossRef]
- Bouvart, F.; Prieur, A. Comparison of Life Cycle GHG Emissions and Energy Consumption of combined Electricity and H2 production pathways with CCS: Selection of technologies with Natural Gas, Coal and Lignite as fuel for the European HYPOGEN Programme. Energy Procedia 2009, 1, 3779–3786. [Google Scholar] [CrossRef]
- Liu, B. Current Status and Example Analysis of CO2 Reservoir Burial Technology. Master’s Thesis, Northeastern Petroleum University, Xi’an, China, 2016. (In Chinese). [Google Scholar]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Zhang, W.; Li, Y.; Omambia, A. Reactive transport modeling of effects of convective mixing on long-term CO2 geological storage in deep saline formations. Int. J. Greenh. Gas Control 2011, 5, 241–256. [Google Scholar] [CrossRef]
- De Silva, P.N.K.; Ranjith, P.G.; Choi, S.K. A study of methodologies for CO2 storage capacity estimation of coal. Fuel 2012, 91, 1–15. [Google Scholar] [CrossRef]
- Wei, W.; Su, Y.M.; Guo, D.B.; Li, F.Y.; Su, H.; Jiang, F.H.; Tan, M.X.; Wu, C.B.J. Carbonate cement diagenesis and formation mechanism of the Lower Cretaceous sandstone reservoir in the Chagan Depression. Geochemistry 2015, 44, 590–599. (In Chinese) [Google Scholar]
- Zhang, L. Effect of temperature on corrosion behavior of five typical steels under supercritical CO2 conditions. Oil Gas Storage Transp. 2020, 39, 1031–1036. (In Chinese) [Google Scholar]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Zhu, P.Y. Geological sequestration of CO2 in brackish water formations and progress in its application. Clean Coal Technol. 2021, 27, 33–38. (In Chinese) [Google Scholar]
- Guo, J.Q.; Wen, D.G.; Zhang, S.Q.; Xu, T.F.; Diao, Y.J.; Jia, X.F. Evaluation of geological storage potential of carbon dioxide in China and demonstration project. Geol. Surv. China 2015, 36–46. (In Chinese) [Google Scholar] [CrossRef]
- Nuttal, B.C.; Eble, C.; Bustin, R.M.; Drahovzal, J.A. Analysis of Devonian black shales in Kentucky for potential carbon dioxide sequestration and enhanced natural gas production. In Greenhouse Gas Control Technologies 7, Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 2225–2228. [Google Scholar] [CrossRef]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.; Raven, M.; Stanjek, H.; Krooss, B. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.B.; Hou, L.H.; Chen, C.; Guo, J.Y.; Yang, C.L.; Wang, Y.F.; Li, Z.S.; Cui, Z.S.; Cui, H.Y.; et al. Geochemical characteristics and resource potential of shale gas in the Sichuan Basin. Nat. Gas Geosci. 2021, 32, 1093–1106. (In Chinese) [Google Scholar] [CrossRef]
- Curtis, J. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Wang, W.F.; Liu, P.; Chen, C.; Wang, H.L.; Jiang, S.; Zhang, Z.C. Shale gas reservoir formation theory and resource evaluation method. Nat. Gas Geosci. 2013, 24, 429–438. (In Chinese) [Google Scholar]
- Zhang, J.C.; Jin, Z.J.; Yuan, M.S. Mechanisms and distribution of shale gas reservoirs. Nat. Gas Ind. 2004, 24, 15–18, 131–132. (In Chinese) [Google Scholar]
- Zhang, J.C.; Xue, H.; Zhang, D.M.; Xue, J. Shale gas and its reservoir formation mechanism. Mod. Geol. 2003, 4, 466. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=XDDZ200304019&DbName=CJFQ2003 (accessed on 31 August 2022). (In Chinese).
- Jarvie, D.; Hill, R.; Ruble, T.; Pollastro, R. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Hill, R.; Zhang, E.; Katz, B.; Tang, Y. Modeling of gas generation from the Barnett Shale, Fort Worth Basin, Texas. AAPG Bull. 2007, 91, 501–521. [Google Scholar] [CrossRef]
- Pu, B.L. Analysis of Shale Gas Formation Conditions in the Sichuan Basin. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2008. (In Chinese). [Google Scholar]
- Dong, B.X.; Cheng, Y.F.; Liu, Y.C.; Yi, X.B.; Yang, L.; Wu, L.Y.; Wang, B. Petrophysical properties of shale gas reservoirs. J. Xi’an Univ. Pet. (Nat. Sci. Ed.) 2013, 28, 25–28. (In Chinese) [Google Scholar]
- Zhang, F. Analysis of shale gas reservoir formation conditions and development. Petrochem. Technol. 2021, 28, 120–121. (In Chinese) [Google Scholar]
- Zou, C.N. Unconventional Oil and Gas Geology; Geological Publishing: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Zhang, J.C.; Shi, S.; Wang, D.S.; Tong, Z.Y.; Hou, X.D.; Niu, J.L.; Li, X.Q.; Li, Z.M.; Zhang, P.; Huang, Y.Q. Shale gas exploration areas and development directions in China. Nat. Gas Ind. 2021, 41, 69–80. (In Chinese) [Google Scholar]
- Liu, Y.F.; Liu, D.R.; Peng, C.; Zhang, R.G. Current status of shale gas exploration and development in China and progress of key technologies. Mod. Chem. Ind. 2022, 42, 16–20. (In Chinese) [Google Scholar]
- Cao, C.H.; Li, L.W.; Liu, Y.H.; Du, L.; Li, P.Z.; He, J. Factors Affecting Shale Gas Chemistry and Stable Isotope and Noble Gas Isotope Composition and Distribution: A Case Study of Lower Silurian Longmaxi Shale Gas, Sichuan Basin. Energies 2020, 13, 5981. [Google Scholar] [CrossRef]
- Dai, J.; Zou, C.; Dong, D.; Ni, Y.; Wu, W.; Gong, D.; Wang, Y.; Huang, S.; Huang, J.; Fang, C.; et al. Geochemical characteristics of marine and terrestrial shale gas in China. Mar. Pet. Geol. 2016, 76, 444–463. [Google Scholar] [CrossRef]
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale gas in China: Characteristics, challenges and prospects (I). Pet. Explor. Dev. 2015, 42, 753–767. [Google Scholar] [CrossRef]
- Dai, J.; Zou, C.; Liao, S.; Dong, D.; Ni, Y.; Huang, J.; Wu, W.; Gong, D.; Huang, S.; Hu, G. Geochemistry of the extremely high thermal maturity Longmaxi shale gas, southern Sichuan Basin. Org. Geochem. 2014, 74, 3–12. [Google Scholar] [CrossRef]
- Long, S.X.; Lu, T.; Li, Q.W.; Yang, G.Q.; Li, D.H. On the development ideas and targets of China’s shale gas in the 14th Five-Year Plan. Nat. Gas Ind. 2021, 41, 1–10. (In Chinese) [Google Scholar]
- China’s Shale Gas Industry Production in 2019, Domestic Shale Gas Production to Reach 100 Billion Cubic Meters in 2030. Available online: https://baijiahao.baidu.com/s?id=1646855542896658376&wfr=spider&for=pc (accessed on 15 June 2022).
- Shale Gas Industry 2019 In-Depth Research Report. Available online: https://www.doc88.com/p-69339033387114.html (accessed on 15 June 2022).
- Competitive Pattern of China’s Shale Gas Industry in 2018: Two Dominant Players in CNPC and Sinopec. Available online: https://free.chinabaogao.com/nengyuan/201901/01153930912019.html (accessed on 15 June 2022).
- Formation Conditions and Key Technologies for Exploration and Development of Marine Shale Gas Fields in the Changning-Weiyuan Block of the Sichuan Basin. Available online: https://www.sohu.com/a/501445155_121123791 (accessed on 15 June 2022).
- Chongqing Fuling Shale Gas Field Daily Production Maintained at 19 Million Cubic Meters. Available online: https://www.sohu.com/a/498807864_362042 (accessed on 15 June 2022).
- China’s Cumulative New Proven Geological Reserves of Shale Gas Exceed Trillion Cubic Meters. Available online: http://energy.people.com.cn/n1/2018/0711/c71661-30139464.html (accessed on 15 June 2022).
- Analysis of the Current Situation of Shale Gas Development and Utilization in China in 2020, with Domestic Production Exceeding 20 Billion Cubic Meters 2022. Available online: https://www.sohu.com/a/518110173_120113054 (accessed on 15 June 2022).
- Westphal, D.; Weijermars, R. Economic appraisal and scoping of geothermal energy extraction projects using depleted hydrocarbon wells. Energy Strategy Rev. 2018, 22, 348–364. [Google Scholar] [CrossRef]
- Zou, C.N.; Dong, D.Z.; Wang, S.J.; Li, J.Z.; Li, X.J.; Wang, Y.M.; Li, D.H.; Cheng, K.M. Geological characteristics, formation mechanism and resource potential of shale gas in China. Pet. Explor. Dev. 2010, 37, 641–653. (In Chinese) [Google Scholar] [CrossRef]
- Chen, M.; Ge, H.K.; Zhao, J.Z.; Yao, J. The Key Fundamentals for the Efficient Exploitation of Shale Oil and Gas and Its Related Challenges. Pet. Drill. Tech. 2015, 43, 7–14. (In Chinese) [Google Scholar]
- He, X.P.; Zhang, P.X.; Fang, D.Z.; Mei, J.W.; He, G.S.; Lu, B. Production characteristics of normal pressure shale gas in Pengshui-Wulong area, southeast Chongqing. Pet. Geol. Recovery Effic. 2018, 25, 72–79. (In Chinese) [Google Scholar]
- Liu, X.W.; Liao, R.G.; Zhang, Y.; Gao, D.W.; Zhang, H.L.; Li, T.; Zhang, C. Application of surface-downhole combined microseismic monitoring technology in the Fuling shale gas field and its enlightenment. Nat. Gas Ind. 2016, 36, 56–62. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.W. Discussion on Evaluation-analysis Method of Volume Modification after Shale Gas Well Fracturing. J. Jianghan Pet. Univ. Staff Work. 2020, 33, 34–37. (In Chinese) [Google Scholar]
- Shu, Z.G.; Liu, L.; Liang, B.; Lu, Y.Q.; Zheng, A.W.; Bao, H.Y. Study on productivity evaluation method of shale gas well based on material balance principle. Nat. Gas Geosci. 2021, 32, 262–267. (In Chinese) [Google Scholar]
- Liu, X.L. Well test analysis and evaluation after shale-gas volume fracturing stimulation. Nat. Gas Ind. 2016, 36, 66–72. (In Chinese) [Google Scholar]
- Wang, Y.Y.; Liu, H.; Wang, W.H.; Hu, X.H.; Guo, Y.D.; Dai, C. Evaluation of shale gas well fracturing performance based on flowback water production data. Pet. Geol. Recovery Effic. 2019, 26, 125–131. (In Chinese) [Google Scholar]
- Chen, Z.; Liao, X.; Zhao, X.; Li, X. Appraising Carbon Geological-Storage Potential in Unconventional Reservoirs: Engineering-Parameters Analysis. SPE Reserv. Eval. Eng. 2017, 21, 476–488. [Google Scholar] [CrossRef]
- Klewiah, I.; Berawala, D.; Walker, H.C.; Andersen, P.; Nadeau, P.H. Review of Experimental Sorption Studies of CO2 and CH4 in Shales. J. Nat. Gas Sci. Eng. 2020, 73, 103045. [Google Scholar] [CrossRef]
- Rani, S.; Padmanabhan, E.; Prusty, B. Review of gas adsorption in shales for enhanced methane recovery and CO2 storage. J. Pet. Sci. Eng. 2018, 175, 634–643. [Google Scholar] [CrossRef]
- Liu, D.Q. Study on the CO2 Enhanced Shale Gas Recovery Technology in Ordos Basin, China. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2017. (In Chinese). [Google Scholar]
- Tinni, A.; Sondergeld, C.; Rai, C. Hydrocarbon Storage Mechanism in Shale Reservoirs and Impact on Hydrocarbon Production. In Proceedings of the Unconventional Resources Technology Conference, Austin, TX, USA, 24–26 July 2017. [Google Scholar] [CrossRef]
- Sui, H.G.; Yao, J. The molecular modelling of CO2/CH4 competitive adsorption patterns in Kerogen. J. China Univ. Pet. 2016, 40, 147–154. (In Chinese) [Google Scholar]
- Sui, H.G.; Yao, J. The CO2/CH4 Competitive Adsorption in Kerogen: A Molecular Simulation. Sci. Technol. Eng. 2016, 16, 128–131, 135. (In Chinese) [Google Scholar]
- Coussy, O. Poromechanics; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Brochard, L.; Vandamme, M.; Pellenq, R. Poromechanics of microporous media. J. Mech. Phys. Solids 2012, 60, 606–622. [Google Scholar] [CrossRef]
- Brochard, L.; Vandamme, M.; Pellenq, R.; Fen-Chong, T. Adsorption-Induced Deformation of Microporous Materials: Coal Swelling Induced by CO2-CH4 Competitive Adsorption. Langmuir 2011, 28, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, H.; Qi, N.; Li, Y. Molecular Insights into the Enhanced Shale Gas Recovery by Carbon Dioxide in Kerogen Slit-Nanopores. J. Phys. Chem. C 2017, 121, 10233–10241. [Google Scholar] [CrossRef]
- Ortiz Cancino, O.; Pérez, D.; Pozo, M.; Bessières, D. Adsorption of pure CO2 and a CO2/CH4 mixture on a black shale sample: Manometry and microcalorimetry measurements. J. Pet. Sci. Eng. 2017, 159, 307–313. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.S.; Li, J.; Chen, K.F.; Zhang, Z.X.; Li, P.P.; Zhang, Y.H. Adsorption characteristics of CH4 and CO2 on shale and its application to binary mixture adsorption under high-pressure conditions: A case study of the Longmaxi Formation shale in Jiaoshiba area of Sichuan Basin. Geochimica 2019, 48, 580–589. (In Chinese) [Google Scholar]
- Shuo, D.; Gu, M.; Du, X.; Xian, X. Adsorption Equilibrium of CO2 and CH4 and Their Mixture on Sichuan Basin Shale. Energy Fuels 2016, 30, 2248–2256. [Google Scholar]
- Billemont, P.; Coasne, B.; De Weireld, G. Adsorption of Carbon Dioxide, Methane, and Their Mixtures in Porous Carbons: Effect of Surface Chemistry, Water Content, and Pore Disorder. Langmuir 2013, 29, 3328–3338. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.F.; Wang, Q.; Qin, H.B.; Ye, H.T.; Zhang, W.T.; Li, Z.Y.; Sun, Y.D. Effect of moisture on CH4/CO2 adsorption on kerogen: A molecular simulation study. Pet. Sci. Bull. 2017, 2, 422–430. (In Chinese) [Google Scholar]
- Lopes, F.; Grande, C.; Ribeiro, A.; Loureiro, J.; Evaggelos, O.; Nikolakis, V.; Rodrigues, A. Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 2009, 44, 1045–1073. [Google Scholar] [CrossRef]
- Billemont, P.; Coasne, B.; De Weireld, G. An Experimental and Molecular Simulation Study of the Adsorption of Carbon Dioxide and Methane in Nanoporous Carbons in the Presence of Water. Langmuir 2011, 27, 1015–1024. [Google Scholar] [CrossRef]
- Jin, Z.; Firoozabadi, A. Phase behavior and flow in shale nanopores from molecular simulations. Fluid Phase Equilibria 2016, 430, 156–168. [Google Scholar] [CrossRef]
- Jing, S.S. Molecular Simulation of CO2/CH4 Mass Transfer Process in Sandstone Micropores. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2015. (In Chinese). [Google Scholar]
- Xiong, J.; Liu, X.J.; Liang, L.X. Adsorption of methane in quartz by Grand Canonical Monte Carlo simulation. Nat. Gas Geosci. 2016, 27, 1532–1540. (In Chinese) [Google Scholar]
- Jin, Z.; Firoozabadi, A. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations. Fluid Phase Equilibria 2014, 382, 10–20. [Google Scholar] [CrossRef]
- Kadoura, A.; Nair, A.; Sun, S. Adsorption of Carbon Dioxide, Methane, and Their Mixture by Montmorillonite in the Presence of Water. Microporous Mesoporous Mater. 2016, 225, 331–341. [Google Scholar] [CrossRef]
- Yang, N.; Liu, S.; Yang, X. Molecular simulation of preferential adsorption of CO2 over CH4 in Na-montmorillonite clay material. Appl. Surf. Sci. 2015, 356, 1262–1271. [Google Scholar] [CrossRef]
- Lu, X.-C.; Li, F.-C.; Watson, A. Adsorption Measurements in Devonian Shales. Fuel 1995, 74, 599–603. [Google Scholar] [CrossRef]
- Zhang, M.H.; Guo, P.; Jiang, W.; Chen, H. Researches on Adsorption Property of CO2 and CH4 in Illite. World Sci-Tech R & D 2016, 38, 950–954. (In Chinese) [Google Scholar]
- Ou, Z.P. Molecular Dynamics Study of Methane Diffusion in Nanopores. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2014. (In Chinese). [Google Scholar]
- Wang, J. Methane Afsorption in Illite by Molecular Simulation. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2015. (In Chinese). [Google Scholar]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Song, Z.P.; Zhang, B.; Kang, T.H. Molecular Simulation of CO2/CH4 Competitive Adsorption in Kaolinite Based on Adsorption Potential Theory. Bull. Miner. Petrol. Geochem. 2018, 37, 724–730. (In Chinese) [Google Scholar]
- Kalantari-Dahaghi, A. Numerical Simulation and Modeling of Enhanced Gas Recovery and CO2 Sequestration in Shale Gas Reservoirs: A Feasibility Study. In Proceedings of the 2010 SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010. [Google Scholar]
- Zhang, J. Adsorption and Desorption of Continental Shale Gas in Fuxian District of Ordos Basin. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2013. (In Chinese). [Google Scholar]
- Tao, Z.; Clarens, A. Estimating the Carbon Sequestration Capacity of Shale Formations Using Methane Production Rates. Environ. Sci. Technol. 2013, 47, 11318–11325. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Sun, H.; Yao, J.; Gao, S.-H.; Fan, D.-Y.; Wang, C.-C.; Sun, Z. Numerical study of CO2 enhanced natural gas recovery and sequestration in shale gas reservoirs. Int. J. Greenh. Gas Control 2013, 19, 406–419. [Google Scholar] [CrossRef]
- Zhou, J.P.; Xian, X.F.; Lu, Y.Y.; Jiang, Y.D.; Liu, Z.F. Carbon dioxide sequestration and potential estimation in shale gas reservoirs. In Proceedings of the 4th Symposium on Underground Waste Disposal, Nanchang, China, 23–27 September 2012. [Google Scholar]
- Lu, Y.Y.; Zhou, J.P.; Xian, X.F.; Tang, J.R.; Zhou, L.; Jiang, Y.D.; Xia, B.W.; Wang, X.Z.; Kang, Y. Research progress and prospect of the integrated supercritical CO2 enhanced shale gas recovery and geological sequestration. Nat. Gas Ind. 2021, 41, 60–73. (In Chinese) [Google Scholar]
- Tang, J.R.; Zhang, J.; Lu, Y.Y.; Wang, X.C.; Chen, X.Y.; Zhou, J.K.; Lu, Z.H. Absolute adsorption capacity of shale on CO2 and its influencing factors. J. China Coal Soc. 2020, 45, 2838–2845. (In Chinese) [Google Scholar]
- Ning, Y.J. Research on the Factors Influencing Porosity in the Process of CO2 Storage in the Saline Aquifer. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2020. (In Chinese). [Google Scholar]
- Tang, L.R.; Jiang, Y.; Yan, J.; Li, G.H.; Wang, Y.; He, Y.W.; Qin, J.Z.; Tang, Y. Study on calculation method of CO2 storage potential in depleted gas reservoir. Pet. Reserv. Eval. Dev. 2021, 11, 858–863. (In Chinese) [Google Scholar]
- Bond, L.P.L. Applications of carbon dioxide in enhanced oil recovery. Energy Convers. Manag. J. Can. Pet. Technol. 2003, 33, 579–586. [Google Scholar] [CrossRef]
- Xu, H.J.; Sang, S.X.; Yang, J.F.; Liu, H.H. CO2 storage capacity of anthracite coal in deep burial depth conditions and its potential uncertainty analysis: A case study of the No. 3 coal seam in the Zhengzhuang Block in Qinshui Basin, China. Geosci. J. 2021, 5, 715–729. [Google Scholar] [CrossRef]
- Zhang, J.C.; Xu, B.; Nie, H.K.; Wang, Z.Y.; Lin, T.; Jiang, S.L.; Song, X.W.; Zhang, Q.; Wang, G.Y.; Zhang, P.X. Exploration potential of shale gas resources in China. Nat. Gas Ind. 2008, 6, 136–140. [Google Scholar]
- Yang, P.; Yin, F.; Yu, Q.; Wang, Z.J.; Liu, J.H.; Zhang, D.; Zhang, D.G. Evolution Anomaly of Organic Matter and Characteristics of Palaeogeothermal Field in the Southeast Edge of Sichuan Basin. Nat. Gas Geosci. 2015, 7, 1299–1309. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).