Overexpression of Ent-Kaurene Synthase Genes Enhances Gibberellic Acid Biosynthesis and Improves Salt Tolerance in Anoectochilus roxburghii (Wall.) Lindl.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Gene Cloning
2.3. Bioinformatic Analysis
2.4. Vector Construction
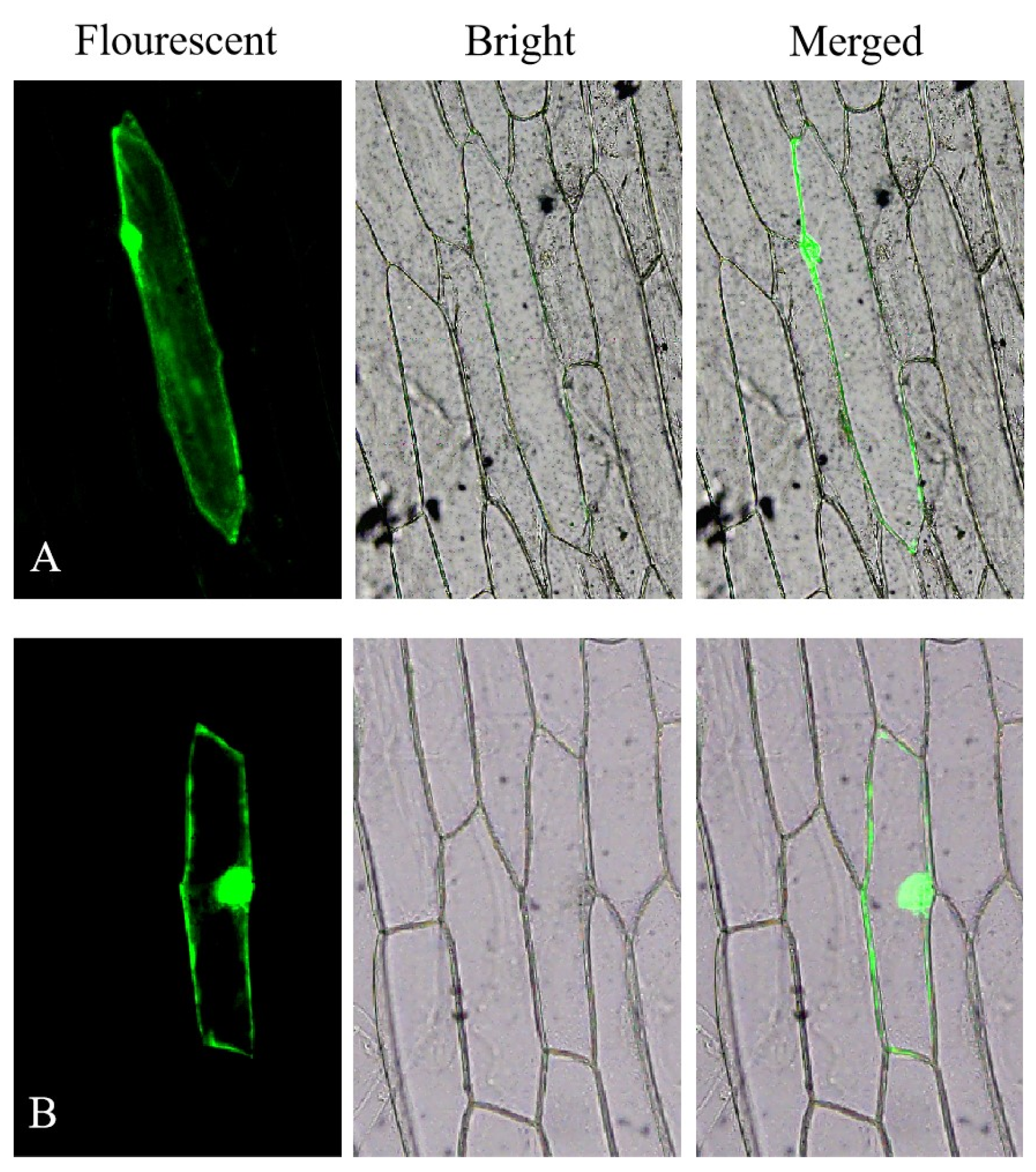
2.5. Subcellular Localization
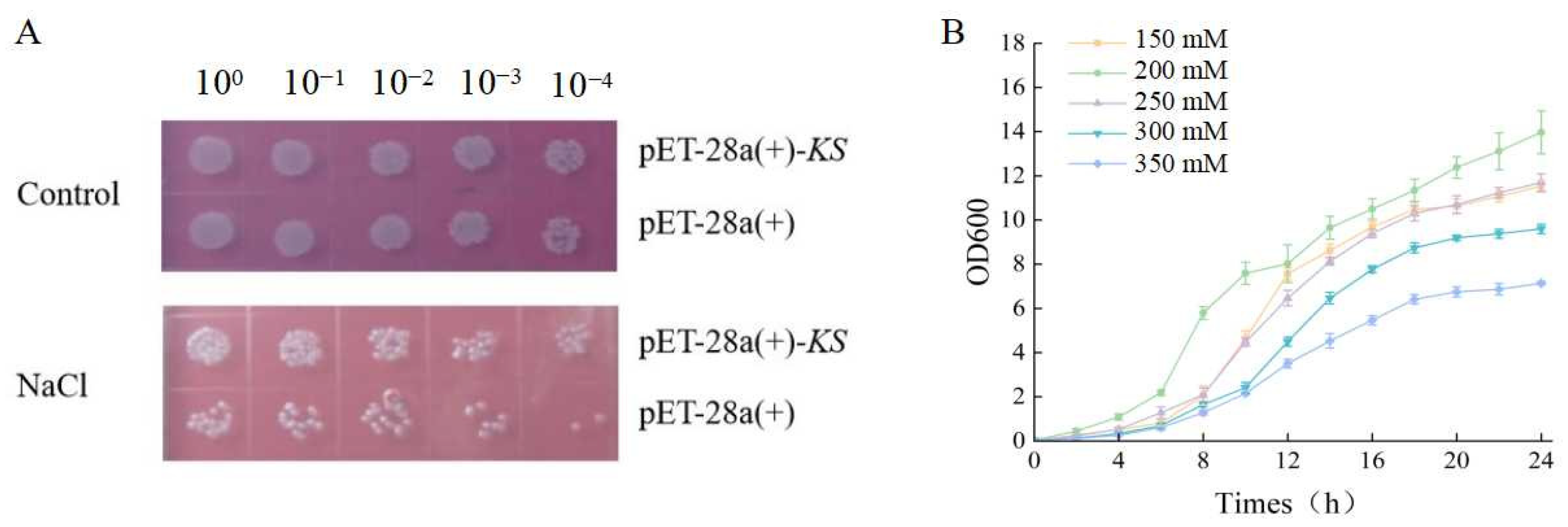
2.6. Prokaryotic Expression
2.7. RT-qPCR
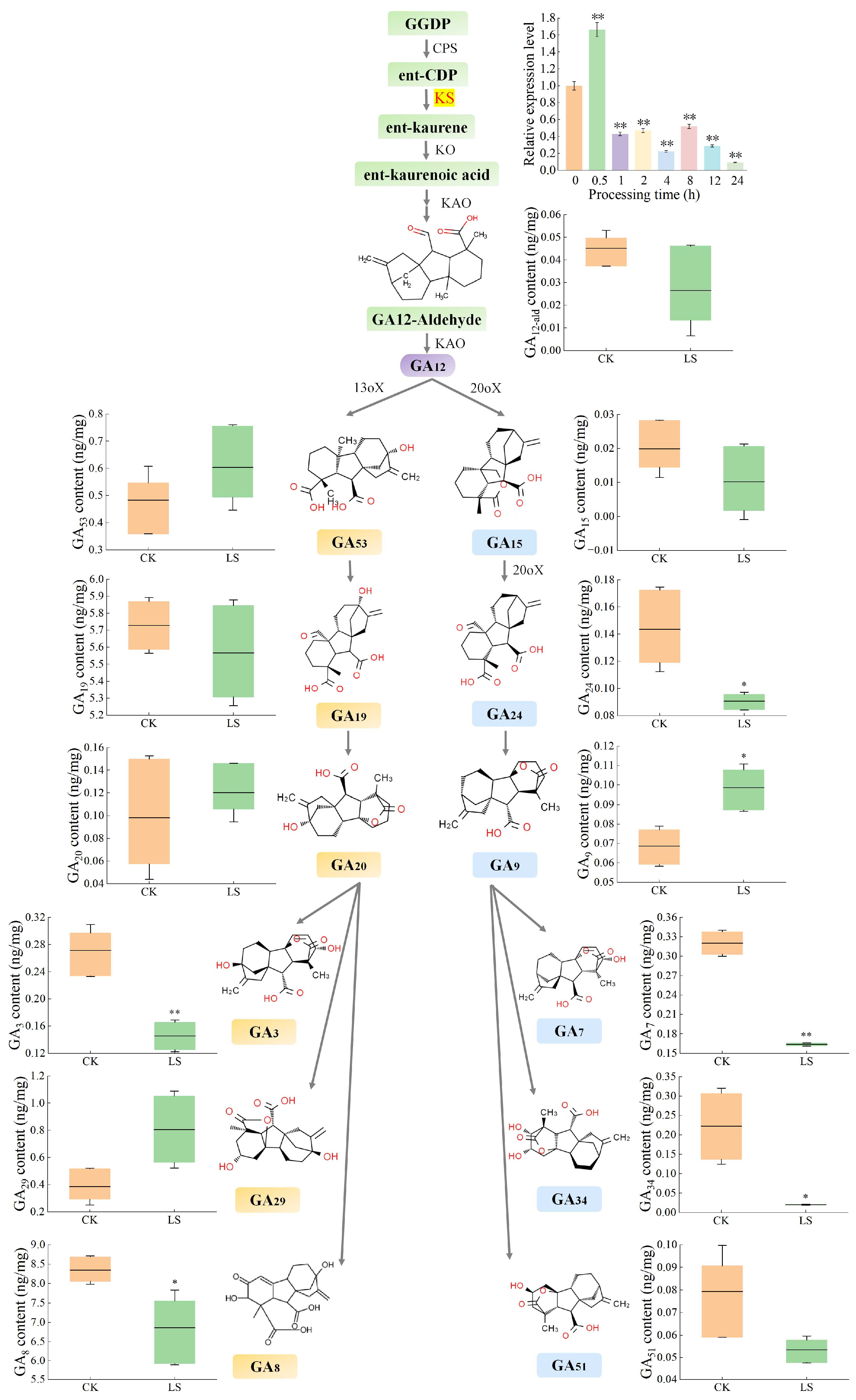
2.8. Quantification GAs
2.9. Measurement of Related Physiological Indicators
3. Results
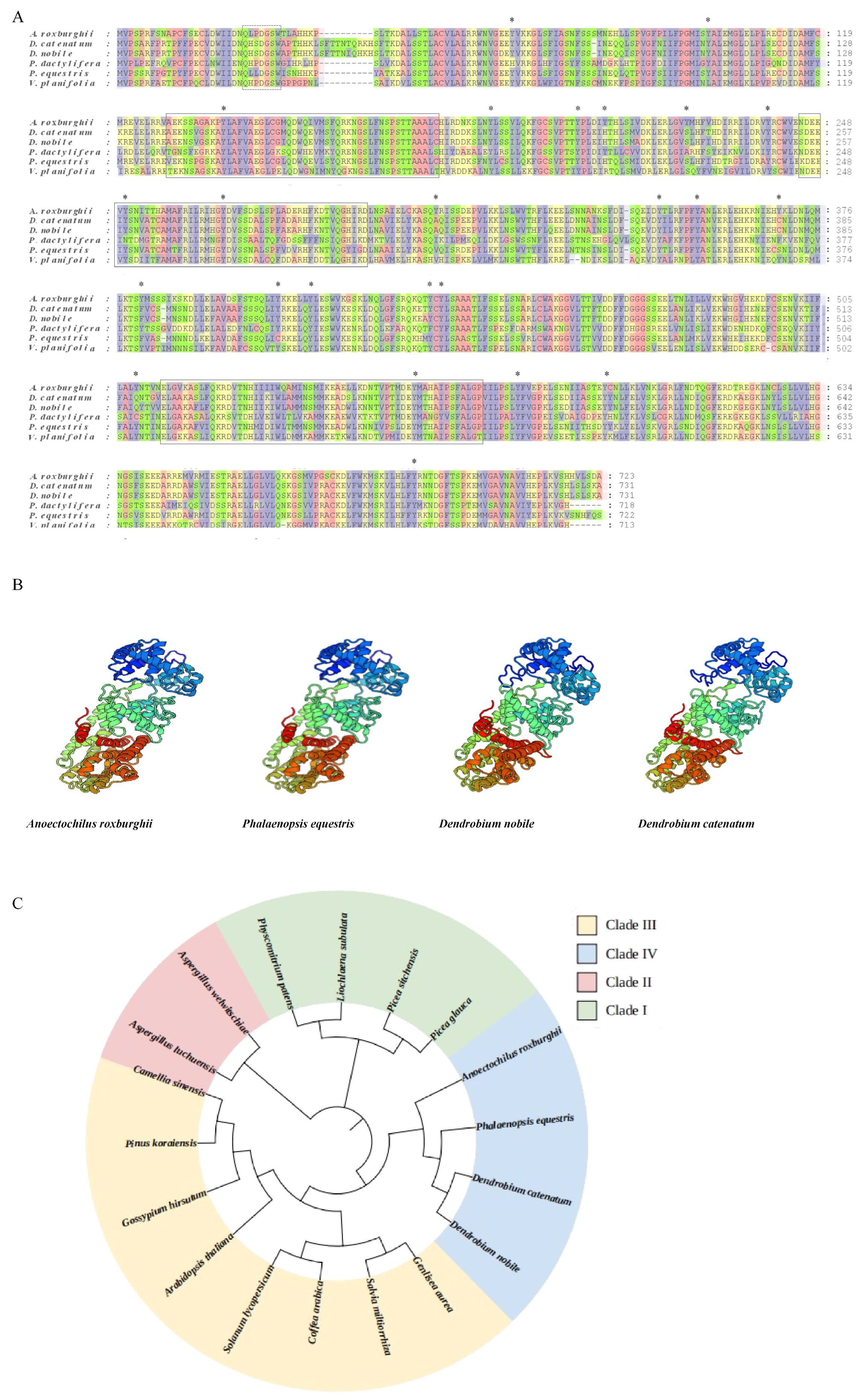
3.1. Open Reading Frame and Putative Proteins
3.2. Multiple Sequence Alignment
3.3. Subcellular Localization of KS Proteins
3.4. Overexpression of KS Gene
3.5. Relative Expression Levels and GA Accumulation Under Salinity Stress
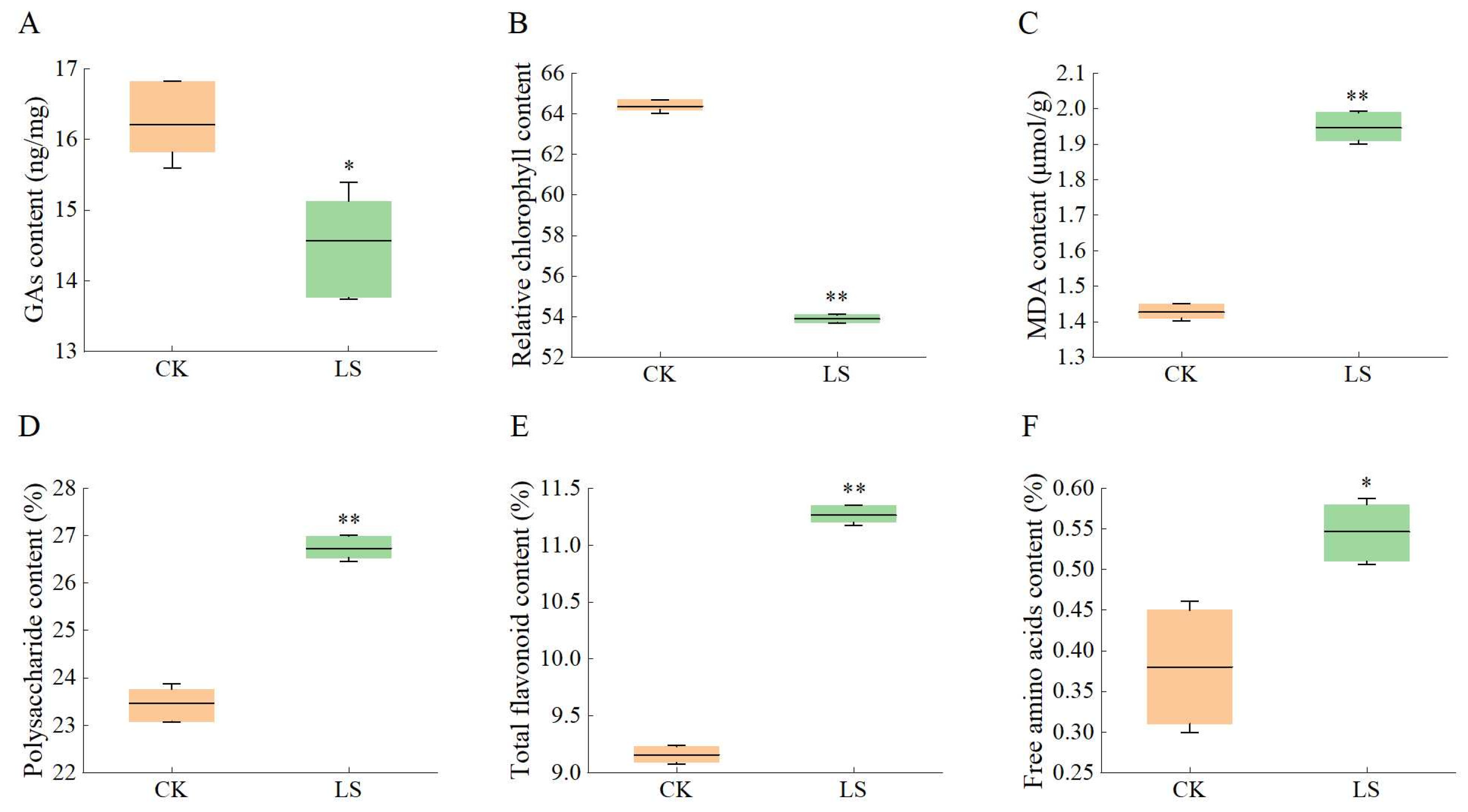
3.6. Response of Related Physiological Indicators to Salinity Stress
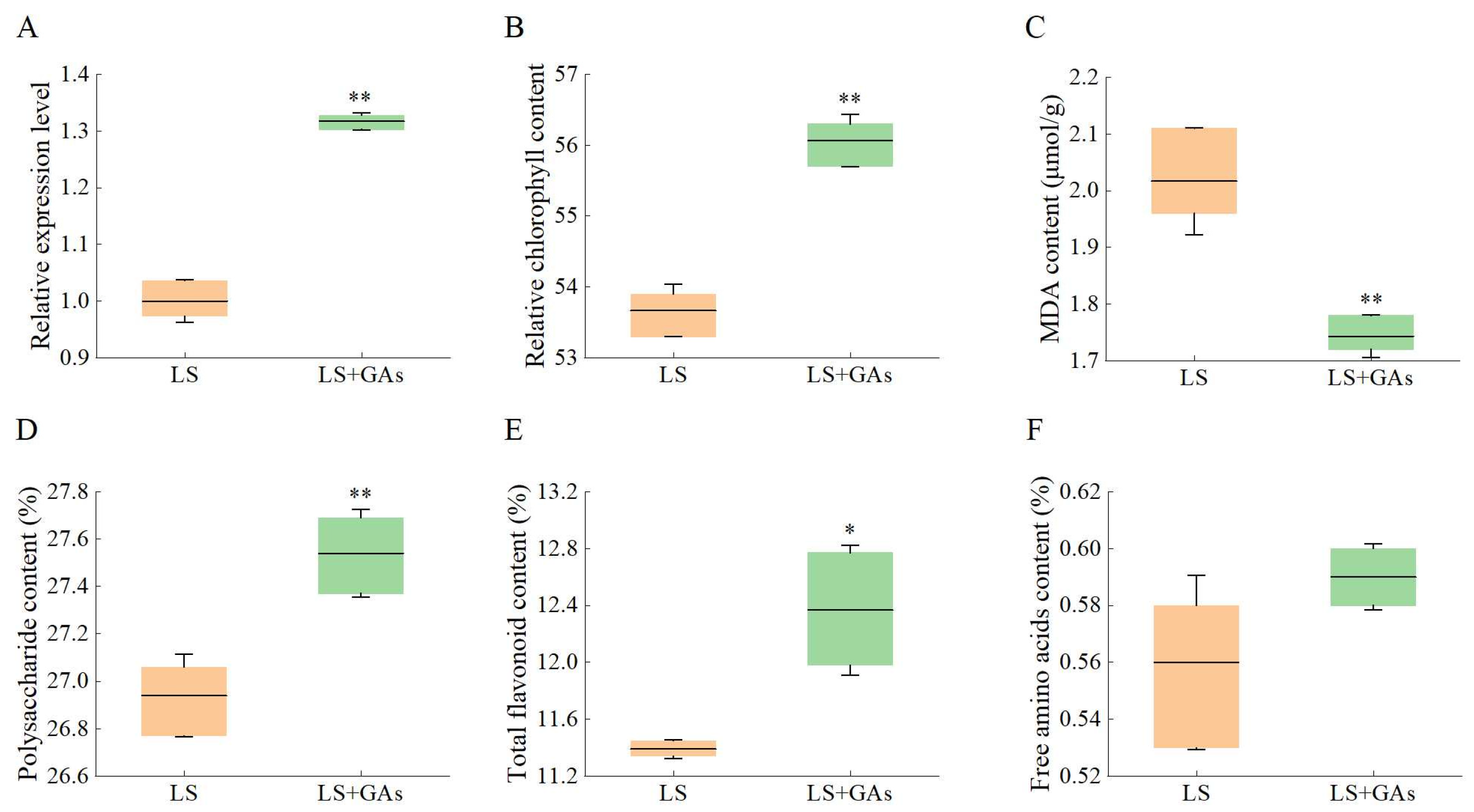
3.7. Relative Expression Levels of KS, Relative Chlorophyll, MDA, and Active Ingredient Content Under Salt Stress with Exogenous GAs Compensation
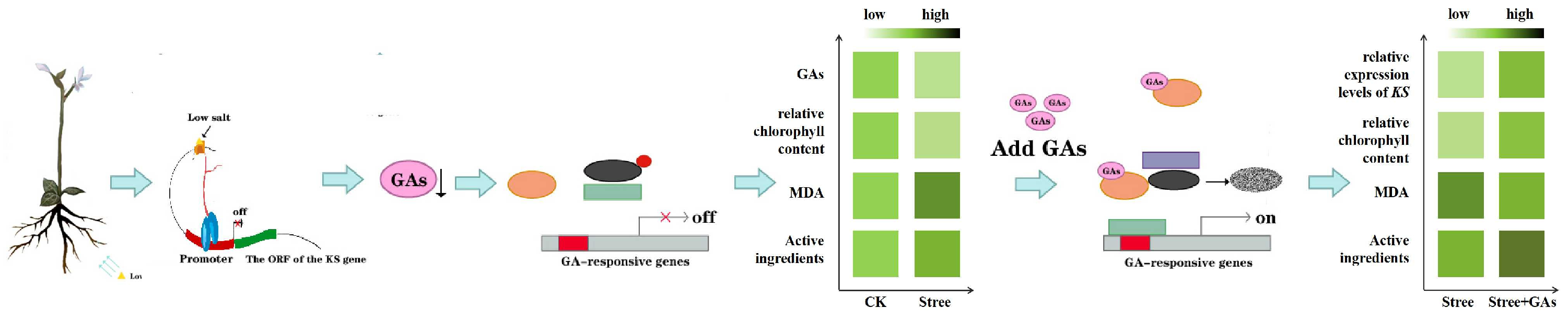
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.P.; Jermakow, A.; Swain, S.M. Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 2002, 14, 3133–3147. [Google Scholar] [CrossRef]
- Tezuka, D.; Ito, A.; Mitsuhashi, W.; Toyomasu, T.; Imai, R. The rice ent-kaurene synthase like 2 encodes a functional ent-beyerene synthase. Biochem. Biophys. Res. Commun. 2015, 460, 766–771. [Google Scholar] [CrossRef]
- Sarkaria, A.S.; Chinna, G.S. Effect of gibberellic acid and potassium nutrition on yield and potassium uptake of potato. Agric. Res. J. 2021, 58, 146–149. [Google Scholar] [CrossRef]
- Yang, M.; Liu, G.H.; Yamamura, Y.; Chen, F.; Fu, J.Y. Divergent evolution of the diterpene biosynthesis pathway in tea plants (Camellia sinensis) caused by single amino acid variation of ent-kaurene synthase. J. Agric. Food Chem. 2020, 68, 9930–9939. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Lin, Y.M.; Qiu, Y.Y.; Li, W.Y.; Shen, Y.T.; Huang, L.L. Identification and functional characterization of the diterpene synthase family in Pogostemon cablin (Blanco) Benth. Plant Physiol. Biochem. 2024, 216, 109190. [Google Scholar] [CrossRef]
- Brandle, J.E.; Telmer, P.G. Steviol glycoside biosynthesis. Phytochemistry 2007, 68, 1855–1863. [Google Scholar] [CrossRef]
- Hayashi, K.; Kawaide, H.; Notomi, M.; Sakigi, Y.; Matsuo, A.; Nozaki, H. Identification and functional analysis of bifunctional ent-kaurene synthase from the moss Physcomitrella patens. FEBS Lett. 2006, 580, 6175–6181. [Google Scholar] [CrossRef]
- Kong, M.K.; Kang, H.L.; Kim, J.H.; Oh, S.H.; Lee, P.C. Metabolic engineering of the Stevia rebaudiana ent-kaurene biosynthetic pathway in recombinant Escherichia coli. J. Biotechnol. 2015, 214, 95–102. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Saito, T.; Abe, H.; Yamane, H.; Kamiya, Y. Molecular cloning and characterization of a cDNA encoding the gibberellin biosynthetic enzyme ent-kaurene synthase B from pumpkin (Cucurbita maxima L.). Plant J. 1996, 10, 203–213. [Google Scholar] [CrossRef]
- Yamamura, Y.; Taguchi, Y.; Ichitani, K.; Umebara, I.; Ohshita, A.; Kurosaki, F.; Lee, J.B. Characterization of ent-kaurene synthase and kaurene oxidase involved in gibberellin biosynthesis from Scoparia dulcis. J. Nat. Med. 2018, 72, 456–463. [Google Scholar] [CrossRef]
- Toyomasu, T.; Miyamoto, K.; Shenton, M.R.; Sakai, A.; Sugawara, C.; Horie, K.; Kawaide, H.; Hasegawa, M.; Chuba, M.; Mitsuhashi, W.; et al. Characterization and evolutionary analysis of ent-kaurene synthase like genes from the wild rice species Oryza rufipogon. Biochem. Biophys. Res. Commun. 2016, 480, 402–408. [Google Scholar] [CrossRef]
- Zerbe, P.; Chiang, A.; Bohlmann, J. Mutational analysis of white spruce (Picea glauca) ent-kaurene synthase (PgKS) reveals common and distinct mechanisms of conifer diterpene synthases of general and specialized metabolism. Phytochemistry 2012, 74, 30–39. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, L.Y.; Wu, Y.X.; Wang, D.H.; He, X.Q. Gibberellin promotes cambium reestablishment during secondary vascular tissue regeneration after girdling in an auxin-dependent manner in Populus. J. Integr. Plant Biol. 2024, 66, 86–102. [Google Scholar] [CrossRef]
- Teng, Y.T.; Wang, Y.W.; Zhang, Y.T.; Xie, Q.Y.; Zeng, Q.Z.; Cai, M.H.; Chen, T. Genome-wide Identification and expression analysis of ent-kaurene synthase-like gene family associated with abiotic stress in rice. Int. J. Mol. Sci. 2024, 25, 5513. [Google Scholar] [CrossRef]
- Phosuwan, S.; Nounjan, N.; Theerakulpisut, P.; Siangliw, M.; Charoensawan, V. Comparative quantitative trait loci analysis framework reveals relationships between salt stress responsive phenotypes and pathways. Front. Plant Sci. 2024, 15, 18. [Google Scholar] [CrossRef]
- Kong, C.C.; Ren, C.G.; Li, R.Z.; Xie, Z.H.; Wang, J.P. Hydrogen peroxide and strigolactones signaling are involved in alleviation of salt stress induced by Arbuscular Mycorrhizal Fungus in Sesbania cannabina seedlings. J. Plant Growth Regul. 2017, 36, 734–742. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, S.N.; Qu, G.Y.; Fu, W.; Zhang, M.D.; Liu, Z.Y.; Feng, H. The mutation of ent-kaurene synthase, a key enzyme involved in gibberellin biosynthesis, confers a non-heading phenotype to Chinese cabbage (Brassica rapa L. ssp. pekinensis). Hortic. Res. 2020, 7, 178. [Google Scholar] [CrossRef]
- Lang, K.Y.; Chen, X.Q.; Luo, Y.B.; Zhu, G.H. Angiospermae. In Flora of China; The Editorial Board of Flora of China, Ed.; Science Press: Beijing, China, 1999; Volume 17, pp. 220–227. [Google Scholar]
- Yu, X.; Lin, S.; Zhang, J.; Huang, L.; Yan, H.; Li, S. Purification of polysaccharide from artificially cultivated Anoectochilus roxburghii (Wall.) Lindl. by high-speed counter current chromatography and its antitumor activity. J. Sep. Sci. 2017, 40, 4338–4346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Liu, Q.; Liu, Z.L.; Li, L.; Yi, L.T. Antihyperglycemic activity of Anoectochilus roxburghii polysaccharose in diabetic mice induced by high-fat diet and streptozotocin. J. Ethnopharmacol. 2015, 164, 180–185. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.C.; Fu, F.L.; Qu, J.T.; Sun, F.A.; Yu, H.Q.; Zhang, J.C. Characterization of phenylalanine ammonia-lyase genes facilitating flavonoid biosynthesis from two species of medicinal plant Anoectochilus. Peer J. 2022, 10, e13614. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.C.; Qu, J.T.; He, G.; Yu, H.Q.; Li, W.C.; Fu, F.L. Expression response of chalcone synthase gene to inducing conditions and its effect on flavonoids accumulation in two medicinal species of Anoectochilus. Sci. Rep. 2019, 9, 20171. [Google Scholar] [CrossRef]
- Yang, L.; Dai, L.W.; Zhang, H.Y.; Su, F.A.; Tang, X.C.; Feng, W.Q.; Yu, H.Q.; Zhang, J.C. Molecular and functional analysis of trehalose-6-phosphate synthase genes enhancing salt tolerance in Anoectochilus roxburghii (Wall.) Lindl. Molecules 2023, 28, 5139. [Google Scholar] [CrossRef]
- He, S.; Tan, L.L.; Hu, Z.L.; Chen, G.P.; Wang, G.X.; Hu, T.Z. Molecular characterization and functional analysis by heterologous expression in E. coli under diverse abiotic stresses for OsLEA5, the atypical hydrophobic LEA protein from Oryza sativa L. Mol. Genet. Genom. 2012, 287, 39–54. [Google Scholar] [CrossRef]
- Udawat, P.; Mishra, A.; Jha, B. Heterologous expression of an uncharacterized uni-versal stress protein gene (SbUSP) from the extreme halophyte, Salicornia brachiata, which confers salt and osmotic tolerance to E. coli. Gene 2014, 536, 163–170. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Sun, T.P.; Kamiya, Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell 1994, 6, 1509–1518. [Google Scholar]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Yang, D.B.; Yan, X.J.; Cui, L.; Wang, Z.Y.; Yuan, H.Z. The role of gibberellins in improving the resistance of tebuconazole-coated maize seeds to chilling stress by microencapsulation. Sci. Rep. 2016, 6, 35447. [Google Scholar] [CrossRef]
- Tsuchida-Mayama, T.; Sakai, T.; Hanada, A.; Uehara, Y.; Asami, T.; Yamaguchi, S. Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 2010, 62, 653–662. [Google Scholar] [CrossRef]
- Reid, J.B.; Botwright, N.A.; Smith, J.J.; O’neill, D.P.; Kerckhoffs, L.H. Control of gibberellin levels and gene expression during de-etio-lation in pea. Plant Physiol. 2002, 128, 734–741. [Google Scholar] [CrossRef]
- Toyomasu, T.; Kawaide, H.; Mitsuhashi, W.; Inoue, Y.; Kamiya, Y. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol. 1998, 118, 1517–1523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Sun, F.; Zhao, S.; Zhang, H.; Yu, H.; Zhang, J.; Yang, C. Overexpression of Ent-Kaurene Synthase Genes Enhances Gibberellic Acid Biosynthesis and Improves Salt Tolerance in Anoectochilus roxburghii (Wall.) Lindl. Genes 2025, 16, 914. https://doi.org/10.3390/genes16080914
Yang L, Sun F, Zhao S, Zhang H, Yu H, Zhang J, Yang C. Overexpression of Ent-Kaurene Synthase Genes Enhances Gibberellic Acid Biosynthesis and Improves Salt Tolerance in Anoectochilus roxburghii (Wall.) Lindl. Genes. 2025; 16(8):914. https://doi.org/10.3390/genes16080914
Chicago/Turabian StyleYang, Lin, Fuai Sun, Shanyan Zhao, Hangying Zhang, Haoqiang Yu, Juncheng Zhang, and Chunyan Yang. 2025. "Overexpression of Ent-Kaurene Synthase Genes Enhances Gibberellic Acid Biosynthesis and Improves Salt Tolerance in Anoectochilus roxburghii (Wall.) Lindl." Genes 16, no. 8: 914. https://doi.org/10.3390/genes16080914
APA StyleYang, L., Sun, F., Zhao, S., Zhang, H., Yu, H., Zhang, J., & Yang, C. (2025). Overexpression of Ent-Kaurene Synthase Genes Enhances Gibberellic Acid Biosynthesis and Improves Salt Tolerance in Anoectochilus roxburghii (Wall.) Lindl. Genes, 16(8), 914. https://doi.org/10.3390/genes16080914





