Impact of Dietary Inputs on Carbapenem Resistance Gene Dynamics and Microbial Safety During Bioconversion of Agri-Food Waste and Anaerobic Digestate by Hermetia illucens Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Sampling
2.2. Viable Counts
2.3. DNA Extraction and qPCR Quantification of Carbapenem Resistance Genes
2.4. Statistical Analysis
3. Results
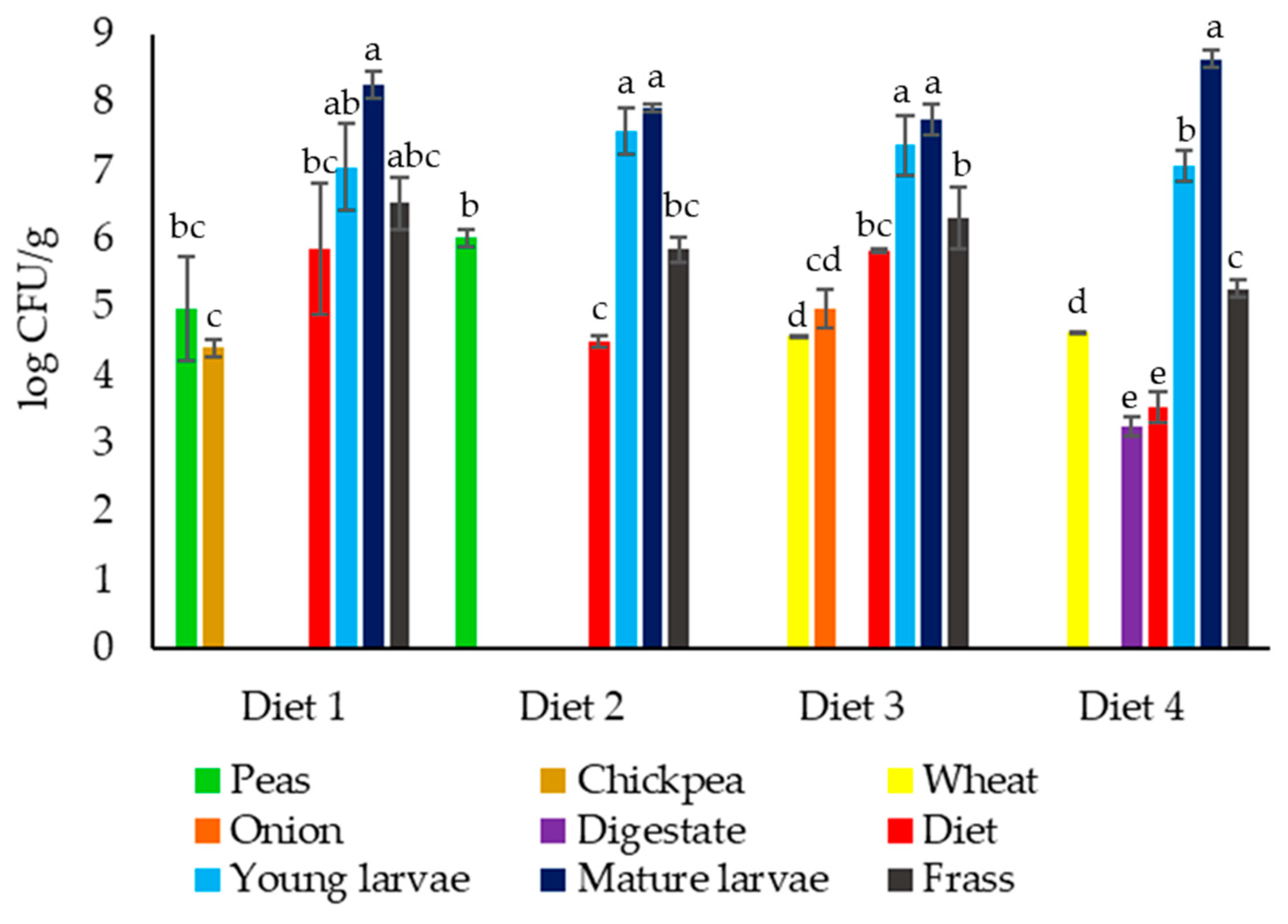
3.1. Viable Counts Results
3.2. qPCR Quantification of Carbapenem Resistance Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Antibiotic Resistance |
| CRGs | Carbapenem Resistance Genes |
| qPCR | Quantitative Polymerase Chain Reaction |
| EU | European Union |
| EFSA | European Food Safety Authority |
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Urugo, M.M.; Teka, T.A.; Gemede, H.F.; Mersha, S.; Tessema, A.; Woldemariam, H.W.; Admassu, H. A Comprehensive Review of Current Approaches on Food Waste Reduction Strategies. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70011. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Brulé, L.; Misery, B.; Baudouin, G.; Yan, X.; Guidou, C.; Trespeuch, C.; Foltyn, C.; Anthoine, V.; Moriceau, N.; Federighi, M.; et al. Evaluation of the Microbial Quality of Hermetia illucens Larvae for Animal Feed and Human Consumption: Study of Different Type of Rearing Substrates. Foods 2024, 13, 1587. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Yuwono, N.W.; Nisa’, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black Soldier Fly Larvae (BSFL) and Their Affinity for Organic Waste Processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Lomonaco, G.; Franco, A.; De Smet, J.; Scieuzo, C.; Salvia, R.; Falabella, P. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects 2024, 15, 293. [Google Scholar] [CrossRef]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture: A Review. Agron. Sustain. Dev. 2021, 41, 56. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and Its Suitability as Animal Feed—A Review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- da-Silva, W.C.; Silva, É.B.R.; Silva, J.A.R.; Martorano, L.G.; Belo, T.S.; Sousa, C.E.L.; Camargo-Júnior, R.N.C.; Andrade, R.L.; Santos, A.G.S.; Carvalho, K.C.; et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects 2024, 15, 619. [Google Scholar] [CrossRef]
- Govorushko, S. Global Status of Insects as Food and Feed Source: A Review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Osuch, B.; Barszcz, M.; Tomaszewska-Zaremba, D. The Potential of Black Soldier Fly (Hermetia illucens L.) Larvae in Chicken and Swine Nutrition: A Review. J. Anim. Feed Sci. 2024, 33, 454–468. [Google Scholar] [CrossRef]
- Mohan, K.; Sathishkumar, P.; Rajan, D.K.; Rajarajeswaran, J.; Ganesan, A.R. Black Soldier Fly (Hermetia illucens) Larvae as Potential Feedstock for Biodiesel Production: Recent Advances and Challenges. Sci. Total Environ. 2023, 860, 160235. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risk Profile Related to Production and Consumption of Insects as Food and Feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Milanović, V.; Cardinali, F.; Aquilanti, L.; Maoloni, A.; Garofalo, C.; Zarantoniello, M.; Olivotto, I.; Riolo, P.; Ruschioni, S.; Isidoro, N.; et al. Quantitative Assessment of Transferable Antibiotic Resistance Genes in Zebrafish (Danio rerio) Fed Hermetia illucens-Based Feed. Anim. Feed Sci. Technol. 2021, 277, 114978. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Guerrero-Barrera, A.L.; Avelar-González, F.J. An Overview of Carbapenem-Resistant Organisms from Food-Producing Animals, Seafood, Aquaculture, Companion Animals, and Wildlife. Front. Vet. Sci. 2023, 10, 2023. [Google Scholar] [CrossRef]
- Carpentier, J.; Martin, C.; Luttenschlager, H.; Vandeweyer, D.; Zels, S.; Cerstiaens, A.; Verheyen, G.; Crauwels, S.; Van Campenhout, L.; Smagghe, G. Common soluble carbohydrates affect the growth, survival, and fatty acid profile of black soldier fly larvae (Hermetia illucens; Stratiomyidae). Sci. Rep. 2024, 14, 28157. [Google Scholar] [CrossRef]
- Schneider, L.; Kisinga, B.; Stoehr, N.; Cord-Landwehr, S.; Schulte-Geldermann, E.; Moerschbacher, B.M.; Eder, K.; Jha, R.; Dusel, G. Dietary protein levels in isoenergetic diets affect the performance, nutrient utilization and retention of nitrogen and amino acids of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae. Insects 2025, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Roncolini, A.; Garofalo, C.; Aquilanti, L.; Pasquini, M.; Tavoletti, S.; Vignaroli, C.; Canonico, L.; Ciani, M.; et al. Investigation of the Dominant Microbiota in Ready-to-Eat Grasshoppers and Mealworms and Quantification of Carbapenem Resistance Genes by qPCR. Front. Microbiol. 2018, 9, 3036. [Google Scholar] [CrossRef]
- Garofalo, C.; Cesaro, C.; Milanović, V.; Belleggia, L.; Matricardi, T.; Osimani, A.; Aquilanti, L.; Cardinali, F.; Rampanti, G.; Simoni, S.; et al. Search for Carbapenem-Resistant Bacteria and Carbapenem Resistance Genes along Swine Food Chains in Central Italy. PLoS ONE 2024, 19, e0296098. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, Y.; Glaeser, S.P.; Mvie, J.; Bartz, J.-O.; Müller, A.; Gutzeit, H.O.; Vilcinskas, A.; Kämpfer, P. The Gut and Feed Residue Microbiota Changing during the Rearing of Hermetia illucens Larvae. Antonie Van Leeuwenhoek 2020, 113, 1323–1344. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Mašková, Z.; Hleba, L.; Kolesár, E.; Urminská, D.; Ivanišová, E.; Medo, J.; Tančinová, D.; Urminská, J.; Mrvová, M.; Barboráková, Z. Microbiological and Nutritional Aspects of Black Soldier Fly (Hermetia illucens) Larvae after Bioconversion of Different Food Wastes. J. Microbiol. Biotechnol. Food Sci. 2025, 14, e12266. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the Microbiota of Black Soldier Fly Larvae (Hermetia illucens) Reared on Organic Waste Streams on Four Different Locations at Laboratory and Large Scale. Microb. Ecol. 2018, 77, 913–930. [Google Scholar] [CrossRef]
- Zheng, L.; Crippen, T.L.; Singh, B.; Tarone, A.M.; Dowd, S.; Yu, Z.; Wood, T.K.; Tomberlin, J.K. A Survey of Bacterial Diversity from Successive Life Stages of Black Soldier Fly (Diptera: Stratiomyidae) by Using 16S rDNA Pyrosequencing. J. Med. Entomol. 2013, 50, 647–658. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The Core Gut Microbiome of Black Soldier Fly (Hermetia illucens) Larvae Raised on Low-Bioburden Diets. Front. Microbiol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Jiang, C.L.; Jin, W.Z.; Tao, X.H.; Zhang, Q.; Zhu, J.; Feng, S.Y.; Xu, X.H.; Li, H.Y.; Wang, Z.H.; Zhang, Z.J. Black Soldier Fly Larvae (Hermetia illucens) Strengthen the Metabolic Function of Food Waste Biodegradation by Gut Microbiome. Microb. Biotechnol. 2019, 12, 528–543. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Rossi, P.; Gorrens, E. The Intestinal Microbiota of Hermetia illucens Larvae Is Affected by the Rearing Substrate. Front. Microbiol. 2019, 10, 301. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2020, 9, 543. [Google Scholar] [CrossRef]
- Almou, A.A.; Alio, S.A.; Yaou, C.; Rabiou, M.M.; Mahamane, I.I.A.; Sabo, H.S.; Sadou, H. Assessment of Risk Factors of Bacteriological Contamination of Vegetables Sold on the Markets of Niamey, Niger. South Asian J. Res. Microbiol. 2023, 17, 38–49. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Druilhe, C.; Repérant, E.; Boscher, E.; Rouxel, S.; Le Roux, S.; Poëzévara, T.; Ziebal, C.; Houdayer, C.; Nagard, B.; et al. Evaluation of the Occurrence of Sporulating and Nonsporulating Pathogenic Bacteria in Manure and in Digestate of Five Agricultural Biogas Plants. Microbiol. Open 2019, 8, e872. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fatla, A.; Farian, E.; Kowalczyk, K.; Sroka, J.; Skowron, P.; Siebielec, G.; Zdybel, J.M.; Jadczyszyn, T.; Cencek, T. Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens 2024, 13, 1056. [Google Scholar] [CrossRef]
- Osimani, A.; Ferrocino, I.; Corvaglia, M.R.; Roncolini, A.; Milanović, V.; Garofalo, C.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Jamshidi, E.; et al. Microbial Dynamics in Rearing Trials of Hermetia illucens Larvae Fed Coffee Silverskin and Microalgae. Food Res. Int. 2021, 140, 110028. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-Based Biogas Fermentation Residues–Friend or Foe of Soil Fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal Sludge Management with the Larvae of the Black Soldier Fly (Hermetia illucens)—From a Hygiene Aspect. Sci. Total Environ. 2013, 458, 312–318. [Google Scholar] [CrossRef]
- Gold, M.; Egert, M.; Hellwig, E.; Singer, D. A Review on the Microbiota Associated with Black Soldier Fly Larvae (Hermetia illucens L.) and Its Impact on the Rearing Environment. Curr. Opin. Insect Sci. 2020, 40, 53–60. [Google Scholar]
- European Commission. Regulation (EU) 2021/1925 of 5 November 2021 Amending Certain Annexes to Regulation (EU) No 142/2011 as Regards the Requirements for Placing on the Market of Certain Insect Products and the Adaptation of a Containment Method. Off. J. Eur. Union 2021, L 393, 4–8. [Google Scholar]
- Van Looveren, N.; Vandeweyer, D.; Van Campenhout, L. Impact of Heat Treatment on the Microbiological Quality of Frass Originating from Black Soldier Fly Larvae (Hermetia illucens). Insects 2022, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Off. J. Eur. Union 2017, L 138, 92–116. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=IT (accessed on 1 June 2025).
- European Commission. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 on Making Fertilising Products Available on the Market. Off. J. Eur. Union 2019, 190, 1–83. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar] [CrossRef] [PubMed]
- OIE. List of Antimicrobial Agents of Veterinary Medicine; World Organization for Animal Health: Paris, France, 2015; Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/Eng_OIE_List_antimicrobials_May2015.pdf (accessed on 1 June 2025).
- Zhang, Y.-J.; Hu, H.-W.; Chen, Q.-L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.-Z. Transfer of Antibiotic Resistance from Manure-Amended Soils to Vegetable Microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef] [PubMed]
| Diet | Substrates (Fresh + Dry) | Ratio (Fresh/Dry) |
|---|---|---|
| 1 | peas + chickpea | 3.5:1 |
| 2 | peas + wheat | 3.5:1 |
| 3 | onion + wheat | 3:1 |
| 4 | liquid digestate + wheat | 2:1 |
| Diet | Sample | Carbapenem Resistance Genes (log Gene Copies/g ± Standard Deviation) | ||||
|---|---|---|---|---|---|---|
| blaKPC | blaOXA-48 | blaNDM | blaGES | blaVIM | ||
| 1 | Peas | n.d. | n.d. | n.d. | n.d. | n.d. |
| Chickpea | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Diet (peas + chickpea) | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Young H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Frass | n.d. | n.d. | n.d. | n.d. | 8.05 ± 0.02 a | |
| Mature H. illucens larvae | n.d. | 5.44 ± 0.02 | n.d. | 7.65 ± 0.01 | 6.67 ± 0.02 b | |
| 2 | Peas | n.d. | n.d. | n.d. | n.d. | n.d. |
| Wheat | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Diet 2 (peas + wheat) | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Young H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Frass | 8.17 ± 0.02 | n.d. | n.d. | 8.66 ± 0.01 a | n.d. | |
| Mature H. illucens larvae | n.d. | 5.43 ± 0.02 | n.d. | 8.14 ± 0.06 b | n.d. | |
| 3 | Onion | n.d. | n.d. | n.d. | n.d. | n.d. |
| Wheat | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Diet 3 (onion + wheat) | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Young H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Frass | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Mature H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 4 | Digestate | n.d. | n.d. | n.d. | n.d. | n.d. |
| Wheat | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Diet 4 (digestate + wheat) | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Young H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Frass | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Mature H. illucens larvae | n.d. | n.d. | n.d. | n.d. | n.d. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelli, A.; Ilari, A.; Milanović, V.; Foppa Pedretti, E.; Boakye-Yiadom, K.A.; Cardinali, F.; Rampanti, G.; Osimani, A.; Garofalo, C.; Aquilanti, L. Impact of Dietary Inputs on Carbapenem Resistance Gene Dynamics and Microbial Safety During Bioconversion of Agri-Food Waste and Anaerobic Digestate by Hermetia illucens Larvae. Genes 2025, 16, 907. https://doi.org/10.3390/genes16080907
Marcelli A, Ilari A, Milanović V, Foppa Pedretti E, Boakye-Yiadom KA, Cardinali F, Rampanti G, Osimani A, Garofalo C, Aquilanti L. Impact of Dietary Inputs on Carbapenem Resistance Gene Dynamics and Microbial Safety During Bioconversion of Agri-Food Waste and Anaerobic Digestate by Hermetia illucens Larvae. Genes. 2025; 16(8):907. https://doi.org/10.3390/genes16080907
Chicago/Turabian StyleMarcelli, Andrea, Alessio Ilari, Vesna Milanović, Ester Foppa Pedretti, Kofi Armah Boakye-Yiadom, Federica Cardinali, Giorgia Rampanti, Andrea Osimani, Cristiana Garofalo, and Lucia Aquilanti. 2025. "Impact of Dietary Inputs on Carbapenem Resistance Gene Dynamics and Microbial Safety During Bioconversion of Agri-Food Waste and Anaerobic Digestate by Hermetia illucens Larvae" Genes 16, no. 8: 907. https://doi.org/10.3390/genes16080907
APA StyleMarcelli, A., Ilari, A., Milanović, V., Foppa Pedretti, E., Boakye-Yiadom, K. A., Cardinali, F., Rampanti, G., Osimani, A., Garofalo, C., & Aquilanti, L. (2025). Impact of Dietary Inputs on Carbapenem Resistance Gene Dynamics and Microbial Safety During Bioconversion of Agri-Food Waste and Anaerobic Digestate by Hermetia illucens Larvae. Genes, 16(8), 907. https://doi.org/10.3390/genes16080907








