Abstract
Ménière’s Disease (MD) is a chronic inner ear disorder defined by recurring episodes of vertigo, fluctuating sensorineural hearing loss, tinnitus, and/or fullness in the ear. Its prevalence varies by region and ethnicity, with scarce epidemiological data in the Brazilian population. Although most MD cases are sporadic, familial MD (FMD) is observed in 5% to 20% of European cases. Through exome sequencing, we have found a rare missense variant in the OTOG gene in a Brazilian individual with MD with probable European ancestry (chr11:17599671C>T), which was previously reported in a Spanish cohort. Two additional rare missense heterozygous OTOG variants were found in the same proband. Splice Site analysis showed that chr11:17599671C>T may lead to substantial changes generating exonic cis regulatory elements, and protein modelling revealed structural changes in the presence of chr11:17599671C>T, chr11:17576581G>C, and chr11:17594108C>T, predicted to highly destabilize the protein structure. The manuscript aims to replicate genes previously reported in a Spanish cohort, and the main finding is that a Brazilian patient with MD also has variants previously reported in familial MD, supporting OTOG as the most frequently mutated gene in MD.
1. Introduction
Ménière’s Disease (MD, MIM 156000) is a rare chronic inner ear disorder that manifests through recurrent episodes of vertigo, fluctuating sensorineural hearing loss (SNHL), tinnitus, and aural fullness. Its prevalence varies across regions and ethnic groups worldwide, ranging from 34 to 190 per 100,000 adults, with familial aggregation and higher occurrence among women and Caucasians [,]. Epidemiological studies in Western European and East Asian populations have shown significant differences in familial aggregation and associated co-morbidities in MD [,,]. However, the lack of epidemiological data in the Brazilian population makes genetic studies an arduous task.
Although most MD cases are considered sporadic, familial Meniere’s Disease (FMD) has been reported in 5–20% of cases of European descent []. The current diagnostic approach relies on clinical criteria outlined by the Barany Society in the 2015 international guidelines, emphasizing clinical stratification and the identification of incomplete phenotypes []. Exome and genome sequencing studies combining gene burden tests and segregation analyses support MD as a polygenic condition [,,]. Around 20 genes have been linked to familial MD [], including SNHL genes demonstrating autosomal dominant, recessive, and digenic inheritance patterns [,], supporting the concept of genetic heterogeneity in this condition.
The OTOG gene, encoding Otogelin, an extracellular protein in the tectorial membrane in the organ of Corti [,], is emerging as a key gene in familial MD []. Several missense variants in OTOG have been identified in Spanish families with MD, showing aggregation in the coding sequence of constrained regions, which may explain the higher MD prevalence in Europeans [,].
This exome sequencing study aims to identify novel candidate genes in Brazilian patients with MD. Our results replicate a rare missense variant chr11:17599671C>T in the OTOG gene previously reported in a Spanish cohort in a Brazilian MD patient, supporting the role of OTOG in the molecular pathophysiology of MD.
2. Materials and Methods
2.1. Hearing Profile
Audiograms from the worse ear were obtained from patients and analyzed in R Studio (v.4.4.2) using a regression equation to estimate hearing onset and outcome. The coefficient of determination (R2) and p-values validated the model.
2.2. Whole Exome Sequencing (WES)
WES was performed on DNA samples from 23 unrelated Brazilian Menière’s Disease (MD) patients. Libraries were prepared with Nextera Rapid Exome Enrichment Kit® (Illumina, Inc., San Diego, CA, USA) and sequenced (2 × 100 base pairs paired end, ≥100× coverage) on HiSeq™ 2500 (Illumina). Reads were aligned to the GRCh38/hg38 using Burrows–Wheeler Aligner (BWA), and variants were called with DeepVariant and filtered for minor allele frequency (MAF) < 0.001 (Genome Aggregation Database, gnomAD v4.1.0) and CADD score > 20. The Integrative Genomics Viewer (IGV) assessed read quality.
2.3. Rare Variant Identification
Rare variants were retrieved from previously published sporadic MD (SMD, n = 511) [] and familial MD (FMD, n = 100) [] datasets (non-Finnish European MAF < 0.001, CADD score > 20). Pathogenicity was assessed per American College of Medical Genetics and Genomics (ACMG) and Association for Molecular Pathology (AMP) guidelines [] applied for Genetic Hearing Loss []. Missense variant density in the OTOG coding sequence was determined using a 200 base pair (bp) sliding window template previously reported [], identifying high-density regions (HDRs) and low-density regions (LDRs), with the latter also called constraint coding regions (CCRs), per gnomAD v2.1.
2.3.1. Splice Site Prediction
Human Splice Finder Pro [] was used to predict donor/acceptor sites, exonic and intronic splicing enhancers (ESEs/ISEs), exonic and intronic splicing silencers (ESSs/ISSs), and branch points.
2.3.2. Protein Modelling
Canonical UniProt sequences were modelled using Modeller [] v10.5 through homology-based reconstruction using a template of the WT protein previously reported []. Model validation included Discrete Optimized Protein Energy (DOPE), Genetic Algorithm 341 (GA341), and molecular probability density function (molpdf). Additional validation was performed via the Structural Analysis and Verification Server (SAVES v6.1; https://saves.mbi.ucla.edu/) (accessed on 25 October 2024 and 30 October 2024) for stereochemical quality and atomic interactions [,]. Structural analysis and images were obtained in PyMOL v3.0 [].
DynaMut2 [] and MuPro [] assessed variant impact on protein stability (ΔΔG). MuPro confidence score (CS) ranged from −1 to 1, with CS < 0 indicating destabilization and CS > 0 indicating stabilization. DynaMut2 classified variants as destabilizing for negative predicted free energy change (ΔΔG_pred) values and stabilizing for positive ΔΔG_pred values.
3. Results
3.1. Clinical Description
This study focuses on a male proband who meets definitive MD diagnostic criteria outlined by the Barany Society, with a 13-year disease course characterized by recurrent vertigo attacks (25–30 episodes), persistent left-sided tinnitus, fluctuating SNHL, and aural fullness. A comprehensive neurotological evaluation, incorporating video head impulse testing (vHIT) and serial audiometry, was conducted (Table 1), which confirmed bilateral SNHL without vestibular hypofunction. His medical history included well-controlled hypertension and autoimmune disease, with no familial history of auditory or vestibular disorders.

Table 1.
Summary of the phenotype observed in the patient with rare variants in OTOG.
Hearing Profile
The hearing profile of the Brazilian proband and three Spanish patients carrying the OTOG variant chr11:17599671C>T showed no significant hearing loss progression at any frequency (Supplementary Figure S1 and Table S1).
3.2. Rare OTOG Variant Replicated in Brazilian Sample
The missense variant chr11:17599671C>T (NFE MAF = 0.005) was replicated in both cohorts. Two additional rare OTOG missense variants were identified in the Brazilian proband, chr11:17576581G>C (NFE MAF = 0.00001) and chr11:17594108C>T (NFE MAF = 0.0004). The three of them were classified as Likely Pathogenic per ACMG guidelines, and chr11:17599671C>T showed a lower allelic frequency, while chr11:17576581G>C and chr11:17594108C>T showed a higher allelic frequency in the Brazilian population (ABraOM) when compared to non-Finnish (gnomAD_AF) and Spanish (CSVS_AF) populations (Table 2).

Table 2.
OTOG missense variants found in the proband. Chr11:17599671C>T was replicated in the Spanish cohort.
Variants chr11:17599671C>T and chr11:17576581G>T were predicted to destabilize the protein in both in silico methods employed, while chr11:17594108C>T showed conflicting results. When analyzed together within Otogelin, mimicking the proband’s protein, DynaMut2 stability prediction was −1.9 kcal mol−1, indicating a higher degree of destabilization (Supplementary Table S2).
Furthermore, the selected variants are located in biologically constrained low-density regions (LDRs), suggesting low protein tolerance to variation (Figure 1). We were unable to determine whether they are cis or trans-variants, as it was not possible to obtain samples from the patient’s parents, as they are deceased.
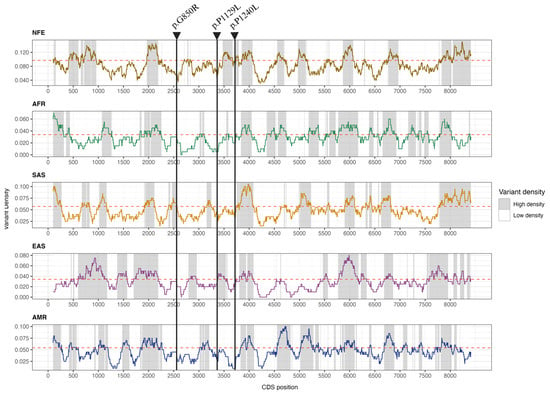
Figure 1.
Variant density profile along the OTOG CDS in the NFE, AFR, SAS, EAS, and AMR populations calculated with a 201 bp sliding window. The threshold density for each population based on the anticipated number of variants within CDS is indicated by the red dashed line.
Using HSF Pro, analysis of the three OTOG variants showed significant exonic cis-element alterations for chr11:17599671C>T, including the disruption of five ESEs and the formation of two ESEs and seven ESSs (Supplementary Table S3).
All three missense variants are located in and around the von Willebrand factor type D 3 (VWFD3) domain. The VWFD, EGF-like, and TIL domains are found in the head of the protein, while the CTCK domain is located in the tail (Figure 2a,b).
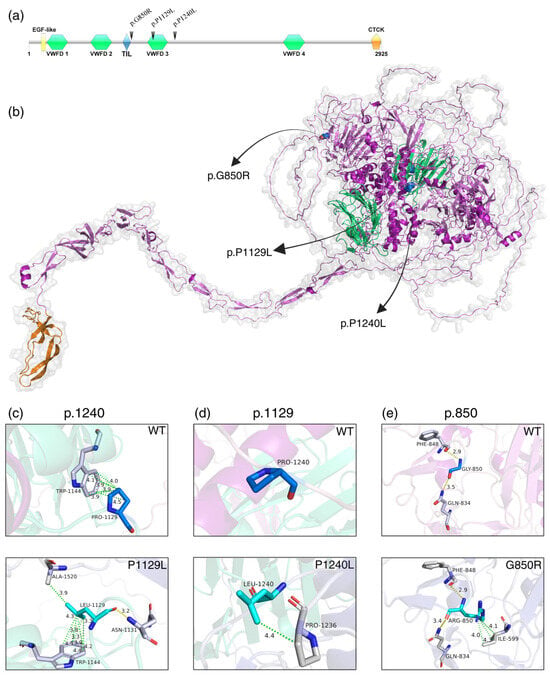
Figure 2.
(a) Otogelin protein domains: EGF-like (epidermal growth factor), VWFD (von Willebrand factor type D), TIL (trypsin inhibitor-like, cysteine-rich domain) and CTCK (C-terminal cystine knot) (created with IBS v.1.0.3 software). (b) Modelled Otogelin protein showing the three selected variants. (c–e) Structural differences between wild-type (WT, in blue) and mutated (in cyan) Otogelin residues at positions p.850, p.1129, and p.1240. Interactions between amino acids are represented by yellow (polar contacts) and green (hydrophobic) dotted lines, with respective distances measured in Ängstrom. It is possible to observe that all mutated amino acids create new bonds with neighbouring residues (in grey).
In silico analysis revealed that both wild-type and mutated amino acids are in loop regions. The variant chr11:17599671C>T (p.P1240L) was predicted not to form any contacts in the WT structure; however, the mutated amino acid formed a hydrophobic contact with the Proline at p.1236 (Figure 2c).
For chr11:17594108 C>T (p.P1129L), WT Proline forms several hydrophobic contacts with p.W1144, while the variant Leucine creates new hydrophobic and polar contacts with p.W1144, p.A1520, and p.N1131 (Figure 2d).
The variant chr11:17576581G>T (p.G850R) replaced Glycine with Arginine at position 850, which is predicted to create three hydrophobic contacts with p.I599 (Figure 2e) and alter polar interactions, leading to a short α-helix at p.841–843 (Supplementary Figure S2).
4. Discussion
This study replicates a missense variant in a Brazilian patient with SMD previously reported in two individuals and one family with MD in Spain.
There is an overload of missense variants in the OTOG gene in FMD []. The variant chr11:17599671C>T has been associated with moderate-to-severe flat hearing loss (60 dB) from early onset, involving all frequencies with minor low-frequency variations over time. Interestingly, it has not been reported in non-European individuals. The Brazilian proband, a male with no MD family history and unknown European ancestry, was screened for additional OTOG variants. Two rare heterozygous missense variants, chr11:17599671C>T and chr11:17594108C>T, were identified, neither previously associated with the disease.
The tectorial membrane (TM), an acellular layer above the organ of Corti, plays a key role in sound transmission by enhancing cochlear feedback []. The TM is generated by cochlear supporting cells [], and it is organized on the apical membrane of the supporting cells facing the lumen of the scala media []. Structurally, it consists of four collagen proteins (II, V, IX, XI), that forms the main structural framework and several non-glycoproteins, and proteoglycans, including α-tectorin, β-tectorin, otogelin, otogelin-like and CEACAM16 [,]. Otogelin, encoded by OTOG, as a key non-collagenous glycoprotein []. OTOG knockout mice have revealed its importance in auditory and balance functions, showing phenotypes like detachment of otoconial membranes, structural defects in the TM’s fibrillar network, and reduced TM resistance to sound stimuli []. Otogelin stabilizes the TM by interacting with its constituent fibres, and OTOG mutations can cause moderate nonsyndromic SNHL [].
When a protein residue mutates, new bonds replace existing ones, significantly altering the protein’s structure if the new residue’s properties differ greatly. These changes affect the conformation and bonding network around the mutation site, depending on the residues and their environment [].
All variants are located around (chr11:17599671C>T and chr11:17576581G>T) and within (chr11:17594108 C>T) the VWFD 3 domain in Otogelin protein. The TM likely regulates Ca2+ levels near hair cell stereocilia and in mechanoelectric transduction channel adaptation, with the VFWD domains of Otogelin binding Ca2+ ions as a reservoir []. Rare variations in Otogelin may create new electrostatic interactions, altering their 3D structure [].
Our study has a limitation since we could not obtain DNA samples from the proband’s relatives for segregation analysis, but the finding of three rare variants in the OTOG gene in the same proband cannot be explained by chance.
5. Conclusions
We have found a patient with MD with three rare missense variants in the OTOG gene, which were predicted to destabilize the protein and alter its structure. This is the first time the variant chr11:17599671C>T has been reported in non-Spanish patients with MD. These results support the OTOG gene as a key player in the pathophysiology of MD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes16060654/s1, Figure S1: Scattered plot showing hearing thresholds and the duration of the disease for each frequency in the four patients carrying the chr11:17599671C>T variant in OTOG gene. Green dots represent the SMD Brazilian proband; Magenta and Violet represent the two SMD Spanish patients; Yellow dots represent the FMD Spanish patient; Regression equation is represented by the blue dotted lines. The worst ear data was used for the plot. There were no available data for the SMD Spanish patients at 3 kHz (magenta dot) and 6 kHz (violet dot); Figure S2: Changes in Otogelin 3D structure. Polar interactions between amino acids are represented by yellow dotted lines, with respective distances measured in Ängstrom. (a) Wild type and mutated Otogelin structures overlapped, showing the creation of a α-helix at p.841–843. (b) Wild type Otogelin amino acids arrange and polar contacts at p.840–843. (c) Mutated Otogelin amino acids arrange and polar contacts at p.840–843. The polar contact formed within p.Asn842 in the WT structure no longer exists in the mutated one; Table S1: Coefficient of determination R2 and p value for each scattered plot; Table S2: Model quality validation of predicted structural models for selected variants in Otogelin; Table S3: Human Splicing Finder PRO predictions for chr11:17599671C>T variant in OTOG gene.
Author Contributions
G.B.-B.: conceptualization, formal analysis, data curation, investigation, methodology, validation, visualization. G.B.-B., K.d.C.L. and T.d.A.L.S.V.: writing of original draft. G.A.-C.: formal analysis, methodology. H.F.-S.: data curation. A.M.P.-P., K.d.C.L., T.d.A.L.S.V., F.F.G., J.C.A.-D. and A.S.-V.: resources. J.A.L.-E. and E.L.S.: conceptualization, funding acquisition, project administration, resources, supervision, validation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; grant number 2014/50897-0). GBB was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant number 141196/2021-1) and in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, Brazil (Finance Code 001). JALE has received funds to support research on MD from the University of Sydney (K7013_B3413 Grant), Asociación Sindrome de Meniere España (ASMES)and Meniere’s Society, UK.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Campinas Faculty of Medical Sciences (CAAE: 82809524.0.0000.5404).
Informed Consent Statement
Written informed consent was obtained from all individual participants included in this study.
Data Availability Statement
The OTOG variants chr11:17599671C>T, chr11:17576581G>T and chr11:17594108 C>T have been deposited in ClinVar (SUB15056944, accession number pending).
Acknowledgments
We would like to acknowledge all patients for donating blood to contribute to this study. We thank the members of CQMED-UNICAMP for their support. We thank the staff of the Life Sciences Core Facility (LaCTD) at UNICAMP for the Genomics analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic Criteria for Menière’s Disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ohmen, J.D.; White, C.H.; Li, X.; Wang, J.; Fisher, L.M.; Zhang, H.; Derebery, M.J.; Friedman, R.A. Genetic evidence for an ethnic diversity in the susceptibility to Ménière‘s disease. Otol. Neurotol. 2013, 34, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.S.; Whinney, D.J.; Ukoumunne, O.C.; Fleming, L.E.; Osborne, N.J. Prevalence, associated factors, and comorbid conditions for Ménière‘s disease. Ear Hear. 2014, 35, e162–e169. [Google Scholar] [CrossRef]
- Kim, M.H. Population-based study for the comorbidities and associated factors in Ménière‘s disease. Sci. Rep. 2022, 12, 8266. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Song, Y.; Kong, L.; Zhang, N.; Liu, J.; Li, G.; Fan, Z.; Lyu, Y.; Zhang, D.; et al. Ménière‘s disease and allergy: Epidemiology, pathogenesis, and therapy. Clin. Exp. Med. 2023, 23, 3361–3371. [Google Scholar] [CrossRef]
- Parra-Perez, A.M.; Lopez-Escamez, J.A. Types of Inheritance and Genes Associated with Familial Meniere Disease. J. Assoc. Res. Otolaryngol. JARO 2023, 24, 269–279. [Google Scholar] [CrossRef]
- Dai, Q.; Long, L.; Zhao, H.; Wang, R.; Zheng, H.; Duan, M. Genetic Advances in Meniere Disease. Mol. Biol. Rep. 2023, 50, 2901–2908. [Google Scholar] [CrossRef]
- Fisch, K.M.; Rosenthal, S.B.; Mark, A.; Sasik, R.; Nasamran, C.A.; Clifford, R.; Derebery, M.J.; Boussaty, E.; Jepsen, K.; Harris, J.; et al. The Genomic Landscape of Ménière’s Disease: A Path to Endolymphatic Hydrops. BMC Genom. 2024, 25, 646. [Google Scholar] [CrossRef]
- Gallego-Martinez, A.; Requena, T.; Roman-Naranjo, P.; Lopez-Escamez, J.A. Excess of Rare Missense Variants in Hearing Loss Genes in Sporadic Meniere Disease. Front. Genet. 2019, 10, 76. [Google Scholar] [CrossRef]
- Roman-Naranjo, P.; Moleon, M.D.C.; Aran, I.; Escalera-Balsera, A.; Soto-Varela, A.; Bächinger, D.; Gomez-Fiñana, M.; Eckhard, A.H.; Lopez-Escamez, J.A. Rare Coding Variants Involving MYO7A and Other Genes Encoding Stereocilia Link Proteins in Familial Meniere Disease. Hear. Res. 2021, 409, 108329. [Google Scholar] [CrossRef]
- Roman-Naranjo, P.; Gallego-Martinez, A.; Soto-Varela, A.; Aran, I.; Moleon, M.d.C.; Espinosa-Sanchez, J.M.; Amor-Dorado, J.C.; Batuecas-Caletrio, A.; Perez-Vazquez, P.; Lopez-Escamez, J.A. Burden of Rare Variants in the OTOG Gene in Familial Meniere’s Disease. Ear Hear. 2020, 41, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- El-Amraoui, A.; Cohen-Salmon, M.; Petit, C.; Simmler, M.C. Spatiotemporal expression of otogelin in the developing and adult mouse inner ear. Hear. Res. 2001, 158, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Avan, P.; Le Gal, S.; Michel, V.; Dupont, T.; Hardelin, J.-P.; Petit, C.; Verpy, E. Otogelin, Otogelin-like, and Stereocilin Form Links Connecting Outer Hair Cell Stereocilia to Each Other and the Tectorial Membrane. Proc. Natl. Acad. Sci. USA 2019, 116, 25948–25957. [Google Scholar] [CrossRef] [PubMed]
- Parra-Perez, A.M.; Gallego-Martinez, A.; Lopez-Escamez, J.A. An Overload of Missense Variants in the OTOG Gene May Drive a Higher Prevalence of Familial Meniere Disease in the European Population. Hum. Genet. 2024, 143, 423–435. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Oza, A.M.; DiStefano, M.T.; Hemphill, S.E.; Cushman, B.J.; Grant, A.R.; Siegert, R.K.; Shen, J.; Chapin, A.; Boczek, N.J.; Schimmenti, L.A.; et al. Expert Specification of the ACMG/AMP Variant Interpretation Guidelines for Genetic Hearing Loss. Hum. Mutat. 2018, 39, 1593–1613. [Google Scholar] [CrossRef]
- Desmet, F.-O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An Online Bioinformatics Tool to Predict Splicing Signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of Protein Structures: Patterns of Nonbonded Atomic Interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for Checking the Quality of Protein Structures Solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- PyMOL v3.0. Available online: https://pymolwiki.org/index.php/Main_Page (accessed on 5 May 2025).
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. DynaMut2: Assessing Changes in Stability and Flexibility upon Single and Multiple Point Missense Mutations. Protein Sci. 2021, 30, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Randall, A.; Baldi, P. Prediction of Protein Stability Changes for Single-Site Mutations Using Support Vector Machines. Proteins 2006, 62, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Moradi, Z.; Mohammadi, M.; Lagzian, M.; Asgharzade, S. Prediction and Interpretation of Rare Missense Variant in OTOG Associated with Hearing Loss. Genomics 2021, 113, 2793–2799. [Google Scholar] [CrossRef]
- Legan, P.K.; Lukashkina, V.A.; Goodyear, R.J.; Kössi, M.; Russell, I.J.; Richardson, G.P. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 2000, 28, 273–285. [Google Scholar] [CrossRef]
- Goodyear, R.J.; Richardson, G.P. Structure, Function, and Development of the Tectorial Membrane: An Extracellular Matrix Essential for Hearing. Curr. Top. Dev. Biol. 2018, 130, 217–244. [Google Scholar] [CrossRef]
- Rueda, J.; Cantos, R.; Lim, D.J. Distribution of glycoconjugates during cochlea development in mice: Light microscopic lectin study. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 274, 923–933. [Google Scholar] [CrossRef]
- Niazi, A.; Kim, J.A.; Kim, D.K.; Lu, D.; Sterin, I.; Park, J.; Park, S. Microvilli control the morphogenesis of the tectorial membrane extracellular matrix. Dev. Cell 2025, 60, 679–695.e8. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, J.A.; Park, J.; Niazi, A.; Almishaal, A.; Park, S. The release of surface-anchored α-tectorin, an apical extracellular matrix protein, mediates tectorial membrane organization. Sci. Adv. 2019, 5, eaay6300. [Google Scholar] [CrossRef]
- Schraders, M.; Ruiz-Palmero, L.; Kalay, E.; Oostrik, J.; del Castillo, F.J.; Sezgin, O.; Beynon, A.J.; Strom, T.M.; Pennings, R.J.E.; Seco, C.Z.; et al. Mutations of the Gene Encoding Otogelin Are a Cause of Autosomal-Recessive Nonsyndromic Moderate Hearing Impairment. Am. J. Hum. Genet. 2012, 91, 883–889. [Google Scholar] [CrossRef]
- Fang, J. A Critical Review of Five Machine Learning-Based Algorithms for Predicting Protein Stability Changes upon Mutation. Brief. Bioinform. 2020, 21, 1285–1292. [Google Scholar] [CrossRef]
- Strimbu, C.E.; Prasad, S.; Hakizimana, P.; Fridberger, A. Control of hearing sensitivity by tectorial membrane calcium. Proc. Natl. Acad. Sci. USA 2019, 116, 5756–5764. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).