Molecular Sexing in Owls (Aves, Strigiformes) and the Unique Genetic Structure of the Chromodomain Helicase DNA-Binding Protein 1 (CHD1) Gene on Chromosome W
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. PCR Amplification of the CHD1 and COI Genes
2.3. DNA Sequencing
2.4. Phylogenetic Analysis
2.5. Ethics Statement
3. Results and Discussion
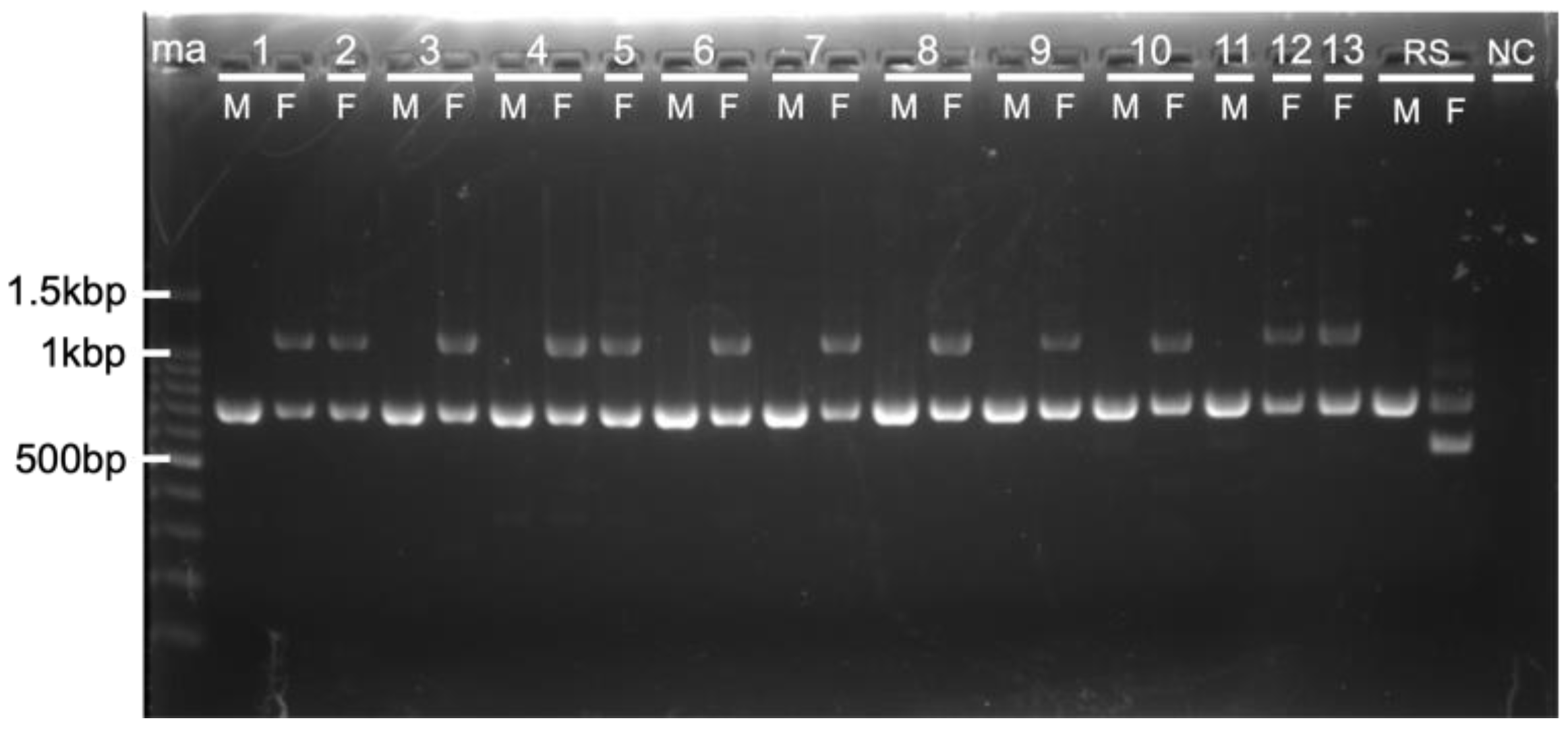
3.1. Molecular Sexing of Four Wild Owls of Unknown Sex
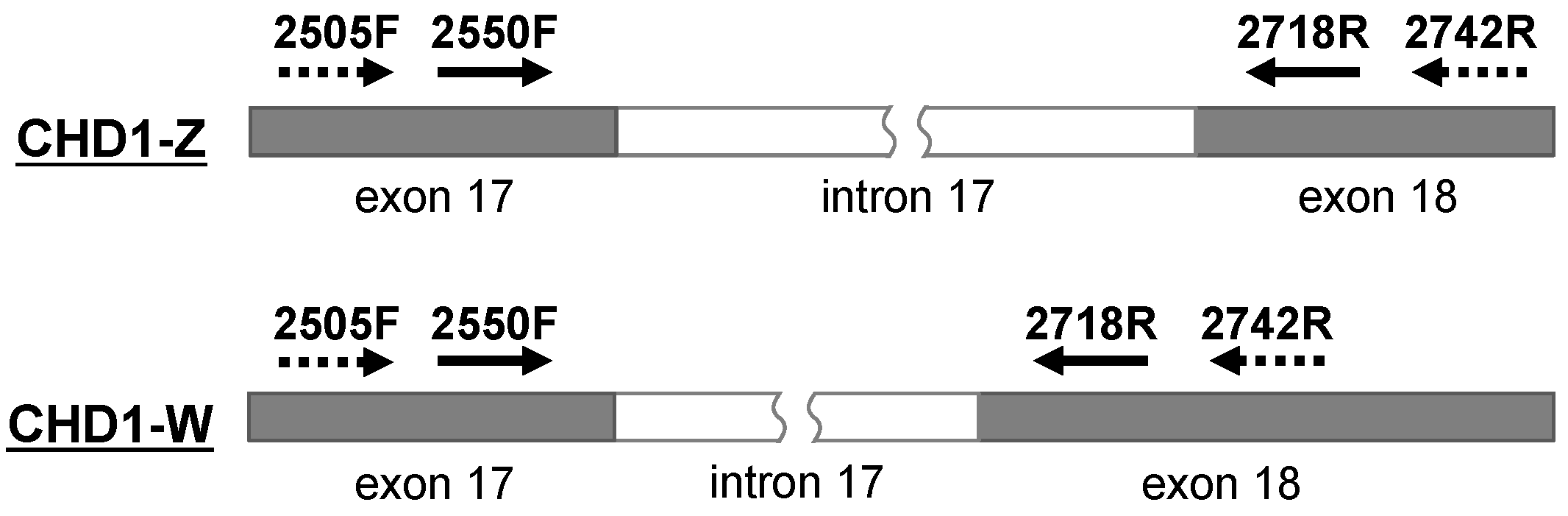
3.2. Application of a New Primer Set 2505F/2742R in Owls
3.3. Confirmation of the Origin of the CHD1 Gene with Phylogenetic Analyses
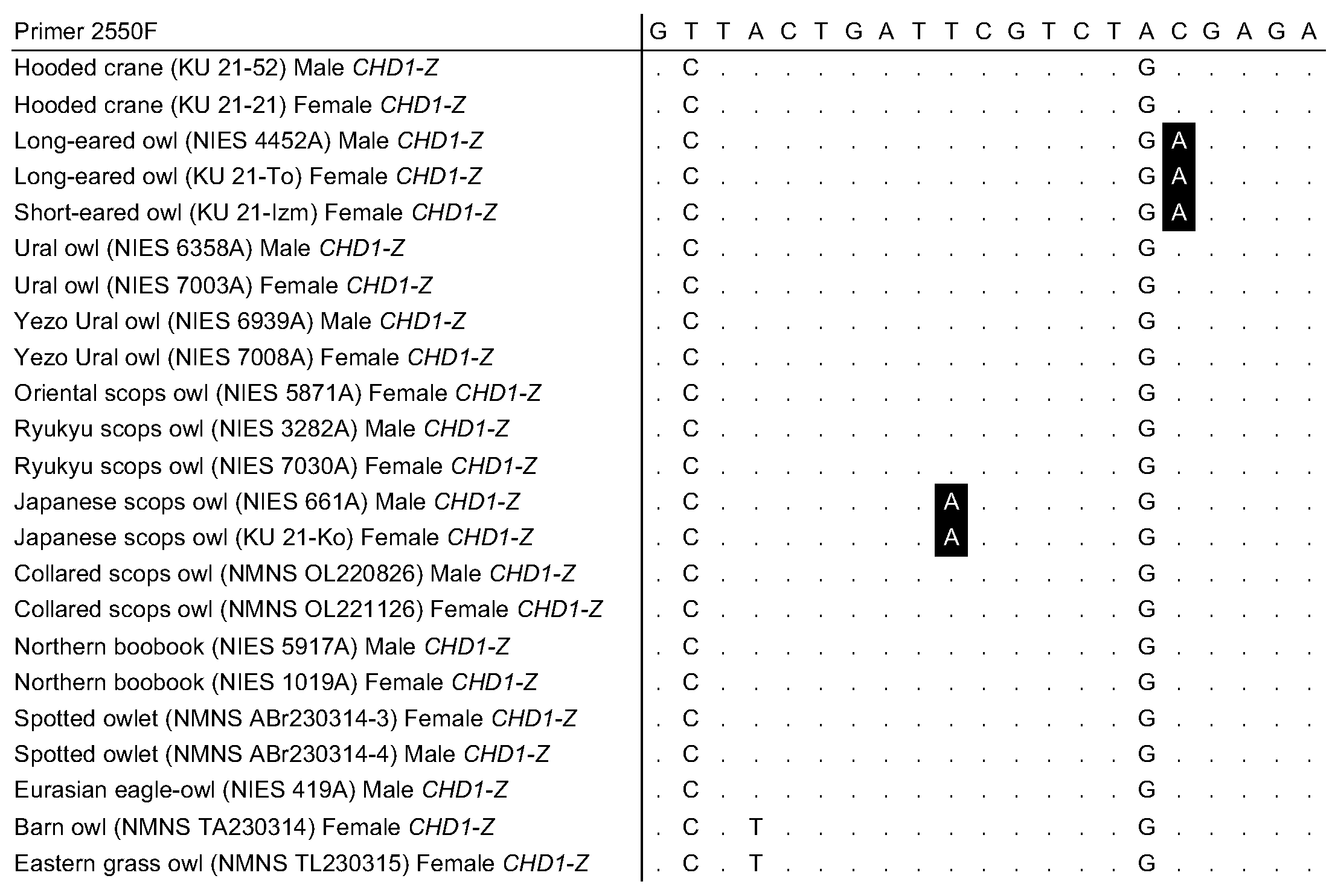
3.4. Alignment of CHD1 Genes in Owls
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, H.; Sudo-Yamaji, A.; Abe, M.; Murase, T.; Tsubota, T. Sex identification by alternative polymerase chain reaction methods in Falconiformes. Zool. Sci. 2003, 20, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.I.; Tsai, L.C.; Hwa, P.Y.; Chan, C.L.; Huang, A.; Chin, S.C.; Wang, L.-C.; Lin, J.-T.; Linacre, A.; Hsieh, H.-M. A novel strategy for avian species and gender identification using the CHD gene. Mol. Cell. Probes 2010, 24, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef]
- Dawson, D.A.; dos Remedios, N.; Horsburgh, G.J. A new marker based on the avian spindlin gene that is able to sex most birds, including species problematic to sex with CHD markers. Zoo Biol. 2016, 35, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R. Sex identification in birds. Semin. Avian Exot. Pet Med. 2000, 9, 14–26. [Google Scholar] [CrossRef]
- Morinha, F.; Cabral, J.A.; Bastos, E. Molecular sexing of birds: A comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 2012, 78, 703–714. [Google Scholar] [CrossRef]
- Wang, L.C.; Chen, C.T.; Lee, H.Y.; Li, S.H.; Lir, J.T.; Chin, S.C.; Pu, C.-E.; Wang, C.-H. Sexing a wider range of avian species based on two CHD1 introns with a unified reaction condition. Zoo Biol. 2007, 26, 425–431. [Google Scholar] [CrossRef]
- Ong, A.H.K.; Vellayan, S. An evaluation of CHD-specific primer sets for sex typing of birds from feathers. Zoo Biol. 2008, 27, 62–69. [Google Scholar] [CrossRef]
- Ellegren, H.; Sheldon, B.C. New tools for sex identification and the study of sex allocation in birds. Trends Ecol. Evol. 1997, 12, 255–259. [Google Scholar] [CrossRef]
- Esaki, M.; Ito, G.; Tokorozaki, K.; Matsui, T.; Masatani, T.; Amano, K.; Ozawa, M. Prevalence and organ tropism of crane-associated adenovirus 1 in cranes overwintering on the Izumi plain, Japan. Transbound. Emerg. Dis. 2022, 69, e2800-7. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Vucicevic, M.; Stevanov-Pavlovic, M.; Stevanovic, J.; Bosnjak, J.; Gajic, B.; Aleksic, N.; Stanimirovic, Z. Sex Determination in 58 Bird Species and Evaluation of CHD Gene as a Universal Molecular Marker in Bird Sexing. Zoo Biol. 2013, 32, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Yuda, P.; Saputra, A.W. Eggshell membrane for DNA sexing of the endangered Maleo (Macrocephalon maleo). F1000Research 2021, 9, 599. [Google Scholar] [CrossRef]
- Çakmak, E.; Akın Pekşen, Ç.; Bilgin, C.C. Comparison of three different primer sets for sexing birds. J. Vet. Diagn. Investig. 2017, 29, 59–63. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Nakamura, Y.; Konaka, T.; Nishida, S.; Kriangwanich, W.; Uematsu, K.; Tsuchida, S. Molecular sexing in Japanese murrelet (Synthliboramphus wumizusume) and a tandem-repeat polymorphism on the W chromosome. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Brommer, J.E.; Karell, P.; Pihlaja, T.; Painter, J.N.; Primmer, C.R.; Pietiäinen, H. Ural owl sex allocation and parental investment under poor food conditions. Oecologia 2003, 137, 140–147. [Google Scholar] [CrossRef]
- Hörnfeldt, B.; Hipkiss, T.; Fridolfsson, A.K.; Eklund, U.; Ellegren, H. Sex ratio and fledging success of supplementary-fed Tengmalm’s owl broods. Mol. Ecol. 2000, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Severinghaus, L.L.; Chen, C.T.; Liu, L.Y.; Pan, C.H.; Huang, D.; Lee, H.Y.; Lir, J.T.; Chin, S.C.; Pu, C.; et al. Sex identification of owls (family Strigidae) using oligonucleotide microarrays. J. Hered. 2008, 99, 187–192. [Google Scholar] [CrossRef][Green Version]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of birds through DNA barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, I.I.; Awumbila, B.; Jeffcoate, I.A.; Robinson, J.E.; Obese, F.Y. Sexing in Guinea fowls (Numida meleagris). Poult. Sci. 2015, 94, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kaewhom, P.; Srikijkasemwat, K. Efficacy of two primer sets used in the sex identification of rufous-winged buzzard (Butastur liventer). Open Vet. J. 2021, 11, 581–586. [Google Scholar] [CrossRef]
- Wink, M.; El-Sayed, A.; Sauer-Gürth, H.; Gonzalez, J. Molecular Phylogeny of Owls (Strigiformes) Inferred from DNA Sequences of the Mitochondrial Cytochrome b and the Nuclear RAG-1 gene. Ardea 2009, 97, 581–591. [Google Scholar] [CrossRef]
- Salter, J.; Oliveros, C.; Hosner, P.; Manthey, J.; Robbins, M.; Moyle, R.; Brumfield, R.; Faircloth, B. Extensive paraphyly in the typical owl family (Strigidae). The Auk 2020, 137, 1–15. [Google Scholar] [CrossRef]
- Stehlíková Sovadinová, S.; Mekadim, C.; Korpimäki, E.; Mrázek, J.; Kouba, M. Comparison three primer pairs for molecular sex determination in Eurasian pygmy owls (Glaucidium passerinum). Sci. Rep. 2024, 14, 16397. [Google Scholar] [CrossRef]
- Liu, C.W.; Hou, H.Y.; Hsieh, H.I.; Jang-Liaw, N.H. Sex identification of birds in Taipei Zoo. Zoo Biol. 2024, 43, 268–275. [Google Scholar] [CrossRef]
- Kulibaba, R.O.; Liashenko, Y.V. Analysis of CHD Gene Polymorphism as a Model Object for Molecular Sexing of Eurasian Eagle-Owl (Bubo bubo). Cytol. Genet. 2021, 55, 324–330. [Google Scholar] [CrossRef]


| Species | Sample No. | Sample ID | Sex | Confirmation of Sex |
|---|---|---|---|---|
| Tyto alba (Barn owl) | 1 | NMNS-TA230314 | Female | -* |
| Tyto longimembris (Eastern grass owl) | 1 | NMNS-TL230315 | Female | - |
| Asio otus (Long-eared owl) | 2 | NIES-4452A | Male | - |
| KU-21-To | Female | - | ||
| Asio flammeus (Short-eared owl) | 1 | KU-21-Izm | Female | Necropsy |
| Strix uralensis hondoensis (Ural owl) | 2 | NIES-6358A | Male | Necropsy |
| NIES-7003A | Female | Necropsy | ||
| Strix uralensis japonica (Yezo Ural owl) | 2 | NIES-6939A | Male | Necropsy |
| NIES-7008A | Female | Necropsy | ||
| Otus sunia (Oriental scops owl) | 1 | NIES-5871A | Female | Necropsy |
| Otus elegans interpositus (Ryukyu scops owl) | 2 | NIES-3282A | Male | Necropsy |
| NIES-7030A | Female | Necropsy | ||
| Otus semitorques (Japanese scops owl) | 2 | NIES-661A | Male | - |
| KU-21-Ko | Female | - | ||
| Otus lettia (Collared scops owl) | 2 | NMNS-OL220826 | Male | - |
| NMNS-OL221126 | Female | - | ||
| Ninox japonica (Northern boobook) | 2 | NIES-5917A | Male | Necropsy |
| NIES-5916A | Female | - | ||
| Athene brama (Spotted owlet) | 2 | NMNS-ABr230314-4 | Male | - |
| NMNS-ABr230314-3 | Female | - | ||
| Bubo bubo (Eurasian eagle-owl) | 1 | NIES-419A | Male | - |
| Grus monacha (Hooded crane) | 2 | KU-21-51 | Male | Necropsy |
| KU-21-21 | Female | Necropsy |
| Primer Name | Nucleotide Sequence (5′ to 3′) | Reference |
|---|---|---|
| 2550F | GTTACTGATTCGTCTACGAGA | Fridolfsson and Ellergen, 1999 [13] |
| 2718R | ATTGAAATGATCCAGTGCTTG | |
| 2505F | GTAGCATTTAATACGTAGCAG | Designed in this study |
| 2742R | ATACCATACCTCTGATCCTTC |
| Species | Gene | Accession No. | Length (bp) |
|---|---|---|---|
| T. alba (Barn owl) | CHD1-Z | LC841896 | 639 |
| CHD1-W | LC841909 | 1073 | |
| T. longimembris (Eastern grass owl) | CHD1-Z | LC841897 | 639 |
| CHD1-W | LC841910 | 1073 | |
| A. otus (Long-eared owl) | CHD1-Z | LC841884 | 646 |
| CHD1-W | LC841898 | 1057 | |
| A. flammeus (Short-eared owl) | CHD1-Z | LC841885 | 646 |
| CHD1-W | LC841899 | 1052 | |
| S. uralensis hondoensis (Ural owl) | CHD1-Z | LC841886 | 641 |
| CHD1-W | LC841900 | 1051 | |
| S. uralensis japonica (Yezo Ural owl) | CHD1-Z | LC841887 | 641 |
| CHD1-W | LC841901 | 1052 | |
| O. sunia (Oriental scops owl) | CHD1-Z | LC841888 | 643 |
| CHD1-W | LC841902 | 1058 | |
| O. elegans interpositus (Ryukyu scops owl) | CHD1-Z | LC841889 | 643 |
| CHD1-W | LC841903 | 1056 | |
| O. semitorques (Japanese scops owl) | CHD1-Z | LC841890 | 646 |
| CHD1-W | LC841904 | 1065 | |
| O. lettia (Collared scops owl) | CHD1-Z | LC841891 | 648 |
| CHD1-W | LC841905 | 1055 | |
| N. japonica (Northern boobook) | CHD1-Z | LC841892 | 649 |
| CHD1-W | LC841906 | 1051 | |
| A. brama (Spotted owlet) | CHD1-Z | LC841893 | 654 |
| CHD1-W | LC841907 | 1033 | |
| B. bubo (Eurasian eagle-owl) | CHD1-Z | LC841895 | 641 |
| G. monacha (Hooded crane) | CHD1-Z | LC841834 | 637 |
| CHD1-W | LC841835 | 462 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esaki, M.; Momohara, K.; Haga, A.; Narahashi, M.; Aung, M.M.; Tokorozaki, K.; Haraguchi, Y.; Okuya, K.; Nishiumi, I.; Onuma, M.; et al. Molecular Sexing in Owls (Aves, Strigiformes) and the Unique Genetic Structure of the Chromodomain Helicase DNA-Binding Protein 1 (CHD1) Gene on Chromosome W. Genes 2025, 16, 653. https://doi.org/10.3390/genes16060653
Esaki M, Momohara K, Haga A, Narahashi M, Aung MM, Tokorozaki K, Haraguchi Y, Okuya K, Nishiumi I, Onuma M, et al. Molecular Sexing in Owls (Aves, Strigiformes) and the Unique Genetic Structure of the Chromodomain Helicase DNA-Binding Protein 1 (CHD1) Gene on Chromosome W. Genes. 2025; 16(6):653. https://doi.org/10.3390/genes16060653
Chicago/Turabian StyleEsaki, Mana, Kenki Momohara, Atsushi Haga, Maria Narahashi, Mu Mu Aung, Kaori Tokorozaki, Yuko Haraguchi, Kosuke Okuya, Isao Nishiumi, Manabu Onuma, and et al. 2025. "Molecular Sexing in Owls (Aves, Strigiformes) and the Unique Genetic Structure of the Chromodomain Helicase DNA-Binding Protein 1 (CHD1) Gene on Chromosome W" Genes 16, no. 6: 653. https://doi.org/10.3390/genes16060653
APA StyleEsaki, M., Momohara, K., Haga, A., Narahashi, M., Aung, M. M., Tokorozaki, K., Haraguchi, Y., Okuya, K., Nishiumi, I., Onuma, M., & Ozawa, M. (2025). Molecular Sexing in Owls (Aves, Strigiformes) and the Unique Genetic Structure of the Chromodomain Helicase DNA-Binding Protein 1 (CHD1) Gene on Chromosome W. Genes, 16(6), 653. https://doi.org/10.3390/genes16060653







