Abstract
Blumea balsamifera (L.) DC., an important economic and medicinal herb, has a long history of being used as a traditional Chinese medicine. Its leaves have always been used as a raw material for the extraction of essential oils, comprising large amounts of terpenoids, which have good therapeutic effects on many diseases, such as eczema, bacterial infection, and hypertension. However, the genetic basis of terpenoid biosynthesis in this plant is virtually unknown on account of the lack of genomic data. Here, a combination of next-generation sequencing (NGS) and full-length transcriptome sequencing was applied to identify genes involved in terpenoid biosynthesis at five developmental stages. Then, the main components of essential oils in B. balsamifera were identified using GC–MS. Overall, 16 monoterpenoids and 20 sesquiterpenoids were identified and 333,860 CCS reads were generated, yielding 65,045 non-redundant transcripts. Among these highly accurate transcripts, 59,958 (92.18%) transcripts were successfully annotated using NR, eggNOG, Swissprot, KEGG, KOG, COG, Pfam, and GO databases. Finally, a total of 56 differently expressed genes (DEGs) involved in terpenoid biosynthesis were identified, including 38 terpenoid backbone genes and 18 TPSs, which provide a significant amount of genetic information for B. balsamifera. These results build a basis for resource protection, molecular breeding, and the metabolic engineering of this plant.
1. Introduction
B. balsamifera (L.) DC., a perennial Compositae plant, has long been used as an ethnic medicine in southwest China, including in Guizhou, Guangxi, Yunan, and Hainan Provinces [1,2]. B. balsamifera located in Luodian County of Guizhou Province has become a geographical indication protection product, and its planting area has reached as much as 30,000 hectares. Since 2010, the Pharmacopoeia of the People’s Republic of China recorded B. balsamifera as the only plant source for “Ai Pian”, which can induce resuscitation, reduce a fever, and relive pain. Due to its high quantity of essential oils, B. Balsamifera is also named “Ai Na Xiang” and “Da Feng Ai” in Chinese and has become an important economic crop in the local area [3]. Moreover, the essential oils of B. balsamifera display new physiological activities, such as anti-tumor [4,5,6], anti-fungal [7,8], radical-scavenging [9], and anti-influenza virus properties [10].
The major active compounds of essential oils in B. balsamifera are monoterpenoids and sesquiterpenoids, such as L-borneol, linalool, camphor, and pinene. The biosynthesis pathways of terpenoids are well understood in many plants [11,12]. Generally, the mevalonate acid pathway (MVA) and the methylerythritol pathway (MEP) are responsible for the biosynthesis of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), respectively. IPP and DMAPP can be condensed into geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), geranyl geranyl pyrophosphate (GGPP), and geranyl farnesyl pyrophosphate (GFPP) [13,14,15]. With the catalysis of terpenoid synthases (TPSs), GPP is further transformed into mono-, di-, and tetra-terpenoids, while FPP is transferred into sesquiterpenoids and polyterpenoids [16,17].
However, the biosynthesis pathways of the terpenoids in B. balsamifera are rarely reported. Importantly, no genomic information for B. balsamifera is available. This is unfavorable for the elucidation of the regulatory mechanisms of the growth and development of B. balsamifera, especially hub genes regulating the biosynthesis of terpenoids in essential oils, which restricts the development of molecular breeding and the precision cultivation of B. balsamifera. The advent of full-length transcriptome sequencing opens a path for the mining of genetic information of many medicinal plants [18,19,20,21,22,23]. In this study, a combination of NGS and full-length transcriptome sequencing was used to obtain a more comprehensive transcriptome. Meanwhile, DETs involved in the biosynthesis of monoterpenoids and sesquiterpenoids were also identified. Moreover, the metabolomics of the leaves of B. balsamifera were also detected. This study provides novel insights into terpenoid biosynthesis in B. balsamifera.
2. Results
2.1. Terpenoids of the Essential Oils in B. balsamifera
The essential oils extracted from the leaves of B. balsamifera were identified using GC–MS/MS based on the MWGC database. As shown in Table 1, two major types of terpenoids were detected, including 16 monoterpenoids and 20 sesquiterpenoids (Table 1). For monoterpenoids, L(-)-borneol showed the highest content followed by (-)-terpinen-4-ol, linalool, (-)-orthodene, and comphene. For sesquiterpenoids, 2-methylene-4,8,8-trimethyl-4-vinylbicyclo [5.2.0] nonane showed the highest content followed by 1R,4R,7R,11R-1,3,4,7-Tetramethyltricyclo[5.3.1.0(4,11)]undec-2-ene, β-copaene, (-)-α- gurjunene, and (-)-epi-bicyclosesquiphellandrene. These results showed that monoterpenoids and sesquiterpenoids are the main components of these essential oils.

Table 1.
Summary of metabolomics analysis using GC–MS.
2.2. Transcriptome Assembly from NGS and SMRT Sequencing of B. balsamifera
To deeply mine the genetic information of B. balsamifera, transcriptome sequencing was performed using the PacBio and Illumina platforms simultaneously. For RNA-Seq, 15 mRNA samples from the leaves of B. balsamifera at five different developmental stages (each in triplicate) were used to construct libraries for NGS using Illumina HiSeq. After trimming and filtering, 26,306,866 (BBLI), 27,130,069 (BBLII), 22,917,945 (BBLIII), 27,682,295 (BBLIV), and 23,295,190 (BBLV) clean reads were obtained. The average GC percentage and Q30 of all libraries were 44.97% and 95.48%, respectively (Table S1). The integrity of the transcriptome was also assessed and illustrated in Figure S1.
For full-length transcriptome sequencing, 1–6 kb full-length cDNA libraries containing BBLI, BBLII, BBLIII, BBLIV, BBLV, BBR, and BBS samples were constructed and applied for SMRT sequencing using the PacBio platform. A total of 333,860 reads of inserts (ROIs) were generated with an average length of 2244 bp. After circular consensus sequence (CCS) generation and filtering for full-length read classification, 298,671 full-length non-chimeric (FLNC) reads were retained. The FLNC reads were clustered into consensus clusters based on RS IsoSeq. Then, 116,656 high-quality (HQ) and consensus isoforms were merged into a total of 116,639 final consensus sequences (Table 2). Finally, a total of 65,045 non-redundant full-length transcripts from B. balsamifera were generated using CD-HIT.

Table 2.
Summary of PacBio single-molecule real-time (SMRT) sequencing.
2.3. Functional Annotation of Full-Length Transcriptome
A total of 65,045 complete ORFs were obtained using TransDecoder. As illustrated in Figure 1, only a minority (29 CDSs) had more than 2100 base pairs (bp), and 86.68% of the CDSs appeared with a length ranging from 100 to 2100 bp. Then, all non-redundant unigenes were blasted against eight biological databases. The results showed that 59,958 unigenes were functionally annotated (Figure 2). Among them, 25,569 (39.41%), 27,306 (41.98%), 39,064 (60.06%), 42,504 (65.35%), 43,396 (66.72%), 49,022 (75.37%), 57,793 (88.85%), and 59,703 (91.79%) unigenes were annotated in COG, KEGG, KOG, GO, Swissprot, Pfam, eggNOG, and Nr, respectively (Table S2).
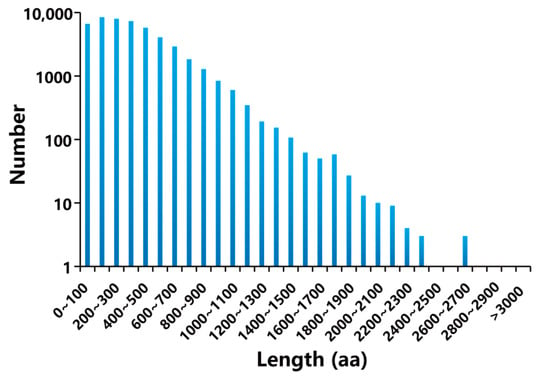
Figure 1.
The length distribution of CDSs.
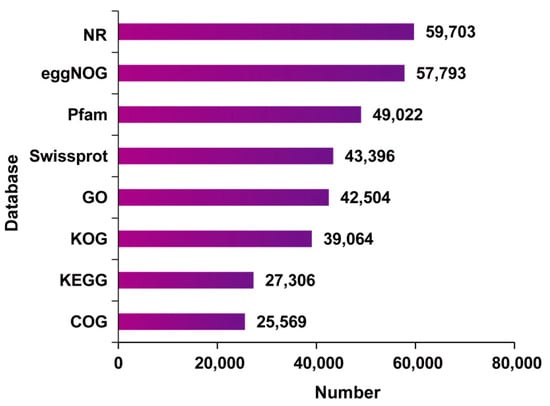
Figure 2.
The number of annotated unigenes with various databases.
By aligning the obtained sequences with the NR database, species homologous to B. balsamifera were analyzed (Figure 3). As a result, 47.61%, 24.94%, and 19.48% of the sequences were mapped to the genes of Helianthus annuus (Arsteraceae), Lactuca sativa (Arsteraceae), and Cynara cardunculus (Arsteraceae), respectively.
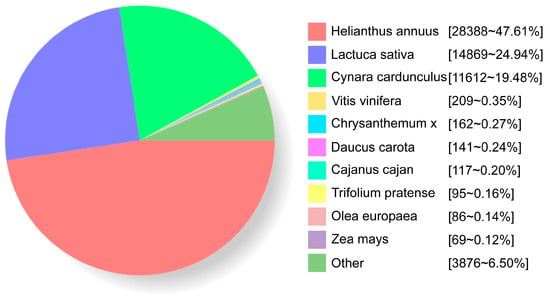
Figure 3.
Species homologous to B. balsamifera.
Furthermore, 25,569, 39,064, and 57,793 unigenes were assigned using the COG, KOG, and eggNOG databases, respectively, and classified into 25 functional clusters (Figure 4; Table S3). In the COG functional annotation, “signal transduction mechanisms” (3132 unigenes) was the most common annotation followed by “general function prediction” (3032 unigenes) and “translation, ribosomal structure and biogenesis” (2620 unigenes). In the KOG functional annotation, the most common annotation was “general function prediction” (8455 unigenes) followed by “signal transduction mechanisms” (4268 unigenes) and “posttranslational modification, protein turnover, chaperones” (4235 unigenes). Although the eggNOG annotation had the most annotation numbers, the most common annotation was “function unknown” (27,338 unigenes).
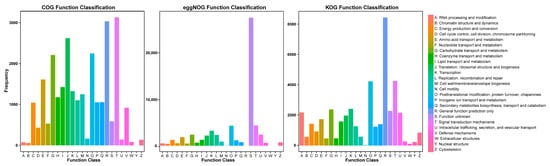
Figure 4.
COG, eggNOG, and KOG functional annotation.
For the GO analysis, 42,504 unigenes were annotated into three categories—molecular function, cellular component, and biological process (Figure 5; Table S5). Most transcripts clustered into the “catalytic activity” (22,152 unigenes), “binding” (21,725 unigenes), “metabolic process” (21,522 unigenes), and “cellular process” (20,014 unigenes) categories. In addition, the KEGG annotation is also illustrated in Table S4.
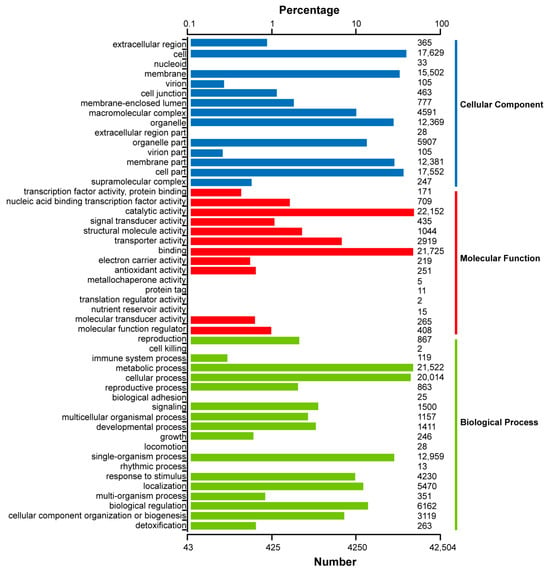
Figure 5.
GO functional classification of the consensus sequence.
2.4. Identification of Differentially Expressed Transcripts (DETs)
Overall, 30,556 transcripts were simultaneously expressed in the leaves of five different stages (Figure 6). Meanwhile, 4529 DETs exhibited stage-specific expression, with 1230, 215, 681, 980, and 1423 transcripts specifically expressed in BBLI, BBLII, BBLIII, BBLIV, and BBLV, respectively. To identify the key genes involved in terpenoid biosynthesis in B. balsamifera, we identified 25,200 DETs in 10 combinations (BBLI vs. BBLII, BBLI vs. BBLIII, BBLI vs. BBLIV, BBLI vs. BBLV, BBLII vs. BBLIII, BBLII vs. BBLIV, BBLII vs. BBLV, BBLIII vs. BBLIV, BBLIII vs. BBLV, and BBLIV vs. BBLV) based on NGS sequencing data. Among all of the DETs, the highest number of transcripts (12,828)—comprising 5884 up-regulated genes and 6944 down-regulated genes—were differentially expressed between BBLI and BBLV. This suggested a large biological difference between BBLI and BBLV. However, only a few of the transcripts (470) were differentially expressed between BBLII and BBLIII (Figure 7).
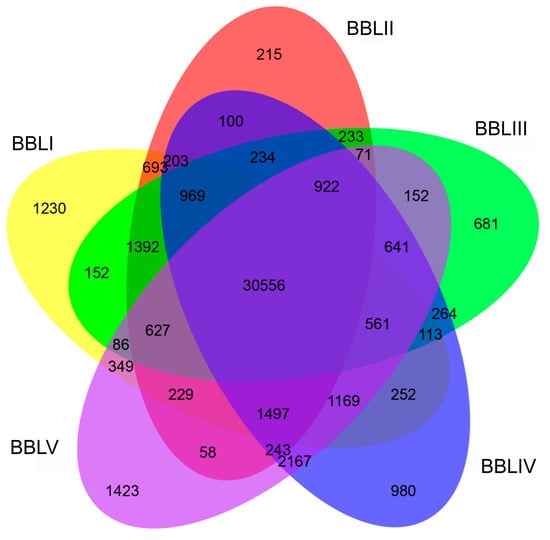
Figure 6.
Venn diagram of DETs for five developmental stages.
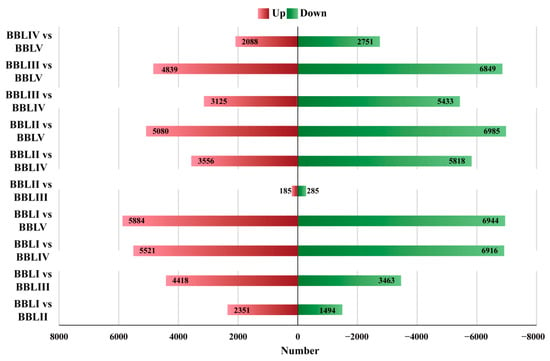
Figure 7.
Up-regulated and down-regulated DETs in different comparisons.
2.5. Identification of TFs—1–2000
TFs are a kind of protein that can bind with specific motifs of DNA regulating transcription efficiency, which can affect cell growth, differentiation, and function. In the full-length transcriptome of B. balsamifera, 2423 TFs belonging to 67 different TF families were annotated. After removing the TFs with extremely low expression (FPKM < 1), 119 C3Hs, 109 bHLHs, 110 bZIPs, 102 MYB-related TFs, 105 AP2s, and 100 C2H2s were the top six TF families (Table 3). MYB, bHLH, WRKY, and bZIP TFs play a vital role in the regulation of terpenoid biosynthesis in many plant species, such as Artemisia annua, Arabidopsis thaliana, and Catharanthus roseus [24,25,26,27,28,29]. In B. balsamifera, most of the bZIPs, bHLHs, and WRKYs had the highest expression level in BBLV. However, nearly half of 88 MYBs showed the highest expression in BBLI. These results reveal that the MYBs play an important role in terpenoid biosynthesis.

Table 3.
Top 21 TFs in B. balsamifera.
2.6. Identification of Hub Genes Involved in Terpenoid Biosynthesis
After removing genes with extremely low expression (FPKM < 1), a total of 116 genes were considered as DETs involved in terpenoid biosynthesis and require extensive investigation in the future. In the MVA pathway, there were 14 genes were identified as DETs related to IPP biosynthesis, including one BbAACT, 1 BbHMGCS, 8 BbHMGCRs, 1 BbMVK, 2 BbPMKs, and 1 BbMVD. As the first key gene involved in the MVA pathway, BbAACT showed a higher expression level at four developmental stages except for BBLIII, and BbHMGCS showed the highest expression in BBLI and the lowest expression in BBLIII. Almost all BbHMGCRs and one BbMVK had similar expression trends, with the highest expression in BBLI and the lowest expression in BBLIV. However, BbHMGCR3 had a different expression trend, with the highest expression in BBLV, which was similar to two BbPMKs and one BbMVK (Figure 8, Table S6). In the MEP pathway, a total of 24 DETs related to DMAPP biosynthesis were identified, comprising 7 BbDXSs, 3 BbDXRs, 1 BbMCT, 2 BbCMKs, 2 BbICSs, 2 BbHDSs, and 7 BbIDSs. There was no significant expression trend in the seven BbDXSs, but most showed the lowest expression level in BBLIV or BBLV. Three BbDXRs had relatively higher expression levels at four developmental stages except for BBLV. BbMCT showed a significant downward trend from BBLI to BBLV; however, two BbCMKs had an increasing trend from BBLI to BBLIV, with the lowest expression in BBLV. In contrast, two BbICSs showed the lowest expression in BBLI and the highest expression in BBLIII or BBLIV. The seven BbIDSs, BbIDS3, BbIDS5, BbIDS6, and BbIDS7 displayed the highest expression in BBLIV and BBLV, whereas BbIDS1, BbIDS2 and BbIDS4 displayed the lowest expression in BBLIV or BBLV. Meanwhile, four BbIDIs, four BbFPPSs, and two GPPSs were also identified and their expression is illustrated in Figure 8 and Table S6.
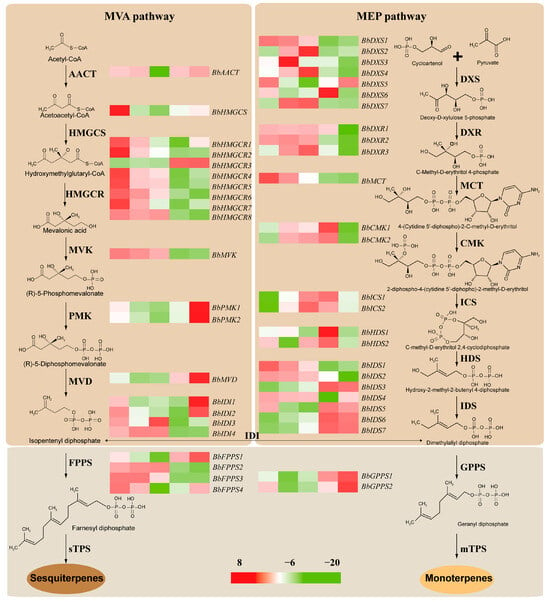
Figure 8.
Heatmap of DETs involved in terpenoid backbone biosynthesis.
Monoterpenoids and sesquiterpenoids are the main active compounds of the essential oils in B. balsamifera. Until now, monoterpenoid synthases (mTPSs) and sesquiterpenoid synthases (sTPSs) in B. balsamifera have rarely been reported. In this study, 7 mTPSs and 11 sTPSs were identified, respectively. Most mTPSs and sTPSs showed a gradually decreasing trend from BBLI to BBLV and the highest expression in BBLI or BBLII. Only one mTPS displayed the lowest expression in BBLI, which was different from the other mTPSs (Figure 9, Table S6). However, three sTPSs showed completely different expression trends, with the highest expression in BBLIV or BBLV and the lowest expression in BBLII. These genes may play special roles in the biosynthesis of terpenoids in B. balsamifera.
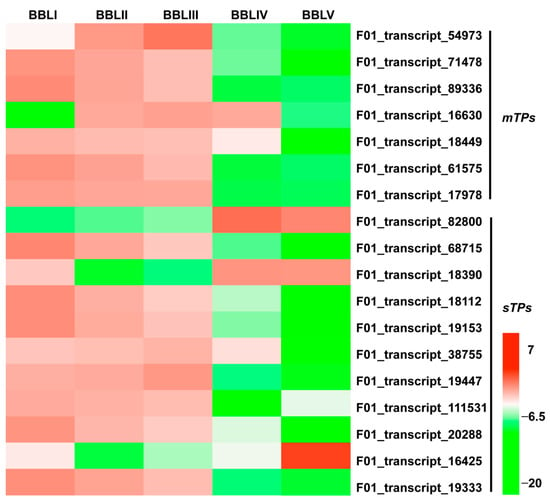
Figure 9.
Heatmap of mTPSs and sTPSs in five developmental stages.
2.7. RT-qPCR Validation of DETs
Eight DETs were randomly selected to verify the gene expression levels using a real-time qPCR (RT-qPCR) test. The results showed that the relative expression levels of BbPMK2, BbFPPS1, and BbsTPS2 were higher in BBLI, and the relative expression levels of BbDXR3, BbCMK2, BbIDS1, and BbsTPS1 were higher in BBLII. Meanwhile, BbACCT showed greater expression in BBLV than in other stages (Figure 10). The RT-qPCR data were in good agreement with the FPKM value in the transcriptome, which proves the accuracy of the transcriptome data.
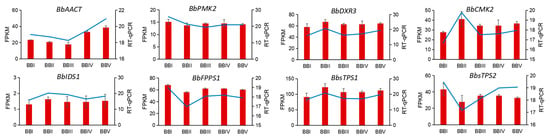
Figure 10.
RT-qPCR validation of eight randomly selected genes in B. balsamifera. Red bar represents FPKM value, blue line represents RT-PCR results.
3. Discussion
According to previous reports, the essential oils in B. balsamifera leaves are mainly composed of monoterpenoids and sesquiterpenoids, such as (E)-caryophyllene, L-borneol, longifolene, camphor, γ-gurjunene [30,31,32,33]. In this study, the analysis revealed that monoterpenoids and sesquiterpenoids are the main compounds of the essential oils. Among these, the highest levels of monoterpenoids and sesquiterpenoids are L-borneol and 2-methylene-4,8,8-trimethyl-4-vinylbicyclo[5.2.0]nonane, respectively. However, the molecular mechanism of terpenoid biosynthesis in B. balsamifera is still not clear. To our knowledge, the reference genome of B. balsamifera has not yet been published, and transcriptome information for B. balsamifera has been rarely reported, which has restricted the development of the molecular biology of B. balsamifera. Due to its fast speed, high precision, and low cost, RNA-Seq has become a popular sequencing technology; therefore, RNA-Seq has been used for the transcriptome sequencing of B. balsamifera based on the HiSeq 2000 platform, which yielded 100,341 unigene fragments [34]. However, RNA-Seq technology often fails to obtain or assemble complete transcripts. In addition, SMRT technology could provide longer reads of transcripts; however, the accuracy of transcripts generated using SMRT is lower than that using RNA-Seq. In this study, a combination of NGS and full-length transcriptome sequencing was first used to generate high-quality transcriptome data for B. balsamifera. This yielded 333,860 CCS reads with a mean read length of 2514 bp, and 65,045 non-redundant transcript isoforms were also obtained. After blasting with eight public databases, 59,958 (92.18%) transcripts were successfully functionally annotated.
TFs play an important role in the transcriptional regulation of monoterpenoids and sesquiterpenoids. For example, bHLH4 and bHLH6 can improve the production of monoterpenoids in butterfly orchids [35], WRKY 1 can promote the biosynthesis of sesquiterpenoids artemisinin in Artemisia annua [36], and MsMYB can suppress the expression of geranyl diphosphate synthase [37]. In this study, 2423 TFs distributed in different TF families were identified, including 88 MYBs, 109 bHLHs, 110 bZIPs, and 81 WRKYs. Interestingly, half of the MYBs were mainly expressed in BBLI, which was different from bHLH, bZIP, and WRKY. Therefore, future research should focus on the role of MYBs in the regulation of terpenoid biosynthesis in B. balsamifera.
In comparison with the gene expression levels of leaves at different developmental stages, 4529 shared DETs were identified, and further exploration was required to discover their functions. To reveal the structural genes responsible for terpenoid biosynthesis, some candidate transcripts were identified using KEGG pathway annotation. The results revealed that 38 transcripts were assigned to “terpenoid backbone biosynthesis”, among which 14 and 24 transcripts are involved in the MVA and MEP pathways, respectively. AACT is the first enzyme of the MVA pathway and is essential for terpenoid backbone biosynthesis [38]. Only one BbAACT was identified, which showed higher expression levels at four developmental stages except for BBLIII in B. balsamifera. For the other genes involved in the MVA pathway, almost all decreased gradually during the development of leaves in B. balsamifera, including one BbHMGCs, seven BbHMGCRs, and one BbMVK. However, the expression levels of two BbPMKs increased gradually in the late development stages, consistent with MiPMK in mango during ripening [39]. One BbMVD also displayed a similar expression trend from BBLII to BBLV. In the MEP pathway, all related genes showed no obvious expression trend. It is worth noting that four BbIDSs showed higher expression levels in BBLIV or BBLV. Based on previous studies, IDS enzymes function at an important branch point in the biosynthesis of different terpenoids, such as monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, and tetraterpenoids [40]. Thus, BbIDSs may play an important role in determining the type of terpenoids.
In addition, as the key enzymes for terpenoid biosynthesis, TPSs can catalyze DMAPP and IPP to form various terpenoids. Recently, more and more TPSs have been identified in medicinal plants. For example, 11 AaTPSs were identified in Angelica archangelica using RNA-Seq by Suenaga-Hiromori M. et al. [41]; AaTPSs1-AaTPSs5, AaTPSs6-AaTPSs10, and AaTPSs11 were responsible for monoterpenoid, sesquiterpenoids, and diterpenoids, respectively. In this study, a total of 18 transcripts were identified as TPSs, wherein 11 transcripts were involved in “sesquiterpenoid and triterpenoid biosynthesis” and seven transcripts related to “monoterpenoid biosynthesis”. All these transcripts play important roles in terpenoid biosynthesis in B. balsamifera and can potentially be used as target markers for breeding programs aimed at increasing terpenoid production in B. balsamifera.
4. Materials and Methods
4.1. Plant Materials
The fresh leaves from five different developmental stages (named BBLI, BBLII, BBLIII, BBLIV, and BBLV) and the root (BBR) and stem (BBS) of B. balsamifera were collected from Red River (Luodian County, Guizhou Province, China). BBLI, BBLII, BBLIII, BBLIV, and BBLV represent leaf buds, small leaves, young leaves, mature leaves, and old leaves, respectively. Each sample was washed with flowing water, surface-dried with sterile filter paper, quick-frozen in liquid nitrogen, and then stored at −80 °C until RNA isolation.
4.2. GC–MS Analysis of B. balsamifera Essential Oils
The essential oils from the B. balsamifera leaves were extracted using headspace solid-phase microextraction (HS-SPME) after grinding with liquid nitrogen in a mortar, equipped with a 100 μm polydimethylosiloxane (PDMS) fiber (Supelco, Bellefonte, PA, USA) and analyzed using a 7890B-7000D gas chromatograph (Agilent Technologies, Santa Clara, CA, USA). The instrument was equipped with an Agilent DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm film) and helium was used as the carrier gas at a flow rate of 1.0 mL/min. The GC oven was maintained at 40 °C for 5 min, gradually increased to 280 °C at a rate of 6 °C/min, and then held for 5 min. The original data obtained were first extracted using Mass Hunter software (Agilent) to obtain the mass-to-charge ratio, retention time, peak area, and other information for the characteristic peak. Then, the quality and quantity of the metabolites were analyzed based on the MWGC database.
4.3. RNA Extraction and Sequencing
A total of 21 RNA samples (each in triplicate) were extracted using Trizol (Sangon Biotech, Shanghai, China). The quality and quantity of the extracted RNA were determined using agarose gel electrophoresis and the Nanodrop 2000 system. For NGS sequencing, mRNA was enriched with magnetic beads from the total RNA of BBLI, BBLII, BBLIII, BBLIV, and BBLV, then the cDNAs were synthesized using random hexamers and purified using AMPure XP beads. Fifteen cDNA libraries were constructed using PCR enrichment. The concentration and quality of these cDNA fragments were measured using an Agilent 2100 bioanalyzer. After passing the quality inspection, 15 libraries were sequenced using the Illumina platform (Biomarker Technologies, Beijing, China). In addition, the library used for the full-length transcriptome sequencing was composed using equal quantities of total RNA from all 21 individual samples. The full-length transcriptome sequencing was completed using the PacBio platform (Biomarker Technologies, Beijing, China) based on SMRT technology.
4.4. Full-Length Transcriptome Analysis
For full-length transcriptome analysis, raw reads were processed into circular consensus (CCS) reads according to having full passes ≥ 3 and an accuracy of sequence > 90%. The full-length non-chimeric reads in the CCS were screened and clustered into consensus isoforms. Then, non-redundant isoforms were generated using CD-HIT software and the integrity was assessed using BUSCO software (version 5.4.7, https://busco.ezlab.org/, accessed on 2 February 2020) [42]. Subsequently, the coding sequence (CDS), SSR types, and transcription factors (TFs) were also analyzed using TransDecoder (version 5.5.0, https://github.com/TransDecoder/, accessed on 3 February 2020), MISA (version 2.1, https://webblast.ipk-gatersleben.de/misa/index.php?action=1, accessed on 3 February 2020), and iTAK (version 1.6, http://itak.feilab.net/cgi-bin/itak/index.cgi, accessed on 3 February 2020) [43], respectively. Meanwhile, the lncRNAs were also predicted using the Coding Potential Calculator (CPC2, version 2.0 β, http://cpc2.gao-lab.org/, accessed on 3 February 2020) [44], the Coding-Non-Coding Index (CNCI, https://github.com/www-bioinfo-org/CNCI#install-cnci, accessed on 5 February 2020) [45], Protein family (Pfam, http://pfam.xfam.org/, accessed on 5 February 2020) [46], and the Coding Potential Assessment Tool (CPAT, http://lilab.research.bcm.edu/cpat/, accessed on 5 February 2020) [47].
4.5. Functional Annotation of Non-Redundant Isoforms
The functional annotation of non-redundant isoforms was performed by blasting with various databases, such as the Non-Redundant (NR) Protein Sequence Database [48], Swissprot, Gene Ontology (GO) [49], Clusters of Orthologous Groups (COG) [50], euKaryotic Ortholog Groups (KOG) [51], Pfam, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) [52].
4.6. Identification of Differentially Expressed Transcripts (DETs)
For the NGS analysis, clean data were obtained from raw data after a series of quality-control procedures, such as primer deletion and lowest-quality-read filtering. Gene expression levels were identified by calculating the total numbers of the fragments mapping to each transcript and normalization using fragments per kilobase of transcript per million mapped reads (FPKM). Differential expression analysis of two groups was performed using DESeq2. The resulting p-values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate; genes with an FDR < 0.01 and a fold change ≥ 2 were assigned as DETs.
4.7. Real Time-Quantitative PCR (RT-qPCR) Validation
Eight random genes were selected for RT-qPCR analysis to confirm the RNA-Seq results. The total RNA of the leaves at five different developmental stages was isolated with Trizol, and from each sample, ~0.5 µg of RNA was used to synthesize the first-strand cDNAs. The specific primers used are listed in Table S7. Each PCR reaction comprised of template cDNA (1 µL), forward primer and reverse primer (0.8 µL), 2× TransStart® Green qPCR SuperMix (10 µL), and nuclease-free water (9.2 µL). The PCR amplification was carried out as follows: 60 s at 95 °C followed by 40 cycles of 5 s at 95 °C and 60 s at 60 °C. After normalization with the actin gene as an internal reference, gene expression levels were calculated using the 2−∆∆Ct method. Three replicates were conducted for each sample.
5. Conclusions
In this study, transcriptomic and metabolomic data for B. balsamifera were first obtained using a combination of NGS and full-length transcriptome sequencing and GC–MS. Through structural analysis and functional annotation, a large amount of accurate and complete transcripts was identified, including DETs and TFs at five different developmental stages in B. balsamifera. These results reveal that the regulatory mechanism of terpenoid biosynthesis is complicated and needs further research. These data provide a valuable basis for gene discovery, molecular breeding, and the metabolic engineering of B. balsamifera.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15030285/s1. Figure S1: The integrity of transcriptome. Table S1: Overview of assembly and quality evaluation of the B. balsamifera RNA-Seq clean data. Table S2: Annotation of transcripts against eight different public databases. Table S3: KOG pathway annotation for B. balsamifera. Table S4: GO annotation of the B. balsamifera transcripts. Table S5: KEGG pathway annotation for B. balsamifera. Table S6: Differentially expressed genes involved in mono- and sesquiterpenoid biosynthesis. Table S7: Primers used in the validation experiment of gene expression using RT-qPCR.
Author Contributions
Conceptualization, Z.J. and Y.Z.; formal analysis, H.S. and W.Z.; data curation and writing—original draft preparation, Z.J. and L.L.; writing—review and editing, W.S.; visualization, L.L. and H.S.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Guizhou Science and Technology Department (2018[1011] and [2019]1019), the Research and Demonstration on Key Technologies for Conservation and Innovative Utilization of Germplasm Resources of Important Southern Medicine in Guangdong Province (Grant No. [2021]163), and the ‘Thousand’ level Innovative Talents Project in Guizhou Province [Grant No. ZQ2018004].
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pang, Y.; Wang, D.; Fan, Z.; Chen, X.; Yu, F.; Hu, X. Blumea balsamifera—A phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef]
- Yuan, Y.; Pang, Y.X.; Wang, W.Q.; Zhang, Y.B.; Yu, J.B. Investigation on the plant resources of Blumea balsamifera (L.) DC. in China. J. Trop. Biol. 2011, 2, 78–82. [Google Scholar]
- Guan, L.L.; Pang, Y.X.; Wang, D.; Zhang, Y.B.; Wu, K.Y. Research progress on Chinese Minority Medicine of Blumea balsamifera L. DC. J. Plant Genet. Res. 2012, 13, 695–698. [Google Scholar]
- Hasegawa, H.; Yamada, Y.; Komiyama, K.; Hayashi, M.; Ishibashi, M.; Yoshida, T.; Sakai, T.; Koyano, T.; Kam, T.S.; Murata, K.; et al. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood 2006, 107, 679–688. [Google Scholar] [CrossRef]
- Saewan, N.; Koysomboon, S.; Chantrapromma, K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J. Med. Plants Res. 2011, 5, 1018–1025. [Google Scholar]
- Chen, X.; Su, H.Z.Z. Detailed studies on the anticancer action of rosmarinic acid in human Hep-G2 liver carcinoma cells: Evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. Off. J. Balk. Union Oncol. 2020, 25, 2011–2016. [Google Scholar]
- Li, J.; Zhao, G.Z.; Chen, H.H.; Wang, H.B.; Qin, S.; Zhu, W.Y.; Xu, L.H.; Jiang, C.L.; Li, W.J. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett. Appl. Microbiol. 2008, 47, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Co, A.L.K.C.; Rideout, J.A. Antifungal metabolites from Blumea balsamifera. Nat. Prod. Res. 2005, 19, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Nessa, F.; Ismail, Z.; Mohamed, N.; Haris, M.R.H.M. Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem. 2004, 88, 243–252. [Google Scholar] [CrossRef]
- Xiong, Y.; Yi, P.; Li, Y.; Gao, R.; Chen, J.; Hu, Z.; Lou, H.; Du, C.; Zhang, J.; Zhang, Y.; et al. New sesquiterpeniod esters form Blumea balsamifera (L.) DC. and their anti-influenza virus activity. Nat. Prod. Res. 2022, 36, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Chappll, J. Isoprenoid biosynthesis in plant: Carbon partitioning within the cytoplasmic pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Ward, B.L.; Bostock, R.M. Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell 1992, 4, 1333–1344. [Google Scholar] [PubMed]
- Zhao, S.; Wang, L.; Liu, L.; Liang, Y.; Sun, Y.; Wu, J. Both the mevalonate and the non-mevalonate pathways are involved in ginsenoside biosynthesis. Plant Cell Rep. 2014, 33, 393–400. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Chen, M.X.; Ye, N.H.; Qiao, W.M.; Gao, B.; Law, W.K.; Tian, Y.; Zhang, D.; Zhang, D.; Liu, T.Y.; et al. Comparative performance of the BGISEQ-500 and Illumina HiSeq4000 sequencing platforms for transcriptome analysis in plants. Plant Methods 2018, 14, 69. [Google Scholar] [CrossRef]
- Xu, Z.; Peters, R.J.; Weirather, J.; Luo, H.; Liao, B.; Zhang, X.; Zhu, Y.; Ji, A.; Zhang, B.; Hu, S.; et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 2015, 82, 951–961. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, X.; Wang, J.; Shang, Z.; Zhang, Z.; Zhou, Z.; Zhang, K. Full-Length Transcriptome Analysis Reveals Candidate Genes Involved in terpenoids Biosynthesis in Artemisia argyi. Front. Genet. 2021, 12, 659962. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Yang, Y.; Liu, X.; Lv, H.; Song, B.H. Transcriptome profiling reveals the spatial-temporal dynamics of gene expression essential for soybean seed development. BMC Genom. 2021, 22, 453. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, C.; Zhao, S.; Zhang, S.; Wang, W.; Fu, H. De novo transcriptome and phytochemical analyses reveal differentially expressed genes and characteristic secondary metabolites in the original oolong tea (Camellia sinensis) cultivar ‘Tieguanyin’ compared with cultivar ‘Benshan’. BMC Genom. 2019, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Kohnen, M.V.; Prasad, K.V.S.K.; Gu, L.; Reddy, A.S.N. Analysis of Transcriptome and Epitranscriptome in Plants Using PacBio Iso-Seq and Nanopore-Based Direct RNA Sequencing. Front. Genet. 2019, 10, 253. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, H.; Xie, L.; Hao, X.; Kayani, S.I.; Liu, H.; Qin, W.; Chen, T.; Pan, Q.; Liu, P.; et al. Basic Helix-Loop-Helix Transcription Factors AabHLH2 and AabHLH3 Function Antagonistically with AaMYC2 and Are Negative Regulators in Artemisinin Biosynthesis. Front. Plant Sci. 2022, 13, 885622. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–4268. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef]
- Paul, P.; Singh, S.K.; Patra, B.; Sui, X.; Pattanaik, S.; Yuan, L. A differentially regulated AP2/ERF transcription factor gene cluster acts downstream of a MAP kinase cascade to modulate terpenoids indole alkaloid biosynthesis in Catharanthus roseus. New Phytol. 2017, 213, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoids indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, L.; Yu, Q.; Zhang, H.; Zhang, T.; Zeng, J. Artemisinin biosynthesis promoting kinase 1 positively regulates artemisinin biosynthesis through phosphorylating AabZIP1. J. Exp. Bot. 2018, 69, 1109–1123. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Yu, F.L.; Wang, D.; Xie, X.L.; Pang, Y.X.; Chen, H.F. Composition and Antibacterial Activity of Blumea balsamifera Extracts. J. Fujian Agric. 2021, 36, 1131–1138. [Google Scholar]
- Wang, Y.H.; Wang, H.X.; Tian, H.Y.; Ma, C.Y.; Chen, T.; Zou, C.L.; Wang, X. Headspace Solid-Phase Microextraction Coupled with GC-MS for Analysis of Aromatic Components in Leaves of Blumea balsamifera (L.) DC. in Different Seasons. Food Sci. 2012, 33, 166–170. [Google Scholar]
- Xie, X.L.; Chen, Z.X.; Yu, F.L.; Pang, Y.X.; Hu, X.; Chen, H.F. Discussion on the Industry Development Status of Blumea balsamifera Based on Patent Analysis. China Pharm. 2021, 32, 1158–1164. [Google Scholar]
- Yuan, C.; Wang, H.F.; Hu, X.; Pang, Y.X. Rapid Identification on Chemical Constituents of Antimicrobial Fractions from Blumea balsamifera (L.) DC. by UPLC-Q-TOF-MSE. Nat. Prod. Res. Dev. 2018, 30, 1904–1912. [Google Scholar]
- Guan, L.L.; Xia, Q.F.; Pang, Y.X.; Zhao, Z.; Hu, Y.P.; Bai, L.; Wang, H.F.; Liu, H.C. Analysis of metabolic pathway of terpenoids in Blumea balsamifera. China J. Chin. Mater. Medica 2016, 41, 1585–1591. [Google Scholar]
- Chuang, Y.C.; Hung, Y.C.; Tsai, W.C.; Chen, W.H.; Chen, H.H. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. J. Exp. Bot. 2018, 69, 4363–4377. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Shen, Q.; Lu, X.; Pan, Q.; Huang, Y.; Tang, Y.; Fu, X.; Liu, M.; Jiang, W.; et al. Glandular trichome-specific WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol. 2017, 214, 304–316. [Google Scholar] [CrossRef]
- Reddy, V.A.; Wang, Q.; Dhar, N.; Kumar, N.; Venkatesh, P.N.; Rajan, C.; Panicker, D.; Sridhar, V.; Mao, H.Z.; Sarojam, R. Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS.LSU). Plant Biotech. J. 2017, 15, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Zhang, Q.; Chai, J.; Peng, Y.; Cai, X. Identification and cytochemical immunolocalization of acetyl-CoA acetyltransferase involved in the terpenoids mevalonate pathway in Euphorbia helioscopia laticifers. Bot. Stud. 2017, 58, 62. [Google Scholar] [CrossRef] [PubMed]
- Pathak, G.; Dudhagi, S.S.; Raizada, S.; Singh, R.K.; Sane, A.P.; Sane, V.A. Phosphomevalonate kinase regulates the MVA/MEP pathway in mango during ripening. Plant Physiol. Bioch. 2023, 196, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Berasategui, A.; Paetz, C.; Gershenzon, J.; Schmidt, A. Overexpression of an isoprenyl diphosphate synthase in spruce leads to unexpected terpene diversion products that function in plant defense. Plant Physiol. 2014, 164, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Suenaga-Hiromori, M.; Mogi, D.; Kikuchi, Y.; Tong, J.; Kurisu, N.; Aoki, Y.; Amano, H.; Furutani, M.; Shimoyama, T.; Waki, T.; et al. Comprehensive identification of terpene synthase genes and organ-dependent accumulation of terpenoids volatiles in a traditional medicinal plant Angelica archangelica L. Plant Biotechnol. 2022, 39, 391–404. [Google Scholar] [CrossRef]
- Felipe, A.S.; Robert, M.W.; Panagiotis, I.; Evgenia, V.K.; Evgeny, M.Z. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. iTAK: A program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein family database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Wang, L.G.; Park, H.J.; Dasari, S.; Kocher, J.P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression mode. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.P.; Chen, Y.W.; He, F.C. Integrated NR Database in Protein Annotation System and Its Localization. Comput. Eng. 2006, 32, 71–74. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A. The COG database: A tool for genome scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).