Abstract
The genus Limonium Mill. (sea lavenders) includes species with sexual and apomixis reproductive strategies, although the genes involved in these processes are unknown. To explore the mechanisms beyond these reproduction modes, transcriptome profiling of sexual, male sterile, and facultative apomictic species was carried out using ovules from different developmental stages. In total, 15,166 unigenes were found to be differentially expressed with apomictic vs. sexual reproduction, of which 4275 were uniquely annotated using an Arabidopsis thaliana database, with different regulations according to each stage and/or species compared. Gene ontology (GO) enrichment analysis indicated that genes related to tubulin, actin, the ubiquitin degradation process, reactive oxygen species scavenging, hormone signaling such as the ethylene signaling pathway and gibberellic acid-dependent signal, and transcription factors were found among differentially expressed genes (DEGs) between apomictic and sexual plants. We found that 24% of uniquely annotated DEGs were likely to be implicated in flower development, male sterility, pollen formation, pollen-stigma interactions, and pollen tube formation. The present study identifies candidate genes that are highly associated with distinct reproductive modes and sheds light on the molecular mechanisms of apomixis expression in Limonium sp.
1. Introduction
Apomixis (agamospermy), the asexual seed production found in less than 1% of angiosperms [1], can be either independent of or dependent on pollination [2,3]. Most natural apomicts produce meiotic-reduced pollen involved in the fertilization of the polar nuclei in the embryo sac (pseudogamy) [2,3], but others reproduce independently of pollination for the initiation of the embryo or endosperm formation (autonomous apomicts) [4,5].
Different from sexual reproduction, apomixis is characterized by alterations in the developmental program during the formation and development of the female germline [2,6]. Apomicts can reproduce via gametophytic apomixis, involving the formation of an unreduced embryo sac (apomeiosis) that gives rise to a parthenogenetic embryo and a functional endosperm without the 2 maternal: 1 parental genome ratio [2,6]. The unreduced gametophytes can develop via restitutional meiosis or mitotic division (diplospory) or by a somatic, unreduced cell of the nucellus, which develops into an embryonic sac (apospory) [2,6]. In sporophytic apomicts (adventitious embryonies), the embryos originate from somatic tissues of the nucellus and/or integument cells. The formation of these apomictic embryos usually occurs in parallel with the formation of sexual embryos [7].
In gametophytic apomicts, pseudogamy is prevalent among aposporous apomicts, whereas autonomous endosperm formation seems to be prevalent in diplosporous apomicts [2,8]. These latter apomicts seem to be tolerant of deviations from a 2 maternal: 1 paternal genome contribution in the endosperm [4,5]. Autonomous apomicts tend to produce pollen with low viability and can even be male-sterile [9,10,11,12,13]. These apomicts include species from Asteraceae (e.g., Crepis, Taraxacum [9,10,11]), Plumbaginaceae (Limonium [12,13], Melastomataceae (Miconia [14], Poaceae (Calamagrostis [15]), and Rosaceae (Alchemilla [16]) genera, among others.
The emergence of apomixis in natural systems has been a long-standing topic of debate. It was hypothesized that the different types of apomixis are caused by different mutations that destabilize meiosis (megasporogenesis), the gametophyte (embryo sac), and egg formation [2,6]. Loci genetically linked to components of apomixis have been identified in various species, and sequencing of these loci has revealed several genes with the potential to play critical roles in apomixis [17,18]. It was hypothesized that apomixis could be caused by asynchronously expressed germline genes in the ovules of certain hybrids [19]. Transcriptome comparisons show that the genetic control of apomixis in gametophytic apomicts has been related to a wide range of mechanisms regulating gene expression, including protein degradation, transcription, cell cycle control, stress response, hormonal pathways, cell-to-cell signaling, and epigenetic mechanisms [18,20,21]. Several common genes found to be differentially expressed in multiple stages of apomictic and sexual seed production support the view that sexual and apomictic reproduction are closely related developmental pathways [22]. Recent studies present substantial evidence in support of a polyphenic condition for meiosis and determine that polyphenic shifts from apomeiosis to meiosis and vice versa are regulated by metabolic states [23].
The genus Limonium is a remarkable case study that could help with the identification of genetic-molecular factors potentially underlying apomixis [24]. The genus comprises c. 350 species with sexual and apomixis reproductive modes [25], having triploid and tetraploid apomicts with very large distributions in the Mediterranean region and the Atlantic coast [26,27,28]. The mode of speciation in the genus has been hypothesized to combine a polymorphic sexual system, hybridization under alloploid conditions, polyploidy combining autoploidy (unreduced pollen), allopolyploidy, and apomixis [13,24,25,29,30,31]. The polymorphic sexual system is associated with flower polymorphisms (ancillary pollen and stigma and/or heterostyly) and self-incompatibility (SI) under sporophytic control [29,30]. Coarsely reticulate pollen grains germinate on papillose stigmas and finely reticulate pollen grains germinate on cob-like stigmas, while the reverse combinations result in unsuccessful fertilization. Dimorphic SI species have two pollen stigma combinations and reproduce sexually as in diploids (Limonium ovalifolium, 2n = 16 chromosomes). Monomorphic self-compatible species present self-fertile combinations, while monomorphic SI species show only one pollen-stigma combination and produce seeds through apomixis as in tetraploids (Limonium multiflorum; Limonium dodartii, 2n = 35, 36) [12,28]. Sexual species form meiotically reduced tetrasporic embryo sacs [32,33,34] as in L. ovalifolium [13]. Whereas triploid and tetraploid facultative apomicts originate both reduced and unreduced, diplosporic apomictic embryo sacs (Limonium oleifolium (syn. Statice oleaefolia) [33]; Limonium transwallianum [34]. Pollen in Limonium apomitics ranges from low to high fertility or is not produced at all [12,25,31,35]. In the agamospermous species of the L. binervosum group (e.g., Limonium binervosum s.s., L. dodartii, and L. multiflorum), male sterility, i.e., lack of viable pollen, is widespread, and male sterile colonies are confined to defined taxa/areas [12,13,26,28,36]. Male-sterile L. multiflorum plants from diverse colonies present aborted pollen with collapsed morphology without the typical exine patterns, pointing to a sporophytic defect [12]. The elevated number of seeds with high viability formed by this species seem to be the result of autonomous apomixis [13].
In this study, we aim to characterize the genetic factors implicated in Limonium reproductive modes by identifying the genes that are differentially expressed in ovules during sexual and apomixis seed production. Ovules in different stages of development were extracted to compare sexual (Limonium auriculifolium (syn. Limonium nydeggeri [37]), L. ovalifolium), putative facultative apomictic (L. dodartii), and male-sterile (L. multiflorum) plants, previously characterized cytogenetically, cytometrically, genetically, and reproductively [12,13,24,31] and used for profiling through RNA sequencing (RNA-Seq). This technology allows the identification of DEGs and the inference of their expression with high accuracy [38]. Downstream analysis, including functional annotation of the assembled transcriptome and GO enrichment analysis of the annotated DEGs, was used to provide meaningful biological insights that contribute to a better understanding of the molecular mechanisms and pathways that control the switch from sexual reproduction to apomixis in the organisms under study. Our experimental approach was designed to overcome difficulties due to the occurrence of differing patterns among reproductive modes. The specific goals of this study were to: (1) identify transcripts showing differential expression between male-sterile L. multiflorum and sexual plants; (2) partition DEGs into groups whose fold-changes reflect genes potentially involved in flower development; and (3) frame our findings according to previous and ongoing studies to understand apomixis regulation in Limonium.
2. Materials and Methods
2.1. Plant Material
Plants from four Limonium species were selected to represent different reproductive modes, i.e., diploid sexual as L. auriculifolium (n = 2) and L. ovalifolium (n = 2; 2n = 2x = 16 chromosomes) [12,13], tetraploid apomictic as L. multiflorum (n = 2; 2n = 4x = 35), and the facultative apomictic L. dodartii (n = 1; 2n = 4x = 35) [13,24]. These plants, grown from seedlings raised from seed collected in the wild, were maintained at the ex situ collection in a semi-closed greenhouse at the Instituto Superior de Agronomia, Lisbon (Table 1).

Table 1.
List of all tested and control samples of Limonium compared in the differential expression analysis, according to species, reproductive strategy, stage (S), and number of replicates (Rep). Number of all and annotated significantly differentially expressed genes (DEGs) detected by edgeR in Limonium plants, namely apomictic L. multiflorum (M), sexual L. auriculifolium (A/a) and L. ovalifolium (O/o), and facultative apomictic L. dodartii (d), in either stage S1 (1), S2 (2), or S3/S4 (4). All DEGs represent the number of significant genes found to be differently expressed between each test and control sample. DEG annotation was performed according to A. thaliana reference genome. [Comparisons: uppercase refers to test samples, lowercase refers to control samples, and numbers refer to respective stages].
2.2. Ovule Extraction
Flower buds at distinct developmental stages were sampled prior to anthesis according to their size (between 2 and 5 mm) based on cytoembryological observations as in [13], at standardized times (between 9:00 a.m. and 12:00 p.m.). The ovules were selected with respect to the timing of apomeiosis (Stage 1—S1), megagametogenesis (embryo sac, Stage 2—S2), parthenogenesis, and endosperm formation (Stages 3/4—S3/S4), detailed in [13]. Dissection of ovules was performed in a sterile laminar air flow cabinet under a stereoscopic microscope (Stemi 2000-C, Zeiss) with the aid of tweezers and pencil-point spinal needles (Transmed). In total, 280 ovules were extracted from each ovary, containing one ovule each, and about ten to twenty ovules per stage were isolated and placed in a sterile Petri dish with B5 medium [39] to maintain hydration before RNA extraction. This procedure generated a total of 18 samples, including nine samples of ovules from apomictic plants (four biological replicates in stage S1 and five biological replicates in stage S2) and three samples from each of the remaining species (sexual: two species x three stages—S1, S2, and S3/S4; facultative apomictic: three biological replicates in stage S3/S4) (Table 1).
2.3. Total RNA Extraction and Library Preparation
Total RNA was extracted from all samples using the Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, USA). Nevertheless, some modifications were required for obtaining high-quality RNA, as detailed. Samples were collected in 150 µL of lysis solution and grounded with the help of a micropestle in the microfuge tube. Then, another 300 μL of lysis solution supplemented with β-mercaptoethanol was added to the tube, which was vortexed vigorously and incubated at 56 °C for 5 min. The lysate was centrifuged at 14,000 rpm for 2 min, and then the supernatant was transferred into a filter column and centrifuged for 1 min at 14,000 rpm. The clarified flow-through lysate was transferred to a new tube, and 250 μL of Binding Solution was added. The mixture was applied to a binding column and centrifuged for 1 min at 14,000 rpm. The remaining steps followed the manufacturer’s instructions. The quantity of RNA was determined using a BioDrop cuvette (BioDrop, Cambridge, UK) and electrophoresis on a 1% agarose gel. The RNA integrity number (RIN) was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and ranged from 9.19 to 9.45. The messenger RNA (mRNA) libraries were constructed with the Illumina “TruSeq Stranded mRNA Sample Preparation kit” (Illumina, San Diego, CA, USA) and sequenced on an Illumina NovaSeq6000 2× 100 bp at Macrogen facilities (Macrogen, Geumcheon-gu, Seoul, Korea).
2.4. Processing, Mapping, and Quantification of Illumina Reads
All raw reads have been deposited in the NCBI Sequence Read Archive (SRA), BioProject accession PRJNA752506. Quality control of the raw reads, including contaminants survey, was performed using FastQC version 0.11.9 [40] and FastQ Screen version 0.14.0 [41], which ran against the genomes of their default pre-indexed species and adaptors. Then, since all raw reads presented a quality base score over 36, Trimmomatic version 0.39 [42] was used to eliminate adaptors and filter reads of length below 36 base pairs (bp). A de novo transcriptome assembly was performed using Trinity version 2.11.0 [43], in which cleaned reads from all samples were combined to generate one global assembly since this software has shown consistent performance and has a high read alignment rate [44]. The assembly was assessed for completeness using BUSCO version 5 [45] through gVolante2 [46]. After alignment against the transcriptome using Bowtie2 aligner version 2.3.5 [47], sequences were quantified at gene-level expression with RSEM version 1.3.3 [48] through the Trinity pipeline. A principal component analysis (PCA) was performed to survey the relatedness of normalized gene counts using the function plotPCA in R Studio version 4.0.2 [49].
2.5. Differentially Expressed Genes Detection
To study significant differences between apomictic and sexual plants, differential expression analysis was performed with edgeR version 3.30.3 [50], which is a flexible empirical Bayes approach that uses weighted likelihood methods to estimate gene-specific variation even with very few or no replicates [51]. Overall, when studying differences between reproductive strategies, apomictic plants were set as the samples to test, while sexual and facultative apomictic plants were set as the controls, according to each comparison (Table 1). As such, up-regulated DEGs are more expressed in apomictic than in sexual plants, while down-regulated DEGs are less expressed in apomictic and more expressed in sexual plants.
Genes with a normalized |log2 fold change (log2 FC)| > 2 were defined as differentially expressed and used in the downstream analysis. In the comparison between apomictic and facultative apomictic plants, in which all samples have at least 3 replicates, DEGs were previously filtered by p < 0.01. Venn diagrams were used to plot DEGs between different comparisons through matplotlib version 3.3.3 [52] in Python version 3.9.0 (Python Software Foundation 2020). Additionally, DEGs commonly triggered by more than one comparison were searched for opposite regulation.
2.6. Functional Annotation
Functional annotation of DEGs was performed with the Basic Local Alignment Search Tool (BLAST) version 2.10.1 command-line tool from the NCBI C++ Toolkit (National Center for Biotechnology Information 2020). Blastx was used to map DEGs to A. thaliana homologs against a local Swissprot database, filtering gene hits by a maximum E-value of 1.0E−3 and a minimum identity of 40%. [53]. Then, to avoid duplicated results, DEGs annotated to the same A. thaliana homolog were filtered by identities and sequence length, keeping the transcripts with the highest values.
Housekeeping genes (HKGs) are typically required for the maintenance of basal cellular functions that are essential for its existence, regardless of their specific role in the tissue or organism. Since these genes are usually highly conserved, genes stably transcribed in all comparisons were filtered. Additionally, DEGs were searched for the most common housekeeping homolog genes in A. thaliana to study their potential role in reproduction in Limonium plants.
In addition, DEGs were searched for sRNA biogenesis, which is known to play pivotal roles in reproductive development [54,55], and oxidative stress-related genes, which negatively affect reproductive development in plants [56]. DEGs associated with tryptophan and ethylene metabolism were investigated, which are associated with the biosynthesis and regulation of the phytohormone auxin, a vital component of plant reproduction since it regulates both male and female reproductive organs [57,58]. Moreover, DEGs related to aminoacyl-tRNA metabolism and lysine degradation, which are respectively essential to produce ribosomes and proteins, and epigenetic processes through DNA methylation [59,60] were searched.
2.7. Transcription Factors (TFs) Involved in Plant Reproduction
TFs can be engaged in plant reproduction, namely in flower development such as APETALA (AP genes: AP1, AP2, and AP3), PISTILLATA (PI), SEPALLATA (SEP genes: SEP1, SEP3), and other MADS-box TFs [61,62,63], and in male sterility (e.g., ROS1, DMC1, MS2, POP1, and 4CLL1; [64,65]. As such, these genes, along with a list of A. thaliana TFs retrieved from the Plant Transcription Factor and Protein Kinase Identifier and Classifier database (iTAK v18.12) [66], were searched among DEGs to find if they were down-regulated in apomictic plants. Next, KEGG and WikiPathways enrichment analysis was performed with gProfiler to find relevant metabolic pathways among these TFs, following the same parameters mentioned for GO analysis (see below). Furthermore, uniquely annotated lists of DEGs were searched for GO terms related to pollen, such as the direct and child terms of the biological processes “microsporocyte differentiation” (GO:0010480), “pollen development” (GO:0009555), “pollen wall” (GO:0043667), “pollen coat” (GO:0070505), “pollination” (GO:0009856), and the “cellular components pollen tube” (GO:0090406), according to UniProtKB and StringDB.
2.8. Enrichment Analysis
Uniquely annotated DEGs were characterized with GO terms using the REST API on the UniprotKB website (The Uniprot Consortium, 2019). Finally, GO enrichment analyses were applied to log2FC-ordered lists of DEGs through an over-representation analysis (ORA) using the g:GOSt functional profiling tool from the gProfiler website [67], with the g:SCS tailored algorithm under FDR < 0.01, using a predefined A. thaliana custom background including only genes expressed by the samples in analysis. Enrichment results were summarized using REVIGO [68] through the removal of redundant GO terms with allowed similarity = 0.5 and then plotted with the R ggplot2 version 3.3.2 library [69]. To better understand the differences between the initial and final stages of both sexual and apomictic plants, ORA results were filtered by specificity to a particular stage.
3. Results
3.1. Gene Expression
The assembled transcriptome generated a total of 162520 trinity unigenes with a 43% GC content and a contig N50 of 2128 (Supplementary Table S1) According to BUSCO, 90% completeness was achieved in the de novo assembled transcriptome, indicating that we have generated a high-quality transcriptome assembly that could be used for further downstream analysis (Supplementary Table S1). The total number of expressed unigenes among Limonium samples was highest in ovules from L. multiflorum (apomictic) in stage S2 (115775), followed by L. dodartii (facultative apomictic) in stage S4 (103345), and varying from 20133 to 76395 in sexual plants (Table 2). Among these, the number of expressed unigenes in L. auriculifolium was higher than that in L. ovalifolium in stages S3/S4, but lower in the remaining stages (Table 2). In the PCA, PC1, which accounted for 71% of the variance, revealed a clear cluster of sexual plants on the right side of the graph (Supplementary Figure S1). The PC2 separated sexual ovules in the S1 and S2 stages (upper-right quadrant) from sexual ovules in the S3/S4 stage (lower-right quadrant). Moreover, PC1 grouped all samples from apomictic and facultative apomictic plants with ovules in stage S1 (Supplementary Figure S1, left), showing a higher dispersion in the remaining stages of these ovules.

Table 2.
Total number of genes expressed by Limonium samples from apomictic L. multiflorum (M), facultative apomictic Limonium dodartii (d), and sexual L. auriculifolium (A/a) and L. ovalifolium (O/o) ovules in stages S1, S2, and S3/S4.
Several HKGs were found in all comparisons of Limonium plants, regardless of species, reproductive strategy or stage, namely: ACT domain-containing proteins (ACR3, ACR8, and ACR9), actin (ACT2, ACT4, and ACT7), actin-depolymerizing factors (ADF1 and ADF10), actin-interacting proteins (AIP1-1, AIP1-2), actin-related proteins (ARP2, ARP6, and ARP8), cytosolic Fe-S cluster assembly factor NBP35, expansins (EXPA2 and EXPA9), glyceraldehyde-3-phosphate dehydrogenase GAPA1, heat shock proteins (HSP90-4 and HSP90-5), NADH dehydrogenase [ubiquinone] flavoprotein 2 (NDUFV2), phosphoglycerate kinase 1 (PGK1), polyubiquitin 3 (UBQ3), TATA-box-binding protein 2 (TBP2), tubby-related proteins (TULP1 and TULP3) and tubulins (TUB1, TUBB1, TUBB4, and TUBB7).
3.2. Overall Differential Expression Analysis
The highest number of annotated DEGs (3544) was found in M2o4, followed by M2a4 (1174) (Table 1). In intraspecies comparisons, L. ovalifolium showed the lowest number of DEGs in O2o1 (693), followed by O4o2 (1650), with the major differences being observed in O4o1 (2067; Supplementary Figures S2B and S3C,D). Conversely, L. auriculifolium showed the lowest differences in A4a1 (351), followed by A2A1 (796), thus presenting the major differences in A4a2 (1346; Supplementary Figures S2A and S3A,B). Notably, DEGs showed a proportion of 24% to 35% unannotated genes across samples (Table 1). On the other hand, M1o1 and M1o2 showed just slightly more DEGs than M1a2, while M2m1 presented more DEGs than M2d4. When comparing the two sexual plants, there was an increasing number of DEGs with the progression of stages, which varied from 616 to 1611 (Table 1; Supplementary Figure S2C).
When comparing DEGs between apomictic and sexual species, among sexual plants in S1, 47% (602) were found both in M1a1 and M1o1, while in S2, 32% (407) were found both in M1a2 and M1o2 (Figure 1A,B). The number of specific DEGs was higher in M1a1 than in M1o1 (478 vs. 207), but lower in M1a2 than in M1o2 (256 vs. 611). Moreover, the number of specific DEGs was much higher in M2o4 than in M2a4 (2562 vs. 272), with the lowest in M2d4 (71; Figure 1C).
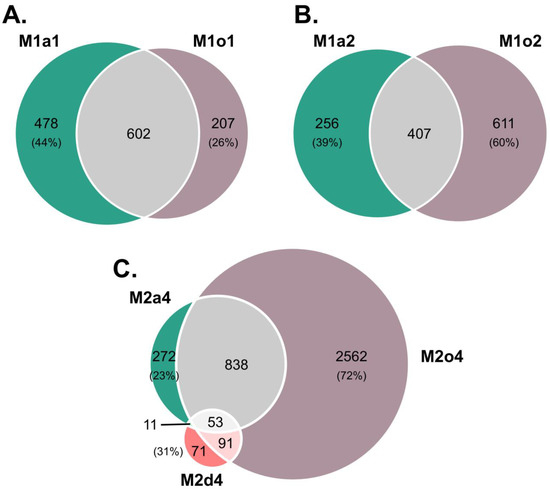
Figure 1.
Weighted Venn diagrams of specific and overlapping differentially expressed genes (DEGs) found in the ovules of apomictic L. multiflorum (M), facultative apomictic L. dodartii (d), and sexual L. auriculifolium (a) and L. ovalifolium (o). DEGs were filtered by |log2 fold-change (log2FC)| > 2. Number of overlapping and specific DEGs in: A. L. multiflorum in S1 relative to L. auriculifolium in S1 (M1a1; green) and to L. ovalifolium in stage S1 (M1o1; purple); B. L. multiflorum in S1 relative to L. auriculifolium in S2 (M2a2; green) and to L. ovalifolium in S2 (M1o2; purple); C. L. multiflorum in S2 relative to L. auriculifolium in S3/S4 (M2a4; green), to L. ovalifolium (M2o4; purple) and to L. dodartii in S4 (M2d4; red).
Some DEGs were detected in more than one developmental phase, although they presented opposite regulations (Supplementary Table S2; Supplementary Figure S4). Only four DEGs were down-regulated in M1a1 and up-regulated in M1o1, including FPF1, associated with flower development. Furthermore, 124 DEGs were down-regulated in M2a4, including ABCG28, ADPG2, AGL66, ATXR5, CALS5, CNGC18, LRL1, PRK3, PS1, SHT, TIP5-1, WRKY2 associated to anther and pollen development, but up-regulated in M2o4, while 5 DEGs presented the opposite regulation, namely SEU, which is involved in flower development. Additionally, 11 DEG were up-regulated in M2a4 but down-regulated in M2d4, being mainly linked to stress responses. Moreover, 44 DEGs were up-regulated in M2o4 but down-regulated in M2d4, which included QRT2, related to anther and pollen.
3.3. DEGs Potentially Implicated in Apomixis Regulation
Some common HKGs in A. thaliana were found to be differentially expressed in apomictic Limonium plants, namely A1, ACR11, two ACT, seven EXPA, two GAPC, HSP90-6, RALFL19, four TUBB, and eight UBC genes. While EXPA and TUBB DEGs were mainly down-regulated in apomictic plants, the remaining genes were mostly up-regulated (Table 3).

Table 3.
List of common housekeeping genes in A. thaliana (gene name) that were differentially expressed (DEGs) in the ovules of apomictic L. multiflorum (M) in S1 and S2, and sexual L. auriculifolium (a) and L. ovalifolium (o) in S1, S2, and S3/S4. DEGs were filtered by |log2 fold-change (log2FC)| >2 (red: up-regulated DEGs; blue: down-regulated DEGs).
Oxidative stress-related DEGs were found to be mainly up-regulated in apomictic plants, namely ACO3, CDSP32, GSH1, GSTU20, ABC1K8, and APX6 (Supplementary Figure S2). However, GR1, GASA14 (GASA—GA-stimulated transcripts), and GSTF11, which are also related to oxidative stress, were down-regulated. Some genes presented mixed regulation, with GSTU19 being down-regulated in M1o2 but up-regulated in M1a2 and M2o4, and MIOX being up-regulated in all comparisons except M2d4, which was down-regulated (Supplementary Figure S5A).
Analyzing DEGs associated with sRNA biogenesis showed an up-regulation of various genes like AGO1, AGO4, AGO5, AGO7, AGO8, AGO9, DML2, and DNMT2 in M2o4. Nevertheless, in the remaining comparisons between apomictic and sexual ovules, while there was a down-regulation of AGO5 and AGO10 in apomictic ovules, DML2 was up-regulated in the same plants (Supplementary Figure S5B).
3.4. Floral-Related DEGs
Globally, the most differentially expressed genes between apomictic and sexual ovules in the early stages were associated with floral development (Supplementary Tables S3 and S4). In M1a1, top-DEGs included a down-regulation of AP3, PI, and PEX4 (log2FC: −8.66, −8.50, and −8.44), while AP1 and SEP1 were among the top down-regulated DEGs in M1a2 (log2FC: −7.24 and −7.09) (Supplementary Table S3). In M1o1, top down-regulated DEGs included SEP1 and GASA7 (log2FC: −7.56 and −7.43), while AP3 was down-regulated in top-DEGs in M1o2 (log2FC: −8.55) (Supplementary Table S4). Overall, top-DEGs from M2a4, M2o4, and M2d4 were implicated in general molecular functions, such as binding, a structural constituent of the ribosome, and catalytic, transporter, structural molecule, ATP-dependent, and transcription regulator activities, presenting a higher log2FC variation in M2a4 (−8.30 to 12.35) and M2o4 (−8.52 to 16.87) than in M2d4 (−5.91 to 8.57) (Supplementary Tables S3–S5). Moreover, among top-DEGs in M2m1, two genes presented opposite regulation, with GASA1 being down-regulated and AGL15 being up-regulated (log2FC: −4.61 to 8.81) (Supplementary Table S6). In the remaining comparisons, there was also a predominance of general molecular functions (Supplementary Tables S7–S9).
In the early stages, among all DEGs between apomictic and sexual ovules, there was a predominant down-regulation of AP, PI, and SEP in apomictic plants. Additionally, it was found that AGAMOUS (AG) genes (e.g., AGL42) were up-regulated, as were other MADS-box genes, namely ANR1, FLC, SOC1, and SVP. However, M2o4 showed both up- and down-regulation of AP genes. Other MADS-box genes were also both up- and down-regulated in M2a4 and M2o4 (Figure 2 and Table 4).
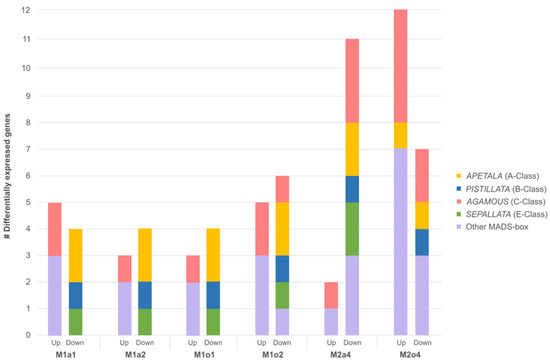
Figure 2.
Distribution of differentially expressed genes related to floral development in ovules from apomictic L. multiflorum (M), sexual L. auriculifolium (a) and L. ovalifolium (o), and facultative apomictic L. dodartii (d). Differentially expressed MADS-box genes APETALA (A-class), PISTILLATA (B-class), AGAMOUS (C-class), SEPALLATA (E-class), and other MADS-box genes are represented. DEGs were found in apomictic ovules in S1 relative to sexual ovules in S1 (M1a1 and M1o1) and S2 (M1a2 and M1o2), and relative to sexual ovules in S3/S4 (M2a4 and M2o4), and facultative apomictic in S4 (M2d4).

Table 4.
List of florally differentially expressed genes (DEGs) in ovules of Limonium plants, namely apomictic L. multiflorum (M), sexual L. auriculifolium (a), and L. ovalifolium (o). DEGs were found in apomictic ovules in S1 relative to sexual ovules in S1 (M1a1 and M1o1) and S2 (M1a2 and M1o2), and in apomictic ovules in S2 relative to sexual ovules in S3/S4 (M2a4 and M2o4); (red: up-regulated DEGs; blue: down-regulated DEGs).
In all comparisons, TFs potentially related to male sterility were mostly associated with down-regulated DEGs (Figure 3). These TFs were classified into 10 major families, namely AP2/ERF, bHLH, bZIP, C2C2, C2H2, HB, MADS, MYB, NAC, and WRKY, from which the most representative families in all comparisons were WRKY and MYB. These TFs were particularly abundant in DEGs in M2o4 and particularly low in M2d4. Furthermore, TFs from apomictic DEGs showed an enrichment of metabolic pathways in the KEGG database. “Plant hormone signal transduction” (KEGG:04075) was up-regulated in M1o1 and M2o4, showing both up- and down-regulation in M1o2 and in M2a4, involving two AHK, ARF1, two ARR, BZR2, DPBF3, EIN4, ERF2, ETR1, GBF4, four IAA, MYC2, NPR5 and three TGA. Up-regulation of “Lysine degradation” (KEGG:00310) and “MAPK signaling pathway—plant” (KEGG:04016) were only enriched in M2o4. The first was associated with ASHR1, two ATXR, EZA1, two SUVH and SUVR3, while the second involved EIN4, ETR1, MYC2 and two WRKY. Moreover, WikiPathways “flower development” (WP:WP618) and “flower development (initiation)” (WP:WP2108) were enriched among up-DEGs across comparisons, being related to AG, three AP, PI, RAP2-7, two SEP, SOC1, and SVP (Supplementary Table S10).
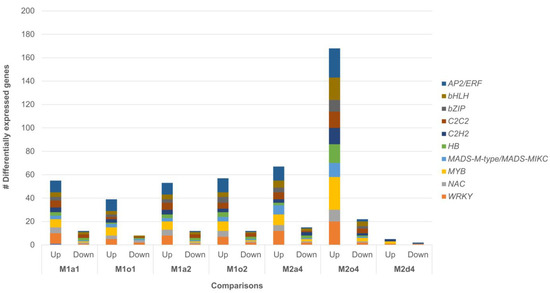
Figure 3.
Distribution of differentially expressed transcription factors potentially related to male sterility is classified into the 10 families with the highest number of differentially expressed genes (DEGs) in ovules: Apomictic L. multiflorum (M), sexual L. auriculifolium (a) and L. ovalifolium (o), and facultative apomictic L. dodartii: AP2/ERF, bHLH, bZIP, C2C2, C2H2, HB, MADS, MYB, NAC, and WRKY.
Additionally, four DEGs were found to be associated with tryptophan metabolism, namely TAA1, TSB2, and AT3G04600, which were up-regulated in M2o4 and TAR2, which was down-regulated in most comparisons (Table 5). Moreover, 29 DEGs were found to be related to ethylene, namely AIL5, ANT, CRF2, EIN2, 14 ERF, ERS1, five RAP2, RTE1, SHN3, TINY, WR11, and AT4G13040, the majority of which were up-regulated in apomictic plants, especially in M2o4. Furthermore, 12 DEGs were found to be related to aminoacyl-tRNA, namely AO, EDD1, GDH2, two GLDP, GRDP2, PSS1, three RBG, RZ1A, and UGLYAH, which showed a similar regulation. Furthermore, 29 DEGs were found to be related to lysine, namely AATL1, ASHR1, three ATX, four ATXR, ELF6, EMB3003, EZA1, three JMJ, two LHT, LTA2, OVA5, two SUVH, three SUVR, AT1G25530, AT4G26910, AT5G55070, AT4G35180, and AT3G11710, presenting mostly up-regulation in apomictic plants (Table 5).

Table 5.
List of differentially expressed genes (DEGs) in ovules of Limonium plants, namely apomictic L. multiflorum (M) relative to sexual L. auriculifolium (a) and L. ovalifolium (o), in different stages, which are involved in tryptophan, ethylene, aminoacyl-tRNA, or lysine metabolism. DEGs were filtered by |log2 fold-change (log2FC)| > 2. DEGs were mapped to their respective A. thaliana homolog (gene name) and annotated according to its reference genome (red: up-regulated DEGs; blue: down-regulated DEGs).
3.5. General GO Enrichment
DEGs between apomictic and sexual ovules in early stages were found to be enriched in seven main ancestor terms (Figure 4A). Most biological regulation terms (GO:0032501, GO:0048580, GO:2000241) were mainly enriched in up-DEGs in M1o2, although “regulation of flower development” (GO:0009909) was enriched in down-DEGs (Figure 4A). Cellular processes (GO:0007015, GO:0030029) were enriched among down-DEGs of M1o1 and M1o2. Although some developmental processes (GO:0009653, GO:0010623) were enriched among down-DEGs, “flower development” (GO:0009908) was enriched among up-DEGs. Localization terms related to biomolecules (GO:0015800, GO:0008643) were down-regulated, while transmembrane transports (GO:1903959, GO:0055085) were up-regulated in M1a1 and M1a2, but mainly down-regulated in M1o1 and M1o2. Although several metabolic processes (GO:0006631, GO:0045490, GO:0009699, GO:0000272, GO:0006468) were down-regulated, photosynthesis-related processes (GO:0005996, GO:0015979, GO:0019684) were up-regulated. Most responses to stimuli (GO:0009416, GO:0014070, GO:0006979) were enriched in up-DEGs. Finally, signaling terms (GO:0023052, GO:0007166) were mainly up-regulated (Figure 4A).
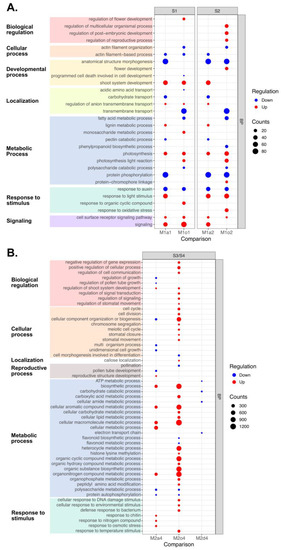
Figure 4.
Over-representation analysis (ORA) performed by gProfiler of differentially expressed genes (DEGs) in ovules from apomictic L. multiflorum (M), sexual L. auriculifolium (a) and L. ovalifolium (o), and facultative apomictic L. dodartii. DEGs were filtered by |log2 fold-change (log2FC)| >2. A.s thaliana, the most similar homolog of each differentially expressed gene (DEG) was mapped to the respective functional annotation, and enriched terms were summarized using REVIGO. Significantly (FDR < 0.01), gene ontology (GO) and biological processes (BP) terms are among DEGs from (A) apomictic in S1 relative to sexual ovules in S1 (M1a1 and M1o1) or S2 (M1a2 and M1o2), and from (B) apomictic in S2 relative to sexual ovules in S3/S4 (M2a4 and M2o4), and facultative apomictic in S4 (M2d4). The dot’s size indicates the number of DEGs annotated with each term (counts), and the color shows the differential expression (red: up-regulated; blue: down-regulated). Enriched terms are grouped by their respective ancestors (ontology level 2).
DEGs from M2a4 and M2o4 showed many enriched terms, especially in the up-DEGs of the latter. DEGs from M2d4 presented only six enriched metabolic process terms associated with down-regulated DEGs (Figure 4B). Most biological regulation terms (GO:0010629, GO:0048522, GO:0010646, GO:0009966, GO:0023051, GO:0010119) were enriched among up-regulated DEGs from M2o4, while “regulation of pollen tube growth” (GO:0080092) was down-regulated in M2a4. Although most cellular process terms were up-regulated in M2o4 (GO:0007049, GO:0051301, GO:0071840, GO:0007059, GO:0051321, GO:0090332, GO:0010118), they were down-regulated in M2a4 (GO:0071840, GO:0032501, GO:0009826). “Callose localization” (GO:0052545) was only enriched for down-DEGs in M2o4. Reproductive processes presented both types of regulation, where “pollination” (GO:0009856) and “pollen tube development” (GO:0080092) were down-regulated in M2o4 and M2a4, respectively, but “reproductive structure development” (GO:0048868) was enriched among up-DEGs in M2a4. Metabolic process and response to stimulus terms were mainly enriched in up-DEGs from apomictic ovules relative to both sexual species (Figure 4B).
3.6. GO Enrichment in Floral and Pollen-Related DEGs
Among all DEGs, 49 were found to be floral-related, which were mainly up-regulated for flowering and gibberellin-related terms (GO:0009908, GO:0010228, GO:0009685, GO:0010077, GO:0009739, GO:0048573, GO:0048437; Figure 5). Conversely, there was a predominance of down-regulation in DEGs related to brassinosteroid, ovule and inflorescence development, and floral organ identity (GO:0010268, GO:0009741, GO:0048481, GO:0010229, GO:0010093).
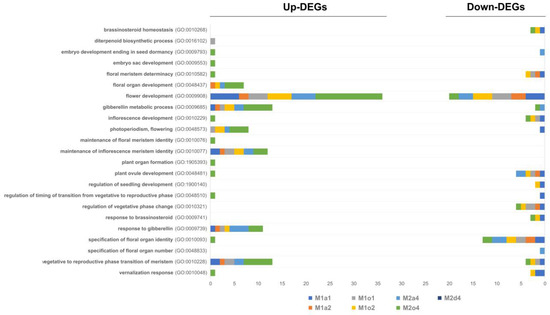
Figure 5.
Regulation of up- and down-regulated differentially expressed genes (DEGs) in ovules in stages S1 (1), S2 (2), and S3/S4 (4) from apomictic L. multiflorum (M), sexual L auriculifolium (a) and L. ovalifolium (o), and facultative apomictic L. dodartii (d), annotated with female-related Gene Ontology (GO) terms. DEGs represent the number of significant genes found to be differently expressed in each comparison.
Overall, among DEGs annotated with pollen-related GO terms, the majority were downregulated in all comparisons (Figure 6; Table 6). However, “pollen development” (GO:0009555), “pollen maturation” (GO:0010152), “pollen tube” (GO:0090406), “pollen tube growth” (GO:0009860) and “pollen tube guidance” (GO:0010183) were up-regulated in M2o4. Globally, both up- and down-regulated DEGs were especially related to pollen development. Noticeably, down-regulated DEGs were also related to pollen wall assembly and pollen tube development and growth. Specifically, the EX5 gene was found to be down-regulated in M1a1 and M1o2.
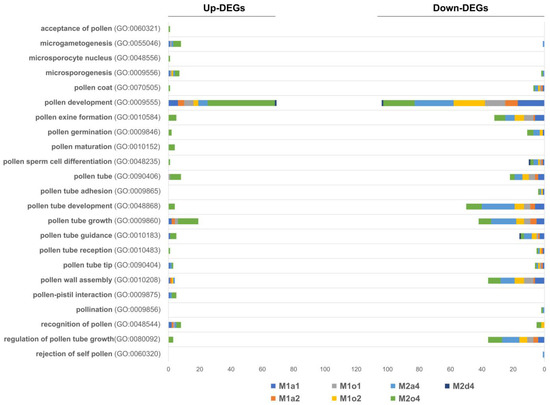
Figure 6.
Regulation of up- and down-regulated differentially expressed genes (DEGs) in ovules in stages S1 (1), S2 (2), and S3/S4 (4) from apomictic L. multiflorum (M), sexual L auriculifolium (a) and L. ovalifolium (o), and facultative apomictic L. dodartii (d), annotated with Gene Ontology (GO) terms related to pollen. DEGs represent the number of significant genes found to be differently expressed in each comparison.

Table 6.
Changes in expression of uniquely annotated differentially expressed genes (DEGs) related to pollen from ovules of apomictic L. multiflorum (M) in S1 (1) relative to sexual L. auriculifolium (a) and L. ovalifolium (o) in either S1 (1) or S2 (2), and L. multiflorum (M) in S2 (2) relative to L. auriculifolium (a), L. ovalifolium (o), and facultative apomictic L. dodartii (d) in S3/S4 (4). Annotated DEGs according to A. thaliana homologs (gene names) were searched in biological process (BP), molecular function (MF), and cellular component (CC) Gene Ontology (GO) terms related to pollen (red: up-regulated DEGs; blue: down-regulated DEGs).
4. Discussion
An increasing number of molecular studies have identified several candidate genes implicated in the shift from sexual to apomixis reproduction [18,21,22,70]. In different species, apomixis has been found to arise due to the action or deregulation of different genes associated with the normal sexual pathway [23,71,72,73]. Nevertheless, it is still not fully understood how these genes alter reproductive pathways to establish apomixis.
In most apomictic wild species. Implementing omics approaches can be particularly challenging as complete genomic sequences are not available and, therefore, genome annotation information is not available. Additionally, obtaining plant material for transcriptomic studies can be an experimentally challenging task in Limonium since each plant presents a single ovary, enclosed in a calyx and inner, medium, and outer bracts that yield just a single basal ovule [13]. In the current study, we performed a comparative transcriptome analysis between sexual and asexual plants and identified candidate genes that are specifically or differentially expressed between reproductive modes and among stages of ovule development. This approach allowed us to disclose differential regulation of both HKGs as well as genes specifically involved in flower development, male sterility, and pollen recognition, besides major pathways potentially central to apomixis, including protein degradation, transcription, stress response, hormonal signaling, signal transduction, and epigenetic regulation.
4.1. Differential Regulation of HKG and Metabolic Pathways in Sexual and Apomictic Plants
In this study, the total number of expressed unigenes among samples was higher in ovules from apomictic plants than in those from sexual plants, together with a differential regulation of genes, particularly in the later stages of ovule development (Table 1). Previous studies between sexual and asexual plants provided support for the deregulation of reproductive pathways, including HKGs in, e.g., Boechera holboellii complex [72], Brachiaria [74], Cenchrus ciliaris [75], and Ranunculus [73], among others. In this study, although many homolog genes in Arabidopsis were stably expressed in Limonium, such as ACT domain-containing proteins. Cytosolic Fe-S cluster assembly factors NBP35, NADH dehydrogenase [ubiquinone] flavoprotein 2, phosphoglycerate kinase 1, polyubiquitin 3, TATA-box binding protein 2, and other HKGs were found to be differentially expressed. These include genes related to the ubiquitin degradation process, such as ubiquitin-conjugating enzymes, tubulin, actin, and elongation factor-1 α as found in other sexual and apomictic plants’ complexes above referred. Therefore, some of the HKGs identified (Table 3) in our study can be potentially used as reference genes to be validated in future quantitative gene expression studies using different developmental stages of specific tissue types or different reproductive modes.
A differential representation of DEGs in Limonium sexual and apomictic plants associated with the oxidative stress response was found. In apomictic plants, some of these DEGs (Supplementary Figure S5), e.g., ACO3, CDSP32, GSH1, etc., were up-regulated while others were down-regulated (GR1, GASA14, and GSTF11) in both the initial (apomeiosis) and later (parthenogenesis) stages of ovule development. Nonetheless, apomicts present more up-regulated DEGs regarding oxidative stress than sexual plants, supporting the involvement of redox reactions in this reproductive mode. Alteration of homeostasis-based processes of stress perception and attenuation in sexual species of several genera would induce apomeiotic spores and gametophyte formation [23]. Apomeiosis occurs when the redox balance is more toward H2O2 catabolism, and the transition from meiosis to apomeiosis can be changed by a disturbance in this homeostasis [23].
Among DEGs between sexual and Limonium apomicts, most were up-regulated in the latter stages of ovule development (parthenogenesis), such as ATRX genes coding for chromatin remodelling proteins as well as multiple histone methylation genes concerning epigenetic developmental mechanisms in plants [76] (Table 5 and Table 6). These DEGs were also previously found to be upregulated, for instance, in parthenogenetic eggs of Cenchrus ciliaris [77]. Moreover, other DEGs implicated in small RNA biogenesis and DNA-methylation pathways, such as the AGO9 and AGO4 homolog genes in Arabidopsis mutants found to be associated with phenotypes reminiscent of apospory or diplospory [78], were also detected in our study. In Boechera apomicts, AGO9 was found at low levels in the megaspore mother cell itself, becoming an apomictic initial cell [79]. However, in our study, AGO4 and AGO9 seem to have more specific roles in ovules at later stages of development (parthenogenesis), likely being involved in eggs assuming a parthenogenesis fate. MAPK signaling and aminoacyl-tRNA biosynthesis pathways that perform roles in translational regulation, RNA splicing, and tRNA proofreading [80] also showed transcriptional changes at this stage in apomictic Limonium plants (Table 5 and Table 6).
DEGs were also remarkably enriched in genes implicated in hormonal signaling, such as the ethylene signaling pathway, in which the apomictic gametophytes overexpressed 26 ethylene-responsive transcription factors (Table 5 and Table 6). For example, in Cenchrus ciliaris, EIN2 (ethylene insensitive 2) together with 14 ethylene responsive transcription factors were up-regulated in parthenogenetic eggs [77], although in our study both genes showed contrasting expression patterns in the same developmental stage (parthenogenesis). Moreover, in the parthenogenetic ovules, overexpressed genes were related to tryptophan metabolism, such as TAA1 (l-tryptophan pyruvate aminotransferase), which converts tryptophan to indole-pyruvic acid, a direct biosynthetic precursor of the auxin in Arabidopsis (IAA [81]). Crosstalk between ethylene signaling and auxin pathways is involved in the regulation of developmental processes [82].
4.2. Feminization of Apomicts Is Related to Down-Regulation of Floral Genes Specifying Stamens
Besides auxin, other hormones like gibberellic acid (GA) contribute to flower development, the development of male and female gametophytes, and seed germination [83.84]. The GASA genes as well as the GA biosynthesis genes in Arabidopsis, implicated in controlling floral induction, seed maturation, and germination [83,84], were differentially expressed between apomictic and sexual plants in our study (Table 4). One of the targets of GA signaling are the floral homeotic genes encoding MADS-box transcription factors involved in floral development in accordance with the ABCDE model [85]. In our study, among the top DEGs between sexual and apomictic plants and between the different ovule stages, MADS-box transcription factors were identified, including floral homeotic genes with a MADS-box domain. In A. thaliana, the MADS-box from A-class genes (AP1; AP2) specifies the formation of sepals; the combination of A- and B-class genes (AP3; PI) determines petal’ development; the B-, C- (AGL), and E-class (SEP) genes specify stamens; and the C- and E-class genes specify carpels. Only the expression of genes from class C specifies carpel formation. Class E genes (SEP3) are associated with the formation of all flower whorls. The gene classes A and C are expressed antagonistically; the A gene class is expressed in sepals and petals, and the C gene class is expressed in stamens and carpels [86,87].
GA promotes reproductive development by upregulating expression of the floral meristem identity gene LEAFY (LFY), which in turn upregulates expression of AP3 and AGL that, in conjunction with PI and SEP3, regulate floral organ identity [87]. In our study, we found changes in the expression of MADS-box genes in the different stages of ovule development between sexual and apomictic plants, particularly PI, SEP1, and AP3 genes, which were downregulated in apomicts. The PI/AP3 genes have a role in sexual dimorphism and have been identified as masculinizing factors in spinach [88]. In dioecious plants such as Populus, constitutive overexpression of PI/AP3 produces male flowers, but in female flowers the presence of a feminizing factor F downregulates PI/AP3, diverting development to a female developmental pathway, inhibiting stamens, and allowing carpels to form [89]. Therefore, it could be hypothesized that male sterile Limonium plants with homeotic changes in floral organs lack the gene function of the corresponding class B genes.
4.3. Male Sterility Appears to Be Linked with Downregulation of Genes Connected to Pollen Wall Formation and Assembly and Pollen Tube Growth
Various genes are involved in pollen wall development and assembly, which is a specialized extracellular cell wall matrix that encases the male gametophytes [90]. A specific cell wall polymer also known as β-glucan is synthesized by callose synthases in pollen mother cells and microspore tetrads that acts as a template for primexine, thus providing a structural basis for exine formation [90]. Callose synthase 5 (CSL5) is a key isoform of callose synthases responsible for the formation of the callose wall, which is essential for the accumulation of callose in the tube wall and the callose plug in growing pollen tubes [91]. In CSL5 mutants, the viability of pollen grains is greatly reduced in Arabidopsis [91,92] and rice [93]. In our study in Limonium, CSL5 is downregulated in the initial (apomeiosis) and later phases of development (parthenogenesis) in the apomicts. One of the characteristic features of plants in the L. binervosum group is the widespread existence of male sterility, i.e., a lack of pollen [12,13,26,36]. Electron microscopy studies showed that L. multiflorum plants had many flowers with empty anthers and sometimes flowers with no pollen at all; the few microspores that formed showed collapsed morphology and lacked the typical exine patterns [12]. In L. multiflorum apomicts, after anther dehiscence releases pollen, the plants never undergo their first mitosis, only attaining the ‘‘ring vacuolate’’ stage, and the male germ unit is not produced [12]. Interestingly, in this study, callose synthase isoforms such as CSL9 or CSL11 that were upregulated in the apomicts have a role in Arabidopsis pollen mitosis by disrupting pollen mitosis and producing pollen with only one or two nuclei, the generative cell being degenerated, undifferentiated, or mislocalized [94,95], as found here in Limonium.
Moreover, in our study, the gene PEX4 that codes for extracellular glycoproteins that belong to the hydroxyproline-rich glycoprotein family and have a role in pollen germination and pollen tube growth in A. thaliana [96,97]. was downregulated in Limonium apomicts (apomeiosis, M1a1). The PEX4 mutants have an excessive deposition of callose [96,97], leading to abnormal pollen tubes that develop bulges and burst [97]. While in L. multiflorum apomicts, pollen tubes are never observed since unicellular pollen never undergoes its first mitosis. In L. ovalifolium sexual plants, pollen grains follow a first asymmetric mitotic division, producing a generative cell within the vegetative pollen grain cell in the binucleate pollen stage [12]. Therefore, our results support a role for PEX4 in pollen tube growth. Perhaps this gene, along with other unknown genes, might create a terminal combination that does not allow the development of pollen tubes.
4.4. Pollen-Stigma Interactions
Pollen-pistil interactions can be viewed as a major prezygotic pollination reproductive barrier and are active systems of pollen rejection [98]. Some of these systems act at the level of the stigma, with a genetic control independent from embryo sac development involving S-alleles [99]. In the Brassicaceae family, the SI is controlled sporophytically by a single S locus that incorporates stigma-expressed and anther-expressed genes composed of multiple alleles or variants [100]. In various gametophytic apomicts, non-functional pollen can cause a weakening of SI and a breakdown of the sporophytic SI system (mentor effects [9]).
Limonium species show a polymorphic sexual system associated with flower polymorphisms and a sporophytic self-incompatibility that prevents self- and intramorph mating [29.30]. Limonium gametophytic apomicts that form diplosporic (apomictic) embryo sacs of Rudbeckia type like in L. multiflorum [13] show abnormal and non-functional pollen due to a sporophytic defect [12]. In our study, in the GO term “recognition of pollen” (GO:0048544; Table 6). All DEGs were lectin receptor kinases (LECRKs) [101], except for AT3G49500. These kinases belong to the class of G-LECRKs [101], particularly the S-locus Receptor Kinase (SRK), known for its role in self-incompatibility [101] and potentially of high interest in our studied species. In our study, DEGs from this LECRKs complex were detected under the GO term “recognition of pollen”, namely AT1G61380, AT1G65800, AT5G24080, AT4G27300, AT4G21380, and AT4G27290. Four DEGs of the SRK were overexpressed, namely in the initial stages of ovule development (apomeiosis), such as AT1G61380 (M1a1 and M1a2) and AT1G65800 (M1a1), as well as in later ovule stages (M2a4 and M2o4), AT4G27300 (M2o4), and AT4G27290 (M2o4). These findings indicate that the genes implicated in pollen recognition in Limonium were already expressed at earlier ovule stages. This implies that the fate of the gametophytic apomict male spores is decided by the maternal genes in the initial ovule stages. Nonetheless, a genetic linkage between SI and apomixis cannot be easily assumed, since the breakdown of SI mostly affects the pollen and the stigma, while apomixis affects the development of the embryo sac.
4.5. Up-Regulation of Specific Genes Related with Embryo Formation in the Apomicts
In Arabidopsis, flowering can be promoted by repressing the transcription of the central flowering repressor and vernalization regulatory gene FLC (FLOWERING LOCUS C), which belongs to the MADS-box class of transcription factors [102]. The FLC seems to regulate several transcription factors involved in important biological processes such as reproductive and embryonic development [103]. FLC impedes the floral transition by inhibiting the expression of the floral primordium identity genes such as FT (FLOWERING LOCUS T), SOC1/AGL20 (SUPPRESSOR OF CONSTANS OVEREXPRESSION 1/AG20), LFY, AP1, and floral organ identity AG and AP3 genes [104]. Another gene involved in the genetic control of flowering time in Arabidopsis is FPF1 (FLOWERING PROMOTING FACTOR), which modulates the acquisition of competence to flower in the apical meristem and is expressed earlier than AP1 [105]. In our study, the FPF1 and SOC1 showed higher levels of regulation in apomicts than in sexual plants, whereas AP1 showed reduced levels in the first ones. These results indicate differences in the regulation of major genes controlling the transition from a vegetative to a reproductive mode in the apomict’s apical meristem. Interestingly, in our study, both FLC, AGL6, and AGL15, as well as other MADS-box transcription factors, were specifically upregulated in the later stages of development. In A. thaliana AGL6 functions in the early stages of the flowering signal transduction pathway by inhibiting the transcription of FLC genes [106]. In Brachiaria brizantha, AGL6 is differentially expressed during embryo sac formation in apomictic and sexual plants [107]. In our study, AGL15, which plays an essential role during early zygotic embryogenesis in Arabidopsis [108], in Brassica napus, and in soyabean somatic embryos [109], was specifically upregulated in the later phase of ovule development in Limonium apomicts. The AGL15 is a component of the SERK protein complex [110] that is part of a molecular network linked to zygotic and somatic embryogenesis. However, in this study, we were unable to find any differential expression of SERK-like genes.
Some genes related to the bioactive gibberellins’ deactivation reaction from the GA2OX family were differentially expressed. For example, GA2OX6 was up-regulated in both the initial and later stages of ovule development. While in A. thaliana, GA2OX6 expression is activated by AGL15 during embryogenesis [111], in our study, AGL15 was down-regulated in M2a4 and up-regulated in M2o4, suggesting differences among species. Moreover, GA2OX6 is found to be expressed in sepals, stigmas, and immature anthers. Regarding seed development, it was only expressed in the antipodal cells before the 8-cell stage, suggesting that this gene is a negative regulator of seed germination [111]. Remarkably, from the same family, GA2OX8, which is exclusively expressed in stomatal cells in A. thaliana but is not expressed or has distinct expression patterns in flower tissues or seed development [111], was down-regulated in Limonium apomicts in later stages of ovule development (parthenogenesis). This finding supports the idea that this gene can have other or different roles in Limonium.
5. Conclusions
This study sheds light on genes involved in Limonium sexual and apomictic reproduction. The findings substantiate the deregulation of gene expression in the regular sexual pathway. While several HKGs are found to be differentially expressed between sexual and asexual plants, other genes are found to be stably expressed. These can be potentially used as reference genes to be validated for specific tissue types (vegetative and reproductive) or reproductive modes (sexual and apomictic) in future quantitative gene expression studies. Our findings reveal that the latter stage of ovule development (parthenogenesis) was the most contrasting phase in terms of differential gene expression between asexual and sexual plants. Among them, the MADS-box domain TFs are central players in many developmental processes, including control of flowering time, homeotic regulation of floral organogenesis, fruit development, and seed pigmentation.
Since L. multiflorum male sterile plants form parthenogenetic ovule sacs, it could be interesting to analyze candidate genes such as PEX4 in pollen tube development and the function of AGL genes (e.g., AGL6) specifically modulated in the latter stages of development (parthenogenesis). Nonetheless, given the high number of 71% unannotated genes in Limonium, other studies are required to clarify the regulatory roles of these genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14040901/s1. Figure S1: Principal component (PC) analysis of all rlog-transformed gene expression data; Figure S2: Weighted Venn diagrams of specific and overlapping differentially expressed genes (DEGs) found in the ovules of sexual L. auriculifolium and L. ovalifolium; Figure S3: Weighted Venn diagrams of specific and overlapping differentially expressed genes (DEGs) found in different stages of apomictic L. multiflorum, sexual L. auriculifolium, and L. ovalifolium ovules; Figure S4: Number of differentially expressed genes (DEGs) common to different comparisons with opposite regulations; Figure S5: Distribution of differentially expressed genes (DEGs) related to oxidative stress; Table S1: Quantification of basic quality and completeness metrics of Limonium de novo transcriptome assembly. Table S2: List of annotated differentially expressed genes (DEGs) shared between two comparisons with opposite regulation in ovules from Limonium plants; Table S3: Uniquely annotated top down- and up-regulated differentially expressed genes (DEGs) in different ovule stages in L. multiflorum relative to L. auriculifolium; Table S4: Uniquely annotated top down- and up-regulated differentially expressed genes (DEGs) in different ovule stages in L. multiflorum relative to L. ovalifolium; Table S5: Uniquely annotated top down- and up-regulate differentially expressed genes (DEGs) in different ovule stages in L. multiflorum relative to L. dodartii; Table S6: Uniquely annotated top down- and up-regulate differentially expressed genes (DEGs) in ovules from apomictic L. multiflorum; Table S7: Uniquely annotated top down- and up-regulated differentially expressed genes (DEGs) in ovules from sexual Limonium auriculifolium; Table S8: Uniquely annotated top down- and up-regulate differentially expressed genes (DEGs) in ovules from sexual L. ovalifolium; Table S9: Uniquely annotated top down- and up-regulate differentially expressed genes (DEGs) in ovules from sexual L ovalifolium (O) relative to the control L. auriculifolium; Table S10: Over-representation analysis (ORA) performed by gProfiler of transcription factors (TFs) and differentially expressed genes (DEGs).
Author Contributions
Conceptualization and supervision A.D.C. and O.S.P. methodology and investigation S.I.R.C., I.F., I.M. and A.S.R.; software I.F.; graphical abstract A.S.R.; writing- original draft preparation A.D.C., I.F., I.M., S.I.R.C. and O.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds through FCT (Fundaçãoo para a Ciência e a Tecnologia, I.P.) (https://www.fct.pt/ (accessed on 1 March 2023). Portugal, in the scope of project PTDC/AGRPRO/4285/2014, research units references UIDB/04129/2020 and UIDB/00239/2020 (CEF), grant PTDC/AGRPRO/4285/BM/2014, and PhD Scholarship UI/BD/153730/2022 attributed to S.I.R.C. and Scientific Employment Stimulus—Individual contract 2021.01107.CEECIND/CP1689/CT0001 attributed to I.M.
Data Availability Statement
Raw data are deposited in the NCBI Sequence Read Archive (SRA), under BioProject accession number PRJNA752506. Pipelines and tailored scripts created during this project were made publicly available at https://github.com/ziisabel/CoBiG2/tree/cobig2 (accessed on 1 March 2023).
Acknowledgments
Thanks to Technician Maria João Fernandes (LEAF) for lab help.
Conflicts of Interest
The authors declare no conflict of interest. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Hojsgaard, D.; Greilhuber, J.; Pellino, M.; Paun, O.; Sharbel, T.F.; Hörandl, E. Emergence of apospory and bypass of meiosis via apomixis after sexual hybridisation and polyploidisation. New Phytol. 2014, 204, 1000–1012. [Google Scholar] [CrossRef]
- Asker, S.E.; Jerling, L. Apomixis in Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Richards, A.J. Plant Breeding Systems; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Koltunow, A.M.; Grossniklaus, U. Apomixis: A developmental perspective. Annu. Rev. Plant Biol. 2003, 54, 547–574. [Google Scholar] [CrossRef]
- Talent, N.; Dickinson, T.A. Endosperm formation in aposporous Crataegus. Rosaceae, Spiraeoideae, tribe Pyreae): Parallels to Ranunculaceae and Poaceae. New Phytol. 2007, 173, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Nogler, G.A. Gametophytic apomixis. In Embryology of Angiosperms, Johri, B.M., Ed.; Springer-Verlag: Berlin, Germany, 1984; pp. 475–518. [Google Scholar]
- Naumova, T.N.; Mershchikova, I. Apomixis in Angiosperms: Nucellar and Integumentary Embryony; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Whitton, J.; Sears, C.J.; Baack, E.; Otto, S.P. The dynamic nature of apomixis in the angiosperms. Int. J. Plant Sci. 2008, 169, 169–182. [Google Scholar] [CrossRef]
- Tas, I.C.; Van Dijk, P.J. Crosses between sexual and apomictic dandelions (Taraxacum). I. The inheritance of apomixis. Heredity 1999, 83, 707–714. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Den Nijs, J.C.; Van Tienderen, P.H. Male sterility in triploid dandelions: Asexual females vs. asexual hermaphrodites. Heredity 2006, 96, 45–52. [Google Scholar] [CrossRef]
- Thompson, S.L.; Choe, G.; Ritland, K.; Whitton, J. Cryptic sex within male-sterile polyploid populations of the easter daisy, Townsendia hookeri. Int. J. Plant Sci. 2008, 169, 183–193. [Google Scholar] [CrossRef]
- Róis, A.S.; Teixeira, G.; Sharbel, T.F.; Fuchs, J.; Martins, S.; Espírito-Santo, D.; Caperta, A.D. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders (Limonium sp., Plumbaginaceae) reveal diversity in reproduction modes. Sex Plant Reprod. 2012, 25, 305–318. [Google Scholar] [CrossRef]
- Róis, A.S.; Sádio, F.; Paulo, O.S.; Teixeira, G.; Paes, A.P.; Espírito-Santo, D.; Caperta, A.D. Phylogeography and modes of reproduction in diploid and tetraploid halophytes of Limonium species (Plumbaginaceae): Evidence for a pattern of geographical parthenogenesis. Ann. Bot. 2016, 117, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Caetano, A.P.S.; Cortez, P.A.; Teixeira, S.P.; Oliveira, P.E.; Carmello-Guerreiro, S.M. Unusual diversity of apomictic mechanisms in a species of Miconia, Melastomataceae. Plant Syst. Evol. 2018, 304, 343–355. [Google Scholar] [CrossRef]
- Nygren, A. The genesis of some Scandinavian species of Calamagrostis. Hereditas 1946, 32, 131–262. [Google Scholar] [CrossRef] [PubMed]
- Czapik, R. Problems of apomictic reproduction in the families Compositae and Rosaceae. Folia Geobot. 1996, 31, 381–387. [Google Scholar] [CrossRef]
- Barcaccia, G.; Albertini, E. Apomixis in plant reproduction: A novel perspective on an old dilemma. Plant Reprod. 2013, 26, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Hand, M.L.; Koltunow, A.M. The genetic control of apomixis: Asexual seed formation. Genetics 2014, 197, 441–450. [Google Scholar] [CrossRef]
- Carman, J.G. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linn. Soc. 1997, 61, 51–94. [Google Scholar] [CrossRef]
- León-Martínez, G.; Vielle-Calzada, J.P. Apomixis in flowering plants: Developmental and evolutionary considerations. Curr. Top. Dev. 2019, 131, 565–604. [Google Scholar] [CrossRef]
- Schmidt, A. Controlling apomixis: Shared features and distinct characteristics of gene regulation. Genes 2020, 11, 329. [Google Scholar] [CrossRef]
- Brukhin, V. Molecular and genetic regulation of apomixis. Russ. J. Genet. 2017, 53, 943–964. [Google Scholar] [CrossRef]
- Mateo de Arias, M.; Gao, L.; Sherwood, D.A.; Dwivedi, K.K.; Price, B.J.; Jamison, M.; Kowalis, B.M.; Carman, J.G. Whether gametophytes are reduced or unreduced in angiosperms might be determined metabolically. Genes 2020, 11, 1449. [Google Scholar] [CrossRef]
- Conceição, S.I.R.; Róis, A.S.; Caperta, A.D. Limonium homoploid and heteroploid intra- and interspecific crosses unveil seed anomalies and neopolyploidy related to sexual and/or apomictic reproduction. Taxon 2018, 67, 1153–1162. [Google Scholar] [CrossRef]
- Erben, M. Die gattung Limonium im südwestmediterranen Raum. Mitt. Bot. Staatssamml. Münch. 1978, 14, 361–626. [Google Scholar]
- Ingrouille, M.J.; Stace, C.A. Pattern of variation of agamospermous Limonium (Plumbaginaceae) in the British Isles. Nordic J. Bot. 1985, 5, 113–125. [Google Scholar] [CrossRef]
- Cowan, R.; Ingrouille, M.J.; Lledó, M.D. The taxonomic treatment of agamosperms in the genus Limonium Mill. (Plumbaginaceae). Folia Geobot. 1998, 33, 353–366. [Google Scholar] [CrossRef]
- Caperta, A.D.; Conceição, S.I.; Róis, A.S.; Loureiro, J.; Castro, S. Cytogenetic features of sexual and asexual Limonium taxa (Plumbaginaceae). Taxon 2018, 67, 1143–1152. [Google Scholar] [CrossRef]
- Baker, H.G. Dimorphism and monomorphism in the Plumbaginaceae: II. Pollen and stigmata in the genus Limonium. Ann. Bot. 1953, 17, 433–446. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution 1966, 20, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Conceição, S.I.R.; Fernandes, J.; Borges da Silva, E.; Caperta, A.D. Reproductive output and insect behavior in hybrids and apomicts from Limonium ovalifolium and L. binervosum complexes (Plumbaginaceae) in an open cross-pollination experiment. Plants 2021, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, F. Contributo all’embriologia delle Plumbaginaceae. Nuov. Giorn. Bot. Ital. 1940, 47, 349–382. [Google Scholar] [CrossRef]
- D’Amato, F. Triploidia e apomissia in Statice oleaefolia Scop. var. confusa Godr. Caryologia 1949, 2, 71–84. [Google Scholar] [CrossRef]
- Hjelmqvist, H.; Grazi, E. Studies on variation in embryo sac development. Bot. Not. 1964, 118, 329–360. [Google Scholar]
- Conceição, S.I.R.; Róis, A.S.; Caperta, A.D. Nonreduction via meiotic restitution and pollen heterogeneity may explain residual male fertility in triploid marine halophyte Limonium algarvense (Plumbaginaceae). Caryologia 2019, 72, 53–62. [Google Scholar] [CrossRef]
- Ingrouille, M.J.; Stace, C.A. The Limonium binervosum aggregate (Plumbaginaceae) in the British Isles. Bot. J. Linn. Soc. 1986, 92, 177–217. [Google Scholar] [CrossRef]
- Del Guacchio, E.; Erben, M.; Caputo, P. The neglected name Statice auriculifolia (Plumbaginaceae) and its related names: A long history of nomenclatural intricacy. Taxon 2019, 68, 1093–1100. [Google Scholar] [CrossRef]
- Soneson, C.; Delorenzi, M.A. Comparison of methods for differential expression analysis of RNA-seq data. BMC Bioinform. 2013, 14, 91. [Google Scholar] [CrossRef]
- Morgan, E.R.; Burge, G.K.; Seelye, J.F.; Hopping, M.E.; Grant, J.E. Production of inter-specific hybrids between Limonium perezii (Stapf) Hubb. and Limonium sinuatum (L.) Mill. Euphytica 1998, 102, 109–115. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 8 September 2020).
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Wang, S.; Gribskov, M. Comprehensive evaluation of de novo transcriptome assembly programs and their effects on differential gene expression analysis. Bioinformatics 2016, 33, 327–333. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Seppey, M.; Simão, F.A.; Manni, M.; Ioannidis, P.; Klioutchnikov, G.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 2018, 31, 3210–3212. [Google Scholar] [CrossRef]
- Nishimura, O.; Hara, Y.; Kuraku, S. gVolante for standardizing completeness assessment of genome and transcriptome assemblies. Bioinformatics 2017, 33, 3635–3637. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010; Available online: https://www.R-project.org/ (accessed on 7 September 2020).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. Differential expression analysis of complex RNA-seq experiments using edgeR. In Statistical Analysis of Next Generation Sequencing Data. Frontiers in Probability and the Statistical Sciences; Datta, S., Nettleton, D., Eds.; Springer: Cham, Switzerland, 2014; pp. 51–74. [Google Scholar] [CrossRef]
- Caswell, T.A.; Droettboom, M.; Lee, A.; Hunter, J.H.; Sales de Andrade, E.; Hoffman, T.; Stansby, D.; Klymak, J.; Varoquaux, N.; Hedegaard, N.J.; et al. matplotlib/matplotlib: REL: v3.3.3 (Version v3.3.3). Zenodo 2020. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Wu, C.H. Protein Bioinformatics Databases and Resources. Methods Mol. Biol. 2017, 1558, 3–39. [Google Scholar] [CrossRef]
- Van Ex, F.; Jacob, Y.; Martienssen, R.A. Multiple roles for small RNAs during plant reproduction. Curr. Opin. Plant Biol. 2011, 14, 588–593. [Google Scholar] [CrossRef]
- Petrella, R.; Cucinotta, M.; Mendes, M.A.; Underwood, C.J.; Colombo, L. The emerging role of small RNAs in ovule development, a kind of magic. Plant Reprod. 2021, 34, 335–351. [Google Scholar] [CrossRef]
- Zinta, G.; Khan, A.; Abd Elgawad, H.; Verma, V.; Srivastava, A.K. Unveiling the redox control of plant reproductive development during abiotic stress. Front. Plant Sci. 2016, 7, 700. [Google Scholar] [CrossRef]
- Sundberg, E.; Østergaard, L. Distinct and dynamic auxin activities during reproductive development. Cold Spring Harb. Perspect. Biol. 2009, 1, a001628. [Google Scholar] [CrossRef]
- Larsson, E.; Vivian-Smith, A.; Offringa, R.; Sundberg, E. Auxin homeostasis in Arabidopsis ovules is anther-dependent at maturation and changes dynamically upon fertilization. Front. Plant Sci. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Dambacher, S.; Hahn, M.; Schotta, G. Epigenetic regulation of development by histone lysine methylation. Heredity 2010, 105, 24–37. [Google Scholar] [CrossRef]
- Rose, N.R.; Klose, R.J. Understanding the relationship between DNA methylation and histone lysine methylation. BBA 2014, 1839, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Agarwal, P.; Ray, S.; Singh, A.K.; Singh, V.P.; Tyagi, A.K.; Kapoor, S. MADS-box gene family in rice: Genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genom. 2007, 8, 242. [Google Scholar] [CrossRef]
- Castelán-Muñoz, N.; Herrera, J.; Cajero-Sánchez, W.; Arrizubieta, M.; Trejo, C.; García-Ponce, B.; de la Sánchez, M.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. MADS-Box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Front. Plant Sci. 2019, 10, 853. [Google Scholar] [CrossRef]
- Shah, L.; Sohail, A.; Ahmad, R.; Cheng, S.; Cao, L.; Wu, W. The roles of MADS-Box genes from root growth to maturity in Arabidopsis and rice. Agronomy 2022, 12, 582. [Google Scholar] [CrossRef]
- Figueroa, P.; Browse, J. Male sterility in Arabidopsis induced by overexpression of a MYC5-SRDX chimeric repressor. Plant J. 2015, 81, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Khouider, S.; Borges, F.; LeBlanc, C.; Ungru, A.; Schnittger, A.; Martienssen, R.; Colot, V.; Bouyer, D. Male fertility in Arabidopsis requires active DNA demethylation of genes that control pollen tube function. Nat. Commun. 2021, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. iTAK: A program for genome-wide prediction and classification of plant transcription factors, transcriptional regulators, and protein kinases. Mol. Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J.G. Profiler: A web server for functional enrichment analysis and conversions of gene lists. Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO summarizes and visualizes long lists of Gene Ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Pupilli, F.; Barcaccia, G. Cloning plants by seeds: Inheritance models and candidate genes to increase fundamental knowledge for engineering apomixis in sexual crops. J. Biotechnol. 2012, 159, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Polegri, L.; Calderini, O.; Arcioni, S.; Pupilli, F. Specific expression of apomixis-linked alleles revealed by comparative transcriptomic analysis of sexual and apomictic Paspalum simplex Morong flowers. JXB 2010, 61, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Sharbel, T.F.; Voigt, M.L.; Corral, J.M.; Galla, G.; Kumlehn, J.; Klukas, C.; Schreiber, F.; Vogel, H.; Rotter, B. Apomictic and sexual ovules of Boechera display heterochronic global gene expression patterns. Plant Cell 2010, 22, 655–671. [Google Scholar] [CrossRef]
- Pellino, M.; Hojsgaard, D.; Hörandl, E.; Sharbel, T.F. Chasing the apomictic factors in the Ranunculus auricomus complex: Exploring gene expression patterns in microdissected sexual and apomictic ovules. Genes 2020, 11, 728. [Google Scholar] [CrossRef]
- Silveira, E.D.; Alves-Ferreira, M.; Guimarães, L.A.; Silva, F.R.; Carneiro, V.T.C. Selection of reference genes for quantitative real-time PCR expression studies in the apomictic and sexual grass Brachiaria brizantha. BMC Plant Biol. 2009, 9, 84. [Google Scholar] [CrossRef]
- Simon, B.; Conner, J.A.; Ozias-Akins, P. Selection and validation of reference genes for gene expression analysis in apomictic and sexual Cenchrus ciliaris. BMC Res. Notes 2013, 6, 397. [Google Scholar] [CrossRef]
- Duc, C.; Benoit, M.; Détourné, G.; Simon, L.; Poulet, A.; Jung, M.; Veluchamy, A.; Latrasse, D.; Le Goff, S.; Cotterell, S.; et al. Arabidopsis ATRX modulates H3.3 occupancy and fine-tunes gene expression. Plant Cell 2017, 29, 1773–1793. [Google Scholar] [CrossRef]
- Ke, Y.; Podio, M.; Conner, J.; Ozias-Akins, P. Single-cell transcriptome profiling of buffelgrass (Cenchrus ciliaris) eggs unveils apomictic parthenogenesis signatures. Sci. Rep. 2021, 11, 9880. [Google Scholar] [CrossRef]
- Olmedo-Monfil, V.; Durán-Figueroa, N.; Arteaga-Vázquez, M.; Demesa-Arévalo, E.; Autran, D.; Grimanelli, D.; Slotkin, R.K.; Martienssen, R.A.; Vielle-Calzada, J.-P. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 2010, 464, 628–632. [Google Scholar] [CrossRef]
- Schmidt, A.; Schmid, M.W.; Klostermeier, U.C.; Qi, W.; Guthörl, D.; Sailer, C.; Waller, M.; Rosenstiel, P.; Grossniklaus, U. Apomictic and sexual germline development differ with respect to cell cycle, transcriptional, hormonal and epigenetic regulation. PLoS Genet. 2014, 10, e1004476. [Google Scholar] [CrossRef]
- Berg, M.; Rogers, R.; Muralla, R.; Meinke, D. Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J. 2005, 44, 866–878. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Ann. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Zemlyanskaya, E.V.; Omelyanchuk, N.A.; Ubogoeva, E.V.; Mironova, V.V. Deciphering auxin-ethylene crosstalk at a systems level. Int. J. Mol. Sci. 2018, 19, 4060. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Plackett, A.R.; Thomas, S.G.; Wilson, Z.A.; Hedden, P. Gibberellin control of stamen development: A fertile field. Trends Plant Sci. 2011, 16, 568–578. [Google Scholar] [CrossRef]
- Liu, Z.; Mara, C. Regulatory mechanisms for floral homeotic gene expression. Semin. Cell Dev. Biol. 2010, 21, 80–86. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Airoldi, C.A. Determination of sexual organ development. Sex. Plant Reprod. 2010, 23, 53–62. [Google Scholar] [CrossRef]
- Sather, D.N.; Jovanovic, M.; Golenberg, E.M. Functional analysis of B and C class floral organ genes in spinach demonstrates their role in sexual dimorphism. BMC Plant Biol. 2010, 10, 46. [Google Scholar] [CrossRef]
- Cronk, Q.; Müller, N.A. Default sex and single gene sex determination in dioecious plants. Front. Plant Sci. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.-J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Dong, X.; Hong, Z.; Sivaramakrishnan, M.; Mahfouz, M.; Verma, D.P.S. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005, 42, 315–328. [Google Scholar] [CrossRef]
- Nishikawa, S.; Zinkl, G.M.; Swanson, R.J.; Maruyama, D.; Preuss, D. Callose (beta-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005, 5, 22. [Google Scholar] [CrossRef]
- Shi, X.; Sun, X.; Zhang, Z.; Han, L.; Wu, J.; Lu, T. GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol. 2015, 56, 497–509. [Google Scholar] [CrossRef]
- Töller, A.; Brownfield, L.; Neu, C.; Twell, D.; Schulze-Lefert, P. Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J. 2008, 54, 911–923. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.Y.; Rim, Y.; Han, X.; Cho, W.K.; Kim, S.-W.; Kim, J.-Y. Arabidopsis glucan synthase-like 10 functions in male gametogenesis. J. Plant Physiol. 2009, 166, 344–352. [Google Scholar] [CrossRef]
- Fabrice, T.N.; Vogler, H.; Draeger, C.; Munglani, G.; Gupta, S.; Herger, A.G.; Knox, P.; Grossniklaus, U.; Ringli, C. LRX proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol. 2018, 176, 1981–1992. [Google Scholar] [CrossRef]
- Sede, A.R.; Borassi, C.; Wengier, D.L.; Mecchia, M.A.; Estevez, J.M.; Muschietti, J.P. Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 2018, 592, 233–243. [Google Scholar] [CrossRef]
- Charlesworth, D.; Vekemans, X.; Castric, V.; Glémin, S. Plant self-incompatibility systems: A molecular evolutionary perspective. New Phytol. 2005, 168, 61–69. [Google Scholar] [CrossRef]
- de Nettancourt, D. The basic features of self-Incompatibility. In Incompatibility and Incongruity in Wild and Cultivated Plants, 2nd ed.; Springer: Berlin, Germany, 2001; pp. 1–24. [Google Scholar] [CrossRef]
- Kusaba, M.; Dwyer, K.; Hendershot, J.; Vrebalov, J.; Nasrallah, J.B.; Nasrallah, M.E. Self-incompatibility in the genus Arabidopsis: Characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell 2001, 13, 627–643. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Rajewski, A.; He, J.; Castaneda, O.G.; Litt, A.; Kaloshian, I. Classification and phylogenetic analyses of the Arabidopsis and tomato G-type lectin receptor kinases. BMC Genom. 2018, 19, 239. [Google Scholar] [CrossRef]
- Cheng, Z.; Ge, W.; Li, L.; Hou, D.; Ma, Y.; Liu, J.; Bai, Q.; Li, X.; Mu, S.; Gao, J. Analysis of MADS-Box gene family reveals conservation in floral organ ABCDE model of Moso bamboo (Phyllostachys edulis). Front. Plant Sci. 2017, 656. [Google Scholar] [CrossRef]
- Deng, W.; Ying, H.; Helliwell, C.A.; Taylor, J.M.; Peacock, W.J.; Denis, E.S. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. PNAS 2011, 108, 6680–6685. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Weigel, D. Move on up, it’s time for change–Mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007, 21, 2371–2384. [Google Scholar] [CrossRef]
- Melzer, S.; Kampmann, G.; Chandler, J.; Apel, K. FPF1 modulates the competence to flowering in Arabidopsis. Plant J. 1999, 18, 395–405. [Google Scholar] [CrossRef]
- Koo, S.C.; Bracko, O.; Park, M.S.; Schawb, R.; Chun, H.J.; Park, K.M.; Seo, J.S.; Grbic, V.; Balasubramanian, S.; Schmid, M.; et al. Control of lateral organ development and flowering time by the Arabidopsis thaliana MADS-box Gene AGAMOUS-LIKE6. Plant J. 2010, 62, 807–816. [Google Scholar] [CrossRef]
- Guimarães, L.A.; de Dusi, D.M.A.; Masiero, S.; Resentini, F.; Gomes, A.C.M.M.; Silveira, E.D.; Florentino, L.H.; Rodrigues, J.C.M.; Colombo, L.; Carneiro, V.T. BbrizAGL6 Is differentially expressed during embryo sac formation of apomictic and sexual Brachiaria brizantha plants. Plant Mol. Biol. Rep. 2013, 31, 1397–1406. [Google Scholar] [CrossRef]
- Perry, S.E.; Lehti, M.D.; Fernandez, D.E. The MADS-domain protein AGAMOUS-like 15 accumulates in embryonic tissues with diverse origins. Plant Physiol. 1999, 120, 121–130. [Google Scholar] [CrossRef]
- Thakare, D.; Tang, W.; Hill, K.; Perry, S.E. The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol. 2008, 146, 1663–1672. [Google Scholar] [CrossRef]
- Karlova, R.; Boeren, S.; Russinova, E.; de Vries, S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes Brassinosteroid-Insensitive1. Plant Cell 2006, 18, 626–638. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Wu, Y.; Gu, A.; Zhang, J.; Luo, S.; Gao, X.; Zhao, J.; Pan, X.; Shen, S. Gene characterization and molecular pathway analysis of reverse thermosensitive genic male sterility in eggplant (Solanum melongena L.). Hortic. Res. 2019, 6, 118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).