Abstract
In this review, we discuss reports of genotype-dependent interindividual differences in phenotypic neurobehavioral responses to total sleep deprivation or sleep restriction. We highlight the importance of using the candidate gene approach to further elucidate differential resilience and vulnerability to sleep deprivation in humans, although we acknowledge that other omics techniques and genome-wide association studies can also offer insights into biomarkers of such vulnerability. Specifically, we discuss polymorphisms in adenosinergic genes (ADA and ADORA2A), core circadian clock genes (BHLHE41/DEC2 and PER3), genes related to cognitive development and functioning (BDNF and COMT), dopaminergic genes (DRD2 and DAT), and immune and clearance genes (AQP4, DQB1*0602, and TNFα) as potential genetic indicators of differential vulnerability to deficits induced by sleep loss. Additionally, we review the efficacy of several countermeasures for the neurobehavioral impairments induced by sleep loss, including banking sleep, recovery sleep, caffeine, and naps. The discovery of reliable, novel genetic markers of differential vulnerability to sleep loss has critical implications for future research involving predictors, countermeasures, and treatments in the field of sleep and circadian science.
1. Introduction
Located in the suprachiasmatic nuclei of the anterior hypothalamus, the biological clock, among other physiological processes, regulates the timing of sleep and wakefulness as well as waking behavior, creating circadian rhythmicity in neurobehavioral variables such as cognitive performance and sleepiness [1,2]. The two-process model of sleep regulation posits that a homeostatic process (Process S) and a circadian process (Process C) interact to modulate the timing of sleep onset and offset, as well as the stability of waking neurobehavioral functions [3,4,5,6,7]. Process S (the drive for sleep) increases while awake and decreases while asleep. Sleep onset occurs when the homeostatic drive increases above a certain threshold, and wakefulness is induced when it decreases below a different threshold [1]. Process C (the cycle of sleep and wakefulness) represents the daily oscillatory modulation of these thresholds and promotes wakefulness at certain times [8].
It is well established that sleep loss induces decrements in neurobehavioral functioning [4,9,10], and that there are robust, trait-like interindividual phenotypic differences related to the magnitude of such decrements, whereby some individuals are minimally affected by insufficient sleep (i.e., resilient) and others are greatly affected (i.e., vulnerable) [11,12,13,14,15,16,17,18].
This review explores the genetic underpinnings of phenotypic individual differences related to sleep deprivation, particularly in relation to differential neurobehavioral resilience and vulnerability. It also discusses the efficacy of various mitigation strategies for sleep loss-induced deficits including caffeine, naps, and recovery sleep, and examines candidate gene studies utilizing caffeine as a countermeasure. The review culminates with a discussion of future directions. Please refer to Figure 1 for a flowchart highlighting the main concepts discussed in this article and their relationships.
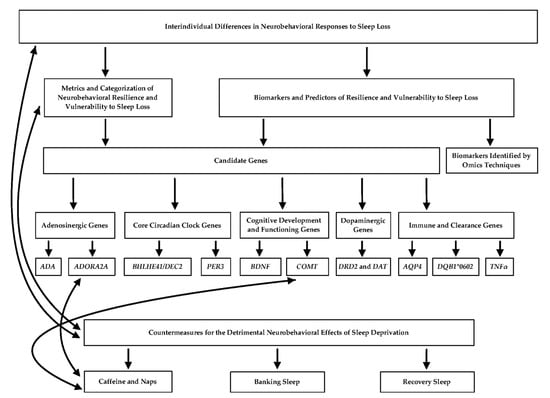
Figure 1.
Flowchart depicting the relationships between the main concepts presented in the review. Solid black arrows indicate an established connection between the topics. Connections between genes and countermeasures are associative and not causal, thus warranting the need for further investigation.
2. Interindividual Differences in Neurobehavioral Responses to Sleep Loss
2.1. Metrics and Categorization of Neurobehavioral Resilience and Vulnerability to Sleep Loss
Common metrics of neurobehavioral functioning for classifying vulnerable and resilient individuals include behavioral attention tasks such as the Psychomotor Vigilance Test (PVT) [19], cognitive throughput tasks such as the Digit Symbol Substitution Test [20], working memory tests such as n-back tasks [21] and the Digit Span Task [22], measures of self-rated sleepiness such as the Karolinska Sleepiness Scale (KSS) [23], and measures of self-rated fatigue, vigor, and mood such as those derived from the Profile of Mood States (POMS) [24]. Importantly, numerous studies have reported that an individual’s resilience or vulnerability to sleep loss when assessing performance on objective and self-rated metrics are not related [12,17,18,25,26], making the determination of reliable categorization methods more complex. The use of a variety of neurobehavioral tests when conducting individual differences research mitigates this issue and allows for better cognitive endophenotyping (accurately capturing the complete essence of a cognitive phenotype through targeted measurements) [27].
Several approaches have been used to classify individuals as resilient or vulnerable in past research, although the optimal methods to do so remain unknown. The most common prior approaches either utilized raw performance or self-rated scores on neurobehavioral tasks during sleep deprivation [15,28,29,30,31] or utilized difference scores that accounted for baseline performance [32,33,34,35,36,37,38]. Intraindividual variance, which considers time-of-day variation in performance [9,39,40,41,42,43,44], has been posited as another potential method to characterize resilience or vulnerability to sleep deprivation. However, further research is needed regarding this approach, since only one published study using two commonly used cognitive measures has explicitly investigated intraindividual variation as a categorization method [45]. Furthermore, the threshold used to divide individuals into resilient and vulnerable groups has also varied in prior research—studies have utilized a median split [28,29,30,32,35,36,46,47,48,49,50], a tertile split [15,34,38,51], a quartile split [31,52], or other numeric divisions of neurobehavioral performance [33,53]. Nevertheless, although more research is needed to determine consistent categorizations and predictors of resilience and vulnerability based on neurobehavioral performance, it remains important to explore possible biological indicators of such characteristics.
2.2. Biomarkers and Predictors of Resilience and Vulnerability to Sleep Loss
While definitive predictors of neurobehavioral resilience and vulnerability to sleep loss have yet to be discovered, genetic and omics (e.g., epigenomic, transcriptomic, metabolomic, and proteomic) techniques have identified biomarkers (objective proxies of biological processes that allow for remote detection of such processes, regardless of their mechanistic role in the assessed condition [54]) to differentiate an individual’s response to chronic sleep restriction (SR; several consecutive nights with a reduction in total sleep time) or total sleep deprivation (TSD; one or more nights without sleep) [4]. While numerous biomarkers and other factors, such as neurobehavioral performance, are considered potential predictors of differential responses to sleep loss, genetic polymorphisms (variants in DNA sequence) are perhaps one of the most studied indicators (see Figure 1).
3. Genetic Polymorphisms Related to Differential Neurobehavioral Vulnerability to Sleep Loss
Neurobehavioral vulnerability to sleep loss is a heritable and stable trait. One twin study found substantial differential neurobehavioral vulnerability to acute TSD (as measured by PVT performance), with 56.2% of the total variance in monozygotic twins, and only 14.5% of the variance in dizygotic twins, attributable to variance between pairs of twins [55]. This study [55] and other studies using unrelated participants [56] support the notion that an individual’s neurobehavioral response to sleep deprivation is a genetically determined and phenotypic trait. Additionally, although neurobehavioral performance during sleep loss is typically normally distributed [13,57] and suggests a polygenetic phenotype [4], previous candidate gene studies have found several specific genetic polymorphisms that are associated with differential neurobehavioral responses to sleep loss. The candidate gene approach is useful for determining the influence of genetic variants on neurobehavioral performance during sleep-deprived and rested conditions. Genome-wide association (GWA) studies have also revealed findings in support of trait-like interindividual differences in sleep parameters and may be useful in determining neurobehavioral vulnerability in response to sleep deprivation (reviewed in [58]). Furthermore, techniques that incorporate a perturbation of the system (e.g., enforced sleep deprivation), theory-driven genotyping, selective sampling, and cognitive endophenotyping are particularly valuable for studying differential vulnerability in relatively small samples [27]. Below, we summarize the genetic underpinnings of differential neurobehavioral responses to sleep loss that explain a portion of the related interindividual variance in resilience and vulnerability. We refer the reader to Goel [58], Yamazaki and Goel [59], Dutta [60], Bolsius et al. [61], and Garfield [62] for reviews of candidate gene and GWA studies related to sleep parameters during rested conditions, normal sleep, and circadian rhythm sleep-wake disorders, and we refer the reader to Goel [54], Mullington et al. [63], and Uyhelji et al. [64] for reports describing additional biomarker and omics techniques used in the context of sleep loss, which are beyond the scope of this article.
3.1. Adenosinergic Genes
3.1.1. ADA
The enzyme adenosine deaminase (ADA) breaks down and regulates intra- and extracellular adenosine levels. Studies have demonstrated differential neurobehavioral vulnerability to sleep deprivation associated with the ADA G22A polymorphism (single nucleotide polymorphism (SNP) ID: rs73598374). Bachmann et al. [65] found that G/A participants had worse vigilant attention, as measured by PVT lapses (>500 ms reaction time) and PVT response speed, and greater self-reported sleepiness and fatigue compared to participants with the G/G genotype [65]. However, in a large twin sample, this ADA polymorphism did not relate to differential vulnerability to TSD, as defined by PVT performance [55]. Another study [66] used a protocol consisting of two sleep conditions (either 40 h TSD or 40 h multiple naps) to investigate the influence of this ADA polymorphism on neurobehavioral measures under conditions of high versus low sleep pressure. The authors found that G/A individuals reported greater subjective sleepiness than G/G individuals only during TSD when sleep pressure was high. G/A individuals also reported worse subjective wellbeing during TSD than during the nap condition and exhibited worse working memory performance when sleep pressure was high [66]. Furthermore, at the end of the night, G/G individuals performed better on the PVT during the nap condition, when sleep pressure was low, than during TSD [66].
3.1.2. ADORA2A
The T1083C polymorphism (SNP-ID: rs5751876) in the adenosine A2A receptor (ADORA2A) gene also contributes to an individual’s neurobehavioral vulnerability to sleep loss: a recent study found that T allele carriers had worse vigilant attention performance (greater number of PVT lapses) than C/C individuals after 32 h TSD [67]. Similarly, another study showed that C/T carriers exhibited greater PVT performance resilience during chronic SR compared with T/T carriers [68]. Additionally, out of the eight distinct haplotypes created by eight SNPs in ADORA2A (SNP-IDs: rs5751862, rs5760405, rs2298383, rs3761422, rs2236624, rs5751876, rs35320474, and rs4822492), HT4 allele carriers performed better on the PVT after a night of TSD than those without the HT4 haplotype [69].
Moreover, the ADORA2A polymorphism is particularly integral to an individual’s sensitivity to caffeine, and accordingly plays a role in whether caffeine may be an especially effective mitigation strategy for maintaining neurobehavioral functioning during extended wakefulness. Caffeine is structurally similar to adenosine, thus contributing to its stimulating properties during sleep loss [70]. One study found that caffeinated coffee mitigated performance deficits on tasks of vigilant attention and executive control, but not self-rated sleepiness, induced by five consecutive nights of SR in C/C allele carriers [71]. Furthermore, caffeine counteracted 40 h TSD-induced decrements in PVT performance in non-HT4 allele carriers but was not effective in individuals with the HT4 haplotype [69], although another study showed that the ADORA2A genotype did not influence the mitigatory effect of caffeine on PVT performance during 38 h TSD [67]. In addition, although A1 adenosine receptor (A1AR) availability has been linked to the mitigatory effects of recovery sleep on neurobehavioral performance following 52 h TSD, this effect was not moderated by the ADORA2A genotype [72]. These studies have particularly important implications for determining whether mitigation strategies involving caffeine consumption may be more beneficial to certain individuals based on their ADORA2A genotype. Please see the Section 4 entitled, “Countermeasures for the Detrimental Neurobehavioral Effects of Sleep Deprivation” for further discussion of this concept (also see Figure 1).
3.2. Core Circadian Clock Genes
3.2.1. BHLHE41/DEC2
The BHLHE41/DEC2 gene is involved in the circadian regulation of sleep quantity, and also contributes to differential vulnerability to sleep loss. Variants of BHLHE41/DEC2 have been reported to be involved in responses to chronic SR and acute TSD [73]. In this study with one pair of twins, the presence of the Y362H variant (c.1084C>T; no SNP-ID available) was associated with fewer lapses on the PVT during TSD, reduced sleep duration, and less recovery sleep following TSD, though non-rapid eye movement sleep duration did not differ between the twins [73]. The Y362H variant also decreased the ability of BHLHE41/DEC2 to suppress in vitro CLOCK/BMAL1 and NPAS2/BMAL1 transactivation [73]. The differential neurobehavioral resilience to sleep deprivation related to BHLHE41/DEC2 may be a consequence of a decreased need for sleep, as the P384R mutation (SNP-ID: rs121912617) has been implicated in familial natural short sleep [74].
3.2.2. PER3
PER3, a core circadian clock gene, has a 54-nucleotide coding region variable-number tandem repeat (VNTR) (SNP-ID: rs57875989) repeating in either four or five units that is associated with differential vulnerability to sleep deprivation. TSD studies have shown that individuals with the PER35/5 genotype (five-repeat allele) had worse cognitive performance [75], poorer executive function performance selectively in the early morning [76], and greater decrements in sustained attention [77] compared to PER34/4 (four-repeat allele) individuals. However, the PER3 genotype did not differentially influence neurobehavioral vulnerability to acute TSD in twins [55] or to chronic SR in unrelated individuals [78]. In contrast to Goel et al. [78], two other studies found individual differences in neurobehavioral responses to SR related to this PER3 VNTR polymorphism [68,79].
The PER3 C7827519G SNP (SNP-ID: rs228697) has also been studied in relation to interindividual differences in responses to sleep loss. However, a study involving 38 h TSD found neither an association between PER3 genotype and neurobehavioral response to TSD, nor an interaction between PER3 genotype, TSD, and caffeine on PVT performance at any point throughout the protocol [67].
3.3. Cognitive Development and Functioning Genes
3.3.1. BDNF
The brain-derived neurotrophic factor (BDNF) gene is essential for proper neuronal development and neuronal plasticity, including as applied to neurobehavioral functioning. The BDNF Val66Met polymorphism (SNP-ID: rs6265) has been found to impact an individual’s vulnerability to sleep deprivation, as studies have shown that Met carriers performed more poorly on neurobehavioral metrics than Val/Val individuals during extended wakefulness. One study demonstrated that Met carriers had worse response inhibition performance on the Stroop Task, which assesses cognitive flexibility, than Val/Val homozygotes after 20 h of wakefulness, suggesting differential impairment related to this BDNF polymorphism [80]. Similarly, Met carriers also performed more poorly than Val/Val homozygotes on a verbal two-back working memory task after 40 h of wakefulness [81]. However, this BDNF genotype did not differentially impact sustained attention, or self-rated sleepiness or wellbeing [81]. In contrast, a study involving simulated night shift schedules demonstrated that the Val/Met BDNF genotype may allow for greater flexibility to adapt to circadian misalignment, since heterozygotes showed fewer lapses on the PVT toward the end of the night shifts in the second simulation bout as compared to the first bout; Val/Val homozygotes did not show a performance difference between night shift bouts [82]. Taken together, these findings suggest possible genotypically regulated differences in BDNF protein expression during sleep loss, which also may be important for determining neurobehavioral resilience and vulnerability [83].
3.3.2. COMT
The catechol-O-methyltransferase gene (COMT) encodes the COMT protein, which is responsible for breaking down catecholamines including epinephrine, norepinephrine, and dopamine. The functional COMT Val158Met polymorphism (SNP-ID: rs4680) has been found to be associated with neurobehavioral responses to sleep deprivation. After a night of TSD, individuals with the Val allele demonstrated poorer performance on tasks of behavioral attention [84] and adaptive decision making [85] than Met carriers. During chronic SR, however, this COMT polymorphism did not differentially impact cognitive performance or subjective or physiological sleepiness [86].
Additionally, this COMT polymorphism was shown to regulate the effect of modafinil, a pharmacological stimulant, on behavioral attention, self-reported wellbeing, and executive functioning, yet was not associated with subjective sleepiness [87,88]. A recent study also investigated the potential effect of this COMT polymorphism on performance impairment during TSD both independent from, and related to, the mitigatory effect of caffeine. While no main effect of COMT genotype was observed on PVT performance or KSS scores overall, the study found that, after 20 h TSD, but not after 26 h or 32 h TSD, Met allele carriers had more PVT lapses than Val/Val individuals in the placebo condition [67]. Notably, this genotypic performance difference was not observed when participants were provided caffeine, suggesting its beneficial impact [67]. Please see the Section 4 entitled, “Countermeasures for the Detrimental Neurobehavioral Effects of Sleep Deprivation” for more details about the potential link between genetic polymorphisms and mitigation strategies (also see Figure 1).
3.4. Dopaminergic Genes
DRD2 and DAT
The dopamine D2 receptor gene (DRD2) C957T polymorphism (SNP-ID: rs6277) and the dopamine transporter gene (DAT1) 3′-UTR VNTR polymorphism (SNP-ID: rs28363170) have also been implicated in differential cognitive vulnerability to sleep deprivation, both separately and in combination with each other. During 38 h TSD, DRD2 C/C individuals were particularly resilient to the effects of sleep loss on cognitive flexibility, whereas T/T individuals were particularly vulnerable; however, no genotypic influence was found on PVT performance resilience [89]. Another TSD study showed that DRD2 T/T homozygotes demonstrated greater declines in performance with increased time spent performing the PVT than C allele carriers, suggesting that the DRD2 genotype predicts the magnitude of this time-on-task (TOT) effect [90]. Similarly, the DAT1 genotype was also found to modulate the TOT effect on the PVT during TSD, as 10-repeat allele (10R) homozygotes showed less severe TOT deficits compared to nine-repeat allele (9R) carriers [91]. Additionally, when examining the combination of these two polymorphisms, individuals with the DAT1-DRD2 10R/10R-C/T or 9R-C/C genotypes showed greater PVT performance resilience from TSD-induced decrements than individuals with other genotype combinations [92]. DAT1-DRD2 9R-C/C individuals were also most resistant to self-reported sleepiness [92]. Collectively, these studies exemplify the influence of multiple different genetic polymorphisms on individual responses to sleep deprivation, as well as suggest a modulatory effect of dopaminergic pathways on some neurobehavioral responses to sleep loss.
3.5. Immune and Clearance Genes
3.5.1. AQP4
Aquaporin 4 (AQP4) is an astrocytic water channel that facilitates the flow of cerebrospinal fluid (CSF) throughout the brain. The AQP4 gene has several SNPs that modulate the expression of AQP4, and which were recently associated with differential responses to 40 h TSD (SNP-IDs: rs162007, rs162008, rs63514, rs455671, rs335931, rs335930, rs335929, and rs16942851). Ulv Larsen et al. [93] reported that individuals with the low-AQP4-expressing HtMi variant haplotype (carriers of the minor allele) had less of a reduction in PVT response speed and less of an increase in self-rated sleepiness than individuals with the HtMa haplotype (carriers of the major allele) during prolonged wakefulness, suggesting that HtMi individuals may be more neurobehaviorally resilient to sleep deprivation [93]. These findings are particularly important for the relationship between CSF flow and brain clearance since these are essential for protecting against the development of neurodegenerative diseases.
3.5.2. DQB1*0602
The human leukocyte antigen DQB1*0602 allele, which relates to narcolepsy [94,95], has been investigated in relation to differential vulnerability to sleep loss. During five consecutive SR nights, DQB1*0602-positive individuals reported greater subjective sleepiness than DQB1*0602-negative individuals [96]. Similarly, DQB1*0602-positive individuals reported greater self-rated sleepiness and fatigue during baseline [96]. Additionally, during SR, DQB1*0602-positive individuals exhibited differentially greater increases in subjective fatigue [96]. Notably, carrying the DQB1*0602 allele did not differentially influence cumulative decrements in neurobehavioral performance induced by SR, as total decreases in cognitive performance and increases in physiological sleepiness were comparable between both positive and negative groups [96].
3.5.3. TNFα
The tumor necrosis factor alpha gene (TNFα) encodes TNFα, a proinflammatory cytokine, and has a G308A polymorphism (SNP-ID: rs1800629) in its promoter region. This polymorphism has been associated with differential resilience to sleep-loss-induced deficits on the PVT, as A allele carriers demonstrated greater performance resilience during TSD [97,98]. Additionally, one study demonstrated a greater sensitivity of TNFα A allele carriers to the effects of caffeine than G/G carriers during TSD; however, this effect may be related to greater TSD performance degradation in A allele carriers since PVT lapses did not differ as a function of genotype [67]. However, Skeiky et al. [98] did not find an interaction of TNFα genotype and caffeine (either 200 or 300 mg doses) on PVT performance during 48 h TSD, despite demonstrating a genetic influence on PVT performance variance alone.
3.6. Strengths and Weaknesses of the Candidate Gene Approach
The candidate gene approach for studying differential vulnerability to sleep deprivation, largely driven by the existence of phenotypic individual differences in neurobehavioral responses to sleep loss, has been commonly used to investigate the influence of genetic variants on such trait-like responses [4]. This approach is useful for investigating the association between specific genetic polymorphisms and phenotypic responses to sleep loss, particularly in a laboratory setting with relatively small sample sizes. Although the candidate gene approach is useful, no published studies have used this approach to explicitly determine causality rather than associations between genotype and phenotype. Nevertheless, previous work has demonstrated that the influence of some of the aforementioned SNPs, individually or in combination, may explain a substantial portion of the variance in neurobehavioral responses to sleep loss (reviewed in Satterfield et al. [27]). Thus, it is important to continue using the candidate gene approach to examine the contribution of specific genes to differential neurobehavioral vulnerability.
4. Countermeasures for the Detrimental Neurobehavioral Effects of Sleep Deprivation
The neurobehavioral and physiological effects of sleep loss are detrimental yet often are undetected by sleep-deprived individuals. Although the optimal way to protect against poorer neurobehavioral performance and adverse health outcomes that are associated with sleep deprivation is to consistently obtain sufficient sleep aligned with an individual’s circadian rhythms [99], societal and work demands often make this difficult to achieve. This is especially true for populations such as shift workers, students pulling “all-nighters”, on-call medical personnel, transmeridian travelers, and individuals whose jobs require extended wakefulness [9]. Sleep deprivation also directly impacts driving and accident risk—sleepiness-related crashes exhibit similar injury and fatality rates as alcohol-related crashes [100,101,102], though they are often underestimated [9,103,104,105,106]. Thus, mitigation strategies to combat the severity of such negative effects, including banking sleep, recovery sleep, caffeine, and naps, are critical. It is particularly important to investigate the efficacy of countermeasures in relation to the aforementioned candidate genes, as doing so may offer more definitive recommendations as to which individuals would benefit most from certain mitigation strategies based on their genetic make-up (see Figure 1).
4.1. Effects of Banking Sleep on Neurobehavioral Performance
Banking sleep—increasing sleep duration to 8–9 h per night for several consecutive nights—has been demonstrated to mitigate neurobehavioral decrements resulting from subsequent sleep loss, including diminished performance on sustained attention tasks [107,108] and high-cognitive-load decision tasks [109]. Banking sleep also has been reported to effectively manage fatigue, stress, and excessive daytime sleepiness [110,111], which may be useful in applied settings such as military operations [112] and shift work [113]. The beneficial effects of banking sleep have been found to persist during a recovery sleep opportunity following sleep deprivation [107,108]. Though promising, further research is needed to establish whether this strategy can reliably maintain neurobehavioral performance during sleep-deprived conditions and subsequent recovery, especially in relation to interindividual phenotypic and genotypic differences. Further research is also necessary to determine whether the utility of banking sleep may be greater for certain individuals; for example, although not yet examined, the BHLHE41/DEC2 polymorphism has been implicated in short sleep [74], which suggests that increasing sleep through banking may not be as effective for individuals with the P384R mutation.
4.2. Effects of Recovery Sleep on Neurobehavioral Performance
Recovery sleep—increased nightly sleep opportunity following a period of sleep deprivation—has also been proposed as a mitigation strategy to facilitate the restoration of several neurobehavioral measures after sleep loss. Some studies have shown that recovery sleep improved cognitive performance, reduced sleepiness, fatigue, and sleep propensity, increased alertness, and improved mood [114,115,116,117]. However, other studies have found that recovery sleep failed to completely reverse sleep deprivation-induced performance impairments on vigilance and working memory tasks, worsened inhibition as defined by a pinball task, and decreased self-rated vigor as defined by the POMS [10,117,118]. While reliable biomarkers of response to recovery sleep have yet to be discovered, interindividual differences may account for some of the discrepancies in research related to recovery sleep, since differential vulnerability could impact the amount of recovery sleep needed for certain aspects of neurobehavioral functioning to return to baseline levels [117]. Thus, it is important to further investigate biomarkers and genetic polymorphisms that may underlie differences in the effectiveness of recovery sleep.
4.3. Effects of Caffeine and Napping on Neurobehavioral Performance
The efficacy of caffeine in attenuating neurobehavioral performance deficits induced by sleep loss has been well established [119,120,121,122,123,124]. Acute caffeine consumption (using doses from <80 mg to 600 mg) has been shown to mitigate performance declines in sleep-deprived individuals in a variety of domains, including on attention, memory, information processing, executive functioning, and driving tasks (reviewed in [125]). As aforementioned, the efficacy of caffeine has also been linked to the ADORA2A [69,71] and COMT [67] genotypes, thus, further evincing caffeine’s biological utility. Notably, caffeine becomes less effective at preventing performance declines as the pressure for sleep increases during extended wakefulness [4]. In addition, robust individual differences in response to both sleep deprivation and caffeine confound the effectiveness of this mitigation strategy [4,123,124].
Napping during the day is another effective countermeasure to prevent performance declines in conditions of increased sleepiness and decreased alertness [126]. Although naps are beneficial, rest opportunities are typically followed by sleep inertia (a period of grogginess and diminished performance) upon awakening [127]. This has traditionally been thought to be especially true after longer naps during which slow-wave sleep is reached, though recent reports showed mixed findings [128]. Since naps alone are also unable to prevent the negative effects of sleep deprivation under all conditions [4,123,127] and across all neurobehavioral domains [129,130], the combination of caffeine consumption and a short nap may provide maximum protection against sleep-loss-induced decrements [127,131].
5. Conclusions and Perspectives
Adequate sleep is a biological imperative, essential for maintaining waking neurobehavioral performance, though it is often difficult to achieve. Thus, determining reliable predictors of differential vulnerability to sleep loss is crucial, given that diminished neurobehavioral functioning may negatively impact productivity and performance in a variety of real-world settings. As discussed, past research has identified several candidate genes and genetic polymorphisms related to circadian factors, neurotransmitter transmission, and immune and cognitive functioning, among others, which are associated with neurobehavioral resilience and vulnerability to sleep deprivation [67,80,82,84,85,89,90,91,93,98] (also see review [4]), and which are important components of individual differences research. Notably, some studies have also identified genetic polymorphisms involved in the efficacy of specific countermeasures used for sleep loss-induced deficits such as caffeine [67,69,71,98] (also see review [4]), suggesting that particular countermeasures may be more effective for certain individuals based on their own genetic profile. Importantly, establishing causality between specific genes and mitigation strategies through the candidate gene approach will enable the implementation of more individualized approaches for countering sleep loss-induced deficits, which is especially important for maximizing neurobehavioral functioning in applied settings.
It also is important to investigate the genetic determinants of resilience and vulnerability in diverse demographic and clinical populations. Studies have reported the influence of ethnicity and/or race on sleep characteristics [132,133,134,135], which are likely impacted by genetic ancestry and social and environmental pressures. Additionally, although associations between various candidate genes (e.g., ADA, ADORA2A, PER3) and clinical and/or sub-clinical symptomology and conditions have been shown [136,137,138,139,140], genotypic relationships between such symptomology and neurobehavioral performance have not been directly examined in the context of sleep loss. Interindividual differences in self-rated personality traits have also been proposed as factors contributing to differences in sleep characteristics [141,142] and to differential vulnerability to sleep loss [143]; however, the polygenetic and complex nature of personality makes it difficult to conduct genetic studies exploring this relationship. Further research on the genetic underpinnings of neurobehavioral responses to sleep deprivation is necessary to create a more generalizable framework of resilience and vulnerability to sleep loss-induced decrements. Overall, investigating such topics will lead to the development of personalized countermeasures and treatments based on an individual’s genetic and neurobehavioral performance profiles, which is critical for optimizing functioning in applied settings involving extended wakefulness.
Author Contributions
Writing—original draft preparation, C.E.C. and N.G.; writing—review and editing, C.E.C. and N.G.; funding acquisition, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by National Aeronautics and Space Administration (NASA) grant NNX14AN49G and grant 80NSSC20K0243 (to N.G.), and National Institutes of Health grant NIH R01DK117488 (to N.G.). The APC was funded by a research account from Rush University Medical Center (to N.G.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the following: preparation, review, or approval of the manuscript.
References
- Goel, N.; Basner, M.; Dinges, D.F. Phenotyping of neurobehavioral vulnerability to circadian phase during sleep loss. Methods Enzymol. 2015, 552, 285–308. [Google Scholar] [CrossRef]
- Achermann, P.; Dijk, D.J.; Brunner, D.P.; Borbély, A.A. A model of human sleep homeostasis based on EEG slow-wave activity; quantitative comparison of data and simulations. Brain Res. Bull. 1993, 31, 97–113. [Google Scholar] [CrossRef]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Goel, N. Neurobehavioral effects and biomarkers of sleep loss in healthy adults. Curr. Neurol. Neurosci. Rep. 2017, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Basner, M.; Rao, H.; Dinges, D.F. Circadian rhythms, sleep deprivation, and human performance. Prog. Mol. Biol. Transl. Sci. 2013, 119, 155–190. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.B.S.; Jewett, M.E.; Duffy, J.F.; Czeisler, C.A. The timing of the human circadian clock is accurately represented by the core body temperature rhythm following phase shifts to a three-cycle light stimulus near the critical zone. J. Biol. Rhythms 2000, 15, 524–530. [Google Scholar] [CrossRef]
- Van Dongen, H.P.A.; Dinges, D.F. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioral performance. J. Sleep Res. 2003, 12, 181–187. [Google Scholar] [CrossRef]
- Edgar, D.M.; Dement, W.C.; Fuller, C.A. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J. Neurosci. 1993, 13, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Rao, H.; Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Belenky, G.; Wesensten, N.J.; Thorne, D.R.; Thomas, M.L.; Sing, H.C.; Redmond, D.P.; Russo, M.B.; Balkin, T.J. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J. Sleep Res. 2003, 12, 1–12. [Google Scholar] [CrossRef]
- Dijkman, M.; Sachs, N.; Levine, E.; Mallis, M.; Carlin, M.M.; Gillen, K.A.; Powell, J.W.; Samuel, S.; Mullington, J.; Rosekind, M.R.; et al. Effects of reduced stimulation on neurobehavioral alertness depend on circadian phase during human sleep deprivation. Sleep Res. 1997, 26, 265. [Google Scholar]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, H.P.; Baynard, M.D.; Maislin, G.; Dinges, D.F. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep 2004, 27, 423–433. [Google Scholar]
- Van Dongen, H.P.; Belenky, G. Individual differences in vulnerability to sleep loss in the work environment. Ind. Health 2009, 47, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.C.; Yeo, S.C.; Lee, I.T.; Tan, L.C.; Lau, P.; Cai, S.; Zhang, X.; Puvanendran, K.; Gooley, J.J. Sustained attention performance during sleep deprivation associates with instability in behavior and physiologic measures at baseline. Sleep 2014, 37, 27–39. [Google Scholar] [CrossRef]
- Rupp, T.L.; Wesensten, N.J.; Balkin, T.J. Trait-like vulnerability to total and partial sleep loss. Sleep 2012, 35, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.E.; Wohl, R.J.; Selame, L.A.; Goel, N. Healthy adults display long-term trait-like neurobehavioral resilience and vulnerability to sleep loss. Sci. Rep. 2017, 7, 14889. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.M.; Goel, N. Robust stability of trait-like vulnerability or resilience to common types of sleep deprivation in a large sample of adults. Sleep 2021, 43, zsz292. [Google Scholar] [CrossRef]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Wechsler, D. The Measurement of Adult Intelligence; The Williams and Wilkins Company: Baltimore, MD, USA, 1939. [Google Scholar]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised; The Psychological Corporation: San Antonio, TX, USA, 1981. [Google Scholar]
- Åkerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual: Profile of Mood States; EdITS: San Diego, CA, USA, 1971. [Google Scholar]
- Leproult, R.; Colecchia, E.F.; Berardi, A.M.; Stickgold, R.; Kosslyn, S.M.; Van Cauter, E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R280–R290. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.; Dinges, D.F. Interindividual variability in neurobehavioral response to sleep loss: A comprehensive review. Neurosci. Biobehav. Rev. 2018, 89, 29–48. [Google Scholar] [CrossRef]
- Satterfield, B.C.; Stucky, B.; Landolt, H.P.; Van Dongen, H.P.A. Unraveling the genetic underpinnings of sleep deprivation-induced impairments in human cognition. Prog. Brain Res. 2019, 246, 127–158. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.L.; Schroeder, V.M.; Kunkle, C.L.; Stephenson, H.G. Differential effects of modafinil on performance of low-performing and high-performing individuals during total sleep deprivation. Pharmacol. Biochem. Behav. 2020, 196, 172968. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; von Scheven, G.; Feiveson, A.; Bürkle, A.; Wu, H.; Goel, N. The degree of radiation-induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep 2018, 41, zsy067. [Google Scholar] [CrossRef] [PubMed]
- Patanaik, A.; Kwoh, C.K.; Chua, E.C.; Gooley, J.J.; Chee, M.W. Classifying vulnerability to sleep deprivation using baseline measures of psychomotor vigilance. Sleep 2015, 38, 723–734. [Google Scholar] [CrossRef]
- Chua, E.C.; Sullivan, J.P.; Duffy, J.F.; Klerman, E.B.; Lockley, S.W.; Kristal, B.S.; Czeisler, C.A.; Gooley, J.J. Classifying attentional vulnerability to total sleep deprivation using baseline features of Psychomotor Vigilance Test performance. Sci. Rep. 2019, 9, 12102. [Google Scholar] [CrossRef]
- Chee, M.W.; Tan, J.C. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. Neuroimage 2010, 51, 835–843. [Google Scholar] [CrossRef]
- Chee, M.W.; Chuah, L.Y.; Venkatraman, V.; Chan, W.Y.; Philip, P.; Dinges, D.F. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage 2006, 31, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Asplund, C.L.; Ling, A.; Chee, M.W. Increased automaticity and altered temporal preparation following sleep deprivation. Sleep 2015, 38, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.Y.; Chong, D.L.; Chen, A.K.; Rekshan, W.R., III; Tan, J.C.; Zheng, H.; Chee, M.W. Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. Sleep 2009, 32, 999–1010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.; Zhu, Y.; Fu, C.; Sun, J.; Li, H.; Yang, X.; Li, W.; Qin, W.; Shi, D.; Tian, J. Frontal metabolic activity contributes to individual differences in vulnerability toward total sleep deprivation-induced changes in cognitive function. J. Sleep Res. 2016, 25, 169–180. [Google Scholar] [CrossRef]
- Riontino, L.; Cavallero, C. Individual differences in working memory efficiency modulate proactive interference after sleep deprivation. Psychol. Res. 2021, 85, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Patanaik, A.; Zagorodnov, V.; Kwoh, C.K.; Chee, M.W.L. Predicting vulnerability to sleep deprivation using diffusion model parameters. J. Sleep. Res. 2014, 23, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep. Med. 2007, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.M.; Van Dongen, H.P.; Dinges, D.F. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch. Ital. Biol. 2001, 139, 253–267. [Google Scholar]
- Schmidt, C.; Collette, F.; Cajochen, C.; Peigneux, P. A time to think: Circadian rhythms in human cognition. Cogn. Neuropsychol. 2007, 24, 755–789. [Google Scholar] [CrossRef] [PubMed]
- Blatter, K.; Cajochen, C. Circadian rhythms in cognitive performance: Methodological constraints, protocols, theoretical underpinnings. Physiol. Behav. 2007, 90, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Rétey, J.V.; Khatami, R.; Landolt, H.P. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep 2006, 29, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Rabbitt, P.; Osman, P.; Moore, B.; Stollery, B. There are stable individual differences in performance variability, both from moment to moment and from day to day. Q. J. Exp. Psychol. A 2001, 54, 981–1003. [Google Scholar] [CrossRef] [PubMed]
- Brieva, T.E.; Casale, C.E.; Yamazaki, E.M.; Antler, C.A.; Goel, N. Cognitive throughput and working memory raw scores consistently differentiate resilient and vulnerable groups to sleep loss. Sleep 2021, zsab197. [Google Scholar] [CrossRef]
- Michael, L.; Passmann, S.; Becker, R. Electrodermal lability as an indicator for subjective sleepiness during total sleep deprivation. J. Sleep Res. 2012, 21, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Salfi, F.; Lauriola, M.; Tempesta, D.; Calanna, P.; Socci, V.; De Gennaro, L.; Ferrara, M. Effects of total and partial sleep deprivation on reflection impulsivity and risk-taking in deliberative decision-making. Nat. Sci. Sleep 2020, 12, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Rocklage, M.; Williams, V.; Pacheco, J.; Schnyer, D.M. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep 2009, 32, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.T.; Tandi, J.; Chee, M.W.L. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 2015, 111, 147–158. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J.; Wagner, U. Sleep enhances false memories depending on general memory performance. Behav. Brain Res. 2010, 208, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Galli, O. Predictors of Interindividual Differences in Vulnerability to Neurobehavioral Consequences of Chronic Partial Sleep Restriction. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2020. [Google Scholar]
- St Hilaire, M.A.; Kristal, B.S.; Rahman, S.A.; Sullivan, J.P.; Quackenbush, J.; Duffy, J.F.; Barger, L.K.; Gooley, J.J.; Czeisler, C.A.; Lockley, S.W. Using a single daytime performance test to identify most individuals at high-risk for performance impairment during extended wake. Sci. Rep. 2019, 9, 16681. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.J.; Badia, P.; Wright, K.P., Jr. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J. Sleep Res. 2004, 13, 305–315. [Google Scholar] [CrossRef]
- Goel, N. “Omics” approaches for sleep and circadian rhythm research: Biomarkers for identifying differential vulnerability to sleep loss. Curr. Sleep Med. Rep. 2015, 1, 38–46. [Google Scholar] [CrossRef][Green Version]
- Kuna, S.T.; Maislin, G.; Pack, F.M.; Staley, B.; Hachadoorian, R.; Coccaro, E.F.; Pack, A.I. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep 2012, 35, 1223–1233. [Google Scholar] [CrossRef]
- Goel, N.; Dinges, D.F. Sleep deprivation: Biomarkers for identifying and predicting individual differences in response to sleep loss. In Sleepiness: Causes, Consequences and Treatment; Thorpy, M.J., Billiard, M., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 101–110. [Google Scholar]
- Van Dongen, H.P.; Maislin, G.; Dinges, D.F. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: Importance and techniques. Aviat. Space Environ. Med. 2004, 75, A147–A154. [Google Scholar]
- Goel, N. Genetic markers of sleep and sleepiness. Sleep Med. Clin. 2017, 12, 289–299. [Google Scholar] [CrossRef]
- Yamazaki, E.M.; Goel, N. Genetics of circadian and sleep measures in adults: Implications for sleep medicine. Curr. Sleep Med. Rep. 2020, 6, 32–45. [Google Scholar] [CrossRef]
- Dutta, R. Do genes matter in sleep?—A comprehensive update. J. Neurosci. Neurol. Disord. 2020, 4, 014–023. [Google Scholar] [CrossRef]
- Bolsius, Y.G.; Zurbriggen, M.D.; Kim, J.K.; Kas, M.J.; Meerlo, P.; Aton, S.J.; Havekes, R. The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 2021, 191, 114493. [Google Scholar] [CrossRef] [PubMed]
- Garfield, V. Sleep duration: A review of genome-wide association studies (GWAS) in adults from 2007 to 2020. Sleep Med. Rev. 2021, 56, 101413. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Abbott, S.M.; Carroll, J.E.; Davis, C.J.; Dijk, D.J.; Dinges, D.F.; Gehrman, P.R.; Ginsburg, G.S.; Gozal, D.; Haack, M.; et al. Developing biomarker arrays predicting sleep and circadian-coupled risks to health. Sleep 2016, 39, 727–736. [Google Scholar] [CrossRef]
- Uyhelji, H.A.; Kupfer, D.M.; White, V.L.; Jackson, M.L.; Van Dongen, H.P.A.; Burian, D.M. Exploring gene expression biomarker candidates for neurobehavioral impairment from total sleep deprivation. BMC Genom. 2018, 19, 341. [Google Scholar] [CrossRef]
- Bachmann, V.; Klaus, F.; Bodenmann, S.; Schäfer, N.; Brugger, P.; Huber, S.; Berger, W.; Landolt, H.P. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb. Cortex 2012, 22, 962–970. [Google Scholar] [CrossRef]
- Reichert, C.F.; Maire, M.; Gabel, V.; Viola, A.U.; Kolodyazhniy, V.; Strobel, W.; Götz, T.; Bachmann, V.; Landolt, H.P.; Cajochen, C.; et al. Insights into behavioral vulnerability to differential sleep pressure and circadian phase from a functional ADA polymorphism. J. Biol. Rhythms 2014, 29, 119–130. [Google Scholar] [CrossRef]
- Erblang, M.; Sauvet, F.; Drogou, C.; Quiquempoix, M.; Van Beers, P.; Guillard, M.; Rabat, A.; Trignol, A.; Bourrilhon, C.; Erkel, M.C.; et al. Genetic determinants of neurobehavioral responses to caffeine administration during sleep deprivation: A randomized, cross over study (NCT03859882). Genes 2021, 12, 555. [Google Scholar] [CrossRef]
- Rupp, T.L.; Wesensten, N.J.; Newman, R.; Balkin, T.J. PER3 and ADORA2A polymorphisms impact neurobehavioral performance during sleep restriction. J. Sleep Res. 2013, 22, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Bodenmann, S.; Hohoff, C.; Freitag, C.; Deckert, J.; Rétey, J.V.; Bachmann, V.; Landolt, H.P. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br. J. Pharmacol. 2012, 165, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.M.; Lange, D.; Elmenhorst, E.M.; Elmenhorst, D.; Bauer, A.; Aeschbach, D.; Landolt, H.P. Coffee effectively attenuates impaired attention in ADORA2A C/C-allele carriers during chronic sleep restriction. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110232. [Google Scholar] [CrossRef]
- Elmenhorst, D.; Elmenhorst, E.M.; Hennecke, E.; Kroll, T.; Matusch, A.; Aeschbach, D.; Bauer, A. Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain. Proc. Natl. Acad. Sci. USA 2017, 114, 4243–4248. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Kavakli, I.H.; Goel, N.; Cardinale, C.J.; Dinges, D.F.; Kuna, S.T.; Maislin, G.; Van Dongen, H.P.; Tufik, S.; Hogenesch, J.B.; et al. A novel BHLHE41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep 2014, 37, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.M.; Fu, Y.H. Recent advances in sleep genetics. Curr. Opin. Neurobiol. 2021, 69, 19–24. [Google Scholar] [CrossRef]
- Viola, A.U.; Archer, S.N.; James, L.M.; Groeger, J.A.; Lo, J.C.; Skene, D.J.; von Schantz, M.; Dijk, D.J. PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 2007, 17, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Groeger, J.A.; Viola, A.U.; Lo, J.C.; von Schantz, M.; Archer, S.N.; Dijk, D.J. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep 2008, 31, 1159–1167. [Google Scholar] [PubMed]
- Maire, M.; Reichert, C.F.; Gabel, V.; Viola, A.U.; Strobel, W.; Krebs, J.; Landolt, H.P.; Bachmann, V.; Cajochen, C.; Schmidt, C. Sleep ability mediates individual differences in the vulnerability to sleep loss: Evidence from a PER3 polymorphism. Cortex 2014, 52, 47–59. [Google Scholar] [CrossRef]
- Goel, N.; Banks, S.; Mignot, E.; Dinges, D.F. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS ONE 2009, 4, e5874. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Groeger, J.A.; Santhi, N.; Arbon, E.L.; Lazar, A.S.; Hasan, S.; von Schantz, M.; Archer, S.N.; Dijk, D.J. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS ONE 2012, 7, e45987. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.K.; Cain, S.W.; Chang, A.M.; Saxena, R.; Czeisler, C.A.; Anderson, C. Impaired cognitive flexibility during sleep deprivation among carriers of the Brain Derived Neurotrophic Factor (BDNF) Val66Met allele. Behav. Brain Res. 2018, 338, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, V.; Klein, C.; Bodenmann, S.; Schäfer, N.; Berger, W.; Brugger, P.; Landolt, H.P. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 2012, 35, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Savenkova, M.I.; Karatsoreos, I.N.; Jackson, M.L.; Belenky, G.; Van Dongen, H.P.A. Interleukin-6 (IL-6) response to a simulated night-shift schedule is modulated by brain-derived neurotrophic factor (BDNF) genotype. Chronobiol. Int. 2020, 37, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.A.; Wellman, L.L.; Sanford, L.D. Progressive increase in the complexity and translatability of rodent testing to assess space-radiation induced cognitive impairment. Neurosci. Biobehav. Rev. 2021, 126, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Valomon, A.; Holst, S.C.; Borrello, A.; Weigend, S.; Müller, T.; Berger, W.; Sommerauer, M.; Baumann, C.R.; Landolt, H.P. Effects of COMT genotype and tolcapone on lapses of sustained attention after sleep deprivation in healthy young men. Neuropsychopharmacology 2018, 43, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Hinson, J.M.; Whitney, P.; Schmidt, M.A.; Wisor, J.P.; Van Dongen, H.P.A. Catechol-O-methyltransferase (COMT) genotype affects cognitive control during total sleep deprivation. Cortex 2018, 99, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Banks, S.; Lin, L.; Mignot, E.; Dinges, D.F. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS ONE 2011, 6, e29283. [Google Scholar] [CrossRef] [PubMed]
- Bodenmann, S.; Xu, S.; Luhmann, U.F.; Arand, M.; Berger, W.; Jung, H.H.; Landolt, H.P. Pharmacogenetics of modafinil after sleep loss: Catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin. Pharmacol. Ther. 2009, 85, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Bodenmann, S.; Landolt, H.P. Effects of modafinil on the sleep EEG depend on Val158Met genotype of COMT. Sleep 2010, 33, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Whitney, P.; Hinson, J.M.; Satterfield, B.C.; Grant, D.A.; Honn, K.A.; Van Dongen, H.P.A. Sleep deprivation diminishes attentional control effectiveness and impairs flexible adaptation to changing conditions. Sci. Rep. 2017, 7, 16020. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.A.; Van Dongen, H.P.A.; Schmidt, M.A.; Wisor, J.P.; Layton, M.E.; DePriest, D.M.; Honn, K.A.; Satterfield, B.C. DRD2 C957T genotype modulates the time-on-task effect during total sleep deprivation. Chronobiol. Int. 2020, 37, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Wisor, J.P.; Schmidt, M.A.; Van Dongen, H.P.A. Time-on-task effect during sleep deprivation in healthy young adults is modulated by dopamine transporter genotype. Sleep 2017, 40, zsx167. [Google Scholar] [CrossRef]
- Holst, S.C.; Müller, T.; Valomon, A.; Seebauer, B.; Berger, W.; Landolt, H.P. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci. Rep. 2017, 7, 45982. [Google Scholar] [CrossRef]
- Ulv Larsen, S.M.; Landolt, H.P.; Berger, W.; Nedergaard, M.; Knudsen, G.M.; Holst, S.C. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 2020, 18, e3000623. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Young, T.; Lin, L.; Finn, L. Nocturnal sleep and daytime sleepiness in normal subjects with HLA-DQB1*0602. Sleep 1999, 22, 347–352. [Google Scholar]
- Dauvilliers, Y.; Tafti, M. Molecular genetics and treatment of narcolepsy. Ann. Med. 2006, 38, 252–262. [Google Scholar] [CrossRef]
- Goel, N.; Banks, S.; Mignot, E.; Dinges, D.F. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology 2010, 75, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Wisor, J.P.; Field, S.A.; Schmidt, M.A.; Van Dongen, H.P.A. TNFα G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behav. Immun. 2015, 47, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Skeiky, L.; Brager, A.J.; Satterfield, B.C.; Petrovick, M.; Balkin, T.J.; Capaldi, V.F.; Ratcliffe, R.H.; Van Dongen, H.P.A.; Hansen, D.A. TNFα G308A genotype, resilience to sleep deprivation, and the effect of caffeine on psychomotor vigilance performance in a randomized, double-blind, placebo-controlled, crossover study. Chronobiol. Int. 2020, 37, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Dawson, D.; Reid, K. Fatigue, alcohol and performance impairment. Nature 1997, 388, 235. [Google Scholar] [CrossRef]
- Fairclough, S.H.; Graham, R. Impairment of driving performance caused by sleep deprivation or alcohol: A comparative study. Hum. Factors 1999, 41, 118–128. [Google Scholar] [CrossRef]
- Williamson, A.M.; Feyer, A.M. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup. Environ. Med. 2000, 57, 649–655. [Google Scholar] [CrossRef]
- Connor, J.; Whitlock, G.; Norton, R.; Jackson, R. The role of driver sleepiness in car crashes: A systematic review of epidemiological studies. Accid. Anal. Prev. 2001, 33, 31–41. [Google Scholar] [CrossRef]
- Horne, J.; Reyner, L. Vehicle accidents related to sleep: A review. Occup. Environ. Med. 1999, 56, 289–294. [Google Scholar] [CrossRef]
- McCartt, A.T.; Ribner, S.A.; Pack, A.I.; Hammer, M.C. The scope and nature of the drowsy driving problem in New York State. Accid. Anal. Prev. 1996, 28, 511–517. [Google Scholar] [CrossRef]
- Philip, P.; Taillard, J.; Micoulaud-Franchi, J.A. Sleep restriction, sleep hygiene, and driving safety: The importance of situational sleepiness. Sleep Med. Clin. 2019, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Arnal, P.J.; Sauvet, F.; Leger, D.; van Beers, P.; Bayon, V.; Bougard, C.; Rabat, A.; Millet, G.Y.; Chennaoui, M. Benefits of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and recovery. Sleep 2015, 38, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.L.; Wesensten, N.J.; Bliese, P.D.; Balkin, T.J. Banking sleep: Realization of benefits during subsequent sleep restriction and recovery. Sleep 2009, 32, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Alger, S.E.; Brager, A.J.; Balkin, T.J.; Capaldi, V.F.; Simonelli, G. Effect of cognitive load and emotional valence of distractors on performance during sleep extension and subsequent sleep deprivation. Sleep 2020, 43, zsaa013. [Google Scholar] [CrossRef]
- Mantua, J.; Skeiky, L.; Prindle, N.; Trach, S.; Doty, T.J.; Balkin, T.J.; Brager, A.J.; Capaldi, V.F.; Simonelli, G. Sleep extension reduces fatigue in healthy, normally-sleeping young adults. Sleep Sci. 2019, 12, 21–27. [Google Scholar] [CrossRef]
- Ebben, M.R. Nonpharmacologic management of excessive daytime sleepiness. Sleep Med. Clin. 2017, 12, 479–487. [Google Scholar] [CrossRef]
- Parker, R.S.; Parker, P. The impact of sleep deprivation in military surgical teams: A systematic review. J. R. Army Med. Corps 2017, 163, 158–163. [Google Scholar] [CrossRef]
- Patterson, P.D.; Ghen, J.D.; Antoon, S.F.; Martin-Gill, C.; Guyette, F.X.; Weiss, P.M.; Turner, R.L.; Buysse, D.J. Does evidence support “banking/extending sleep” by shift workers to mitigate fatigue, and/or to improve health, safety, or performance? A systematic review. Sleep Health 2019, 5, 359–369. [Google Scholar] [CrossRef]
- Drummond, S.P.; Paulus, M.P.; Tapert, S.F. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 2006, 15, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Kaplan, K.A.; Kezirian, E.J.; Dement, W.C. The impact of extended sleep on daytime alertness, vigilance, and mood. Sleep Med. 2004, 5, 441–448. [Google Scholar] [CrossRef]
- Mantua, J.; Brager, A.J.; Alger, S.E.; Adewale, F.; Skeiky, L.; Balkin, T.J.; Capaldi, V.F.; Simonelli, G. Self-reported sleep need, subjective resilience, and cognitive performance following sleep loss and recovery sleep. Psychol. Rep. 2021, 124, 210–226. [Google Scholar] [CrossRef]
- Yamazaki, E.M.; Antler, C.A.; Lasek, C.R.; Goel, N. Residual, differential neurobehavioral deficits linger after multiple recovery nights following chronic sleep restriction or acute total sleep deprivation. Sleep 2021, 44, zsaa224. [Google Scholar] [CrossRef]
- Taub, J.M.; Globus, G.G.; Phoebus, E.; Drury, R. Extended sleep and performance. Nature 1971, 233, 142–143. [Google Scholar] [CrossRef]
- Stepan, M.E.; Altmann, E.M.; Fenn, K.M. Caffeine selectively mitigates cognitive deficits caused by sleep deprivation. J. Exp. Psychol. Learn. Mem. Cogn. 2021. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Kamimori, G.H. Multiple caffeine doses maintain vigilance, attention, complex motor sequence expression, and manual dexterity during 77 hours of total sleep deprivation. Neurobiol. Sleep Circadian Rhythms 2020, 9, 100051. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.A.; Ramakrishnan, S.; Satterfield, B.C.; Wesensten, N.J.; Layton, M.E.; Reifman, J.; Van Dongen, H.P.A. Randomized, double-blind, placebo-controlled, crossover study of the effects of repeated-dose caffeine on neurobehavioral performance during 48 h of total sleep deprivation. Psychopharmacology 2019, 236, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Paech, G.M.; Vedova, C.D.; Pajcin, M.; Grant, C.; Kamimori, G.; Banks, S. Caffeine has minimal effects on daytime recovery sleep following severe sleep deprivation. Sleep Biol. Rhythms 2016, 14, 149–156. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Goel, N.; Dinges, D.F. Cumulative neurobehavioral and physiological effects of chronic caffeine intake: Individual differences and implications for the use of caffeinated energy products. Nutr. Rev. 2014, 72, 34–47. [Google Scholar] [CrossRef]
- Wesensten, N.J.; Balkin, T.J.; Belenky, G. Countermeasures for mitigating fatigue in motor vehicle operators. Rev. Hum. Factors Ergon. 2015, 10, 115–137. [Google Scholar] [CrossRef]
- Irwin, C.; Khalesi, S.; Desbrow, B.; McCartney, D. Effects of acute caffeine consumption following sleep loss on cognitive, physical, occupational and driving performance: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 108, 877–888. [Google Scholar] [CrossRef]
- Faraut, B.; Andrillon, T.; Vecchierini, M.F.; Leger, D. Napping: A public health issue. From epidemiological to laboratory studies. Sleep Med. Rev. 2017, 35, 85–100. [Google Scholar] [CrossRef]
- Trotti, L.M. Waking up is the hardest thing I do all day: Sleep inertia and sleep drunkenness. Sleep Med. Rev. 2017, 35, 76–84. [Google Scholar] [CrossRef]
- Hilditch, C.J.; McHill, A.W. Sleep inertia: Current insights. Nat. Sci. Sleep 2019, 11, 155–165. [Google Scholar] [CrossRef]
- Kurniawan, I.T.; Cousins, J.N.; Chong, P.L.; Chee, M.W. Procedural performance following sleep deprivation remains impaired despite extended practice and an afternoon nap. Sci. Rep. 2016, 6, 36001. [Google Scholar] [CrossRef]
- Pomares, F.B.; Cross, N.; Jegou, A.; Nguyen, A.; Perrault, A.; Lee, K.; Smith, D.; Aydin, U.; Grova, C.; Dang-Vu, T. Cognitive performance and brain activation recovery after a nap following total sleep deprivation. Sleep Med. 2019, 64, S305. [Google Scholar] [CrossRef]
- Centofanti, S.; Banks, S.; Coussens, S.; Gray, D.; Munro, E.; Nielsen, J.; Dorrian, J. A pilot study investigating the impact of a caffeine-nap on alertness during a simulated night shift. Chronobiol. Int. 2020, 37, 1469–1473. [Google Scholar] [CrossRef]
- Johnson, D.A.; Jackson, C.L.; Williams, N.J.; Alcántara, C. Are sleep patterns influenced by race/ethnicity—A marker of relative advantage or disadvantage? Evidence to date. Nat. Sci. Sleep 2019, 11, 79–95. [Google Scholar] [CrossRef]
- Emmanuel, P.; von Schantz, M. Absence of morningness alleles in non-European populations. Chronobiol. Int. 2018, 35, 1758–1761. [Google Scholar] [CrossRef]
- Matthews, K.A.; Hall, M.H.; Lee, L.; Kravitz, H.M.; Chang, Y.; Appelhans, B.M.; Swanson, L.M.; Neal-Perry, G.S.; Joffe, H. Racial/ethnic disparities in women’s sleep duration, continuity, and quality, and their statistical mediators: Study of Women’s Health Across the Nation. Sleep 2019, 42, zsz042. [Google Scholar] [CrossRef]
- Prasad, B.; Saxena, R.; Goel, N.; Patel, S.R. Genetic ancestry for sleep research: Leveraging health inequalities to identify causal genetic variants. Chest 2018, 153, 1478–1496. [Google Scholar] [CrossRef]
- Hohoff, C.; Kroll, T.; Zhao, B.; Kerkenberg, N.; Lang, I.; Schwarte, K.; Elmenhorst, D.; Elmenhorst, E.M.; Aeschbach, D.; Zhang, W.; et al. ADORA2A variation and adenosine A1 receptor availability in the human brain with a focus on anxiety-related brain regions: Modulation by ADORA1 variation. Transl. Psychiatry 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.; Sletten, T.L.; Murray, J.M.; Gordon, C.J.; Lovato, N.; Bartlett, D.J.; Kennaway, D.J.; Lockley, S.W.; Lack, L.C.; Grunstein, R.R.; et al. Delayed Sleep on Melatonin (DelSoM) Study Group. A PERIOD3 variable number tandem repeat polymorphism modulates melatonin treatment response in delayed sleep-wake phase disorder. J. Pineal Res. 2020, 69, e12684. [Google Scholar] [CrossRef]
- Ozsoy, F.; Yigit, S.; Nursal, A.F.; Kulu, M.; Karakus, N. The impact of PER3 VNTR polymorphism on the development of schizophrenia in a Turkish population. Cytol. Genet. 2021, 55, 188–193. [Google Scholar] [CrossRef]
- Weiss, C.; Woods, K.; Filipowicz, A.; Ingram, K.K. Sleep quality, sleep structure, and PER3 genotype mediate chronotype effects on depressive symptoms in young adults. Front. Psychol. 2020, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Tartar, J.L.; Hiffernan, F.S.; Freitas, K.E.; Fins, A.I.; Banks, J.B. A functional adenosine deaminase polymorphism associates with evening melatonin levels and sleep quality. J. Circadian Rhythms 2021, 19, 5. [Google Scholar] [CrossRef]
- Križan, Z.; Hisler, G. Personality and sleep: Neuroticism and conscientiousness predict behaviourally recorded sleep years later. Eur. J. Pers. 2019, 33, 133–153. [Google Scholar] [CrossRef]
- Križan, Z.; Hisler, G.; Krueger, R.F.; McGue, M. Why is personality tied to sleep quality? A biometric analysis of twins. J. Res. Pers. 2021, 90, 104048. [Google Scholar] [CrossRef]
- Killgore, W.D.; Richards, J.M.; Killgore, D.B.; Kamimori, G.H.; Balkin, T.J. The trait of introversion-extraversion predicts vulnerability to sleep deprivation. J. Sleep Res. 2007, 16, 354–363. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).