Abstract
Sculpin fishes belonging to the family Cottidae represent a large and complex group, inhabiting a wide range of freshwater, brackish-water, and marine environments. Numerous studies based on analysis of their morphology and genetic makeup frequently provided controversial results. In the present work, we sequenced complete mitochondrial (mt) genomes and fragments of nuclear ribosomal DNA (rDNA) of the fourhorn sculpin Myoxocephalus quadricornis and some related cottids to increase the power of phylogenetic and taxonomic analyses of this complex fish group. A comparison of the My. quadricornis mt genomes obtained by us with other complete mt genomes available in GenBank has revealed a surprisingly low divergence (3.06 ± 0.12%) with Megalocottus platycephalus and, at the same time, a significantly higher divergence (7.89 ± 0.16%) with the species of the genus Myoxocephalus. Correspondingly, phylogenetic analyses have shown that My. quadricornis is clustered with Me. platycephalus but not with the Myoxocephalus species. Completely consistent patterns of divergence and tree topologies have been obtained based on nuclear rDNA. Thus, the multi-gene data in the present work indicates obvious contradictions in the relationships between the Myoxocephalus and Megalocottus species studied. An extensive phylogenetic analysis has provided evidence for a closer affinity of My. quadricornis with the species of the genus Megalocottus than with the species of the genus Myoxocephalus. A recombination analysis, along with the additional GenBank data, excludes introgression and/or incorrect taxonomic identification as the possible causative factors responsible for the observed closer affinity between the two species from different genera. The above facts necessitate realignment of the genera Myoxocephalus and Megalocottus. The genetic data supports the two recognized genera, Myoxocephalus and Megalocottus, but suggests changing their compositions through transferring My. quadricornis to the genus Megalocottus. The results of the present study resolve the relationships within a complex group of sculpin fishes and show a promising approach to phylogenetic systematics (as a key organizing principle in biodiversity research) for a better understanding of the taxonomy and evolution of fishes and for supplying relevant information to address various fish biodiversity conservation and management issues.
1. Introduction
The genus Myoxocephalus Tilesius 1811 comprises 15 or 16 species [1,2,3,4] distributed widely from the northern Pacific and Arctic Oceans to the northern Atlantic Ocean [5,6,7,8,9]. Members of this genus have diverse morphologies but are clearly differentiated from other taxa in the family Cottidae [5,6,10,11,12,13]. Morpho-anatomical [5,6,14,15] and genetic [16,17,18,19] studies have shown that the genus Myoxocephalus is closely related to the genus Megalocottus Gill 1861 represented by the only species, the belligerent sculpin Megalocottus platycephalus (Pallas 1814). Mitochondrial DNA (mtDNA) markers [20] have revealed a genetic heterogeneity associated with the spatial distribution of the species which might result in the emergence of two subspecies known for Me. platycephalus: Me. p. platycephalus (Pallas 1814) and Me. p. taeniopterus (Kner 1868). Using osteological and myological characters, Yabe [13] found that the Myoxocephalus, Microcottus Schmidt 1940, Argyrocottus Herzenstein 1892, and Porocottus Gill 1859 constitute a group of closely related genera, which are referred to as the Myoxocephalus group. Further genetic studies confirmed the Yabe’s [13] classification [19,21,22], except Argyrocottus, which still remains unstudied by genetic methods.
The fourhorn sculpin Myoxocephalus quadricornis (Linnaeus 1758) is an arctic euryhaline species with circumpolar distribution, inhabiting shallow Arctic waters south to the Gulf of Anadyr, waters off St. Lawrence Island, northern Bristol Bay, and the Bering Sea in the Pacific, and from off northern Greenland to the Baltic Sea in the Atlantic [5,6,7,8,9]. The species is represented by several ecological forms, which complicates its taxonomic classification [23]. The taxonomic nomenclature of fourhorn sculpin based on morphological characters has been subject to various changes with multiple known synonyms. Formerly, this fish was referred to as Triglopsis quadricornis (Linnaeus 1758), Cottus hexacornis Richardson 1823, Oncocottus hexacornis (Richardson 1823), etc. [2,3,4].
Little is known about the population genetics and phylogenetics of My. quadricornis; available data are limited mostly to allozymes [24] and mitochondrial markers [25,26,27,28]. Using 30 enzyme loci, Gyllensten and Ryman [24] detected a low genetic heterogeneity between the Baltic freshwater and brackish-water populations with no evidence for deviations from the Hardy–Weinberg expectation, or linkage disequilibrium. In addition, the authors detected a statistically significant spatial and temporal allele frequency heterogeneity between the Baltic populations, with the most pronounced temporal allele frequency shifts found at highly polluted localities [24].
Using the cytb, atp6, and atp8 mt genes, Kontula and Väinölä [25] investigated the spatial structure of My. quadricornis from the Arctic coastal waters and from ‘glacial relict’ populations in Nearctic and Palearctic postglacial lakes. The authors showed a principal phylogeographical split that separates the North American continental deepwater sculpin My. q. thompsonii from a lineage of the Arctic marine and North European landlocked populations of the fourhorn sculpin My. quadricornis. However, the Baltic Sea populations and the Fennoscandian lacustrine populations of My. quadricornis exhibit a significant genetic similarity [25]. Kontula et al. [26] used the cytb, atp6, and atp8 mt sequences of Triglopsis quadricornis (a synonym of My. quadricornis, see above) and Malacocottus zonurus as outgroups to investigate the endemic diversification of the cottoid fishes from Lake Baikal. Hubert et al. [27] and Mecklenburg et al. [28] assessed the degree to which the COI gene-based DNA barcoding discriminates marine and freshwater fishes, including My. quadricornis. The authors concluded that fish species can be efficiently identified through DNA barcoding, and that the COI library can subsequently find application in ecology and systematics. McCusker et al. [29] noted, however, that sculpins (along with eelpouts and rocklings) proved to be among the most challenging groups for DNA barcoding, which could be explained by a number of factors including incomplete lineage sorting, introgressive hybridization, or erroneous taxonomic identifications.
Previously, we investigated complete mt genomes and taxonomic relationships of sculpin fishes including Cottus szanaga, C. volki, C. amblystomopsis, C. czerskii, My. polyacanthocephalus, My. jaok, and Me. platycephalus [30,31,32,33,34,35,36]. In the present study, the molecular phylogenetic analyses have been carried out using mitochondrial and nuclear DNA datasets and based on extensive taxon sampling, including most of the constituent species in the genera Myoxocephalus and Megalocottus (My. quadricornis, My. ochotensis, My. scorpius, My. scorpioides, My. octodecemspinosus, My. aenaeus, My. q. thompsonii, My. polyacanthocephalus, My. brandtii, My. stelleri, My. jaok, and Me. platycephalus) and related sculpin fishes (Microcottus sellaris, Argyrocottus zanderi, Porocottus allisi, P. minutus, and P. japonicus), with two main objectives: (1) investigate the phylogenetic affinities of different species of Myoxocephalus and (2) clarify the Myoxocephalus–Megalocottus taxonomic interrelationships within the family Cottidae, which would allow assessment of circumscription for the genera.
2. Materials and Methods
2.1. Fish Specimens and Sequencing
The environmental status of the fourhorn sculpin My. quadricornis is considered as stable; it is listed under Least Concern on the International Union for Conservation of Nature’s (IUCN) Red List of Threatened Species [37]. The other sculpin fishes studied here are not included in the IUCN Red List of Threatened Species; they are not listed as endangered, vulnerable, rare, or protected species in the Russian Federation, either. The sampling points are located beyond any protected areas. The sites of the field studies are not privately-owned or protected.
The specimens of My. quadricornis were collected from Sukhoe More Bay, Dvina Bay, White Sea (March 18, 2017; 64°55′53″ N, 40°17′19″ E). The specimens of Argyrocottus zanderi Herzenstein 1892, Me. platycephalus, Myoxocephalus polyacanthocephalus (Pallas 1814), Myoxocephalus brandtii (Steindachner 1867), Myoxocephalus stelleri Tilesius 1811, Myoxocephalus jaok (Cuvier 1829), Microcottus sellaris (Gilbert 1896), Porocottus minutus (Pallas 1814), Porocottus allisi (Jordan and Starks 1904), and Porocottus japonicus Schmidt 1935 were collected from the Sea of Japan and the Sea of Okhotsk (for localities and coordinates, see Table S1). The genomic DNA was extracted using a KingFisher Flex System and a set of reagents of a MagMAX DNA Multi-Sample Kit (ThermoFisher Scientific, Waltham, MA, USA).
The complete mt genome and rDNA sequences were amplified in five and three overlapping fragments, respectively, using the Phusion High-Fidelity DNA Polymerase (ThermoFisher Scientific). Libraries were prepared using an Ion Plus Fragment Library Kit and unique adapters (Ion Xpress, Waltham, MA, USA) with pre-fragmentation on a Covaris M220 Focused ultrasonicator. Ready libraries were sequenced on an Ion S5 sequencing platform (ThermoFisher Scientific) at the Far Eastern Federal University (Vladivostok, Russia). The complete mt genomes obtained were initially annotated using the MitoFish Web Server [38] and further manually adjusted in MEGA 7 [39] by comparing them with mt genomes of other sculpin fishes. The rDNA sequences were aligned using SPAdes 3.14.1 [40] with a correction of IonTorrent data using the IonHammer tool available in the SPAdes software and annotated using sequences of other Scorpaeniformes fishes available in GenBank. The nucleotide sequences were deposited in GenBank under the accession numbers MT303953–MT303954, MT890585–MT890586, MT906795–MT906796, MT909822–MT909823 (mt genomes) and MT497857–MT497873 (rDNA). Additional complete mt genomes and separate COI gene sequences of sculpin fishes were accessed from the GenBank National Center for Biotechnology Information (NCBI) genetic sequence database [41] (see Table S2 for accession numbers).
2.2. DNA Sequence Analysis
The nucleotide sequences were aligned using the MUSCLE [42] and MAFFT v. 7 [43] software. The alignments were analyzed for evidence of recombination using various recombination detection methods provided in the RDP4 program [44]. The DnaSP v. 5 [45], PROSEQ v. 2.9 [46], and MEGA 7 [39] programs were used for interspecific analysis of divergence. Phylogeny reconstructions were based on the mt genome, rDNA, and COI gene alignments using the maximum-likelihood methods available in IQ-TREE [47,48]. ModelFinder [49] or jModelTest [50] were used to find the best-fit model of nucleotide substitution under the maximum likelihood criterion. The General Time Reversible + gamma + invariant sites (GTR + G +I; [51]), Tamura-Nei (TN93; [52]), and Hasegawa-Kishino-Yano (HKY; [53]) models showed the lowest Akaike Information Criterion (AIC; [54]) value (189,144.4265, 22,798.8538, and 6315.5052) and Bayesian information criterion (BIC; [55]) scores (189,529.5437, 23,075.7355, and 6625.4694) for the mt genome, rDNA, and COI gene alignments, respectively, and they were selected for further phylogenetic reconstructions. The ultrafast maximum likelihood bootstrap analysis [56] consisted of 1000 replicates. Partitioned analyses were performed in PartitionFinder 2.1.1 [57] that allowed the overall rate to vary between partitions including each protein-coding gene (see Table S3 for the best model of substitution under the maximum likelihood criterion).
The complete dataset was also analyzed by the Bayesian inference using MrBayes v. 3.2.7a (released March 6, 2019; [58]) under the GTR + G + I model. Analyses were performed as two independent runs, each with four incrementally heated Metropolis-coupled Monte-Carlo Markov Chains. Output trees and data were sampled for every 500 generations. Likelihood values reached a plateau within 15,000–25,000 generations. A total of 4002 trees in two files were read and 3002 of them were sampled. For the mt genomes, the log likelihood values increased from below −148,989.341 to around −94,582.436 in the first 5000 generations and then to around −94,479.814 after 1,000,000 generations. The likelihood of the best state for “cold” chain of run 1 was −94,452.62, and the likelihood of the best state for “cold” chain of run 2 was −94,452.71. The average standard deviation of split frequencies was 0.000824 after 1,000,000 generations, indicating stationary conditions. In convergence diagnostic, the Potential Scale Reduction Factor (PSRF) [59] was between 1.000 and 1.011 for all parameters, thus, indicating a good sample from the posterior probability distribution. The log likelihood values and standard deviations of split frequencies, as well as the values of convergence diagnostic for the rDNA and COI genes, are provided in the Text S1 file.
The tree topologies for the sculpin fishes obtained by the maximum-likelihood method and by the Bayesian inference were very similar. The close congruence can be explained by the fact that the three separate datasets were relatively straightforward and included the complete mt genomes, rDNA, and COI sequences of sculpin fishes only. The quality of alignment was high, and the length of alignment was long enough (a total of 19.1 kb for the mt genomes, 6.3 kb for the rDNA, and 1.1 kb for the COI gene). As has been shown by many authors, the relative efficiencies of different methods applied for obtaining correct tree topology are very close under these conditions [60]. The difference between the methods applied to the sculpin fishes (our data) resulted in the slightly different topologies and bootstrap values and did not cause changes in the relationships between My. quadricornis and its close relatives. To be conservative, we here provide the lowest bootstrap values obtained by the maximum-likelihood method.
3. Results
3.1. Complete Mitochondrial Genomes
The mt genomes (120× coverage) of My. quadricornis, P. allisi, and A. zanderi are 16,682 bp, 16,367–16,370, and 16,608, respectively. For Microcottus sellaris, only the main fragment (12,442) of the mt genome has been sequenced. The gene arrangement, composition, and size are very similar to those of the sculpin fish genomes published previously. A comparison of the mt genomes obtained by us with other complete mt genomes of several sculpin genera available in GenBank (Myoxocephalus Tilesius 1811, Megalocottus Gill 1861, Enophrys Swainson 1839, Gymnocanthus Swainson 1839, Icelus Krøyer 1845, Cottiusculus Schmidt in Jordan and Starks 1904, Hemilepidotus Cuvier 1829, Comephorus Lacepède 1800, Mesocottus Gratzianov 1907, Trachidermus Heckel 1837, and Clinocottus Gill 1861) has revealed a surprisingly close affinity (Dxy = 0.0306 ± 0.0012) of My. quadricornis to a non-congeneric sculpin species, Me. platycephalus (Figure 1; Cluster 1). The level of divergence between My. quadricornis and Me. platycephalus (3.06 ± 0.12%) matches the average value of divergence between different species within the same genus (intrageneric level) of cottids [16,17,18].
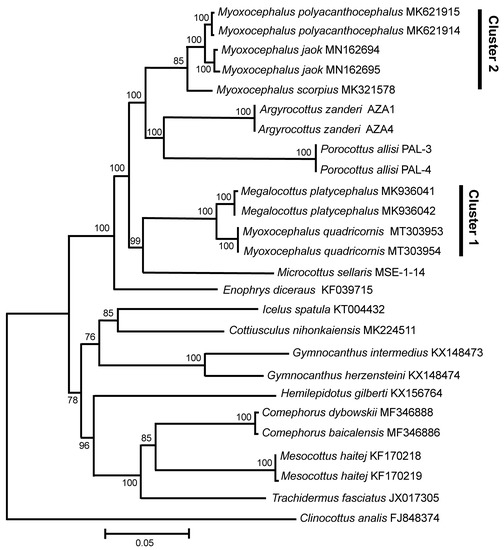
Figure 1.
Maximum likelihood tree for the fourhorn sculpin Myoxocephalus quadricornis and members of the family Cottidae from GenBank inferred from the complete mitochondrial genomes. The tree is based on the General Time Reversible + gamma + invariant sites (GTR + G + I) model of nucleotide substitution. The numerals at the nodes are bootstrap percentage probability values based on 1000 replications (values below 75% are omitted).
Another group of sequences (Figure 1; Cluster 2) contains the Myoxocephalus species only, including My. polyacanthocephalus, My. jaok, and My. scorpius. The level of divergence (Dxy = 0.0314 ± 0.0012) between the sequences within this group is slightly higher, but still matches the value of divergence for different species within the same genus (intrageneric level). The level of divergence between clusters 1 and 2 is significantly higher (Dxy = 0.0789 ± 0.0016), matching the values of divergence, albeit the lowest ones, between different genera within the family Cottidae (intrafamily level) [16,17,18]. Comparable values of divergence based on complete mt genomes have been obtained in the pairwise comparisons between the other genera of the cottids studied (Figure 1) such as Megalocottus–Microcottus (Dxy = 0.0829 ± 0.0024), Myoxocephalus–Microcottus (Dxy = 0.0865 ± 0.0015), Myoxocephalus–Enophrys (Dxy = 0.0898 ± 0.0025), Myoxocephalus–Argyrocottus (Dxy = 0.0910 ± 0.0020), Megalocottus–Enophrys (Dxy = 0.0970 ± 0.0023), Megalocottus–Argyrocottus (Dxy = 0.0987 ± 0.0019), Myoxocephalus–Icelus (Dxy = 0.1025 ± 0.0023), and Myoxocephalus–Porocottus (Dxy = 0.1032 ± 0.0021).
The species belonging to the genera Argyrocottus and Porocottus are clustered with the genus Myoxocephalus (Figure 1). The level of divergence (Dxy) between A. zanderi and P. allisi (0.1093 ± 0.0021), as well as between these two species and the Myoxocephalus cluster 2 (0.0950 ± 0.0017), matches the value of divergence between different genera within the family Cottidae (intrafamily level). The species belonging to the genus Microcottus is clustered with the genus Megalocottus (Figure 1). The level of divergence between M. sellaris and the Megalocottus cluster 1 (0.0829 ± 0.0025) also matches the intrafamily level.
3.2. Nuclear Ribosomal Genes
The 6.3 kb fragment of the nuclear rDNA sequences of the genera Myoxocephalus and Megalocottus shows completely consistent patterns of divergence and tree topologies with those observed for the mt genomes. There are two clusters containing Myoxocephalus species (Figure 2; Cluster 1 and Cluster 2). The first cluster contains both My. quadricornis and Me. platycephalus with the difference between them being very low (Dxy = 0.0018 ± 0.0005). The second cluster contains the Myoxocephalus species only, including My. jaok, My. stelleri, My. brandtii, and My. polyacanthocephalus, with the average distance (Dxy) equal to 0.0037 ± 0.0005. The difference between Clusters 1 and 2 is significantly higher (Dxy = 0.0071 ± 0.0010). The members of the genera Microcottus and Porocottus form separate clusters outside of both Myoxocephalus and Megalocottus, with relatively high genetic distances (Dxy) varying from 0.0122 ± 0.0015 to 0.0160 ± 0.0017 and a significant bootstrap support (Figure 2). The tree topologies obtained for the Myoxocephalus + Megalocottus and Microcottus + Porocottus clusters are slightly different when based on the rDNA fragments (Figure 2) and mtDNA (Figure 1), which can reflect specific evolutionary trends in nuclear and mitochondrial genomes. The phylogenetic discordance between nuclear and mitochondrial genomes is not a rare phenomenon among fishes [61] and many other organisms [62].
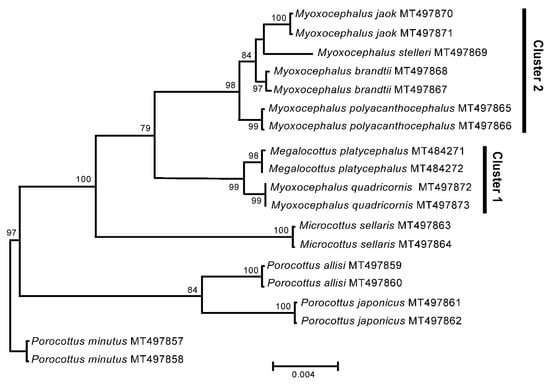
Figure 2.
Maximum likelihood tree for the fourhorn sculpin Myoxocephalus quadricornis and members of the family Cottidae from GenBank inferred from the 6332 bp ribosomal DNA. The tree is based on the Tamura-Nei (TN93) model of nucleotide substitution. For other comments, see Figure 1.
Nuclear ribosomal genes are frequently used in molecular systematics [63,64]. Both small (18S) and large (28S) ribosomal genes, characterized by slow rate of evolution, have been used for phylogenetic studies on distantly related species to resolve macroevolutionary issues such as the origin of tetrapods and general vertebrate phylogeny [65]. Despite rDNA genes are less sensitive than mtDNA genes in analysis of close groups (species or genus level), nevertheless, data obtained using nuclear ribosomal markers are important for comparative purposes. As an independent source of genetic information, our rDNA data confirms the results obtained based on the mtDNA sequences.
3.3. GenBank COI Gene Dataset
As in the case of mt genome and rDNA data, the COI gene cluster 1 contains both the Myoxocephalus species (My. scorpioides, My. octodecemspinosus, My. aenaeus, and My. quadricornis) and Me. platycephalus (represented by two subspecies, Me. p. taeniopterus and Me. p. platycephalus; see [20]) (Figure 3). The average difference (Dxy) between the species of Cluster 1 is 0.0387 ± 0.0047 (using three randomly picked sequences per species). The COI gene Cluster 2 contains the species belonging to the genus Myoxocephalus only, including My. stelleri, My. ochotensis, My. jaok, My. polyacanthocephalus, My. brandtii, and My. scorpius (Figure 3). The average difference (Dxy) between the species of Cluster 2 is 0.0265 ± 0.0037. The average levels of divergence within both clusters are relatively low, varying within 2.7–3.9%, which fits into the range of interspecific divergence for sculpin fishes [16,17,18,36]. The level of divergence between Clusters 1 and 2 is significantly higher, Dxy = 0.0826 ± 0.0091, indicating the intergeneric level of divergence for cottids [16,17,18] (see also the Section 3.1). Thus, the COI dataset containing multiple GenBank sequences shows a significantly higher divergence (8.3%) between the clusters than within them (2.7–3.9%), which agrees with the analyses based on the complete mt genomes and the rDNA sequences.
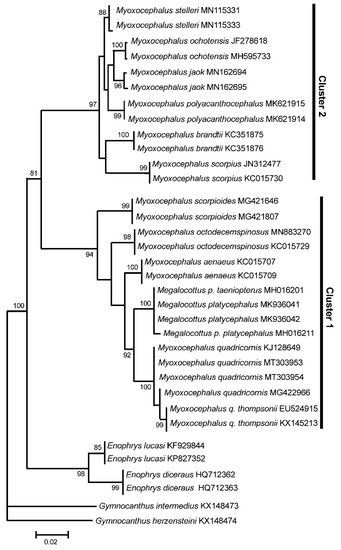
Figure 3.
Maximum likelihood tree for the fourhorn sculpin Myoxocephalus quadricornis and members of the family Cottidae from GenBank inferred from the mitochondrial COI gene. The tree is based on the Hasegawa-Kishino-Yano + gamma + invariant sites (HKY + G + I) model of nucleotide substitution. Species of the genera Enophrys and Gymnocanthus are used as outgroups. For other comments, see Figure 1.
The GenBank COI sequences demonstrate also a close similarity of the three other Myoxocephalus species, My. aenaeus, My. octodecemspinosus, and My. scorpioides, to the My. quadricornis plus Me. platycephalus cluster (Figure 3, Cluster 1). This suggests that the above-listed three species may also belong to the genus Megalocottus. The close affinity between My. cf. scorpiodes and Me. platycephalus was also reported previously by Knope [19] based on mitochondrial (cytb) and nuclear (the first intron of the nuclear S7 ribosomal protein) markers. However, the available data are still insufficient to draw any reliable conclusion.
3.4. Alternative Hypotheses: Recombination and Species Misidentification
The phylogenetic inconsistency that we detected may reflect the historical hybridization event(s) between the Myoxocephalus and Megalocottus species which could result in interspecific recombination of their mtDNA (as it has been found in other organisms including fishes; see, e.g., [66,67]), or it may be due to incorrect taxonomic identification. We, therefore, analyzed the mt genome alignments for evidence of recombination using various recombination detection methods provided in the RDP4 program [44]. All the methods failed to reveal any signal of recombination between the mt genomes of the Myoxocephalus and Megalocottus species, thus rejecting hybridization as a possible explanation for the anomalous similarity between the My. quadricornis and Me. platycephalus mt genomes.
To exclude the probability of species misidentification, we collected all available GenBank COI sequences for the genera Myoxocephalus and Megalocottus (Table S2) and, again, obtained two significantly diverged groups of sequences containing the Myoxocephalus and Megalocottus species (Figure 3; Cluster 1 and Cluster 2). It is important that the My. quadricornis sequences (MT303953 and MT303954) obtained in the present study are identical or very similar to the GenBank COI sequences (KJ128649, MG422966, EU524915, KX145213; Figure 3, see also Table S2 for additional sequences absent in Figure 3) reported by many other authors (Table S2), which also rejects misidentification as the possible causative factor responsible for the observed close affinity between My. quadricornis and Me. platycephalus.
4. Discussion
The mt genome and nuclear rDNA data have revealed a mixed composition of the genus Myoxocephalus, presumably including also a Megalocottus species. The observed inconsistencies are supported by the GenBank COI sequences representing all the available Myoxocephalus and Megalocottus species. Both mitochondrial and nuclear markers indicate close evolutionary relationships between the genera Myoxocephalus and Megalocottus (as well as other studied sculpins belonging to the genera Microcottus, Argyrocottus, and Porocottus). Close associations between these genera were also inferred from morphological [5,6,13,14,15] and genetic [16,17,18,19,21,22,68] studies. However, the present genetic data does not support the synonymization of Megalocottus and Myoxocephalus, as was proposed by a number of authors (e.g., [10,11,69,70,71]). The genetic distance between them is significantly larger than the average interspecific distance within the genus Myoxocephalus (see above) and fits into the range of intergeneric distances detected for sculpin fishes [16,17,18], nevertheless, having the lowest values (see also the Results section). Instead, our genetic data indicate that the composition of the genera Myoxocephalus and Megalocottus needs revision. In particular, the closer affinity of My. quadricornis to the genus Megalocottus than to the genus Myoxocephalus, detected using both mt genomes and rDNA genes, strongly suggests the necessity of generic realignment by transferring My. quadricornis to the genus Megalocottus. The morphological, ecological, and life-history traits of the species do not contradict the genetic data obtained and also support the necessity of generic realignment. My. quadricornis and Me. platycephalus share a number of common features, including some ecological preferences and morphological traits, that distinguish them from the Myoxocephalus species [5,6,8,11,12]. In particular, the topography of the seismosensory system, which is a highly specific character used in fish taxonomy [6,12], is similar between My. quadricornis and Me. platycephalus on the one hand and differs them from the rest of the Myoxocephalus species on the other hand [6].
Our genetic data are consistent with the earlier reports of Cowan [12,72] who investigated relationships within the genus Myoxocephalus based on 45 morphological and 35 biochemical (isozymes) characters. The study placed My. quadricornis in the most basal position of the trees containing 13 Myoxocephalus species and constructed using morphological [12] and combined morphological and biochemical [72] data. Our results also agree with the data obtained by Knope [19], who showed that Myoxocephalus is not a monophyletic genus. The six Myoxocephalus species included in that study formed a monophyletic clade with high statistical support, except for one specimen identified as Triglops quadricornis (an unaccepted name of My. quadricornis; see, e.g., [4]), which grouped with a sister clade including My. cf. scorpioides and Me. platycephalus [19]. Smith and Busby [21] (p. 345), referring to a previous study by Kontula and Väinölä [25], indicated that “…Myoxocephalus may need minor revision.”
Using the cytb, atp6, and atp8 mt genes, Kontula and Väinölä [25] found that the five marine Myoxocephalus species, included in their phylogenetic assessment, formed two main clades, while My. quadricornis was deeply nested within the Myoxocephalus genus. Based on these observations, the authors claimed that My. quadricornis (as well as its subspecies, the deepwater sculpin My. q. thompsonii) should be retained in Myoxocephalus without placing it in a separate genus, as it was proposed, for instance, by Neyelov [6]. Mecklenburg et al. [9] (Figure 4E, p. 118) and Mecklenburg and Steinke [28] (Figure 12, p. 176) obtained very similar tree topologies using the COI gene and also concluded that My. quadricornis should remain in Myoxocephalus.
The datasets of Kontula and Väinölä [25], Mecklenburg et al. [9], and Mecklenburg and Steinke [28] were limited only to the Myoxocephalus species and contained neither members of the genus Megalocottus nor other close sculpin genera (e.g., Microcottus, Argyrocottus, and Porocottus). The two divergent Myoxocephalus clades, revealed in their works, completely correspond to our Clusters 1 and 2 (Figure 1, Figure 2 and Figure 3). However, due to insufficient sample of species, the authors could not match the clades to representatives of Myoxocephalus and Megalocottus and recognize Myoxocephalus as a paraphyletic group containing both Myoxocephalus and Megalocottus species. When a dataset is represented by an insufficient sample of species, the hypothesis of monophyly can be mistakenly supported (see, e.g., [73], p. 298). Consequently, the conclusion of Kontula and Väinölä [25], Mecklenburg et al. [9], and Mecklenburg and Steinke [28] is not supported by our extended dataset containing species of fourteen genera (including Myoxocephalus and Megalocottus) and obtained based on both complete mt genomes (16.68 kb) and a long fragment of rDNA (6.33 kb), which represent more reliable markers than the short mtDNA fragments (0.65–1.98 kb) used by those authors.
Recently, Mecklenburg et al. [74] (Vol. 1, p. 210) noted that “DNA barcodes place M. platycephalus as a sister species to Myoxocephalus quadricornis in a larger all-inclusive clade of Myoxocephalus species”. The data obtained in the present study agrees with the first part of the statement made by Mecklenburg et al. [74]. Indeed, we show that Me. platycephalus and My. quadricornis are sister species, but we also show that Myoxocephalus is not an “all-inclusive clade”. Instead, it consists of two evolutionary distinct lineages at the intergeneric level of divergence (see Figure 1, Figure 2 and Figure 3), which is clearly evidenced by a comparative analysis of interspecific and intergeneric distances for Myoxocephalus, Megalocottus, and other sculpins (see the Results section). No such analyses were reported in the works of Kontula and Väinölä [25], Mecklenburg et al. [9,74], and Mecklenburg and Steinke [28].
The observed discordance may be explained in part by incorrect taxonomic identification, as was noted by McCusker et al. [29]. For instance, Smith and Busby [21] reported that Myoxocephalus was paraphyletic relative to Microcottus. We reanalyzed Smith and Busby’s data and found that the Mi. sellaris specimen was misidentified and actually represented My. scorpius. The very low distance (0.86%) between the My. scorpius CytB nucleotide sequence (GenBank accession number MK321578) and the corresponding sequence (KM057906) of Smith and Busby’s “Microcottus” clearly indicates that these authors misidentified Mi. sellaris. The two other sequences of Mi. sellaris obtained independently by Togashi [75] (LC125750) and Balakirev, Kravchenko, and Semenchenko (present study) show a very substantial divergence (around 11%) between Mi. sellaris and My. scorpius, which explains the clear resolution of these species (Figure 1). Thus, the observation of Smith and Busby [21] that Microcottus is nested within Myoxocephalus is erroneous, being based on incorrect species identification (other examples of incorrect taxonomic identification see, e.g., in [28,33]). Another problem potentially causing different tree topologies arises from DNA barcoding, which is still used and recommended for fish identifications [9,27,28,29]. However, the DNA barcoding approach is frequently not enough to obtain reliable resolution. This problem is highly debated (for recent reviews see, e.g., [76,77]).
In accordance with the principle of priority in the International Code of Zoological Nomenclature (ICZN; Article 23) [78], Neyelov [6] proposed to place My. quadricornis in a separate monotypic genus, Triglopsis Girard 1851 (which is currently an unaccepted junior synonym of the genus Myoxocephalus Tilesius 1811 [2,3,4]), based mostly on the seismosensory system topography and other morphological characters. In this case, Me. platycephalus should also be transferred to the genus Triglopsis due to the genetic data obtained in the present work and the principle of priority, because Triglopsis Girard 1851 precedes Megalocottus Gill 1861. However, this hypothetic transfer contradicts the classification of Neyelov [6], who, in spite of the close relationship between My. quadricornis and Me. platycephalus [5,6,8,11,12], did not include both species in the same genus Triglopsis [6].
The type species of the genus Triglopsis is the deepwater sculpin T. thompsonii Girard 1851 (currently a subspecies of My. quadricornis [25]) inhabiting the North American continental lakes. This sculpin is a highly specialized form adapted to freshwater habitats. It originated from an Arctic marine lineage of the fourhorn sculpin My. quadricornis that was driven south to freshwater habitats by early glacial advances within the Early to Middle Pleistocene or earlier [79,80]. The divergence time between T. q. thompsonii and My. quadricornis is estimated at around one million years (Myr) ago [25,81] based on the COI, cytb, atp6, and atp8 mt genes. In turn, the Arctic My. quadricornis lineage diverged from the ancient Myoxocephalus forms from the Pacific, including closely related Megalocottus lineage, in the late Pliocene (see [5,6,14,82] and references therein), which agrees well with the estimated divergence time (≈7.9 Myr ago) between the Pacific and Arctic–Atlantic Myoxocephalus species [81].
In accordance with the principle of priority in the International Code of Zoological Nomenclature (ICZN; Article 23) [78], both species, My. quadricornis and Me. platycephalus, should be placed in the genus Triglopsis Girard 1851. However, this would contradict the basic assumption of phylogenetic taxonomy [73,83,84], because Triglopsis represents a derived form relative to the ancestral Myoxocephalus and Megalocottus forms. Thus, at the moment, we find it unreasonable to resurrect the genus Triglopsis for both My. quadricornis and Me. Platycephalus; instead, we consider the transfer of My. quadricornis to the genus Megalocottus as a feasible alternative, which is consistent with the evolutionary history of both Pacific (by origin) forms [5,6,14,82].
Taking in account the above circumstances, it would be premature to provide the diagnosis, description, and classification of My. quadricornis within the traditional Linnaean system. The compositions of the genera Myoxocephalus and Megalocottus inferred in the present work are delimited based on a phylogenetic approach according to the concept of phylogenetic systematics [73,84]. Further studies using complete mt genomes and nuclear genes, along with a more detailed morphological analysis and representative sample of species, are necessary to build a natural classification of these sculpin fishes that would satisfy the requirements of both the traditional Linnaean system and the phylogenetic systematics.
5. Conclusions
The present data, obtained through the analysis of pairwise genetic distances and phylogenetic reconstructions with high bootstrap support, indicates an intergeneric level of divergence between Megalocottus, Myoxocephalus, Microcottus, Argyrocottus, and Porocottus. It also shows some obvious inconsistencies in the relationships among the sculpin fishes belonging to the genera Myoxocephalus and Megalocottus (Figure 1, Figure 2 and Figure 3), which has always been a controversial and long-debated issue [6,9,10,11,28,71,74]. The multi-gene approach provides evidence for a close affinity of My. quadricornis with the species of the genus Megalocottus (but not with Myoxocephalus) and strongly suggests My. quadricornis to be placed in the genus Megalocottus.
We believe that the proposed taxonomic realignment based upon both mitochondrial and nuclear genes is robust. However, we also expect that the genera Myoxocephalus and Megalocottus, as well as other cottids, will continue to be subject to taxonomic changes in the future. In particular, the GenBank COI sequences suggest that three other Myoxocephalus species (besides My. quadricornis), My. aenaeus, My. octodecemspinosus, and My. scorpioides, may also belong to the genus Megalocottus (and, consequently, be removed from the genus Myoxocephalus; see above). To resolve this issue, a large-scale genetic analysis including complete mt genomes and nuclear genes is necessary. The next-generation sequencing (NGS) provides extensive full nuclear genome data, which is highly efficient for identifying speciation events and taxonomic relationships among non-model fish species. It turned out that a few candidate adaptive genes may demonstrate high divergence and create a barrier for the gene flow leading to reproductive isolation between species, which may be identical for the rest part of the nuclear genome [85,86,87,88]. A targeted NGS approach makes it possible to identify a suite of loci that can be used in future research to investigate the genomic basis of adaptation, differentiation, speciation, and phylogenetic relationships between sculpins, the most challenging group of fish for genetic and taxonomic studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/9/1071/s1, Table S1: Sites of specimen collection for the study, Table S2: Species names and GenBank accession numbers, Table S3: The best model of substitution under the maximum likelihood criterion, Text S1: Values of log likelihood, standard deviations of split frequencies, and convergence diagnostic for the rDNA and COI genes.
Author Contributions
E.S.B. conceived and designed the research. A.Y.K. and A.A.S. performed the sequencing procedures. E.S.B., A.A.S., and A.Y.K. analyzed the obtained data. A.A.S. provided reagents/materials/analysis tools. E.S.B. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The present study received a budgetary support within the framework of the Research Work no. 14 “Biodiversity of the World Ocean: Composition and distribution of biota” (State registry no. 115081110047, Ministry of Science and Higher Education of the Russian Federation, no. 0268-2019-0007), A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia.
Acknowledgments
We are grateful to Igor Plakhin (Northern Administration for Hydrometeorology and Environmental Monitoring, Arkhangelsk, Russia) and Pavel A. Saveliev (A.V. Zhirmunsky National Scientific Center of Marine Biology FEB RAS, Vladivostok, Russia) for the help with collecting fish specimens. We cordially thank E.P. Shvetsov (A.V. Zhirmunsky National Scientific Center of Marine Biology FEB RAS, Vladivostok, Russia) for proofreading the manuscript and useful comments. Special thanks are also due to two anonymous reviewers for their helpful comments on the previous versions of the manuscript.
Conflicts of Interest
The authors acknowledge no financial interest or benefit from any direct applications of this research. The authors declare no conflict of interest.
References
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: http://www.fishbase.org/ (accessed on 6 July 2020).
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) The Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 6 July 2020).
- The Integrated Taxonomic Information System (ITIS). Available online: https://www.itis.gov/ (accessed on 6 July 2020).
- The World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/index.php (accessed on 6 July 2020).
- Andriashev, A.P. Fishes of the Northern Seas of the USSR; Publishing house of the Academy of Sciences of USSR: Moscow/Leningrad, Russia, 1954. [Google Scholar]
- Neyelov, A.V. Seismosensory System and Classification of Sculpins (Cottidae: Myoxocephalinae, Artedellinae); Nauka: Leningrad, Russia, 1979. [Google Scholar]
- Fedorov, V.V. Cottidae. In Fishes of the North Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J.G., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1243–1260. [Google Scholar]
- Mecklenburg, C.W.; Mecklenburg, T.A.; Thorsteinson, L.K. Fishes of Alaska; American Fisheries Society: Bethesda, MD, USA, 2002; p. 1116. [Google Scholar]
- Mecklenburg, C.W.; Møller, P.R.; Steinke, D. Biodiversity of Arctic marine fishes – taxonomy and zoogeography. Mar. Biodivers. 2011, 41, 109–140. [Google Scholar] [CrossRef]
- Taranetz, A.Y. On the origin and taxonomy of the Cottoid fishes. Bull. Acad. Sci. USSR Biol. Sci. Dept. (Izv. Akad. Nauk SSSR, Otdel. Biol. Nauk) 1941, 3, 427–447. [Google Scholar]
- Berg, L.S. Freshwater Fish of the USSR and Adjacent Countries, 4th ed.; Publishing house of the Academy of Sciences of USSR: Moscow, Russia, 1949; pp. 929–1383. [Google Scholar]
- Cowan, G.I.M. Comparative morphology of the cottid genus Myoxocephalus based on meristic, morphometric, and another anatomical characters. Can. J. Zool. 1971, 49, 1479–1496. [Google Scholar] [CrossRef]
- Yabe, M. Comparative osteology and myology of the superfamily Cottoidea (Pisces: Scorpaeniformes), and its phylogenetic classification. Mem. Fac. Fish. Hokkaido Univ. 1985, 32, 1–130. [Google Scholar]
- Schmidt, P.Y. Fishes of the Sea of Okhotsk. Transact. Pac. Commit. Acad. Sci. USSR 1950, 6, 1–370. [Google Scholar] [CrossRef]
- Lindberg, G.U.; Krasyukova, Z.V. Fishes of the Sea of Japan and the Adjacent Areas of the Sea of Okhotsk and Yellow Sea; Part 5; Akad. Nauk. SSSR: Leningrad, Russia, 1987. [Google Scholar]
- Kartavtsev, Y.P.; Sharina, S.N.; Goto, T.; Balanov, A.A.; Hanzawa, N. Sequence diversity at cytochrome oxidase 1 (Co1) gene among Sculpins (Scorpaeniformes, Cottidae) and some other scorpionfish of Russia Far East with phylogenetic and taxonomic insights. Genes Genom. 2009, 31, 183–197. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P.; Rozhkovan, K.V.; Masalkova, N.A. Phylogeny based on two mtDNA genes (Co-1, Cyt-B) among sculpins (Scorpaeniformes, Cottidae) and some other scorpionfish in the Russian Far East. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 2225–2240. [Google Scholar] [CrossRef]
- Podlesnykh, A.V.; Moreva, I.N. Variability and relationships of the Far Eastern species of sculpins Myoxocephalus and Megalocottus (Cottidae) based on mtDNA markers and karyological data. Russ. J. Genet. 2014, 50, 949–956. [Google Scholar] [CrossRef]
- Knope, M.L. Phylogenetics of the marine sculpins (Teleostei: Cottidae) of the North American Pacific Coast. Mol. Phylogenet. Evol. 2013, 66, 341–349. [Google Scholar] [CrossRef]
- Radchenko, O.A.; Petrovskaya, A.V. Molecular-genetic differentiation of the belligerent sculpin Megalocottus platycephalus (Pallas, 1814) (Scorpaeniformes: Cottidae). Russ. J. Mar. Biol. 2019, 45, 56–66. [Google Scholar] [CrossRef]
- Smith, W.L.; Busby, M.S. Phylogeny and taxonomy of sculpins, sandfishes, and snailfishes (Perciformes: Cottoidei) with comments on the phylogenetic significance of their early-life-history specializations. Mol. Phylogenet. Evol. 2014, 79, 332–352. [Google Scholar] [CrossRef] [PubMed]
- Moreva, I.N. The karyotype of the brightbelly sculpin Microcottus sellaris (Gilbert, 1896) (Cottidae: Myoxocephalinae). Russ. J. Mar. Biol. 2020, 46, 29–33. [Google Scholar] [CrossRef]
- Nyman, L.; Westin, L. On the problem of sibling species and possible intraspecific variation in Fourhorn sculpin, Myoxocephalus quadricornis (L.). Rep. Inst. Freshwater Res. 1968, 48, 57–66. [Google Scholar]
- Gyllensten, U.; Ryman, N. Biochemical genetic variation and population structure of fourhorn sculpin (Myoxocephalus quadricornis; Cottidae) in Scandinavia. Hereditas 1988, 108, 179–185. [Google Scholar] [CrossRef]
- Kontula, T.; Väinölä, R. Relationships of Palearctic and Nearctic ‘glacial relict’ Myoxocephalus sculpins from mitochondrial DNA data. Mol. Ecol. 2013, 12, 3179–3184. [Google Scholar] [CrossRef]
- Kontula, T.; Kirilchik, S.V.; Väinöä, R. Endemic diversification of the monophyletic cottoid fish species flock in Lake Baikal explored with mtDNA sequencing. Mol. Phylogen. Evol. 2003, 27, 143–155. [Google Scholar] [CrossRef]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E. Identifying Canadian freshwater fishes through DNA Barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef]
- Mecklenburg, C.W.; Steinke, D. Ichthyofaunal baselines in the Pacific Arctic region and RUSALCA study area. Oceanography 2015, 28, 158–189. [Google Scholar] [CrossRef]
- McCusker, M.R.; Denti, D.; Van Guelpen, L.; Kenchington, E.; Bentzen, P. Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol. Ecol. Res. 2012, 13, 177–188. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Saveliev, P.A.; Ayala, F.J. Complete mitochondrial genome of the Amur sculpin Cottus szanaga (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2016, 1, 337–338. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Saveliev, P.A.; Ayala, F.J. Complete mitochondrial genome of the Volk’s sculpin Cottus volki (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2017, 2, 185–186. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Saveliev, P.A.; Ayala, F.J. Complete mitochondrial genome of the Sakhalin sculpin Cottus amblystomopsis (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2017, 2, 244–245. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Saveliev, P.A.; Ayala, F.J. Complete mitochondrial genomes of the Cherskii’s sculpin Cottus czerskii and Siberian taimen Hucho taimen reveal GenBank entry errors: Incorrect species identification and recombinant mitochondrial genome. Evolut. Bioinform. 2017, 13, 1176934317726783. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Kravchenko, A.Y.; Saveliev, P.A.; Semenchenko, A.A.; Ayala, F.J. Complete mitochondrial genome of the great sculpin Myoxocephalus polyacanthocephalus (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2019, 4, 2361–2362. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Kravchenko, A.Y.; Cherepkova, E.V.; Saveliev, P.A.; Semenchenko, A.A.; Ayala, F.J. Complete mitochondrial genome of the belligerent sculpin Megalocottus platycephalus (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2019, 4, 2980–2981. [Google Scholar] [CrossRef]
- Balakirev, E.S.; Kravchenko, A.Y.; Cherepkova, E.V.; Saveliev, P.A.; Semenchenko, A.A.; Ayala, F.J. Complete mitochondrial genome of the plain sculpin Myoxocephalus jaok (Cottoidei: Cottidae). Mitochondrial DNA Part B Res. 2020, 5, 1295–1296. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 6 July 2020).
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M. MitoFish and MitoAnnotator: A mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. Lect. Notes Comput. Sci. 2013, 7821, 158–170. [Google Scholar]
- The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 6 July 2020).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Filatov, D.A. PROSEQ: A software for preparation and evolutionary analysis of DNA sequence data sets. Mol. Ecol. Notes 2002, 2, 621–624. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, 232–235. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Hasegawa, M.; Kishino, H.; Taka-aki, Y. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G.E. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 1–4. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–511. [Google Scholar] [CrossRef]
- Douady, C.J.; Delsuc, F.; Boucher, Y.; Doolittle, W.F.; Douzery, E.J. Comparison of Bayesian and maximum likelihood bootstrap measures of phylogenetic reliability. Mol. Biol. Evol. 2003, 20, 248–254. [Google Scholar] [CrossRef]
- Graham, P.; Wallis, G.P.; Cameron-Christie, S.R.; Kennedy, H.L.; Palmer, G.; Tessa, R.; Sanders, T.R.; Winter, D.J. Interspecific hybridization causes long-term phylogenetic discordance between nuclear and mitochondrial genomes in freshwater fishes. Mol. Ecol. 2017, 26, 3116–3127. [Google Scholar]
- Toews, D.; Brelsford, A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]
- Hillis, D.M.; Dixon, M.T. Ribosomal DNA: Molecular evolution and phylogenetic inference. Q. Rev. Biol. 1991, 66, 411–453. [Google Scholar] [CrossRef]
- Meyer, A. Molecular phylogenetic studies of fish. In Genetics and Evolution of Aquatic Organisms; Beaumont, A.R., Ed.; Chapman and Hall: London, UK, 1994; pp. 219–249. [Google Scholar]
- Hillis, D.M.; Dixon, M.T. Vertebrate phylogeny: Evidence from 28S ribosomal DNA sequences. In Hierarchy of Life: Molecules and Morphology in Phylogenetic Analysis; Fernholm, B., Bremer, K., Brudin, L., Jornvall, H., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1989; pp. 355–367. [Google Scholar]
- Balakirev, E.S.; Krupnova, T.N.; Ayala, F.J. DNA variation in the phenotypically diverse brown alga Saccharina japonica. BMC Plant Biol. 2012, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, E.S.; Romanov, N.S.; Mikheev, P.B.; Ayala, F.J. Mitochondrial DNA variation and introgression in Siberian taimen Hucho taimen. PLoS ONE 2013, 8, e71147. [Google Scholar] [CrossRef] [PubMed]
- Moreva, I.N.; Borisenko, S.A. The karyotype of the flathead sculpin Megalocottus platycephalus (Pallas, 1814) (Pisces: Cottidae) from Odyan Bay, Sea of Okhotsk. Russ. J. Mar. Biol. 2014, 40, 125–130. [Google Scholar] [CrossRef]
- Taranetz, A.Y. Handbook for identification of fishes of Soviet Far East and adjacent waters. Izv. TINRO 1937, 11, 1–200. [Google Scholar]
- Taranetz, A.Y. Material to the knowledge of the ichthyofauna of Soviet Sakhalin. Izv. TINRO 1937, 12, 5–50. [Google Scholar]
- Walters, V. Fishes of western arctic America and eastern arctic Siberia: Taxonomy and zoogeography. Bull. Am. Mus. Nat. Hist. 1955, 10, 255–368. [Google Scholar]
- Cowan, G.I.M. Relationships within the genus Myoxocephalus (Pisces: Cottidae) based on morphological and biochemical data using numerical and conventional method of analyses. Ibid 1972, 50, 671–682. [Google Scholar] [CrossRef]
- Wägele, J.-W. Foundations of Phylogenetic Systematics; Verlag Dr. Friedrich Pfeil: München, Germany, 2005. [Google Scholar]
- Mecklenburg, C.W.; Lynghammar, A.; Johannesen, E.; Byrkjedal, I.; Christiansen, J.S.; Dolgov, A.V.; Karamushko, O.V.; Møller, P.R.; Steinke, D.; Wienerroither, R.M. Marine Fishes of the Arctic Region; Monitoring Series Report 28; Conservation of Arctic Flora and Fauna: Akureyri, Iceland, 2018; Volume 1, pp. 1–454. [Google Scholar]
- Togashi, K.; Yamazaki, A.; Awata, S.; Koya, Y.; Abe, T.; Tsuruoka, S.; Takeshima, H.; Malkevich, A.; Munehara, H. Molecular phylogeny and Adaptive radiation of Cottoidei. Unpublished. Uploaded to GenBank on 14/02/2019.
- Naciri, Y.; Linder, P. Species delimitation and relationships: The dance of the seven veils. Taxon 2015, 64, 3–16. [Google Scholar] [CrossRef]
- Balakirev, E.S. Conservation genetics of marine biological resources: Methodological aspects with the sea urchin Strongylocentrotus intermedius as an example. In Studies of Marine Organisms in the Far East: Biodiversity, Monitoring, and Rational Management of Resources; Malakhov, V.V., Chernyshev, A.V., Eds.; Far Eastern Federal University Press: Vladivostok, Russia, 2020; pp. 36–103. [Google Scholar]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Dadswell, M.J. Distribution, ecology, and postglacial dispersal of certain crustaceans and fishes in eastern North America. Nat. Mus. Nat. Sci. Publ. Zool. 1974, 11, 1–110. [Google Scholar]
- McAllister, D.E. The origin and status of the deepwater sculpin, Myoxocephalus thompsonii, a Nearctic glacial relict. Bull. Nat. Mus. Can. Contrib. Zool. 1961, 172, 44–65. [Google Scholar]
- Yamazaki, A.; Nishimiya, Y.; Tsuda, S.; Togashi, K.; Munehara, H. Gene expression of antifreeze protein in relation to historical distributions of Myoxocephalus fish species. Mar. Biol. 2018, 165, 181. [Google Scholar] [CrossRef]
- Gurjanova, E.F. The peculiar properties of the Arctic Ocean fauna and their value for the understanding the history of its developments. In The Arctic Ocean and Its Coast in Cainozoe; Gidrometeoizdat: Leningrad, Russia, 1970; pp. 126–161. [Google Scholar]
- De Queiroz, K.; Gauthier, J. Toward a phylogenetic system of biological nomenclature. Trends Ecol. Evol. 1994, 9, 27–31. [Google Scholar] [CrossRef]
- Wiley, E.O.; Lieberman, B.S. Phylogenetics: Theory and Practice of Phylogenetic Systematic, 2nd ed.; Wiley-Blackwell: New York, NY, USA, 2011. [Google Scholar]
- Mateus, C.S.; Stange, M.; Berner, D.; Roesti, M.; Quintella, B.R.; Alves, M.J.; Almeida, P.R.; Salzburger, W. Strong genome-wide divergence between sympatric European river and brook lampreys. Curr. Biol. 2013, 23, R649–R650. [Google Scholar] [CrossRef]
- Bernardi, G.; Nelson, P.; Paddack, M.; Rulmal, J., Jr.; Crane, N. Genomic islands of divergence in the Yellow Tang and the Brushtail Tang Surgeonfishes. Ecol. Evol. 2018, 8, 8676–8685. [Google Scholar] [CrossRef]
- Whitney, J.L.; Bowen, B.W.; Karl, S.A. Flickers of speciation: Sympatric colour morphs of the arc-eye hawkfish, Paracirrhites arcatus, reveal key elements of divergence with gene flow. Mol. Ecol. 2018, 27, 1479–1493. [Google Scholar] [CrossRef]
- Hench, K.; Vargas, M.; Höppner, M.P.; McMillan, W.O.; Puebla, O. Inter-chromosomal coupling between vision and pigmentation genes during genomic divergence. Nat. Ecol. Evol. 2019, 3, 657–667. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).