Abstract
Sirtuin1 (Sirt1) has a NAD (+) binding domain and modulates the acetylation status of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) and Fork Head Box O1 transcription factor (Foxo1) according to the nutritional status. Sirt1 is decreased in obese patients and increased in weight loss. Its decreased expression explains part of the pathomechanisms of the metabolic syndrome, diabetes mellitus type 2 (DT2), cardiovascular diseases and nonalcoholic liver disease. Sirt1 plays an important role in the differentiation of adipocytes and in insulin signaling regulated by Foxo1 and phosphatidylinositol 3′-kinase (PI3K) signaling. Its overexpression attenuates inflammation and macrophage infiltration induced by a high fat diet. Its decreased expression plays a prominent role in the heart, liver and brain of rat as manifestations of fetal programming produced by deficit in vitamin B12 and folate during pregnancy and lactation through imbalanced methylation/acetylation of PGC1α and altered expression and methylation of nuclear receptors. The decreased expression of Sirt1 produced by impaired cellular availability of vitamin B12 results from endoplasmic reticulum stress through subcellular mislocalization of ELAVL1/HuR protein that shuttles Sirt1 mRNA between the nucleus and cytoplasm. Preclinical and clinical studies of Sirt1 agonists have produced contrasted results in the treatment of the metabolic syndrome. A preclinical study has produced promising results in the treatment of inherited disorders of vitamin B12 metabolism.
1. Introduction
Sirtuin1 (Sirt1) is one of the seven mammalian proteins belonging to the silent information regulator 2 (Sir2) proteins/Sirtuin family with highly conserved catalytic and nicotinamide adenine dinucleotide (NAD+) binding domain [1]. Sirtuins (Sirt) catalyze histone and non-histone lysine deacetylation in a NAD+ dependent manner [2,3]. Sirt1 has been shown to play an important role in increasing longevity in a number of lower animals like worms and flies [4,5,6]. Sirt1 is localized in the cytosol and the nucleus, between which it shuttles in response to different pathological and physiological environmental stimuli [2] including cellular stress and metabolic energy dysregulation. Sirt1 regulates cellular energy metabolism through deacetylation of transcription factors and co-factors of energy metabolism [4,7]. Beside its role in metabolic redox electron transfer, NAD+ is an essential Sirt1-dependent cell sensor of energy metabolism, insulin secretion and adaptation to cell stress [8].
1.1. Role of Sirt1 on the Regulation of Energy Metabolism by Peroxisome Proliferator-Activated Receptor-Γ Coactivator-1α and Peroxisome Proliferator Activated Receptors
Calorie restriction regulates the expression levels of Sirt1 in a tissue-dependent manner [9]. Sirt1 regulates tissue glucose homeostasis, fatty acid beta oxidation and energy metabolism by modulating the acetylation status of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α), Fork Head Box O1 transcription factor (Foxo1) and cAMP response element-binding protein (CREB), according to the nutritional status and fasting state. Sirt1 enhances PGC1α activity by deacetylating its lysine residues [10,11,12]. During fasting, Sirt1 activates PGC1α, which then induces the transcription of genes encoding gluconeogenic enzymes and suppresses the transcription of glycolytic genes in the liver [11]. Deacetylation of Foxo1 by Sirt1 regulates thyroid hormone mediated transcription of gluconeogenic genes like glucose-6-phosphatase and phospho-enoyl pyruvate carboxy kinase in mouse and human hepatic cells [13,14]. Activated Foxo1 binds to insulin response elements (IRE) promoters of these genes to induce their transcription [13,15]. Peroxisome proliferator activated receptors (PPARs) interact with co-regulators, including PGC1α in the regulation of energy homeostasis, insulin sensitivity, inflammation and adipogenesis [16]. Sirt1 regulates lipid metabolism of the liver in response to energy deprivation through deacetylation of PPARα. PPARα target genes encode fatty acid beta oxidation enzymes, carnitine palmitoyltransferase I, medium-chain acyl-CoA dehydrogenase and fatty acyl CoA synthase [17]. Liver specific ablation of Sirt1 induces the decreased transcription of PPARα target genes of fatty acid oxidation [18]. Similarly, the decreased expression of Sirt1 induces dysregulated energy metabolism through imbalanced acetylation and methylation of PGC1α in the myocardium of weaning animals exposed to methyl donor deficiency [19]. Sirt1 inhibits the expression of uncoupling protein gene 2 (UCP2), while Sirt1 silencing produces a mirrored effect in knock out mice [20]. Taken together, these data show that Sirt1 plays a prominent role on the regulation of energy metabolism through the deacetylation of PGC1α and Foxo1.
1.2. The Role of Sirt1 in Metabolic Syndrome and Insulin Resistance
The metabolic syndrome is a global public health problem related to overnutrition. It is defined as a cluster of cardio-metabolic abnormalities that includes obesity, insulin resistance or diabetes mellitus type 2 (DT2), hypertension and dyslipidemia [21,22]. The prevalence and incidence of the metabolic syndrome is increasing dramatically worldwide [21,23]. Systemic overexpression of Sirt1 has been reported to have protective effects against physiological damage in mice exposed to a high fat diet [24]. The decreased expression of Sirt1 explains part of the consequences of fatty acid enriched diets on the metabolic syndrome, DT2, cardiovascular diseases, nonalcoholic liver disease [25,26,27,28] and metabolic syndrome-associated cancers [29] (Figure 1). The decreased expression and activity of Sirt1 is involved in the pathogenesis of insulin resistance, DT2 and liver steatosis [30,31]. Decreased Sirt1 expression produces overexpression of miR-180a and impaired insulin signaling in hepatocytes miR-180a targets the 3′ UTR Sirt1 mRNA and is increased in insulin resistant hepatocytes and serum of diabetic patients [32]. Conversely, the overexpression of Sirt1 improves insulin sensitivity of hepatocytes in genetically obese ob/ob mice [33]. Insulin sensitivity is improved through inhibition of endoplasmic reticulum (ER) stress and decreased activity of mammalian/mechanistic target of Rapamycin complex 1 (mTORC1) in these animals [33]. Taken together, these data highlight the decreased activity of Sirt1 as a key component of the molecular mechanisms that produce the outcomes of metabolic syndrome and insulin resistance.
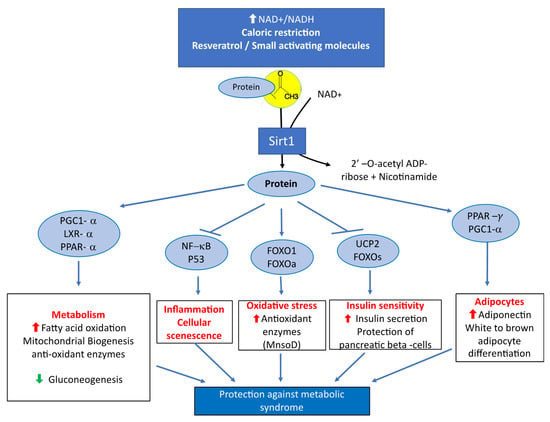
Figure 1.
Main metabolic effects of Sirtuin1 related to its protective influence against the manifestations of metabolic syndrome.
1.3. Role of Sirt1 in Pancreatic Beta Cells, Adipose Tissue and Skeletal Muscle
Secretion of insulin by pancreatic beta cells is enhanced by Sirt1 in response to glucose stimulation [34,35,36]. Conversely, decreased expression of Sirt1 impairs glucose-stimulated insulin secretion and expression of mitochondrial genes that control metabolic coupling. Genetic or pharmacologic activation of Sirt1 protects beta cells against lipotoxicity of circulating lipids [37,38]. Furthermore, the specific deletion of Sirt1 in beta cells decreases the expression of glucose transporters and ER chaperones involved in an unfolded protein response [39]. The treatment of human isolated islets with gamma butyric-acid (GABA) enhances Sirt1 activity and attenuates drug-induced apoptosis, suggesting an anti-apoptotic role of Sirt1 [40].
Clinical studies evidenced decreased Sirt1 expression in adipose tissues of obese patients [41,42] and increased expression in progressive weight loss [43,44]. Adipocyte specific ablation of Sirt1 induces increased adiposity and manifestation of metabolic dysfunction including insulin resistance [25]. Overexpression of Sirt1 in adipose tissue in mice enhances glucose homeostasis and prevents age-induced decline in insulin sensitivity [45]. Chronic exposure to a high fat diet accelerates glucose intolerance and hyperinsulinemia in mice with adipocyte specific knockout of Sirt1 [46]. High fat diet induces cleavage of Sirt1 via Caspase 1 activation in adipose tissues [25]. Sirt1 induces browning remodeling of white adipose tissue by deacetylation of PPARγ [47,48]. PPARγ is highly expressed in adipocytes where it acts as a key regulator of lipid metabolism, insulin sensitivity and adipocyte differentiation [41,42,49]. Adiponectin is an adipocyte hormone associated with obesity and type 1 diabetes [50]. Sirt1 regulates the secretion of adiponectin through PPARγ upregulation of ER oxidoreductase α (Erol-Lα) [51]. Sirt1 also upregulates adiponectin secretion by activating Foxo1 and increasing the interaction between Foxo1 and C/EBP α [52].
Sirt1 plays an important role in the differentiation of skeletal muscle [53]. Insulin signaling is regulated by the Foxo1-Sirt1 pathway in skeletal muscle [54]. Sirt1 enhances insulin sensitivity through PI3K signaling in response to caloric restriction [55]. Conversely, Sirt1 silencing decreases insulin sensitivity in Sirt1 KO mice. Wild type mice fed with a caloric restriction diet have a dramatic decreased acetylation of p53 and PGC1α, compared to Sirt1 KO mice [55]. In contrast, the overexpression of Sirt1 in skeletal muscle in vivo does not improve insulin resistance and had little impact on mitochondrial metabolism, suggesting that the overexpression of Sirt1 protein is not the single factor involved in insulin sensitization of skeletal muscle [56]. PPARγ- PGC1α couple is crucial in the regulation of energy metabolism in skeletal muscle. Its inhibition induces insulin resistance in C2C12 skeletal muscle cells, while its overexpression attenuates insulin resistance and enhanced glucose uptake [57]. In summary, Sirt1 acts as a sensor of the nutritional status on molecular mechanisms related with metabolic syndrome and insulin resistance, in pancreatic beta cells, adipose tissue and skeletal muscle.
1.4. Anti-Inflammatory Role of Sirt1 and PPAR in Metabolic Syndromes and Related Diseases
Visceral low grade inflammation triggered by adipocytes contributes to the pathogenesis of obesity, insulin resistance and other outcomes of the metabolic syndrome [58,59,60]. Besides dysregulated energy metabolism, increasing evidences link inflammation to pathogenesis of metabolic syndromes and related disorders, including diabetes and obesity [59,60,61]. Inflammation of the pancreatic beta cells in the islets is one of the prominent mechanisms of diabetes type 1 (DT1) and DT2. Adipocyte Sirt1 controls systemic insulin sensitivity through its effects on macrophages of adipose tissue [46,62]. Sirt1 knockdown exhibits elevated expression of Tumor Necrosis Factor α (TNF α) in adipocytes, induces macrophage infiltration and inflammation and increases cytokines levels of interleukin 1 beta (IL-1B), Interleukin 10 (IL-10), Interleukin 4 (IL-4) and TNF α in mice fed with high fat diet [63]. Conversely, overexpression of Sirt1 attenuates the adipose tissue inflammation and macrophage infiltration induced by a high fat diet [63]. Sirt1 inhibits inflammation in adipose tissue via mTOR/p70 ribosomal protein kinase 1 (S6k1) and Akt2 interacting pathways [64].
Converging evidences support an antagonistic crosstalk between Sirt1 and nuclear transcription factor Kappa B (NF-κB) [65]. Damaging effect of proinflammatory cytokines on beta cells is attenuated by the overexpression of Sirt1 in isolated rat islets through the deacetylation of P35 subunit of NF-κB [66]. Sirt1 deacetylates lysine 310 in the RelA/P65 subunit of NF-κB [67,68]. As a consequence, deacetylated RelA/P65 impairs methylation of lysine 314 and 315 residues, leading to ubiquitination and degradation [68,69]. Sirt1 knockdown in 3T3-L1 adipocytes activates the NF-κB signaling pathway by increased acetylation of NF-kB components and impaired interaction with promoters of matrix metalloproteinases and monocyte chemoattractant protein 1 (MCP1) [70].
Mice with hepatocyte specific knock out of Sirt1 (Sirt1LKO) exposed to a high fat diet develop hepatic inflammation and ER stress [18]. Liver inflammation of Sirt1 knock out mice results from increased expression of proinflammatory cytokine including TNF-α and IL-1B and macrophage infiltration. Liver ER stress of Sirt1LKO mice results from increased phosphorylation of translation initiation factor (elf2-α) and C-Jun N-terminal (JNK) [18]. PPARα confers protection against cellular stress and inflammation through various mechanisms related to Sirt1 [71]. PPARα agonist fenofibrate upregulates Sirt1 expression, suppresses CD40 and decreases acetylation of NF-κB-P65 in TNF-α treated 3T3-L1 adipocytes [72]. Chronic LPS stimulation of PPARγ deficient macrophages results in increased production of proinflammatory cytokines and decreased expression of anti-inflammatory cytokine IL-10 [73]. Furthermore, PPARγ deficiency causes delayed monocyte kinetic differentiation into macrophages [73]. In addition, PPARγ plays a crucial role in maturation of alternative phenotype in adipose tissues [74]. In summary, Sirt1 exerts protective effects against cellular stress and inflammation through complementary molecular and cellular mechanisms in adipose tissue, pancreatic islets and liver.
1.5. Antioxidant Role of Sirt1 and PPAR against Metabolic Syndromes and Related Diseases
Oxidative stress is associated with pathogenesis of complex metabolic diseases. Sirt1 activates Foxo transcription factors via the feedback loop [75,76]. Activation of Foxo3a and Foxo1a by Sirt1 deacetylation induces the transcription of catalase and manganese superoxide dismutase (MnSOD). Moderate overexpression of Sirt1 is protective against oxidative stress in the mice heart by upregulating the expression of catalase through Foxo1a-dependent mechanisms [77]. In contrast, high levels of Sirt1 increase oxidative stress and cardiomyopathy [77]. A decreased expression of Sirt1, increased expression of NADPH oxidases (P42Phox) and increased superoxide production is observed in monocytes of DT1 patients [78]. The protective role of Sirt1 against oxidative stress is linked to the deacetylation of check point kinase 2, which increases cell death under oxidative stress [79]. We and others have shown a link between Sirt1 and RNA binding proteins like HUR in response to cellular stress [80,81]. Mitochondrion is one of the main organelle involved in ROS production [82]. PGC1α induces expression of superoxide dismutase 2 (SOD2) and glutathione peroxidase involved in ROS detoxification [12,83]. These data illustrate the important role of Sirt1 in oxidative stress homeostasis.
1.6. Role of Sirt1 in Fetal Programming and Nutritional and Inherited Disorders of Vitamin B12 Metabolism
The fetal programming hypothesis [84], also named “developmental origins of health and disease hypothesis” (DOHaD), proposes that unfavorable intrauterine life, including intrauterine growth restriction (IUGR), predicts the risk of postnatal complex diseases, including insulin resistance, DT2 and other outcomes of pathological obesity. We have shown that the maternal deficiency in methyl donors (MDD, vitamin B12 and folate) during pregnancy and lactation of rodents produces a low birth weight and an epigenomic Sirt1-dependent dysregulation of mitochondrial energy production and fatty acid oxidation in offspring [85,86]. It is noteworthy that the decreased expression of Sirt1 observed in fetal programming of maternal MDD is also a hallmark of overnutrition and pathological obesity (Figure 2). Moreover, population studies have highlighted an association between maternal methyl donor status and manifestations of fetal programming. In India and Nepal, many babies are thin with central obesity. There is a higher prevalence of mothers with low serum vitamin B12, folate deficiency and intrauterine growth restriction, compared to Europe [87]. In these two countries, the most insulin resistant children are born to mothers who have the lowest serum vitamin B12 at the first trimester of pregnancy [88,89]. A variant of adenosyl-methionine decarboxylase is also associated with childhood obesity, in India [90]. Folate seems to influence the metabolic consequences of fetal programming in Europe, despite contrasted results among population studies [91]. In France, a genetic polymorphism (677C > T, relatively common) of methylenetetrahydrofolate reductase (MTHFR) was associated with low birth weight and high insulin resistance in morbidly obese adolescents [92].
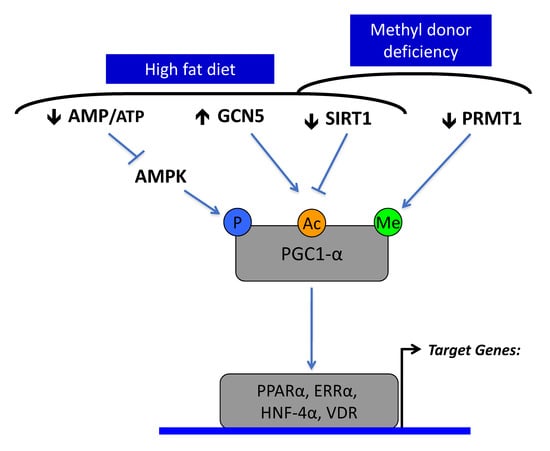
Figure 2.
The decreased expression and activity of Sirtuin1 is a common molecular hallmark in a high fat diet and methyl donor deficiency (MDD). Sirtuin1 targets the acetylation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) and has a complementary role with GCN5 acetylase, AMP kinase and protein arginine methyltransferase (PRMT1) in the regulation of PGC1α activation of nuclear receptors, including PPARα, ERRα, HNF-4α and VDR. PGC1α is phosphorylated and acetylated under the control of AMP kinase (AMPK), GCN5 acetylase and SIRT1 deacetylase. High fat diet and over nutrition decrease activity of AMP kinase, through high intracellular ATP levels, leading to decreased phosphorylation of PGC-1 α. It produces hyperacetylation of PGC-1α through increased expression of GCN5 and decreased expression and activity of SIRT1. MDD produces similar effects through decreased SIRT1 and PRMT1.
The decreased expression of Sirt1 plays a prominent role in the pathomechanisms of fetal programming produced by MDD in rats (vitamin B12 and folate) [19,81,93,94]. Vitamin B12 is metabolized into two active cofactors, methyl-cobalamin (Me-Cbl) and adenosyl-cobalamin (Ado-Cbl), the cofactors for cytoplasmic methionine synthase (MS/MTR) and mitochondrial methyl malonyl CoA mutase, respectively [95]. Me-Cbl and methyl folate are needed for the transmethylation of homocysteine into endogenous methionine, which is catalyzed by methionine synthase. Methionine is the direct precursor of S-adenosyl methionine (SAM), the universal methyl donor needed for transmethylation reactions involved in epigenomic regulatory mechanisms [86].
MDD during pregnancy and lactation induces impaired fatty acid oxidation, reduced activity of complexes I and II, cardiac hypertrophy with enlargement of cardiomyocyte and liver steatosis in weaning rat pups [19,96]. These observations are linked to the hyperacetylation and hypomethylation of PGC1α and dissociation of PGC1α from PPARα through decreased expression of Sirt1 and protein arginine methyltransferase 1 (PMRT1; Figure 2 and Figure 3) [19]. MDD weakens the activator activity of PGC1α for other nuclear receptors, including estrogen receptor-α (ER-α), estrogen-related receptor-α, hepatocyte nuclear factor 4 (HNF-4) and vitamin D receptor (VDR). The liver steatosis of pups born from mothers with MDD during pregnancy and lactation resulted predominantly from hypomethylation of PGC1α, the decreased binding with its partners, including PPARα and HNF4 and the subsequent impaired mitochondrial fatty acid oxidation [96]. The effects of fetal programming on the liver of rats born from MDD mothers are worsened when pups are subsequently subjected to a high fat diet (HF) after d50 [97]. The MDD/HF animals have hallmarks of steato-hepatitis, with increased markers of inflammation and fibrosis, insulin resistance and key genes triggering the pathomechanisms of non-alcoholic steato-hepatitis (NASH; transforming growth factor beta super family, angiotensin and angiotensin receptor type 1). These data show that MDD during pregnancy is a risk factor of NASH in populations subsequently exposed to a HF diet. The deactivation of PGC1α is also involved in the brain manifestations of MDD fetal programming in rats. MDD during gestation and lactation alters the cerebellum plasticity in offspring, with a lower expression of synapsins. The altered neuroplasticity results from decreased expression and methylation of ER-α and subsequent decreased ER-α/PPAR-γ coactivator 1 α (PGC-1α) interaction in the deficiency condition. The impaired ER-α pathway leads to decreased expression of synapsins through a decreased EGR-1/Zif-268 transcription factor and Src-dependent phosphorylation of synapsins [98]. Deficiencies in methyl donors and in vitamin D are independently associated with altered bone development. In young rats, MDD decreases the total body bone mineral density, reduced tibia length and impaired growth plate maturation, and in preosteoblasts, MDD slows cellular proliferation. MDD produces a decreased expression of VDR, estrogen receptor-α, PGC1α, PRMT1 and Sirt1 and decreased nuclear VDR-PGC1α interaction [99]. The weaker VDR-PGC1α interaction is attributed to the reduced expression and imbalanced methylation/acetylation of PGC1α and the nuclear VDR sequestration by heat shock protein 90 (HSP90). These mechanisms together compromise bone development, as reflected by lowered bone alkaline phosphatase and increased proadipogenic PPARγ, adiponectin and estrogen-related receptor-α expression [99].
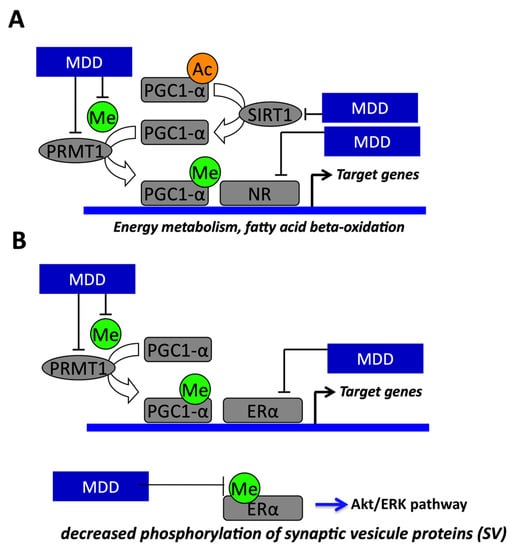
Figure 3.
Molecular mechanisms demonstrating the link between methyl donor deficiency (MDD) and fetal programming and energy metabolism in liver and heart and regulation of synapsin expression in neuron. (A) The effects of fetal programming on the heart, liver and brain of rats born from methyl donor deficient (MDD) mothers are related to impaired PGC-1α activity through decreased expression of Sirtuin1 (Sirt1) and protein arginine methyltransferase (PRMT1) and decreased synthesis of the universal methyl donor (Me) methyl S- adenosyl methionine (SAM). The decreased activity of PGC-1α results from imbalanced methylation and acetylation. PGC-1α is a regulator of lipid metabolism and fatty acid oxidation through its role as coactivator of PPARα in heart and liver. (B) MDD induces decreased phosphorylation of synaptic vesicle proteins through impaired ERα activity linked to decreased SAM levels, PMRT1 expression and PGC-1α activity. PGC-1α is a regulator of synapsin expression and vesicle transport through its role as a coactivator of ERα in the brain.
The impaired cellular availability of vitamin B12 leads to ER stress related to decreased expression of Sirt1. ER stress is evidenced by increased expression and activation of elf2-α and activating transcription factor 6 (ATF6) and may be favored by decreased expression of heat shock proteins (HSP). Decreased Sirt1 impairs the transcription of HSP by increasing the acetylation of heat shock protein factor 1 (HSF1; Figure 4) [93]. Conversely, overexpression of Sirt1 and HSF1 and activation of Sirt1 by SRT1720 as well as addition of vitamin B12 induce a dramatic decrease of ER stress in NIE115 neuronal cells with impaired cellular B12 availability [93]. Similarly, ER stress is increased in fibroblasts of cblC, cblG and cblG* patients with inherited disorders of cellular vitamin B12 metabolism [100]. Some of the molecular mechanisms that underlie the neurological manifestations of patients with inherited disorders of vitamin B12 metabolism are related to transcriptomic changes of genes involved in RNA metabolism and ER stress. The transcriptomic changes result from the subcellular mislocalization of several RNA binding proteins (RBP), including the ELAVL1/HuR protein implicated in neuronal stress and HnRNPA1 and RBM10, in patient fibroblasts and Cd320 knockout mice with impaired cellular uptake of vitamin B12. The decreased interaction of ELAVL1/HuR with the CRM1/exportin protein of the nuclear pore complex and its subsequent mislocalization result from hypomethylation by decreased SAM and protein methyl transferase CARM1 and dephosphorylation by increased protein phosphatase PP2A. The mislocalization of ELAVL1/HuR triggers the decreased expression of Sirt1 deacetylase and other genes involved in brain development, neuroplasticity, myelin formation and brain aging [81,100]. In summary, Sirt1 plays a prominent role in the pathomechanisms produced by MDD through its role on the acetylation of PGC1α, the transcription of HSP and the subcellular localization of RNA binding proteins (RBP).
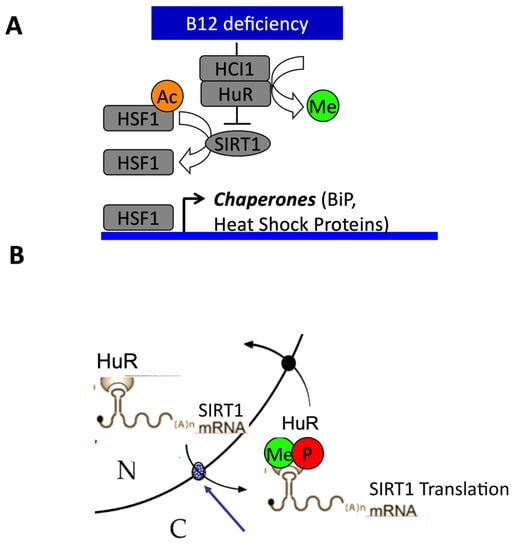
Figure 4.
Influence of vitamin B12 cellular availability on endoplasmic reticulum (ER) stress-related decreased expression of Sirtuin1 (Sirt1). (A) The decreased cellular availability in B12 activates ER stress pathways and decreases the expression of heat shock proteins through decreased expression of Sirt1 and subsequent hyperacetylation of heat shock factor 1 (HSF1). (B) The decreased cellular availability in B12 produces the subcellular mislocalization of the ELAVL1/HuR RNA binding protein implicated in response to ER stress through hypomethylation by decreased synthesis of methyl S-adenosyl methionine (SAM) and dephosphorylation by increased protein phosphatase PP2A. The blue arrow shows the nuclear pore complex. The mislocalization of ELAVL1/HuR triggered the decreased expression of Sirt1 by altered Sirt1 mRNA export from nucleus to cytoplasm.
1.7. Sirt1 Is a Target for the Treatment of Complex and Hereditary Metabolic Diseases
Preclinical and clinical studies in the treatment of complex and inherited metabolic diseases have produced contrasted results in the evaluation of health benefits of activation of Sirt1 by natural and pharmaceutical small activating molecules.
Resveratrol is a natural polyphenolic compound found in red wine and grape and in plants and fruits like berries and peanuts. Resveratrol activates Sirt1 and mimics the caloric restriction status known to be protective against the metabolic syndrome. For decades, the therapeutic use of resveratrol has been considered in regard to its anti-inflammatory, antioxidant, antiaging and anticancer properties [101,102,103]. It reduces oxidative stress, hepatic steatosis and hypertension in high fat diet induced obesity in rodents and non-human primates [104,105,106,107,108]. In addition, resveratrol protects against high fat diet induced hepatic steatosis and endoplasmic stress [104,108,109] by decreasing the expression of proinflammatory cytokines, including TNF- α, IL-1β and IL-6 and increasing antioxidant enzymes like SOD2 and catalase [110]. Treatment of human monocytes in hyperglycemic condition with resveratrol induces upregulated expression of Sirt1, decreased P42Phox expression and upregulates the expression and activation of Foxo3a, which together lead to decreased production of superoxide [78]. Resveratrol has been proposed as a potential therapeutic agent against cardiovascular diseases [107,111,112,113]. It reduces hypertension [114,115] and cardiac hypertrophy via LBK1-AMPK1-eNOS signaling pathways in hypertensive animals [114].
Given the health benefits of resveratrol in experimental animal studies, clinical trials have been carried out to evaluate its effects in the prevention and treatment of metabolic syndrome. A preliminary exploratory trial showed that administration of 500 mg resveratrol twice per day for 60 days in type 1 diabetic patients decreased fasting plasma sugar (FPS) and hemoglobin A1C [116]. A prospective open-label randomized controlled trial reported that a 30 days resveratrol supplementation improves hemoglobin A1c, total cholesterol and systolic blood pressure and insulin sensitivity in patients with diabetes type 2 [117,118]. A meta-analysis of nine randomized clinical trials with 283 participants concluded that resveratrol improves insulin sensitivity (HOMA-IR index) in DT2 patients, and had no effects on hemoglobin A1c and the lipid profile [119]. Similar results were observed in another meta-analysis of 29 randomized clinical trials involving 1069 participants [120]. In contrast, a one month randomized clinical double-blinded crossover administration of 150 mg/day of resveratrol had no effect on peripheral and hepatic insulin sensitivity [121]. This contrasted result could be due to the interaction of metformin and resveratrol in the DT2 recruited patients. Resveratrol has been also evaluated in cardiovascular diseases, neurodegenerative diseases and cancers [113,122,123]. For example, it ameliorates the endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ (PPARδ) [124].
Activators of Sirt1 such as SRT1720, SRT2183 and SRT1460 are 1000-fold more active than resveratrol. SRT1720 and resveratrol improve insulin sensitivity in nutritionally and genetically induced obesity and DT2 in mice [125]. SRT1720 treatment improved insulin sensitivity of liver, skeletal muscle and adipose tissue in insulin resistance Zucker fa/fa rats [126]. SRT1720 enhances fatty acid oxidation through direct deacetylation of PGC1α, Foxo1 and indirect activation of the AMP activated protein kinase (AMPK) pathway [126]. Furthermore, SRT1720 extends the lifespan of mice fed a high fat diet, decreases hepatic steatosis, increases insulin sensitivity and reverses inflammation and oxidative stress markers in adult obese mice subjected to a high fat diet [127]. SRT1720 impairs lipopolysaccharide stimulated inflammatory pathways and TNF-α secretion in macrophages and adipose tissues of Zucker fatty rats [70]. SRT1720 also activates AMPK in a Sirt1-independent manner. However, SRT1720 cannot be used in humans because of potential toxicity. Metformin regulates gluconeogenesis in ob/ob mice by upregulating hepatic Sirt1 and GCN5 [128]. A computational study confirmed that metformin directly activates Sirt1 [129]. At low NAD+ concentration, leucine and low dose of metformin synergistically activate Sirt1 and AMPK, with enhanced energy metabolism and insulin sensitivity in muscle cells and hepatic cells in vitro [130]. Recently two novel Sirt1 activators with high affinity for Sirt1, SC1C2 and SC1C2.1 attenuated doxorubicin induced DNA damage via deacetylation of P53 and cellular senescence in HepG2 and H9c2 cell lines [131].
Phase 1 clinical trials showed that SRT2104, another small molecule activator of Sirt1 is safe and tolerable in healthy volunteers, including the elderly [132,133]. However, in a prospective double blinded random placebo control crossover study, the daily administration of 2.0 g of SRT2104 for 28 days had a neutral cardiovascular effect in DT2 patients, even if it induced a loss in body weight [134]. Lipid profile of healthy smokers was improved after 28 days SRT2104 administration but again the cardiovascular effects were neutral [134,135].
Some severe forms of inherited disorders of intracellular metabolism of vitamin B12 are resistant to conventional treatments. Decreased Sirt1 activity plays a central role in some of the pathomechanisms of these disorders. We therefore evaluated the effect of Sirt1 agonists in a preclinical study in fibroblasts from patients with cblG and cblC inherited defects of vitamin B12 metabolism and an original transgenic mouse model of methionine synthase deficiency specific to neuronal cells. Patient fibroblasts with cblC and cblG defects of vitamin B12 metabolism presented with endoplasmic reticulum stress, altered subcellular localization of HuR, HnRNPA1 and RBM10, global mRNA mislocalization and increased HnRNPA1-dependent skipping of interferon regulatory factor 3 (IRF3) exons. SRT1720 inhibited ER stress and rescued RBP and mRNA mislocalization and IRF3 splicing. Furthermore, Sirt1 activation by SRT1720 partially restored the methylation and phosphorylation of these RBPs in patients’ fibroblasts. Interestingly, SRT1720, vitamin B12 and SAM treatment improved cognitive functions in conditional MTR-KO mice with brain specific invalidation of Mtr gene encoding MS enzyme [100]. In particular, SRT1720 improved the deficient hippocampo-dependent learning ability of the mice.
In summary, preclinical and clinical studies of Sirt1 agonists have produced contrasted results in the treatment of the metabolic syndrome. A preclinical study has produced promising results in the treatment of inherited disorders of vitamin B12 metabolism.
2. Conclusions
Numerous experimental evidence show a strong link between decreased Sirt1 and the pathological manifestations of metabolic syndrome by synergistic cellular and molecular mechanisms. It is noteworthy that over nutrition and MDD both produce a decrease of Sirt1. There are additive and synergistic effects of the MDD fetal programming and subsequent exposure to a high fat diet in adult life. The therapeutic prospects for using activators of Sirt1 in the treatment of disease outcomes of metabolic syndrome have not been conclusive to date. However, pharmacological activation of Sirt1 opens promising perspectives for the treatment of rare diseases of vitamin B12 metabolism with particular effects on reticulum stress and mislocalization of RBPs.
Author Contributions
Main drafting of the manuscript, V.J.K. and J.-L.G.; partial drafting of specific parts, R.-M.G.-R. and D.C.; critical revision of the manuscript, V.J.K., D.C., R.-M.G.-R. and J.-L.G.; study supervision, J.-L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FHU ARRIMAGE and the French PIA project “Lorraine Université d’Excellence”, reference ANR-15-IDEX-04-LUE.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
Abbreviations
SIRT1: Sirtuin 1; PPARs, Peroxisome proliferator activated receptors; PPARα, Peroxisome proliferator-activated receptor alpha; PPARγ, Peroxisome proliferator-activated receptor gamma; PGC1α, peroxisome proliferator-activated receptor-γ coactivator-1α; DT1, diabetes type 1; DT2, diabetes mellitus type 2; Foxo1, Forkhead Box O1; PI3K, phosphatidylinositol 3′-kinase; NAD, Nicotinamide adenine dinucleotide; CREB, cAMP response element-binding protein; IRE, insulin response element; UCP2, uncoupling protein gene 2; ER, endoplasmic reticulum; mTORC1, mammalian/mechanistic target of Rapamycin complex 1; GABA, gamma butyric-acid; Erol-Lα, ER oxidoreductase α; C/EBP α, CCAAT/enhancer binding protein alpha; TNF α, Tumor Necrosis Factor alpha, IL- 1B, Interleukin 1 beta; IL- 10, Interleukin 10; IL- 4, Interleukin 4; S6K1, p70 ribosomal protein kinase 1; NF-κB, nuclear transcription factor Kappa B; Sirt1LKO, hepatocyte specific knock out of Sirt1; MCP1, monocyte chemoattractant protein 1; JNK, C-Jun N-terminal; LPS, lipopolysaccharide; MnSOD, manganese superoxide dismutase; DOHaD, Developmental origins of health and disease hypothesis; IUGR, intra-uterine growth restriction, MDD, methyl donors deficiency; MTHFR, methylenetetrahydrofolate reductase; Me-Cbl, methyl-cobalamin; Ado-Cbl, adenosyl-cobalamin; MS/MTR, methionine synthase, SAM, S- adenosyl methionine, PMRT1, protein arginine methyltransferase; HNF-4, hepatocyte nuclear factor 4; VDR, vitamin D receptor HF, high fat diet; NASH, non-alcoholic steato-hepatitis; ER-α,estrogen receptor-α; ERRα, estrogen related receptor-α; HSP, heat shock protein; HSF1, heat shock protein factor 1; ATF6, activating transcription factor 6; RBP, RNA binding proteins, AMPK, AMP activated protein kinase; FPS, fasting plasma sugar, IRF3, interferon regulatory factor 3.
References
- Frye, R. Phylogenetic Classification of Prokaryotic and Eukaryotic Sir2-like Proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic Shuttling of the NAD+-dependent Histone Deacetylase SIRT1. J. Biol. Chem. 2006, 282, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.; Sutton, A.; Tafrov, S.T.; Heller, R.C.; Stebbins, J.; Pillus, L.; Sternglanz, R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA 2000, 97, 5807–5811. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef]
- Rogina, B.; Helfand, S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2012, 45, 51–60. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Canto, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2009, 31, 194–223. [Google Scholar] [CrossRef]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.-J.; Cheng, H.-L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef]
- Nakae, J.; Cao, Y.; Daitoku, H.; Fukamizu, A.; Ogawa, W.; Yano, Y.; Hayashi, Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J. Clin. Investig. 2006, 116, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Sinha, R.A.; Zhou, J.; Xie, S.Y.; You, S.-H.; Gauthier, K.; Yen, P.M. FoxO1 Deacetylation Regulates Thyroid Hormone-induced Transcription of Key Hepatic Gluconeogenic Genes*. J. Biol. Chem. 2013, 288, 30365–30372. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.K.; Suwanichkul, A.; Scheimann, A.O.; Yee, D.; Jackson, J.G.; Barr, F.G.; Powell, D.R. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology 1999, 140, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Lamichane, S.; Lamichane, B.D.; Kwon, S.-M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef]
- Feingold, K.R.; Wang, Y.; Moser, A.; Shigenaga, J.K.; Grunfeld, C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J. Lipid Res. 2008, 49, 2179–2187. [Google Scholar] [CrossRef]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-Specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef]
- Garcia, M.M.; Guéant-Rodriguez, R.-M.; Pooya, S.; Brachet, P.; Alberto, J.-M.; Jeannesson, E.; Maskali, F.; Gueguen, N.; Marie, P.-Y.; Lacolley, P.; et al. Methyl donor deficiency induces cardiomyopathy through altered methylation/acetylation of PGC-1α by PRMT1 and SIRT1. J. Pathol. 2011, 225, 324–335. [Google Scholar] [CrossRef]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.E.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2005, 4, e31. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Sigit, F.S.; Tahapary, D.L.; Trompet, S.; Sartono, E.; Van Dijk, K.W.; Rosendaal, F.R.; De Mutsert, R. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: A cross-sectional analysis of two population-based studies. Diabetol. Metab. Syndr. 2020, 12, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.; Elakoum, R.; Ziegler, O.; Coelho, D.; Feigerlova, E.; Daval, J.-L.; Gueant-Rodriguez, R.-M. Nutritional models of foetal programming and nutrigenomic and epigenomic dysregulations of fatty acid metabolism in the liver and heart. Pflüg. Arch. Eur. J. Physiol. 2013, 466, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-Fat Diet Triggers Inflammation-Induced Cleavage of SIRT1 in Adipose Tissue to Promote Metabolic Dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Von Frankenberg, A.D.; Marina, A.; Song, X.; Callahan, H.S.; Kratz, M.; Utzschneider, K.M. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur. J. Nutr. 2017, 56, 431–443. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Liu, G.; Chen, Y.; Wu, T.; Feng, F.; Chao, S. Sirt1 decreased adipose inflammation by interacting with Akt2 and inhibiting mTOR/S6K1 pathway in mice. J. Lipid Res. 2016, 57, 1373–1381. [Google Scholar] [CrossRef]
- Herranz, D.; Muñoz-Martin, M.; Cañamero, M.; Mulero, F.; Martinez-Pastor, B.; Fernandez-Capetillo, O.; Serrano, M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010, 1, 1–8. [Google Scholar] [CrossRef]
- Wang, R.H.; Kim, H.S.; Xiao, C.; Xu, X.; Gavrilova, O.; Deng, C.X. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Investig. 2011, 121, 4477–4490. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Liang, Y.; Vanhoutte, P.M. SIRT1 in metabolic syndrome: Where to target matters. Pharmacol. Ther. 2012, 136, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, C.; Qi, W.; Zhang, Y.; Zhang, F.; Wu, J.X.; Hu, Y.N.; Wu, D.M.; Liu, Y.; Yan, T.T.; et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetology 2012, 55, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Luo, Z.; Walsh, K.; et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Roy, U.; Garg, S.; Ghosh, S.; Pathak, S.; Kolthur-Seetharam, U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic beta-islets. FEBS J. 2011, 278, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.; Ogawa, O.; Kubo, S.; Mitsuhashi, N.; Onuma, T.; Kawamori, R. Ankle brachial pressure index and carotid intima-media thickness as atherosclerosis markers in Japanese diabetics. Diabetes Res. Clin. Pract. 2004, 66, 269–275. [Google Scholar] [CrossRef]
- Luu, L.; Dai, F.F.; Prentice, K.J.; Huang, X.; Hardy, A.B.; Hansen, J.B.; Liu, Y.; Joseph, J.W.; Wheeler, M.B. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia 2013, 56, 2010–2020. [Google Scholar] [CrossRef]
- Desai, T.; Koulajian, K.; Ivovic, A.; Breen, D.M.; Luu, L.; Tsiani, E.L.; Wheeler, M.B.; Giacca, A. Pharmacologic or genetic activation of SIRT1 attenuates the fat-induced decrease in beta-cell function in vivo. Nutr. Diabetes 2019, 9, 11. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Lu, Y.; Zhang, J.; Jian, F.; Liu, Y.; Li, F.; Wenyi, L.; Xiao, W.; Li, G. Activation of SIRT1 protects pancreatic beta-cells against palmitate-induced dysfunction. Biochim. Biophys. Acta 2012, 1822, 1815–18125. [Google Scholar] [CrossRef]
- Pinho, A.V.; Bensellam, M.; Wauters, E.; Rees, M.; Giry-Laterriere, M.; Mawson, A.; Ly, L.Q.; Biankin, A.V.; Wu, J.; Laybutt, D.R.; et al. Pancreas-Specific Sirt1-Deficiency in Mice Compromises Beta-Cell Function without Development of Hyperglycemia. PLoS ONE 2015, 10, e0128012. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y.; Udovyk, O.; Hasilo, C.; Paraskevas, S.; Wang, Q. GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochem. Biophys. Res. Commun. 2014, 452, 649–654. [Google Scholar] [CrossRef]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gómez, G.; Gray, S.L.; Yetukuri, L.; Shimomura, K.; Virtue, S.; Campbell, M.; Curtis, R.K.; Jimenez-Liñan, M.; Blount, M.; Yeo, G.S.H.; et al. PPAR gamma 2 Prevents Lipotoxicity by Controlling Adipose Tissue Expandability and Peripheral Lipid Metabolism. PLoS Genet. 2007, 3, e64. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Fiore, D.; Persichetti, A.; Basciani, S.; Lubrano, C.; Poggiogalle, E.; Genco, A.; Donini, L.M.; Gnessi, L. Circulating SIRT1 Increases After Intragastric Balloon Fat Loss in Obese Patients. Obes. Surg. 2015, 26, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Rappou, E.; Jukarainen, S.; Rinnankoski-Tuikka, R.; Kaye, S.; Heinonen, S.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Saunavaara, V.; Rissanen, A.; et al. Weight Loss Is Associated With Increased NAD(+)/SIRT1 Expression But Reduced PARP Activity in White Adipose Tissue. J. Clin. Endocrinol. Metab. 2016, 101, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bai, B.; Fan, P.; Cai, Y.; Huang, B.; Law, I.K.; Liu, L.; Xu, A.; Tung, C.; Li, X.; et al. Selective overexpression of human SIRT1 in adipose tissue enhances energy homeostasis and prevents the deterioration of insulin sensitivity with ageing in mice. Am. J. Transl. Res. 2013, 5, 412–426. [Google Scholar]
- Hui, X.; Zhang, M.; Gu, P.; Li, K.; Gao, Y.; Wu, D.; Wang, Y.; Xu, A. Adipocyte SIRT 1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 2017, 18, 645–657. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARgamma in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, H.; Farmer, S.R. Adiponectin Secretion Is Regulated by SIRT1 and the Endoplasmic Reticulum Oxidoreductase Ero1-Lα. Mol. Cell. Biol. 2007, 27, 4698–4707. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Shao, J. SIRT1 Regulates Adiponectin Gene Expression through Foxo1-C/Enhancer-binding Protein Transcriptional Complex. J. Biol. Chem. 2006, 281, 39915–39924. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Schiltz, R.; Iezzi, S.; King, M.; Zhao, P.; Kashiwaya, Y.; Hoffman, E.; Veech, R.L.; Sartorelli, V. Sir2 Regulates Skeletal Muscle Differentiation as a Potential Sensor of the Redox State. Mol. Cell 2003, 12, 51–62. [Google Scholar] [CrossRef]
- Sin, T.K.; Yung, B.Y.; Siu, P.M. Modulation of SIRT1-Foxo1 Signaling axis by Resveratrol: Implications in Skeletal Muscle Aging and Insulin Resistance. Cell. Physiol. Biochem. 2015, 35, 541–552. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.E.; Tid-Ang, J.; Wright, L.E.; Stuart, E.; Suryana, E.; Bentley, N.; Turner, N.; Cooney, G.J.; Ruderman, N.B.; Kraegen, E.W. Overexpression of SIRT1 in Rat Skeletal Muscle Does Not Alter Glucose Induced Insulin Resistance. PLoS ONE 2015, 10, e0121959. [Google Scholar] [CrossRef]
- Verma, N.K.; Singh, J.; Dey, C.S. PPAR-gamma expression modulates insulin sensitivity in C2C12 skeletal muscle cells. Br. J. Pharmacol. 2004, 143, 1006–1013. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of Chronic State of Inflammation as Mediators That Link Obese Adipose Tissue and Metabolic Syndrome. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Porro, S.; Nigro, P.; Cignarelli, A.; Caccioppoli, C.; Genchi, V.A.; Martines, G.; De Fazio, M.; Capuano, P.; Natalicchio, A.; et al. Reduced SIRT1 and SIRT2 expression promotes adipogenesis of human visceral adipose stem cells and associates with accumulation of visceral fat in human obesity. Int. J. Obes. 2019, 44, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Gillum, M.P.; Kotas, M.E.; Erion, D.M.; Kursawe, R.; Chatterjee, P.; Nead, K.T.; Muise, E.S.; Hsiao, J.J.; Frederick, D.W.; Yonemitsu, S.; et al. SirT1 Regulates Adipose Tissue Inflammation. Diabetes 2011, 60, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Patil, I.Y.; Jiang, T.; Sancheti, H.; Walsh, J.P.; Stiles, B.L.; Yin, F.; Cadenas, E. High-Fat Diet Induces Hepatic Insulin Resistance and Impairment of Synaptic Plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Song, M.-Y.; Song, E.-K.; Kim, E.-K.; Moon, W.S.; Han, M.-K.; Park, J.-W.; Kwon, K.-B.; Park, B.-H. Overexpression of SIRT1 Protects Pancreatic β-Cells against Cytokine Toxicity by Suppressing the Nuclear Factor-κB Signaling Pathway. Diabetes 2008, 58, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; Mu, Y.; Greene, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002, 21, 6539–6548. [Google Scholar] [CrossRef]
- Yang, X.-D.; Tajkhorshid, E.; Chen, L.-F. Functional Interplay between Acetylation and Methylation of the RelA Subunit of NF-κB. Mol. Cell. Biol. 2010, 30, 2170–2180. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Milne, J.C.; Imamura, T.; Schenk, S.; Sonoda, N.; Babendure, J.L.; Lu, J.-C.; Smith, J.J.; Jirousek, M.R.; Olefsky, J. SIRT1 Exerts Anti-Inflammatory Effects and Improves Insulin Sensitivity in Adipocytes. Mol. Cell. Biol. 2008, 29, 1363–1374. [Google Scholar] [CrossRef]
- Fuentes, E.; Guzman, L.; Moore-Carrasco, R.; Palomo, I. Role of PPARs in inflammatory processes associated with metabolic syndrome (Review). Mol. Med. Rep. 2013, 8, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, Q.; Lin, R.; Zhang, J.; Ren, F.; Zhang, J.; Meixi, J.; Li, Y. PPARalpha agonist fenofibrate attenuates TNF-alpha-induced CD40 expression in 3T3-L1 adipocytes via the SIRT1-dependent signaling pathway. Exp. Cell Res. 2013, 319, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Heming, M.; Gran, S.; Jauch, S.L.; Fischer-Riepe, L.; Russo, A.; Klotz, L.; Hermann, S.; Schäfers, M.; Roth, J.; Barczyk-Kahlert, K. Peroxisome Proliferator-Activated Receptor-gamma Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front. Immunol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Red Eagle, A.; Vats, D.; Morel, C.R.; Goforth, M.H.; Subramanianet, V.; Mukundan, L.; Ferrante, A.W.; Chawla, A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008, 7, 496–507. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, J.F.A.; Patrushev, N.; Alexander, R.W. FoxO1 Mediates an Autofeedback Loop Regulating SIRT1 Expression. J. Biol. Chem. 2010, 286, 5289–5299. [Google Scholar] [CrossRef]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J.-I. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Yun, J.-M.; Chien, A.; Jialal, I.; Devaraj, S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: Mechanistic insights. J. Nutr. Biochem. 2012, 23, 699–705. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, S.; Kim, Y.-N.; Lee, I.H. Deacetylation of CHK2 by SIRT1 protects cells from oxidative stress-dependent DNA damage response. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Pullmann, R.; Lal, A.; Kim, H.H.; Galban, S.; Yang, X.; Blethrow, J.D.; Walker, M.; Shubert, J.; Gillespie, D.A.; et al. Phosphorylation of HuR by Chk2 Regulates SIRT1 Expression. Mol. Cell 2007, 25, 543–557. [Google Scholar] [CrossRef]
- Battaglia-Hsu, S.-F.; Ghemrawi, R.; Coelho, D.; Dreumont, N.; Mosca, P.; Hergalant, S.; Gauchotte, G.; Sequeira, J.M.; Ndiongue, M.; Houlgatte, R.; et al. Inherited disorders of cobalamin metabolism disrupt nucleocytoplasmic transport of mRNA through impaired methylation/phosphorylation of ELAVL1/HuR. Nucleic Acids Res. 2018, 46, 7844–7857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.S.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef]
- Blaise, S.; Alberto, J.-M.; Audonnet-Blaise, S.; Guéant, J.; Daval, J.-L. Influence of preconditioning-like hypoxia on the liver of developing methyl-deficient rats. Am. J. Physiol. Metab. 2007, 293, E1492–E1502. [Google Scholar] [CrossRef]
- Guéant, J.; Namour, F.; Gueant-Rodriguez, R.-M.; Daval, J.-L. Folate and fetal programming: A play in epigenomics. Trends Endocrinol. Metab. 2013, 24, 279–289. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshmukh, U.S. Fetal programming: Maternal nutrition and role of one-carbon metabolism. Rev. Endocr. Metab. Disord. 2012, 13, 121–127. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshmukh, U.S. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev. Endocr. Metab. Disord. 2008, 9, 203–211. [Google Scholar] [CrossRef]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P. Low Maternal Vitamin B-12 Status Is Associated with Offspring Insulin Resistance Regardless of Antenatal Micronutrient Supplementation in Rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef]
- Tabassum, R.; Jaiswal, A.; Chauhan, G.; Dwivedi, O.P.; Ghosh, S.; Marwaha, R.K.; Tandon, N.; Bharadwaj, D. Genetic Variant of AMD1 Is Associated with Obesity in Urban Indian Children. PLoS ONE 2012, 7, e33162. [Google Scholar] [CrossRef]
- Lewis, S.J.; Leary, S.; Smith, G.D.; Ness, A. Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br. J. Nutr. 2009, 102, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Frelut, M.-L.; Nicolas, J.-P.; Guilland, J.-C.; De Courcy, G.P. Methylenetetrahydrofolate reductase 677 C->T polymorphism: A link between birth weight and insulin resistance in obese adolescents. Pediatr. Obes. 2011, 6, e312–e317. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Pooya, S.; Lorentz, S.; Gauchotte, G.; Arnold, C.; Guéant, J.; Battaglia-Hsu, S.-F. Decreased vitamin B12 availability induces ER stress through impaired SIRT1-deacetylation of HSF1. Cell Death Dis. 2013, 4, e553. [Google Scholar] [CrossRef] [PubMed]
- Melhem, H.; Hansmannel, F.; Bressenot, A.; Battaglia-Hsu, S.-F.; Billioud, V.; Alberto, J.M.; Guéant, J.; Peyrin-Biroulet, L. Methyl-deficient diet promotes colitis and SIRT1-mediated endoplasmic reticulum stress. Gut 2015, 65, 595–606. [Google Scholar] [CrossRef]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef]
- Pooya, S.; Blaise, S.; Garcia, M.M.; Giudicelli, J.; Alberto, J.-M.; Gueant-Rodriguez, R.-M.; Jeannesson, E.; Gueguen, N.; Bressenot, A.; Nicolas, B.; et al. Methyl donor deficiency impairs fatty acid oxidation through PGC-1α hypomethylation and decreased ER-α, ERR-α, and HNF-4α in the rat liver. J. Hepatol. 2012, 57, 344–351. [Google Scholar] [CrossRef]
- Bison, A.; Marchal-Bressenot, A.; Li, Z.; Elamouri, I.; Feigerlova, E.; Peng, L.; Houlgatte, R.; Beck, B.; Pourié, G.; Alberto, J.-M.; et al. Foetal programming by methyl donor deficiency produces steato-hepatitis in rats exposed to high fat diet. Sci. Rep. 2016, 6, 37207. [Google Scholar] [CrossRef]
- Pourié, G.; Martin, N.; Bossenmeyer-Pourié, C.; Akchiche, N.; Gueant-Rodriguez, R.-M.; Geoffroy, A.; Jeannesson, E.; Chehadeh, S.E.H.; Mimoun, K.; Brachet, P.; et al. Folate- and vitamin B12–deficient diet during gestation and lactation alters cerebellar synapsin expression via impaired influence of estrogen nuclear receptor α. FASEB J. 2015, 29, 3713–3725. [Google Scholar] [CrossRef]
- Feigerlova, E.; Demarquet, L.; Melhem, H.; Ghemrawi, R.; Battaglia-Hsu, S.F.; Ewu, E.; Alberto, J.M.; Helle, D.; Weryha, G.; Guéant, J.G. Methyl donor deficiency impairs bone development via peroxisome proliferator-activated receptor-gamma coactivator-1alpha-dependent vitamin D receptor pathway. FASEB J. 2016, 30, 3598–3612. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Arnold, C.; Battaglia-Hsu, S.-F.; Pourié, G.; Trinh, I.; Bassila, C.; Rashka, C.; Wiedemann, A.; Flayac, J.; Robert, A.; et al. SIRT1 activation rescues the mislocalization of RNA-binding proteins and cognitive defects induced by inherited cobalamin disorders. Metabilisem 2019, 101, 153992. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; D’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol:In VitroandIn VivoStudies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Jung, U.J.; Choi, M.-S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.-S.; Maudsley, S.; An, S.S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef]
- Pan, Q.-R.; Ren, Y.-L.; Liu, W.-X.; Hu, Y.-J.; Zheng, J.-S.; Xu, Y.; Wang, G. Resveratrol prevents hepatic steatosis and endoplasmic reticulum stress and regulates the expression of genes involved in lipid metabolism, insulin resistance, and inflammation in rats. Nutr. Res. 2015, 35, 576–584. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Paraíso, A.F.; De Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; De Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutriens 2014, 30, 915–919. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; Gonzalez, A.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Nekooeian, A.A.; Mashghoolozekr, E.; Panjehshahin, M.R. The cardioprotective effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension. Nat. Prod. Commun. 2015, 10, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Lisboa, P.C.; Lima, N.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutriens 2020, 12, 161. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Guo, X.-F.; Li, J.-M.; Tang, J.; Li, D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 58, 3016–3029. [Google Scholar] [CrossRef]
- Timmers, S.; De Ligt, M.; Phielix, E.; Van De Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, W.; Fang, J.; Wang, W.; He, P.; Lei, J.; Wang, C. Efficacy of Resveratrol Supplementation against Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Placebo-Controlled Clinical Trials. PLoS ONE 2016, 11, e0161792. [Google Scholar] [CrossRef] [PubMed]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Feige, J.N.; Lagouge, M.; Canto, C.; Strehle, A.; Houten, S.M.; Milne, J.C.; Lambert, P.D.; Mataki, C.; Elliott, P.J.; Auwerx, J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008, 8, 347–358. [Google Scholar] [CrossRef]
- Minor, R.K.; Baur, J.A.; Gomes, A.P.; Ward, T.M.; Csiszar, A.; Mercken, E.M.; Abdelmohsen, K.; Shin, Y.-K.; Canto, C.; Scheibye-Knudsen, M.; et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011, 1, 70. [Google Scholar] [CrossRef]
- Caton, P.W.; Nayuni, N.K.; Kieswich, J.; Khan, N.Q.; Yaqoob, M.M.; Corder, R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J. Endocrinol. 2010, 205, 97–106. [Google Scholar] [CrossRef]
- Cuyàs, E.; Verdura, S.; Llorach-Pares, L.; Fernández-Arroyo, S.; Joven, J.; Martin-Castillo, B.; Bosch-Barrera, J.; Brunet, J.; Nonell-Canals, A.; Sanchez-Martinez, M.; et al. Metformin Is a Direct SIRT1-Activating Compound: Computational Modeling and Experimental Validation. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Banerjee, J.; Bruckbauer, A.; Zemel, M. Activation of the AMPK/Sirt1 pathway by a leucine–metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolisam 2016, 65, 1679–1691. [Google Scholar] [CrossRef]
- Scisciola, L.; Sarno, F.; Carafa, V.; Cosconati, S.; Di Maro, S.; Ciuffreda, L.; De Angelis, A.; Stiuso, P.; Feoli, A.; Sbardella, G.; et al. Two novel SIRT1 activators, SCIC2 and SCIC2.1, enhance SIRT1-mediated effects in stress response and senescence. Epigenetics 2020, 15, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Wald, J.; Lavu, S.; Roberts, J.; Beaumont, C.; Haddad, J.; Elliott, P.; Westphal, C.; Jacobson, E. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br. J. Clin. Pharmacol. 2012, 75, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Libri, V.; Brown, A.P.; Gambarota, G.; Haddad, J.; Shields, G.S.; Dawes, H.; Pinato, D.J.; Hoffman, E.; Elliot, P.J.; Vlasuk, G.P.; et al. A Pilot Randomized, Placebo Controlled, Double Blind Phase I Trial of the Novel SIRT1 Activator SRT2104 in Elderly Volunteers. PLoS ONE 2012, 7, e51395. [Google Scholar] [CrossRef] [PubMed]
- Noh, R.M.; Venkatasubramanian, S.; Daga, S.; Langrish, J.; Mills, N.L.; Lang, N.N.; Hoffmann, E.; Waterhouse, B.; Newby, D.E.; Frier, B.M. Cardiometabolic effects of a novel SIRT1 activator, SRT2104, in people with type 2 diabetes mellitus. Open Heart 2017, 4. [Google Scholar]
- Venkatasubramanian, S.; Noh, R.M.; Daga, S.; Langrish, J.P.; Joshi, N.V.; Mills, N.L.; Hoffmann, E.; Jacobson, E.W.; Vlasuk, G.P.; Waterhouse, B.R.; et al. Cardiovascular Effects of a Novel SIRT1 Activator, SRT2104, in Otherwise Healthy Cigarette Smokers. J. Am. Heart Assoc. 2013, 2, e000042. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).