Abstract
The KDM4 histone demethylase subfamily is constituted of yeast JmjC domain-containing proteins, such as Gis1, and human Gis1 orthologues, such as KDM4A/B/C. KDM4 proteins have important functions in regulating chromatin structure and gene expression in response to metabolic and nutritional stimuli. Heme acts as a versatile signaling molecule to regulate important cellular functions in diverse organisms ranging from bacteria to humans. Here, using purified KDM4 proteins containing the JmjN/C domain, we showed that heme stimulates the histone demethylase activity of the JmjN/C domains of KDM4A and Cas well as full-length Gis1. Furthermore, we found that the C-terminal regions of KDM4 proteins, like that of Gis1, can confer heme regulation when fused to an unrelated transcriptional activator. Interestingly, biochemical pull-down of Gis1-interacting proteins followed by mass spectrometry identified 147 unique proteins associated with Gis1 under heme-sufficient and/or heme-deficient conditions. These 147 proteins included a significant number of heterocyclic compound-binding proteins, Ubl-conjugated proteins, metabolic enzymes/proteins, and acetylated proteins. These results suggested that KDM4s interact with diverse cellular proteins to form a complex network to sense metabolic and nutritional conditions like heme levels and respond by altering their interactions with other proteins and functional activities, such as histone demethylation.
1. Introduction
Histone lysyl demethylases (KDMs) play important roles in chromatin remodeling and gene regulation by catalyzing the demethylation of methylated lysine residues on the N-terminal tails of histones [1]. The majority of KDMs are JmjC domain-containing proteins, which are dioxygenases that use α-ketoglutarate and Fe2+ to oxidize various substrates [2,3,4]. The KDM4 family, containing five members, i.e., KDM4A-E, accepts mono-, di- and trimethylated lysines as substrates [5,6]. KDM4A/B/C (Figure 1), but not KDM4D or E, contain the JmjC domain in the N-terminus as well as Tudor and plant homeodomain (PHD) domains in the C-terminus. KDM4A/B/C are widely expressed, and their orthologues are found among all vertebrates [7,8]. KDM4A/B/C share more than 50% sequence identity (Figure 1) and catalyze the demethylation of tri- and di-methylated forms of both histone H3 lysine 9 (H3K9me3/me2) and lysine 36 (H3K36me3/me2) [3,9,10,11,12,13]. KDM4 proteins play important roles in diverse biological processes, including oncogenesis, inflammation, and hematopoiesis [8,14,15,16]. It is worth noting that the histone demethylase activity of KDM4s requires α-ketoglutarate, O2, and Fe2+, which are important nutritional and metabolic indicators. Thus, histone demethylase activity is linked to and is highly responsive to nutrient status [17,18,19,20,21,22].
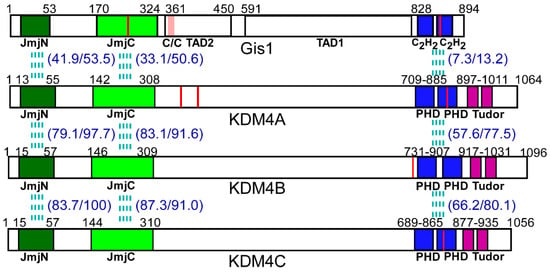
Figure 1.
The structure and alignment of Gis1 and KDM4A/B/C domains. The domains of Gis1 and KDM4s are shown. Gis1 contains the JmjN + JmjC (JmjN/C) domain, the coiled-coil domain (C/C), two C2H2 type zinc fingers (ZnFs), and two transcription activation domains (TAD1 and TAD2). KDM4A/B/C proteins contain the JmjN/C domain, two PHD domains (each containing a conserved C4HC3 or C4HC2H motif), and two Tudor domains. The red lines represent cysteine-proline (CP) motifs, black lines indicate the start and end of the shown domains, and the pink box in the Gis1 structure denotes the coiled-coil domain. The % amino acid identity and similarity between Gis1 and KDM4s in JmjN/C and zinc finger domains are shown in parentheses.
The yeast JmjC domain-containing protein Gis1 is an orthologue of KDM4 proteins. Previous data, including our own recent data, showed that Gis1 possesses histone demethylase activity at H3K36 [23,24,25,26]. In yeast, Gis1 is one of the key regulators mediating transcriptional reprogramming of carbon metabolism and stress responses during diauxic and post-diauxic shifts [27,28,29,30,31,32]. Gis1 binds to the post-diauxic shift (PDS) DNA element and can both activate and repress transcription [33,34,35]. It acts downstream of TOR, RAS/cAMP, and AKT/Sch9 signaling pathways in response to nutrient status [28,30,32]. Gis1 contains multiple domains, including a JmjN region, a JmjC region, a coiled-coil domain, two C2H2 type zinc fingers (ZnFs), and two transcription activation domains (TADs) (Figure 1) [30,36,37]. JmjN and JmjC interact physically to form a structural unit or a domain [37]. The JmjN/C domain presumably confers histone demethylase activity, but is dispensable for transcriptional activation by Gis1 [36]. This Gis1 JmjN/C domain is highly homologous to those of KDM4A/B/C, while the C-terminal regions of Gis1 and KDM4A/B/C share low homology (Figure 1). The Gis1 zinc fingers allow Gis1 to bind to DNA and regulate transcription [30,36,37]. The C-terminal regions of KDM4A/B/C proteins contain two PHDs and two Tudor domains (Figure 1). The PHD domain is a protein module containing ~50 amino acid residues with a conserved C4HC3 or C4HC2H motif that coordinates two zinc ions in a cross-brace configuration [38]. The Tudor domain typically contains ~60 amino acids that comprise 4–5 antiparallel β-strands to form a barrel-like structure [39]. Both PHD and Tudor domains are histone reader domains that recognize post-translationally modified histones, such as methylated H3 [40,41,42,43]. Although the JmjN/C domains of Gis1 and KDM4s are highly conserved, the C-terminal domain sequences of Gis1 are quite different, and there is no homology between the TADs of Gis1 and the corresponding regions of KDM4s (Figure 1). Thus, we do not expect that KDM4s can substitute for Gis1 in yeast.
Interestingly, our recent studies showed that heme promotes both transcriptional and histone demethylase activities of Gis1 [23]. Heme can bind to both the JmjN/C and ZnF domains, however, heme binding to the JmjN/C domain is not necessary for heme regulation of Gis1 activity. In contrast, heme binding to the ZnF domain promotes heme activation of both transcriptional and histone demethylase activities [23]. Under heme-deficient conditions, Gis1 is free of heme and is inactive. Its activities are repressed by the ZnF domain, which likely cooperates with other cellular proteins. When heme levels increase, heme binds to the JmjN/C and ZnF domains, presumably causing other cellular proteins to dissociate from Gis1 and alter Gis1 conformation, thereby leading to full activation of Gis1 transcriptional and demethylase activities. Heme acts as a prosthetic group or cofactor in proteins and enzymes required for oxygen utilization and metabolism, such as globins and cytochromes [44,45,46,47]. Heme serves as a signaling molecule regulating diverse processes ranging from gene transcription to circadian rhythm [48,49,50]. Heme directly binds to and modulates the activities of certain cellular proteins, such as the yeast heme-regulatory protein Hap1 [51,52] and the mammalian nuclear receptor Rev-erbα [49,53]. Experimental studies have increasingly shown that heme is a central signaling molecule in an array of physiological and pathological processes, including neurodegeneration, tumorigenesis, and adipogenesis [54,55,56].
Because Gis1 can sense heme and change its transcriptional and histone demethylase activities in response to heme, we asked if heme also regulates the activity of the human KDM4 family members, particularly, KDM4A/B/C which, like Gis1, not only contain the conserved JmjN/C domain, but also the C-terminal region with PHD zinc fingers (Figure 1). Interestingly, our data showed that heme strongly activates histone demethylase activity of KDM4A and C, but not KDM4B, while heme binds to all three. Furthermore, we identified other cellular proteins that are critical for heme regulation of Gis1. We found that 147 proteins could bind to Gis1 in vitro, notably, the transcriptional regulator Mot3 [57,58], which binds to Gis1 and is required for heme activation of Gis1 activity. Our data showed that heme is a conserved regulatory factor for KDM4 proteins and that heme regulation of Gis1 and likely other KDM4s involves a network of cellular proteins.
2. Materials and Methods
2.1. Yeast Strains and Plasmids
The yeast strains used were BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), MHY101 (MATa ura3-52 leu2-3,112 his4-519 ade1-100 hem1-Δ100 URA3::PDS-lacZ), MHY101Δgis1 (MATa ura3-52 leu2-3,112 his4-519 ade1-100 hem1-Δ100 URA3::PDS-lacZ gis1::LEU2 ura3::Kanr), MHY101Δgis1Δmot3 (MATa ura3-52 leu2-3,112 his4-519 ade1-100 hem1-Δ100 URA3::PDS-lacZ gis1::LEU2 mot3::HIS4 ura3::Kanr), and BWG1-7bΔgis1 (MATa ura3-52leu2-3,112 his4-159 ade1-100 URA3::PDS-lacZ gis1::LEU2 ura3::Kanr). The Yeast Tandem Affinity Purification (TAP) Fusion Library (scTAP) comprised of 4247 Open Reading Frames (ORFs), was purchased from Open Biosystems (Cat. No. YSC1177).
To delete the GIS1 gene, MHY101 cells were transformed with a PCR product containing the LEU2 gene in the middle and 44 bps sequences flanking the open reading frame sequence of GIS1 on both sides. Knockout strains were confirmed by PCR and β-galactosidase assay. To delete the MOT3 gene, MHY101Δgis1 cells were transformed with a PCR product containing the HIS4 gene in the middle and 44 bps sequences flanking the open reading frame sequence of MOT3 on both sides. Knockout strains were confirmed by PCR. The PDS element-driven LEU2-lacZ reporter was introduced by transforming yeast strains with the with linearized, NcoI-cut pLS9-PDS plasmid, as described previously [34]. In the MHY101Δgis1 strain, the URA3 gene was deleted by transformation with a PCR product containing Kanr sequence in the middle and 44 bps of sequence flanking the open reading frame sequence of URA3 on both sides.
The PDS element-driven LEU2-lacZ reporter plasmid pLS9-PDS was as described [34], and was provided by Dr. Claudio de Virgilio’s lab (University of Fribourg, Fribourg, Switzerland). The pET-15b bacterial expression vector for expressing His6-tagged Gis1 from the T7 lac promoter was as described [59], and was provided by Dr. George M. Carman’s lab (Rutgers University, New Brunswick, NJ, USA). The pET-15b bacterial expression vector for expression of the His6-tagged JmjN/C domain of Gis1 from the T7 lac promoter was as described [23]. The JmjN/C domains of KDM4A/B/C were cloned into the T7 expression vector pET-15b cut with NdeI/BamHI, NdeI/XhoI, or XhoI/BamHI respectively. The coding sequences were confirmed by DNA sequencing (Eurofins MWG operon, Louisville, KY, USA). The yeast expression vectors for full-length Gis1 (pYY53), Gis1ΔJmjC (pYY54), and Gis1ΔZnF (pYY55) were as described [36], and were provided by Dr. Rolf Sternglanz’s lab (Stony Brook University, Stony Brook, NY, USA). The expression vector for Gis1ΔJmjN/C was as described [37,60], and was provided by Dr. Nianshu Zhang’s lab (University of Cambridge, UK). The expression vectors for the fusion proteins Hap1-GisΔZnF, Hap1-Gis1, Hap1-KDM4A, Hap1-KDM4B, and Hap1-KDM4C were constructed by inserting the coding sequences for the fusion proteins to the yeast expression vector SD5-HAP1 [61]. The DNA containing the coding sequences for fusion proteins was generated by overlapping PCR, as described previously [62]. The sequences of fusion proteins were confirmed by DNA sequencing using Eurofins MWG operon USA.
2.2. Cell Growth and β-Galactosidase Assays
Yeast cells were grown in rich yeast extract peptone dextrose (YPD) or synthetic complete media, as described previously [63,64]. Cell density was determined by measuring optical density at 600 nm. To determine β-galactosidase levels from reporter genes in Δhem1 cells bearing the PDS element-driven LEU2-lacZ reporter or the Hap1-driven UAS1-CYC1-lacZ reporter, cells were grown in synthetic complete medium containing a limiting amount of the heme precursor 5-aminolevulinic acid (ALA, 2.5 μg/mL) or a high amount of ALA (250 μg/mL). Cells were collected after they reached an optical density (600 nm) of approximately 1.0–1.5 to measure Gis1 and Hap1 activities. Collected cells were then subjected to chloroform permeabilization and β-galactosidase assays. The activities were measured and calculated in Miller units, as described previously [65,66].
2.3. Purification of KDM4 and Gis1 proteins, Spectroscopic Analyses, and Protein Binding to Heme–Agarose Beads
To purify the JmjN/C domains of KDM4A/B/C or full-length JmjN/C of Gis1 from Escherichia coli, BL21(DE3) bearing the pET-15b expression plasmids were grown to A0.5, then induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 2 h at 25 °C. Cells were collected and lysed with a French Press. The His6-tagged proteins were purified with Ni Sepharose 6 fast flow columns (GE Healthcare, Chicago, IL, USA), according to the manufacturer’s protocol. The eluted His6-Gis1 or His6-KDM4A/B/C proteins were desalted with PD-10 desalting columns (GE healthcare, Chicago, IL, USA), then cleaved with 0.005 U thrombin per μg of protein. Proteins without the tag were then purified from the mixture by passing them through the Ni Sepharose 6 fast flow columns again. To overcome nonspecific Ni binding, a stepped elution was performed with elution buffer containing increasing concentrations of imidazole (0, 40, 80, and 250 mM). All eluates were analyzed on SDS-PAGE gels.
Heme absorbance spectra were measured with a Varian Cary® 50 UV-Vis Spectrophotometer. Samples contained 10 µM protein, 5 µM heme, and 0, 10, 20, 40, 80, or 160 mM imidazole. Protein and heme were prepared in 20 mM Tris (pH 8.0) and 500 mM NaCl; the imidazole stock was adjusted to pH 8.0 with HCl. Each sample was incubated for 30 s after the addition of heme prior to absorbance measurement.
To detect protein binding to heme with heme–agarose beads (Sigma, St. Louis, MO, USA), purified proteins were incubated with the beads in the heme binding buffer (20 mM Tris (pH 8.0), 500 mM NaCl, and 1% TritonX-100) for 1 h at 4 °C. After incubation, the beads were pelleted by centrifugation, and the supernatant was collected. The beads were then washed twice with heme-binding buffer. Subsequently, proteins bound to the beads and in the supernatant were electrophoresed on SDS polyacrylamide gels and visualized by Coomassie blue staining.
2.4. Measurement of KDM4A/B/C and Gis1 Demethylase Activity
The demethylase activity of purified KDM4A/B/C proteins was measured using an Epigenase Demethylase Activity/Inhibition Assay Kit (EpiGentek, P3081-48), according to the manufacturer’s protocol. Briefly, 50 ng of the trimethyl histone H3K9 peptide (Cat. No. R-1028), which was derived from residues within 1–100 of human histone H3, was stably coated onto 96-well microplate wells. Enzymatic reactions were set up for blank (without protein) and sample (with 300 ng, about 3 pmol, of purified KDM4A/B/C proteins) in a total volume of 50 μL, in the presence or absence of 2 μM heme. The microplate strip wells were covered with adhesive covering film and incubated at 37 °C for 120 min. To detect the amount of demethylated product, the wells were washed and incubated with a capture antibody (anti-H3K9 me1) at room temperature for 60 min followed by incubation with a detection antibody at room temperature for 30 min. The amount of demethylated product was fluorometrically measured with a BioTek Cytation 5-plate reader with excitation and emission wavelengths of 530 nm and 590 nm, respectively, after adding a fluorescence development solution. Demethylase activity of the protein was calculated in Relative Fluorescence Units (RFU) /min/mg, as described in the manufacturer’s protocol.
The demethylase activity of purified Gis1 proteins was measured using a custom-made Epigenase Demethylase Activity/Inhibition Assay Kit (EpiGentek, P3081-48 CUSTOM) with dimethyl histone H3K36 peptide (Cat. No. R-1038) anti-H3K36me1 capture antibody, as described above.
2.5. Identification of Gis1 Interactors, Mass Spectrometry (MS), and Proteomic Data Analysis
Yeast cell extracts were prepared according to previously established protocols [67,68]. Briefly, yeast cells were grown to an optical density (OD) of 1.0–1.5 in rich medium containing glucose under normoxic, hypoxic, intermediate, and high-heme conditions. The hypoxic (~10 ppb O2) growth condition was created using a hypoxia chamber (Coy Laboratory, Inc.) and it with a mixture of 5% H2 and 95% N2 in the presence of a palladium catalyst [69]. Intermediate and high heme conditions were achieved by growing Δhem1 yeast cells in the presence of intermediate (50 μg/mL) or high (250 μg/mL) levels of ALA. Cells were harvested and resuspended in three packed cell volumes of buffer (20 mM Tris, 10 mM MgCl2, 1 mM Ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 1 mM dithiothreitol (DTT), 0.3 M NaCl, 1 mM phenylmethylsulfonyl fluoride, 1X protease inhibitors). Cells were then permeabilized by agitation with four packed cell volumes of glass beads and extracts were collected, as previously described [68].
His6-Gis1 was pre-bound to a Ni Sepharose 6 Fast Flow column (GE Healthcare, Chicago, IL). To identify the Gis1 interactors, the crude yeast protein extract was loaded onto a Gis1-bound column and incubated at 4 °C for 2 h. The complexes were then eluted from the column and separated by SDS-PAGE using 12% polyacrylamide gels followed by Coomassie blue staining. A total of 19 protein bands (see Supplemental Figure S2) were excised with sterile blades and destained twice with 100 mM NH4HCO3 in 50% acetonitrile. The samples were prepared following the Penn State Hershey Mass Spectrometry Core facility standard protocol. The Mass Spectrometry (MS) spectra were taken by ABSciex 5600 TripleTOF using samples separated by Eksigent NanoLC-Ultra-2D Plus and Eksigent cHiPLC Nanoflex via 200 µm × 0.5 mm chromatography. Multiple proteins were often identified in each band.
ProteinPilot software version 5.0 was used to perform protein identification by searching MS spectra against the species-specific (Saccharomyces. cerevisiae) National Center for Biotechnology Information (NCBI) database sequences, which contains 36,621 protein sequences plus 156 common lab contaminants. Bioinformatics analyses were performed using the paragon algorithm in ProteinPilot 5.0 software (Framingham, MA, USA) to identify the potential proteins interacting with Gis1 by ranking them according to their unused scores. Protein identifications must have a ProteinPilot Unused Score >1.3 (>95% confidence interval) in order to be accepted. In addition, the only protein IDs accepted must have a “Local False Discovery Rate” estimation of no higher than 5%, which is a more stringent criterion than the often-used 1% Global False Discovery Rate. The identified protein list was then uploaded to CRAPome to eliminate potential false positives [70]. Proteins with p values equal to 0 were selected and further analyzed. The total number of unique proteins identified was 148, including Gis1. The proteins were then grouped based on the conditions in which they were identified into various classes (high ALA only, intermediate ALA only, hypoxia only, air only, and a combination of these classes). Further Gene Ontology (GO) analysis of the identified proteins was performed using STRING (http://string-db.org/). Human orthologues of the yeast proteins were identified using YeastMine (https://yeastmine.yeastgenome.org/).
2.6. Preparation of Yeast Extracts, TAP-Pulldown, and Western Blotting
Yeast cells expressing various TAP-tagged proteins and full-length Gis1 or Gis1 deletion proteins were grown in synthetic complete media to an optical density (600 nm) of approximately 1.0–1.5. Cells were harvested and extracts were prepared as described previously [71]. For TAP-pulldown, protein extracts were incubated with IgG-Sepharose beads to capture the target proteins. After this binding reaction, the beads were washed to remove unbound proteins, as described [72]. All steps were carried out in the cold room at 4 °C. Input and protein complexes bound to the beads were detected by Western blotting, where 75 μg of proteins from each treatment condition were electrophoresed on 10% SDS–Polyacrylamide gels, then transferred onto an Immuno-Blot PVDF Membrane (Bio-Rad, Hercules, CA, USA). The membranes were probed with antibodies, followed by detection with a chemiluminescent Western blotting kit (Roche Diagnostics, Mannheim, Germany). The signals were detected with a Carestream image station 4000MM Pro and quantitation was performed with the Carestream molecular imaging software version 5.0.5.30 (Carestream Health, Inc., Rochester, NY, USA).
3. Results
3.1. Heme Binds to the JmjN/C Domain of KDM4A/B/C and Regulates the Histone Demethylase Activity of KDM4A and C, but not KDM4B
Previously, we showed that the purified JmjN/C domain of Gis1 binds to heme [23]. Here, we purified the JmjN/C domains (Figure 1) of KDM4A/B/C. We used previously well-established heme bead binding and heme spectrum methods [23,51,73] to determine if the JmjN/C domains of KDM4A/B/C bind to heme. First, using heme–agarose beads, we showed that the JmjN/C domains of KDM4A/B/C all bound to heme (Figure 2). For controls, we showed that carbonic anhydrase did not bind to heme agarose (lanes 1–3, Figure 2), as expected [74]. In contrast, both human serum albumin (has) (lanes 4–6, Figure 2) and bovine serum albumin (BSA) (lanes 7–9, Figure 2) bound to heme beads, because they bind to heme directly [74,75]. The JmjN/C domains of KDM4A/B/C all bound to heme beads strongly (lanes 10–18, Figure 2). When the JmjN/C domains of KDM4A/B/C were mixed in equal molar amounts with BSA and incubated with heme beads, they bound more strongly than BSA (lanes 19–24, Figure 2). Note that heme attached to the beads via the propionate group, thus, KDM4s do not interact with heme via the propionate group. Second, we examined heme binding to JmjN/C domains by detecting their effect on the major heme absorption band, the Soret band, which is around 400 nm. Like the JmjN/C domain of Gis1 (Figure 3A), JmjN/C domains of KDM4A/B/C (Figure 3B–D) bound to heme and shifted the Soret peak to longer wave lengths (Figure 3A–D, compare lines 1 and 2). The JmjN/C domains of Gis1 and KDM4A/B/C appeared to be purified without heme (Figure 3A–D), while full-length Gis1, which has higher heme-binding affinity than the JmjN/C domain, appeared to be purified with some heme [23]. As shown previously [51], imidazole chelated heme and shifted the Soret peak from about 386 nm to about 434 nm, and it also enhanced the spectral changes caused by the binding of the JmjN/C domain of Gis1 or KDM4A/B/C (compare lines 3 and 4 in Figure 3A–D). Specifically, in the presence of 10 mM imidazole, binding of the JmjN/C domains shifted the heme absorption peak from around 434 nm to 411 nm (compare lines 3 and 4 in Figure 3A–D). Figure 3E shows that 10–160 mM imidazole induced very similar heme spectral changes. Using imidazole titration as described previously [23], we found that similar concentrations of imidazole were required to shift the Soret peak to 434 nm in the presence of JmjN/C domains of KDM4-C compared to that of Gis1 (Supplemental Figure S1). The difference spectra shown in Figure 3A–D further illustrate the change caused by the binding of the JmjN/C domains to heme, particularly in the presence of imidazole, suggesting that the JmjN/C domains of KDM4A/B/C bound to heme with comparable affinity to the Gis1 JmjN/C domain.
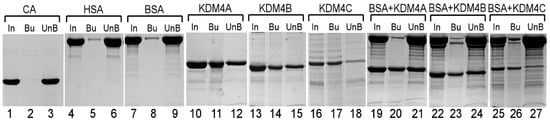
Figure 2.
The pull-down of KDM4 JmjN/C domains by heme–agarose beads. Samples containing 500 pm of carbonic anhydrase (CA) (lanes 1–3), human serum albumin (HSA) (lanes 4–6), and bovine serum albumin (BSA) (lanes 7–9), and the JmjN/C domain of KDM4A (lanes 10–12), KDM4B (lanes 13–15), or KDM4C (lanes 16–18), or a mixture of the indicated proteins (500 pm each, lines 19–27) were incubated with heme–agarose beads. The input (In), bound (Bu), and unbound proteins (UnB) were analyzed by SDS-PAGE and are shown here.
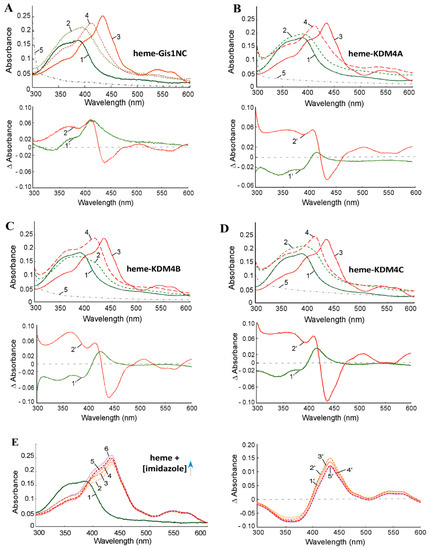
Figure 3.
Absorption spectra of heme in the absence and presence of KDM4 JmjN/C domains. (A) Heme absorption spectra in the presence of the Gis1 JmjN/C domain (shown for comparison). (B) Heme absorption spectra in the presence of the KDM4A JmjN/C domain. (C) Heme absorption spectra in the presence of the KDM4B JmjN/C domain. (D) Heme absorption spectra in the presence of the KDM4C JmjN/C domain. (E) The effect of increasing concentrations of imidazole on the heme absorption spectrum. (A–D) Line 1: 5 μM heme; line 2: 5 μM heme + 10 μM JmjN/C domain of Gis1 or KDM4A/B/C; line 3: 5 μM heme + 10 mM imidazole; line 4: 5 μM heme + 10 μM JmjN/C domain of Gis1 or KDM4A/B/C + 10 mM imidazole; line 5: 10 μM JmjN/C domain of Gis1 or KDM4A/B/C. (E) Line 1: 5 μM heme; line 2: 5 μM heme + 10 mM imidazole; line 3: 5 μM heme + 20 mM imidazole; line 4: 5 μM heme + 40 mM imidazole; line 5: 5 μM heme + 80 mM imidazole; line 6: 5 μM heme + 160 mM imidazole. The difference spectra are shown under the heme absorption spectra. (A–D) Line 1′: (heme + protein) – heme; line 2′: (heme + imid + protein) – (heme + imid). E: Line 1′: (heme + 10 mM imidazole) – heme; line 2′: (heme + 20 mM imidazole) – heme; line 3′: (heme + 40 mM imidazole) – heme; line 4′: (heme + 80 mM imidazole) – heme; line 5′: (heme + 160 mM imidazole) – heme.
To determine whether heme binding to KDM4 JmjN/C domains affects their functions, we detected the effect of heme on histone demethylase activity using the well-established Epigenase Demethylase Activity/Inhibition Assay Kit (EpiGentek, Farmingdale, NY, USA). We detected the effect of heme on the activity of purified JmjN/C domains of KDM4A/B/C on H3K9me3. We found that heme strongly potentiated the activity of KDM4A and C, but not KDM4B (Figure 4). For comparison, we showed that heme stimulated the demethylase activity of full-length Gis1, but not the JmjN/C domain, as reported previously [23]. Clearly, the JmjN/C domains of KDM4A and C can mediate heme regulation of their histone demethylase activity, whereas those of KDM4B and Gis1 cannot.
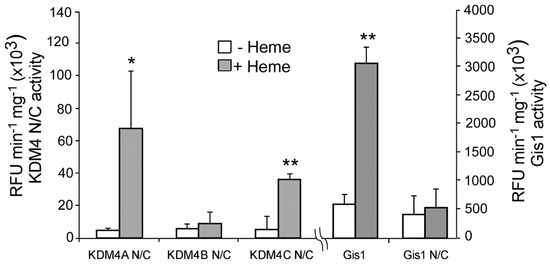
Figure 4.
Heme stimulates histone demethylase activities of KDM4A and C, but not KDM4B. The H3K9me3 demethylase activities of purified KDM4 JmjN/C domains were measured as described in Section 2. The assays were repeated multiple times. The data plotted here are averages from at least three replicates. For statistical analysis, the activity in the presence of heme was compared to the activity in the absence of heme with a Welch 2-sample t-test. * p value < 0.05; ** p value < 0.005. The demethylase activities of full-length Gis1 and the Gis1 N/C domain are shown for comparison.
3.2. The C-terminal Regions of KDM4A/B/C, like that of Gis1, Have the Potential to Modulate the Activity of the JmjN/C Domain and Heme Regulation
Previously, we showed that the C-terminal region of Gis1 containing the zinc finger domain promoted heme regulation when inserted between the DNA-binding and activation domains of a heterologous transcriptional activator Hap1 [23], as shown in Figure 5A,B (see Gis1-Hap1). We also inserted the C-terminal regions containing the PHD and Tudor domains of KDM4A/B/C between the DNA-binding and activation domains of Hap1 (Figure 5A,B). We found that the C-terminal regions of KDM4A, like that of Gis1, strongly promoted heme activation of transcriptional activity, while those of KDM4B and C somewhat promoted heme activation of transcriptional activity (Figure 5B). These results suggested that the C-terminal regions of KDM4A, like that of Gis1, and to a lesser extent, KDM4B and C, have the potential to modulate the activity of the JmjN/C domains and heme regulation.
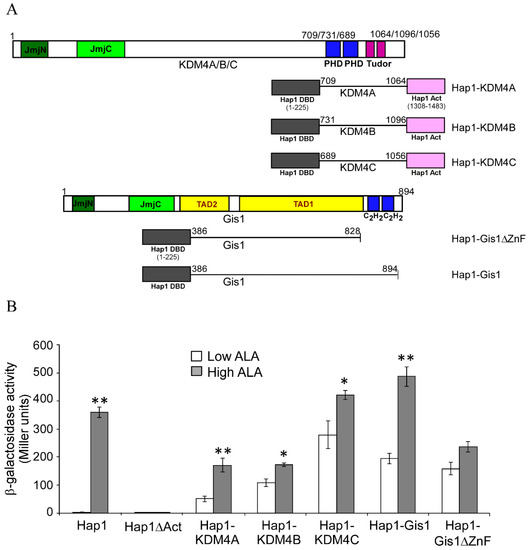
Figure 5.
The C-terminal domains of KDM4 proteins can confer heme regulation of transcriptional activity via an unrelated transcription factor. (A) The domain maps of the hybrid proteins are shown. (B) The transcriptional activities of hybrid proteins in yeast cells. Yeast Δgis1Δhem1 cells bearing the expression vector for wild type Hap1, Hap1ΔAct lacking the activation domain, Hap1-KDM4A, Hap1-KDM4B, and Hap1-KDM4C fusion protein, as well as the Hap1-binding UAS1-CYC1-lacZ reporter, were grown in the presence of low (2.5 μg/mL) or high (250 μg/mL) levels of ALA. Cells were then collected and β-galactosidase activities were measured. For statistical analysis, the levels in heme-deficient cells were compared to the levels in heme-sufficient cells with a Welch 2-sample t-test. * p value < 0.05; ** p value <0.005. The activities of Hap1-Gis1 and Hap1-Gis1ΔZnF are shown for comparison.
3.3. Mass Spectrometry (MS) Identified 147 Proteins that can Interact with Gis1 In Vitro
Gis1, like other KDM4 proteins, is known to interact with many proteins [76]. These proteins may influence Gis1 histone demethylase and transcriptional activities and may promote heme regulation. Furthermore, Gis1 is likely to interact with different proteins under heme-deficient and heme-sufficient conditions. We therefore systematically identified the proteins that interact with Gis1 under heme-deficient and heme-sufficient conditions by coupling biochemical pull-down with mass spectrometry. To this end, we used yeast strains that were fully functional in oxygen and heme signaling [64]. We prepared extracts from Δgis1 (to limit preformed Gis1 complexes) cells grown in air or under hypoxic conditions, and Δgis1Δhem1 cells grown at high or intermediate heme levels. We used extracts prepared from hypoxic cells which could not synthesize heme [77] in lieu of heme-deficient cells because proteins are very labile in extracts prepared from heme-deficient cells. To identify Gis1-interacting proteins, extracts were loaded onto columns bound by full-length Gis1 purified from E. coli, and bound proteins were collected and subjected to SDS-PAGE. Protein bands that were retained specifically and only on Gis1-bound beads were excised (see bands shown in Supplemental Figure S2) and identified with mass spectrometry (MS). After extensive statistical and computational analyses (see Section 2), we identified 147 Gis1-interacting protein candidates (see Figure 6A). Several classes of the candidate proteins were worth noting, including 58 proteins that were identified only under hypoxic conditions that may suppress Gis1 activities in heme-deficient cells (see Table 1), 54 proteins that were identified only under high or intermediate levels of ALA or in air (heme-sufficient) that may promote Gis1 activities in heme-sufficient cells (see Table 2), and 36 proteins that were identified under both hypoxic/heme-deficient and heme-sufficient conditions that may both suppress and promote Gis1 activities depending on the heme levels (see Table 3). Seven of the identified Gis1-interacting proteins, Mig1, Snf1, Msc1, Bck1, Kcs1, Rvs167, and Cyr1 interact with Gis1 genetically [34,78,79,80,81], strongly supporting the validity of the biochemistry-MS approach used here. To further confirm the results from MS, we carried out co-immunoprecipitation for several identified Gis1-interacting protein candidates. Figure 6B shows that Mot3, Oye2, and PbP4 can be pulled down together specifically with Gis1, which further validates the results from the MS analysis. Interestingly, Figure 6C also shows that the ZnF domain of Gis1 is required for interaction with Mot3, while the JmjN/C domain is not necessary for interaction with Mot3. The lower band of the doublets shown in Figure 6B,C are presumably shorter, degraded fragments of the detected proteins lacking part of the N-terminal sequences (tags here are at the C-terminus).
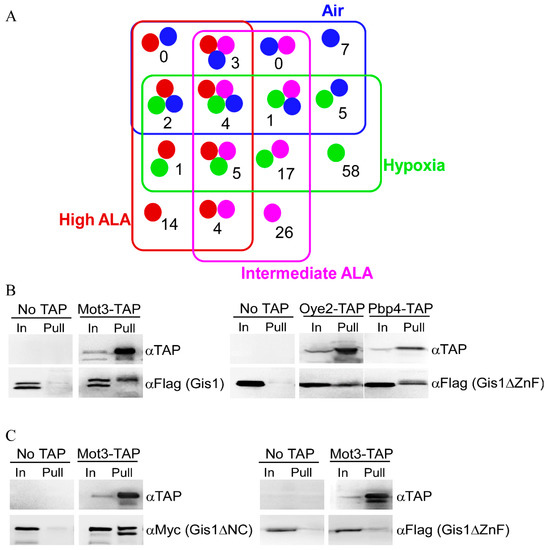
Figure 6.
(A) Venn diagrams showing overlaps for Gis1-bound proteins under various conditions. The numbers of proteins bound to Gis1 identified from extracts prepared from cells grown under various conditions are shown here. (B) TAP pull-down confirms that Mot3, Oye2, and Pbp4 interact with Gis1. Extracts prepared from cells expressing TAP-tagged Mot3, Pbp4, or Oye2 and FLAG-tagged Gis1 were incubated with IgG-Sepharose 6 beads. Input proteins (In) and pulled-down (Pull) proteins were electrophoresed and detected by anti-TAP or anti-FLAG antibodies. Blots were probed with anti-TAP antibodies and then stripped and probed with anti-Flag antibodies. (C) The Gis1 ZnF domain, but not the JmjN/C domain, is required for interaction with Mot3. Extracts prepared from cells expressing TAP-tagged Mot3 and FLAG-tagged Gis1ΔZnF or Myc-tagged Gis1ΔNC were incubated with gG-Sepharose 6 beads. Input proteins (In) and pulled-down (Pull) proteins were electrophoresed and detected by anti-TAP, anti-Myc, or anti-FLAG antibodies. Blots were probed with anti-TAP antibodies and then stripped and probed with anti-Flag or anti-Myc antibodies.

Table 1.
List of bound proteins found only in extracts from hypoxic cells.

Table 2.
List of bound proteins found only in extracts from normoxic or heme-sufficient cells.

Table 3.
List of bound proteins found in extracts from both hypoxic and normoxic or heme-sufficientcells.
Gene Ontology (GO) analysis of the identified Gis1-interacting proteins revealed several major, statistically significant functional classes, including 68 proteins, such as Gpd2 and Cpa1, that are able bind to heterocyclic compounds, such as nucleotides and nicotinamide adenine dinucleotide (Table 1, Table 2 and Table 3), 32 Gis1-interacting proteins that are involved in metabolism, such as the glycolytic pathway, the tricarboxylic acid cycle (TCA) cycle, and fatty acid metabolism (see Table 1, Table 2 and Table 3), 20 interacting proteins that are involved in ubiquitin-like protein (Ubl) conjugation (Table 1, Table 2 and Table 3), and 22 interacting proteins that are involved in acetylation (Table 1, Table 2 and Table 3). These results suggest that Gis1 interacts with proteins of many different pathways and potentially influences many molecular and cellular processes.
3.4. Mot3 is Essential for Heme Activation of Gis1 Transcriptional Activity
Mot3 is a known transcriptional regulator involved in the regulation of a subset of hypoxic genes [57,58,82,83]. Thus, we further ascertained its functional importance in heme regulation of Gis1 activity. Note that in wild type cells, which express both Gis1 and Mot3, Gis1 transcriptional activity was stimulated in heme-sufficient cells (high ALA, Figure 7A) relative to heme-deficient cells (low ALA). When the MOT3 gene was deleted (Δmot3, Figure 7A), Gis1 transcriptional activity in heme-deficient cells remained at a similar level as in wild type cells. However, Gis1 activity in heme-sufficient cells was no longer higher than in heme-deficient cells in Δmot3 cells. Figure 7A shows that deletion of MOT3 did not affect Gis1 activity in heme-deficient cells, but abolished heme activation of Gis1 activity in heme-sufficient cells. Next, we examined the effect of MOT3 deletion on the transcriptional activities of various Gis1 deletion mutants in heme-sufficient cells (cells grown in the presence of high levels of ALA, Figure 7B). We found that deletion of MOT3 also strongly reduced the activity of Gis1 deletion proteins lacking JmjN and/or JmjC domains (Figure 7B). This result was consistent with previous studies showing that the JmjN/C domain does not affect Gis1 transcriptional activity [36]. Together, our results showed that Mot3 specifically interacts with Gis1 and is essential for heme activation of Gis1 transcriptional activity.

Figure 7.
Mot3 is required for heme activation of Gis1 transcriptional activity in vivo. (A) Deletion of the MOT3 gene reduces Gis1 transcriptional activity in heme-sufficient cells. Wild type Δhem1 (WT) or Δmot3Δhem1 (Δmot3) cells bearing the PDS-lacZ reporter and expressing Gis1 were grown at a low (2.5 μg/mL) or high levels (250 μg/mL) of ALA. β-galactosidase activities (mean+SD) were measured from at least three independent cultures. For statistical analysis, the activities in wild type cells were compared to those in Δmot3 cells at the same heme levels with a Welch 2-sample t-test. ** p value, 0.0001. (B) Deletion of the Gis1 JmjN/C domain does not abolish the requirement of Mot3 for heme activation. β-galactosidase activities (mean + SD) were measured from at least three independent cultures of yeast wild type (HEM1 deletion) or Δmot3 cells bearing the PDS-lacZ reporter and expressing Gis1 full-length or deletion proteins grown at high levels (250 μg/mL) of ALA. For statistical analysis, the activities in wild type cells were compared to those in Δmot3 cells using a Welch 2-sample t-test. ** p value, 0.0001.
4. Discussion
The JmjC domain-containing proteins are highly conserved from yeast to humans, with 32 found in humans [8,11]. These proteins are dioxygenases and often possess histone demethylase activity [2,3,4]. The human JMJB2/KDM4 subfamily of histone demethylases has five functional members, namely, KDM4A–4E [8,11]. KDM4A, B, and C are broadly expressed in normal human tissues, while KDM4D and E (containing only JmjN+JmjC domain) are predominantly expressed in the testes in humans [13,84]. The functional domains of KDM4 proteins share high homology with Gis1 domains (Figure 1). KDM4 proteins, like Gis1, are responsive to nutritional and metabolic signals, in part due to their requirement for α-ketoglutarate (AKG), Fe2+, and O2 for activity [17,18,19,20,21]. KDM4 proteins play important roles in diverse biological processes, and their altered expression or function has been implicated in multiple cancers, cardiovascular diseases, and mental retardation [8,15,85,86,87,88].
Heme acts as a prosthetic group or cofactor in proteins and enzymes required for oxygen utilization and metabolism, such as globins and cytochromes [44,45,46,47]. Heme serves as a signaling molecule regulating diverse processes ranging from gene transcription to circadian rhythm [48,49,50]. Heme directly binds to and modulates the activities of certain cellular proteins, such as the yeast heme-regulatory protein Hap1 [51,52] and the mammalian nuclear receptor Rev-erbα [49,53]. In humans, heme deficiency causes serious diseases such as anemia and porphyria [89]. Conversely, high heme intake is associated with increased risk of cancer, type 2 diabetes, and coronary heart disease [90]. In yeast and mammals, intracellular heme levels are influenced by multiple factors including carbon sources, oxygen levels, heme availability, and circadian rhythm [77,89,91,92].
In this work, our data provide a direct link between KDM4 activities and heme, enabling the coordination of the regulation of histone demethylation, chromatin structure, and gene regulation with changes in heme levels and cellular stimuli and factors influencing heme levels. This link also provides an additional level of regulation of KDM4 activities by metabolic status relating to oxidative metabolism. The metabolic status influences KDM4 demethylase activity at two levels. The first level involves the requirement of α-ketoglutarate, Fe2+, and O2 for demethylase activity. α-ketoglutarate is a metabolite of the TCA cycle, which is a key part of oxidative metabolism. Interestingly, α-ketoglutarate is also a source of succinyl-CoA for heme synthesis [93]. Fe2+ and O2 are important nutrients and metabolic substrates which are required for heme synthesis. Heme synthesis also requires succinyl CoA, which is a metabolite of the TCA cycle. The second level of regulation is directly mediated by heme regulation of KDM4 histone demethylase activity. These two levels of regulation provide a tight regulatory scheme to coordinate KDM4 histone demethylase activity with the status of oxidative metabolism, oxygen, iron, and heme. Interestingly, although the JmjN/C domains of Gis1 and KDM4A/B/C are highly homologous (Figure 1) and can all bind to heme (Figure 2 and Figure 3), only those of KDM4A and C can mediate heme regulation of histone demethylase activity (Figure 4). Further structure–function analyses of these domains may shed light on the molecular codes underpinning heme regulation of histone demethylase activity.
The KDM4 proteins, particularly KDM4A/B/C and Gis1, contain multiple functional domains that are known to be versatile in their interactions with other macromolecules. A total of 195 protein–protein interactions (physical and genetic) were identified for Gis1, with 149 unique interactions (see https://thebiogrid.org/32151/summary/saccharomyces-cerevisiae/gis1.html). These interactions can be mediated by the JmjN/C domain, the C-terminal ZnF domain, or any other domains (Figure 1). PHD and Tudor domains of KDM4A/B/C are histone reader domains that interact with post-translationally modified histones, as well as other proteins and DNA [40,41,42,43]. These domains, like the JmjN/C domain, have high potential to interact with many protein partners. Indeed, most of the Gis1-interacting proteins have human orthologues (see Table 1, Table 2 and Table 3). These human orthologues are likely to interact with KDM4A/B/C proteins and influence diverse processes and pathways.
Interestingly, GO analysis of the Gis1-interacting proteins identified several prominent classes (Table 1: hypoxic conditions; Table 2: heme-sufficient conditions; Table 3: hypoxic and heme-sufficient conditions). The first class includes those proteins that bind to heterocyclic compounds, including 25 from hypoxic conditions, 19 from heme-sufficient conditions, and 24 from hypoxic and heme-sufficient conditions. Heterocyclic compounds are effective inhibitors of KDM4 activity [94,95], thus, it is fitting that many Gis1-interacting proteins can interact with heterocyclic compounds. The second class include those involved in metabolism, including nine from hypoxic conditions, 13 from heme-sufficient conditions, and 10 from hypoxic and heme-sufficient conditions. Gis1 and KDM4 proteins require the metabolites α-ketoglutarate, Fe2+, and O2 for activity. Furthermore, heme levels are directly linked to metabolic and nutritional conditions. Gis1 and KDM4 can sense heme levels and metabolic changes. The interactions of proteins involved in metabolism with Gis1 and perhaps KDM4 proteins are likely to facilitate the response of Gis1 and KDM4 proteins to changes in metabolic and nutritional conditions. The third class are those that can be conjugated by ubiquitin-like (Ubl) proteins, including seven from hypoxic conditions, four from heme-sufficient conditions, and nine from hypoxic and heme-sufficient conditions. Previous studies showed that ubiquitination and proteasomes are involved in controlling the levels of Gis1 and KDM4 proteins [60,96,97]. The association of Gis1 and likely KDM4 proteins with ubiquitination machinery probably allows them to associate with other ubiquitin-modified proteins. The fourth class include those that are acetylated, including twelve from hypoxic conditions, seven from heme-sufficient conditions, and three from hypoxic and heme-sufficient conditions. Methylation and acetylation are two important histone modifications that strongly influence chromatin structure and gene regulation. Previous studies indicated that crosstalk existed between acetylation and demethylation [98,99]. Our results in this work, which showed that many Gis1-interacting partners are acetylated, suggested that many potential crosstalk pathways exist between demethylation and acetylation.
It is also worth noting that C-terminal regions of KDM4 proteins, like the ZnF domain of Gis1, can promote heme regulation of unrelated transcriptional activators (Figure 5). This finding, alongside the data showing the effect of heme on histone demethylase activity of KDM4A and C, demonstrates that heme is a global regulator of KDM4 proteins. Furthermore, over 100 Gis1-interacting partners with diverse cellular functions exist, and these proteins often have human orthologues (Table 1, Table 2 and Table 3). Thus, the Gis1-KDM4 regulatory network appears to consist of many proteins and is well organized to respond to changes in metabolic and nutritional conditions. It is highly likely that human KDM4 proteins can interact with many proteins to influence diverse cellular pathways and processes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/3/773/s1, Figure S1: Absorption spectra of heme bound to the JmjN/C domain of Gis1 or KDM4A/B/C in the presence of increasing concentrations of imidazole; Figure S2: Proteins pulled down with His6-tagged Gis1 in the presence of yeast extracts prepared from cells grown under normoxic conditions (A), hypoxic conditions (B), intermediate amounts of heme precursor ALA (C), or high amounts of ALA (D).
Author Contributions
Conceptualization, L.Z., P.C.K., and T.W.; methodology, L.Z., P.C.K., and T.W.; data acquisition, P.C.K., T.W., and N.S.; writing—original draft preparation, L.Z., P.C.K., and T.W.; writing—review and editing, L.Z., P.C.K., T.W., and N.S.; supervision, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the National Institute of General Medical Sciences [R15GM107717 to L.Z.].
Acknowledgments
We are very grateful to Drs. Claudio De Virgilio (University of Fribourg, Switzerland), George M. Carman (Rutgers University, New Jersey), Rolf Sternglanz (Stony Brook University, New York), and Nianshu Zhang (University of Cambridge, UK) for their generous support of Gis1 expression and reporter plasmids. We are indebted to Drs. Lei Wang and Elisabeth D. Martinez (UT Southwestern Medical Center, Texas) for kindly providing us with information and advice about demethylase assays. We thank Dr. Zhenyu Xuan (UT Dallas, Texas) for assistance in bioinformatics analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Shi, Y. Histone lysine demethylases: Emerging roles in development, physiology and disease. Nat. Rev. Genet. 2007, 8, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Zhang, Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 307–318. [Google Scholar] [CrossRef]
- Chen, Z.; Zang, J.; Whetstine, J.; Hong, X.; Davrazou, F.; Kutateladze, T.G.; Simpson, M.; Mao, Q.; Pan, C.H.; Dai, S.; et al. Structural insights into histone demethylation by JMJD2 family members. Cell 2006, 125, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Tumber, A.; Che, K.; Cain, P.; Nowak, R.; Gileadi, C.; Oppermann, U. The roles of Jumonji-type oxygenases in human disease. Epigenomics 2014, 6, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Walport, L.J.; Hopkinson, R.J.; Madden, S.K.; Chowdhury, R.; Schofield, C.J.; Kawamura, A. Studies on the catalytic domains of multiple JmjC oxygenases using peptide substrates. Epigenetics 2014, 9, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Trojer, P.; Zhang, J.; Yonezawa, M.; Schmidt, A.; Zheng, H.; Jenuwein, T.; Reinberg, D. Dynamic Histone H1 Isotype 4 Methylation and Demethylation by Histone Lysine Methyltransferase G9a/KMT1C and the Jumonji Domain-containing JMJD2/KDM4 Proteins. J. Biol. Chem. 2009, 284, 8395–8405. [Google Scholar] [CrossRef]
- Hillringhaus, L.; Yue, W.W.; Rose, N.R.; Ng, S.S.; Gileadi, C.; Loenarz, C.; Bello, S.H.; Bray, J.E.; Schofield, C.J.; Oppermann, U. Structural and evolutionary basis for the dual substrate selectivity of human KDM4 histone demethylase family. J. Biol. Chem. 2011, 286, 41616–41625. [Google Scholar] [CrossRef]
- Labbe, R.M.; Holowatyj, A.; Yang, Z.Q. Histone lysine demethylase (KDM) subfamily 4: Structures, functions and therapeutic potential. Am. J. Transl. Res. 2014, 6, 1–15. [Google Scholar]
- Cloos, P.A.; Christensen, J.; Agger, K.; Maiolica, A.; Rappsilber, J.; Antal, T.; Hansen, K.H.; Helin, K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 2006, 442, 307–311. [Google Scholar] [CrossRef]
- Wissmann, M.; Yin, N.; Muller, J.M.; Greschik, H.; Fodor, B.D.; Jenuwein, T.; Vogler, C.; Schneider, R.; Gunther, T.; Buettner, R.; et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat. Cell Biol. 2007, 9, 347–353. [Google Scholar] [CrossRef]
- Klose, R.J.; Kallin, E.M.; Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006, 7, 715–727. [Google Scholar] [CrossRef]
- Whetstine, J.R.; Nottke, A.; Lan, F.; Huarte, M.; Smolikov, S.; Chen, Z.; Spooner, E.; Li, E.; Zhang, G.; Colaiacovo, M.; et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 2006, 125, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Janknecht, R. Diversity within the JMJD2 histone demethylase family. Biochem. Biophys. Res. Commun. 2007, 353, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Agger, K.; Nishimura, K.; Miyagi, S.; Messling, J.E.; Rasmussen, K.D.; Helin, K. The KDM4/JMJD2 histone demethylases are required for hematopoietic stem cell maintenance. Blood 2019, 134, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Filiu-Braga, L.D.C.; Serejo, T.R.T.; Lucena-Araujo, A.R.; Neves, F.A.R.; de Carvalho, J.L.; Rego, E.M.; Saldanha-Araujo, F. Unraveling KDM4 histone demethylase expression and its association with adverse cytogenetic findings in chronic lymphocytic leukemia. Med. Oncol 2018, 36, 3. [Google Scholar] [CrossRef]
- Kang, M.K.; Mehrazarin, S.; Park, N.H.; Wang, C.Y. Epigenetic gene regulation by histone demethylases: Emerging role in oncogenesis and inflammation. Oral Dis 2017, 23, 709–720. [Google Scholar] [CrossRef]
- Teperino, R.; Schoonjans, K.; Auwerx, J. Histone methyl transferases and demethylases; can they link metabolism and transcription? Cell Metab. 2010, 12, 321–327. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr. Cancer and altered metabolism: Potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 335–345. [Google Scholar] [CrossRef]
- Gut, P.; Verdin, E. The nexus of chromatin regulation and intermediary metabolism. Nature 2013, 502, 489–498. [Google Scholar] [CrossRef]
- Carbonneau, M.; Gagné, L.M.; Lalonde, M.E.; Germain, M.A.; Motorina, A.; Guiot, M.C.; Secco, B.; Vincent, E.E.; Tumber, A.; Hulea, L.; et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat. Commun. 2016, 7, 12700. [Google Scholar] [CrossRef]
- Filipp, F.V. Crosstalk between epigenetics and metabolism-Yin and Yang of histone demethylases and methyltransferases in cancer. Brief. Funct. Genomics 2017, 16, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Hiltunen, M.; Kaarniranta, K. Krebs cycle intermediates regulate DNA and histone methylation: Epigenetic impact on the aging process. Ageing Res. Rev. 2014, 16, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Comer, J.M.; Konduri, P.C.; Shah, A.; Wang, T.; Lewis, A.; Shoffner, G.; Guo, F.; Zhang, L. Heme promotes transcriptional and demethylase activities of Gis1, a member of the histone demethylase JMJD2/KDM4 family. Nucleic Acids Res. 2018, 46, 215–228. [Google Scholar] [CrossRef]
- Kwon, D.W.; Ahn, S.H. Role of yeast JmjC-domain containing histone demethylases in actively transcribed regions. Biochem. Biophys. Res. Commun. 2011, 410, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sein, H.; Varv, S.; Kristjuhan, A. Distribution and maintenance of histone H3 lysine 36 trimethylation in transcribed locus. PLoS ONE 2015, 10, e0120200. [Google Scholar] [CrossRef]
- Tu, S.; Bulloch, E.M.; Yang, L.; Ren, C.; Huang, W.C.; Hsu, P.H.; Chen, C.H.; Liao, C.L.; Yu, H.M.; Lo, W.S.; et al. Identification of histone demethylases in Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 14262–14271. [Google Scholar] [CrossRef]
- Cameroni, E.; Hulo, N.; Roosen, J.; Winderickx, J.; De Virgilio, C. The novel yeast PAS kinase Rim 15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle 2004, 3, 462–468. [Google Scholar] [CrossRef]
- Roosen, J.; Engelen, K.; Marchal, K.; Mathys, J.; Griffioen, G.; Cameroni, E.; Thevelein, J.M.; De Virgilio, C.; De Moor, B.; Winderickx, J. PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability. Mol. Microbiol. 2005, 55, 862–880. [Google Scholar] [CrossRef]
- Cheng, C.; Fabrizio, P.; Ge, H.; Longo, V.D.; Li, L.M. Inference of transcription modification in long-live yeast strains from their expression profiles. BMC Genomics 2007, 8, 219. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, J.; Oliver, S.G. Gis1 is required for transcriptional reprogramming of carbon metabolism and the stress response during transition into stationary phase in yeast. Microbiology 2009, 155, 1690–1698. [Google Scholar] [CrossRef]
- Wei, M.; Fabrizio, P.; Madia, F.; Hu, J.; Ge, H.; Li, L.M.; Longo, V.D. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009, 5, e1000467. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski Westholm, J.; Tronnersjo, S.; Nordberg, N.; Olsson, I.; Komorowski, J.; Ronne, H. Gis1 and Rph1 regulate glycerol and acetate metabolism in glucose depleted yeast cells. PLoS ONE 2012, 7, e31577. [Google Scholar] [CrossRef] [PubMed]
- Boorstein, W.R.; Craig, E.A. Regulation of a yeast HSP70 gene by a cAMP responsive transcriptional control element. EMBO J. 1990, 9, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Pedruzzi, I.; Burckert, N.; Egger, P.; De Virgilio, C. Saccharomyces cerevisiae Ras/cAMP pathway controls post-diauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 2000, 19, 2569–2579. [Google Scholar] [CrossRef]
- Jang, Y.K.; Wang, L.; Sancar, G.B. RPH1 and GIS1 are damage-responsive repressors of PHR1. Mol. Cell Biol. 1999, 19, 7630–7638. [Google Scholar] [CrossRef]
- Yu, Y.; Neiman, A.M.; Sternglanz, R. The JmjC domain of Gis1 is dispensable for transcriptional activation. FEMS Yeast Res. 2010, 10, 793–801. [Google Scholar] [CrossRef]
- Quan, Z.; Oliver, S.G.; Zhang, N. JmjN interacts with JmjC to ensure selective proteolysis of Gis1 by the proteasome. Microbiology 2011, 157, 2694–2701. [Google Scholar] [CrossRef]
- Pascual, J.; Martinez-Yamout, M.; Dyson, H.J.; Wright, P.E. Structure of the PHD zinc finger from human Williams-Beuren syndrome transcription factor. J. Mol. Biol. 2000, 304, 723–729. [Google Scholar] [CrossRef]
- Lu, R.; Wang, G.G. Tudor: A versatile family of histone methylation ‘readers’. Trends Biochem. Sci. 2013, 38, 546–555. [Google Scholar] [CrossRef]
- Musselman, C.A.; Kutateladze, T.G. PHD fingers: Epigenetic effectors and potential drug targets. Mol. Interv. 2009, 9, 314–323. [Google Scholar] [CrossRef]
- Musselman, C.A.; Kutateladze, T.G. Handpicking epigenetic marks with PHD fingers. Nucleic Acids Res. 2011, 39, 9061–9071. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Zhou, M.M. The PHD finger: A versatile epigenome reader. Trends Biochem. Sci. 2011, 36, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Adams-Cioaba, M.A.; Min, J. Structure and function of histone methylation binding proteins. Biochem. Cell Biol. 2009, 87, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W.; De Matteis, F.; Aldridge, W.N. Heme and Hemoproteins; Springer: Berlin/Heidelberg, Germany, 1978; pp. 49–80. [Google Scholar]
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Hemes in Biology. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2009; pp. 240–249. [Google Scholar]
- Padmanaban, G.; Venkateswar, V.; Rangarajan, P.N. Haem as a multifunctional regulator. Trends Biochem. Sci. 1989, 14, 492–496. [Google Scholar] [CrossRef]
- Sassa, S. Novel effects of heme and heme-related compounds in biological systems. Current Med. Chem. 1996, 3, 273–290. [Google Scholar]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Weitz, S.H.; Gong, M.; Barr, I.; Weiss, S.; Guo, F. Processing of microRNA primary transcripts requires heme in mammalian cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1861–1866. [Google Scholar] [CrossRef]
- Zhang, L.; Guarente, L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995, 14, 313–320. [Google Scholar] [CrossRef]
- Zhang, L.; Hach, A. Molecular mechanism of heme signaling in yeast: The transcriptional activator Hap1 serves as the key mediator. Cell Mol. Life Sci. 1999, 56, 415–426. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, D.; Fiorito, V.; Petrillo, S.; Tolosano, E. Unraveling the Role of Heme in Neurodegeneration. Front. Neurosci. 2018, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, S.; Ghosh, P.; Wang, T.; Kalainayakan, S.P.; Vidal, C.; Dey, S.; Konduri, P.C.; Zhang, L. Elevated Heme Synthesis and Uptake Underpin Intensified Oxidative Metabolism and Tumorigenic Functions in Non-Small Cell Lung Cancer Cells. Cancer Res. 2019, 79, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Galmozzi, A.; Kok, B.P.; Kim, A.S.; Montenegro-Burke, J.R.; Lee, J.Y.; Spreafico, R.; Mosure, S.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.V.; Rothenberg, M.; Downs, M.A.; Blumer, K.J. Mot3, a Zn finger transcription factor that modulates gene expression and attenuates mating pheromone signaling in Saccharomyces cerevisiae. Genetics 1998, 149, 879–892. [Google Scholar] [PubMed]
- Martinez-Montanes, F.; Rienzo, A.; Poveda-Huertes, D.; Pascual-Ahuir, A.; Proft, M. Activator and repressor functions of the Mot3 transcription factor in the osmostress response of Saccharomyces cerevisiae. Eukaryot. Cell 2013, 12, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, J.; Han, G.S.; Iwanyshyn, W.M.; Conover, K.; Carman, G.M. Regulation of the yeast DPP1-encoded diacylglycerol pyrophosphate phosphatase by transcription factor Gis1p. J. Biol. Chem. 2003, 278, 31495–31503. [Google Scholar] [CrossRef]
- Zhang, N.; Oliver, S.G. The transcription activity of Gis1 is negatively modulated by proteasome-mediated limited proteolysis. J. Biol. Chem. 2010, 285, 6465–6476. [Google Scholar] [CrossRef]
- Turcotte, B.; Guarente, L. HAP1 positive control mutants specific for one of two binding sites. Genes Dev. 1992, 6, 2001–2009. [Google Scholar] [CrossRef]
- Zhang, L.; Guarente, L. The C6 zinc cluster dictates asymmetric binding by HAP1. EMBO J. 1996, 15, 4676–4681. [Google Scholar] [CrossRef]
- Hon, T.; Lee, H.C.; Zhang, L. The actions of Hsp90 and Hsp70 molecular chaperones in heme signaling in eukaryotes. In Recent Research Developments in Molecular and Cellular Biology; Fagan, J., Shimizu, N., Davidson, J.N., Eds.; Research Signpost: Trivandrum, India, 2003; pp. 11–21. [Google Scholar]
- Kundaje, A.; Xin, X.; Lan, C.; Lianoglou, S.; Zhou, M.; Zhang, L.; Leslie, C. A predictive model of the oxygen and heme regulatory network in yeast. PLoS Comput. Biol. 2008, 4, e1000224. [Google Scholar] [CrossRef] [PubMed]
- Hon, T.; Hach, A.; Lee, H.C.; Chen, T.; Zhang, L. Functional analysis of heme regulatory elements of the transcriptional activator Hap1. Biochem. Biophys Res. Commun. 2000, 273, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Hon, T.; Hach, A.; Tamalis, D.; Zhu, Y.; Zhang, L. The yeast heme-responsive transcriptional activator Hap1 is a preexisting dimer in the absence of heme. J. Biol. Chem. 1999, 274, 22770–22774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guarente, L. HAP1 is nuclear but is bound to a cellular factor in the absence of heme. J. Biol. Chem. 1994, 269, 14643–14647. [Google Scholar] [PubMed]
- Zhang, L.; Hach, A.; Wang, C. Molecular mechanism governing heme signaling in yeast: A higher-order complex mediates heme regulation of the transcriptional activator HAP1. Mol. Cell Biol. 1998, 18, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, R.; Hooda, J.; Shah, A.; Cao, T.M.; Henke, R.M.; Zhang, L. The nuclear localization of SWI/SNF proteins is subjected to oxygen regulation. Cell Biosci. 2012, 2, 30. [Google Scholar] [CrossRef]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A contaminant repository for affinity purification-mass spectrometry data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef]
- Hon, T.; Lee, H.C.; Hach, A.; Johnson, J.L.; Craig, E.A.; Erdjument-Bromage, H.; Tempst, P.; Zhang, L. The Hsp70-Ydj1 Molecular Chaperone Represses the Activity of the Transcriptional Activator Hap1 in the Absence of Heme. Mol. Cell Biol. 2001, 21, 7923–7932. [Google Scholar] [CrossRef]
- Gerace, E.; Moazed, D. Affinity Purification of Protein Complexes Using TAP Tags. Methods Enzymol. 2015, 559, 37–52. [Google Scholar]
- Ishimori, K.; Watanabe, Y. Unique Heme Environmental Structures in Heme-regulated Proteins Using Heme as the Signaling Molecule. Chem. Lett. 2014, 43, 1680–1689. [Google Scholar] [CrossRef]
- Yao, X.; Balamurugan, P.; Arvey, A.; Leslie, C.; Zhang, L. Heme controls the regulation of protein tyrosine kinases Jak2 and Src. Biochem. Biophys Res. Commun. 2010, 403, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.K.; Simpson, R.J.; Kwan, A.H.; Crofts, L.A.; Loughlin, F.E.; Matthews, J.M.; Crossley, M.; Mackay, J.P. Zinc fingers as protein recognition motifs: Structural basis for the GATA-1/friend of GATA interaction. Proc. Natl. Acad. Sci. USA 2005, 102, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Tronnersjo, S.; Hanefalk, C.; Balciunas, D.; Hu, G.Z.; Nordberg, N.; Muren, E.; Ronne, H. The jmjN and jmjC domains of the yeast zinc finger protein Gis1 interact with 19 proteins involved in transcription, sumoylation and DNA repair. Mol. Genet. Genomics 2007, 277, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hon, T.; Dodd, A.; Dirmeier, R.; Gorman, N.; Sinclair, P.R.; Zhang, L.; Poyton, R.O. A mechanism of oxygen sensing in yeast. Multiple oxygen-responsive steps in the heme biosynthetic pathway affect Hap1 activity. J. Biol. Chem. 2003, 278, 50771–50780. [Google Scholar] [CrossRef] [PubMed]
- Balciunas, D.; Ronne, H. Yeast genes GIS1-4: Multicopy suppressors of the Gal- phenotype of snf1 mig1 srb8/10/11 cells. Mol. Gen. Genet. 1999, 262, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Mehta, M.; Kuo, D.; Sung, M.K.; Chuang, R.; Jaehnig, E.J.; Bodenmiller, B.; Licon, K.; Copeland, W.; Shales, M.; et al. Rewiring of genetic networks in response to DNA damage. Science 2010, 330, 1385–1389. [Google Scholar] [CrossRef]
- Costanzo, M.; Baryshnikova, A.; Bellay, J.; Kim, Y.; Spear, E.D.; Sevier, C.S.; Ding, H.; Koh, J.L.; Toufighi, K.; Mostafavi, S.; et al. The genetic landscape of a cell. Science 2010, 327, 425–431. [Google Scholar] [CrossRef]
- Costanzo, M.; VanderSluis, B.; Koch, E.N.; Baryshnikova, A.; Pons, C.; Tan, G.; Wang, W.; Usaj, M.; Hanchard, J.; Lee, S.D.; et al. A global genetic interaction network maps a wiring diagram of cellular function. Science 2016, 353, aaf1420. [Google Scholar] [CrossRef]
- Kastaniotis, A.J.; Zitomer, R.S. Rox1 mediated repression. Oxygen dependent repression in yeast. Adv. Exp. Med. Biol. 2000, 475, 185–195. [Google Scholar]
- Lai, L.C.; Kosorukoff, A.L.; Burke, P.V.; Kwast, K.E. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryot Cell 2006, 5, 1468–1489. [Google Scholar] [CrossRef]
- Krupp, M.; Marquardt, J.U.; Sahin, U.; Galle, P.R.; Castle, J.; Teufel, A. RNA-Seq Atlas--a reference database for gene expression profiling in normal tissue by next-generation sequencing. Bioinformatics 2012, 28, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.L.; Janknecht, R. KDM4/JMJD2 histone demethylases: Epigenetic regulators in cancer cells. Cancer Res. 2013, 73, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Chen, H.Z.; Wang, L.; Liu, D.P.; Hill, J.A.; Liu, Z.P. The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J. Clin. Invest. 2011, 121, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Young, L.C.; Hendzel, M.J. The oncogenic potential of Jumonji D2 (JMJD2/KDM4) histone demethylase overexpression. Biochem. Cell Biol. 2013, 91, 369–377. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, G.W.; Jeon, Y.H.; Yoo, J.; Lee, S.W.; Kwon, S.H. Advances in histone demethylase KDM4 as cancer therapeutic targets. FASEB J. 2020, 34, 3461–3484. [Google Scholar] [CrossRef]
- Bishop, D.F.; Anderson, K.E.; Desnick, R.J.; Sassa, S. Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. In The Online Metabolic and Molecular Bases of Inherited Disease; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2016; pp. 2991–3062. [Google Scholar]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef]
- Mattoon, J.; Lancashire, W.; Sanders, H.; Carvajal, E.; Malamud, D.; Braz, G.; Panek, A. Oxygen and catabolite regulation of hemoprotein biosynthesis in the yeast Saccharomyces cerevisiae. In Biosynthesis of Heme and Cholorophylls; Caughey, W.J., Ed.; Academic Press: Cambridge, MA, USA, 1979; pp. 421–435. [Google Scholar]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef]
- Burch, J.S.; Marcero, J.R.; Maschek, J.A.; Cox, J.E.; Jackson, L.K.; Medlock, A.E.; Phillips, J.D.; Dailey, H.A., Jr. Glutamine via alpha-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood 2018, 132, 987–998. [Google Scholar] [CrossRef]
- Leurs, U.; Clausen, R.P.; Kristensen, J.L.; Lohse, B. Inhibitor scaffold for the histone lysine demethylase KDM4C (JMJD2C). Bioorg Med. Chem. Lett. 2012, 22, 5811–5813. [Google Scholar] [CrossRef]
- Metzger, E.; Stepputtis, S.S.; Strietz, J.; Preca, B.T.; Urban, S.; Willmann, D.; Allen, A.; Zenk, F.; Iovino, N.; Bronsert, P.; et al. KDM4 Inhibition Targets Breast Cancer Stem-like Cells. Cancer Res. 2017, 77, 5900–5912. [Google Scholar] [CrossRef]
- Tan, M.K.; Lim, H.J.; Harper, J.W. SCF(FBXO22) regulates histone H3 lysine 9 and 36 methylation levels by targeting histone demethylase KDM4A for ubiquitin-mediated proteasomal degradation. Mol. Cell Biol. 2011, 31, 3687–3699. [Google Scholar] [CrossRef] [PubMed]
- Ipenberg, I.; Guttmann-Raviv, N.; Khoury, H.P.; Kupershmit, I.; Ayoub, N. Heat shock protein 90 (Hsp90) selectively regulates the stability of KDM4B/JMJD2B histone demethylase. J. Biol. Chem. 2013, 288, 14681–14687. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Vasilatos, S.N.; Bhargava, R.; Fine, J.L.; Oesterreich, S.; Davidson, N.E.; Huang, Y. Functional interaction of histone deacetylase 5 (HDAC5) and lysine-specific demethylase 1 (LSD1) promotes breast cancer progression. Oncogene 2017, 36, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Seo, J.; Lee, H.S.; Stanton, L.W.; Kim, D.; Choi, J.K. Global mapping of the regulatory interactions of histone residues. FEBS Lett. 2015, 589, 4061–4070. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).