Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance
Abstract
1. Introduction
2. The Unique Biology of Vγ9Vδ2 γδ T Cells
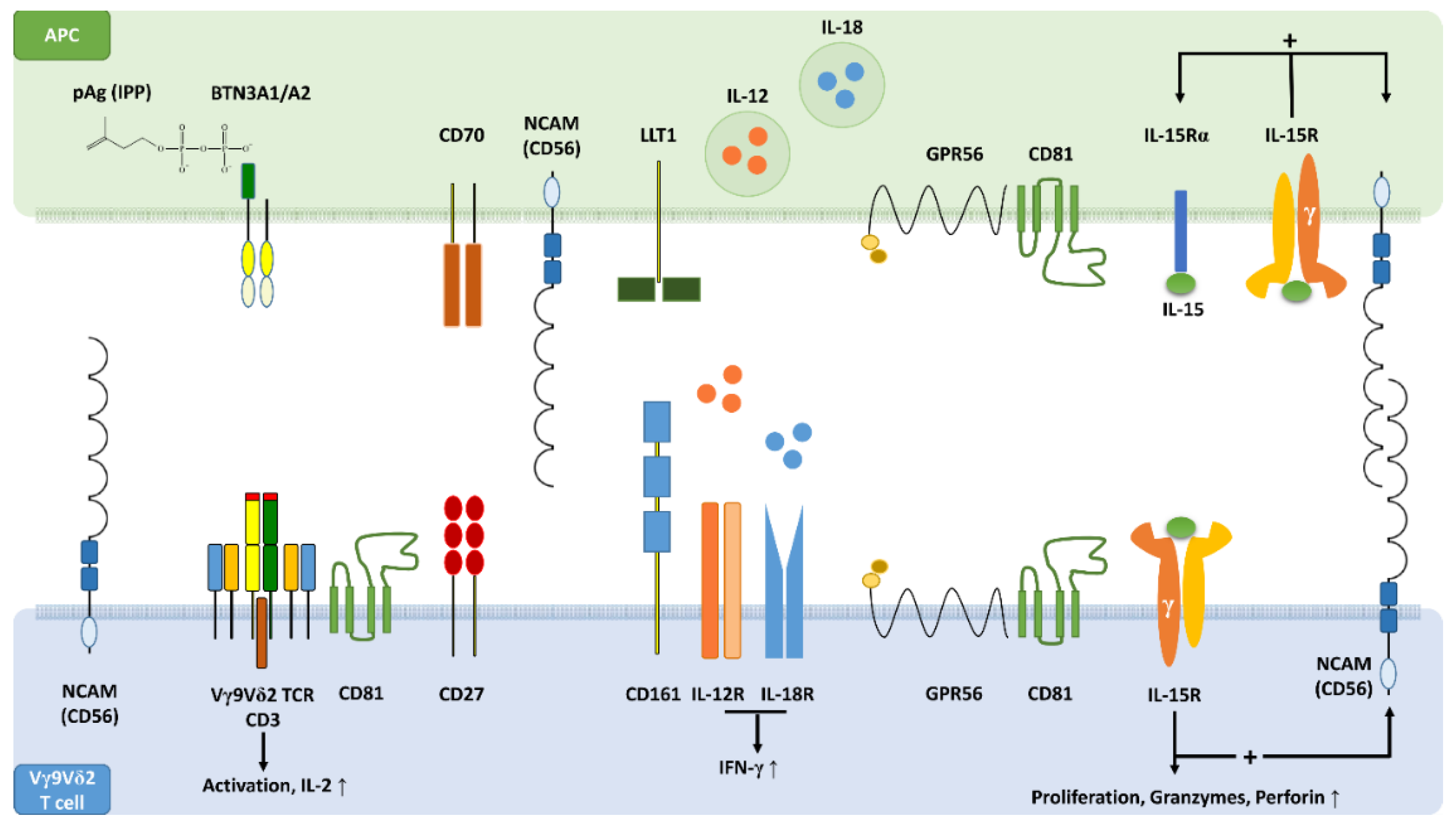
3. Vγ9Vδ2 T Cell Phenotypes
4. CD161 Marks a Functional Phenotype of γδ T Cells Mediating Innate-Like Responses
5. CD56+ γδ T Cells Participate in Lymphoid Stress Surveillance
6. CD56+ γδ T Cells Have a Distinct Surface Phenotype and Display Increased Cytolytic Potential
7. CD56+ Antigen-Presenting Cells Activate CD56+ γδ T Cells
8. CD56+ DC-Like Cells Have a Distinct Phenotype and Express CD161 and CD122
9. CD56+ DC-Like Cells Express the G Protein-Coupled Receptor GPR56
10. GPR56 Is Expressed by Cytotoxic Human Lymphocytes Including γδ T Cells
11. CD161, CD56 and GPR56 May Be Critical Components of an Innate-Like Immunological Synapse
12. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C.; Saito, H.; Gillies, S.D.; Kranz, D.M.; Tanigawa, G.; Eisen, H.N.; Tonegawa, S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell 1985, 40, 259–269. [Google Scholar] [CrossRef]
- Rast, J.P.; Anderson, M.K.; Strong, S.J.; Luer, C.; Litman, R.T.; Litman, G.W. alpha, beta, gamma, and delta T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity 1997, 6, 1–11. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Correa, I.; Bix, M.; Liao, N.S.; Zijlstra, M.; Jaenisch, R.; Raulet, D. Most gamma delta T cells develop normally in beta 2-microglobulin-deficient mice. Proc. Natl. Acad. Sci. USA 1992, 89, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Dimova, T.; Brouwer, M.; Gosselin, F.; Tassignon, J.; Leo, O.; Donner, C.; Marchant, A.; Vermijlen, D. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc. Natl. Acad. Sci. USA 2015, 112, E556–E565. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C. [gamma][delta] cells: A right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef]
- Barbee, S.D.; Woodward, M.J.; Turchinovich, G.; Mention, J.J.; Lewis, J.M.; Boyden, L.M.; Lifton, R.P.; Tigelaar, R.; Hayday, A.C. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3330–3335. [Google Scholar] [CrossRef]
- Di Marco Barros, R.; Roberts, N.A.; Dart, R.J.; Vantourout, P.; Jandke, A.; Nussbaumer, O.; Deban, L.; Cipolat, S.; Hart, R.; Iannitto, M.L.; et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell 2016, 167, 203–218 e217. [Google Scholar] [CrossRef]
- Mohamed, R.H.; Sutoh, Y.; Itoh, Y.; Otsuka, N.; Miyatake, Y.; Ogasawara, K.; Kasahara, M. The SKINT1-like gene is inactivated in hominoids but not in all primate species: Implications for the origin of dendritic epidermal T cells. Plos ONE 2015, 10, e0123258. [Google Scholar] [CrossRef]
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; et al. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018, 19, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Willcox, C.R.; Vantourout, P.; Salim, M.; Zlatareva, I.; Melandri, D.; Zanardo, L.; George, R.; Kjaer, S.; Jeeves, M.; Mohammed, F.; et al. Butyrophilin-like 3 Directly Binds a Human Vgamma4(+) T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 2019, 51, 813–825 e814. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 2009, 31, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C. gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J. Immunol. 2019, 203, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.C.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Strid, J. Working in “NK Mode”: Natural Killer Group 2 Member D and Natural Cytotoxicity Receptors in Stress-Surveillance by gammadelta T Cells. Front. Immunol. 2018, 9, 851. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cauvi, G.; Witherden, D.A.; Havran, W.L. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 2004, 172, 3573–3579. [Google Scholar] [CrossRef]
- Komori, H.K.; Witherden, D.A.; Kelly, R.; Sendaydiego, K.; Jameson, J.M.; Teyton, L.; Havran, W.L. Cutting edge: Dendritic epidermal gammadelta T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J. Immunol. 2012, 188, 2972–2976. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Karunakaran, M.M.; Gobel, T.W.; Starick, L.; Walter, L.; Herrmann, T. Vgamma9 and Vdelta2 T cell antigen receptor genes and butyrophilin 3 (BTN3) emerged with placental mammals and are concomitantly preserved in selected species like alpaca (Vicugna pacos). Immunogenetics 2014, 66, 243–254. [Google Scholar] [CrossRef]
- Morita, C.T.; Jin, C.; Sarikonda, G.; Wang, H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Bender, A.; Prospero, T.; Wesselborg, S.; Janssen, O.; Pechhold, K. The primary response of human gamma/delta + T cells to Mycobacterium tuberculosis is restricted to V gamma 9-bearing cells. J. Exp. Med. 1991, 173, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Zaidi, I.; Loizon, S.; Mercereau-Puijalon, O.; Dechanet-Merville, J.; Mamani-Matsuda, M. Human Vgamma9Vdelta2 T Lymphocytes in the Immune Response to P. falciparum Infection. Front. Immunol. 2018, 9, 2760. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Meyer, C.; Bonneville, M. gammadelta T Cells: First Line of Defense and Beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Puan, K.J.; Jin, C.; Wang, H.; Sarikonda, G.; Raker, A.M.; Lee, H.K.; Samuelson, M.I.; Marker-Hermann, E.; Pasa-Tolic, L.; Nieves, E.; et al. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int. Immunol. 2007, 19, 657–673. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Nussbaumer, O.; Gander, H.; Steiner, B.; Leonhartsberger, N.; Thurnher, M. Stress-related and homeostatic cytokines regulate Vgamma9Vdelta2 T-cell surveillance of mevalonate metabolism. Oncoimmunology 2014, 3, e953410. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Gander, H.; Rahm, A.; Idzko, M.; Nussbaumer, O.; Thurnher, M. Ecto-ATPase CD39 Inactivates Isoprenoid-Derived Vgamma9Vdelta2 T Cell Phosphoantigens. Cell Rep. 2016, 16, 444–456. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Brandes, M.; Willimann, K.; Moser, B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef]
- Kenna, T.; Golden-Mason, L.; Norris, S.; Hegarty, J.E.; O’Farrelly, C.; Doherty, D.G. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 2004, 113, 56–63. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigne, C.M.; Monkkonen, H.; Monkkonen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Laing, A.; Woodward, M.J.; Zlatareva, I.; Apolonia, L.; Jones, A.W.; Snijders, A.P.; Malim, M.H.; Hayday, A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, A.; Peigne, C.M.; Leger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Sachleben, J.R.; Boughter, C.T.; Nawrocka, W.I.; Borowska, M.T.; Tarrasch, J.T.; Skiniotis, G.; Roux, B.; Adams, E.J. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vgamma9Vdelta2 T cell activation. Proc. Natl. Acad. Sci. USA 2017, 114, E7311–E7320. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, M.M.; Willcox, C.R.; Salim, M.; Paletta, D.; Fichtner, A.S.; Noll, A.; Starick, L.; Nohren, A.; Begley, C.R.; Berwick, K.A.; et al. Butyrophilin-2A1 Directly Binds Germline-Encoded Regions of the Vgamma9Vdelta2 TCR and Is Essential for Phosphoantigen Sensing. Immunity 2020. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.M.; Cruz, J.; Costanzo, A.; Terajima, M.; Ennis, F.A. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell. Immunol. 2010, 264, 71–77. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Mizuno, H.; Zhao, X.; Langerod, A.; Moon, S.H.; Rodriguez-Barrueco, R.; Barsotti, A.; Chicas, A.; Li, W.; Polotskaia, A.; et al. Mutant p53 Disrupts Mammary Tissue Architecture via the Mevalonate Pathway. Cell 2012, 148, 244–258. [Google Scholar] [CrossRef]
- Thurnher, M.; Gruenbacher, G.; Nussbaumer, O. Regulation of mevalonate metabolism in cancer and immune cells. Biochim. Biophys. Acta 2013, 1831, 1009–1015. [Google Scholar] [CrossRef]
- Thurnher, M.; Nussbaumer, O.; Gruenbacher, G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin. Cancer Res. 2012, 18, 3524–3531. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef]
- Castella, B.; Kopecka, J.; Sciancalepore, P.; Mandili, G.; Foglietta, M.; Mitro, N.; Caruso, D.; Novelli, F.; Riganti, C.; Massaia, M. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vgamma9Vdelta2 T cell activation by dendritic cells. Nat. Commun. 2017, 8, 15663. [Google Scholar] [CrossRef] [PubMed]
- Sireci, G.; Champagne, E.; Fournie, J.J.; Dieli, F.; Salerno, A. Patterns of phosphoantigen stimulation of human Vgamma9/Vdelta2 T cell clones include Th0 cytokines. Hum. Immunol. 1997, 58, 70–82. [Google Scholar] [CrossRef]
- Caccamo, N.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Todaro, M.; Stassi, G.; Sireci, G.; Fournie, J.J.; Dieli, F. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood 2011, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Zeng, X.; Prinz, I. The natural and the inducible: Interleukin (IL)-17-producing gammadelta T cells. Trends Immunol. 2013, 34, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Lalor, S.J.; McLoughlin, R.M. Memory gammadelta T Cells-Newly Appreciated Protagonists in Infection and Immunity. Trends Immunol. 2016, 37, 690–702. [Google Scholar] [CrossRef]
- Dieli, F.; Gebbia, N.; Poccia, F.; Caccamo, N.; Montesano, C.; Fulfaro, F.; Arcara, C.; Valerio, M.R.; Meraviglia, S.; Di Sano, C.; et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003, 102, 2310–2311. [Google Scholar] [CrossRef]
- DeBarros, A.; Chaves-Ferreira, M.; d’Orey, F.; Ribot, J.C.; Silva-Santos, B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human gammadelta peripheral blood lymphocytes. Eur. J. Immunol. 2011, 41, 195–201. [Google Scholar] [CrossRef]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; La Mendola, C.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. [Google Scholar] [CrossRef]
- Coscia, M.; Vitale, C.; Peola, S.; Foglietta, M.; Rigoni, M.; Griggio, V.; Castella, B.; Angelini, D.; Chiaretti, S.; Riganti, C.; et al. Dysfunctional Vgamma9Vdelta2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012, 120, 3271–3279. [Google Scholar] [CrossRef][Green Version]
- Rincon-Orozco, B.; Kunzmann, V.; Wrobel, P.; Kabelitz, D.; Steinle, A.; Herrmann, T. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005, 175, 2144–2151. [Google Scholar] [CrossRef]
- Lanier, L.L.; Chang, C.; Phillips, J.H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994, 153, 2417–2428. [Google Scholar] [PubMed]
- Fergusson, J.R.; Smith, K.E.; Fleming, V.M.; Rajoriya, N.; Newell, E.W.; Simmons, R.; Marchi, E.; Bjorkander, S.; Kang, Y.H.; Swadling, L.; et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep. 2014, 9, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, H.; Prod’homme, V.; Dumaurier, M.J.; Retiere, C.; Poupon, G.; Cazareth, J.; Bihl, F.; Braud, V.M. Cutting edge: Lectin-like transcript 1 is a ligand for the CD161 receptor. J. Immunol. 2005, 175, 7791–7795. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, A.; Cosgrove, C.; Simoni, Y.; van Wilgenburg, B.; Geremia, A.; Bjorkander, S.; Sverremark-Ekstrom, E.; Thurnheer, C.; Gunthard, H.F.; Khanna, N.; et al. CD161 Defines a Functionally Distinct Subset of Pro-Inflammatory Natural Killer Cells. Front. Immunol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Jabri, B.; Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 2015, 15, 771–783. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef]
- Lanier, L.L.; Testi, R.; Bindl, J.; Phillips, J.H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J. Exp. Med. 1989, 169, 2233–2238. [Google Scholar] [CrossRef]
- Lanier, L.L.; Chang, C.; Azuma, M.; Ruitenberg, J.J.; Hemperly, J.J.; Phillips, J.H. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56). J. Immunol. 1991, 146, 4421–4426. [Google Scholar]
- Alexander, A.A.; Maniar, A.; Cummings, J.S.; Hebbeler, A.M.; Schulze, D.H.; Gastman, B.R.; Pauza, C.D.; Strome, S.E.; Chapoval, A.I. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4232–4240. [Google Scholar] [CrossRef]
- Pittet, M.J.; Speiser, D.E.; Valmori, D.; Cerottini, J.C.; Romero, P. Cutting edge: Cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J. Immunol. 2000, 164, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Rujkijyanont, P.; Neale, G.; Yang, J.; Bari, R.; Das Gupta, N.; Holladay, M.; Rooney, B.; Leung, W. Multiplex and genome-wide analyses reveal distinctive properties of KIR+ and CD56+ T cells in human blood. J. Immunol. 2013, 191, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Hasler, R.; Wesch, D.; Kabelitz, D. Human Vdelta2 T cells are a major source of interleukin-9. Proc. Natl. Acad. Sci. USA 2016, 113, 12520–12525. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Meyer, A.; Kouakanou, L.; Feder, J.; Schricker, T.; Lettau, M.; Janssen, O.; Wesch, D.; Kabelitz, D. TGF-beta enhances the cytotoxic activity of Vdelta2 T cells. Oncoimmunology 2019, 8, e1522471. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Gander, H.; Rahm, A.; Nussbaumer, W.; Romani, N.; Thurnher, M. CD56+ human blood dendritic cells effectively promote TH1-type gammadelta T-cell responses. Blood 2009, 114, 4422–4431. [Google Scholar] [CrossRef]
- Milush, J.M.; Long, B.R.; Snyder-Cappione, J.E.; Cappione, A.J., 3rd; York, V.A.; Ndhlovu, L.C.; Lanier, L.L.; Michaelsson, J.; Nixon, D.F. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood 2009, 114, 4823–4831. [Google Scholar] [CrossRef]
- Papewalis, C.; Jacobs, B.; Wuttke, M.; Ullrich, E.; Baehring, T.; Fenk, R.; Willenberg, H.S.; Schinner, S.; Cohnen, M.; Seissler, J.; et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J. Immunol. 2008, 180, 1462–1470. [Google Scholar] [CrossRef]
- Tsuda, J.; Li, W.; Yamanishi, H.; Yamamoto, H.; Okuda, A.; Kubo, S.; Ma, Z.; Terada, N.; Tanaka, Y.; Okamura, H. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human gammadelta T cells. J. Immunol. 2011, 186, 2003–2012. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Gander, H.; Nussbaumer, O.; Nussbaumer, W.; Rahm, A.; Thurnher, M. IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56+ dendritic cells. Cancer Res. 2010, 70, 9611–9620. [Google Scholar] [CrossRef]
- Bryant, C.; Fitzgerald, K.A. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009, 19, 455–464. [Google Scholar] [CrossRef]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Thurnher, M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by gammadelta T lymphocytes. Blood 2011, 118, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.M.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; Di Pucchio, T.; Belardelli, F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Lion, E.; Tel, J.; de Vries, I.J.; Coudere, K.; Fromm, P.D.; Van Tendeloo, V.F.; Smits, E.L.; Berneman, Z.N. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. Plos ONE 2012, 7, e51851. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; De Reu, H.; Berneman, Z.N.; Smits, E.L.; Van Tendeloo, V.F. Interleukin-15-Cultured Dendritic Cells Enhance Anti-Tumor Gamma Delta T Cell Functions through IL-15 Secretion. Front. Immunol. 2018, 9, 658. [Google Scholar] [CrossRef]
- Caligiuri, M.A.; Murray, C.; Levine, H.; Longtine, J.A.; Ritz, J. Clonal evidence for the induction of NKH1 on activated human thymocytes. Functional changes associated with antigen expression. Eur. J. Immunol. 1989, 19, 1735–1739. [Google Scholar] [CrossRef]
- Yona, S.; Lin, H.H.; Siu, W.O.; Gordon, S.; Stacey, M. Adhesion-GPCRs: Emerging roles for novel receptors. Trends Biochem. Sci. 2008, 33, 491–500. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef]
- Bond, R.A.; Ijzerman, A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 2006, 27, 92–96. [Google Scholar] [CrossRef]
- Iguchi, T.; Sakata, K.; Yoshizaki, K.; Tago, K.; Mizuno, N.; Itoh, H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J. Biol. Chem. 2008, 283, 14469–14478. [Google Scholar] [CrossRef]
- Little, K.D.; Hemler, M.E.; Stipp, C.S. Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: Central role of CD81 in facilitating GPR56-Galpha q/11 association. Mol. Biol. Cell 2004, 15, 2375–2387. [Google Scholar] [CrossRef]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.M.; van de Garde, M.D.; Cheng, K.F.; Baars, P.A.; Remmerswaal, E.B.; van Lier, R.A.; Mackay, C.R.; Lin, H.H.; Hamann, J. Specific expression of GPR56 by human cytotoxic lymphocytes. J. Leukoc. Biol. 2011, 90, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Babor, M.; Lane, J.; Schulten, V.; Patil, V.S.; Seumois, G.; Rosales, S.L.; Fu, Z.; Picarda, G.; Burel, J.; et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017, 8, 1473. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Perugini, V.; Zamai, M.; Gonzalez-Granado, J.M.; Barreiro, O.; Tejera, E.; Yanez-Mo, M.; Caiolfa, V.R.; Sanchez-Madrid, F. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol. Cell. Biol. 2013, 33, 3644–3658. [Google Scholar] [CrossRef]
- Tu, W.; Zheng, J.; Liu, Y.; Sia, S.F.; Liu, M.; Qin, G.; Ng, I.H.; Xiang, Z.; Lam, K.T.; Peiris, J.S.; et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J. Exp. Med. 2011, 208, 1511–1522. [Google Scholar] [CrossRef]
- Nussbaumer, O.; Koslowski, M. The emerging role of γδ T cells in cancer immunotherapy. Immuno.-Oncol. Technol. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Fisher, J.; Anderson, J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1409. [Google Scholar] [CrossRef]
- Lamb, L.S., Jr.; Bowersock, J.; Dasgupta, A.; Gillespie, G.Y.; Su, Y.; Johnson, A.; Spencer, H.T. Engineered drug resistant gammadelta T cells kill glioblastoma cell lines during a chemotherapy challenge: A strategy for combining chemo- and immunotherapy. Plos ONE 2013, 8, e51805. [Google Scholar] [CrossRef]
- Oberg, H.H.; Peipp, M.; Kellner, C.; Sebens, S.; Krause, S.; Petrick, D.; Adam-Klages, S.; Rocken, C.; Becker, T.; Vogel, I.; et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014, 74, 1349–1360. [Google Scholar] [CrossRef]
- Fournie, J.J.; Sicard, H.; Poupot, M.; Bezombes, C.; Blanc, A.; Romagne, F.; Ysebaert, L.; Laurent, G. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 2013, 10, 35–41. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nussbaumer, O.; Thurnher, M. Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance. Cells 2020, 9, 772. https://doi.org/10.3390/cells9030772
Nussbaumer O, Thurnher M. Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance. Cells. 2020; 9(3):772. https://doi.org/10.3390/cells9030772
Chicago/Turabian StyleNussbaumer, Oliver, and Martin Thurnher. 2020. "Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance" Cells 9, no. 3: 772. https://doi.org/10.3390/cells9030772
APA StyleNussbaumer, O., & Thurnher, M. (2020). Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance. Cells, 9(3), 772. https://doi.org/10.3390/cells9030772





