IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Polyclonal B Cell Stimulation
2.3. B Cell ImmunoSpot® Assays
2.4. ELISA Assays
2.5. Statistical Methods
3. Results
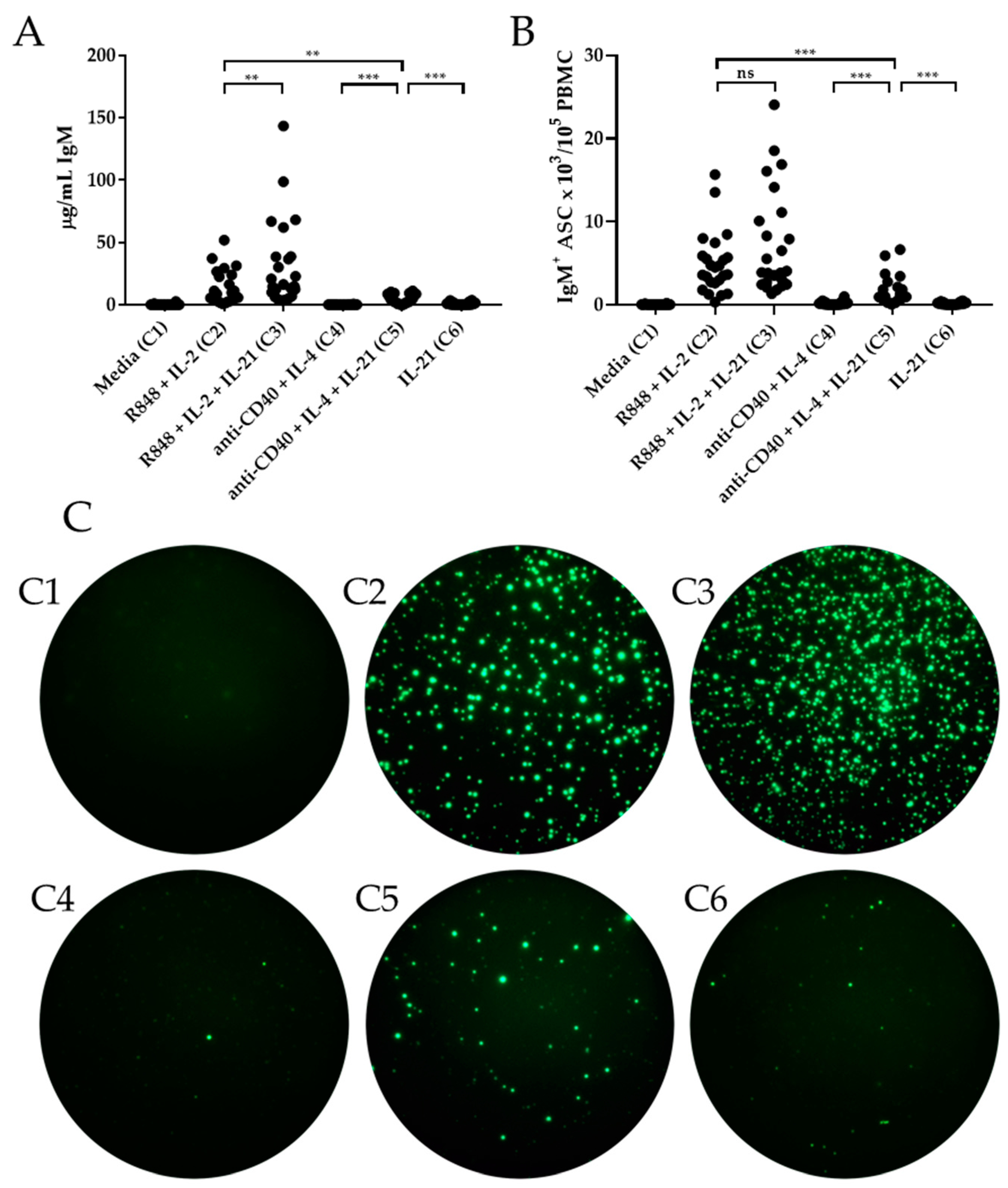
3.1. IL-21 Has Marginal Effects on Polyclonal IgM Production
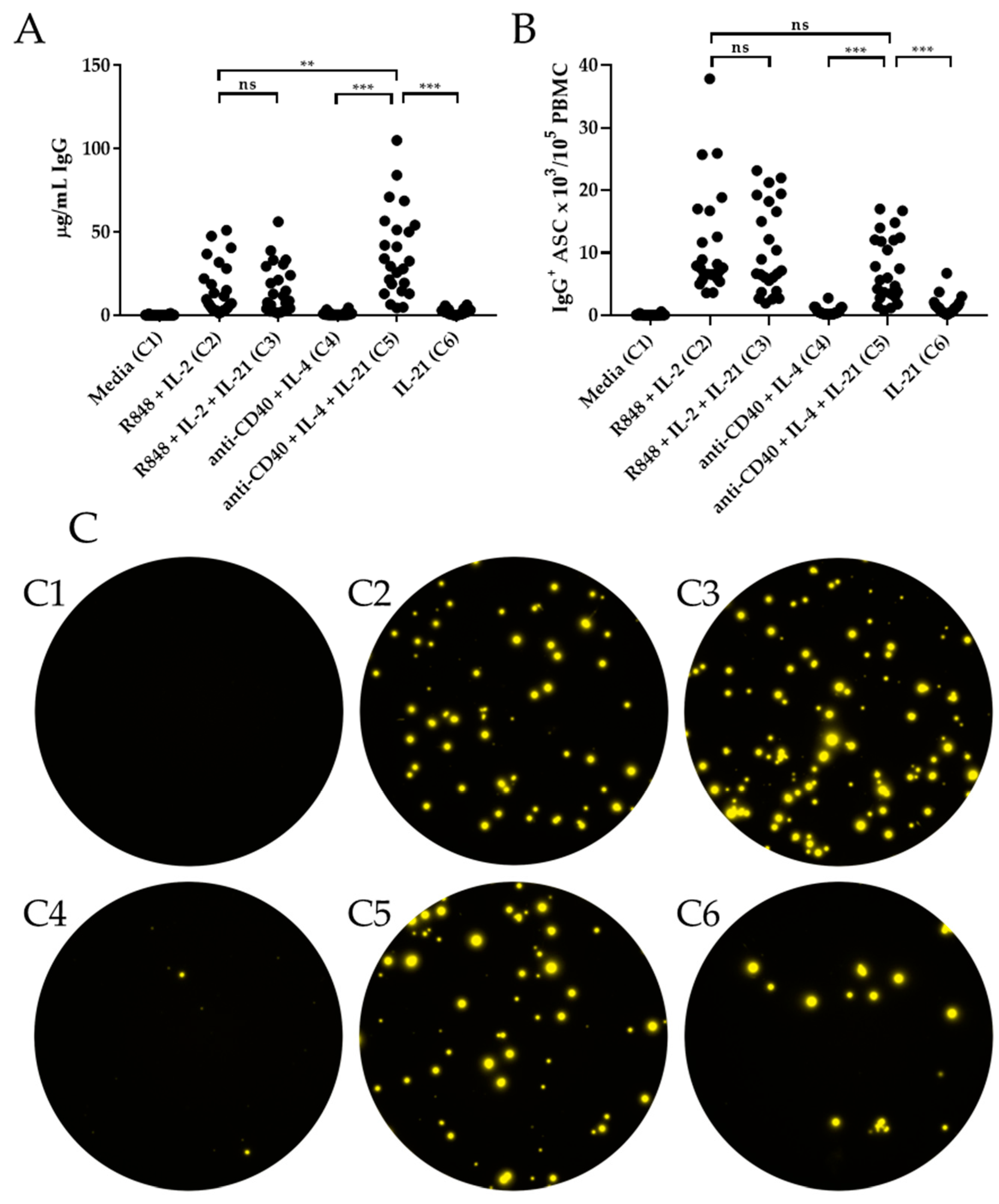
3.2. IL-21 Triggers IgG and IgA Production in Conjunction with anti-CD40 + IL-4
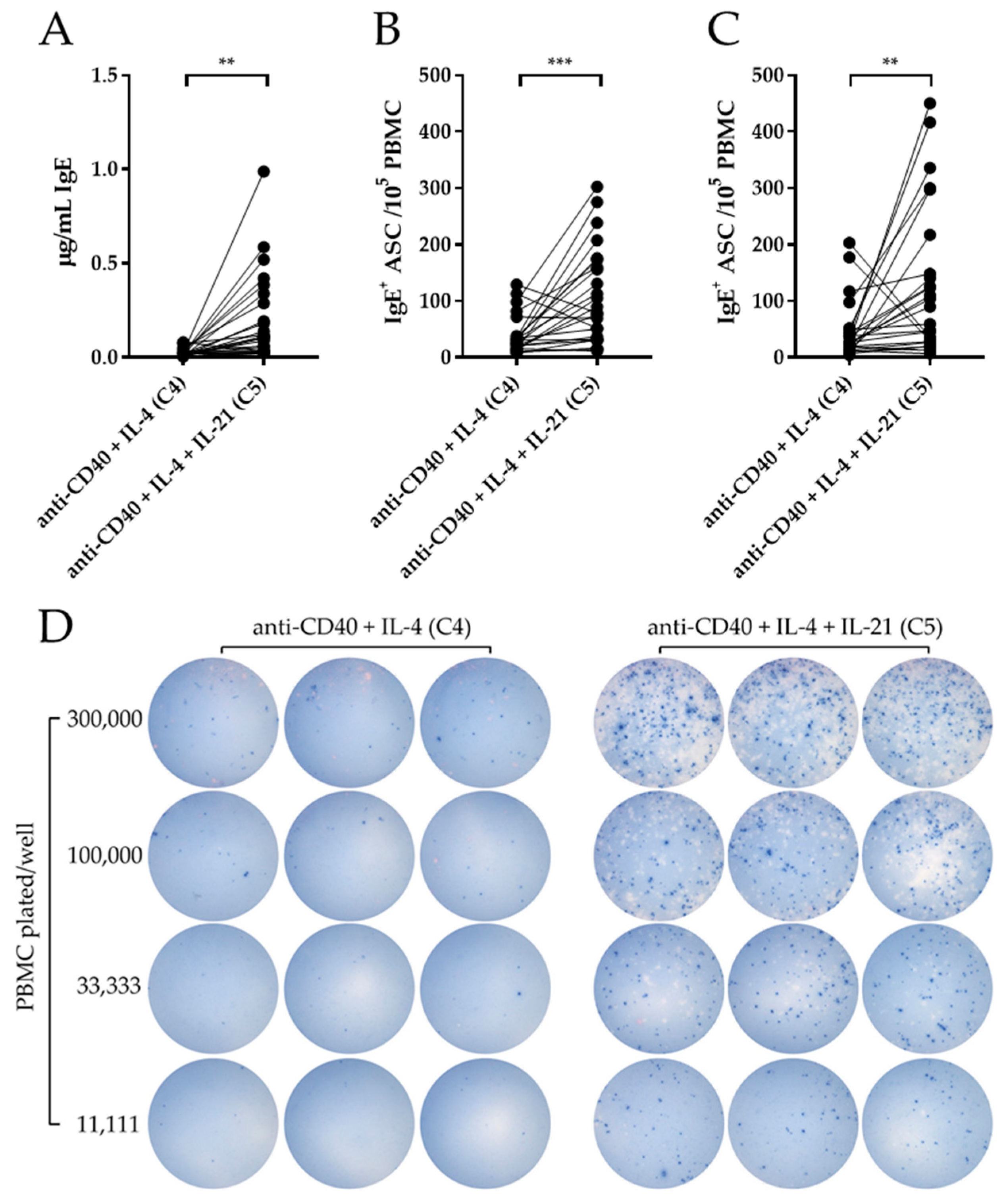
3.3. IL-21 Potentiates IgE Production Elicited by Anti-CD40 + IL-4
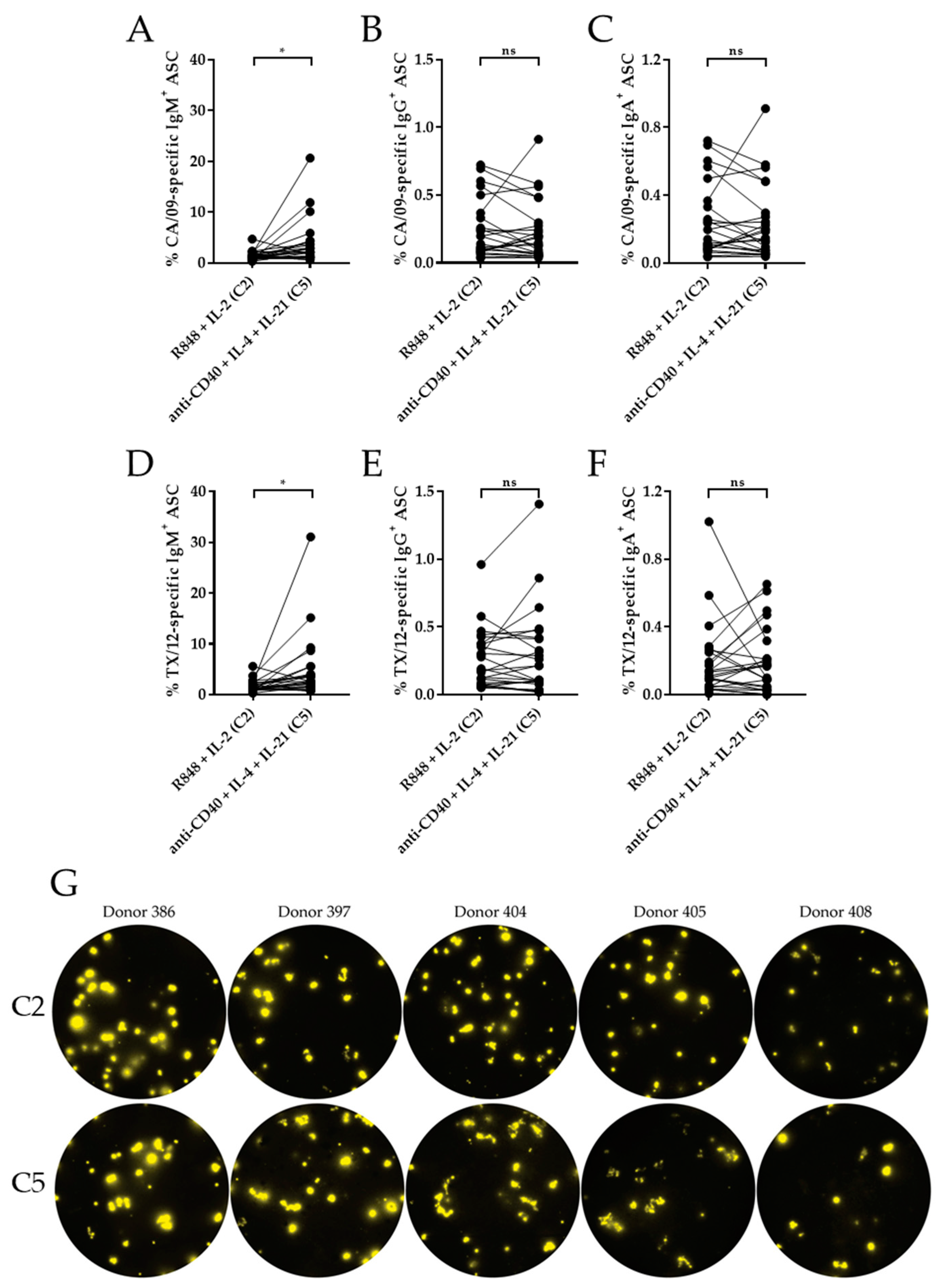
3.4. Effect of IL-21 on the Antigen-Specific B Cell Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tangye, S. Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Parmigiani, A.; Pahwa, S. Role of IL-21 and IL-21 receptor on B cells in HIV infection. Crit. Rev. Immunol. 2012, 32, 173–195. [Google Scholar] [CrossRef] [PubMed]
- King, C. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 2009, 9, 757–766. [Google Scholar] [CrossRef]
- Chtanova, T.; Tangye, S.G.; Newton, R.; Frank, N.; Hodge, M.R.; Rolph, M.S.; Mackay, C.R. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004, 173, 68–78. [Google Scholar] [CrossRef]
- Armitage, R.J.; Macduff, B.M.; Spriggs, M.K.; Fanslow, W.C. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J. Immunol. 1993, 150, 3671–3680. [Google Scholar]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Tangye, S.G.; Ma, C.S.; Brink, R.; Deenick, E.K. The good, the bad and the ugly—TFH cells in human health and disease. Nat. Rev. Immunol. 2013, 13, 412–426. [Google Scholar] [CrossRef]
- Banchereau, J.; Rousset, F. Human B Lymphocytes: Phenotype, Proliferation, and Differentiation. Adv. Immunol. 1992, 52, 125–262. [Google Scholar] [CrossRef]
- Defrance, T.; Vanbervliet, B.; Durand, I.; Briolay, J.; Banchereau, J. Proliferation and differentiation of human CD5+ and CD5- B cell subsets activated through their antigen receptors or CD40 antigens. Eur. J. Immunol. 1992, 22, 2831–2839. [Google Scholar] [CrossRef]
- Jelinek, D.F.; Lipsky, P.E. Inhibitory influence of IL-4 on human B cell responsiveness. J. Immunol. 1988, 141, 164–173. [Google Scholar]
- Defrance, T.; Vanbervliet, B.; Pene, J.; Banchereau, J. Human recombinant IL-4 induces activated B lymphocytes to produce IgG and IgM. J. Immunol. 1988, 141, 2000–2005. [Google Scholar]
- Rousset, F.; Garcia, E.; Banchereau, J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J. Exp. Med. 1991, 173, 705–710. [Google Scholar] [CrossRef]
- Splawski, J.B.; Fu, S.M.; Lipsky, P.E. Immunoregulatory role of CD40 in human B cell differentiation. J. Immunol. 1993, 150, 1276–1285. [Google Scholar]
- Vernino, L.; McAnally, L.M.; Ramberg, J.; Lipsky, P.E. Generation of nondividing high rate Ig-secreting plasma cells in cultures of human B cells stimulated with anti-CD3-activated T cells. J. Immunol. 1992, 148, 404–410. [Google Scholar]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef]
- Rousset, F.; Garcia, E.; Defrance, T.; Peronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef]
- Briere, F.; Servet-Delprat, C.; Bridon, J.M.; Saint-Remy, J.M.; Banchereau, J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 1994, 179, 757–762. [Google Scholar] [CrossRef]
- Rousset, F.; Peyrol, S.; Garcia, E.; Vezzio, N.; Andujar, M.; Grimaud, J.A.; Banchereau, J. Long-term cultured CD40-activated B lymphocytes differentiate into plasma cells in response to IL-10 but not IL-4. Int. Immunol. 1995, 7, 1243–1253. [Google Scholar] [CrossRef]
- Dubois, B.; Massacrier, C.; Vanbervliet, B.; Fayette, J.; Briere, F.; Banchereau, J.; Caux, C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998, 161, 2223–2231. [Google Scholar]
- Punnonen, J.; Aversa, G.; Cocks, B.G.; McKenzie, A.N.; Menon, S.; Zurawski, G.; de Waal Malefyt, R.; de Vries, J.E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc. Natl. Acad. Sci. USA 1993, 90, 3730–3734. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.J.; Macduff, B.M.; Eisenman, J.; Paxton, R.; Grabstein, K.H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 1995, 154, 483–490. [Google Scholar] [PubMed]
- Defrance, T.; Vanbervliet, B.; Briere, F.; Durand, I.; Rousset, F.; Banchereau, J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 1992, 175, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Hanna, N.; Fast, L.D.; Kouttab, N.; Shank, P.R.; Vazquez, A.; Sharma, S. Novel diversity in IL-4-mediated responses in resting human naive B cells versus germinal center/memory B cells. J. Immunol. 2000, 165, 5573–5579. [Google Scholar] [CrossRef]
- Arpin, C.; Dechanet, J.; Van Kooten, C.; Merville, P.; Grouard, G.; Briere, F.; Banchereau, J.; Liu, Y.J. Generation of memory B cells and plasma cells in vitro. Science 1995, 268, 720–722. [Google Scholar] [CrossRef]
- Mingari, M.C.; Gerosa, F.; Carra, G.; Accolla, R.S.; Moretta, A.; Zubler, R.H.; Waldmann, T.A.; Moretta, L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature 1984, 312, 641–643. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hirano, T.; Nakagawa, N.; Yoshizaki, K.; Kishimoto, T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J. Immunol. 1985, 134, 959–966. [Google Scholar]
- Gauchat, J.F.; Aversa, G.; Gascan, H.; de Vries, J.E. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int. Immunol. 1992, 4, 397–406. [Google Scholar] [CrossRef]
- Lundgren, M.; Persson, U.; Larsson, P.; Magnusson, C.; Smith, C.I.; Hammarstrom, L.; Severinson, E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur. J. Immunol. 1989, 19, 1311–1315. [Google Scholar] [CrossRef]
- Pène, J.; Rousset, F.; Brière, F.; Chrétien, I.; Bonnefoy, J.Y.; Spits, H.; Yokota, T.; Arai, N.; Arai, K.; Banchereau, J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1988, 85, 6880–6884. [Google Scholar] [CrossRef]
- Vercelli, D.; Jabara, H.H.; Arai, K.-I.; Yokota, T.; Geha, R.S. Endogenous interleukin 6 plays an obligatory role in interleukin 4-dependent human IgE synthesis. Eur. J. Immunol. 1989, 19, 1419–1424. [Google Scholar] [CrossRef]
- Good, K.L.; Bryant, V.L.; Tangye, S.G. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 2006, 177, 5236–5247. [Google Scholar] [CrossRef]
- Dam, E.M.; Maier, A.C.; Hocking, A.M.; Carlin, J.; Ng, B.; Buckner, J.H. Corrigendum: Increased Binding of Specificity Protein 1 to the IL21R Promoter in B Cells Results in Enhanced B Cell Responses in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1122. [Google Scholar] [CrossRef]
- Kuchen, S.; Robbins, R.; Sims, G.P.; Sheng, C.; Phillips, T.M.; Lipsky, P.E.; Ettinger, R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 2007, 179, 5886–5896. [Google Scholar] [CrossRef]
- Spensieri, F.; Borgogni, E.; Zedda, L.; Bardelli, M.; Buricchi, F.; Volpini, G.; Fragapane, E.; Tavarini, S.; Finco, O.; Rappuoli, R.; et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc. Natl. Acad. Sci. USA 2013, 110, 14330–14335. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Pilakka Kanthikeel, S.; Silva, S.Y.; Fischl, M.; Pahwa, R.; Pahwa, S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J. Immunol. 2011, 186, 6173–6181. [Google Scholar] [CrossRef]
- Pinna, D.; Corti, D.; Jarrossay, D.; Sallusto, F.; Lanzavecchia, A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur. J. Immunol. 2009, 39, 1260–1270. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Kesa, G.; Netterlid, E.; Buisman, A.M.; Thorstensson, R.; Ahlborg, N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J. Immunol. Methods 2013, 391, 50–59. [Google Scholar] [CrossRef]
- Gascan, H.; Gauchat, J.F.; Aversa, G.; Van Vlasselaer, P.; de Vries, J.E. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J. Immunol. 1991, 147, 8–13. [Google Scholar]
- Wrammert, J.; Ahmed, R. Maintenance of serological memory. Biol. Chem. 2008, 389, 537–539. [Google Scholar] [CrossRef]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef]
- Caspell, R.; Lehmann, P.V. Detecting all Immunoglobulin Classes and Subclasses in a Multiplex 7 Color ImmunoSpot((R)) Assay. Methods Mol. Biol. 2018, 1808, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012, 1, 313–324. [Google Scholar] [CrossRef]
- Croce, M.; Rigo, V.; Ferrini, S. IL-21: A pleiotropic cytokine with potential applications in oncology. J. Immunol. Res. 2015, 2015, 696578. [Google Scholar] [CrossRef]
- Liu, S.M.; King, C. IL-21-producing Th cells in immunity and autoimmunity. J. Immunol. 2013, 191, 3501–3506. [Google Scholar] [CrossRef]
- Kumar, S.; Mohan, A.; Guleria, R. Prognostic implications of circulating anti-p53 antibodies in lung cancer--a review. Eur. J. Cancer Care (Engl) 2009, 18, 248–254. [Google Scholar] [CrossRef]
- Pene, J.; Gauchat, J.F.; Lecart, S.; Drouet, E.; Guglielmi, P.; Boulay, V.; Delwail, A.; Foster, D.; Lecron, J.C.; Yssel, H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immuno. 2004, 172, 5154–5157. [Google Scholar] [CrossRef]
- Ettinger, R.; Sims, G.P.; Fairhurst, A.M.; Robbins, R.; da Silva, Y.S.; Spolski, R.; Leonard, W.J.; Lipsky, P.E. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005, 175, 7867–7879. [Google Scholar] [CrossRef]
- Ozaki, K.; Spolski, R.; Ettinger, R.; Kim, H.P.; Wang, G.; Qi, C.F.; Hwu, P.; Shaffer, D.J.; Akilesh, S.; Roopenian, D.C.; et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 2004, 173, 5361–5371. [Google Scholar] [CrossRef]
- Bryant, V.L.; Ma, C.S.; Avery, D.T.; Li, Y.; Good, K.L.; Corcoran, L.M.; de Waal Malefyt, R.; Tangye, S.G. Cytokine-Mediated Regulation of Human B Cell Differentiation into Ig-Secreting Cells: Predominant Role of IL-21 Produced by CXCR5+ T Follicular Helper Cells. J. Immunol. 2007, 179, 8180–8190. [Google Scholar] [CrossRef]
- Ding, B.B.; Bi, E.; Chen, H.; Yu, J.J.; Ye, B.H. IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J. Immunol. 2013, 190, 1827–1836. [Google Scholar] [CrossRef]
- Marasco, E.; Farroni, C.; Cascioli, S.; Marcellini, V.; Scarsella, M.; Giorda, E.; Piano Mortari, E.; Leonardi, L.; Scarselli, A.; Valentini, D.; et al. B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur. J. Immunol. 2017, 47, 131–143. [Google Scholar] [CrossRef]
- Hasbold, J.; Corcoran, L.M.; Tarlinton, D.M.; Tangye, S.G.; Hodgkin, P.D. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 2004, 5, 55–63. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Jackson, K.J.; Kissick, H.T.; Nakaya, H.I.; Davis, C.W.; Roskin, K.M.; McElroy, A.K.; Oshansky, C.M.; Elbein, R.; Thomas, S.; et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016, 17, 1226–1234. [Google Scholar] [CrossRef]
- Milovanovic, M.; Drozdenko, G.; Weise, C.; Babina, M.; Worm, M. Interleukin-17A promotes IgE production in human B cells. J. Invest. Derm. 2010, 130, 2621–2628. [Google Scholar] [CrossRef]
- Avery, D.T.; Ma, C.S.; Bryant, V.L.; Santner-Nanan, B.; Nanan, R.; Wong, M.; Fulcher, D.A.; Cook, M.C.; Tangye, S.G. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood 2008, 112, 1784–1793. [Google Scholar] [CrossRef]
- Hoof, I.; Schulten, V.; Layhadi, J.A.; Stranzl, T.; Christensen, L.H.; de la Mata, S.H.; Seumois, G.; Vijayanand, P.; Lundegaard, C.; Niss, K.; et al. Allergen-specific IgG+ memory B cells are temporally linked to IgE memory responses. J. Allergy Clin. Immunol. 2019, S0091-6749, 32602–32608. [Google Scholar] [CrossRef]
- Adam, A.; Woda, M.; Kounlavouth, S.; Rothman, A.L.; Jarman, R.G.; Cox, J.H.; Ledgerwood, J.E.; Gromowski, G.D.; Currier, J.R.; Friberg, H.; et al. Multiplexed FluoroSpot for the Analysis of Dengue Virus- and Zika Virus-Specific and Cross-Reactive Memory B Cells. J. Immunol. 2018, 201, 3804–3814. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Ellenbogen, Y.; Bruton, K.; Spill, P.; Sommer, D.D.; Lima, H.; Waserman, S.; Patil, S.U.; Shreffler, W.G.; Jordana, M. Human BCR analysis of single-sorted, putative IgE+ memory B cells in food allergy. J. Allergy Clin. Immunol. 2019, 144, 336–339.e6. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke, F.; Kirchenbaum, G.A.; Kuerten, S.; Lehmann, P.V. IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells. Cells 2020, 9, 433. https://doi.org/10.3390/cells9020433
Franke F, Kirchenbaum GA, Kuerten S, Lehmann PV. IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells. Cells. 2020; 9(2):433. https://doi.org/10.3390/cells9020433
Chicago/Turabian StyleFranke, Fridolin, Greg A. Kirchenbaum, Stefanie Kuerten, and Paul V. Lehmann. 2020. "IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells" Cells 9, no. 2: 433. https://doi.org/10.3390/cells9020433
APA StyleFranke, F., Kirchenbaum, G. A., Kuerten, S., & Lehmann, P. V. (2020). IL-21 in Conjunction with Anti-CD40 and IL-4 Constitutes a Potent Polyclonal B Cell Stimulator for Monitoring Antigen-Specific Memory B Cells. Cells, 9(2), 433. https://doi.org/10.3390/cells9020433








