Abstract
Lysosomes are central effectors of cellular maintenance, integrating the degradation of damaged organelles and protein aggregates with macromolecule recycling and metabolic signaling. In neurons, lysosomes are particularly crucial due to the cells’ long lifespan, polarized architecture, and high metabolic demands. Proper regulation of lysosomal function is essential to sustain proteostasis, membrane turnover, and synaptic integrity. Although lysosomal dysfunction has been extensively studied in neurodegenerative diseases, far less is known about how lysosomal capacity and function are maintained—or fail to be maintained—with age in non-diseased neurons. In this review, we summarize current understanding of neuronal lysosomal dynamics, discuss methodological challenges in assessing lysosomal capacity and function, and highlight recent advances that reveal age-associated decline in neuronal lysosomal competence.
1. Introduction
Lysosomes are membrane-bound degradative and signaling organelles essential for maintaining cellular homeostasis [1,2]. They contain an array of hydrolases within an acidic lumen that break down proteins, lipids, and nucleic acids delivered through the endocytic, autophagic, and phagocytic pathways. The resulting metabolites are released into the cytoplasm and recycled into biosynthetic and energetic pathways, linking lysosomal degradation to cellular metabolism and renewal. Beyond these canonical roles, lysosomes also act as organizing centers for metabolic signaling and stress response pathways such as mTORC1, AMPK, and calcium signaling [3,4]. Within individual cells, the balance between cellular growth and catabolism fluctuates in response to environmental and physiological conditions, necessitating the adjustment of lysosomal degradative capacity to meet these dynamic demands [1,5]. Because lysosomes continuously fuse with endosomal and autophagic compartments and then reform from endolysosomes and autolysosomes, a suite of lysosomes and related compartments of heterogeneous size, luminal pH, and molecular composition together define a cell’s lysosomal capacity [6,7,8]. For clarity, we use “lysosomal capacity” to mean the product of (A) the abundance of competent degradative lysosomal compartments and (B) their per-organelle degradative potential; “lysosomal function” refers to operational readouts including luminal pH, hydrolase activity, fusion/fission competence, cargo flux, and trafficking.
In neurons, proper lysosomal function is both especially important and unusually challenging. It is important because most neurons are generated during development and maintained throughout the lifespan of the animal in which they reside. This necessitates robust proteostasis and organelle quality control, including degradation and replacement. The exceptional morphological complexity of neurons contributes to the challenge of generating adequate lysosomal function: degradative lysosomes predominantly reside in the soma, but lysosomal degradative cargo resides throughout the neuron, including the distal axons and dendrites [9,10]. This necessitates the sustained transport of endosomes, autophagosomes, and other lysosomal compartments to the neuron cell body. Likely due to a combination of these circumstances, neurons are particularly sensitive to impairments in lysosomal function [9,11].
Much of the research on neuronal lysosomes has centered around the study of neurodegenerative diseases [12,13,14]. In Alzheimer’s disease (AD), Parkinson’s disease (PD), and other age-associated neurodegenerative diseases, lysosomal and autophagic defects contribute to the buildup of toxic proteins and organelle damage [15]. These pathological contexts highlight the critical role of lysosomal function in neuronal maintenance. Further, lysosomal decline is not limited to disease states. It has long been appreciated that neuronal lysosomal structure and function change in aging [16]. Age-associated neuronal lysosomal phenotypes include increased size and heterogeneity, altered positioning within neurites, and accumulation of undegraded substrates such as lipofuscin [17]. These changes in neurons parallel age-associated lysosomal alterations in a range of other tissue types from C. elegans to humans [18,19,20,21]. As described below, recent research building on the momentum of interest and methodological improvements has made it increasingly clear that lysosomal decline is a physiological feature of neuronal aging even in the absence of disease.
How neuronal lysosomal capacity is maintained across the lifespan and what mechanisms drive neuronal lysosomal decline are important questions of ongoing research. Recent work highlights the roles of transcriptional regulators, including Transcription Factor EB (TFEB), signaling pathways that tune lysosomal activity to metabolic state, regulation of lysosomal compartment trafficking, and mechanisms to sense and ameliorate lysosomal stress [1,22,23,24,25].
In this review, we synthesize current knowledge of neuronal lysosomal functions, the evidence for their decline during aging, and the transcriptional and signaling mechanisms that regulate their capacity. We feature emerging insights from experimental organisms and recent methodological advances that allow neuronal lysosomal function to be visualized and quantified.
2. Lysosomal Functions in Neurons
2.1. Catabolism and Recycling
Degradative lysosomes are the terminal organelles for a wide range of substrates, including proteins, lipids and organelles [26]. Their degradative capacity, which is generated by both the abundance of lysosomes and their functionality, is particularly critical in neurons, where turnover of neuronal proteins, organelles, and other macromolecules sustains functionality across an animal’s lifespan.
One major route of cargo delivery to lysosomes is the endo-lysosomal pathway (Figure 1). In addition to endocytosis, cytosolic proteins can enter this pathway through microautophagy, in which late endosomes and lysosomes directly engulf cytosolic material [27]. Following endocytosis, early endosomes function as sorting stations that direct cargo either toward degradation or back to the plasma membrane [28,29,30]. Recycling cargo can be returned to the cell via retromer-mediated retrieval subdomains or by bulk membrane flow [31,32,33]. By contrast, cargo destined for degradation is recognized by the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery, which binds ubiquitinated transmembrane proteins and sorts them into intraluminal vesicles (ILVs) within maturing endosomes, generating multivesicular bodies (MVBs) [32,34,35]. Some ILVs are released to the extracellular space as exosomes [36,37,38], whereas others remain within the organelle as it acidifies and transitions from a RAB5-positive early endosome to a RAB7-positive late endosome [39]. Late endosomes subsequently fuse with pre-existing lysosomes to form acidic, cathepsin-active endolysosomes, a process mediated by SNARE proteins including VAMP7 [40,41]. For example, during synaptic vesicle cycling, synaptic vesicle proteins retrieved from the plasma membrane often enter early endosomes, where most are recycled back into synaptic vesicles, but a subset is trafficked into late endosomes and delivered to lysosomes for degradation [42,43,44].
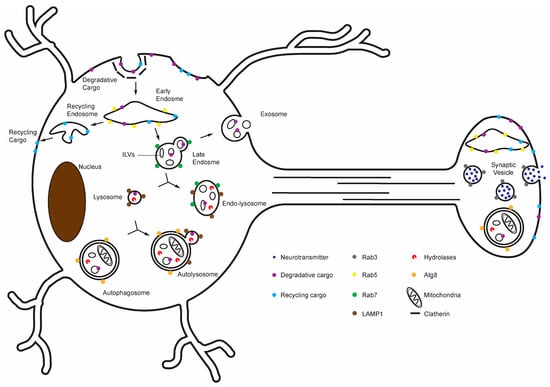
Figure 1.
Overview of the delivery of lysosomal degradative cargoes via endo-lysosomal and autophagic pathways in the neuron. Surveillance and sorting of lysosomal degradative cargoes occur throughout the neuron, but degradative lysosomes predominantly reside in the soma.
Autophagy provides several parallel routes for cargo degradation. In macroautophagy, autophagosomes form throughout dendrites and presynaptic axonal regions, where they engulf cytoplasmic material [45]. These autophagosomes fuse with lysosomal compartments in the neurites, which promotes retrograde trafficking, and in the soma to complete degradation [7,46,47,48,49]. Macroautophagy is particularly important in neurons for removing damaged mitochondria (mitophagy) and protein aggregates that cannot be refolded (aggrephagy) [45,50,51,52,53]. Fusion of autophagosomes with lysosomes is mediated by the BORC complex, the HOPS complex, and the autophagosomal SNARE syntaxin 17, whereas lysosomal trafficking regulator (LYST) facilitates lysosome reformation from autolysosomes [54,55,56]. Defects in autophagosome–lysosome fusion lead to the accumulation of autophagosomes, a phenotype commonly observed in neurodegenerative disease models [57,58]. The term macroautophagy encompasses the entire process from autophagosome initiation through autolysosomal degradation and lysosome reformation. Dysfunction of macroautophagy is considered a hallmark of aging, meaning that its occurrence correlates with age, and it drives functional decline in aging [59,60,61,62]. Different studies pinpoint impairments at distinct stages of macroautophagy in aging—some upstream of lysosomes, others that involve lysosomal steps. This distinction has implications for therapeutic strategies and is considered below. Chaperone-Mediated Autophagy (CMA) is another route of lysosome-mediated protein degradation, and it involves the translocation of degradative cargo proteins across the lysosomal membrane [63,64].
In addition to continuous fusion and reformation with endosomes and autophagosomes, lysosomes undergo fission, which adjusts lysosomal compartment number, size and positioning [65]. Spatacsin and spastizin, which interact with AP-5 and canonical fission factors such as clathrin and dynamin, drive putative lysosome-specific fission events [66,67,68]. Large BEACH domain-containing proteins such as LYST1 are recruited to lysosomal membranes and promote appropriate lysosomal fission [69,70]. The lysosomal Ca2+ channel TRPML1 is also required for lysosomal fission and fusion, and mutations in TRPML1 disturb neuronal lysosome trafficking and lead to lysosomal storage disorders (LSDs) [71].
Together, endo-lysosomal sorting and autophagy provide neurons with a constant degradative flux into lysosomal compartments, and lysosomal fission and fusion restore the number and size of lysosomes. Lysosomal degradative capacity directly controls the rate at which cargoes delivered from these two pathways are degraded. The resulting amino acids, short peptides, and other monomeric subunits are exported back into the cytosol via efflux transporters for reuse, and they also serve as signaling molecules to regulate metabolic responses. Thus, degradative flux into lysosomes (from endocytosis and autophagy) and the ability of lysosomes to clear that flux together define neuronal lysosomal capacity—a parameter that appears to change with age (Section 3) and is tuned by TFEB-family signaling (Section 4).
2.2. Signaling and Regulatory Roles
Lysosomes act as signaling platforms. The nutrient-sensitive kinase complex mTORC1 is recruited to the cytoplasmic side of lysosomal membranes in response to amino acid availability, where it integrates inputs to promote anabolic processes and inhibit autophagy [4,72,73]. Conversely, AMPK can be activated at lysosomes during energetic stress, promoting catabolic processes and enhancing autophagy [74,75].
Lysosomal compartments participate in intracellular Ca2+ signaling. Endolysosomes are a major storage compartment for Ca2+: the luminal concentration of free Ca2+ is thought to be ~500 μM when the luminal pH is ~4–5, whereas the cytosolic concentration is ~100 nM [76,77,78]. Still, the ER and mitochondria each store more of the cell’s total Ca2+ [79]. The lysosomal Ca2+ pool is readily releasable through channels including MCOLN1/TRPML1, TPCs, and inositol triphosphate receptor (IP3R) [80,81,82]. Indeed, through the IP3R, endolysosomal compartments have been shown to release Ca2+ in response to IP3 in several cell types [81]. Release of Ca2+ through endolysosomal IP3Rs generates local increases in cytosolic Ca2+ concentration, which could be amplified by Ca2+ release from the ER through Ca2+-gated ryanodine receptor (RYR) Ca2+ channels in excitable cells, including neurons [83]. In addition to its involvement in cellular Ca2+ signaling, lysosomal Ca2+ release influences lysosomal fusion, fission, and trafficking [71,84] and serves as a signal during lysosomal stress by activating calcineurin, promoting TFEB nuclear translocation [85].
In neurons, there are hints that these signaling functions are important for activity-dependent processes. For instance, synaptic activity alters local energy demand and can modulate mTORC1 activity, linking lysosomal signaling to plasticity [86,87]. Whether lysosomal signaling is sufficient to drive specific synaptic plasticity programs in vivo remains under investigation.
Lysosomes also regulate other organelles. For instance, at lysosome-mitochondria contact sites, lysosomes regulate mitochondrial fission [88]. Lysosome-Endoplasmic Reticulum contact sites mediate the exchange of lipids, and their disruption is associated with neurological diseases, including hereditary spastic paraplegia and amyotrophic lateral sclerosis [89].
Furthermore, lysosomes are a major storage site of ions beyond Ca2+, including Fe2+, Fe3+, Zn2+, and Cu2+ [90], and it is thought that inter-organellar contact sites mediate transport of ions from lysosomes to other organelles [90,91,92]. Considering the redox-active iron in particular, lysosomes accumulate these cations through degradation of ferritin, heme-containing proteins, and transferrin cargo [80,93]. When this iron is released into the cytosol, such as through lysosomal membrane permeabilization (LMP), it can not only exacerbate cellular damage, including ROS production and lipid peroxidation, but also promote ferroptosis [93,94,95].
The endolysosomal system is involved in intercellular signaling through the secretion of exosomes [96]. Exosomes are the intraluminal vesicles of MVBs that are released into the extracellular space when the MVB fuses with the plasma membrane. Exosomes can regulate neurodevelopment and synaptic function; the field is still in the early stages of understanding where and when exosomes form, what the relevant cargoes are, and the roles of exosomes in intercellular communication [96].
2.3. Transport and Spatial Organization
Neuronal morphology poses unique demands for lysosomal positioning and transport. Degradative lysosomes are enriched in the soma, while distal axons and dendrites are sites of high turnover demand [9]. Accommodating this disconnect, endosomes and autophagosomes form in axons and dendrites, and they undergo retrograde transport to fuse with somatic lysosomes [9,97]. Acidified lysosomal compartments containing degradative enzymes are also present in neurites [98,99]. These lysosomal compartments fuse with autophagosomes and endolysosomes, and this fusion promotes their retrograde trafficking by motor proteins along microtubules [7,46]. These lysosomal transport events are not only a mechanism for lysosomal catabolism and signaling but also generate a distinct lysosomal function in that neuronal lysosomes and late endosomes can also act as transport vehicles: lysosomes carry mRNAs along axons and dendrites to sites of local translation, and they also carry ER tubules along neurites [100,101]. Regarding mRNA transport, this coupling may coordinate the anabolic process of local protein synthesis with the catabolic process of cargo degradation.
Together, these features illustrate the complexity of lysosomal organization and function in healthy neurons. An important question, therefore, is how well these processes are maintained over the course of aging.
3. Neuronal Lysosomes in Aging
Does the functionality of neuronal lysosomal compartments decline in aging? If so, is neuronal lysosomal dysfunction a driver of neuronal dysfunction with age? Addressing these questions requires a working definition of lysosomal functionality that incorporates the various roles of lysosomes in neurons. We propose a multi-pronged definition: adequate abundance and activity of lysosome-resident proteins, including degradative enzymes and transporters, as well as appropriate lysosome fusion, fission, and trafficking machinery; proper sorting of lysosome-resident proteins to lysosomes; adequate functionality of fission, fusion, and sorting machinery; and intact mechanisms for lysosomal repair and quality control. When a cell’s lysosomal functionality is adequate, lysosomal cargo degradation overall is resolved in a timely manner and without over-reliance on backup mechanisms such as lysosome exocytosis.
Lysosomal dysfunction not only disrupts physiological lysosomal roles but also generates additional cellular stresses. LMP is a central example. When the lysosomal limiting membrane becomes damaged, luminal pH rises, impairing lysosomal degradation and recycling activities. LMP also permits leakage of lysosomal hydrolases and ions, including redox-active iron, into the cytoplasm, which can amplify oxidative stress and trigger cell death [93,102]. LMP may be a critical component for the age-associated accumulation of protein aggregates in the brain, as the lysosomal membrane ruptures enable endocytosed protein aggregates to escape the endolysosomal system and enter the cytoplasm [103]. LMP has been documented in several neurodegenerative contexts, including Parkinson’s disease [104,105] and Niemann–Pick disease [106]. Disease-associated protein aggregates themselves can induce LMP [107,108], suggesting a feedback loop in which lysosomal dysfunction and proteotoxic stress exacerbate each other. In addition, failure to retain luminal Ca2+, whether due to LMP or altered flux through lysosomal Ca2+ channels such as MCOLN1, perturbs local Ca2+ signaling and can further impair lysosomal trafficking and repair responses [109,110].
To evaluate whether neuronal lysosomal functionality declines during aging, we first discuss the methods by which neuronal lysosomal function is assessed. We will highlight challenges to quantifying lysosomal functionality; in particular, many endolysosomal phenotypes, such as increased abundance of early endosomes, could be indicative of a variety of underlying phenomena other than lysosomal dysfunction. Still, by assessing a combination of lysosome-related phenotypes, recent studies have painted a picture of inadequate neuronal lysosomal function in aging. Finally, we address whether neuronal lysosomal dysfunction is a driver of neuronal dysfunction with age, drawing on studies in which lysosomal pathways have been genetically or pharmacologically manipulated.
3.1. Approaches to and Challenges of Assessing Lysosomal Functionality
Here, we briefly consider some of the main approaches used to quantify lysosomal functionality, with emphasis on challenges of interpretation when comparing young versus aging neurons (Figure 2). These approaches, and additional methodologies, have been excellently reviewed in depth elsewhere [6].
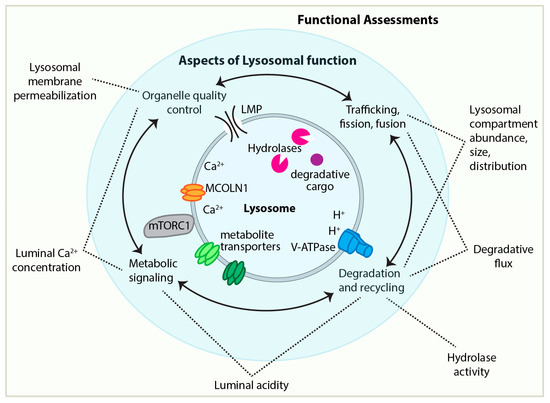
Figure 2.
Functional assessment of lysosomal biology encompasses multiple, interrelated aspects of lysosomal function. (Center) Schematic representation of a lysosome. Four major, interconnected lysosomal functionalities are depicted: three that service the cell—degradation and recycling, metabolic signaling, and trafficking—and a fourth, organelle quality control, which is required for the homeostatic maintenance of lysosomal compartment functionality. (Perimeter) Six categories of approaches to assess lysosomal capacity and function.
Counting lysosomal compartment number and size: With Transmission Electron Microscopy (TEM), lysosomal compartments can be identified based on the single-membrane encapsulation and presence of internal electron dense material and membrane whorls, but the heterogeneity of size and appearance, combined with the presence of other organelles such as autophagosomes that can have a similar appearance, means practice and judgment are necessary [111]. Autophagosomes and endolysosomes can also be identified by their distinctive appearances. Immuno-TEM or correlated light and electron microscopy (CLEM) provides additional information that aids in distinguishing organelles and subpopulations of organelles [112,113,114,115,116]. Volume EM techniques such as serial block-face scanning EM (SBEM) and focused ion beam SEM (FIB-SEM) have increased the throughput of generating 3D EM reconstructions such that sample sizes conducive to quantifying lysosomal compartments are feasible [117,118,119].
For visualizing lysosomal compartments with light microscopy, a challenge is that lysosomal compartments are heterogeneous in size, shape, molecular composition, and pH. Furthermore, lysosomal resident proteins traffic through the secretory pathway at least during biosynthesis. These factors make it challenging to label specific lysosomal compartments. For example, LAMP1, which has been used as a marker for lysosomes, labels not only degradative lysosomes but also late endosomes, autolysosomes, and amphisomes (autophagosomes fused with late endosomes) throughout neurons [120,121,122]. Acidic lysosomal compartments can be visualized using pH-sensitive, live-cell LysoTracker dyes, though the fluorescence intensity cannot be used to measure lysosomal pH [6]. A combinatorial labeling approach helps to identify specific subsets of lysosomal compartments.
Beyond the technical challenges to assessing the number, size and distribution of lysosomal compartments, there is a conceptual challenge for interpreting such data in regard to neuronal lysosomal function, in that the steady-state abundance and localization could mean a variety of things for the cell [123]. For instance, if an aged neuron has increased lysosomal functionality compared to a young neuron, it might have more lysosomes in the soma, enabling faster flux of degradative cargo. On the other hand, if an aged neuron has decreased lysosomal functionality compared to a young neuron, it might likewise have more lysosomes in the soma due to slowed degradation of each load of cargo, leading to a lack of lysosomal fission.
Quantifying lysosomal degradative flux: For cultured neurons, degradative flux can be assessed using fluorescence microscopy by visualizing fluorescent cargoes for lysosomal degradation, typically employed with cargoes internalized through endocytosis. pH-sensitive dyes can help identify when and where these cargoes reach acidified lysosomal lumens. These cargoes can be endocytosed by receptor-mediated endocytosis or bulk endocytosis. For receptor-mediated endocytosis, a challenge is that the dynamics of that specific receptor’s trafficking might change with age in a manner that is specific to the biology of that receptor rather than due to changing endolysosomal flux or lysosomal function. Another consideration is that the rate of endocytosis must be assessed, as that rate will impact the accumulation of degradative flux reporters. Using a combination of both fluorogenic probes, like DQ-BSA, and fluorescent probes, like Texas Red-BSA, distinguishes endocytosis defects and degradation defects [124]. Finally, for dyes that are degraded within lysosomes, the appropriate timing of the experiment is critical so that reduced fluorescence in, for example, aged neurons compared to young neurons can be confidently interpreted as decreased degradative flux rather than increased degradative flux [6].
Degradative flux of a representative lysosomal cargo can be quantified in vivo using genetically encoded tools that incorporate a pulse-chase component, such as Halo tag or the ARGO (Analysis of Red-Green Offset) method [125,126]. However, if turnover is found to be slower in aged animals, additional experiments are required to determine whether the mechanism involves lysosomal dysfunction as opposed to altered regulation of that specific cargo.
Assessing lysosomal acidity: Lysosomal acidity is a good indicator of degradative competence, as most lysosomal hydrolyses operate optimally at low pH [127]. Acidity is also relevant for metabolic signaling because the V-ATPase, which generates the proton gradient across the lysosomal membrane, directly regulates mTORC1 signaling [73].
Relative lysosomal luminal pH can be quantified using ratiometric fluorescence [128]. This can be achieved with dyes such as LysoSensor, which labels all acidic compartments, or with genetically encoded ratiometric reporters in which a lysosomal luminal protein is dually tagged with pH-insensitive mCherry and pH-sensitive GFP [6,21]. Fluid-phase pulse-chase labeling with pH-sensitive and pH-insensitive dyes can also be used, but only compartments actively participating in the endocytic flux will be labeled, limiting the ability to assess total lysosomal capacity [6,129].
Measuring hydrolase activity: In vitro biochemical assays can quantify total lysosomal hydrolase activity in brain lysates [17,20]. These assays provide a general measure of degradative capacity, but they neither distinguish between cell types nor report on subcellular localization. In vitro activity may also overestimate physiological function if lysosomal pH is elevated in situ. Moreover, total hydrolase activity alone does not indicate whether degradative capacity is adequate for cellular needs [17].
In cultured cells, hydrolase activity can be quantified using membrane-permeant fluorogenic substrates [6,130]. Magic Red reagents, for example, contain peptide sequences that confer relative cathepsin selectivity, and fluorescence intensity per cell serves as a proxy for lysosomal function [130].
Lysosome hydrolase activity can also be inferred from the abundance and maturation state of hydrolases. Western blot analysis can quantify total hydrolase protein and the proportion of mature versus pro-enzyme forms [131]. Endoglycosidase H treatment, which degrades proteins without N-linked glycan modifications, can identify immature hydrolases that have not yet trafficked through the Golgi [132]. However, these assays do not report subcellular localization and therefore may miss cases of hydrolase leakage, mis-sorting, or defects in endo-lysosomal fusion.
Hydrolase abundance can be measured in situ using antibodies or fluorescent hydrolase inhibitors such as BODIPY-conjugated probes [6,132,133].
Measuring lysosomal Ca2+ concentration: Lysosomes store Ca2+, and the leakage of Ca2+ from lysosomes into the cytoplasm can be indicative of lysosomal dysfunction [134]. Genetically encoded Ca2+ indicators such as GCaMPs are commonly used for measuring Ca2+ levels in specific subcellular locations; however, the fluorophore is sensitive to acidic environments [135,136]. Cytosolic GCaMP can be used as an indirect indicator of Ca2+ uptake and release from organelles, though whether lysosomes versus other Ca2+ storage organelles are involved is unclear. The development of pH-insensitive, bioluminescent, aequorin-based genetically encoded Ca2+ indicators has enabled Ca2+ measurement of lysosomes [81,135,137]. For cultured cells, another option is chemical Ca2+ sensors, such as the fluorogenic calcium-sensitive dye Cal-520, conjugated to dextran so that they will enter the endolysosomal system through endocytosis [78,138].
Lysosomal membrane permeabilization (LMP): LMP can be indicative of lysosomal dysfunction: if the ions and enzymes residing within the lysosome leak into the cytoplasm in an uncontrolled manner, that would both deplete lysosomal functionality and possibly generate toxicity in the cytoplasm.
LMP can be assessed by cytosolic release of 3–10 kDa fluorescent dextran, which works as a marker for endolysosomal flux and cannot be degraded by lysosomes [139]. The accuracy of these measurements requires functional endocytic machinery to load dextran into lysosomes.
Alternatively, LMP can be quantified with Galectin-3/GAL3 recruitment to lysosomes [140,141]. This occurs when the lysosomal resident glycoproteins, which shield the luminal membrane from the low pH and hydrolases, are exposed to the cytoplasmic side, such as when cells are exposed to strong lysosomal membrane-permeabilizing agents [140,141,142]. The physiological circumstances that cause LMP and/or recruit Galectin-3 to lysosomes are still a developing area of research [6]. There are hints that neurodegenerative disease-associated protein aggregates within the lysosomal lumen can cause LMP, described further in Section 3.2.1 [107,143]. Interestingly, neurons exhibit a degree of basal LMP, observed in cultured human induced neurons, human transduced neurons, and mouse pyramidal neurons in vivo [103,138]. Basal LMP is also observed in astrocytes [144]. In light of this, LMP in neurons may not be exclusively indicative of pathology but rather could be a physiologically regulated phenomenon with a yet-unclear cellular function. Indeed, LMP does have physiological roles in other contexts, such as to promote proper chromosome segregation during mitotic cell division [145].
In summary, a neuron’s lysosomal function can be compromised if the lysosomal luminal acidity is not low enough to maintain activity of degradative enzymes, if the abundance of one or more critical degradative enzymes is too low, if Ca2+ storage or release is disrupted, or if there is inadequate fusion or fission. Considering this list of disparate phenotypes, there is no one key metric by which lysosome dysfunction can be identified. Instead, assessing lysosomal functionality requires a combinatorial assessment of lysosomal compartment and distribution, lysosomal protein abundance, lysosomal acidity, and lysosomal degradative flux. As an illustration, we consider an example from cultured human fibroblasts: in senescent cells, the average number of Galectin puncta per cell and the average lysosomal luminal pH are both increased compared to non-senescent cells, indicative of lysosomal dysfunction [146]. Senescent cells also have more lysosomal compartments and increased abundance of several lysosomal proteins compared to non-senescent cells, and multiple reporters for lysosomal degradative flux indicated no decrease between senescent versus non-senescent cells [146,147]. Considered together, these phenotypes indicate that senescent cells sufficiently upregulate lysosome abundance to maintain their degradative capacity [146]. Finally, for mammalian neurons, lysosomal function can be most thoroughly assessed in cultured cells, but culture conditions do not fully recapitulate neurons’ morphology, function, and cell-extrinsic environments in the intact nervous system. Confidence in the applicability of in-depth analyses from cultured mammalian neurons is bolstered by similar findings from invertebrate and vertebrate animal neurons in situ [123,148].
3.2. Neuronal Lysosomal Dysfunction in Aging: Overview of the Evidence
It has been appreciated for decades that cargoes for neuronal lysosomal degradation accumulate in the brain with age, leading to the hypothesis that neuronal lysosomal function itself declines in aging [17]. Only in the past few years has the question been addressed with the combinatorial methodological approach necessary to generate strong evidence that this is indeed the case.
3.2.1. Hints and Correlations
One lysosomal degradative cargo that accumulates with advanced age is lipofuscin, which is composed mainly of oxidized cross-linked proteins and lipids [149,150]. Lipofuscin largely accumulates within lysosomal compartments, where it is delivered by macroautophagy; it is generally considered poorly degradable, and its accumulation strongly correlates with cellular aging in neurons and other cell types [149,151]. Further, it is postulated to be a cause of lysosomal dysfunction in aging [149,152]. On the other hand, the presence of a subset of non-degradative, non-recycling lysosomal compartments within a neuron does not necessarily imply that the cell’s overall lysosomal capacity is reduced or inadequate—there could still be functional lysosomal compartments working in parallel to the compartments that stably sequester lipofuscin. This notion that a subset of dysfunctional lysosomes could be set aside from a functional lysosomal system within the neuron is speculative.
Another type of cargo for lysosomal degradation that accumulates with age is misfolded protein aggregates, which are cargoes for autophagy [45,153,154]. As these proteins are continuously expressed (as opposed to turned-on in aging), their accumulation could indicate that (1) protein misfolding increases and/or the ubiquitin–proteasome system declines with age, but autophagy is not sufficiently up-regulated and/or not up-regulated at all, (2) autophagic surveillance declines with age such that protein aggregates that would have been identified and degraded in youth no longer are, or (3) autophagic flux is disrupted downstream of the formation of the autophagosome, and neurons and their surroundings accumulate protein aggregates that were ejected or escaped from autophagy. These possibilities are not mutually exclusive. Another aspect of this age-associated accumulation of protein aggregates is that the protein aggregates involved in neurodegenerative diseases, including tau and α-synuclein, propagate between neurons [155]. In this propagation, neurons endocytose protein aggregates [155,156,157]. These aggregates escape the endocytic pathway into the cytosol, where they template misfolding of the neurons’ endogenous proteins, and a major route of escape is from lysosomal compartments through LMP [107,143,158].
A pulse-chase isotope labeling plus mass spectrometry approach to quantify protein half-lives across the proteome in the brains of young adult versus aged mice indicated an overall increase of 20% in protein lifespans in the aged mice [159]. This increase was observed for putative cargoes of both lysosomal and proteasomal degradation, and putative cargoes for lysosomal degradation (identified as such based on their low isoelectric point) were significantly enriched for slowed turnover. This suggests that the flux of lysosomal degradation declines in aging. This could arise from age-associated lysosomal decline in neurons and/or glial cells, though alternate underlying mechanisms such as metabolic adaptations are equally plausible [159].
There is also an abundance of evidence that endosomal, autophagic, and lysosomal compartments change with age in neurons. Aging leads to the accumulation of late endosomes and multivesicular bodies in the presynaptic terminal in mice and D. melanogaster neuromuscular junctions (NMJ) [154,160,161]. In aged mouse hippocampal CA1 and PFC, but not CA3, lysosomal compartment size was increased compared to young adults [148]. This could be consistent with increased endocytic flux, decreased lysosomal degradative flux (leading to a backup of endosomal and autophagic compartments), or both. Indeed, neuronal endocytosis is upregulated with aging in cultured neurons [162], and endocytic proteins, including clathrin, dynamin-1, and Rab5, are up-regulated in aged human brains [163]. The correlation between endocytosis and neuron aging has also been noted in C. elegans: C. elegans can suspend aging while maintaining neuronal function in the dauer state, and in the dauer state, constitutive endocytosis is strongly suppressed [164]. Furthermore, in that context, the suppression of endocytosis contributes to the long-term preservation of dendrite morphology over time, prompting speculation that constitutive endocytosis can promote aging [164].
Whether and how the abundance and functionality of lysosomal proteins change with age in the brain varies between studies. Cathepsin D, the major lysosomal hydrolase, accumulates in the aging rat brain [165,166]. On the other hand, brains from humans without diagnosed neurodegenerative disease exhibited a progressive decline in glucocerebrosidase activity in the substantia nigra with aging [167]. Haploinsufficiency of glucocerebrosidase is one of the most common genetic risk factors of PD, which suggests that the declining glucocerebrosidase activity with age is likely a factor that promotes pathologies of aging rather than an adaptation to aging [168]. In the aging mouse brain, the activity of some lysosomal hydrolases, including beta-glucosidase, is decreased in aged compared to young adults, but the activity of other lysosomal hydrolases is unchanged or increased, complicating the picture [169]. In both the cortex and hippocampus of aged mice (18 months), the level of TFEB protein is reduced compared to younger adults (6 and 12 months), suggestive of decreased lysosomal capacity [170]. Finally, a neuron-specific transcriptional reporter for the activity of HLH-30, the sole C. elegans homolog of TFEB, showed decreased activation within C. elegans neurons by mid-adulthood, indicative of declining neuronal lysosomal capacity in aging [123,171].
CMA can be considered an aspect of lysosomal function, as the rate-limiting step is LAMP2A-mediated translocation of cargoes across the lysosomal membrane. The abundance of LAMP2A and other CMA proteins in the brain is either unchanged or increased in aged mice compared to young adults [169,172]. Still, the CMA flux in aging may be inadequate: genetic knockdown of LAMP2A pan-neuronally or from excitatory neurons causes accelerated molecular aging phenotypes in the mouse brain, including lipofuscin deposits, ubiquitinated protein inclusions, and other indicators of proteostasis collapse, suggesting that inadequate CMA contributes to the same aging phenotypes in the wild-type genetic background [172,173].
Neurons, with their high metabolic activity and exceptional longevity, are particularly vulnerable to cumulative environmental stress. The term exposome refers to the totality of lifetime exposures, including pollutants, pesticides, endocrine disruptors, and dietary metabolites, several of which are linked to oxidative stress and risk for age-related neurodegenerative diseases [174,175]. Chronic oxidative stress can impair neuronal function by oxidizing lipids, proteins and nucleic acids [176,177]. Oxidative stress may directly damage lysosomes through lipid peroxidation or impaired acidification, indirectly disrupt autophagy, or contribute to neuronal dysfunction without overtly altering lysosomal compartments [95,178,179,180,181,182]. Evidence also suggests that reactive oxidative species (ROS) accumulate in aged neurons, and overexpression of extracellular superoxide dismutase (EC-SOD) in aged mice improves learning and memory [183,184,185,186,187]. The “two-hit hypothesis” has been proposed for AD, PD, and several other neurodegenerative disorders, suggesting that both genetic and environmental factors are necessary to trigger these diseases [188,189]. Oxidative stress serves as one of the key environmental factors, is highly associated with the pathology of these neurodegenerative disorders [190,191]. Additional components of the exposome, such as advanced glycation end-products (AGEs), have been detected in Lewy bodies of Parkinson’s disease and in amyloid plaques of Alzheimer’s disease, suggesting involvement in neurodegenerative pathology [192,193,194,195]. Collectively, these findings indicate that environmental exposures can contribute to age-related neuronal disorders, with lysosomal dysfunction representing one potential pathogenic mechanism.
Considering how all these age-associated changes map onto lysosomal function or dysfunction, the important parameter is whether the lysosomal capacity is adequate for the needs of the cell, rather than the total lysosomal capacity per se. Because the need for lysosomal function increases with aging, aged neurons with unchanged or even increased levels of lysosomal proteins compared to young neurons may still have inadequate lysosomal capacity.
3.2.2. Evidence of Neuronal Lysosomal Dysfunction in Aging
Neuronal lysosomal function with aging was assessed in an aging paradigm of cultured primary neurons, wherein neurons at 21 DIV (days in vitro) are considered mature and neurons at 28 DIV are considered aged [148]. Supporting this categorization, 21 DIV neurons had established a maximal number of synapses, and 28 DIV neurons phenocopied several signs of aging in vivo, including accumulation of lipofuscin, but did not show gross morphological changes such as neurite degeneration [148]. Notably, the aged neurons showed decreased lysosomal compartment acidity compared to mature neurons [149]. Furthermore, the DQ-BSA intensity within lysosomal compartments throughout the neurites, but increased intensity of Dextran-647. As Dextran-647 fluoresces constitutively but DQ-BSA only fluoresces upon proteolytic cleavage within lysosomal compartments, these data show that bulk endocytosis is increased, but degradative flux is decreased, in the aging cultured neurons [148]. In addition, compared to the mature neurons, the aged neurons showed a redistribution of lysosomal material. Specifically, the aged neurons had increased size and number of lysosomal compartments labeled by LAMP1 and increased ratio of cathepsin D to LAMP1 in lysosomal compartments containing both, but no obvious change in the total abundance of LAMP1, total cathepsin D, or mature (protease-cleaved) cathepsin D.
The question of how aging impacts neuronal lysosomal function was also recently addressed using cultured human neurons that were transdifferentiated from fibroblasts of young adults (~25 years old) versus older adults (~70 years old) [138]. Compared to those from young adults, tNeurons from older adults showed decreased abundance of lysosomal proteins by proteomic analysis, decreased lysosomal Ca2+, decreased Cathepsin B activity, and no detectable change in lysosomal acidity. They also showed increased basal levels of LMP as well as slower kinetics of repairing LMP induced by exposure to the lysosomotropic drug LLOME (L-leucyl-L-leucine O-methyl ester). Altogether, these results paint a picture of decreased neuronal lysosomal capacity and function in aging [138].
CMA flux within neurons, assessed by genetically expressing the CMA-targeting motif KFERQ tagged to the fluorescent protein Dendra, tended to be reduced within the neurons of aged mouse brain compared to young adults [196]. Indeed, most organs and cell types showed reduced CMA flux in aged mice. The changes were dependent on neuron type and animal sex, though: C1 and DG hippocampal neurons and Purkinje neurons of both males and females showed reduced CMA flux in aged adults, entorhinal cortex neurons showed increased CMA flux in older animals, and somatosensory cortex neurons showed decreased CMA flux in aged males but not females [196].
In C. elegans, neuronal lysosomal compartments show increased luminal pH with aging [123]. They also exhibit an increased proportion of lysosomes with an abnormally high accumulation of a representative cargo for lysosomal degradation, the transmembrane synaptic vesicle protein Synaptotagmin/SNT-1, and slowed turnover of another transmembrane synaptic vesicle protein, Synaptogyrin/SNG-1 [123]. Unlike lipofuscin, Abeta, and similar, these lysosomal degradative cargoes are unlikely to be damaged such that they are challenging to degrade; therefore, this alteration in their degradative flux more directly implicates lysosomal dysfunction [123].
Considering these studies together, the interpretation of age-associated lysosomal dysfunction is bolstered.
3.2.3. Functional Consequences of Neuronal Lysosomal Dysfunction
Does it matter for neuronal health when the lysosomal capacity is inadequate, and if so, in what regard? As described above, lysosomes perform a variety of functions in the neuron; therefore, neuronal lysosomal dysfunction is expected to impact the neuron in multiple aspects. Experiments in which neuronal lysosomal function is genetically or chemically manipulated to assess the outcome on neuron maintenance have shed light on this question. There is a wealth of experimental evidence showing that causing lysosomal dysfunction accelerates neuronal aging phenotypes and functional decline; this indicates that lysosomal function is necessary to prevent aging phenotypes. The most compelling evidence also includes showing that preserving lysosomal function into aging delays age-related neuron dysfunction, as this suggests that lysosomal functionality is limiting for neuronal maintenance. Here, we highlight studies that incorporate that latter genre of evidence.
The accumulation of protein aggregates with age is perhaps the most proximal pathology to arise from age-associated decline in neuronal lysosomal function. In human tNeurons from aged individuals, treatment with compounds that improve lysosomal function led to decreased levels of Aβ42, the accumulation of which contributes to AD, whereas treatment with compounds that exacerbate lysosomal dysfunction led to increased accumulation of Aβ42 [138]. Aged, cultured neurons show reduced synapse numbers compared to younger neurons; pharmacologically restoring lysosomal acidity in the aged, cultured neurons partially restored synapse numbers, while pharmacologically reducing lysosomal acidity caused the decrease in synapse number to occur earlier [148]. These studies from cultured neurons indicate that neuronal lysosomal function is instructive for preventing both pathological protein buildup and declining neurotransmission in aging.
Several studies from intact animals corroborate the key role of neuronal lysosomal function in maintaining neuronal function in aging. Over-expression of TFEB in neurons, which is expected to increase lysosomal capacity and function, caused improved learning and memory in old mice [170]. In D. melanogaster, neuronal over-expression of the CMA effector LAMP2A bolstered climbing performance during aging without extending lifespan [197]. In C. elegans, the impaired neuronal lysosomal acidification that occurs during aging drives structural deterioration of dendrites [123]. Loss of TFEB/HLH-30 likewise accelerated age-associated dendrite morphological defects, whereas over-expression of TFEB/HLH-30 improved them [123].
For the cargo degradation function of lysosomes, it is worth considering that lysosomal degradation need not be resolved cell-intrinsically. Neuronal lysosomal degradative cargoes can be released to the extracellular space through several mechanisms. Lysosomal exocytosis, originally thought to be a specialized function of hematopoietic cells, has been identified in an increasingly wide variety of mammalian cell types [198,199], and it provides a cell-non-autonomous route for removal of cellular garbage [200,201]. A second mechanism is MVB fusion with the plasma membrane, releasing exosomes [96]. Exosome release can serve as a backup for lysosomal dysfunction in that disruption of lysosomal acidity causes increased exosome release [202,203,204]. A third mechanism is through the release of ectosomes, which contain cytosolic material that buds out from the plasma membrane [96]. For instance, in C. elegans, would-be cargoes for lysosomal degradation, including protein aggregates and organelles, can be secreted from neurons as large vesicles called exophers [205]. Neuronal exopher secretion increases upon inhibition of many canonical autophagy genes, and surprisingly, this generates a lifespan extension [206]. Therefore, this is a case where reduced neuronal autophagic flux is beneficial, rather than harmful, for neuronal health [206].
Mammalian neurons employ these backup mechanisms for inadequate lysosomal degradation by secreting pathogenic protein aggregates involved in neurodegenerative diseases. For example, neuron-expressed human α-synuclein is degraded by microglia in the mouse brain, which prevents neurodegeneration [207]. In fact, a few hundred aggregation-prone neuronal proteins can be found within microglia in the aging mouse brain, indicative of widespread neuronal outsourcing of protein degradation [208]. Unfortunately, the outsourcing of neuronal lysosomal degradation, combined with inadequate lysosomal degradation in the cells that uptake the waste, is a mechanism by which pathogenic protein aggregates spread and propagate between neurons, leading to neurodegenerative disease [23,209]. Furthermore, though the degradative functions of neuronal lysosomes can be outsourced to other cells, neuronal lysosomes provide anabolic building blocks and participate in trafficking functions, and outsourcing the degradation of intracellular waste leaves these challenges to resolve.
Another mode by which neuronal lysosomal dysfunction promotes functional decline in the brain is through inflammation. Aged tNeurons generated from healthy individuals as well as those generated from individuals with sporadic AD are more prone to inflammasome activation, and both secrete more pro-inflammatory cytokines, compared to tNeurons from young adults [138]. Connecting that inflammation to lysosomal dysfunction, cytokine secretion can be induced from young adult tNeurons by incubation with a drug that disrupts the lysosomal membrane, LLOME, and cytokine secretion can be ameliorated in the sporadic AD neurons by incubation with a drug that promotes lysosomal function, C381 [138]. These inflammatory signals can cause toxicity to neurons, microglia, and astrocytes [210]. In addition, impaired autophagy can lead to activation of the cyclic GMP-AMP (cGAMP) synthase (cGAS)/stimulator of interferon genes (STING) pathway, which drives inflammation in microglia and neurodegeneration in aging [211,212].
Finally, lysosomal dysfunction also causes or contributes to several neurodegenerative disorders. Mutations that impair lysosomal homeostasis lead to abnormal accumulation of macromolecules and result in LSDs [213]. In the nervous system, these defects promote axonal degeneration, inflammation and neuronal death, and more than half of LSDs present with psychiatric or cognitive symptoms [214,215,216,217]. For instance, a mouse model of Niemann–Pick type C (NPC) disease exhibits transcriptional and functional features consistent with accelerated brain aging [218]. Further, NPC mouse tissue shows transcriptomic similarities to human AD samples [218]. Both aging and AD neurons exhibit constitutive lysosomal damage and impaired lysosome repair, and restoring autophagy or lysosomal function mitigates AD-related defects such as Aβ accumulation and inflammation [139,219,220,221,222]. Lysosomal and autophagic-related abnormalities are also present in PD, where mutations in several common risk genes, including LRRK2, ATP6AP2, and SNCA, have been implicated in impaired lysosomal acidification and abnormal accumulation of undegraded cargo within lysosomes [223,224,225]. In both AD and PD, lysosomal dysfunction co-occurs with age-related hallmarks such as lipofuscin accumulation, oxidative stress, and inflammation [192,226,227,228,229]. Altogether, lysosomal dysfunction not only contributes to neurodegenerative pathology but can also manifest as features resembling accelerated neuronal aging.
3.2.4. Autophagic Dysfunction, Upstream of Lysosomal Function, with Age
There are many mechanisms of neuronal aging that likely function in parallel, at least partially, to lysosomal function [230]. By contrast, macroautophagy and lysosomal function are partially overlapping Venn diagrams. Therefore, we consider here age-associated dysfunction of autophagy upstream of lysosomal function, since the two are so connected. Specifically, there is evidence that insufficient autophagosome formation, as opposed to insufficient lysosomal functionality, is a driver of neuronal pathologies of aging [231].
Macroautophagy becomes dysfunctional in aging neurons in C. elegans, D. melanogaster, and mice [232,233,234,235,236,237,238,239,240]. For mice, work in cultured Dorsal Root Ganglion neurons isolated from adult mice at different ages, combined with characterizations of the aging neuromuscular junction in vivo, showed that the rate of autophagosome formation in distal neurites progressively declines with age, with no obvious defect in acidification nor retrograde transport of autophagosomes in aging [234,235]. Likewise, autophagic flux was reduced in hypothalamic proopiomelanocortin (POMC) neurons in the aged mouse brain [241]. In D. melanogaster, Atg8a loss-of-function causes shortened lifespan and more rapid onset of accumulation of insoluble ubiquitinated proteins, and over-expression of Atg8a in the brain causes a dramatic lifespan extension and reduced accumulation of damaged macromolecules—would-be cargoes for lysosomal degradation—in the brain [242]. ATG8 is involved in autophagosome formation and fusion with lysosomes, and so it is upstream of lysosomal function [243]. Along similar lines, loss-of-function of ER-phagy (selective autophagy of the Endoplasmic Reticulum) receptors leads to neurodegeneration, and neuron-specific up-regulation of the ER-phagy receptors extends lifespan and enhances motor function in aged D. melanogaster [244]. Neuronal over-expression of BNIP3, a mitochondrial protein and inducer of autophagy, was sufficient to partially ameliorate age-associated decline in neuronal mitophagy in D. melanogaster [233]. In C. elegans, activation of neuronal autophagy delays age-associated neurodegeneration and extends lifespan [232,245]. Of note, in D. melanogaster, genetic hyperactivation of autophagy in aging non-cell-autonomously antagonizes age-associated decline in neuron function and extends lifespan [233,246]. That non-cell-autonomous signaling role is likely a parallel mechanism to lysosomal function/dysfunction.
In summary, aging neurons exhibit multiple, partially overlapping failures in maintenance relating to lysosomal degradation, from reduced autophagosome formation to impaired lysosomal acidification. Together, these changes lower the neuron’s effective lysosomal capacity and constrain its ability to recover under stress. Given these age-associated deficits in lysosomal function, a central question is how neurons set and preserve lysosomal capacity across adulthood. We therefore next discuss TFEB and allied pathways that scale lysosomal capacity, as well as mechanisms of lysosomal quality control that preserve lysosomal function.
4. Regulation of Lysosomal Functional Capacity: TFEB and Quality Control
An understanding of how neuronal lysosomal capacity is regulated in healthy, young neurons is useful for assessing and counteracting age-associated neuronal lysosomal dysfunction. Lysosomal biogenesis and renewal rely on the coordination between gene expression, protein synthesis, and secretory pathway dynamics [1]. The Endoplasmic Reticulum (ER), Golgi apparatus and endosomes ensure the delivery of newly synthesized membrane and luminal proteins, including hydrolases, ion channels and transporters, into lysosomes [2]. Generating a cell’s lysosomal capacity involves transcription of lysosome and lysosome-related genes, and protein production and trafficking. It also requires quality control mechanisms to detect and resolve lysosomal stress—circumstances or defects that impair lysosomal function.
4.1. TFEB-Mediated Lysosomal Biogenesis
TFEB promotes transcription of many genes that encode lysosomal and autophagic proteins as a suite, and it is therefore often called a “master regulator” of lysosome biogenesis [247,248] (Figure 3). TFEB activity is regulated by phosphorylation: under nutrient-rich conditions, mTORC1 phosphorylates TFEB, causing its cytoplasmic retention [249,250,251]. During starvation, stress, or lysosomal dysfunction, TFEB is dephosphorylated, allowing it to translocate to the nucleus and activate gene expression [247,252]. TFEB function is regulated by several other signaling mechanisms beyond mTORC1. These include negative regulation from extracellular signal-regulated kinase 2 (ERK2), positive regulation by the phosphatase calcineurin when Ca2+ leaks from the lysosomes during lysosomal stress, positive regulation by AMPK, and positive regulation by protein phosphatase 2A (PP2A) [247]. As an integrator of these signaling pathways, TFEB sits at the intersection of nutrient sensing and lysosomal homeostasis. Furthermore, TFEB can be activated by lysosomal damage/stress, which can include the release of Ca2+ from the lysosome, and it is therefore part of a lysosome quality-control response [134]. In mammals, Transcription Factor E3 (TFE3) and MITF, members of the Mit/TFE family of transcription factors, along with TFEB, play some partially overlapping roles as TFEB in lysosomal biogenesis [253]. Despite their partial functional redundancy, the presence of both TFEB and TFE3 is required for maximal lysosomal biogenesis and autophagy in response to starvation or stress [254].
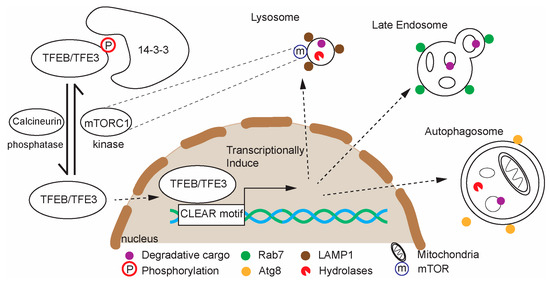
Figure 3.
Schematic of TFEB and TFE3 regulation and function in lysosomal gene expression. TFEB and its homologs regulate genes involved in lysosome, endolysosome, and autophagosome biogenesis. TFEB is most prominently regulated by mTORC1, which phosphorylates TFEB, promoting its cytoplasmic retention.
TFEB has garnered attention as a modulator of neurodegenerative disease progression: overexpression of TFEB can improve cellular and organismal health in several murine neurodegenerative disease models, including models of Parkinson’s, Huntington’s and Alzheimer’s disease, by inducing autophagy to degrade toxic protein aggregates, and depletion of TFEB can cause accelerated disease progression [87,247,255,256,257].
TFEB also promotes neuronal autophagy, including lysosomal biogenesis, in non-diseased neurons. In D. melanogaster, both brain-specific over-expression of TFEB/Mitf and brain-specific knockout cause increased abundance of autophagosomes [258]. A plausible interpretation of these data is that TFEB/Mitf sets the rate of autophagic flux: excessive Mitf leads to increased autophagosome formation, while lack of Mitf causes autophagosomes to accumulate due to diminished lysosomal capacity [258]. In C. elegans, TFEB/HLH-30 appears to be dispensable during neurodevelopment but expands neuronal lysosomal capacity in early adulthood [124]. Furthermore, neuronal TFEB may play a role in regulating synaptic communication in some contexts: in cultured rat primary cortical neurons, chronic suppression of synaptic inactivity activates TFEB, which promotes homeostatic synaptic up-scaling [86].
In addition to TFEB and TFE3, several other transcription factors, transcriptional repressors, and epigenetic regulators contribute to the control of lysosomal biogenesis. In mouse cell culture, deficiency in lysosomal cysteine proteases or increased endocytic substrate load leads to elevated lysosomal oxidative stress, which activates the transcription factor STAT3 and induces expression of lysosomal proteolytic hydrolases [259]. MYC, an E-box-binding transcription factor, acts as a transcriptional repressor of lysosomal biogenesis by occupying promoters of lysosomal genes and antagonizing TFEB/TFE3 activity; overexpression of MYC suppresses lysosomal and autophagic function in pluripotent stem cells and cancer cells [260]. Glucose starvation produces genome-wide demethylation of H3 Arg17, inducing nuclear translocation of the arginine methyltransferase CARM1, which cooperates with TFEB to serve as a transcriptional coactivator of lysosomal and autophagosomal biogenesis [261]. However, to date, there is no direct evidence regarding whether these regulatory mechanisms influence lysosomal biogenesis in neurons.
4.2. Lysosomal Quality Control
The integrity of the lysosomal limiting membrane is continuously under stress: as degradative substrates are delivered, processed, and exported, the osmotic composition of the lumen shifts dynamically, producing fluctuations in membrane tension and lysosomal volume [143]. The high acidity, hydrolytic enzymes, and relatively high concentrations of cations inside the lysosomal lumen also present a peril to the membrane. To withstand these stresses, lysosomal compartments rely on protective mechanisms that broadly fall into four categories: (1) intrinsic membrane-protective components; (2) mechanisms that sense and repair lysosomal damage; (3) mechanisms that expand lysosomal capacity through transcriptional regulation (TFEB and homologs from Section 4.1); and (4) pathways that remove damaged lysosomes. The inducible quality control mechanisms in categories 2–4 can occur simultaneously, and multiple repair pathways within category 2 function in parallel using distinct molecular machinery [262]. We briefly overview lysosomal quality control mechanisms here; several in-depth reviews have been published recently [143,262,263].
- (1)
- Intrinsic protection: The first line of defense against these stresses is intrinsic components of the lysosomal compartments, including the integral membrane glycoproteins, which protect the luminal membrane from damage, and ion channels and transporters [142,264,265].
- (2)
- Sense and repair damage: LMP, Ca2+ efflux, and/or disrupted lysosomal acidity can activate several partially overlapping repair pathways. Ca2+ efflux from the lysosomal lumen is both a hazard and a signal: elevation of local cytosolic Ca2+ rapidly recruits ESCRT machinery to the lysosomal membrane, which promotes repair [266,267,268,269]. Annexin A7 promotes lysosome repair in parallel to ESCRT-III [270]. The mechanisms of both ESCRT- and Annexin A7-mediated repair are not fully understood, but both are thought to involve inducing negative membrane curvature from the cytosolic face of the lysosomal membrane [266,267,268,269,270]. Stress granules form at sites of LMP, stabilizing the membrane and facilitating repair through both ESCRT-dependent and independent mechanisms [271]. LMP can also be repaired by the phosphoinositide-initiated tethering and lipid transport (PITT) pathway, which involves transfer of phosphatidylserine and cholesterol from the ER to the lysosomal membrane [272,273]. Another way in which the ER participates in repairing damaged lysosomes uses the ER-resident, lipid-transport protein VPS13C; the cytosolic side of VPS13C associates with damaged lysosomal membrane, likely to facilitate the transport of lipids from the ER to the lysosome [274].The conjugation of ATG8 to single membranes (CASM) pathway provides another major repair mechanism. CASM is activated by lysosomal deacidification or ionic imbalance to repair the lysosome [25,275]. Activation of CASM at lysosomal compartments can occur through two parallel mechanisms: one involves flipping sphingomyelin from the lysosome luminal to cytosolic side [276], and the other involves V-ATPase recruitment of ATG16L1 to the lysosome [25,277].
- (3)
- Stress-responsive expansion of lysosomal capacity: Efflux of Ca2+ from the lysosomal lumen can activate calcineurin, which dephosphorylates TFEB to promote transcriptional expansion of lysosomal and autophagic capacity [85]. The CASM machinery also facilitates activation of TFEB/TFE3 during lysosomal stress [278].
- (4)
- Removal of damaged lysosomes: When repair mechanisms are insufficient, damaged lysosomes can be selectively eliminated by lysophagy—selective autophagy of the lysosome [266]. To initiate lysophagy, LMP is sensed by cytosolic galectins, which bind lysosomal luminal beta-galactosides that become exposed by the membrane rupture [279,280,281,282]. Some of these galectins induce ubiquitination of the lysosome, triggering lysophagy through a mechanism that involves recruitment of VCP/p97 [108,280,282,283]. Pathogenic mutations in VCP/p97 cause disorders including frontotemporal dementia, amyotrophic lateral sclerosis, and Parkinsonism; in addition to promoting lysophagy, VCP/p97 is also an effector of macroautophagy, and both of these functions likely contribute to neuron pathologies [284,285]. Damaged lysosomes can also be removed from the cell via lysosomal exocytosis [286].
Beyond activating lysosome quality control pathways, lysosomal stress also interfaces directly with metabolic signaling. Lysosomal stress granules formed at sites of LMP repress mTORC1 activity in several cell types, including neurons [287]. In addition, galectins recruited to damaged lysosomes negatively regulate mTORC1 and activate AMPK, which not only activates TFEB to expand lysosomal capacity but also links lysosomal stress to broader shifts in cellular metabolic state [288].
5. Outlook
The body of evidence suggests that neuronal lysosomal function declines with age, and this decline contributes to neuronal dysfunction. The mechanisms that drive this decline remain incompletely defined. Understanding them is important for designing strategies to preserve lysosomal capacity, thereby extending neuronal health-span. A key challenge is to determine what limits a neuron’s ability to sense and repair lysosomal dysfunction to maintain lysosomal capacity [212].
TFEB is one key player that is both necessary and rate-limiting for sustaining neuronal lysosomal capacity [123,170,258]. How TFEB activity is tuned in the context of neuronal aging remains unclear. In principle, TFEB should respond to lysosomal stress, but current evidence indicates that age-associated lysosomal dysfunction does not robustly activate it. Indeed, neuronal HLH-30/TFEB activity appears to decline during aging despite lysosomal impairment [123]. Identifying molecular checkpoints that prevent TFEB reactivation in aging neurons could reveal new therapeutic opportunities.
Lysosomal quality control mechanisms, some of which involve TFEB activation, sustain lysosomal capacity in various cellular contexts. The extent to which these pathways function, adapt, or fail in neurons during adult maintenance is still poorly understood.
Altogether, defining how lysosomal quality-control, metabolic signaling, and transcriptional feedback interact during neuron aging will be crucial for understanding what causes lysosomal capacity to decline and how it might be restored.
Author Contributions
Writing—original draft preparation, R.Z. and C.E.R.; writing—review and editing, R.Z. and C.E.R.; visualization, R.Z. and C.E.R.; supervision, C.E.R.; project administration, C.E.R.; funding acquisition, C.E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health (NIH), grant number R35-GM154869 to and by the United States Department of Agriculture (USDA) grant WIS05016 to C.E.R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT5.1 for the purposes of editing the organization and language for clarity and readability. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the writing of the manuscript or in the decision to publish.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPK | AMP-activated protein kinase |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ILV | Intraluminal vesicle |
| RAB5 | Ras-related protein in brain 5 |
| RAB7 | Ras-related protein in brain 7 |
| VAMP7 | Vesicle-associated membrane protein 7 |
| BORC | BLOC-1-Related Complex |
| HOPS | Homotypic fusion and protein sorting |
| LYST | Lysosomal trafficking regulator |
| TFEB | Transcription factor EB |
| TFE3 | Transcription factor E3 |
| CMA | Chaperone-mediated autophagy |
| BEACH | Beige and Chediak-Higashi domain |
| LSD | Lysosomal storage disorder |
| MCOLN1 | Mucolipin-1 |
| TRPML1 | Transient receptor potential mucolipin 1 |
| IP3 | Inositol Trisphosphate |
| IP3R | Inositol triphosphate receptor |
| RYR | Ryanodine receptor |
| MVB | Multivesicular body |
| TEM | Transmission electron microscopy |
| CLEM | Correlated light and electron microscopy |
| SBEM | Serial block-face electron microscopy |
| FIB-SEM | Focused ion beam scanning electron microscopy |
| LAMP1 | Lysosomal-associated membrane protein 1 |
| BSA | Bovine serum albumin |
| ARGO | Analysis of red-green offset |
| GFP | Green fluorescent protein |
| GCaMP | Genetically encoded calcium indicator |
| LMP | Lysosomal membrane permeabilization |
| NMJ | Neuromuscular junction |
| CA | Cornu ammonis |
| PFC | Prefrontal cortex |
| LAMP2A | Lysosomal-associated membrane protein 2A |
| ROS | Reactive oxidative species |
| EC-SOD | Extracellular superoxide dismutase |
| AGE | Advanced glycation end-products |
| DIV | Days in vitro |
| LLOME | L-leucyl-L-leucine O-methyl ester |
| SNT | Synaptotagmin |
| SNG | Synaptogyrin |
| Aβ | Beta-amyloid |
| STING | Stimulator of interferon genes |
| NPC | Niemann–Pick type C |
| POMC | Proopiomelanocortin |
| ATG8 | Autophagy-related protein 8 |
| ER | Endoplasmic reticulum |
| ERK2 | Extracellular signal-regulated kinase 2 |
| PP2A | Protein phosphatase 2A |
| STAT3 | Signal transducer and activator of transcription 3 |
| MYC | Myelocytomatosis oncogene |
| CARM1 | Coactivator-associated arginine methyltransferase 1 |
| BNIP3 | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 |
| ESCRT | Endosomal sorting complexes required for transport |
| PITT | Phosphoinositide-initiated tethering and lipid transport |
| CASM | Conjugation of ATG8 to single membranes |
References
- Yang, C.; Wang, X. Lysosome Biogenesis: Regulation and Functions. J. Cell Biol. 2021, 220, e202102001. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as Dynamic Regulators of Cell and Organismal Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Lawrence, R.E.; Zoncu, R. The Lysosome as a Cellular Centre for Signalling, Metabolism and Quality Control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Lamming, D.W.; Bar-Peled, L. Lysosome: The Metabolic Signaling Hub. Traffic 2019, 20, 27–38. [Google Scholar] [CrossRef]
- Settembre, C.; Perera, R.M. Lysosomes as Coordinators of Cellular Catabolism, Metabolic Signalling and Organ Physiology. Nat. Rev. Mol. Cell Biol. 2024, 25, 223–245. [Google Scholar] [CrossRef]
- Barral, D.C.; Staiano, L.; Guimas Almeida, C.; Cutler, D.F.; Eden, E.R.; Futter, C.E.; Galione, A.; Marques, A.R.A.; Medina, D.L.; Napolitano, G.; et al. Current Methods to Analyze Lysosome Morphology, Positioning, Motility and Function. Traffic 2022, 23, 238–269. [Google Scholar] [CrossRef]
- Roney, J.C.; Cheng, X.T.; Sheng, Z.H. Neuronal Endolysosomal Transport and Lysosomal Functionality in Maintaining Axonostasis. J. Cell Biol. 2022, 221, e202111077. [Google Scholar] [CrossRef]
- Bond, C.; Hugelier, S.; Xing, J.; Sorokina, E.M.; Lakadamyali, M. Heterogeneity of Late Endosome/Lysosomes Shown by Multiplexed DNA-PAINT Imaging. J. Cell Biol. 2024, 224, e202403116. [Google Scholar] [CrossRef] [PubMed]
- Winckler, B.; Faundez, V.; Maday, S.; Cai, Q.; Guimas Almeida, C.; Zhang, H. The Endolysosomal System and Proteostasis: From Development to Degeneration. J. Neurosci. 2018, 38, 9364–9374. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.Y.; Yang, D.S.; Stavrides, P.; Goulbourne, C.N.; Zheng, P.; Mohan, P.S.; Cataldo, A.M.; Nixon, R.A. Post-Golgi Carriers, Not Lysosomes, Confer Lysosomal Properties to Pre-Degradative Organelles in Normal and Dystrophic Axons. Cell Rep. 2021, 35, 109034. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.M. Neuronal Lysosomes. Neurosci. Lett. 2018, 697, 1–9. [Google Scholar] [CrossRef]
- Udayar, V.; Chen, Y.; Sidransky, E.; Jagasia, R. Lysosomal Dysfunction in Neurodegeneration: Emerging Concepts and Methods. Trends Neurosci. 2022, 45, 184–199. [Google Scholar] [CrossRef]
- Abe, T.; Kuwahara, T. Targeting of Lysosomal Pathway Genes for Parkinson’s Disease Modification: Insights from Cellular and Animal Models. Front. Neurol. 2021, 12, 681369. [Google Scholar] [CrossRef]
- Root, J.; Merino, P.; Nuckols, A.; Johnson, M.; Kukar, T. Lysosome Dysfunction as a Cause of Neurodegenerative Diseases: Lessons from Frontotemporal Dementia and Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2021, 154, 105360. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Rubinsztein, D.C. Mechanisms of Autophagy–Lysosome Dysfunction in Neurodegenerative Diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Takeda, M.; Suzuki, H.; Morita, H.; Tada, K.; Hariguchi, S.; Nishimura, T. Lysosome Instability in Aged Rat Brain. Neurosci. Lett. 1989, 97, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. The Aging Lysosome: An Essential Catalyst for Late-Onset Neurodegenerative Diseases. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140443. [Google Scholar] [CrossRef]
- Gómez-Sintes, R.; Ledesma, M.D.; Boya, P. Lysosomal Cell Death Mechanisms in Aging. Ageing Res. Rev. 2016, 32, 150–168. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. Age-Related Decline in Chaperone-Mediated Autophagy. J. Biol. Chem. 2000, 275, 31505–31513. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-Associated β-Galactosidase Reflects an Increase in Lysosomal Mass during Replicative Ageing of Human Endothelial Cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Zhao, D.; Li, X.; Yang, C.; Wang, X. Lysosome Activity Is Modulated by Multiple Longevity Pathways and Is Important for Lifespan Extension in C. elegans. eLife 2020, 9, e55745. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.J.; La Spada, A.R. TFEB Dysregulation as a Driver of Autophagy Dysfunction in Neurodegenerative Disease: Molecular Mechanisms, Cellular Processes, and Emerging Therapeutic Opportunities. Neurobiol. Dis. 2018, 122, 83–93. [Google Scholar] [CrossRef]
- Amin, S.; Taverna, E. Vesicular Trafficking and Cell-Cell Communication in Neurodevelopment and Neurodegeneration. Front. Cell Dev. Biol. 2025, 13, 1600034. [Google Scholar] [CrossRef]
- Zhang, R.; Vooijs, M.A.; Keulers, T.G.H. Key Mechanisms in Lysosome Stability, Degradation and Repair. Mol. Cell. Biol. 2025, 45, 212–224. [Google Scholar] [CrossRef]
- Kaur, N.; Carlsson, S.R.; Lystad, A.H. Lysosome-Associated CASM: From Upstream Triggers to Downstream Effector Mechanisms. Front. Cell Dev. Biol. 2025, 13, 1559125. [Google Scholar] [CrossRef]
- Mony, V.K.; Benjamin, S.; O’Rourke, E.J. A Lysosome-Centered View of Nutrient Homeostasis. Autophagy 2016, 12, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The Emerging Mechanisms and Functions of Microautophagy. Nat. Rev. Mol. Cell Biol. 2022, 24, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.D.; Donaldson, J.G. Pathways and Mechanisms of Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef]
- Shi, A.; Chen, C.C.H.; Banerjee, R.; Glodowski, D.; Audhya, A.; Rongo, C.; Grant, B.D. EHBP-1 Functions with RAB-10 during Endocytic Recycling in Caenorhabditis elegans. Mol. Biol. Cell 2010, 21, 2930–2943. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K.; Liou, W.; Pant, S.; Harada, A.; Grant, B.D. Regulation of Endocytic Recycling by C. elegans Rab35 and Its Regulator RME-4, a Coated-Pit Protein. EMBO J. 2008, 27, 1183–1196. [Google Scholar] [CrossRef]
- Daly, J.L.; Danson, C.M.; Lewis, P.A.; Zhao, L.; Riccardo, S.; Di Filippo, L.; Cacchiarelli, D.; Lee, D.; Cross, S.J.; Heesom, K.J.; et al. Multi-Omic Approach Characterizes the Neuroprotective Role of Retromer in Regulating Lysosomal Health. Nat. Commun. 2023, 14, 3086. [Google Scholar] [CrossRef]
- Cullen, P.J.; Steinberg, F. To Degrade or Not to Degrade: Mechanisms and Significance of Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2018, 19, 679–696. [Google Scholar] [CrossRef]
- Carosi, J.M.; Hein, L.K.; Sandow, J.J.; Dang, L.V.P.; Hattersley, K.; Denton, D.; Kumar, S.; Sargeant, T.J. Autophagy Captures the Retromer-TBC1D5 Complex to Inhibit Receptor Recycling. Autophagy 2023, 20, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Loncle, N.; Agromayor, M.; Martin-Serrano, J.; Williams, D.W. An ESCRT Module Is Required for Neuron Pruning. Sci. Rep. 2015, 5, 8461. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Chen, X.; Perry, S.; Fan, Z.; Wang, B.; Loxterkamp, E.; Wang, S.; Hu, J.; Dickman, D.; Han, C. Tissue-Specific Knockout in the Drosophila Neuromuscular System Reveals ESCRT’s Role in Formation of Synapse-Derived Extracellular Vesicles. PLoS Genet. 2024, 20, e1011438. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Harris, K.P.; Blanchette, C.R.; Koles, K.; Del Signore, S.J.; Pescosolido, M.F.; Ermanoska, B.; Rozencwaig, M.; Soslowsky, R.C.; Parisi, M.J.; et al. ESCRT Disruption Provides Evidence against Trans-Synaptic Signaling via Extracellular Vesicles. J. Cell Biol. 2024, 223, e202405025. [Google Scholar] [CrossRef]
- Katzmann, D.J.; Odorizzi, G.; Emr, S.D. Receptor Downregulation and Multivesicular-Body Sorting. Nat. Rev. Mol. Cell Biol. 2002, 3, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab Conversion as a Mechanism of Progression from Early to Late Endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Rab GTPases: Master Regulators That Establish the Secretory and Endocytic Pathways. Mol. Biol. Cell 2017, 28, 712–715. [Google Scholar] [CrossRef]
- Bright, N.A.; Davis, L.J.; Luzio, J.P. Endolysosomes Are the Principal Intracellular Sites of Acid Hydrolase Activity. Curr. Biol. 2016, 26, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Uytterhoeven, V.; Kuenen, S.; Wang, Y.C.; Slabbaert, J.R.; Swerts, J.; Kasprowicz, J.; Aerts, S.; Verstreken, P. Reduced Synaptic Vesicle Protein Degradation at Lysosomes Curbs TBC1D24/Sky-Induced Neurodegeneration. J. Cell Biol. 2014, 207, 453–462. [Google Scholar] [CrossRef]
- Uytterhoeven, V.; Kuenen, S.; Kasprowicz, J.; Miskiewicz, K.; Verstreken, P. Loss of Skywalker Reveals Synaptic Endosomes as Sorting Stations for Synaptic Vesicle Proteins. Cell 2011, 145, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.; Zhu, M.; Beskow, A.; Vollmer, C.; Waites, C.L. Activity-Dependent Degradation of Synaptic Vesicle Proteins Requires Rab35 and the ESCRT Pathway. J. Neurosci. 2016, 36, 8668–8686. [Google Scholar] [CrossRef]
- Yamamoto, A.; Yue, Z. Autophagy and Its Normal and Pathogenic States in the Brain. Annu. Rev. Neurosci. 2014, 37, 55–78. [Google Scholar] [CrossRef]
- Maday, S.; Wallace, K.E.; Holzbaur, E.L.F. Autophagosomes Initiate Distally and Mature during Transport toward the Cell Soma in Primary Neurons. J. Cell Biol. 2012, 196, 407–417. [Google Scholar] [CrossRef]
- Cheng, X.T.; Zhou, B.; Lin, M.Y.; Cai, Q.; Sheng, Z.H. Axonal Autophagosomes Recruit Dynein for Retrograde Transport through Fusion with Late Endosomes. J. Cell Biol. 2015, 209, 377–386. [Google Scholar] [CrossRef]
- Kargbo-Hill, S.E.; Colón-Ramos, D.A. The Journey of the Synaptic Autophagosome: A Cell Biological Perspective. Neuron 2020, 105, 961–973. [Google Scholar] [CrossRef]
- Maday, S.; Holzbaur, E.L.F. Compartment-Specific Regulation of Autophagy in Primary Neurons. J. Neurosci. 2016, 36, 5933–5945. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of Basal Autophagy in Neural Cells Causes Neurodegenerative Disease in Mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the Parkin Gene Cause Autosomal Recessive Juvenile Parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Jia, R.; Guardia, C.M.; Pu, J.; Chen, Y.; Bonifacino, J.S. BORC Coordinates Encounter and Fusion of Lysosomes with Autophagosomes. Autophagy 2017, 13, 1648–1663. [Google Scholar] [CrossRef]
- Serra-Vinardell, J.; Sandler, M.B.; De Pace, R.; Manzella-Lapeira, J.; Cougnoux, A.; Keyvanfar, K.; Introne, W.J.; Brzostowski, J.A.; Ward, M.E.; Gahl, W.A.; et al. LYST Deficiency Impairs Autophagic Lysosome Reformation in Neurons and Alters Lysosome Number and Size. Cell. Mol. Life Sci. 2023, 80, 53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Nishimura, T.; Sakamaki, Y.; Itakura, E.; Hatta, T.; Natsume, T.; Mizushima, N. The HOPS Complex Mediates Autophagosome–Lysosome Fusion through Interaction with Syntaxin 17. Mol. Biol. Cell 2014, 25, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Jimenez Sanchez, M.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Gamble, K.L.; Schultheiss, C.E.; Riddle, D.M.; West, A.B.; Lee, V.M.Y. Formation of α-Synuclein Lewy Neurite-like Aggregates in Axons Impedes the Transport of Distinct Endosomes. Mol. Biol. Cell 2014, 25, 4010–4023. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.M.; Zheng, B.; Lu, T.; Yan, Z.; Py, B.F.; Ng, A.; Xavier, R.J.; Li, C.; Yankner, B.A.; Scherzer, C.R.; et al. Genome-Wide Analysis Reveals Mechanisms Modulating Autophagy in Normal Brain Aging and in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 14164–14169. [Google Scholar] [CrossRef]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in Mice Activates Autophagy and Extends Lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Cassidy, L.D.; Young, A.R.J.; Young, C.N.J.; Soilleux, E.J.; Fielder, E.; Weigand, B.M.; Lagnado, A.; Brais, R.; Ktistakis, N.T.; Wiggins, K.A.; et al. Temporal Inhibition of Autophagy Reveals Segmental Reversal of Ageing with Increased Cancer Risk. Nat. Commun. 2020, 11, 307. [Google Scholar] [CrossRef]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The Different Autophagy Degradation Pathways and Neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Saffi, G.T.; Botelho, R.J. Lysosome Fission: Planning for an Exit. Trends Cell Biol. 2019, 29, 635–646. [Google Scholar] [CrossRef]
- Hirst, J.; Itzhak, D.N.; Antrobus, R.; Borner, G.H.H.; Robinson, M.S. Role of the AP-5 Adaptor Protein Complex in Late Endosome-to-Golgi Retrieval. PLoS Biol. 2018, 16, e2004411. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, P.; Gautier-Stein, A.; Riedel, D.; Schomburg, C.; Cerdà, J.; Vollack, N.; Dosch, R. Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis. PLoS Genet. 2014, 10, e1004449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirst, J.; Borner, G.H.H.; Edgar, J.; Hein, M.Y.; Mann, M.; Buchholz, F.; Antrobus, R.; Robinson, M.S. Interaction between AP-5 and the Hereditary Spastic Paraplegia Proteins SPG11 and SPG15. Mol. Biol. Cell 2013, 24, 2558. [Google Scholar] [CrossRef]
- Holland, P.; Torgersen, M.L.; Sandvig, K.; Simonsen, A. LYST Affects Lysosome Size and Quantity, but Not Trafficking or Degradation through Autophagy or Endocytosis. Traffic 2014, 15, 1390–1405. [Google Scholar] [CrossRef]
- Rahman, M.; Haberman, A.; Tracy, C.; Ray, S.; Krämer, H. Drosophila Mauve Mutants Reveal a Role of LYST Homologs Late in the Maturation of Phagosomes and Autophagosomes. Traffic 2012, 13, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rydzewski, N.; Hider, A.; Zhang, X.; Yang, J.; Wang, W.; Gao, Q.; Cheng, X.; Xu, H. A Molecular Mechanism to Regulate Lysosome Motility for Lysosome Positioning and Tubulation. Nat. Cell Biol. 2016, 18, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ratto, E.; Chowdhury, S.R.; Siefert, N.S.; Schneider, M.; Wittmann, M.; Helm, D.; Palm, W. Direct Control of Lysosomal Catabolic Activity by mTORC1 through Regulation of V-ATPase Assembly. Nat. Commun. 2022, 13, 4848. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 Senses Lysosomal Amino Acids through an Inside-out Mechanism That Requires the Vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as Well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Jiang, B.; Li, M.; Zhu, M.; Peng, Y.; Zhang, Y.L.; Wu, Y.Q.; Li, T.Y.; Liang, Y.; Lu, Z.; et al. The Lysosomal V-ATPase-Ragulator Complex Is a Common Activator for AMPK and mTORC1, Acting as a Switch between Catabolism and Anabolism. Cell Metab. 2014, 20, 526–540. [Google Scholar] [CrossRef]
- Christensen, K.A.; Myers, J.T.; Swanson, J.A. pH-Dependent Regulation of Lysosomal Calcium in Macrophages. J. Cell Sci. 2002, 115, 599–607. [Google Scholar] [CrossRef]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick Disease Type C1 Is a Sphingosine Storage Disease That Causes Deregulation of Lysosomal Calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef]
- Morgan, A.J.; Davis, L.C.; Galione, A. Imaging Approaches to Measuring Lysosomal Calcium. Methods Cell Biol. 2015, 126, 159–195. [Google Scholar] [CrossRef]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Dong, X.P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The Type IV Mucolipidosis-Associated Protein TRPML1 Is an Endolysosomal Iron Release Channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Torres-Vidal, P.; Delrio-Lorenzo, A.; Rodriguez, C.; Aulestia, F.J.; Rojo-Ruiz, J.; Callejo, B.; McVeigh, B.M.; Keller, M.; Grimm, C.; et al. Direct Measurements of Luminal Ca2+ with Endo-Lysosomal GFP-Aequorin Reveal Functional IP3 Receptors. J. Cell Biol. 2025, 224, e202410094. [Google Scholar] [CrossRef]
- Morgan, A.J.; Platt, F.M.; Lloyd-Evans, E.; Galione, A. Molecular Mechanisms of Endolysosomal Ca2+ Signalling in Health and Disease. Biochem. J. 2011, 439, 349–374. [Google Scholar] [CrossRef]
- Yuan, Y.; Arige, V.; Saito, R.; Mu, Q.; Brailoiu, G.C.; Pereira, G.J.S.; Bolsover, S.R.; Keller, M.; Bracher, F.; Grimm, C.; et al. Two-Pore Channel-2 and Inositol Trisphosphate Receptors Coordinate Ca2+ Signals between Lysosomes and the Endoplasmic Reticulum. Cell Rep. 2023, 43, 113628. [Google Scholar] [CrossRef]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal Calcium Signalling Regulates Autophagy through Calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Li, J.; Yan, L.; Li, W.; He, X.; Ma, H. Chronic Neuronal Inactivity Utilizes the mTOR-TFEB Pathway to Drive Transcription-Dependent Autophagy for Homeostatic Up-Scaling. J. Neurosci. 2023, 43, 2631–2652. [Google Scholar] [CrossRef] [PubMed]
- Akwa, Y.; Di Malta, C.; Zallo, F.; Gondard, E.; Lunati, A.; Diaz-de-Grenu, L.Z.; Zampelli, A.; Boiret, A.; Santamaria, S.; Martinez-Preciado, M.; et al. Stimulation of Synaptic Activity Promotes TFEB-Mediated Clearance of Pathological MAPT/Tau in Cellular and Mouse Models of Tauopathies. Autophagy 2022, 19, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria-Lysosome Contacts Regulate Mitochondrial Fission via Rab7 GTP Hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef]
- Kim, S.; Coukos, R.; Gao, F.; Krainc, D. Dysregulation of Organelle Membrane Contact Sites in Neurological Diseases. Neuron 2022, 110, 2386–2408. [Google Scholar] [CrossRef]
- Rizzollo, F.; More, S.; Vangheluwe, P.; Agostinis, P. The Lysosome as a Master Regulator of Iron Metabolism. Trends Biochem. Sci. 2021, 46, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-Lysosome Contacts Regulate Mitochondrial Ca2+ Dynamics via Lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, W.; Peng, T.; Wang, X.; Lian, X.; He, J.; Wang, C.; Xie, N. TBC1D15-Regulated Mitochondria–Lysosome Membrane Contact Exerts Neuroprotective Effects by Alleviating Mitochondrial Calcium Overload in Seizure. Sci. Rep. 2024, 14, 23782. [Google Scholar] [CrossRef]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. The Role of Lysosomes in Iron Metabolism and Recycling. Int. J. Biochem. Cell Biol. 2011, 43, 1686–1697. [Google Scholar] [CrossRef]
- Tian, R.; Abarientos, A.; Hong, J.; Hashemi, S.H.; Yan, R.; Dräger, N.; Leng, K.; Nalls, M.A.; Singleton, A.B.; Xu, K.; et al. Genome-Wide CRISPRi/a Screens in Human Neurons Link Lysosomal Failure to Ferroptosis. Nat. Neurosci. 2021, 24, 1020–1034. [Google Scholar] [CrossRef]
- Cañeque, T.; Baron, L.; Müller, S.; Carmona, A.; Colombeau, L.; Versini, A.; Solier, S.; Gaillet, C.; Sindikubwabo, F.; Sampaio, J.L.; et al. Activation of Lysosomal Iron Triggers Ferroptosis in Cancer. Nature 2025, 642, 492–500. [Google Scholar] [CrossRef]
- Mason, A.J.; Deppmann, C.; Winckler, B. Emerging Roles of Neuronal Extracellular Vesicles at the Synapse. Neuroscientist 2024, 30, 199–213. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Neefjes, J. Moving and Positioning the Endolysosomal System. Curr. Opin. Cell Biol. 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Farfel-Becker, T.; Roney, J.C.; Cheng, X.T.; Li, S.; Cuddy, S.R.; Sheng, Z.H. Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity. Cell Rep. 2019, 28, 51–64.e4. [Google Scholar] [CrossRef]
- Goo, M.S.; Sancho, L.; Slepak, N.; Boassa, D.; Deerinck, T.J.; Ellisman, M.H.; Bloodgood, B.L.; Patrick, G.N. Activity-Dependent Trafficking of Lysosomes in Dendrites and Dendritic Spines. J. Cell Biol. 2017, 216, 2499–2513. [Google Scholar] [CrossRef]
- Liao, Y.C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 2019, 179, 147–164.e20. [Google Scholar] [CrossRef]
- Lu, M.; Van Tartwijk, F.W.; Lin, J.Q.; Nijenhuis, W.; Parutto, P.; Fantham, M.; Christensen, C.N.; Avezov, E.; Holt, C.E.; Tunnacliffe, A.; et al. The Structure and Global Distribution of the Endoplasmic Reticulum Network Are Actively Regulated by Lysosomes. Sci. Adv. 2020, 6, eabc7209. [Google Scholar] [CrossRef]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal Membrane Permeabilization and Cell Death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Sanyal, A.; Scanavachi, G.; Somerville, E.; Saminathan, A.; Nair, A.; Bango Da Cunha Correia, R.F.; Aylan, B.; Sitarska, E.; Oikonomou, A.; Hatzakis, N.S.; et al. Neuronal Constitutive Endolysosomal Perforations Enable α-Synuclein Aggregation by Internalized PFFs. J. Cell Biol. 2024, 224, e202401136. [Google Scholar] [CrossRef]
- Dehay, B.; Bové, J.; Rodríguez-Muela, N.; Perier, C.; Recasens, A.; Boya, P.; Vila, M. Pathogenic Lysosomal Depletion in Parkinson’s Disease. J. Neurosci. 2010, 30, 12535–12544. [Google Scholar] [CrossRef]
- Vila, M.; Bové, J.; Dehay, B.; Rodríguez-Muela, N.; Boya, P. Lysosomal Membrane Permeabilization in Parkinson Disease. Autophagy 2011, 7, 98–100. [Google Scholar] [CrossRef][Green Version]
- Gabandé-Rodríguez, E.; Boya, P.; Labrador, V.; Dotti, C.G.; Ledesma, M.D. High Sphingomyelin Levels Induce Lysosomal Damage and Autophagy Dysfunction in Niemann Pick Disease Type A. Cell Death Differ. 2014, 21, 864–875. [Google Scholar] [CrossRef]
- Flavin, W.P.; Bousset, L.; Green, Z.C.; Chu, Y.; Skarpathiotis, S.; Chaney, M.J.; Kordower, J.H.; Melki, R.; Campbell, E.M. Endocytic Vesicle Rupture Is a Conserved Mechanism of Cellular Invasion by Amyloid Proteins. Acta Neuropathol. 2017, 134, 629–653. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kirchner, P.; Bug, M.; Grum, D.; Koerver, L.; Schulze, N.; Poehler, R.; Dressler, A.; Fengler, S.; Arhzaouy, K.; et al. VCP/P97 Cooperates with YOD1, UBXD1 and PLAA to Drive Clearance of Ruptured Lysosomes by Autophagy. EMBO J. 2016, 36, 135–150. [Google Scholar] [CrossRef]
- Duran, J.; Salinas, J.E.; Wheaton, R.p.; Poolsup, S.; Allers, L.; Rosas-Lemus, M.; Chen, L.; Cheng, Q.; Pu, J.; Salemi, M.; et al. Calcium Signaling from Damaged Lysosomes Induces Cytoprotective Stress Granules. EMBO J. 2024, 43, 6410–6443. [Google Scholar] [CrossRef]
- Thakore, P.; Pritchard, H.A.T.; Griffin, C.S.; Yamasaki, E.; Drumm, B.T.; Lane, C.; Sanders, K.M.; Feng Earley, Y.; Earley, S. TRPML1 Channels Initiate Ca2+ Sparks in Vascular Smooth Muscle Cells. Sci. Signal. 2020, 13, eaba1015. [Google Scholar] [CrossRef]
- Eskelinen, E.L. To Be or Not to Be? Examples of Incorrect Identification of Autophagic Compartments in Conventional Transmission Electron Microscopy of Mammalian Cells. Autophagy 2008, 4, 257–260. [Google Scholar] [CrossRef]
- van der Beek, J.; de Heus, C.; Liv, N.; Klumperman, J. Quantitative Correlative Microscopy Reveals the Ultrastructural Distribution of Endogenous Endosomal Proteins. J. Cell Biol. 2022, 221, e202106044. [Google Scholar] [CrossRef]
- Fermie, J.; Liv, N.; ten Brink, C.; van Donselaar, E.G.; Müller, W.H.; Schieber, N.L.; Schwab, Y.; Gerritsen, H.C.; Klumperman, J. Single Organelle Dynamics Linked to 3D Structure by Correlative Live-Cell Imaging and 3D Electron Microscopy. Traffic 2018, 19, 354–369. [Google Scholar] [CrossRef]
- Giepmans, B.N.G.; Deerinck, T.J.; Smarr, B.L.; Jones, Y.Z.; Ellisman, M.H. Correlated Light and Electron Microscopic Imaging of Multiple Endogenous Proteins Using Quantum Dots. Nat. Methods 2005, 2, 743–749. [Google Scholar] [CrossRef]
- Tao-Cheng, J.H.; Crocker, V.; Moreira, S.L.; Azzam, R. Optimization of Protocols for Pre-Embedding Immunogold Electron Microscopy of Neurons in Cell Cultures and Brains. Mol. Brain 2021, 14, 86. [Google Scholar] [CrossRef]
- MacO, B.; Cantoni, M.; Holtmaat, A.; Kreshuk, A.; Hamprecht, F.A.; Knott, G.W. Semiautomated Correlative 3D Electron Microscopy of in Vivo-Imaged Axons and Dendrites. Nat. Protoc. 2014, 9, 1354–1366. [Google Scholar] [CrossRef]
- Bonet-Ponce, L.; Beilina, A.; Williamson, C.D.; Lindberg, E.; Kluss, J.H.; Saez-Atienzar, S.; Landeck, N.; Kumaran, R.; Mamais, A.; Bleck, C.K.E.; et al. LRRK2 Mediates Tubulation and Vesicle Sorting from Lysosomes. Sci. Adv. 2020, 6, eabb2454. [Google Scholar] [CrossRef]
- Biazik, J.; Ylä-Anttila, P.; Vihinen, H.; Jokitalo, E.; Eskelinen, E.L. Ultrastructural Relationship of the Phagophore with Surrounding Organelles. Autophagy 2015, 11, 439–451. [Google Scholar] [CrossRef]
- Bissig, C.; Hurbain, I.; Raposo, G.; van Niel, G. PIKfyve Activity Regulates Reformation of Terminal Storage Lysosomes from Endolysosomes. Traffic 2017, 18, 747–757. [Google Scholar] [CrossRef]
- Yap, C.C.; Digilio, L.; McMahon, L.P.; Garcia, A.D.R.; Winckler, B. Degradation of Dendritic Cargos Requires Rab7-Dependent Transport to Somatic Lysosomes. J. Cell Biol. 2018, 217, 3141–3159. [Google Scholar] [CrossRef]
- Cheng, X.T.; Xie, Y.X.; Zhou, B.; Huang, N.; Farfel-Becker, T.; Sheng, Z.H. Characterization of LAMP1-Labeled Nondegradative Lysosomal and Endocytic Compartments in Neurons. J. Cell Biol. 2018, 217, 3127–3139. [Google Scholar] [CrossRef]
- Kulkarni, V.V.; Maday, S. Neuronal Endosomes to Lysosomes: A Journey to the Soma. J. Cell Biol. 2018, 217, 2977–2979. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, C.E. Expansion of Lysosomal Capacity in Early Adult Neurons Driven by TFEB/HLH-30 Protects Dendrite Maintenance during Aging in Caenorhabditis elegans. PLoS Biol. 2025, 23, e3002957. [Google Scholar] [CrossRef]
- Snead, A.M.; Gowrishankar, S. Loss of MAPK8IP3 Affects Endocytosis in Neurons. Front. Cell. Neurosci. 2022, 16, 828071. [Google Scholar] [CrossRef]
- Shiliaev, N.; Baumberger, S.; Richardson, C.E. Analysis In Vivo Using a New Method, ARGO (Analysis of Red Green Offset), Reveals Complexity and Cell-Type Specificity in Presynaptic Turnover of Synaptic Vesicle Protein Synaptogyrin/SNG-1. bioRxiv 2025. [Google Scholar] [CrossRef]
- Bulovaite, E.; Qiu, Z.; Kratschke, M.; Zgraj, A.; Fricker, D.G.; Tuck, E.J.; Gokhale, R.; Koniaris, B.; Jami, S.A.; Merino-Serrais, P.; et al. A Brain Atlas of Synapse Protein Lifetime across the Mouse Lifespan. Neuron 2022, 110, 4057–4073.e8. [Google Scholar] [CrossRef]
- Falace, A.; Volpedo, G.; Scala, M.; Zara, F.; Striano, P.; Fassio, A. V-ATPase Dysfunction in the Brain: Genetic Insights and Therapeutic Opportunities. Cells 2024, 13, 1441. [Google Scholar] [CrossRef]
- Overly, C.C.; Lee, K.D.; Berthiaume, E.; Hollenbeck, P.J. Quantitative Measurement of Intraorganelle pH in the Endosomal-Lysosomal Pathway in Neurons by Using Ratiometric Imaging with Pyranine. Proc. Natl. Acad. Sci. USA 1995, 92, 3156–3160. [Google Scholar] [CrossRef]
- Johnson, D.E.; Ostrowski, P.; Jaumouillé, V.; Grinstein, S. The Position of Lysosomes within the Cell Determines Their Luminal pH. J. Cell Biol. 2016, 212, 677–692. [Google Scholar] [CrossRef]
- Van Noorden, C.J.F. Imaging Enzymes at Work: Metabolic Mapping by Enzyme Histochemistry. J. Histochem. Cytochem. 2010, 58, 481–497. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, C. Exploring Lysosomal Biology: Current Approaches and Methods. Biophys. Rep. 2024, 10, 111–120. [Google Scholar] [CrossRef]
- Cuddy, L.K.; Mazzulli, J.R. Analysis of Lysosomal Hydrolase Trafficking and Activity in Human iPSC-Derived Neuronal Models. STAR Protoc. 2021, 2, 100340. [Google Scholar] [CrossRef]
- Witte, M.D.; Kallemeijn, W.W.; Aten, J.; Li, K.Y.; Strijland, A.; Donker-Koopman, W.E.; Van Den Nieuwendijk, A.M.C.H.; Bleijlevens, B.; Kramer, G.; Florea, B.I.; et al. Ultrasensitive In Situ Visualization of Active Glucocerebrosidase Molecules. Nat. Chem. Biol. 2010, 6, 907–913. [Google Scholar] [CrossRef]
- Yang, H.; Tan, J.X. Lysosomal Quality Control: Molecular Mechanisms and Therapeutic Implications. Trends Cell Biol. 2023, 33, 749–764. [Google Scholar] [CrossRef]
- Alonso, M.T.; Torres-Vidal, P.; Calvo, B.; Rodriguez, C.; Delrio-Lorenzo, A.; Rojo-Ruiz, J.; Garcia-Sancho, J.; Patel, S. Use of Aequorin-Based Indicators for Monitoring Ca2+ in Acidic Organelles. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119481. [Google Scholar] [CrossRef]
- Nakai, J.; Ohkura, M.; Imoto, K. A High Signal-to-Noise Ca2+ Probe Composed of a Single Green Fluorescent Protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A Novel Ca2+-Mediated Cross-Talk between Endoplasmic Reticulum and Acidic Organelles: Implications for NAADP-Dependent Ca2+ Signalling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef]
- Chou, C.C.; Vest, R.; Prado, M.A.; Wilson-Grady, J.; Paulo, J.A.; Shibuya, Y.; Moran-Losada, P.; Lee, T.T.; Luo, J.; Gygi, S.P.; et al. Proteostasis and Lysosomal Repair Deficits in Transdifferentiated Neurons of Alzheimer’s Disease. Nat. Cell Biol. 2025, 27, 619–632. [Google Scholar] [CrossRef]
- Du Rietz, H.; Hedlund, H.; Wilhelmson, S.; Nordenfelt, P.; Wittrup, A. Imaging Small Molecule-Induced Endosomal Escape of SiRNA. Nat. Commun. 2020, 11, 1809. [Google Scholar] [CrossRef]
- Aits, S.; Kricker, J.; Liu, B.; Ellegaard, A.M.; Hämälistö, S.; Tvingsholm, S.; Corcelle-Termeau, E.; Høgh, S.; Farkas, T.; Jonassen, A.H.; et al. Sensitive Detection of Lysosomal Membrane Permeabilization by Lysosomal Galectin Puncta Assay. Autophagy 2015, 11, 1408–1424. [Google Scholar] [CrossRef]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2019, 52, 69–87.e8. [Google Scholar] [CrossRef]
- Scott, O.; Saran, E.; Freeman, S.A. The Spectrum of Lysosomal Stress and Damage Responses: From Mechanosensing to Inflammation. EMBO Rep. 2025, 26, 1425–1439. [Google Scholar] [CrossRef]
- Freeman, D.; Cedillos, R.; Choyke, S.; Lukic, Z.; McGuire, K.; Marvin, S.; Burrage, A.M.; Sudholt, S.; Rana, A.; O’Connor, C.; et al. Alpha-Synuclein Induces Lysosomal Rupture and Cathepsin Dependent Reactive Oxygen Species Following Endocytosis. PLoS ONE 2013, 8, e62143. [Google Scholar] [CrossRef]
- Rose, K.; Jepson, T.; Shukla, S.; Maya-Romero, A.; Kampmann, M.; Xu, K.; Hurley, J.H. Tau Fibrils Induce Nanoscale Membrane Damage and Nucleate Cytosolic Tau at Lysosomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2315690121. [Google Scholar] [CrossRef]
- Hämälistö, S.; Stahl, J.L.; Favaro, E.; Yang, Q.; Liu, B.; Christoffersen, L.; Loos, B.; Guasch Boldú, C.; Joyce, J.A.; Reinheckel, T.; et al. Spatially and Temporally Defined Lysosomal Leakage Facilitates Mitotic Chromosome Segregation. Nat. Commun. 2020, 11, 229. [Google Scholar] [CrossRef]
- Curnock, R.; Yalci, K.; Palmfeldt, J.; Jäättelä, M.; Liu, B.; Carroll, B. TFEB-dependent Lysosome Biogenesis Is Required for Senescence. EMBO J. 2023, 42, e111241. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Young, A.R.J.; Arakawa, S.; Samarajiwa, S.A.; Nakashima, T.; Yoshida, S.; Hong, S.; Berry, L.S.; Reichelt, S.; Ferreira, M.; et al. Spatial Coupling of mTOR and Autophagy Augments Secretory Phenotypes. Science 2011, 332, 966–970. [Google Scholar] [CrossRef]
- Burrinha, T.; Cunha, C.; Hall, M.J.; Lopes-da-Silva, M.; Seabra, M.C.; Guimas Almeida, C. Deacidification of Endolysosomes by Neuronal Aging Drives Synapse Loss. Traffic 2023, 24, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. Lipofuscin: Mechanisms of Formation and Increase with Age. APMIS 1998, 106, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Terman, A. Lipofuscin: Mechanisms of Age-Related Accumulation and Influence on Cell Function. Free Radic. Biol. Med. 2002, 33, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Baldensperger, T.; Jung, T.; Heinze, T.; Schwerdtle, T.; Höhn, A.; Grune, T. The Age Pigment Lipofuscin Causes Oxidative Stress, Lysosomal Dysfunction, and Pyroptotic Cell Death. Free Radic. Biol. Med. 2024, 225, 871–880. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Koch, S.C.; Nelson, A.; Hartenstein, V. Structural Aspects of the Aging Invertebrate Brain. Cell Tissue Res. 2021, 383, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Hivare, P.; Mujmer, K.; Swarup, G.; Gupta, S.; Bhatia, D. Endocytic Pathways of Pathogenic Protein Aggregates in Neurodegenerative Diseases. Traffic 2023, 24, 434–452. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M.Y. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef]
- Desplats, P.; Lee, H.J.; Bae, E.J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.J. Inclusion Formation and Neuronal Cell Death through Neuron-to-Neuron Transmission of α-Synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef]
- Kakuda, K.; Ikenaka, K.; Kuma, A.; Doi, J.; Aguirre, C.; Wang, N.; Ajiki, T.; Choong, C.J.; Kimura, Y.; Badawy, S.M.M.; et al. Lysophagy Protects against Propagation of α-Synuclein Aggregation through Ruptured Lysosomal Vesicles. Proc. Natl. Acad. Sci. USA 2024, 121, e2312306120. [Google Scholar] [CrossRef]
- Kluever, V.; Russo, B.; Mandad, S.; Kumar, N.H.; Alevra, M.; Ori, A.; Rizzoli, S.O.; Urlaub, H.; Schneider, A.; Fornasiero, E.F. Protein Lifetimes in Aged Brains Reveal a Proteostatic Adaptation Linking Physiological Aging to Neurodegeneration. Sci. Adv. 2022, 8, 4437. [Google Scholar] [CrossRef]
- Wagner, N.; Laugks, U.; Heckmann, M.; Asan, E.; Neuser, K. Aging Drosophila Melanogaster Display Altered Pre- and Postsynaptic Ultrastructure at Adult Neuromuscular Junctions. J. Comp. Neurol. 2015, 523, 2457–2475. [Google Scholar] [CrossRef]
- Boaro, S.N.; Soares, J.C.; König, B. Comparative Structural Analysis of Neuromuscular Junctions in Mice at Different Ages. Ann. Anat. Anat. Anz. 1998, 180, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Burrinha, T.; Martinsson, I.; Gomes, R.; Terrasso, A.P.; Gouras, G.K.; Almeida, C.G. Upregulation of APP Endocytosis by Neuronal Aging Drives Amyloid-Dependent Synapse Loss. J. Cell Sci. 2021, 134, jcs255752. [Google Scholar] [CrossRef] [PubMed]
- Alsaqati, M.; Thomas, R.S.; Kidd, E.J. Proteins Involved in Endocytosis Are Upregulated by Ageing in the Normal Human Brain: Implications for the Development of Alzheimer’s Disease. J. Gerontol. Ser. A 2018, 73, 289–298. [Google Scholar] [CrossRef]
- Richardson, C.E.; Yee, C.; Shen, K. A Hormone Receptor Pathway Cell-Autonomously Delays Neuron Morphological Aging by Suppressing Endocytosis. PLoS Biol. 2019, 17, e3000452. [Google Scholar] [CrossRef]
- Nakanishi, H.; Amano, T.; Sastradipura, D.F.; Yoshimine, Y.; Tsukuba, T.; Tanabe, K.; Hirotsu, I.; Ohono, T.; Yamamoto, K. Increased Expression of Cathepsins E and D in Neurons of the Aged Rat Brain and Their Colocalization with Lipofuscin and Carboxy-Terminal Fragments of Alzheimer Amyloid Precursor Protein. J. Neurochem. 1997, 68, 739–749. [Google Scholar] [CrossRef]
- Jung, H.; Lee, E.Y.; Lee, S.I. Age-Related Changes in Ultrastructural Features of Cathepsin B- and D-Containing Neurons in Rat Cerebral Cortex. Brain Res. 1999, 844, 43–54. [Google Scholar] [CrossRef]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hallett, P.; Isacson, O. Progressive Decline of Glucocerebrosidase in Aging and Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2015, 2, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Burke, D.; Heales, S.J.R.; Cooper, J.M.; Hardy, J.; Wood, N.W.; Schapira, A.H.V. Glucocerebrosidase Deficiency in Substantia Nigra of Parkinson Disease Brains. Ann. Neurol. 2012, 72, 455–463. [Google Scholar] [CrossRef]
- Hallett, P.J.; Huebecker, M.; Brekk, O.R.; Moloney, E.B.; Rocha, E.M.; Priestman, D.A.; Platt, F.M.; Isacson, O. Glycosphingolipid Levels and Glucocerebrosidase Activity Are Altered in Normal Aging of the Mouse Brain. Neurobiol. Aging 2018, 67, 189–200. [Google Scholar] [CrossRef]
- Wang, H.; Muthu Karuppan, M.K.; Devadoss, D.; Nair, M.; Chand, H.S.; Lakshmana, M.K. TFEB Protein Expression Is Reduced in Aged Brains and Its Overexpression Mitigates Senescence-Associated Biomarkers and Memory Deficits in Mice. Neurobiol. Aging 2021, 106, 26–36. [Google Scholar] [CrossRef]
- Lapierre, L.R.; De Magalhaes Filho, C.D.; McQuary, P.R.; Chu, C.C.; Visvikis, O.; Chang, J.T.; Gelino, S.; Ong, B.; Davis, A.E.; Irazoqui, J.E.; et al. The TFEB Orthologue HLH-30 Regulates Autophagy and Modulates Longevity in Caenorhabditis elegans. Nat. Commun. 2013, 4, 2267. [Google Scholar] [CrossRef]
- Zhang, K.K.; Zhang, P.; Kodur, A.; Erturk, I.; Burns, C.M.; Kenyon, C.; Miller, R.A.; Endicott, S.J. LAMP2A, and Other Chaperone-Mediated Autophagy Related Proteins, Do Not Decline with Age in Genetically Heterogeneous UM-HET3 Mice. Aging 2023, 15, 4685–4698. [Google Scholar] [CrossRef]
- Bourdenx, M.; Martín-Segura, A.; Scrivo, A.; Rodriguez-Navarro, J.A.; Kaushik, S.; Tasset, I.; Diaz, A.; Storm, N.J.; Xin, Q.; Juste, Y.R.; et al. Chaperone-Mediated Autophagy Prevents Collapse of the Neuronal Metastable Proteome. Cell 2021, 184, 2696–2714.e25. [Google Scholar] [CrossRef]
- Kalia, V.; Belsky, D.W.; Baccarelli, A.A.; Miller, G.W. An Exposomic Framework to Uncover Environmental Drivers of Aging. Exposome 2022, 2, osac002. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and Unhealthy Aging: Environmental Drivers from Air Pollution to Occupational Exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Wickens, A.P. Ageing and the Free Radical Theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Fulton, R.E.; Pearson-Smith, J.N.; Huynh, C.Q.; Fabisiak, T.; Liang, L.P.; Aivazidis, S.; High, B.A.; Buscaglia, G.; Corrigan, T.; Valdez, R.; et al. Neuron-Specific Mitochondrial Oxidative Stress Results in Epilepsy, Glucose Dysregulation and a Striking Astrocyte Response. Neurobiol. Dis. 2021, 158, 105470. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative Stress and Autophagy in the Regulation of Lysosome-Dependent Neuron Death. Antioxid. Redox Signal. 2009, 11, 481–496. [Google Scholar] [CrossRef]
- Dagda, R.K.; Cherra, S.J.; Kulich, S.M.; Tandon, A.; Park, D.; Chu, C.T. Loss of PINK1 Function Promotes Mitophagy through Effects on Oxidative Stress and Mitochondrial Fission. J. Biol. Chem. 2009, 284, 13843–13855. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, S.J.; Kim, T.Y.; Cho, J.H.; Koh, J.Y. Zinc and 4-Hydroxy-2-Nonenal Mediate Lysosomal Membrane Permeabilization Induced by H2O2 in Cultured Hippocampal Neurons. J. Neurosci. 2008, 28, 3114–3122. [Google Scholar] [CrossRef]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Do Carmo, S. Early Oxidative Stress and DNA Damage in Aβ-Burdened Hippocampal Neurons in an Alzheimer’s-like Transgenic Rat Model. Commun. Biol. 2024, 7, 861. [Google Scholar] [CrossRef]
- Zhang, K.R.; Jankowski, C.S.R.; Marshall, R.; Nair, R.; Gómez, N.M.; Alnemri, A.; Liu, Y.; Erler, E.; Ferrante, J.; Song, Y.; et al. Oxidative Stress Induces Lysosomal Membrane Permeabilization and Ceramide Accumulation in Retinal Pigment Epithelial Cells. DMM Dis. Models Mech. 2023, 16, dmm050066. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Kamsler, A.; Avital, A.; Greenberger, V.; Segal, M. Aged SOD Overexpressing Mice Exhibit Enhanced Spatial Memory While Lacking Hippocampal Neurogenesis. Antioxid. Redox Signal. 2007, 9, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Serrano, F.; Oury, T.D.; Klann, E. Aging-Dependent Alterations in Synaptic Plasticity and Memory in Mice That Overexpress Extracellular Superoxide Dismutase. J. Neurosci. 2006, 26, 3933–3941. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, I.Y.; Bi, X.; Thompson, R.F.; Doctrow, S.R.; Malfroy, B.; Baudry, M. Reversal of Age-Related Learning Deficits and Brain Oxidative Stress in Mice with Superoxide Dismutase/Catalase Mimetics. Proc. Natl. Acad. Sci. USA 2003, 100, 8526–8531. [Google Scholar] [CrossRef]
- Sohal, R.S.; Ku, H.H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative Damage, Mitochondrial Oxidant Generation and Antioxidant Defenses during Aging and in Response to Food Restriction in the Mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s Disease: A Dual-Hit Hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef]
- Zhu, X.; Raina, A.K.; Perry, G.; Smith, M.A. Alzheimer’s Disease: The Two-Hit Hypothesis. Lancet Neurol. 2004, 3, 219–226. [Google Scholar] [CrossRef]
- Ryan, S.D.; Dolatabadi, N.; Chan, S.F.; Zhang, X.; Akhtar, M.W.; Parker, J.; Soldner, F.; Sunico, C.R.; Nagar, S.; Talantova, M.; et al. Isogenic Human iPSC Parkinson’s Model Shows Nitrosative Stress-Induced Dysfunction in MEF2-PGC1α Transcription. Cell 2013, 155, 1351–1364. [Google Scholar] [CrossRef]
- Heinemann, S.D.; Posimo, J.M.; Mason, D.M.; Hutchison, D.F.; Leak, R.K. Synergistic Stress Exacerbation in Hippocampal Neurons: Evidence Favoring the Dual-Hit Hypothesis of Neurodegeneration. Hippocampus 2016, 26, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Morrone Parfitt, G.; Coccia, E.; Goldman, C.; Whitney, K.; Reyes, R.; Sarrafha, L.; Nam, K.H.; Sohail, S.; Jones, D.R.; Crary, J.F.; et al. Disruption of Lysosomal Proteolysis in Astrocytes Facilitates Midbrain Organoid Proteostasis Failure in an Early-Onset Parkinson’s Disease Model. Nat. Commun. 2024, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Uchiki, T.; Weikel, K.A.; Jiao, W.; Shang, F.; Caceres, A.; Pawlak, D.; Handa, J.T.; Brownlee, M.; Nagaraj, R.; Taylor, A. Glycation-Altered Proteolysis as a Pathobiologic Mechanism That Links Dietary Glycemic Index, Aging, and Age-Related Disease (in Non Diabetics). Aging Cell 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Cerami, A. Advanced Glycation End Products Contribute to Amyloidosis in Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1994, 91, 4766–4770. [Google Scholar] [CrossRef]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yan, S.D.; Stern, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard Reaction End Products Are Associated with Alzheimer Disease Pathology. Proc. Natl. Acad. Sci. USA 1994, 91, 5710–5714. [Google Scholar] [CrossRef]
- Khawaja, R.R.; Martín-Segura, A.; Santiago-Fernández, O.; Sereda, R.; Lindenau, K.; McCabe, M.; Macho-González, A.; Jafari, M.; Scrivo, A.; Gomez-Sintes, R.; et al. Sex-Specific and Cell-Type-Specific Changes in Chaperone-Mediated Autophagy across Tissues during Aging. Nat. Aging 2025, 5, 691–708. [Google Scholar] [CrossRef]
- Issa, A.R.; Sun, J.; Petitgas, C.; Mesquita, A.; Dulac, A.; Robin, M.; Mollereau, B.; Jenny, A.; Chérif-Zahar, B.; Birman, S. The Lysosomal Membrane Protein LAMP2A Promotes Autophagic Flux and Prevents SNCA-Induced Parkinson Disease-like Symptoms in the Drosophila Brain. Autophagy 2018, 14, 1898–1910. [Google Scholar] [CrossRef]
- Andrews, N.W. Regulated Secretion of Conventional Lysosomes. Trends Cell Biol. 2000, 10, 316–321. [Google Scholar] [CrossRef]
- Tancini, B.; Buratta, S.; Delo, F.; Sagini, K.; Chiaradia, E.; Pellegrino, R.M.; Emiliani, C.; Urbanelli, L. Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle. Membranes 2020, 10, 406. [Google Scholar] [CrossRef]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Xu, Y.; Du, S.; Marsh, J.A.; Horie, K.; Sato, C.; Ballabio, A.; Karch, C.M.; Holtzman, D.M.; Zheng, H. TFEB Regulates Lysosomal Exocytosis of Tau and Its Loss of Function Exacerbates Tau Pathology and Spreading. Mol. Psychiatry 2020, 26, 5925–5939. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation Controls Exosome Secretion by Promoting Lysosomal Degradation of MVB Proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef]
- Edgar, J.R.; Manna, P.T.; Nishimura, S.; Banting, G.; Robinson, M.S. Tetherin Is an Exosomal Tether. eLife 2016, 5, e17180. [Google Scholar] [CrossRef]
- Guo, H.; Chitiprolu, M.; Roncevic, L.; Javalet, C.; Hemming, F.J.; Trung, M.T.; Meng, L.; Latreille, E.; Tanese de Souza, C.; McCulloch, D.; et al. Atg5 Disassociates the V1V0-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev. Cell 2017, 43, 716–730.e7. [Google Scholar] [CrossRef]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. C. elegans Neurons Jettison Protein Aggregates and Mitochondria Under Neurotoxic Stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Yang, Y.; Arnold, M.L.; Lange, C.M.; Sun, L.H.; Broussalian, M.; Doroodian, S.; Ebata, H.; Choy, E.H.; Poon, K.; Moreno, T.M.; et al. Autophagy Protein ATG-16.2 and Its WD40 Domain Mediate the Beneficial Effects of Inhibiting Early-Acting Autophagy Genes in C. elegans Neurons. Nat. Aging 2024, 4, 198–212. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia Clear Neuron-Released α-Synuclein via Selective Autophagy and Prevent Neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Guldner, I.H.; Wagner, V.P.; Moran-Losada, P.; Shi, S.M.; Chen, K.; Meese, B.T.; Oh, H.; Guen, Y.L.; Lu, N.; Wong, P.S.; et al. Synaptic Proteins That Aggregate and Degrade Slower with Aging Accumulate in Microglia. bioRxiv 2025. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s Disease β-Amyloid Peptides Are Released in Association with Exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. CGAS–STING Drives Ageing-Related Inflammation and Neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. Lysosomes in Senescence and Aging. EMBO Rep. 2023, 24, e57265. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of Lysosomal Storage Disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef]
- Futerman, A.H.; Van Meer, G. The Cell Biology of Lysosomal Storage Disorders. Nat. Rev. Mol. Cell Biol. 2004, 5, 554–565. [Google Scholar] [CrossRef]
- Teixeira, C.A.; Miranda, C.O.; Sousa, V.F.; Santos, T.E.; Malheiro, A.R.; Solomon, M.; Maegawa, G.H.; Brites, P.; Sousa, M.M. Early Axonal Loss Accompanied by Impaired Endocytosis, Abnormal Axonal Transport, and Decreased Microtubule Stability Occur in the Model of Krabbe’s Disease. Neurobiol. Dis. 2014, 66, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Pressey, S.N.R.; Smith, D.A.; Wong, A.M.S.; Platt, F.M.; Cooper, J.D. Early Glial Activation, Synaptic Changes and Axonal Pathology in the Thalamocortical System of Niemann–Pick Type C1 Mice. Neurobiol. Dis. 2012, 45, 1086–1100. [Google Scholar] [CrossRef]
- Kavetsky, L.; Green, K.K.; Boyle, B.R.; Yousufzai, F.A.K.; Padron, Z.M.; Melli, S.E.; Kuhnel, V.L.; Jackson, H.M.; Blanco, R.E.; Howell, G.R.; et al. Increased Interactions and Engulfment of Dendrites by Microglia Precede Purkinje Cell Degeneration in a Mouse Model of Niemann Pick Type-C. Sci. Rep. 2019, 9, 14722. [Google Scholar] [CrossRef]
- Gujjala, V.A.; Abyadeh, M.; Klimek, I.; Tyshkovskiy, A.; Oz, N.; Castro, J.P.; Gladyshev, V.N.; Newton, J.; Kaya, A. Short-Lived Niemann-Pick Type C Mice with Accelerated Brain Aging as a Novel Model for Alzheimer’s Disease Research. Neural Regen. Res. 2025. [Google Scholar] [CrossRef]
- Nixon, R.A. Endosome Function and Dysfunction in Alzheimer’s Disease and Other Neurodegenerative Diseases. Neurobiol. Aging 2005, 26, 373–382. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Wu, Y.; Ferguson, S.M. Impaired JIP3-Dependent Axonal Lysosome Transport Promotes Amyloid Plaque Pathology. J. Cell Biol. 2017, 216, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal Proteolysis and Autophagy Require Presenilin 1 and Are Disrupted by Alzheimer-Related PS1 Mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef]
- Boland, B.; Kumar, A.; Lee, S.; Platt, F.M.; Wegiel, J.; Yu, W.H.; Nixon, R.A. Autophagy Induction and Autophagosome Clearance in Neurons: Relationship to Autophagic Pathology in Alzheimer’s Disease. J. Neurosci. 2008, 28, 6926–6937. [Google Scholar] [CrossRef]
- Colacurcio, D.J.; Nixon, R.A. Disorders of Lysosomal Acidification—The Emerging Role of v-ATPase in Aging and Neurodegenerative Disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Wallings, R.; Connor-Robson, N.; Wade-Martins, R. LRRK2 Interacts with the Vacuolar-Type H+-ATPase Pump A1 Subunit to Regulate Lysosomal Function. Hum. Mol. Genet. 2019, 28, 2696–2710. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Wu, J.W.; Myeku, N.; Figueroa, Y.H.; Herman, M.; Marinec, P.S.; Gestwicki, J.E.; Dickey, C.A.; Yu, W.H.; Duff, K.E. Methylthioninium Chloride (Methylene Blue) Induces Autophagy and Attenuates Tauopathy in Vitro and in Vivo. Autophagy 2012, 8, 609–622. [Google Scholar] [CrossRef]
- Dehkordi, S.K.; Walker, J.; Sah, E.; Bennett, E.; Atrian, F.; Frost, B.; Woost, B.; Bennett, R.E.; Orr, T.C.; Zhou, Y.; et al. Profiling Senescent Cells in Human Brains Reveals Neurons with CDKN2D/P19 and Tau Neuropathology. Nat. Aging 2021, 1, 1107–1116. [Google Scholar] [CrossRef]
- Kaur, K.; Gill, J.S.; Bansal, P.K.; Deshmukh, R. Neuroinflammation—A Major Cause for Striatal Dopaminergic Degeneration in Parkinson’s Disease. J. Neurol. Sci. 2017, 381, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Root, J.; Mendsaikhan, A.; Taylor, G.; Merino, P.; Nandy, S.; Wang, M.; Araujo, L.T.; Ryu, D.; Holler, C.; Thompson, B.M.; et al. Granulins Rescue Inflammation, Lysosome Dysfunction, Lipofuscin, and Neuropathology in a Mouse Model of Progranulin Deficiency. Cell Rep. 2024, 43, 114985. [Google Scholar] [CrossRef]
- Pajares, M.; I Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Liénard, C.; Pintart, A.; Bomont, P. Neuronal Autophagy: Regulations and Implications in Health and Disease. Cells 2024, 13, 103. [Google Scholar] [CrossRef]
- Lim, S.H.Y.; Hansen, M.; Kumsta, C. Molecular Mechanisms of Autophagy Decline during Aging. Cells 2024, 13, 1364. [Google Scholar] [CrossRef]
- Konstantinidis, G.; Tavernarakis, N. Molecular Basis of Neuronal Autophagy in Ageing: Insights from Caenorhabditis elegans. Cells 2021, 10, 694. [Google Scholar] [CrossRef]
- Schmid, E.T.; Pyo, J.H.; Walker, D.W. Neuronal Induction of BNIP3-Mediated Mitophagy Slows Systemic Aging in Drosophila. Nat. Aging 2022, 2, 494–507. [Google Scholar] [CrossRef]
- Stavoe, A.K.H.; Gopal, P.P.; Gubas, A.; Tooze, S.A.; Holzbaur, E.L.F. Expression of WIPI2B Counteracts Age-Related Decline in Autophagosome Biogenesis in Neurons. eLife 2019, 8, e44219. [Google Scholar] [CrossRef]
- Tsong, H.; Holzbaur, E.L.F.; Stavoe, A.K.H. Aging Differentially Affects Axonal Autophagosome Formation and Maturation. Autophagy 2023, 19, 3079–3095. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Oba, M.; Suzuki, M.; Takahashi, A.; Yamamuro, T.; Fujiwara, M.; Ikenaka, K.; Minami, S.; Tabata, N.; Yamamoto, K.; et al. Suppression of Autophagic Activity by Rubicon Is a Signature of Aging. Nat. Commun. 2019, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Kumsta, C.; Hellman, A.B.; Adams, L.M.; Hansen, M. Spatiotemporal Regulation of Autophagy during Caenorhabditis elegans Aging. eLife 2017, 6, e18459. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.; McWilliams, T.G. Mitophagy in the Aging Nervous System. Front. Cell Dev. Biol. 2022, 10, 978142. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, L.; Li, J.; Lan, X.; Lixiang, A.; Lv, X.; Zhang, M.; Chen, L. The Alteration of Autophagy and Apoptosis in the Hippocampus of Rats with Natural Aging-Dependent Cognitive Deficits. Behav. Brain Res. 2017, 334, 155–162. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Kaushik, S.; Arias, E.; Kwon, H.; Lopez, N.M.; Athonvarangkul, D.; Sahu, S.; Schwartz, G.J.; Pessin, J.E.; Singh, R. Loss of Autophagy in Hypothalamic POMC Neurons Impairs Lipolysis. EMBO Rep. 2012, 13, 258–265. [Google Scholar] [CrossRef]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting Basal Levels of Autophagy in the Nervous System Enhances Longevity and Oxidant Resistance in Adult Drosophila. Autophagy 2008, 4, 176–184. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, H. Autophagosome Maturation: An Epic Journey from the ER to Lysosomes. J. Cell Biol. 2019, 218, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Tang, Y.; Huang, Y.; Wu, Z.; Cui, Y. Upregulation of Neuronal ER-Phagy Improves Organismal Fitness and Alleviates APP Toxicity. Cell Rep. 2024, 43, 114255. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, K.; Parker, J.A. Nicotinamide-N-Methyltransferase Controls Behavior, Neurodegeneration and Lifespan by Regulating Neuronal Autophagy. PLoS Genet. 2018, 14, e1007561. [Google Scholar] [CrossRef] [PubMed]
- Bhukel, A.; Beuschel, C.B.; Maglione, M.; Lehmann, M.; Juhász, G.; Madeo, F.; Sigrist, S.J. Autophagy within the Mushroom Body Protects from Synapse Aging in a Non-Cell Autonomous Manner. Nat. Commun. 2019, 10, 1318. [Google Scholar] [CrossRef]
- Napolitano, G.; Ballabio, A. TFEB at a Glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef]
- Sardiello, M.; Palmieri, M.; Di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A Gene Network Regulating Lysosomal Biogenesis and Function. Science (1979) 2009, 325, 473–477. [Google Scholar] [CrossRef]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The Transcription Factor TFEB Links MTORC1 Signaling to Transcriptional Control of Lysosome Homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 Functions as a Transcriptional Regulator of Autophagy by Preventing Nuclear Transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A Lysosome-to-Nucleus Signalling Mechanism Senses and Regulates the Lysosome via MTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- La Spina, M.; Contreras, P.S.; Rissone, A.; Meena, N.K.; Jeong, E.; Martina, J.A. MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front. Cell Dev. Biol. 2021, 8, 609683. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong-A, L.; Patange, S.; Raben, N.; Puertollano, R. The Nutrient-Responsive Transcription Factor TFE3 Promotes Autophagy, Lysosomal Biogenesis, and Clearance of Cellular Debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Mattsson, B.; Weikop, P.; Lundblad, M.; Jakobsson, J.; Björklund, A. TFEB-Mediated Autophagy Rescues Midbrain Dopamine Neurons from α-Synuclein Toxicity. Proc. Natl. Acad. Sci. USA 2013, 110, E1817–E1826. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.A.; Li, H.; Martini-Stoica, H.; Wang, B.; Yang, L.; Xu, Y.; Swartzlander, D.B.; Palmieri, M.; di Ronza, A.; Lee, V.M.; et al. Selective Clearance of Aberrant Tau Proteins and Rescue of Neurotoxicity by Transcription Factor EB. EMBO Mol. Med. 2014, 6, 1142–1160. [Google Scholar] [CrossRef]
- Gu, Z.; Cao, H.; Zuo, C.; Huang, Y.; Miao, J.; Song, Y.; Yang, Y.; Zhu, L.; Wang, F. TFEB in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Neurobiol. Dis. 2022, 173, 105855. [Google Scholar] [CrossRef] [PubMed]
- Bouché, V.; Espinosa, A.P.; Leone, L.; Sardiello, M.; Ballabio, A.; Botas, J. Drosophila Mitf Regulates the V-ATPase and the Lysosomal-Autophagic Pathway. Autophagy 2016, 12, 484–498. [Google Scholar] [CrossRef]
- Martínez-Fábregas, J.; Prescott, A.; van Kasteren, S.; Pedrioli, D.L.; McLean, I.; Moles, A.; Reinheckel, T.; Poli, V.; Watts, C. Lysosomal Protease Deficiency or Substrate Overload Induces an Oxidative-Stress Mediated STAT3-Dependent Pathway of Lysosomal Homeostasis. Nat. Commun. 2018, 9, 5343. [Google Scholar] [CrossRef]
- Annunziata, I.; van de Vlekkert, D.; Wolf, E.; Finkelstein, D.; Neale, G.; Machado, E.; Mosca, R.; Campos, Y.; Tillman, H.; Roussel, M.F.; et al. MYC Competes with MiT/TFE in Regulating Lysosomal Biogenesis and Autophagy through an Epigenetic Rheostat. Nat. Commun. 2019, 10, 3623. [Google Scholar] [CrossRef]
- Shin, H.J.R.; Kim, H.; Oh, S.; Lee, J.G.; Kee, M.; Ko, H.J.; Kweon, M.N.; Won, K.J.; Baek, S.H. AMPK–SKP2–CARM1 Signalling Cascade in Transcriptional Regulation of Autophagy. Nature 2016, 534, 553–557. [Google Scholar] [CrossRef]
- Henn, D.; Yang, X.; Li, M. Lysosomal Quality Control Review. Autophagy 2025, 21, 1413–1432. [Google Scholar] [CrossRef]
- Radulovic, M.; Yang, C.; Stenmark, H. Lysosomal Membrane Homeostasis and Its Importance in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2025, 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Krogsaeter, E.; Butz, E.S.; Li, Y.; Puertollano, R.; Wahl-Schott, C.; Biel, M.; Grimm, C. TRPML2 Is an Osmo/Mechanosensitive Cation Channel in Endolysosomal Organelles. Sci. Adv. 2020, 6, eabb5064. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, Y.; Wang, Y.; Zhu, R.; Chen, W.; Cheng, T.; Zhang, X.; Jia, Y.; Liu, T.; Zhang, W.; et al. Drosophila TMEM63 and Mouse TMEM63A Are Lysosomal Mechanosensory Ion Channels. Nat. Cell Biol. 2024, 26, 393–403. [Google Scholar] [CrossRef]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nähse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-mediated Lysosome Repair Precedes Lysophagy and Promotes Cell Survival. EMBO J. 2018, 37, e99753. [Google Scholar] [CrossRef]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered Recruitment of ESCRT Machinery Promotes Endolysosomal Repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef]
- Shukla, S.; Larsen, K.P.; Ou, C.; Rose, K.; Hurley, J.H. In Vitro Reconstitution of Calcium-Dependent Recruitment of the Human ESCRT Machinery in Lysosomal Membrane Repair. Proc. Natl. Acad. Sci. USA 2022, 119, e2205590119. [Google Scholar] [CrossRef]
- Chen, W.; Motsinger, M.M.; Li, J.; Bohannon, K.P.; Hanson, P.I. Ca2+-Sensor ALG-2 Engages ESCRTs to Enhance Lysosomal Membrane Resilience to Osmotic Stress. Proc. Natl. Acad. Sci. USA 2024, 121, e2318412121. [Google Scholar] [CrossRef] [PubMed]
- Ebstrup, M.L.; Sønder, S.L.; Fogde, D.L.; Heitmann, A.S.B.; Dietrich, T.N.; Dias, C.; Jäättelä, M.; Maeda, K.; Nylandsted, J. Annexin A7 Mediates Lysosome Repair Independently of ESCRT-III. Front. Cell Dev. Biol. 2023, 11, 1211498. [Google Scholar] [CrossRef]
- Bussi, C.; Mangiarotti, A.; Vanhille-Campos, C.; Aylan, B.; Pellegrino, E.; Athanasiadi, N.; Fearns, A.; Rodgers, A.; Franzmann, T.M.; Šarić, A.; et al. Stress Granules Plug and Stabilize Damaged Endolysosomal Membranes. Nature 2023, 623, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. A Phosphoinositide Signalling Pathway Mediates Rapid Lysosomal Repair. Nature 2022, 609, 815–821. [Google Scholar] [CrossRef]
- Radulovic, M.; Wenzel, E.M.; Gilani, S.; Holland, L.K.; Lystad, A.H.; Phuyal, S.; Olkkonen, V.M.; Brech, A.; Jäättelä, M.; Maeda, K.; et al. Cholesterol Transfer via Endoplasmic Reticulum Contacts Mediates Lysosome Damage Repair. EMBO J. 2022, 41, e112677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, P.; Bentley-DeSousa, A.; Hancock-Cerutti, W.; Cai, S.; Johnson, B.T.; Tonelli, F.; Shao, L.; Talaia, G.; Alessi, D.R.; et al. The Bridge-like Lipid Transport Protein VPS13C/PARK23 Mediates ER-Lysosome Contacts Following Lysosome Damage. Nat. Cell Biol. 2025, 27, 776–789. [Google Scholar] [CrossRef]
- Durgan, J.; Florey, O. Many Roads Lead to CASM: Diverse Stimuli of Noncanonical Autophagy Share a Unifying Molecular Mechanism. Sci. Adv. 2022, 8, eabo1274. [Google Scholar] [CrossRef]
- Niekamp, P.; Scharte, F.; Sokoya, T.; Vittadello, L.; Kim, Y.; Deng, Y.; Südhoff, E.; Hilderink, A.; Imlau, M.; Clarke, C.J.; et al. Ca2+-Activated Sphingomyelin Scrambling and Turnover Mediate ESCRT-Independent Lysosomal Repair. Nat. Commun. 2022, 13, 1875. [Google Scholar] [CrossRef]
- Fischer, T.D.; Wang, C.; Padman, B.S.; Lazarou, M.; Youle, R.J. STING Induces LC3B Lipidation onto Single-Membrane Vesicles via the V-ATPase and ATG16L1-WD40 Domain. J. Cell Biol. 2020, 219, e202009128. [Google Scholar] [CrossRef]
- Goodwin, J.M.; Walkup, W.G.; Hooper, K.; Li, T.; Kishi-Itakura, C.; Ng, A.; Lehmberg, T.; Jha, A.; Kommineni, S.; Fletcher, K.; et al. GABARAP Sequesters the FLCN-FNIP Tumor Suppressor Complex to Couple Autophagy with Lysosomal Biogenesis. Sci. Adv. 2021, 7, eabj2485. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, S.; Jain, A.; Ponpuak, M.; Mudd, M.H.; Kimura, T.; Choi, S.W.; Peters, R.; Mandell, M.; Bruun, J.A.; et al. TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-Direct Autophagy in Endomembrane Damage Homeostasis. Dev. Cell 2016, 39, 13–27. [Google Scholar] [CrossRef]
- Hung, Y.H.; Chen, L.M.W.; Yang, J.Y.; Yang, W.Y. Spatiotemporally Controlled Induction of Autophagy-Mediated Lysosome Turnover. Nat. Commun. 2013, 4, 2111. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; Von Muhlinen, N.; Foeglein, Á.; Randow, F. Galectin 8 Targets Damaged Vesicles for Autophagy to Defend Cells against Bacterial Invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Kravic, B.; Meyer, H. Repair or Lysophagy: Dealing with Damaged Lysosomes. J. Mol. Biol. 2020, 432, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Maejima, I.; Takahashi, A.; Omori, H.; Kimura, T.; Takabatake, Y.; Saitoh, T.; Yamamoto, A.; Hamasaki, M.; Noda, T.; Isaka, Y.; et al. Autophagy Sequesters Damaged Lysosomes to Control Lysosomal Biogenesis and Kidney Injury. EMBO J. 2013, 32, 2336–2347. [Google Scholar] [CrossRef]
- Peck, A.; Dadi, A.; Yavarow, Z.; Alfano, L.N.; Anderson, D.; Arkin, M.R.; Chou, T.F.; D’Ambrosio, E.S.; Diaz-Manera, J.; Dudley, J.P.; et al. 2024 VCP International Conference: Exploring Multi-Disciplinary Approaches from Basic Science of Valosin Containing Protein, an AAA+ ATPase Protein, to the Therapeutic Advancement for VCP-Associated Multisystem Proteinopathy. Neurobiol. Dis. 2025, 207, 106861. [Google Scholar] [CrossRef]
- Hill, S.M.; Wrobel, L.; Ashkenazi, A.; Fernandez-Estevez, M.; Tan, K.; Bürli, R.W.; Rubinsztein, D.C. VCP/P97 Regulates Beclin-1-Dependent Autophagy Initiation. Nat. Chem. Biol. 2021, 17, 448–455. [Google Scholar] [CrossRef]
- Samie, M.A.; Xu, H. Lysosomal Exocytosis and Lipid Storage Disorders. J. Lipid Res. 2014, 55, 995–1009. [Google Scholar] [CrossRef]
- Prentzell, M.T.; Rehbein, U.; Cadena Sandoval, M.; De Meulemeester, A.S.; Baumeister, R.; Brohée, L.; Berdel, B.; Bockwoldt, M.; Carroll, B.; Chowdhury, S.R.; et al. G3BPs Tether the TSC Complex to Lysosomes and Suppress MTORC1 Signaling. Cell 2021, 184, 655–674.e27. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins Control MTOR in Response to Endomembrane Damage. Mol. Cell 2018, 70, 120–135.e8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).