Abstract
Cognitive impairment remains one of the most prevalent and debilitating sequelae of traumatic brain injury (TBI), profoundly compromising long-term quality of life. Nevertheless, effective treatment options are limited, as the complexity of post-TBI pathology often exceeds the protective scope of conventional neuroprotective strategies. Accumulating research has revealed regulated cell death (RCD) as a central driver of neuronal loss and cognitive decline post-TBI. Consequently, targeting RCD pathways has emerged as a promising strategic direction for alleviating post-TBI cognitive impairment. This review provides an analysis of the molecular mechanisms underlying five major RCD forms, including apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis. Furthermore, it critically assesses the therapeutic potential of these pathways while examining their complex interplay in post-TBI cognitive impairment. By systematically synthesizing recent advances in targeted therapeutic strategies, we highlight that targeting RCD pathways paves the way for highly effective and precise therapeutic modalities against post-TBI cognitive impairment, although challenges in multi-target combination therapies and brain delivery warrant further investigation.
1. Introduction
Traumatic brain injury (TBI) is defined by the Centers for Disease Control and Prevention (CDC) as a disruption in normal brain function resulting from an external mechanical force to the head, such as a blow, jolt, or penetrating injury []. Globally, an estimated 69 million individuals sustain a TBI annually, with falls and road traffic incidents constituting the major etiologies [].
The pathophysiology of TBI comprises two distinct phases: primary and secondary injury. Primary injury occurs instantaneously at the moment of impact, causing mechanical damage to neurons and vasculature. This leads to direct cell death and activates inflammatory pathways []. Secondary injury begins within hours and continues over days to weeks, driven by molecular cascades such as glutamate excitotoxicity, oxidative stress, and mitochondrial dysfunction, all of which exacerbate neuronal loss through regulated cell death (RCD) processes [,,]. Unlike the uncontrolled accidental cell death from direct trauma, RCD is a genetically programmed process that eliminates compromised cells []. Key RCD modalities, including apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis, contribute significantly to secondary injury []. The activation of these pathways causes delayed and often irreversible neuronal loss in brain regions critical for cognition, establishing RCD as a key driver of the long-term cognitive deficits associated with TBI [].
Among the various sequelae that arise following secondary brain injury, cognitive impairment is one of the most prevalent and debilitating outcomes []. It typically manifests as deficits in multiple domains, including language ability, logical reasoning, and executive control, substantially compromising patients’ daily functioning and quality of life []. Given the restricted efficacy of existing interventions, which predominantly involve conventional neuroprotective approaches, developing effective treatments for TBI-related sequelae represents a critical unmet need []. Therefore, exploring therapeutic strategies that target the mechanisms of RCD, a key driver of secondary injury, holds significant clinical potential for mitigating post-TBI cognitive impairment and merits intensified research.
This review analyzes the molecular mechanisms of RCD in post-TBI cognitive impairment. By integrating this analysis with emerging therapeutic targets and pathway interactions, and considering translational challenges like human TBI heterogeneity, we ultimately aim to propose novel interventions for patients.
2. Molecular Mechanism of Apoptosis
In 1972, Kerr et al. first introduced the concept of apoptosis, a process morphologically characterized by cell shrinkage, nuclear condensation, DNA fragmentation, and the formation of apoptotic bodies under an intact plasma membrane []. Apoptotic execution requires the activation of caspases via two distinct pathways: the intrinsic pathway and the extrinsic pathway []. On the one hand, the intrinsic apoptotic pathway is initiated in response to diverse intracellular stresses, such as toxic insults, DNA damage, or endoplasmic reticulum stress [], which promote the competitive binding of BH3-only proteins to antiapoptotic Bcl-2 family members, leading to the liberation and activation of the proapoptotic effectors BAX and BAK. Thereafter, oligomerized BAX/BAK enhances mitochondrial outer membrane permeability (MOMP), releasing cytochrome c into the cytosol [,,]. The released cytochrome c facilitates the assembly of the apoptosome by binding to apoptosis protease activating factor-1 (Apaf-1), which activates initiator caspase-9 and subsequently cleaves effector caspase-3, thereby initiating the apoptotic cascade []. A caspase-independent intrinsic apoptotic pathway has been identified, which is mediated by apoptosis-inducing factor (AIF) []. Upon proteolytic cleavage, AIF translocates from the mitochondrial inner membrane to the nucleus, where it directly induces chromatin fragmentation []. On the other hand, the extrinsic apoptotic pathway is triggered by the binding of extracellular ligands, such as FasL, to transmembrane death receptors like Fas [,]. This interaction induces the assembly of the death-inducing signaling complex (DISC), leading to the activation of the initiator caspase, caspase-8 []. Subsequently, activated caspase-8 proteolytically cleaves and activates the executioner caspase-3, ultimately culminating in apoptosis [].
3. Therapeutic Potential of Apoptosis in Post-TBI Cognitive Impairment
As early as 1997, Yakovlev et al. identified apoptosis as a critical pathological process underlying cognitive impairment following TBI []. In their model, DNA fragmentation, a biochemical hallmark of apoptosis, was detected, along with a significant increase in both mRNA expression and enzymatic activity of caspase-3 (a central apoptotic protease []) in the lateral cortex and hippocampus following TBI []. These findings indicate that apoptosis, observable even after mild TBI, leads to neuronal loss within cognition-critical regions, thereby directly compromising neurological function []. Accordingly, a study has demonstrated that caspase-3 mediates neuronal death following TBI in rats, and that the caspase-3 inhibitor N-benzyloxycarbonyl-Asp-fluromethyl ketone (z-DEVD-fmk) effectively reduces cerebral tissue damage in TBI models [].
The translocation of AIF, a key mediator of caspase-independent apoptosis, is triggered by the activation of poly(ADP-ribose) polymerase-1 (PARP-1) []. A study demonstrated that administration of the PARP-1 inhibitor INO-1001, an indeno [1,2-c]isoquinolinone derivative, within 24 h post-TBI in mice improved cognitive function. This beneficial effect was attributed to PARP-1 inhibition, which subsequently inhibited the caspase-independent apoptotic pathway mediated by AIF, thereby suggesting AIF as a potential therapeutic target []. Additionally, caspase-8 functions as the apical protease of the extrinsic apoptotic route. Clinical research has revealed that TBI patients with high caspase-8 levels showed higher mortality rates []. Furthermore, a study also indicated that caspase-8 expression is significantly upregulated in TBI mice, and neuron-specific knockdown of caspase-8 attenuates neuronal apoptosis and improves neurological function in TBI mice []. These findings are corroborated by experiments in which pharmacological inhibition of caspase-8 diminished neuronal apoptosis and neurological dysfunction, underscoring its importance in the acute pathophysiology of TBI.
Pharmacological interventions that modulate apoptosis have demonstrated considerable success in ameliorating TBI pathology. Azithromycin exhibits robust neuroprotective effects following TBI by modulating key apoptotic regulators, including the downregulation of caspase-3 and the proapoptotic protein BAX, alongside the upregulation of the antiapoptotic protein Bcl-2, ultimately leading to significant improvements in spatial memory and recognition in rats []. Similarly, Lupeol has been shown to suppress reactive oxygen species generation and lipid peroxidation, reduce the expression of proapoptotic factors such as BAX, cytochrome c release, and caspase-3 activation, ultimately leading to the improvement of hippocampal-dependent cognitive function []. Edaravone improves TBI-induced learning and memory deficits in rats by inhibiting hippocampal neuronal apoptosis through activation of the BDNF/TrkB pathway []. Pifithrin-α and its oxygen analog PFT-α(O) have been shown to ameliorate TBI-induced learning and memory dysfunction in rats by inhibiting p53-dependent apoptosis []. Previous study demonstrated that COG1410, a triggering receptor expressed on myeloid cells 2 (TREM2) activator, improves post-TBI cognitive impairment through activation of the Akt/CREB/BDNF pathway and inhibition of caspase-3-dependent neuronal apoptosis []. Moreover, a recent study demonstrated that the small-molecule drug ZL006 ameliorates cognitive dysfunction in mice following TBI by inhibiting the interaction between nNOS and PSD95, thereby reducing NO-mediated oxidative damage and suppressing caspase-3-dependent neuronal apoptosis []. Recent work revealed that HS38 attenuates neuronal apoptosis and ameliorates cognitive dysfunction in mice following TBI by inhibiting ZIPK activity, thereby reducing DEDD-mediated activation of caspase-3 []. Furthermore, several physical interventions have shown promise in modulating apoptotic pathways to improve cognitive outcomes after TBI. Mild hypothermia therapy, for instance, has been found to downregulate caspase-3 and upregulate Bcl-2, thereby attenuating neuronal apoptosis and exerting protective effects against TBI-induced cognitive deficits []. Similarly, a study demonstrated that hyperbaric oxygen therapy suppresses cleaved caspase-3 levels, preserves mitochondrial respiratory function, and enhances spatial learning in murine TBI models [].
Several pharmacological agents targeting apoptotic pathways have advanced to clinical development. Previously, N-methyl-D-aspartate (NMDA) receptor inhibitors have been confirmed to reduce hippocampal caspase-3 expression, mitigate apoptosis, and alleviate cognitive impairment []. Clinical evaluation further demonstrated that Traxoprodil improved cognitive function in TBI patients []. Experimental models showed that statins modulated the anti/proapoptotic balance and enhanced spatial learning in rodents, while double-blind trials confirmed their efficacy in promoting memory recovery in moderate-to-severe TBI cases [,]. Moreover, preclinical evidence indicated that progesterone mitigated apoptosis and supported cognitive recovery, findings subsequently validated in a Phase II randomized controlled trial []. These results underscore the translational promise of apoptosis-targeted strategies while highlighting the need for expanded clinical investigation.
Collectively, these studies demonstrate that targeted inhibition of apoptotic pathways, whether directed against executioner caspases (such as caspase-3), initiator caspases (e.g., caspase-8), downstream effectors like PARP-1, or upstream regulatory molecules, effectively reduces neuronal apoptosis and ameliorates cognitive deficits following TBI. Therapeutic strategies targeting apoptosis are presented in Figure 1.
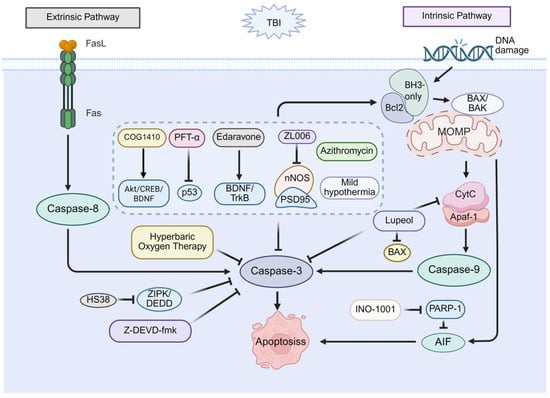
Figure 1.
Therapeutic potential of apoptosis in post-TBI cognitive impairment. Apoptosis occurs through two main pathways. The intrinsic pathway initiates when BH3-only proteins bind and inhibit Bcl-2, leading to BAX activation, mitochondrial outer membrane permeability (MOMP), cytochrome c release, apoptosome formation, and subsequent activation of caspase-9 and caspase-3. The extrinsic pathway begins with FasL-Fas binding, activating caspase-8, and directly cleaving caspase-3. Pharmacological inhibition strategies include: agents that inhibit caspase-3, upregulate Bcl-2, and downregulate BAX (COG1410 via Akt/CREB/BDNF; PFT-α via p53 inhibition; Edaravone via BDNF/TrkB signaling; ZL006 via nNOS-PSD95 disruption; Azithromycin; Mild hypothermia); targeting caspase-3 inhibition (z-DEVD-fmk; HS38 via ZIPK/DEDD; Hyperbaric Oxygen Therapy); and other multi-target agents (Lupeol, which inhibits caspase-3, downregulates BAX, and reduces cytochrome c release; INO-1001, which inhibits PARP-1/AIF). Together, these strategies reduce neuronal death and cognitive impairment.
4. Molecular Mechanism of Necroptosis
Necroptosis, a regulated form of necrotic cell death first formally characterized by Degterev et al. in 2005, is morphologically defined by cellular swelling, organellar distension, plasma membrane rupture, and chromatin condensation [,]. Necroptosis is initiated through the activation of diverse signaling components, including cell surface death receptors, such as tumor necrosis factor receptor 1 (TNFR1) and Toll-like receptors (TLRs), as well as intracellular sensors of RNA and DNA []. The occurrence of necroptosis is contingent upon the sequential activation of receptor-interacting protein kinase 3 (RIPK3) and mixed lineage kinase-like pseudokinase (MLKL) []. RIPK3 activation occurs primarily through three distinct pathways. The first involves TNFR1-mediated activation of receptor-interacting protein kinase 1 (RIPK1), which then engages RIPK3 via mutual RIP (receptor-interacting protein) homology interaction motifs (RHIM) domain interactions []. Alternatively, TLR3 or TLR4 activation triggers the recruitment of RHIM-containing adaptor proteins that directly bind and activate RIPK3 []. A third pathway is initiated by the cytosolic nucleic acid sensor Z-dsDNA/dsRNA-binding protein 1 (ZBP1), which activates RIPK3 through its RHIM domain [,]. Subsequently, activated RIPK3 phosphorylates MLKL, which ultimately oligomerizes to form activated necrosomes that translocate to the plasma membrane, thereby triggering necroptosis [].
5. Therapeutic Potential of Necroptosis in Post-TBI Cognitive Impairment
Research on necroptosis in cognitive impairment after TBI began in 2008. The study demonstrated that treatment of the necroptosis-specific inhibitor necrostatin-1 to TBI mice significantly reduced brain tissue injury volume and improved cognitive function, without decreasing apoptosis-related caspase-3 activity, thereby highlighting the specific contribution of necroptosis to post-TBI cognitive impairment []. Subsequent studies further explore the role of necroptosis in cognitive dysfunction following TBI. RIPK3, a key regulator of necroptosis, has been demonstrated to contribute to TBI-induced cognitive impairment, as evidenced by a study showing that RIPK3 knockout significantly improves spatial learning and memory in TBI mice []. Another study showed that neuron-specific deficiency of RIPK1 or RIPK3 significantly enhances cognitive performance of TBI mice, indicating that RIPK1 or RIPK3 serve as viable therapeutic targets for post-TBI cognitive impairment []. Consistent with this finding, evidence indicated that 2-(2-benzofuranyl)-2-imidazoline (2-BFI) attenuates necroptosis through downregulation of RIPK1 and RIPK3 expression, thereby ameliorating cognitive dysfunction in the TBI rat model []. Emerging evidence indicated that overexpression of charged multivesicular body protein 4B (CHMP4B) reduced RIPK3 levels, markedly diminished necroptosis, and promoted cognitive recovery in TBI mice []. Additionally, a pioneering study analyzing human brain samples provided direct evidence of necroptosis following TBI. The same investigation further demonstrated that both necrostatin-1 and melatonin reduced the levels of RIPK1 and RIPK3, inhibited necroptosis, and subsequently improved cognitive function post-TBI in rats [].
In summary, targeted inhibition of key necroptotic signaling molecules, such as RIPK1, RIPK3, and MLKL, provides a promising therapeutic strategy to protect cognitive function after TBI. Given the established role of necroptosis in secondary injury progression, pharmacologic interventions like necrostatin-1 or novel small-molecule inhibitors offer significant potential for clinical translation, not only as acute neuroprotective treatments but also as preventive measures against long-term cognitive deficits. The major pathways related to necroptosis are illustrated in Figure 2.
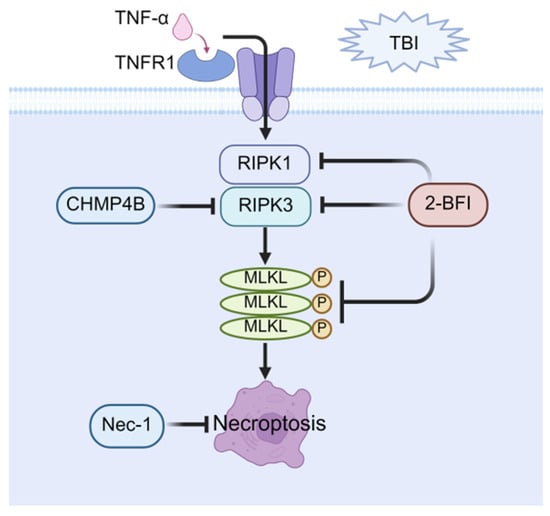
Figure 2.
Therapeutic potential of necroptosis in post-TBI cognitive impairment. Necroptosis is mediated through RIPK1/RIPK3/MLKL activation, resulting in MLKL oligomerization, leading to necroptosis. Key pharmacological interventions targeting this pathway include: direct necroptosis inhibitors, Nec-1; and upstream pathway regulators (2-BFI: suppresses RIPK1/RIPK3/MLKL expression; CHMP4B: blocks RIPK3 activation). These strategies attenuate neuronal death and improve cognitive function after TBI.
6. Molecular Mechanism of Pyroptosis
Pyroptosis is an inflammatory form of cell death hallmarked by rupture of the plasma membrane, cell bubbling, and chromatin condensation [,]. In brief, the pyroptotic signaling cascade comprises two pathways: the canonical pathway mediated by caspase-1 and the non-canonical pathway driven by caspase-4, -5, or -11 []. The canonical pathway is the process by which various pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are detected by cytoplasmic sensor proteins, such as absent in melanoma 2 (AIM2) []. Activated sensor proteins recruit and activate caspase-1 through the caspase recruitment domain (CARD)-containing adaptor apoptosis-associated speck-like protein, thereby assembling the NOD-like receptor family members 3 (NLRP3) inflammasome [,]. Subsequently, activated caspase-1 cleaves gasdermin D (GSDMD) while concurrently driving maturation of IL-1β and IL-18. Cleaved GSDMD liberates the N-terminal pore-forming domain (PFD), which inserts into the plasma membrane, oligomerizes, and forms transmembrane pores that execute pyroptosis []. Conversely, in the noncanonical pathway, bacterial lipopolysaccharide (LPS) directly activates caspase-11 in mice (or caspase-4/5 in humans), which then cleaves GSDMD to perforate the plasma membrane and trigger pyroptosis [].
7. Therapeutic Potential of Pyroptosis in Post-TBI Cognitive Impairment
The association between pyroptosis and post-TBI cognitive impairment was recognized relatively recently. Studies have confirmed the upregulation of NLRP3 inflammasomes and caspase-1 in the cerebral cortex after TBI, even following mild injury [,]. Furthermore, genetic ablation of caspase-1 was shown to suppress pyroptosis and restore cognitive function in murine TBI models []. Building on this observation, subsequent work treated TBI mice with the caspase-1 inhibitor Ac-YVAD-cmk and confirmed that pharmacological blockade of caspase-1 attenuates pyroptosis and promotes cognitive recovery, underscoring the therapeutic potential of targeting caspase-1 for the treatment of post-TBI cognitive impairment []. At the execution level, caspase-1-cleaved GSDMD executes pyroptotic cell lysis. A study has shown that rhein attenuates post-TBI neurological deficits by down-regulating caspase-1 and GSDMD, leading to marked cognitive improvement []. Similarly, more recent research has confirmed that knocking out GSDMD in TBI model mice significantly elevates synaptic protein expression, thereby protecting cognition-related neural circuits and suggesting GSDMD as a promising therapeutic target [].
Moreover, the NLRP3 inflammasome serves as a central regulatory hub within the molecular mechanisms governing pyroptosis. A study developed JC124, a small molecule that specifically targets NLRP3 inflammasomes. The research confirmed that JC124 can effectively inhibit NLRP3 activation and reduce neuronal degeneration and lesion volume in TBI []. This confirms the therapeutic potential of NLRP3-mediated pyroptosis in TBI and prompts multiple interventions targeting NLRP3 to alleviate post-TBI cognitive impairment. First, oridonin, the primary active component of the Chinese herbal medicine Rabdosia rubescens, has been reported to improve cognitive function in TBI mice by inhibiting NLRP3 inflammasome activation []. Similarly, dexmedetomidine has been shown to improve cognitive function in the rat TBI model by inhibiting NLRP3 inflammasome-mediated pyroptosis []. Consistently, several indirect pathways can also achieve similar effects by inhibiting the NLRP3 inflammasome. For example, blocking high mobility group box 1 (HMGB1) can prevent prolonged NLRP3 activation, significantly improving long-term potentiation (LTP) and memory function in TBI mice []. NIMA-related kinase 7 (NEK7) serves as a critical regulator of NLRP3 inflammasome activation, and its downregulation in TBI mice attenuates NLRP3 inflammasome activation, reduces neuronal damage, and ameliorates cognitive dysfunction post-TBI []. Additionally, a recent study has demonstrated that knockdown of galectin-3 inhibits the NLRP3 inflammasome, thereby mitigating pyroptosis and ameliorating neuronal damage and cognitive deficits post-TBI [].
Non-pharmacological interventions also provide therapeutic benefits through modulation of pyroptosis pathways. In one investigation, transcranial pulsed current stimulation (tPCS) promoted orexin-A release, thereby inhibiting NLRP3 inflammasome formation and activation. This suppression reduced ROS production, decreased pyroptosis, and improved cognitive recovery in murine TBI models []. Separately, vagus nerve stimulation (VNS) has been shown to attenuate NLRP3 inflammasome activation and limit the release of cleaved caspase-1, ASC, and GSDMD. Through this modulation of neuroinflammatory cascades, VNS exerts neuroprotective effects and preserves cognitive function in mild TBI models [].
Taken together, the aforementioned studies suggested that targeting key molecules involved in pyroptosis, particularly NLRP3, may represent a highly valuable and potentially therapeutic strategy for ameliorating cognitive dysfunction following TBI. Intervention strategies for pyroptosis are schematized in Figure 3.
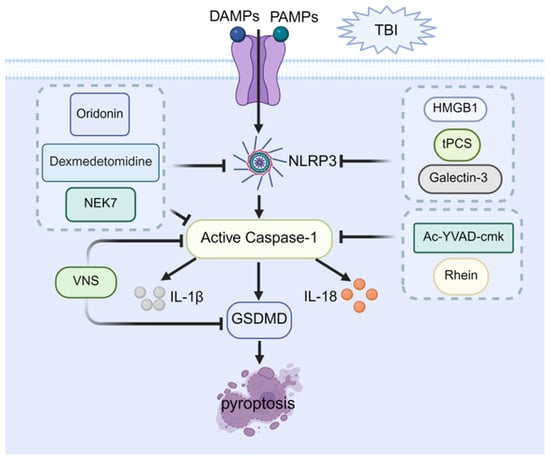
Figure 3.
Therapeutic potential of pyroptosis in post-TBI cognitive impairment. Pyroptosis proceeds through NLRP3 inflammasome assembly and caspase-1 activation, which cleaves gasdermin D (GSDMD) to induce membrane pore formation and promote the maturation and release of IL-1β and IL-18. Pharmacological interventions alleviate neuroinflammation and cognitive impairment through direct targeting of NLRP3 inflammasome inhibition (e.g., by downregulating HMGB1 or galectin-3, and via transcranial Pulsed Current Stimulation (tPCS)); direct targeting of caspase-1 inhibition (using Ac-YVAD-cmk or Rhein); concurrent targeting of both NLRP3 and caspase-1 (via Dexmedetomidine or oridoninor and by downregulating NEK7); and multi-target agents such as Vagus Nerve Stimulation (VNS) (inhibiting NLRP3 and GSDMD).
8. Molecular Mechanism of Ferroptosis
Ferroptosis, a distinct iron-dependent form of regulated cell death first described by Dixon et al., is morphologically characterized by the preservation of plasma membrane integrity, normal nuclear morphology, mitochondrial shrinkage, rupture of the outer mitochondrial membrane, and reduction or loss of mitochondrial cristae []. Ferroptosis is primarily driven by iron overload and disruption of the redox balance []. On the one hand, transferrin (Tf) and transferrin receptor (TfR) form Tf–TfR complexes that facilitate the transport of Fe3+ into cells, where it is reduced to Fe2+. Any disturbance of iron homeostasis or an increase in free Fe2+ induces intracellular iron accumulation []. The accumulated iron, via the Fenton reaction, generates excessive reactive oxygen species (ROS) that trigger ferroptosis []. On the other hand, the SLC7A11/SLC3A2 complex constitutes the cystine (Cys2)-glutamate antiporter system Xc−. This Xc− imports Cys2 to support glutathione (GSH) synthesis, and the GSH-dependent enzyme glutathione peroxidase 4 (GPX4) reduces toxic lipid peroxides to harmless alcohols [,]. Consequently, inhibition of Xc−, depletion of GSH, or suppression of GPX4 activity leads to lipid peroxide accumulation, thereby inducing ferroptosis [].
9. Therapeutic Potential of Ferroptosis in Post-TBI Cognitive Impairment
Since the introduction of ferroptosis, numerous studies have revealed a robust link between iron overload and TBI. Magnetic resonance imaging (MRI) studies have revealed iron deposition in the thalamic region of TBI mice, which is further supported by a significant elevation in serum total iron levels observed as early as day 1 post-TBI [,,]. Notably, ferroptosis can be induced even after mild TBI []. Consistent with these findings, Ji-Yao Jiang et al. reported iron accumulation, reduced GPX4 activity, and elevated ROS levels in TBI mice, with TEM further revealing mitochondrial atrophy—a hallmark of ferroptosis. Crucially, treatment with the ferroptosis-specific inhibitor Fer-1 attenuated iron deposition and neuronal death, while concurrently rescuing cognitive deficits post-TBI []. These findings suggest that ferroptosis serves as a critical driver of post-TBI cognitive impairment, thereby laying a solid foundation for the development of therapeutic strategies.
GPX4 serves as a central regulator of ferroptosis and confers neuroprotection []. Based on a study, the overexpression of GPX4 in the hippocampus of TBI mice reversed ferroptosis and synaptic damage, thereby markedly mitigating cognitive deficits, positioning GPX4 as a promising therapeutic target []. Netrin-1, a secretory laminin implicated in nerve regeneration, has been reported to activate the UNC5B/Nrf2 pathway, thereby upregulating GPX4 expression, suppressing lipid peroxidation, mitigating neuronal loss, and ultimately ameliorating spatial learning and memory impairment in TBI mice []. Furthermore, methyltransferase-like 3 (METTL3) elevated GPX4 levels by enhancing its mRNA stability, thereby suppressing ferroptosis and ameliorating cognitive impairment post-TBI []. Together, these findings consolidate the rationale for targeting GPX4 in cognitive impairment post-TBI therapy.
Certain therapeutic interventions attenuate ferroptosis by suppressing lipid peroxidation and intracellular iron accumulation through the modulation of core regulatory pathways. Specifically, these interventions downregulate TfR-mediated iron uptake, upregulate the SLC7A11 subunit of system Xc−, and enhance GPX4 expression, thereby attenuating neuronal damage and promoting cognitive recovery post-TBI. Anacardic acid (AA) and Fer-1 exert comparable neuroprotective effects against cognitive impairment post-TBI by mitigating ferroptosis through a concerted mechanism involving TfR downregulation and GPX4 upregulation []. Similarly, by downregulating TfR levels, inhibiting lipid peroxidation, and upregulating GPX4 expression, ruxolitinib mitigates neuronal oxidative damage, thereby enhancing spatial learning and memory in TBI mice []. In addition, aminophylline (AMP) markedly improves spatial cognition in TBI mice by downregulating microRNA-128-3p, which enhances SLC7A11 expression and consequently reduces lipid peroxidation []. Likewise, fisetin, a naturally occurring flavonoid, inhibits ferroptosis by elevating the expression of GPX4 and SLC7A11, thereby suppressing lipid peroxidation and iron accumulation, maintaining hippocampal neuronal integrity, and ultimately enhancing spatial memory after TBI []. Beyond pharmacological approaches, non-pharmacological interventions such as lifestyle modifications and physical therapies also have demonstrated considerable therapeutic potential. For example, median nerve stimulation (MNS) has been shown to upregulate GPX4, alleviate oxidative stress, inhibit ferroptosis, and ameliorate cognitive deficits in TBI rats []. Also, a study has demonstrated that moderate-intensity treadmill exercise downregulates TfR expression and upregulates GPX4, rescues ferroptosis, and thereby attenuates cognitive impairment post-TBI []. Furthermore, intermittent fasting has been shown to upregulate GPX4 expression, inhibit ferroptosis, and ameliorate cognitive deficits post-TBI, suggesting another promising therapeutic strategy for similar applications [].
Acyl-CoA synthetase long-chain family members (ACSLs) are central to lipid metabolism and play a critical regulatory role in the process of ferroptosis [,]. Recent studies have demonstrated that modulation of ACSL can ameliorate ferroptosis-related cognitive impairment following TBI. For example, a study reported that prokineticin-2 (Prok2) overexpression attenuates neuronal degeneration and cognitive deficits by inhibiting ferroptosis, a mechanism mediated through the accelerated degradation of ACSL4 and a consequent reduction in lipid peroxidation substrates []. Similarly, a study has demonstrated that the downregulation of tumor necrosis factor-α-induced protein 3 (TNFAIP3) impairs the degradation of ACSL3, thereby suppressing lipid peroxidation and neuronal ferroptosis, which ultimately alleviates cognitive deficits induced by TBI [].
In terms of ferroptosis, these collective findings underscore the therapeutic potential of targeting its pathways to ameliorate post-TBI cognitive deficits. Elucidating these mechanisms further provides a rationale for developing novel TBI therapeutic interventions, as summarized in Figure 4.
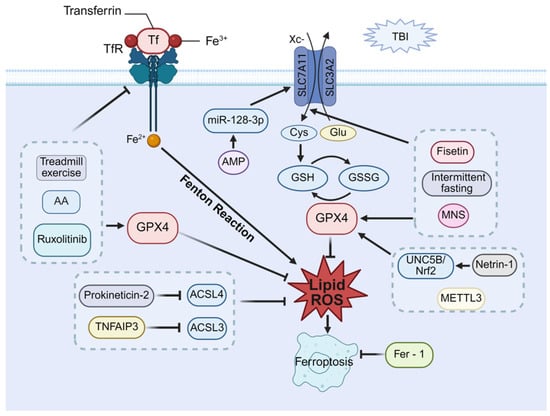
Figure 4.
Therapeutic potential of ferroptosis in post-TBI cognitive impairment. Ferroptosis proceeds through two primary mechanisms: (1) dysregulated iron metabolism via TF/TfR, leading to Fe2+ accumulation and Fenton reaction-mediated lipid peroxidation, and (2) impaired system Xc−-GSH-GPX4 antioxidant axis, resulting in unchecked lipid peroxide accumulation. Therapeutic strategies to inhibit ferroptosis and improve cognitive function include modulation of iron metabolism (via moderate intensity of treadmill exercise, asiatic acid (AA), or Ruxolitinib, which inhibit transferrin receptor and upregulate GPX4); activation of system Xc− (via AMP through the miR-128-3p/SLC7A11 axis, intermittent fasting and median nerve stimulation (MNS), or Fisetin by upregulating SLC7A11 and GPX4); enhancement of the GPX4 axis (via Netrin-1 activating the UNC5B/Nrf2/GPX4 pathway or METTL3); and suppression of lipid peroxidation (via Prokineticin-2 and TNFAIP3, which inhibit ACSL4/3 to reduce lipid peroxides).
10. Molecular Mechanism of Cuproptosis
In 2022, Tsvetkov et al first proposed a copper-dependent mode of cell death, coining the term cuproptosis and describing its cytomorphology by mitochondrial condensation, rupture of the plasma membrane, endoplasmic reticulum damage, and chromatin condensation []. Notably, cuproptosis is not blocked by inhibitors of other known RCD, underscoring its mechanistic uniqueness. In essence, the disruption of copper homeostasis elevates intracellular copper levels, which initiates cuproptosis. In this process, copper carriers facilitate the import of Cu2+ into mitochondria, and this Cu2+ is then reduced to Cu+ by ferredoxin-1 (FDX1) []. Critically, the elevated Cu+ directly targets the acyl-carboxylase subunits dihydrolipoamide S-acetyltransferase (DLAT) and dihydrolipoamide S-succinyltransferase (DLST) within the tricarboxylic acid (TCA) cycle. This targeting promotes their oligomerization into insoluble aggregates, thereby disrupting normal TCA cycle metabolism. At the same time, excess copper destabilizes and degrades Fe-S cluster proteins []. The combined insult, TCA-cycle collapse plus Fe-S loss, generates proteotoxic stress that upregulates heat shock protein 70 (HSP70) and triggers cuproptosis [].
11. Therapeutic Potential of Cuproptosis in Post-TBI Cognitive Impairment
Even before the term cuproptosis was introduced, numerous studies had already reported a correlation between copper overload and mild TBI []. Initially, two studies by Portbury et al. confirmed increased copper concentrations in the ipsilateral cerebral cortex of TBI mouse models. This increase was specifically observed in the region adjacent to the impact zone [,]. Furthermore, a separate study using PET/CT imaging assessed copper ion uptake and demonstrated significantly higher cortical copper levels in the TBI-injured group []. These findings substantiate the phenomenon of copper overload following TBI. However, cuproptosis is a newly recognized pathway, so data on its role in post-TBI cognitive impairment remain scarce and fragmentary. A study demonstrated that elevated copper in the brains of copper-exposed mice triggers a canonical cuproptosis signature. Excess copper further impaired synaptic function, which resulted in significant cognitive dysfunction. Conversely, the copper-selective chelator Bathocuproinedisulfonic acid (BCS) alleviated cuproptosis, underscoring cuproptosis as one of the key drivers of TBI-associated cognitive decline []. As the role of cuproptosis in post-TBI cognitive impairment becomes increasingly elucidated (Figure 5), there is hope for utilizing mechanisms of cuproptosis to treat memory and learning deficits, albeit further exploration remains warranted.
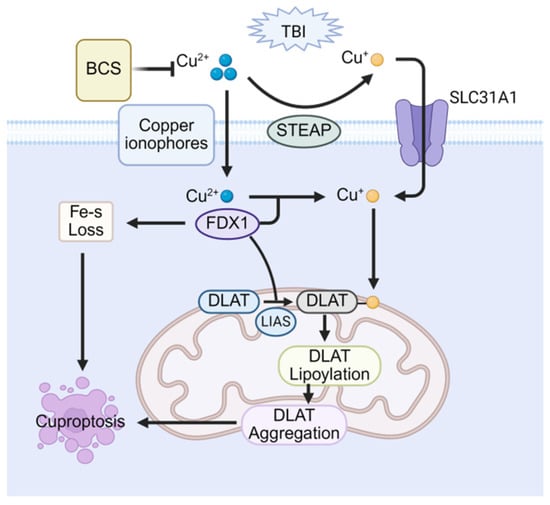
Figure 5.
Therapeutic potential of cuproptosis in post-TBI cognitive impairment. Copper overload triggers cuproptosis via mitochondrial FDX1-mediated reduction of Cu2+ to Cu+, inducing lipoylated protein (e.g., DLAT) aggregation and Fe–S cluster protein loss. The copper chelator BCS attenuates cognitive impairment by sequestering excess copper and inhibiting this process.
12. Interaction Between RCDs in Post-TBI Cognitive Impairment
TBI activates multiple RCD pathways, which do not function in isolation but instead form a highly interdependent regulatory network. Post-TBI insults, such as glutamate excitotoxicity, oxidative stress, and mitochondrial dysfunction, simultaneously engage multiple RCD mechanisms, amplifying neuronal loss and cognitive decline []. For example, oxidative stress induced by glutamate excitotoxicity activates cylindromatosis, which promotes necroptosis through RIPK1/RIPK3 complex formation while also enhancing mitochondrial AIF release to induce apoptosis []. In addition, iron accumulation, exacerbated by oxidative stress, triggers ferroptosis by decreasing GSH and GPX4 activity and generating ROS via the Fenton reaction. This oxidative burst activates the NLRP3 inflammasome, triggering pyroptosis and amplifying apoptosis through caspase-3 []. These findings establish a crucial role for synergistic interactions among RCD pathways in post-TBI cognitive impairment. Notably, key molecules within individual RCD pathways exhibit cross-pathway regulatory capacity. For instance, RIPK3, a central mediator of necroptosis in post-TBI cognitive impairment, not only facilitates necroptosis but also promotes apoptosis through caspase-8 and caspase-3 activation []. Furthermore, RIPK3 deficiency has been shown to redirect cell death signaling from pyroptosis toward ferroptosis through the 15-Lipoxygenase/phosphatidylethanolamine-binding protein 1 (15LOX/PEBP1) complex, highlighting the intricate crosstalk among RCD modalities [].
Importantly, these RCD pathways exhibit distinct temporal dynamics following TBI. In the acute phase, ferroptosis predominates, characterized by rapid iron accumulation and lipid peroxidation. In contrast, apoptosis and pyroptosis markers, such as caspase-3 and NLRP3, show delayed activation, suggesting that ROS from iron-induced ferroptosis trigger subsequent RCD pathways []. During the chronic phase, necroptosis becomes dominant, with sustained ROS production from hemorrhage-derived iron accumulation perpetuating RIPK1/RIPK3 activation. Critically, genetic deletion of RIPK1/RIPK3 significantly reduced chronic lesion expansion by approximately 80%, underscoring the key role of necroptosis in chronic pathology [].
The complexity and temporal dynamics of the post-TBI RCD network highlight the limitations of single-target therapies and underscore the need for multi-pathway, phase-adjusted interventions. Necrostatin-1, previously established as a necroptosis inhibitor, has also been shown to mitigate apoptosis in the context of post-TBI cognitive impairment []. Similarly, angiotensin-converting enzyme 2 (ACE2) has been identified as a key cross-regulatory node linking the renin-angiotensin system and the RCD network. Acute administration of recombinant human ACE2 curbed ferroptotic cascade amplification, whereas chronic delivery of the ACE2 activator diethylisopropylamine (DIZE) sustained ACE2 activity while concurrently blocking necroptosis, apoptosis, and inflammation-driven RCD amplification, exemplifying a phased, multi-pathway therapeutic strategy [].
Overall, future TBI treatment strategies should emphasize multi-target and phase-specific interventions. By precisely targeting dominant RCD pathways at different disease stages, such approaches may achieve comprehensive regulation of the RCD network, thereby more effectively improving cognitive impairment after TBI.
13. Challenges and Prospects
Targeting RCD pathways represents a promising therapeutic strategy for cognitive impairment following TBI. However, its clinical translation faces several critical barriers. A primary challenge lies in the limited translational validity of preclinical models. While animal studies typically employ standardized injuries with single mechanisms, human TBI involves complex secondary pathologies such as hemorrhage, inflammatory cascades, and multi-organ damage. This substantial heterogeneity impedes the clinical replication of neuroprotective effects observed with RCD inhibitors in animal studies []. Furthermore, the blood–brain barrier (BBB) constitutes a major obstacle by restricting brain delivery of most RCD-targeting therapeutics. The dynamic evolution of BBB permeability during TBI progression—from acute disruption that facilitates drug entry to subsequent repair that restricts access—further complicates drug pharmacokinetics and challenges the development of sustained-release formulations [].
To address these limitations, nanocarrier systems offer promising solutions through two complementary strategies. The first employs multitargeting nanoparticles to concurrently address several injury mechanisms, providing broader therapeutic efficacy than conventional drugs with single mechanisms. The second utilizes adaptive nanoparticles that respond to BBB changes, ensuring effective drug delivery during both acute and recovery phases of TBI to maintain therapeutic concentrations in the brain []. These integrated strategies align effectively with the complex pathophysiology of human TBI and establish a solid foundation for translating RCD-targeted therapies into clinical practice.
Human TBI research also confronts distinct challenges in clinical translation. Considerable patient heterogeneity in age, injury mechanisms, and severity leads to diverse RCD activation profiles, complicating the development of standardized treatments. Cognitive assessment is further constrained by the inherent subjectivity of conventional scales and the prolonged interval between initial pathology and observable symptomatic decline []. To overcome these issues, future research should increasingly integrate network pharmacology and artificial intelligence (AI). Network pharmacology was employed to identify potential therapeutic targets of Danshen-Chuanxiong-Honghua for cognitive impairment following TBI []. AI-driven analytics can identify patient subgroups with heightened responsiveness to specific RCD inhibitors—for instance, apolipoprotein E4 carriers or patients with elevated necroptosis activity—thereby advancing stratified precision medicine []. Additionally, machine learning models can dynamically correlate evolving RCD biomarker patterns with long-term cognitive outcomes [].
Looking forward, integrated strategies combining nanomaterial-based delivery platforms, network pharmacology-enabled multitarget screening, and AI-guided patient stratification offer considerable promise for overcoming existing translational barriers in TBI-related cognitive impairment.
14. Conclusions
Cognitive impairment following TBI substantially diminishes patients’ quality of life, and the limited efficacy of existing treatments underscores the need for novel therapeutic strategies. This review systematically outlines key targets and intervention approaches across major RCD pathways, apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis (Table 1). While targeting the interconnected RCD network holds transformative potential, clinical translation faces challenges, including species differences, drug delivery limitations, and assessment standardization. Future research should focus on three pivotal directions: nanotechnology-enhanced drug delivery, network pharmacology for multi-target screening, and AI-driven precision medicine. Integrating RCD mechanism insights with innovative bioengineering approaches will be crucial for developing effective therapies to alleviate post-TBI cognitive impairment.

Table 1.
RCD in the post-TBI cognitive impairment model.
Author Contributions
Y.X. and M.L. contributed equally to this article. They drafted the original manuscript and prepared the figures. Z.C., M.F., Q.P., Y.T., X.L. and P.D. conducted the literature search and contributed to the writing of the paper. J.L. proposed the research design and contributed to the writing of the paper. Additionally, J.L. provided professional advice and revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82401681), the Natural Science Foundation of Hubei Province (Grant No. 2023AFB476), and the Wuhan Natural Science Foundation (Dawn Plan Program, No. 2024040801020367).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Biorender (https://app.biorender.com/ (accessed on 17 November 2025)) for expert assistance in the pattern drawing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Fieggen, G.; Arraez, M.A.; Servadei, F.; Boop, F.A.; Johnson, W.D.; Warf, B.C.; Park, K.B. Global neurosurgery: The current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J. Neurosurg. 2019, 130, 1055–1064. [Google Scholar] [CrossRef]
- Lin, C.-T.; Lecca, D.; Yang, L.-Y.; Luo, W.; Scerba, M.T.; Tweedie, D.; Huang, P.-S.; Jung, Y.-J.; Kim, D.S.; Yang, C.-H.; et al. 3,6’-dithiopomalidomide reduces neural loss, inflammation, behavioral deficits in brain injury and microglial activation. eLife 2020, 9, e54726. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 484040. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.M.; Wu, J.; Faden, A.I.; Sarkar, C. Function and Mechanisms of Autophagy in Brain and Spinal Cord Trauma. Antioxid. Redox Signal. 2015, 23, 565–577. [Google Scholar] [CrossRef]
- Bakaeva, Z.; Goncharov, M.; Krasilnikova, I.; Zgodova, A.; Frolov, D.; Grebenik, E.; Timashev, P.; Pinelis, V.; Surin, A. Acute and Delayed Effects of Mechanical Injury on Calcium Homeostasis and Mitochondrial Potential of Primary Neuroglial Cell Culture: Potential Causal Contributions to Post-Traumatic Syndrome. Int. J. Mol. Sci. 2022, 23, 3858. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liu, Z.; Zhou, J.; Ke, C.; Li, D. Significance of Programmed Cell Death Pathways in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 9947. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics 2020, 17, 446–456. [Google Scholar] [CrossRef]
- Lai, J.-Q.; Shi, Y.-C.; Lin, S.; Chen, X.-R. Metabolic disorders on cognitive dysfunction after traumatic brain injury. Trends Endocrinol. Metab. 2022, 33, 451–462. [Google Scholar] [CrossRef]
- Latella, D.; Maggio, M.G.; De Luca, R.; Maresca, G.; Piazzitta, D.; Sciarrone, F.; Carioti, L.; Manuli, A.; Bramanti, P.; Calabro, R.S. Changes in sexual functioning following traumatic brain injury: An overview on a neglected issue. J. Clin. Neurosci. 2018, 58, 1–6. [Google Scholar] [CrossRef]
- Lucke-Wold, B.; Zasler, N.D.; Ruchika, F.N.U.; Weisman, S.; Le, D.; Brunicardi, J.; Kong, I.; Ghumman, H.; Persad, S.; Mahan, D.; et al. Supplement and nutraceutical therapy in traumatic brain injury. Nutr. Neurosci. 2024, 28, 709–743. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2021, 29, 272–284. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Puthalakath, H.; Strasser, A. Keeping killers on a tight leash: Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002, 9, 505–512. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Serial killers: Ordering caspase activation events in apoptosis. Cell Death Differ. 1999, 6, 1067–1074. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Graham, S.H.; Du, L.; Kochanek, P.M.; Draviam, R.; Guo, F.; Nathaniel, P.D.; Szabó, C.; Watkins, S.C.; et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 2002, 82, 181–191. [Google Scholar] [CrossRef]
- Boldin, M.P.; Goncharov, T.M.; Goltseve, Y.V.; Wallach, D. Involvement of MACH, a Novel MORT1/FADD-Interacting Protease, in Fas/APO-1- and TNF Receptor–Induced Cell Death. Cell 1996, 85, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Kischkel, F.C.; Lawrence, D.A.; Tinel, A.; LeBlanc, H.; Virmani, A.; Schow, P.; Gazdar, A.; Blenis, J.; Arnott, D.; Ashkenazi, A. Death Receptor Recruitment of Endogenous Caspase-10 and Apoptosis Initiation in the Absence of Caspase-8. J. Biol. Chem. 2001, 276, 46639–46646. [Google Scholar] [CrossRef]
- Green, D.R. Apoptotic Pathways: Paper Wraps Minireview Stone Blunts Scissors. Cell 2000, 102, 1–4. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Knoblach, S.M.; Fan, L.; Fox, G.B.; Goodnight, R.; Faden, A.I. Activation of CPP32-Like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 1997, 17, 7415–7424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, P.; Wei, W.; Jiang, W. Apoptosis in cerebrospinal fluid as outcome predictors in severe traumatic brain injury. Medicine 2020, 99, e20922. [Google Scholar] [CrossRef]
- Luo, Y.; Zou, H.; Wu, Y.; Cai, F.; Zhang, S.; Song, W. Mild traumatic brain injury induces memory deficits with alteration of gene expression profile. Sci. Rep. 2017, 7, 10846. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Kochanek, P.M.; Watkins, S.C.; Chen, M.; Dixon, C.E.; Seidberg, N.A.; Melick, J.; Loeffert, J.E.; Nathaniel, P.D.; Jin, K.L.; et al. Caspase-3 Mediated Neuronal Death After Traumatic Brain Injury in Rats. J. Neurochem. 2001, 74, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of Poly(ADP-Ribose) Polymerase-1-Dependent Cell Death by Apoptosis-Inducing Factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Vagni, V.A.; Nathaniel, P.D.; Jenkins, L.W.; Dixon, C.E.; Szabó, C. Local Administration of the Poly(ADP-Ribose) Polymerase Inhibitor INO-1001 Prevents NAD+ Depletion and Improves Water Maze Performance after Traumatic Brain Injury in Mice. J. Neurotrauma 2007, 24, 1399–1405. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Ramos-Gómez, L.; Solé-Violán, J.; Cáceres, J.J.; Ferrer-Moure, C.; Jiménez, A. Low blood caspase-8 levels in survivor patients of traumatic brain injury. Neurol. Sci. 2021, 42, 5065–5070. [Google Scholar] [CrossRef]
- Krajewska, M.; You, Z.; Rong, J.; Kress, C.; Huang, X.; Yang, J.; Kyoda, T.; Leyva, R.; Banares, S.; Hu, Y.; et al. Neuronal Deletion of Caspase 8 Protects against Brain Injury in Mouse Models of Controlled Cortical Impact and Kainic Acid-Induced Excitotoxicity. PLoS ONE 2011, 6, e24341. [Google Scholar] [CrossRef] [PubMed]
- Almikhlafi, M.A.; Abdallah, N.A.; Kumar, A.; Sharma, T.; Khan, Z.; Fadil, H.A.; Althagfan, S.; Aljohani, A.K.B.; Almadani, S.A.; Miski, S.F.; et al. Exploring Azithromycin’s Neuroprotective Role in Traumatic Brain Injury: Insights into Cognitive and Motor Recovery and Neuroinflammatory Modulation. Pharmaceuticals 2025, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khan, A.; Rehman, I.U.; Lee, H.J.; Khan, I.; Kim, M.O. Lupeol Treatment Attenuates Activation of Glial Cells and Oxidative-Stress-Mediated Neuropathology in Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 6086. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.; Kong, W.; Li, T.; Zou, P.; Chen, H. Edaravone attenuates neuronal apoptosis in hippocampus of rat traumatic brain injury model via activation of BDNF/TrkB signaling pathway. Arch. Med. Sci. 2021, 17, 514–522. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Greig, N.H.; Huang, Y.-N.; Hsieh, T.-H.; Tweedie, D.; Yu, Q.-S.; Hoffer, B.J.; Luo, Y.; Kao, Y.-C.; Wang, J.-Y. Post-traumatic administration of the p53 inactivator pifithrin-α oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury. Neurobiol. Dis. 2016, 96, 216–226. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Wang, L.; Li, Z.; Tang, S.; Wang, Y.; Gu, N.; Sun, X.; Li, L. TREM2 activation alleviates neural damage via Akt/CREB/BDNF signalling after traumatic brain injury in mice. J. Neuroinflamm. 2022, 19, 289. [Google Scholar] [CrossRef]
- Qu, W.; Liu, N.-K.; Wu, X.; Wang, Y.; Xia, Y.; Sun, Y.; Lai, Y.; Li, R.; Shekhar, A.; Xu, X.-M. Disrupting nNOS–PSD95 Interaction Improves Neurological and Cognitive Recoveries after Traumatic Brain Injury. Cereb. Cortex 2020, 30, 3859–3871. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; She, F.; Zhang, L.; Kim, G.; Li, R.; Zheng, X.; Wang, Z.; Chen, R.; Wang, L.; Chen, D.; et al. Zipper-interacting protein kinase mediates neuronal cell death and cognitive dysfunction in traumatic brain injury via regulating DEDD. Cell Death Dis. 2025, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, T.-Z.; Wang, L.-N.; Wang, J.-J.; Tu, Y.; Zhao, M.-L.; Zhang, S.; Sun, H.-T.; Li, X.-H. Mild hypothermia facilitates the long-term survival of newborn cells in the dentate gyrus after traumatic brain injury by diminishing a pro-apoptotic microenvironment. Neuroscience 2016, 335, 114–121. [Google Scholar] [CrossRef]
- Sakas, R.; Dan, K.; Edelman, D.; Abu-Ata, S.; Ben-Menashe, A.; Awad-Igbaria, Y.; Francois-Soustiel, J.; Palzur, E. Hyperbaric Oxygen Therapy Alleviates Memory and Motor Impairments Following Traumatic Brain Injury via the Modulation of Mitochondrial-Dysfunction-Induced Neuronal Apoptosis in Rats. Antioxidants 2023, 12, 2034. [Google Scholar] [CrossRef]
- Han, R.-Z.; Hu, J.-J.; Weng, Y.-C.; Li, D.-F.; Huang, Y. NMDA receptor antagonist MK-801 reduces neuronal damage and preserves learning and memory in a rat model of traumatic brain injury. Neurosci. Bull. 2009, 25, 367–375. [Google Scholar] [CrossRef]
- Yurkewicz, L.; Weaver, J.; Bullock, M.R.; Marshall, L.F. The Effect of the Selective NMDA Receptor Antagonist Traxoprodil in the Treatment of Traumatic Brain Injury. J. Neurotrauma 2005, 22, 1428–1443. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Qu, C.S.; Goussev, A.; Jiang, H.; Lu, C.; Schallert, T.; Mahmood, A.; Chen, J.L.; Li, Y.; Chopp, M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma 2007, 24, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aguilar, M.; Tapia-Pérez, J.H.; Sánchez-Rodríguez, J.J.; Viñas-Ríos, J.M.; Martínez-Pérez, P.; de la Cruz-Mendoza, E.; Sánchez-Reyna, M.; Torres-Corzo, J.G.; Gordillo-Moscoso, A. Effect of rosuvastatin on cytokines after traumatic head injury. J. Neurosurg. 2013, 118, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Wei, J.; Yan, W.; Wang, W.; Lu, Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit. Care 2008, 12, R61. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like Receptor 3-mediated Necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.-S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kumari, S.; Kim, C.; Van, T.-M.; Wachsmuth, L.; Polykratis, A.; Pasparakis, M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 2016, 540, 124–128. [Google Scholar] [CrossRef]
- Newton, K.; Wickliffe, K.E.; Maltzman, A.; Dugger, D.L.; Strasser, A.; Pham, V.C.; Lill, J.R.; Roose-Girma, M.; Warming, S.; Solon, M.; et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 2016, 540, 129–133. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Savitz, S.I.; Yang, J.; Degterev, A.; Yuan, J.; Cuny, G.D.; Moskowitz, M.A.; Whalen, M.J. Necrostatin-1 Reduces Histopathology and Improves Functional Outcome after Controlled Cortical Impact in Mice. J. Cereb. Blood Flow Metab. 2008, 28, 1564–1573. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Chen, Q.-X.; Chen, Z.-B.; Tian, D.-F.; Li, M.-C.; Wang, J.-M.; Wang, L.; Liu, B.-H.; Zhang, S.-Q.; Li, F.; et al. RIP3 deficiency protects against traumatic brain injury (TBI) through suppressing oxidative stress, inflammation and apoptosis: Dependent on AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 499, 112–119. [Google Scholar] [CrossRef]
- Wehn, A.C.; Khalin, I.; Duering, M.; Hellal, F.; Culmsee, C.; Vandenabeele, P.; Plesnila, N.; Terpolilli, N.A. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol. Commun. 2021, 9, 138. [Google Scholar] [CrossRef]
- Ni, H.; Rui, Q.; Lin, X.; Li, D.; Liu, H.; Chen, G. 2-BFI Provides Neuroprotection Against Inflammation and Necroptosis in a Rat Model of Traumatic Brain Injury. Front. Neurosci. 2019, 13, 674. [Google Scholar] [CrossRef]
- Zhao, P.; Li, C.; Chen, B.; Sun, G.; Chao, H.; Tu, Y.; Bao, Z.; Fan, L.; Du, X.; Ji, J. Up-regulation of CHMP4B alleviates microglial necroptosis induced by traumatic brain injury. J. Cell. Mol. Med. 2020, 24, 8466–8479. [Google Scholar] [CrossRef]
- Bao, Z.; Fan, L.; Zhao, L.; Xu, X.; Liu, Y.; Chao, H.; Liu, N.; You, Y.; Liu, Y.; Wang, X.; et al. Silencing of A20 Aggravates Neuronal Death and Inflammation After Traumatic Brain Injury: A Potential Trigger of Necroptosis. Front. Mol. Neurosci. 2019, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.H.; Ellis, L.Z.; Fujita, M. Inflammasomes as molecular mediators of inflammation and cancer: Potential role in melanoma. Cancer Lett. 2012, 314, 24–33. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Liu, H.-D.; Li, W.; Chen, Z.-R.; Hu, Y.-C.; Zhang, D.-D.; Shen, W.; Zhou, M.-L.; Zhu, L.; Hang, C.-H. Expression of the NLRP3 Inflammasome in Cerebral Cortex After Traumatic Brain Injury in a Rat Model. Neurochem. Res. 2013, 38, 2072–2083. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Liu, C.; Li, S.; Yang, Z.; Zhang, F.; Chen, X.; Shan, H.; Tao, L.; Zhang, M. Surface-fill H2S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 2022, 154, 259–274. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Meng, J.; Wu, M.; Bi, F.; Chang, C.; Li, H.; Zhang, L. Ablation of caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo. J. Neuroinflamm. 2018, 15, 48. [Google Scholar] [CrossRef]
- Ge, X.; Li, W.; Huang, S.; Yin, Z.; Xu, X.; Chen, F.; Kong, X.; Wang, H.; Zhang, J.; Lei, P. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res. 2018, 1697, 10–20. [Google Scholar] [CrossRef]
- Bi, F.; Ma, H.; Ji, C.; Chang, C.; Liu, W.; Xie, K. Rhein Protects Against Neurological Deficits After Traumatic Brain Injury in Mice via Inhibiting Neuronal Pyroptosis. Front. Pharmacol. 2020, 11, 564367. [Google Scholar] [CrossRef]
- Du, H.; Li, C.-H.; Gao, R.-B.; Cen, X.-Q.; Li, P. Ablation of GSDMD Attenuates Neurological Deficits and Neuropathological Alterations After Traumatic Brain Injury. Front. Cell. Neurosci. 2022, 16, 915969. [Google Scholar] [CrossRef] [PubMed]
- Kuwar, R.; Rolfe, A.; Di, L.; Xu, H.; He, L.; Jiang, Y.; Zhang, S.; Sun, D. A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J. Neuroinflamm. 2019, 16, 81. [Google Scholar] [CrossRef]
- Yan, C.; Yan, H.; Mao, J.; Liu, Y.; Xu, L.; Zhao, H.; Shen, J.; Cao, Y.; Gao, Y.; Li, K.; et al. Neuroprotective Effect of Oridonin on Traumatic Brain Injury via Inhibiting NLRP3 Inflammasome in Experimental Mice. Front. Neurosci. 2020, 14, 557170. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, S.; Ying, Y.; Guo, X.; Li, H.; Xu, L.; Ruan, X. Administration of Dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci. Rep. 2018, 38, BSR20180892. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-W.; Zhao, Y.; Li, P.; Ning, Y.-L.; Huang, Z.-Z.; Yang, N.; Liu, D.; Zhou, Y.-G. HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J. Neuroinflamm. 2021, 18, 241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Bi, F.; Li, H.; Chang, C.; Ji, C.; Liu, W. NEK7 Regulates NLRP3 Inflammasome Activation and Neuroinflammation Post-traumatic Brain Injury. Front. Mol. Neurosci. 2019, 12, 202. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.-Q.; Wang, X.; Li, T.; Han, Y.-L.; Miao, S.-H.; Zhao, R.; Zheng, X.-B.; Qiu, J.-Y.; Jin, W.-X.; et al. Galectin-3 activates microglia and promotes neurological impairment via NLRP3/pyroptosis pathway following traumatic brain injury. Brain Res. 2025, 1855, 149560. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, Q.; Ren, B.; Yang, L.; Bai, Y.; Feng, Z. Transcranial pulsed current stimulation alleviates neuronal pyroptosis and neurological dysfunction following traumatic brain injury via the orexin-A/NLRP3 pathway. Neuropeptides 2025, 110, 102501. [Google Scholar] [CrossRef]
- Ren, B.; Kang, J.; Dong, X.; Huang, L.; Wu, X.; Tang, Y. Vagus Nerve Stimulation Attenuates Cognitive Impairment in Traumatic Brain Injury via the mtDNA/cGAS-STING/NLRP3 Inflammasome Axis. Neurocrit. Care 2025, 1–15. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Conrad, M.; Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2023, 25, 133–155. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2013, 14, 102–108. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Onyszchuk, G.; LeVine, S.M.; Brooks, W.M.; Berman, N.E.J. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: A magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci. Lett. 2009, 452, 204–208. [Google Scholar] [CrossRef]
- Portbury, S.D.; Hare, D.J.; Sgambelloni, C.; Finkelstein, D.I.; Adlard, P.A. A time-course analysis of changes in cerebral metal levels following a controlled cortical impact. Metallomics 2016, 8, 193–200. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, B.; Li, J.; Li, Q.; Luo, C. The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants 2024, 13, 395. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Ge, X.; Yin, Z.; Li, M.; Guo, M.; Hu, T.; Han, Z.; Kong, X.; Li, D.; et al. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J. Neuroinflamm. 2022, 19, 185. [Google Scholar] [CrossRef]
- Xie, B.S.; Wang, Y.Q.; Lin, Y.; Mao, Q.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2018, 25, 465–475. [Google Scholar] [CrossRef]
- Gao, S.-Q.; Liu, J.-Q.; Han, Y.-L.; Deji, Q.-Z.; Zhaba, W.-D.; Deng, H.-J.; Liu, X.-L.; Zhou, M.-L. Neuroprotective role of glutathione peroxidase 4 in experimental subarachnoid hemorrhage models. Life Sci. 2020, 257, 118050. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yuan, Q.; Du, Z.; Zhang, Q.; Yang, L.; Wang, M.; Yang, W.; Yuan, C.; Yu, J.; Wu, G.; et al. Overexpression of GPX4 attenuates cognitive dysfunction through inhibiting hippocampus ferroptosis and neuroinflammation after traumatic brain injury. Free Radic. Biol. Med. 2023, 204, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, J.; Zhao, D.; Ruan, C.; Zhou, J.; Tan, H.; Bao, Y. Netrin-1 upregulates GPX4 and prevents ferroptosis after traumatic brain injury via the UNC5B/Nrf2 signaling pathway. CNS Neurosci. Ther. 2022, 29, 216–227. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Zhang, S.; Zhao, W.; Li, F.; Qin, F. M6A Methyltransferase METTL3 Modulates Traumatic Brain Injury by Targeting Ferroptosis. Front. Biosci.-Landmark 2025, 30, 31304. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, J.; Ma, Y.; Li, J.; Ji, H.; Chen, Z.; Zheng, J. Anacardic acid improves neurological deficits in traumatic brain injury by anti-ferroptosis and anti-inflammation. Exp. Neurol. 2023, 370, 114568. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Yan, Y.N.; Cheng, Z.; Chen, G.; Rui, T.; Luo, C.; Gao, Y.; Wang, T.; Chen, X.; et al. Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Exp. Neurol. 2021, 342, 113762. [Google Scholar] [CrossRef]
- Manrui, L.; Xu, Y.; Liu, J.; Zhang, X.; Yuan, R.; Sun, Y.; Sun, Y.; Yang, Q.; Liao, M.; Lv, M.; et al. Aminophylline targets miR-128-3p/Slc7a11 axis to attenuate neuronal ferroptosis after traumatic brain injury. Cell. Mol. Life Sci. 2025, 82, 87. [Google Scholar] [CrossRef]
- Yang, H.; Hong, Y.; Gong, M.; Cai, S.; Yuan, Z.; Feng, S.; Chen, Q.; Liu, X.; Mei, Z. Fisetin exerts neuroprotective effects in vivo and in vitro by inhibiting ferroptosis and oxidative stress after traumatic brain injury. Front. Pharmacol. 2024, 15, 1480345. [Google Scholar] [CrossRef]
- Zhong, Y.-J.; Liu, L.-L.; Zhao, Y.; Feng, Z.; Liu, Y. Elucidating the molecular mechanisms behind the therapeutic impact of median nerve stimulation on cognitive dysfunction post-traumatic brain injury. Exp. Gerontol. 2024, 194, 112500. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, T.; Yu, D.; Yan, B.; Zhang, Y.; Jin, J.; Yang, Z.; Zhang, B.; Hao, X.; Chen, Z.; et al. Moderate Intensity of Treadmill Exercise Rescues TBI-Induced Ferroptosis, Neurodegeneration, and Cognitive Impairments via Suppressing STING Pathway. Mol. Neurobiol. 2023, 60, 4872–4896. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Liu, J.; Zhang, L.; Yuan, R.; Xu, Y.; Zheng, J.; Cao, S.; Dai, H.; Liao, M.; et al. Intermittent fasting ameliorates neuronal ferroptosis and cognitive impairment in mice after traumatic brain injury. Nutrition 2023, 109, 111992. [Google Scholar] [CrossRef]
- Lin, J.; Lai, Y.; Lu, F.; Wang, W. Targeting ACSLs to modulate ferroptosis and cancer immunity. Trends Endocrinol. Metab. 2025, 36, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Liu, Y.; Chen, B.; Miao, Z.; Tu, Y.; Li, C.; Chao, H.; Ye, Y.; Xu, X.; Sun, G.; et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat. Commun. 2021, 12, 4220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, L.; Yang, J.; Mansuer, M.; Deng, X.; Wang, Y.; Ren, H.; Cui, D.; Jiang, Y.; Gao, L. TNFAIP3 affects ferroptosis after traumatic brain injury by affecting the deubiquitination and ubiquitination pathways of the HMOX1 protein and ACSL3. Free Radic. Biol. Med. 2025, 228, 221–239. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef]
- Qi, X. The potential value of cuprotosis (copper-induced cell death) in the therapy of clear cell renal cell carcinoma. Am. J. Cancer Res. 2022, 12, 3947–3966. [Google Scholar] [CrossRef]
- Juan, S.M.A.; Daglas, M.; Gunn, A.P.; Lago, L.; Adlard, P.A. Characterization of the spatial distribution of metals and profile of metalloprotein complexes in a mouse model of repetitive mild traumatic brain injury. Metallomics 2022, 14, mfac092. [Google Scholar] [CrossRef]
- Portbury, S.D.; Hare, D.J.; Sgambelloni, C.J.; Bishop, D.P.; Finkelstein, D.I.; Doble, P.A.; Adlard, P.A. Age modulates the injury-induced metallomic profile in the brain. Metallomics 2017, 9, 402–410. [Google Scholar] [CrossRef]
- Peng, F.; Muzik, O.; Gatson, J.; Kernie, S.G.; Diaz-Arrastia, R. Assessment of Traumatic Brain Injury by Increased 64Cu Uptake on 64CuCl2 PET/CT. J. Nucl. Med. 2015, 56, 1252–1257. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Lu, L.; Su, Y.; Shi, W.; Zhang, H.; Liu, R.; Pu, Y.; Yin, L. Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling. Nutrients 2023, 15, 972. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Liu, M.; Luo, J.; Zhang, X.; Dai, Z.; Zhang, B.; Chen, H.; Xue, J.; He, M.; Xu, H.; et al. Exosomes derived from bone marrow mesenchymal stem cells attenuate neurological damage in traumatic brain injury by alleviating glutamate-mediated excitotoxicity. Exp. Neurol. 2022, 357, 114182. [Google Scholar] [CrossRef] [PubMed]
- Ganjam, G.K.; Terpolilli, N.A.; Diemert, S.; Eisenbach, I.; Hoffmann, L.; Reuther, C.; Herden, C.; Roth, J.; Plesnila, N.; Culmsee, C. Cylindromatosis mediates neuronal cell death in vitro and in vivo. Cell Death Differ. 2018, 25, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Pang, Q.; Huang, R.; Xu, H.; Guo, H.; Gao, C.; Chen, X.; Wang, Y.; Cao, Q.; Gao, Y.; et al. Stress-mediated Activation of Ferroptosis, Pyroptosis, and Apoptosis Following Mild Traumatic Brain Injury Exacerbates Neurological Dysfunctions. Mol. Neurobiol. 2024, 62, 4055–4075. [Google Scholar] [CrossRef]
- Lamade, A.M.; Wu, L.; Dar, H.H.; Mentrup, H.L.; Shrivastava, I.H.; Epperly, M.W.; St Croix, C.M.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Yang, Q.; et al. Inactivation of RIP3 kinase sensitizes to 15LOX/PEBP1-mediated ferroptotic death. Redox Biol. 2022, 50, 102232. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Wang, X.P.; Hu, Y.F.; Li, W.G.; Zhou, Q.; Xu, F.; Wang, Q.J. Propofol Suppresses Ferroptosis via Modulating eNOS/NO Signaling Pathway to Improve Traumatic Brain Injury. Brain Behav. 2024, 14, e70187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Wang, L.; Zhang, M.-Y.; Wang, T.; Bao, H.-J.; Liu, W.-L.; Dai, D.-K.; Zhang, L.; Chang, P.; Dong, W.-W.; et al. Necrostatin-1 Suppresses Autophagy and Apoptosis in Mice Traumatic Brain Injury Model. Neurochem. Res. 2012, 37, 1849–1858. [Google Scholar] [CrossRef]
- Li, T.; Huang, H.-Y.; Wang, H.-D.; Gao, C.-C.; Liang, H.; Deng, C.-L.; Zhao, X.; Han, Y.-L.; Zhou, M.-L. Restoration of Brain Angiotensin-Converting Enzyme 2 Alleviates Neurological Deficits after Severe Traumatic Brain Injury via Mitigation of Pyroptosis and Apoptosis. J. Neurotrauma 2022, 39, 423–434. [Google Scholar] [CrossRef]
- Chauhan, P.; Yadav, N.; Wadhwa, K.; Ganesan, S.; Walia, C.; Rathore, G.; Singh, G.; Abomughaid, M.M.; Ahlawat, A.; Alexiou, A.; et al. Animal Models of Traumatic Brain Injury and Their Relevance in Clinical Settings. CNS Neurosci. Ther. 2025, 31, e70362. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Zlotnik, A.; Fleidervish, I.; Frenkel, A.; Boyko, M. Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. Int. J. Mol. Sci. 2022, 23, 9628. [Google Scholar] [CrossRef]
- Hwang, N.C.; Lim, D.M.; Goh, T.S.; Kang, J.M.; Kim, J.; Kim, S.; Kim, Y.H.; Kim, D. Recent advances in theranostic nanomaterials for overcoming traumatic brain injury. J. Nanobiotechnol. 2025, 23, 692. [Google Scholar] [CrossRef]
- Mitchell, J.E.; McDonald, S.J.; Sharp, D.J.; Gan, G.; Ponsford, J.L.; Marquand, A.; Wellington, C.; Law, M.; Shultz, S.R.; Spitz, G. The normative modelling framework for traumatic brain injury. Brain 2025, 148, 3817–3832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Y.; Chen, M.; Chen, L.; Mao, Y.; He, R.; Yang, P.; Xu, M.; Yan, H.; Zhao, Q. Danshen-Chuanxiong-Honghua ameliorates neurological function and inflammation in traumatic brain injury in rats via modulating Ghrelin/GHSR. J. Ethnopharmacol. 2025, 345, 119625. [Google Scholar] [CrossRef] [PubMed]
- Schulten, W.; Czaniera, N.J.; Buschheuer, A.L.; Liermann, A.; Wiegand, A.; Kaltschmidt, B.; Kaltschmidt, C. Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response. Int. J. Mol. Sci. 2025, 26, 9283. [Google Scholar] [CrossRef]
- Saludar, C.J.A.; Tayebi, M.; Kwon, E.; McGeown, J.; Schierding, W.; Wang, A.; Fernandez, J.; Holdsworth, S.; Shim, V. Application of Machine Learning in the Diagnosis and Prognosis of Mild Traumatic Brain Injury Using Diffusion Tensor Imaging: A Systematic Review. J. Magn. Reson. Imaging 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).