The Duality of Cdk5: A Master Regulator in Neurodevelopment and a Hijacked Oncogene in Cancer
Highlights
- Cyclin-dependent kinase 5 (Cdk5) exhibits a functional duality: it is an essential regulator of neurodevelopment, but it is frequently repurposed as an oncogene in cancer to drive proliferation, metastasis, and therapeutic resistance.
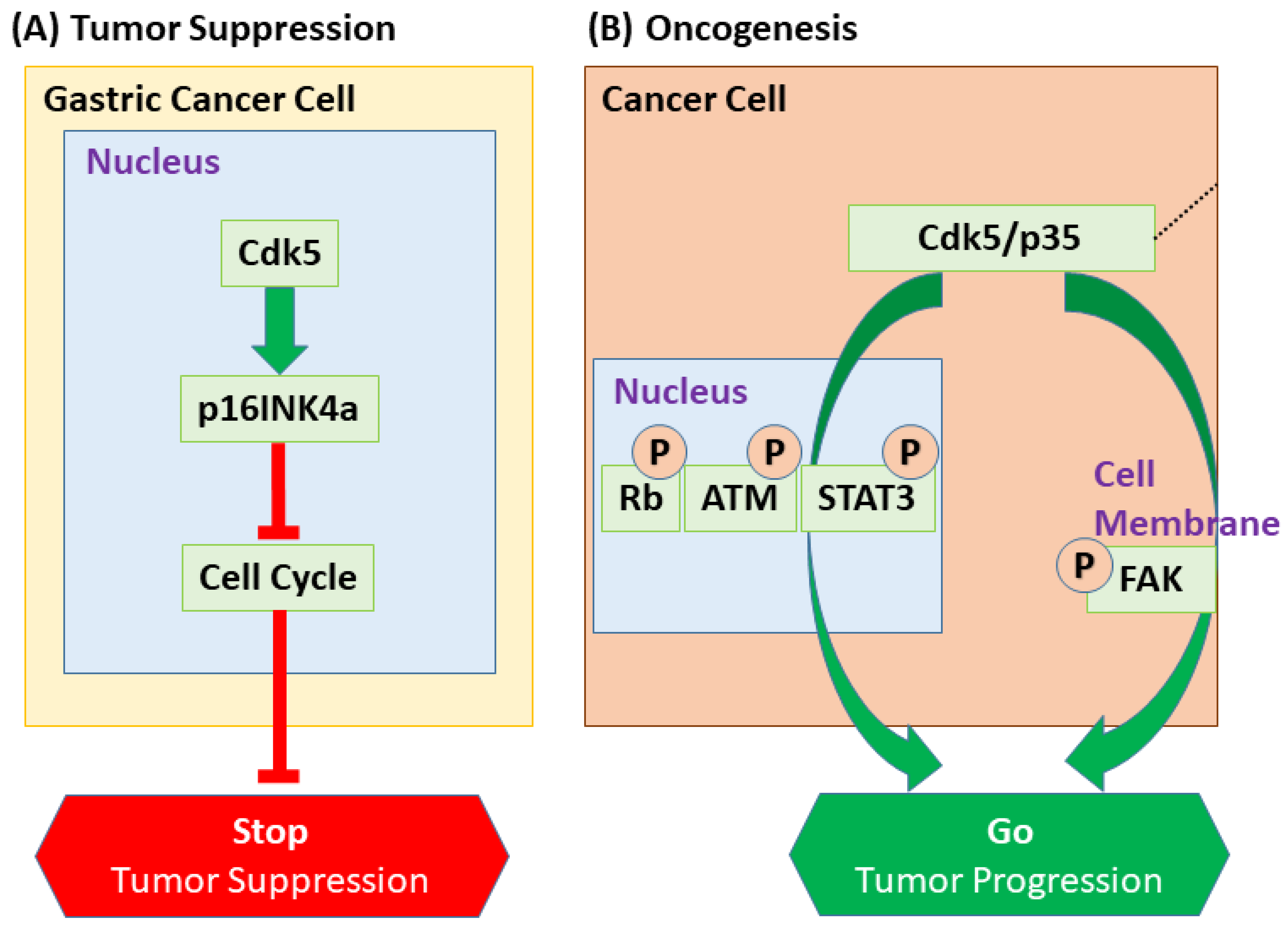
- The functional output of Cdk5 is context-dependent, determined by its upstream regulation, choice of activator, and subcellular localization. Notably, it can act as a nuclear tumor suppressor in gastric cancer, in contrast to its cytoplasmic oncogenic roles.
- The duality of Cdk5 presents an opportunity and a significant challenge for cancer therapy. Novel strategies are needed to distinguish its oncogenic activities from its essential physiological functions, ensuring both efficacy and safety.
- By bridging neurodevelopment and cancer biology, this cross disciplinary synthesis offers a unified framework that clarifies shared mechanisms and supports reciprocal progress in both fields.
Abstract
1. Introduction: The Discovery and Characteristics of the Atypical Cyclin-Dependent Kinase Cdk5
2. Upstream Regulation of Cdk5 Activity
2.1. Transcriptional Control of Activator Expression
2.2. Post-Translational Regulation of Activator Stability and Function
2.3. Post-Translational Modifications of Cdk5
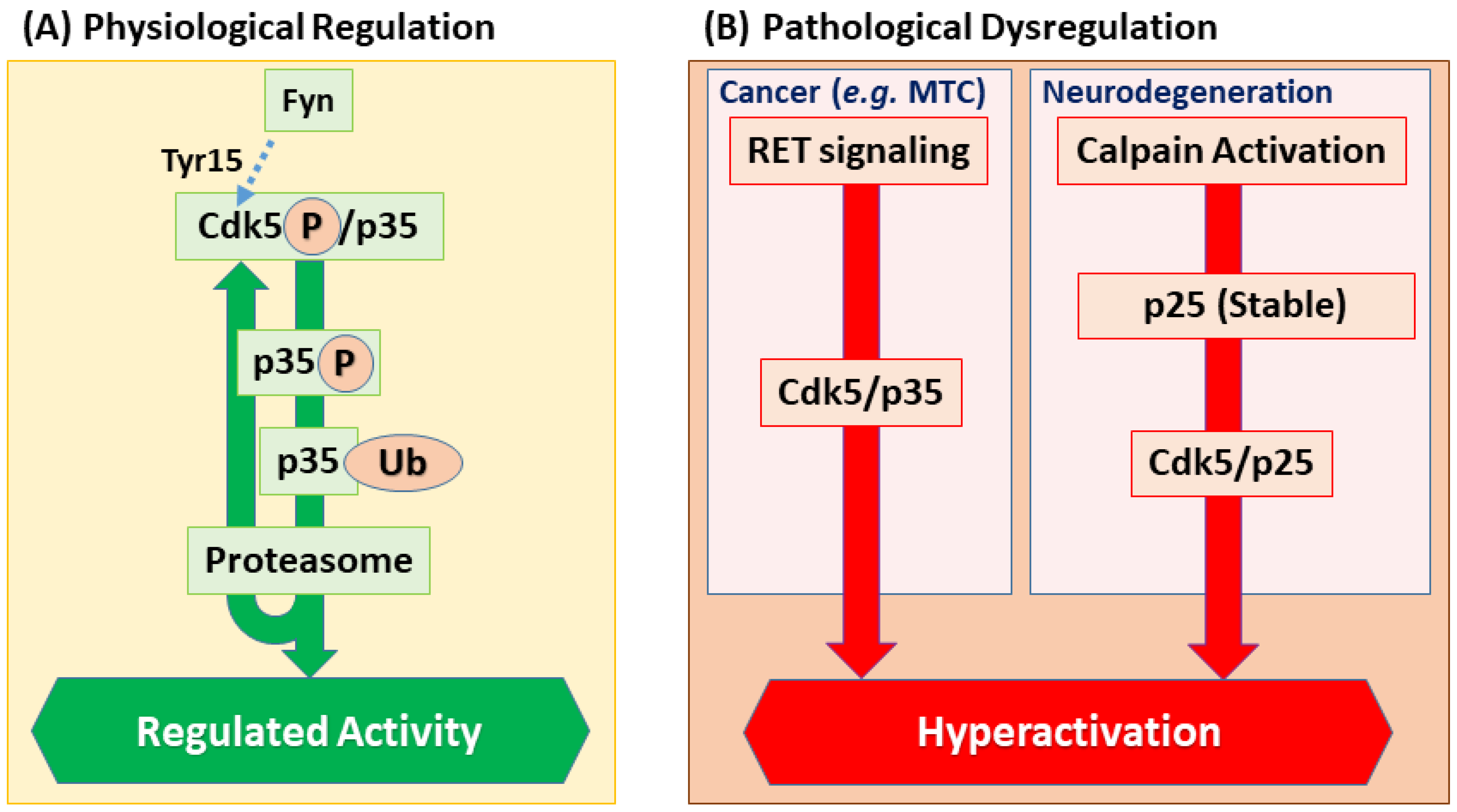
2.4. Negative Regulators of Cdk5 Activity
3. The Role of Cdk5 in Neurodevelopment
3.1. Controlling the Switch from Proliferation to Differentiation in Neural Progenitor Cells
3.2. Multistep Regulation of Neuronal Migration and Layer Formation
3.3. Construction and Maturation of Neural Circuitry
4. The Role of Cdk5 in Cancer
4.1. Promotion of Cancer Cell Proliferation
4.2. Promotion of Invasion and Metastasis
4.3. Cdk5 in the Tumor Microenvironment
4.4. Therapeutic Resistance via DNA Damage Response Activation
4.5. Repurposing the Neurodevelopmental Toolkit in Cancer
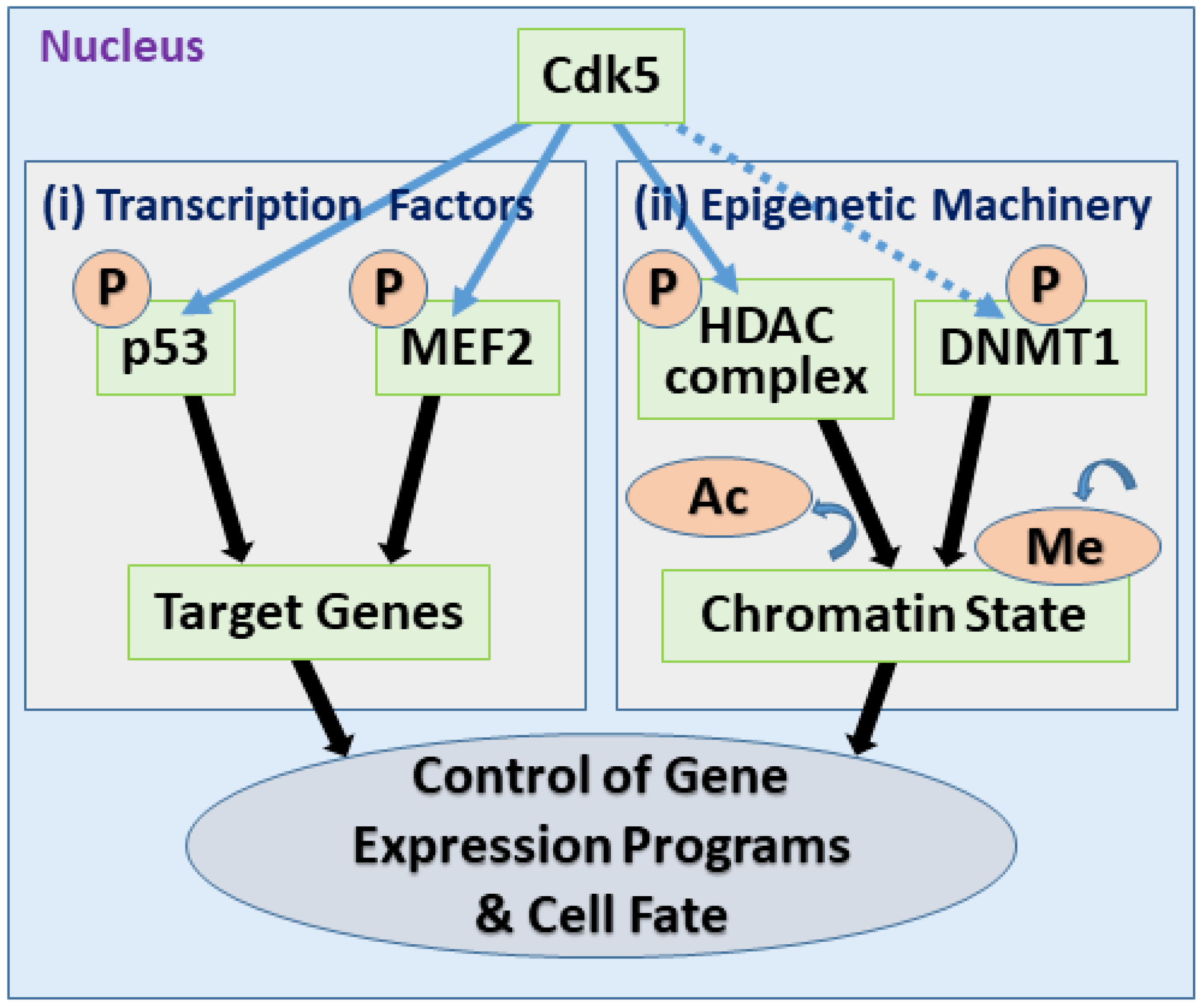
5. Nuclear Functions of Cdk5
5.1. Direct Regulation of Transcription Factors
5.2. Control of the Epigenetic Machinery
6. Conclusions and Future Perspectives: The Duality of Cdk5 and Its Implications for Cancer Therapy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSS2 | Acyl-CoA synthetase short-chain family member 2 |
| AMPAR | AMPA receptor |
| AP-1 | Activating protein 1 |
| AP-2 | Adaptor protein 2 |
| AR | Androgen receptor |
| ATM | Ataxia telangiectasia mutated |
| BBB | Blood–brain barrier |
| bFGF | basic fibroblast growth factor |
| BIN1 | Bridging integrator 1 |
| CAFs | Cancer-associated fibroblasts |
| CAK | Cdk-activating kinase |
| CCNI | Cyclin I |
| CCNI2 | Cyclin I-like |
| Cdk | Cyclin-dependent kinase |
| CMA | Chaperone-mediated autophagy |
| CNTF | Ciliary neurotrophic factor |
| CRF | Corticotropin-releasing factor |
| CRM1 | Chromosome region maintenance 1 |
| CRMP2 | Collapsin response mediator protein 2 |
| Dcx | Doublecortin |
| DDR | DNA damage response |
| DLC1 | Deleted in liver cancer 1 |
| DNMT1 | DNA methyltransferase 1 |
| Drp1 | Dynamin-related protein 1 |
| EGF | Epidermal growth factor |
| EGR1 | Early growth response protein 1 |
| EMT | Epithelial–mesenchymal transition |
| EPRS | Glutamyl-prolyl-tRNA synthetase |
| ERK5 | Extracellular signal-regulated kinase 5 |
| EZH2 | Enhancer of zeste homolog 2 |
| FAK | Focal adhesion kinase |
| GAIT | Gamma interferon-activated inhibitor of translation |
| GSTP1 | Glutathione S-transferase P1 |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HIF-1α | Hypoxia-inducible factor 1α |
| hiPSC | human induced pluripotent stem cell |
| IFN-γ | Interferon-γ |
| LMTK1 | Lemur tyrosine kinase 1 |
| LTP/LTD | Long-term potentiation/depression |
| MAPK | Mitogen-activated protein kinase |
| MCM2 | Minichromosome maintenance protein 2 |
| MEF2 | Myocyte enhancer factor 2 |
| mEPSC | miniature excitatory postsynaptic current |
| MHC-I | Major histocompatibility complex class I |
| MTC | Medullary thyroid carcinoma |
| Nde1 | Nuclear distribution element 1 |
| Ndel1 | Nuclear distribution element-like 1 |
| NES | Nuclear export signal |
| NICD | Notch intracellular domain |
| NLGN | Neuroligin |
| NLS | Nuclear localization signal |
| NMDAR | NMDA receptor |
| NSCLC | Non-small cell lung cancer |
| OGT | O-GlcNAc transferase |
| PD-L1 | Programmed death-ligand 1 |
| PI(3)P | Phosphatidylinositol 3-phosphate |
| PKA | Protein kinase A |
| PKCδ | Protein kinase C delta |
| PRC2 | Polycomb repressive complex 2 |
| PROTAC | Proteolysis-targeting chimera |
| PSD-95 | Postsynaptic density protein-95 |
| Rb | Retinoblastoma protein |
| Rho-GAP | Rho GTPase-activating protein |
| RMS | Rostral migratory stream |
| RPA | Replication protein A |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| ssDNA | single-stranded DNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β1 | Transforming growth factor β1 |
| TME | Tumor microenvironment |
| Treg | regulatory T cell |
| YAP | Yes-associated protein |
References
- Hellmich, M.R.; Pant, H.C.; Wada, E.; Battey, J.F. Neuronal cdc2-like kinase: A cdc2-related protein kinase with predominantly neuronal expression. Proc. Natl. Acad. Sci. USA 1992, 89, 10867–10871. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.; Winkfein, R.J.; Paudel, H.K.; Wang, J.H. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J. Biol. Chem. 1992, 267, 25922–25926. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, M.; Enders, G.H.; Wu, C.L.; Su, L.K.; Gorka, C.; Nelson, C.; Harlow, E.; Tsai, L.H. A family of human cdc2-related protein kinases. EMBO J. 1992, 11, 2909–2917. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Beach, D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71, 505–514. [Google Scholar] [CrossRef]
- Tsai, L.H.; Takahashi, T.; Caviness, V.S., Jr.; Harlow, E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 1993, 119, 1029–1040. [Google Scholar] [CrossRef]
- Lew, J.; Huang, Q.Q.; Qi, Z.; Winkfein, R.J.; Aebersold, R.; Hunt, T.; Wang, J.H. A brain-specific activator of cyclin-dependent kinase 5. Nature 1994, 371, 423–426. [Google Scholar] [CrossRef]
- Tsai, L.H.; Delalle, I.; Caviness, V.S., Jr.; Chae, T.; Harlow, E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 1994, 371, 419–423. [Google Scholar] [CrossRef]
- Uchida, T.; Ishiguro, K.; Ohnuma, J.; Takamatsu, M.; Yonekura, S.; Imahori, K. Precursor of cdk5 activator, the 23 kDa subunit of tau protein kinase II: Its sequence and developmental change in brain. FEBS Lett. 1994, 355, 35–40. [Google Scholar] [CrossRef]
- Tang, D.; Yeung, J.; Lee, K.Y.; Matsushita, M.; Matsui, H.; Tomizawa, K.; Hatase, O.; Wang, J.H. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 1995, 270, 26897–26903. [Google Scholar] [CrossRef]
- Humbert, S.; Dhavan, R.; Tsai, L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci. 2000, 113 Pt 6, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Humbert, S.; Bronson, R.T.; Takahashi, S.; Kulkarni, A.B.; Li, E.; Tsai, L.H. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci. 2001, 21, 6758–6771. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Saito, T.; Hisanaga, S.; Pant, H.C.; Kulkarni, A.B. Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J. Biol. Chem. 2003, 278, 10506–10515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, J.; Li, J.; Wang, J.; Xu, M.; Chen, H.; Zhang, P. S-Nitrosylation of p39 promotes its degradation and contributes to synaptic dysfunction induced by beta-amyloid peptide. Commun. Biol. 2024, 7, 1113. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef]
- Asada, A.; Yamamoto, N.; Gohda, M.; Saito, T.; Hayashi, N.; Hisanaga, S. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J. Neurochem. 2008, 106, 1325–1336. [Google Scholar] [CrossRef]
- Ximerakis, M.; Lipnick, S.L.; Innes, B.T.; Simmons, S.K.; Adiconis, X.; Dionne, D.; Mayweather, B.A.; Nguyen, L.; Niziolek, Z.; Ozek, C.; et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 2019, 22, 1696–1708. [Google Scholar] [CrossRef]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Pippin, J.W.; Hagmann, H.; Krofft, R.D.; Chang, A.M.; Zhang, J.; Terada, Y.; Brinkkoetter, P.; Shankland, S.J. Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am. J. Physiol. Ren. Physiol. 2012, 302, F1161–F1171. [Google Scholar] [CrossRef][Green Version]
- Hagmann, H.; Taniguchi, Y.; Pippin, J.W.; Kauerz, H.M.; Benzing, T.; Shankland, S.J.; Brinkkoetter, P.T. Cyclin I and p35 determine the subcellular distribution of Cdk5. Am. J. Physiol. Cell Physiol. 2015, 308, C339–C347. [Google Scholar] [CrossRef]
- Liu, C.; Zhai, X.; Zhao, B.; Wang, Y.; Xu, Z. Cyclin I-like (CCNI2) is a cyclin-dependent kinase 5 (CDK5) activator and is involved in cell cycle regulation. Sci. Rep. 2017, 7, 40979. [Google Scholar] [CrossRef]
- Zheng, X.F.; Sarkar, A.; Lotana, H.; Syed, A.; Nguyen, H.; Ivey, R.G.; Kennedy, J.J.; Whiteaker, J.R.; Tomasik, B.; Huang, K.; et al. CDK5-cyclin B1 regulates mitotic fidelity. Nature 2024, 633, 932–940. [Google Scholar] [CrossRef]
- Pozo, K.; Castro-Rivera, E.; Tan, C.; Plattner, F.; Schwach, G.; Siegl, V.; Meyer, D.; Guo, A.; Gundara, J.; Mettlach, G.; et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell 2013, 24, 499–511. [Google Scholar] [CrossRef]
- Patrick, G.N.; Zhou, P.; Kwon, Y.T.; Howley, P.M.; Tsai, L.H. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J. Biol. Chem. 1998, 273, 24057–24064. [Google Scholar] [CrossRef]
- Büchner, A.; Krumova, P.; Ganesan, S.; Bahr, M.; Eckermann, K.; Weishaupt, J.H. Sumoylation of p35 modulates p35/cyclin-dependent kinase (Cdk) 5 complex activity. Neuromolecular Med. 2015, 17, 12–23. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, W.Y.; Fu, A.K.; Ip, N.Y. S-nitrosylation-dependent proteasomal degradation restrains Cdk5 activity to regulate hippocampal synaptic strength. Nat. Commun. 2015, 6, 8665. [Google Scholar] [CrossRef]
- Fu, X.; Choi, Y.K.; Qu, D.; Yu, Y.; Cheung, N.S.; Qi, R.Z. Identification of nuclear import mechanisms for the neuronal Cdk5 activator. J. Biol. Chem. 2006, 281, 39014–39021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ko, Y.U.; Chung, Y.; Yun, N.; Kim, M.; Kim, K.; Oh, Y.J. The acetylation of cyclin-dependent kinase 5 at lysine 33 regulates kinase activity and neurite length in hippocampal neurons. Sci. Rep. 2018, 8, 13676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, P.C.; Tsang, A.H.; Chen, Y.; Fu, A.K.; Fu, W.Y.; Chung, K.K.; Ip, N.Y. S-nitrosylation of cyclin-dependent kinase 5 (cdk5) regulates its kinase activity and dendrite growth during neuronal development. J. Neurosci. 2010, 30, 14366–14370. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Cheng, C.; Uchida, Y.; Nakajima, O.; Ohshima, T.; Yagi, T.; Taniguchi, M.; Nakayama, T.; Kishida, R.; Kudo, Y.; et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002, 35, 907–920. [Google Scholar] [CrossRef]
- Kobayashi, H.; Saito, T.; Sato, K.; Furusawa, K.; Hosokawa, T.; Tsutsumi, K.; Asada, A.; Kamada, S.; Ohshima, T.; Hisanaga, S. Phosphorylation of cyclin-dependent kinase 5 (Cdk5) at Tyr-15 is inhibited by Cdk5 activators and does not contribute to the activation of Cdk5. J. Biol. Chem. 2014, 289, 19627–19636. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Amin, N.D.; Albers, R.W.; Pant, H.C. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc. Natl. Acad. Sci. USA 1999, 96, 11156–11160. [Google Scholar] [CrossRef]
- Tarricone, C.; Dhavan, R.; Peng, J.; Areces, L.B.; Tsai, L.H.; Musacchio, A. Structure and regulation of the CDK5-p25(nck5a) complex. Mol. Cell 2001, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Odajima, J.; Wills, Z.P.; Ndassa, Y.M.; Terunuma, M.; Kretschmannova, K.; Deeb, T.Z.; Geng, Y.; Gawrzak, S.; Quadros, I.M.; Newman, J.; et al. Cyclin E constrains Cdk5 activity to regulate synaptic plasticity and memory formation. Dev. Cell 2011, 21, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.H.; Chang, K.H.; Clawson, S.; Ghosh, S.; Mirzaei, H.; Regnier, F.; Shah, K. Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity. J. Neurochem. 2011, 118, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Pollan, S.G.; Huang, F.; Sperger, J.M.; Lang, J.M.; Morrissey, C.; Cress, A.E.; Chu, C.Y.; Bhowmick, N.A.; You, S.; Freeman, M.R.; et al. Regulation of inside-out beta1-integrin activation by CDCP1. Oncogene 2018, 37, 2817–2836. [Google Scholar] [CrossRef]
- Kawauchi, T.; Chihama, K.; Nabeshima, Y.; Hoshino, M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006, 8, 17–26. [Google Scholar] [CrossRef]
- Fang, W.Q.; Chen, W.W.; Fu, A.K.; Ip, N.Y. Axin directs the amplification and differentiation of intermediate progenitors in the developing cerebral cortex. Neuron 2013, 79, 665–679. [Google Scholar] [CrossRef]
- Fang, W.Q.; Ip, J.P.; Li, R.; Ng, Y.P.; Lin, S.C.; Chen, Y.; Fu, A.K.; Ip, N.Y. Cdk5-mediated phosphorylation of Axin directs axon formation during cerebral cortex development. J. Neurosci. 2011, 31, 13613–13624. [Google Scholar] [CrossRef]
- Singh, K.K.; Ge, X.; Mao, Y.; Drane, L.; Meletis, K.; Samuels, B.A.; Tsai, L.H. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 2010, 67, 33–48. [Google Scholar] [CrossRef]
- Ye, T.; Ip, J.P.; Fu, A.K.; Ip, N.Y. Cdk5-mediated phosphorylation of RapGEF2 controls neuronal migration in the developing cerebral cortex. Nat. Commun. 2014, 5, 4826. [Google Scholar] [CrossRef]
- Kawauchi, T.; Sekine, K.; Shikanai, M.; Chihama, K.; Tomita, K.; Kubo, K.; Nakajima, K.; Nabeshima, Y.; Hoshino, M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 2010, 67, 588–602. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Gupta, A.; Zhou, Y.; Nikolic, M.; Tsai, L.H. Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr. Biol. 2000, 10, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Serneo, F.F.; Tseng, H.C.; Kulkarni, A.B.; Tsai, L.H.; Gleeson, J.G. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron 2004, 41, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Shionoya, A.; Ishida, M.; Gambello, M.J.; Yingling, J.; Wynshaw-Boris, A.; Hirotsune, S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 2000, 28, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Doobin, D.J.; Helmer, P.; Carabalona, A.; Bertipaglia, C.; Vallee, R.B. The Role of Nde1 phosphorylation in interkinetic nuclear migration and neural migration during cortical development. Mol. Biol. Cell 2024, 35, ar129. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Moizumi, M.; Nagai, J.; Hatashita, Y.; Cai, T.; Kolattukudy, P.; Inoue, T.; Goshima, Y.; Ohshima, T. Requirement of CRMP2 Phosphorylation in Neuronal Migration of Developing Mouse Cerebral Cortex and Hippocampus and Redundant Roles of CRMP1 and CRMP4. Cereb. Cortex 2022, 32, 520–527. [Google Scholar] [CrossRef]
- Shuang, R.; Zhang, L.; Fletcher, A.; Groblewski, G.E.; Pevsner, J.; Stuenkel, E.L. Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J. Biol. Chem. 1998, 273, 4957–4966. [Google Scholar] [CrossRef]
- Floyd, S.R.; Porro, E.B.; Slepnev, V.I.; Ochoa, G.C.; Tsai, L.H.; De Camilli, P. Amphiphysin 1 binds the cyclin-dependent kinase (cdk) 5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J. Biol. Chem. 2001, 276, 8104–8110. [Google Scholar] [CrossRef]
- Tomizawa, K.; Sunada, S.; Lu, Y.F.; Oda, Y.; Kinuta, M.; Ohshima, T.; Saito, T.; Wei, F.Y.; Matsushita, M.; Li, S.T.; et al. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J. Cell Biol. 2003, 163, 813–824. [Google Scholar] [CrossRef]
- Tan, T.C.; Valova, V.A.; Malladi, C.S.; Graham, M.E.; Berven, L.A.; Jupp, O.J.; Hansra, G.; McClure, S.J.; Sarcevic, B.; Boadle, R.A.; et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 2003, 5, 701–710. [Google Scholar] [CrossRef]
- Montenegro-Venegas, C.; Guhathakurta, D.; Pina-Fernandez, E.; Andres-Alonso, M.; Plattner, F.; Gundelfinger, E.D.; Fejtova, A. Bassoon controls synaptic vesicle release via regulation of presynaptic phosphorylation and cAMP. EMBO Rep. 2022, 23, e53659. [Google Scholar] [CrossRef]
- Su, S.C.; Seo, J.; Pan, J.Q.; Samuels, B.A.; Rudenko, A.; Ericsson, M.; Neve, R.L.; Yue, D.T.; Tsai, L.H. Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron 2012, 75, 675–687. [Google Scholar] [CrossRef]
- Tomizawa, K.; Ohta, J.; Matsushita, M.; Moriwaki, A.; Li, S.T.; Takei, K.; Matsui, H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J. Neurosci. 2002, 22, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.; Sun, M.K.; Zhang, L.; Takahashi, S.; Ma, W.; Vinade, L.; Kulkarni, A.B.; Brady, R.O.; Pant, H.C. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc. Natl. Acad. Sci. USA 2001, 98, 12742–12747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Edelmann, L.; Liu, J.; Crandall, J.E.; Morabito, M.A. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J. Neurosci. 2008, 28, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Plattner, F.; Hernandez, A.; Kistler, T.M.; Pozo, K.; Zhong, P.; Yuen, E.Y.; Tan, C.; Hawasli, A.H.; Cooke, S.F.; Nishi, A.; et al. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron 2014, 81, 1070–1083. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, K.; Li, J.; Wu, J.; Zhang, J.; Chen, L.; Guo, G.; Zhang, J. Phosphorylation of CRMP2 by Cdk5 Negatively Regulates the Surface Delivery and Synaptic Function of AMPA Receptors. Mol. Neurobiol. 2022, 59, 762–777. [Google Scholar] [CrossRef]
- Morabito, M.A.; Sheng, M.; Tsai, L.H. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 2004, 24, 865–876. [Google Scholar] [CrossRef]
- Jeong, J.; Han, W.; Hong, E.; Pandey, S.; Li, Y.; Lu, W.; Roche, K.W. Regulation of NLGN3 and the Synaptic Rho-GEF Signaling Pathway by CDK5. J. Neurosci. 2023, 43, 7264–7275. [Google Scholar] [CrossRef]
- Lehr, A.W.; Nguyen, T.A.; Han, W.; Hong, E.; Badger, J.D., 2nd; Lu, W.; Roche, K.W. Phosphorylation of NLGN4X Regulates Spinogenesis and Synaptic Function. eNeuro 2025, 12, ENEURO.0278-23.2025. [Google Scholar] [CrossRef]
- Nishino, H.; Saito, T.; Wei, R.; Takano, T.; Tsutsumi, K.; Taniguchi, M.; Ando, K.; Tomomura, M.; Fukuda, M.; Hisanaga, S.I. The LMTK1-TBC1D9B-Rab11A Cascade Regulates Dendritic Spine Formation via Endosome Trafficking. J. Neurosci. 2019, 39, 9491–9502. [Google Scholar] [CrossRef]
- Kwak, Y.; Jeong, J.; Lee, S.; Park, Y.U.; Lee, S.A.; Han, D.H.; Kim, J.H.; Ohshima, T.; Mikoshiba, K.; Suh, Y.H.; et al. Cyclin-dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. J. Biol. Chem. 2013, 288, 36878–36889. [Google Scholar] [CrossRef]
- Brenna, A.; Olejniczak, I.; Chavan, R.; Ripperger, J.A.; Langmesser, S.; Cameroni, E.; Hu, Z.; De Virgilio, C.; Dengjel, J.; Albrecht, U. Cyclin-dependent kinase 5 (CDK5) regulates the circadian clock. eLife 2019, 8, e50925. [Google Scholar] [CrossRef]
- Fu, K.; Lin, N.; Xu, Y.; Huang, E.; He, R.; Wu, Z.; Qu, D.; Chen, X.; Huang, T. CDK5-mediated hyperphosphorylation of Tau217 impairs neuronal synaptic structure and exacerbates cognitive impairment in Alzheimer’s disease. Transl. Psychiatry 2025, 15, 302. [Google Scholar] [CrossRef]
- Cicero, S.; Herrup, K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. 2005, 25, 9658–9668. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Li, B.S.; Rudrabhatla, P.; Shukla, V.; Amin, N.D.; Maric, D.; Kesavapany, S.; Kanungo, J.; Pareek, T.K.; Takahashi, S.; et al. Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation. Mol. Biol. Cell 2010, 21, 3601–3614. [Google Scholar] [CrossRef]
- Nguyen, L.; Besson, A.; Heng, J.I.; Schuurmans, C.; Teboul, L.; Parras, C.; Philpott, A.; Roberts, J.M.; Guillemot, F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes. Dev. 2006, 20, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Angevine, J.B., Jr.; Sidman, R.L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 1961, 192, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H.; Nakajima, K. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 2003, 23, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, B.; Brunstrom, J.E.; Grutzendler, J.; Wong, R.O.; Pearlman, A.L. Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 2001, 4, 143–150. [Google Scholar] [CrossRef]
- Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972, 145, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Ward, J.M.; Huh, C.G.; Longenecker, G.; Veeranna; Pant, H.C.; Brady, R.O.; Martin, L.J.; Kulkarni, A.B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 1996, 93, 11173–11178. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, E.C.; Ohshima, T.; Goffinet, A.M.; Kulkarni, A.B.; Herrup, K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 1998, 18, 6370–6377. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Tsai, L.H. A novel disruption of cortical development in p35(-/-) mice distinct from reeler. J. Comp. Neurol. 1998, 395, 510–522. [Google Scholar] [CrossRef]
- Nishimura, Y.V.; Shikanai, M.; Hoshino, M.; Ohshima, T.; Nabeshima, Y.; Mizutani, K.; Nagata, K.; Nakajima, K.; Kawauchi, T. Cdk5 and its substrates, Dcx and p27kip1, regulate cytoplasmic dilation formation and nuclear elongation in migrating neurons. Development 2014, 141, 3540–3550. [Google Scholar] [CrossRef]
- Vinopal, S.; Dupraz, S.; Alfadil, E.; Pietralla, T.; Bendre, S.; Stiess, M.; Falk, S.; Camargo Ortega, G.; Maghelli, N.; Tolic, I.M.; et al. Centrosomal microtubule nucleation regulates radial migration of projection neurons independently of polarization in the developing brain. Neuron 2023, 111, 1241–1263.E16. [Google Scholar] [CrossRef]
- Niethammer, M.; Smith, D.S.; Ayala, R.; Peng, J.; Ko, J.; Lee, M.S.; Morabito, M.; Tsai, L.H. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron 2000, 28, 697–711. [Google Scholar] [CrossRef]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Horesh, D.; Sapir, T.; Francis, F.; Wolf, S.G.; Caspi, M.; Elbaum, M.; Chelly, J.; Reiner, O. Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet. 1999, 8, 1599–1610. [Google Scholar] [CrossRef]
- Kawauchi, T.; Chihama, K.; Nishimura, Y.V.; Nabeshima, Y.; Hoshino, M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem. Biophys. Res. Commun. 2005, 331, 50–55. [Google Scholar] [CrossRef]
- Yamada, M.; Toba, S.; Yoshida, Y.; Haratani, K.; Mori, D.; Yano, Y.; Mimori-Kiyosue, Y.; Nakamura, T.; Itoh, K.; Fushiki, S.; et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 2008, 27, 2471–2483. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.W.; Bremner, K.H.; Vallee, R.B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 2007, 10, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H.; Gleeson, J.G. Nucleokinesis in neuronal migration. Neuron 2005, 46, 383–388. [Google Scholar] [CrossRef]
- Toyo-oka, K.; Shionoya, A.; Gambello, M.J.; Cardoso, C.; Leventer, R.; Ward, H.L.; Ayala, R.; Tsai, L.H.; Dobyns, W.; Ledbetter, D.; et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: A molecular explanation for Miller-Dieker syndrome. Nat. Genet. 2003, 34, 274–285. [Google Scholar] [CrossRef]
- Shinmyo, Y.; Terashita, Y.; Dinh Duong, T.A.; Horiike, T.; Kawasumi, M.; Hosomichi, K.; Tajima, A.; Kawasaki, H. Folding of the Cerebral Cortex Requires Cdk5 in Upper-Layer Neurons in Gyrencephalic Mammals. Cell Rep. 2017, 20, 2131–2143. [Google Scholar] [CrossRef]
- Ohshima, T.; Hirasawa, M.; Tabata, H.; Mutoh, T.; Adachi, T.; Suzuki, H.; Saruta, K.; Iwasato, T.; Itohara, S.; Hashimoto, M.; et al. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development 2007, 134, 2273–2282. [Google Scholar] [CrossRef]
- Nikolic, M.; Dudek, H.; Kwon, Y.T.; Ramos, Y.F.; Tsai, L.H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996, 10, 816–825. [Google Scholar] [CrossRef]
- Mucci, S.; Clas, G.S.; Allio, C.P.; Rodriguez-Varela, M.S.; Isaja, L.; Marazita, M.; Sevlever, G.E.; Scassa, M.E.; Romorini, L. CDK5 Deficiency Does not Impair Neuronal Differentiation of Human Induced Pluripotent Stem Cells but Affects Neurite Outgrowth. Mol. Neurobiol. 2025, 62, 918–934. [Google Scholar] [CrossRef]
- Desbois, M.; Opperman, K.J.; Amezquita, J.; Gaglio, G.; Crawley, O.; Grill, B. Ubiquitin ligase activity inhibits Cdk5 to control axon termination. PLoS Genet. 2022, 18, e1010152. [Google Scholar] [CrossRef]
- Liu, G.T.; Kochlamazashvili, G.; Puchkov, D.; Muller, R.; Schultz, C.; Mackintosh, A.I.; Vollweiter, D.; Haucke, V.; Soykan, T. Endosomal phosphatidylinositol 3-phosphate controls synaptic vesicle cycling and neurotransmission. EMBO J. 2022, 41, e109352. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Choi, Y.; Kim, D.; Jeong, H.J.; Do, Y.H.; Jung, S.; Lee, B.; Choi, H.J.; Kim, S.; Oh, J.M.; et al. Developmental deficits, synapse and dendritic abnormalities in a Clcn4 KO autism mice model: Endophenotypic target for ASD. Transl. Psychiatry 2025, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Vandael, D.; Vints, K.; Baatsen, P.; Sliwinska, M.A.; Gabarre, S.; De Groef, L.; Moons, L.; Rybakin, V.; Gounko, N.V. Cdk5-dependent rapid formation and stabilization of dendritic spines by corticotropin-releasing factor. Transl. Psychiatry 2024, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Tomizawa, K.; Ohshima, T.; Asada, A.; Saito, T.; Nguyen, C.; Bibb, J.A.; Ishiguro, K.; Kulkarni, A.B.; Pant, H.C.; et al. Control of cyclin-dependent kinase 5 (Cdk5) activity by glutamatergic regulation of p35 stability. J. Neurochem. 2005, 93, 502–512. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Yang, P.; Shi, H.X.; Xiong, Y.; Nie, Z.Y.; Yu, J.Q.; Wang, Y.A.; Zhou, R.; Wang, L.Y. Cyclin-dependent Kinase 5 Regulates Cortical Neurotransmission and Neural Circuits Associated with Motor Control in the Secondary Motor Cortex in the Mouse. Neuroscience 2020, 438, 9–24. [Google Scholar] [CrossRef]
- Patzke, H.; Tsai, L.H. Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J. Biol. Chem. 2002, 277, 8054–8060. [Google Scholar] [CrossRef]
- Lee, M.S.; Kwon, Y.T.; Li, M.; Peng, J.; Friedlander, R.M.; Tsai, L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 2000, 405, 360–364. [Google Scholar] [CrossRef]
- Brenna, A.; Borsa, M.; Saro, G.; Ripperger, J.A.; Glauser, D.A.; Yang, Z.; Adamantidis, A.; Albrecht, U. Cyclin-dependent kinase 5 (Cdk5) activity is modulated by light and gates rapid phase shifts of the circadian clock. eLife 2025, 13, e97029. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, M.C.; Chiu, C.Y.; Song, Y.M.; Lin, S.Y. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J. Biol. Chem. 2007, 282, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.N.; Chen, M.C.; Lin, K.C.; Peng, Y.T.; Li, P.C.; Lin, E.; Chiang, M.C.; Hsieh, J.T.; Lin, H. Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor activation through phosphorylation of Ser727 on STAT3 in prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E975–E986. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, J.; Imanishi, S.Y.; Torvaldson, E.; Malinen, M.; Remes, M.; Orn, F.; Palvimo, J.J.; Eriksson, J.E. Cyclin-dependent kinase 5 acts as a critical determinant of AKT-dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol. Biol. Cell 2015, 26, 1971–1984. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Zhou, W.; Jiang, Y.; He, K.; Huang, W.; Feng, Y.; Wu, H.; Liu, L.; Pan, Y.; et al. Prenatal p25-activated Cdk5 induces pituitary tumorigenesis through MCM2 phosphorylation-mediated cell proliferation. Neoplasia 2024, 57, 101054. [Google Scholar] [CrossRef]
- Tung, M.C.; Oner, M.; Soong, S.W.; Cheng, P.T.; Li, Y.H.; Chen, M.C.; Chou, C.K.; Kang, H.Y.; Lin, F.C.; Tsai, S.C.; et al. CDK5 targets p21(CIP1) to regulate thyroid cancer cell proliferation and malignancy in patients. Mol. Med. Rep. 2025, 32, 182. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, L.; Zhang, J.; Wang, W.; Wang, Y. CDK5 positively regulates Notch1 signaling in pancreatic cancer cells by phosphorylation. Cancer Med. 2021, 10, 3689–3699. [Google Scholar] [CrossRef]
- Zhuang, K.; Zhang, J.; Xiong, M.; Wang, X.; Luo, X.; Han, L.; Meng, Y.; Zhang, Y.; Liao, W.; Liu, S. CDK5 functions as a tumor promoter in human colorectal cancer via modulating the ERK5-AP-1 axis. Cell Death Dis. 2016, 7, e2415. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jia, Y.; Liu, T.; Wang, M.; Lv, W.; Zhang, R.; Shi, J.; Liu, L. CDK5 neutralizes the tumor suppressing effect of BIN1 via mediating phosphorylation of c-MYC at Ser-62 site in NSCLC. Cancer Cell Int. 2019, 19, 226. [Google Scholar] [CrossRef]
- Ehrlich, S.M.; Liebl, J.; Ardelt, M.A.; Lehr, T.; De Toni, E.N.; Mayr, D.; Brandl, L.; Kirchner, T.; Zahler, S.; Gerbes, A.L.; et al. Targeting cyclin dependent kinase 5 in hepatocellular carcinoma—A novel therapeutic approach. J. Hepatol. 2015, 63, 102–113. [Google Scholar] [CrossRef]
- Tian, B.; Yang, Q.; Mao, Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 2009, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chiker, S.; Pennaneach, V.; Loew, D.; Dingli, F.; Biard, D.; Cordelieres, F.P.; Gemble, S.; Vacher, S.; Bieche, I.; Hall, J.; et al. Cdk5 promotes DNA replication stress checkpoint activation through RPA-32 phosphorylation, and impacts on metastasis free survival in breast cancer patients. Cell Cycle 2015, 14, 3066–3078. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, G.Z.; Luo, S.Y.; Chen, R.; Zhang, J.X. Cyclin I promotes cisplatin resistance via Cdk5 activation in cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4533–4541. [Google Scholar] [PubMed]
- Lowman, X.H.; McDonnell, M.A.; Kosloske, A.; Odumade, O.A.; Jenness, C.; Karim, C.B.; Jemmerson, R.; Kelekar, A. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol. Cell 2010, 40, 823–833. [Google Scholar] [CrossRef]
- Herzog, J.; Ehrlich, S.M.; Pfitzer, L.; Liebl, J.; Frohlich, T.; Arnold, G.J.; Mikulits, W.; Haider, C.; Vollmar, A.M.; Zahler, S. Cyclin-dependent kinase 5 stabilizes hypoxia-inducible factor-1alpha: A novel approach for inhibiting angiogenesis in hepatocellular carcinoma. Oncotarget 2016, 7, 27108–27121. [Google Scholar] [CrossRef]
- Liang, Q.; Li, L.; Zhang, J.; Lei, Y.; Wang, L.; Liu, D.X.; Feng, J.; Hou, P.; Yao, R.; Zhang, Y.; et al. CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci. Rep. 2013, 3, 2932. [Google Scholar] [CrossRef]
- Huang, C.; Rajfur, Z.; Yousefi, N.; Chen, Z.; Jacobson, K.; Ginsberg, M.H. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 2009, 11, 624–630. [Google Scholar] [CrossRef]
- He, Z.; Sun, J.; Wang, M.; Chen, S.; Mao, G.; Yang, L. Talin1 Ser425 phosphorylation promotes colorectal cancer progression and metastasis. Transl. Cancer Res. 2025, 14, 796–807. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, T.; Michowski, W.; Rebecca, V.W.; Xiao, M.; Ferretti, R.; Suski, J.M.; Bronson, R.T.; Paulo, J.A.; Frederick, D.; et al. Targeting the cyclin-dependent kinase 5 in metastatic melanoma. Proc. Natl. Acad. Sci. USA 2020, 117, 8001–8012. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Qian, X.; Mertins, P.; Wang, D.; Papageorge, A.G.; Carr, S.A.; Lowy, D.R. CDK5 is a major regulator of the tumor suppressor DLC1. J. Cell Biol. 2014, 207, 627–642. [Google Scholar] [CrossRef]
- Bei, Y.; Wang, S.; Wang, R.; Ahmad, O.; Jia, M.; Yao, P.; Ji, J.; Shen, P. CDK5-triggered G6PD phosphorylation at threonine 91 facilitating redox homeostasis reveals a vulnerability in breast cancer. Acta Pharm. Sin. B 2025, 15, 1608–1625. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Ni, W.; Zhou, L.; Tong, Y.; Dai, C.; Zhao, X.; Qiang, Y.; Gao, J.; Xiao, Y.; Liu, W.; et al. Ubiquitin-specific protease 1 facilitates hepatocellular carcinoma progression by modulating mitochondrial fission and metabolic reprogramming via cyclin-dependent kinase 5 stabilization. Cell Death Differ. 2024, 31, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Ouyang, L.; Yu, N.N.; Sun, Z.H.; Gui, Z.K.; Niu, Y.L.; He, Q.Y.; Zhang, J.; Wang, Y. Histone deacetylase inhibitor pracinostat suppresses colorectal cancer by inducing CDK5-Drp1 signaling-mediated peripheral mitofission. J. Pharm. Anal. 2023, 13, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Ciraku, L.; Bacigalupa, Z.A.; Ju, J.; Moeller, R.A.; Le Minh, G.; Lee, R.H.; Smith, M.D.; Ferrer, C.M.; Trefely, S.; Izzo, L.T.; et al. O-GlcNAc transferase regulates glioblastoma acetate metabolism via regulation of CDK5-dependent ACSS2 phosphorylation. Oncogene 2022, 41, 2122–2136. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Lowery, F.J.; Saito, Y.; Yuan, X.; Yao, J.; Duan, Y.; Ding, J.; Acharya, S.; Zhang, C.; Fajardo, A.; et al. Astrocyte-induced Cdk5 expedites breast cancer brain metastasis by suppressing MHC-I expression to evade immune recognition. Nat. Cell Biol. 2024, 26, 1773–1789. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Du, Y.; Yu, Z.; Wang, Y.; Jiang, Y.; Wu, Y.; Le, T.; Li, Z.; Zhang, G.; et al. CDK5 destabilizes PD-L1 via chaperon-mediated autophagy to control cancer immune surveillance in hepatocellular carcinoma. J. Immunother. Cancer 2023, 11, e007529. [Google Scholar] [CrossRef]
- Gao, L.; Xia, L.; Ji, W.; Zhang, Y.; Xia, W.; Lu, S. Knockdown of CDK5 down-regulates PD-L1 via the ubiquitination-proteasome pathway and improves antitumor immunity in lung adenocarcinoma. Transl. Oncol. 2021, 14, 101148. [Google Scholar] [CrossRef]
- Zhang, J.; Krishnamurthy, P.K.; Johnson, G.V. Cdk5 phosphorylates p53 and regulates its activity. J. Neurochem. 2002, 81, 307–313. [Google Scholar] [CrossRef]
- Kim, D.; Frank, C.L.; Dobbin, M.M.; Tsunemoto, R.K.; Tu, W.; Peng, P.L.; Guan, J.S.; Lee, B.H.; Moy, L.Y.; Giusti, P.; et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 2008, 60, 803–817. [Google Scholar] [CrossRef]
- Fu, A.K.; Hung, K.W.; Wong, H.H.; Fu, W.Y.; Ip, N.Y. Cdk5 phosphorylates a component of the HDAC complex and regulates histone acetylation during neuronal cell death. Neurosignals 2013, 21, 55–60. [Google Scholar] [CrossRef]
- Lavoie, G.; St-Pierre, Y. Phosphorylation of human DNMT1: Implication of cyclin-dependent kinases. Biochem. Biophys. Res. Commun. 2011, 409, 187–192. [Google Scholar] [CrossRef]
- Oner, M.; Lin, E.; Chiu, K.Y.; Chen, M.C.; Prince, G.; Lai, C.H.; Hsieh, J.T.; Wang, H.Y.; Lin, H.O. p35/CDK5 Regulates Bladder Cancer Proliferation and Migration and Promotes Higher Tumor Grade and Poor Survival Rate in Patients with Bladder Cancer. Anticancer. Res. 2024, 44, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Lv, P.; Yu, H.; Jiang, X. CDK5 Knockdown inhibits proliferation and induces apoptosis and Cell Cycle Arrest in Human Glioblastoma. J. Cancer 2021, 12, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.H.; Chiu, K.Y.; Tsai, S.C.; Teng, C.J.; Oner, M.; Lai, C.H.; Hsieh, J.T.; Lin, C.C.; Wang, H.Y.; Chen, M.C.; et al. PI3K/Akt inhibition promotes AR activity and prostate cancer cell proliferation through p35-CDK5 modulation. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167568. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Asl, A.; Huang, E.; Irrcher, I.; Rashidian, J.; Ishihara, N.; Lagace, D.C.; Slack, R.S.; Park, D.S. CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death. Hum. Mol. Genet. 2015, 24, 4573–4583. [Google Scholar] [CrossRef]
- Carter, A.M.; Kumar, N.; Herring, B.; Tan, C.; Guenter, R.; Telange, R.; Howse, W.; Viol, F.; McCaw, T.R.; Bickerton, H.H.; et al. Cdk5 drives formation of heterogeneous pancreatic neuroendocrine tumors. Oncogenesis 2021, 10, 83. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Bhandari, D.; Lopez-Sanchez, I.; To, A.; Lo, I.C.; Aznar, N.; Leyme, A.; Gupta, V.; Niesman, I.; Maddox, A.L.; Garcia-Marcos, M.; et al. Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration-proliferation dichotomy. Proc. Natl. Acad. Sci. USA 2015, 112, E4874–E4883. [Google Scholar] [CrossRef]
- Wang, Y.; Kaneko, N.; Asai, N.; Enomoto, A.; Isotani-Sakakibara, M.; Kato, T.; Asai, M.; Murakumo, Y.; Ota, H.; Hikita, T.; et al. Girdin is an intrinsic regulator of neuroblast chain migration in the rostral migratory stream of the postnatal brain. J. Neurosci. 2011, 31, 8109–8122. [Google Scholar] [CrossRef]
- Itoh, Y.; Higuchi, M.; Oishi, K.; Kishi, Y.; Okazaki, T.; Sakai, H.; Miyata, T.; Nakajima, K.; Gotoh, Y. PDK1-Akt pathway regulates radial neuronal migration and microtubules in the developing mouse neocortex. Proc. Natl. Acad. Sci. USA 2016, 113, E2955–E2964. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Kawauchi, T. Cell adhesion and its endocytic regulation in cell migration during neural development and cancer metastasis. Int. J. Mol. Sci. 2012, 13, 4564–4590. [Google Scholar] [CrossRef]
- Xie, Z.; Sanada, K.; Samuels, B.A.; Shih, H.; Tsai, L.H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 2003, 114, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Pozzato, C.; Outeiro-Pinho, G.; Galie, M.; Ramadori, G.; Konstantinidou, G. ERK5 suppression overcomes FAK inhibitor resistance in mutant KRAS-driven non-small cell lung cancer. EMBO Mol. Med. 2024, 16, 2402–2426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Tuszynski, G.P.; Sharma, M.C. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J. Cell Biochem. 2004, 91, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Liebl, J.; Weitensteiner, S.B.; Vereb, G.; Takacs, L.; Furst, R.; Vollmar, A.M.; Zahler, S. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J. Biol. Chem. 2010, 285, 35932–35943. [Google Scholar] [CrossRef]
- de Nigris, F.; Mancini, F.P.; Schiano, C.; Infante, T.; Zullo, A.; Minucci, P.B.; Al-Omran, M.; Giordano, A.; Napoli, C. Osteosarcoma cells induce endothelial cell proliferation during neo-angiogenesis. J. Cell Physiol. 2013, 228, 846–852. [Google Scholar] [CrossRef]
- Merk, H.; Zhang, S.; Lehr, T.; Muller, C.; Ulrich, M.; Bibb, J.A.; Adams, R.H.; Bracher, F.; Zahler, S.; Vollmar, A.M.; et al. Inhibition of endothelial Cdk5 reduces tumor growth by promoting non-productive angiogenesis. Oncotarget 2016, 7, 6088–6104. [Google Scholar] [CrossRef]
- Dorand, R.D.; Nthale, J.; Myers, J.T.; Barkauskas, D.S.; Avril, S.; Chirieleison, S.M.; Pareek, T.K.; Abbott, D.W.; Stearns, D.S.; Letterio, J.J.; et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 2016, 353, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Ren, Y.; Jia, H.H.; Xu, Y.Q.; Zhou, X.; Zhao, X.H.; Wang, Y.F.; Song, X.; Zhu, Z.Y.; Sun, T.; Dou, Y.; et al. Paracrine and epigenetic control of CAF-induced metastasis: The role of HOTAIR stimulated by TGF-ss1 secretion. Mol. Cancer 2018, 17, 5. [Google Scholar] [CrossRef]
- Reiter, V.; Matschkal, D.M.; Wagner, M.; Globisch, D.; Kneuttinger, A.C.; Muller, M.; Carell, T. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012, 40, 6235–6240. [Google Scholar] [CrossRef]
- Lam, E.; Choi, S.H.; Pareek, T.K.; Kim, B.G.; Letterio, J.J. Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727. Mol. Immunol. 2015, 67, 317–324. [Google Scholar] [CrossRef]
- Lam, E.; Pareek, T.K.; Letterio, J.J. Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex. Cell Cycle 2015, 14, 1327–1336. [Google Scholar] [CrossRef][Green Version]
- Arif, A.; Jia, J.; Moodt, R.A.; DiCorleto, P.E.; Fox, P.L. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control. Proc. Natl. Acad. Sci. USA 2011, 108, 1415–1420. [Google Scholar] [CrossRef]
- Liebl, J.; Zhang, S.; Moser, M.; Agalarov, Y.; Demir, C.S.; Hager, B.; Bibb, J.A.; Adams, R.H.; Kiefer, F.; Miura, N.; et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 2015, 6, 7274. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. The DNA-damage response: New molecular insights and new approaches to cancer therapy. Biochem. Soc. Trans. 2009, 37, 483–494. [Google Scholar] [CrossRef]
- Yue, C.H.; Oner, M.; Chiu, C.Y.; Chen, M.C.; Teng, C.L.; Wang, H.Y.; Hsieh, J.T.; Lai, C.H.; Lin, H. RET Regulates Human Medullary Thyroid Cancer Cell Proliferation through CDK5 and STAT3 Activation. Biomolecules 2021, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Xie, J.M.; Li, B.; Sun, Y.H.; Gao, Q.G.; Ding, Z.H.; Wu, H.R.; Qin, Z.H. TIGAR regulates DNA damage and repair through pentosephosphate pathway and Cdk5-ATM pathway. Sci. Rep. 2015, 5, 9853. [Google Scholar] [CrossRef]
- Vigneron, A.; Gamelin, E.; Coqueret, O. The EGFR-STAT3 oncogenic pathway up-regulates the Eme1 endonuclease to reduce DNA damage after topoisomerase I inhibition. Cancer Res. 2008, 68, 815–825. [Google Scholar] [CrossRef]
- Courapied, S.; Sellier, H.; de Carne Trecesson, S.; Vigneron, A.; Bernard, A.C.; Gamelin, E.; Barre, B.; Coqueret, O. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. J. Biol. Chem. 2010, 285, 26765–26778. [Google Scholar] [CrossRef]
- Quintavalle, M.; Elia, L.; Price, J.H.; Heynen-Genel, S.; Courtneidge, S.A. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci. Signal. 2011, 4, ra49. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Mao, W.; Ahmed, A.A.; Yang, H.; Zhou, J.; Jennings, N.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Miranda, R.; et al. CDK5 Regulates Paclitaxel Sensitivity in Ovarian Cancer Cells by Modulating AKT Activation, p21Cip1- and p27Kip1-Mediated G1 Cell Cycle Arrest and Apoptosis. PLoS ONE 2015, 10, e0131833. [Google Scholar] [CrossRef]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef] [PubMed]
- Mimori, K.; Sadanaga, N.; Yoshikawa, Y.; Ishikawa, K.; Hashimoto, M.; Tanaka, F.; Sasaki, A.; Inoue, H.; Sugimachi, K.; Mori, M. Reduced tau expression in gastric cancer can identify candidates for successful Paclitaxel treatment. Br. J. Cancer 2006, 94, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Chakrapani, B.P.; Schwegler, H.; Hofmann, H.D.; Kirsch, M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells 2009, 27, 431–441. [Google Scholar] [CrossRef]
- Smith, P.D.; Mount, M.P.; Shree, R.; Callaghan, S.; Slack, R.S.; Anisman, H.; Vincent, I.; Wang, X.; Mao, Z.; Park, D.S. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 2006, 26, 440–447. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, J.; Zhang, J.; Wu, S.; Yang, X.; Zhao, X.; Li, H.; Luo, M.; Yu, Q.; Lin, G.; et al. Cyclin-dependent kinase 5 decreases in gastric cancer and its nuclear accumulation suppresses gastric tumorigenesis. Clin. Cancer Res. 2015, 21, 1419–1428. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Xie, J.W.; Chen, P.C.; Zheng, C.H.; Li, P.; Wang, J.B.; Lin, J.X.; Lu, J.; Chen, Q.Y.; Cao, L.L.; et al. Low Expression of CDK5 and p27 Are Associated with Poor Prognosis in Patients with Gastric Cancer. J. Cancer 2016, 7, 1049–1056. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Li, H.; Herrup, K. Cdk5 nuclear localization is p27-dependent in nerve cells: Implications for cell cycle suppression and caspase-3 activation. J. Biol. Chem. 2010, 285, 14052–14061. [Google Scholar] [CrossRef]
- Cao, L.L.; Wu, Y.K.; Lin, T.X.; Lin, M.; Chen, Y.J.; Wang, L.Q.; Wang, J.B.; Lin, J.X.; Lu, J.; Chen, Q.Y.; et al. CDK5 promotes apoptosis and attenuates chemoresistance in gastric cancer via E2F1 signaling. Cancer Cell Int. 2023, 23, 286. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Gu, Z.P.; Zhou, Y.A.; Han, Y.; Li, X.F.; Wang, X.P.; Cheng, Q.S.; Mei, Q.B. Enhancement of radiosensitivity by roscovitine pretreatment in human non-small cell lung cancer A549 cells. J. Radiat. Res. 2008, 49, 541–548. [Google Scholar] [CrossRef]
- Rossini, E.; Tamburello, M.; Abate, A.; Zini, S.; Ribaudo, G.; Gianoncelli, A.; Calza, S.; Valcamonico, F.; Suardi, N.R.; Mirabella, G.; et al. The CDK Inhibitor Dinaciclib Improves Cisplatin Response in Nonseminomatous Testicular Cancer: A Preclinical Study. Cells 2024, 13, 368. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Z.; Xiao, L.; Chen, Y.; Liu, X.; Long, D.; Chai, L.; Li, Y.; Tan, C. Dinaciclib exerts a tumor-suppressing effect via beta-catenin/YAP axis in pancreatic ductal adenocarcinoma. Anticancer. Drugs 2024, 35, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.; Poon, E.; Ebus, M.E.; den Hartog, I.J.; van Noesel, C.J.; Jamin, Y.; Hallsworth, A.; Robinson, S.P.; Petrie, K.; Sparidans, R.W.; et al. Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma. Clin. Cancer Res. 2015, 21, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Robb, C.M.; Kour, S.; Contreras, J.I.; Agarwal, E.; Barger, C.J.; Rana, S.; Sonawane, Y.; Neilsen, B.K.; Taylor, M.; Kizhake, S.; et al. Characterization of CDK(5) inhibitor, 20-223 (aka CP668863) for colorectal cancer therapy. Oncotarget 2018, 9, 5216–5232. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.; Pareek, T.; Asad, A.B.; Rajkumar, R.; Cheong, W.F.; Wenk, M.R.; Dawe, G.S.; Chuang, K.H.; et al. Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J. Neurosci. 2013, 33, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Seo, J.; Binukumar, B.K.; Amin, N.D.; Reddy, P.; Grant, P.; Kuntz, S.; Kesavapany, S.; Steiner, J.; Mishra, S.K.; et al. TFP5, a Peptide Inhibitor of Aberrant and Hyperactive Cdk5/p25, Attenuates Pathological Phenotypes and Restores Synaptic Function in CK-p25Tg Mice. J. Alzheimers Dis. 2017, 56, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.D.; Zheng, Y.; Bk, B.; Shukla, V.; Skuntz, S.; Grant, P.; Steiner, J.; Bhaskar, M.; Pant, H.C. The interaction of Munc 18 (p67) with the p10 domain of p35 protects in vivo Cdk5/p35 activity from inhibition by TFP5, a peptide derived from p35. Mol. Biol. Cell 2016, 27, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Wang, H.; Amin, N.; Jung, J.; Appay, R.; Cui, J.; Song, Q.; Cardone, A.; Park, D.M.; Gilbert, M.R.; et al. TP5, a Peptide Inhibitor of Aberrant and Hyperactive CDK5/p25: A Novel Therapeutic Approach against Glioblastoma. Cancers 2020, 12, 1935. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Neklesa, T.K.; Winkler, J.D.; Crews, C.M. Targeted protein degradation by PROTACs. Pharmacol. Ther. 2017, 174, 138–144. [Google Scholar] [CrossRef]

| Biological Process | Molecule | Biological Role | Ref. |
|---|---|---|---|
| Neural Progenitor Regulation | |||
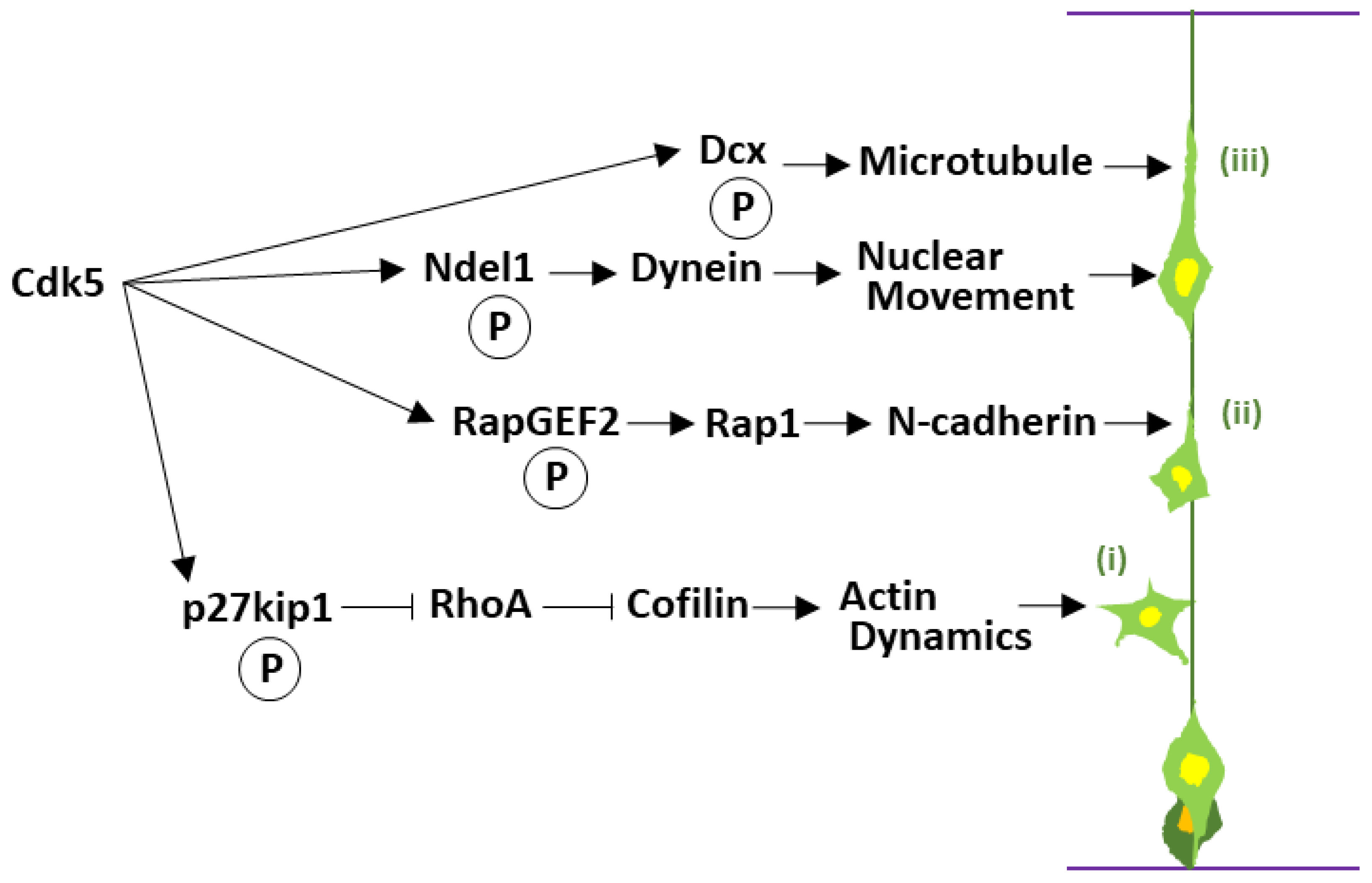
| Cell Cycle Exit and Differentiation | p27kip1 (CDK Inhibitor) | Phosphorylation at Ser10 stabilizes the protein to regulate actin dynamics for neuronal migration. | [36] |
| Axin (Wnt Signaling) | Phosphorylation at Thr485 biases Wnt signaling from proliferation toward differentiation and axon formation. | [37,38] | |
| Dixdc1 (DISC1 Partner; Scaffold) | Phosphorylation at Ser250 enhances Ndel1 binding to promote migration, not affecting DISC1–Wnt/proliferation. | [39] | |
| Neuronal Migration | |||
| Multipolar to Bipolar Transition | RapGEF2 (Guanine Nucleotide Exchange Factor) | Activates Rap1, initiating the process of N-cadherin surface trafficking. | [40] |
| Locomotion and Cytoskeletal Control | N-cadherin (Adhesion Molecule) | Establishes and maintains adhesion to radial glia, providing the physical track for sustained locomotion. | [41] |
| β-catenin (Adhesion Complex) | Phosphorylation negatively regulates N-cadherin-mediated cell adhesion. | [42] | |
| Doublecortin (Dcx) (Microtubule Regulator) | Phosphorylation at Ser297 modulates microtubule binding to ensure cytoskeletal flexibility during movement. | [43] | |
| Ndel1 and Nde1 (Dynein Regulators) | Control the dynein motor complex to drive nuclear translocation (nucleokinesis). | [44,45] | |
| CRMP2 (Microtubule Regulator) | Phosphorylation at Ser522 contributes to microtubule assembly and proper neuronal migration. | [46] | |
| Synapse Maturation, Plasticity, and Homeostasis | |||
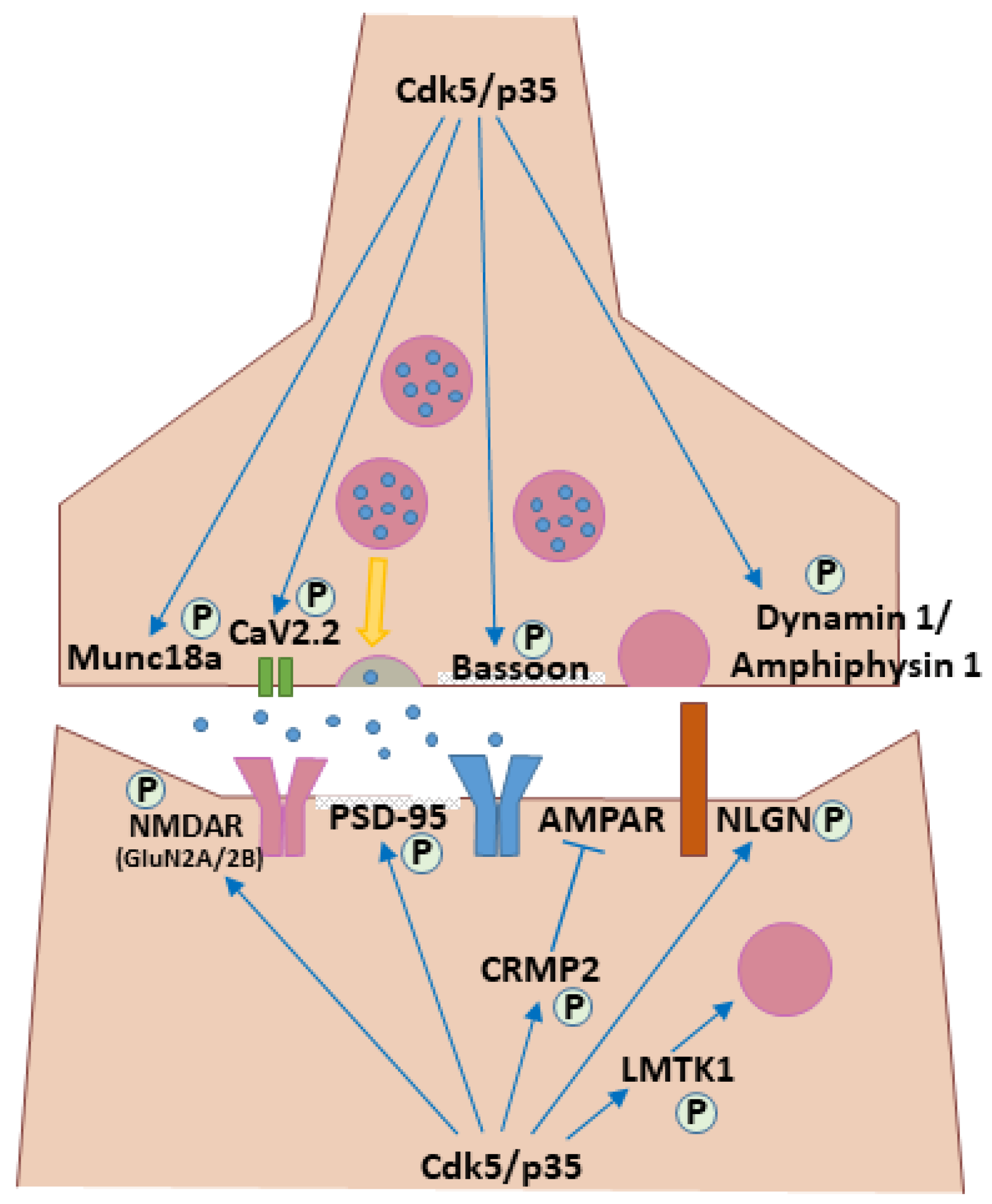
| Presynaptic Function | Munc18a (SNARE Regulator) | Modulates the SNARE complex to regulate neurotransmitter release. | [47] |
| Dynamin 1 (Endocytosis) | Phosphorylation at Ser774/Ser778 regulates synaptic vesicle endocytosis. | [48,49] | |
| Amphiphysin 1 (Endocytosis) | Phosphorylation regulates clathrin-mediated endocytosis for synaptic vesicle recycling. | [49,50] | |
| Bassoon (Scaffold) | Organizes the presynaptic active zone and coordinates Cdk5 and PKA signaling. | [51] | |
| CaV2.2 (N-type Ca2+ Channel) | Phosphorylation increases Ca2+ influx and neurotransmitter release. | [52] | |
| P/Q-type Ca2+ Channels | Phosphorylation reduces channel activity, dampening neurotransmitter release. | [53] | |
| Postsynaptic Function | GluN2A (NMDAR Subunit) | Phosphorylation at Ser1232 directly enhances receptor function, contributing to long-term potentiation (LTP). | [54] |
| GluN2B (NMDAR Subunit) | Promotes endocytosis and reduces surface levels via direct (Ser1116) and indirect (PSD-95/Src) mechanisms. | [55,56] | |
| CRMP2 (Microtubule Regulator) | Phosphorylation at Ser522 negatively regulates the surface delivery of AMPA receptors. | [57] | |
| PSD-95 (Scaffold) | Phosphorylation regulates scaffold dynamics and synaptic structure. | [58] | |
| Neuroligins (NLGN3, NLGN4X) (Synapse Organizers) | Phosphorylation modulates protein interactions, surface expression, and spine morphology. | [59,60] | |
| LMTK1 (Kinase) | Controls endosome trafficking to supply membrane for dendritic spine growth. | [61] | |
| Circadian Rhythm Regulation | CLOCK (Transcription Factor) | Phosphorylation at Thr451/Thr461 promotes nuclear translocation and transcriptional activity. | [62] |
| PER2 (Transcription Factor) | Phosphorylation at Ser394 facilitates nuclear entry and stabilizes the clock. | [63] | |
| Pathological Dysregulation | |||
| Pathological Activation | p25 (Activator Fragment) | Forms a hyperactive and mislocalized complex with Cdk5, driving neurotoxicity. | [14] |
| Pathological Substrate | Tau (Microtubule Regulator) | Aberrant hyperphosphorylation by Cdk5/p25, leading to synaptic damage. | [64] |
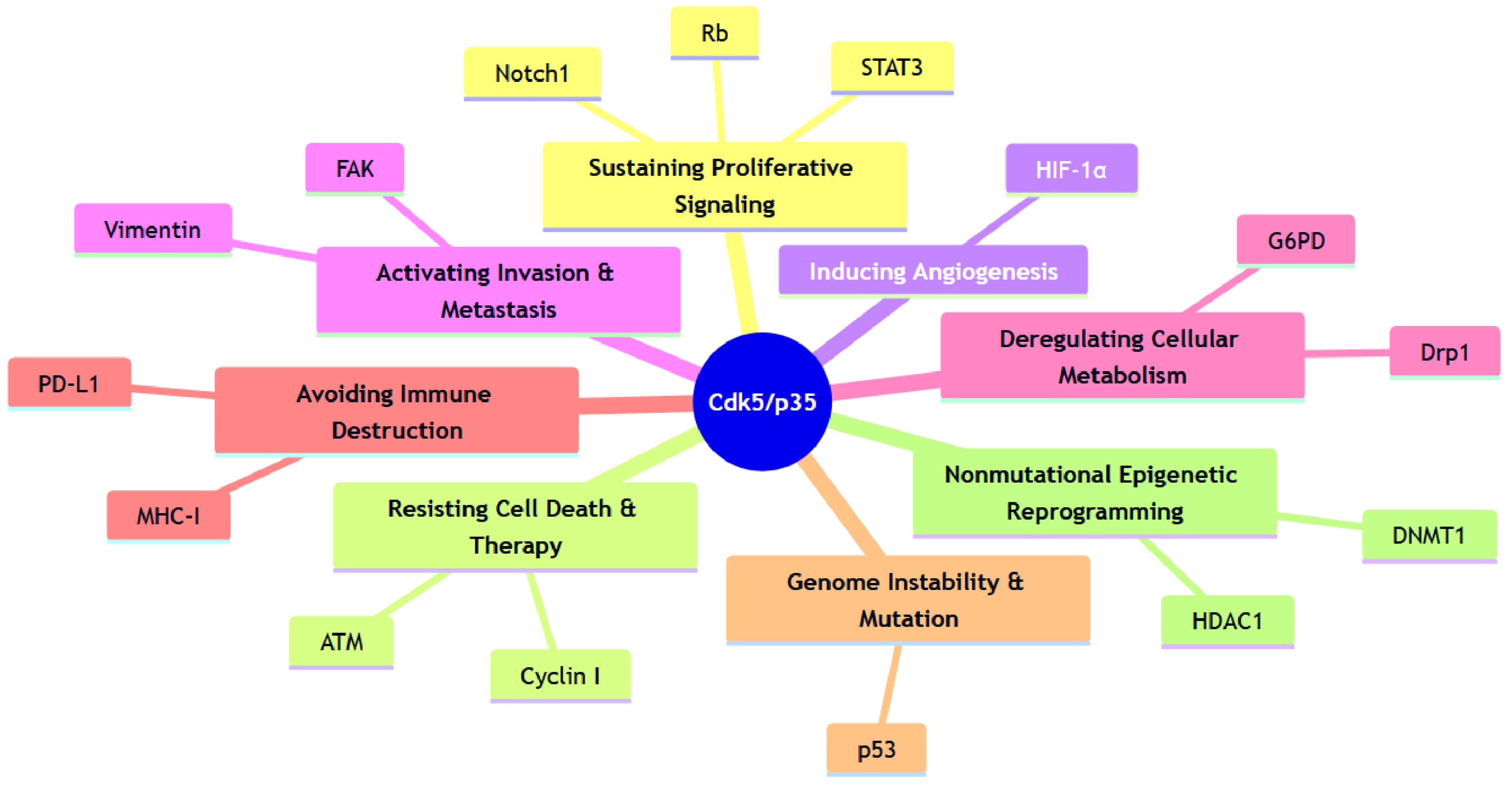
| Hallmark of Cancer | Molecule | Mechanism of Action | Cancer Type | Ref. |
|---|---|---|---|---|
| Sustaining Proliferative Signaling | Rb (Tumor Suppressor) | Phosphorylation inactivates Rb to promote G1/S transition, substituting for Cdk4/6 in some contexts. | Medullary Thyroid | [22] |
| STAT3 (Transcription Factor) | Phosphorylation at Ser727 activates transcription and proliferation. | Prostate, Medullary Thyroid | [102,103] | |
| Androgen Receptor (AR) (Nuclear Receptor) | Phosphorylation increases protein stability and transcriptional activity. | Prostate | [103,104] | |
| MCM2 (DNA Replication Factor) | Phosphorylation promotes DNA replication. | Pituitary | [105] | |
| p21cip1 (CDK Inhibitor) | Phosphorylation inactivates p21cip1 to promote cell cycle progression. | Thyroid | [106] | |
| Notch1 (Signaling Receptor) | Phosphorylation positively regulates Notch1 signaling pathway activity. | Pancreatic | [107] | |
| ERK5 (Kinase) | Phosphorylation at Thr732 enables AP-1 coactivation and c-Myc upregulation. | Colorectal | [108] | |
| c-Myc (Protooncogene) | Phosphorylation at Ser62 prevents the tumor suppressor BIN1 binding, disinhibiting proliferation. | NSCLC | [109] | |
| Resisting Cell Death & Therapy | ATM (DDR Kinase) | Phosphorylation at Ser794 directly activates ATM to initiate the DNA Damage Response (DDR). | Multiple | [110,111] |
| RPA32 (ssDNA-Binding Protein) | Phosphorylation protects single-stranded DNA (ssDNA) during genotoxic stress. | Multiple | [112] | |
| Cyclin I (CDK-binding) | A non-canonical anti-apoptotic complex with Cdk5 confers cisplatin resistance. | Cervical | [113] | |
| NOXA (BH3-only Protein) | Phosphorylation at Ser13 restrains apoptosis while shifting metabolism to the pentose phosphate pathway. | Hematopoietic | [114] | |
| Inducing Angiogenesis | HIF-1α (Transcription Factor) | Phosphorylation stabilizes the protein, leading to upregulation of VEGF. | Multiple | [115] |
| Activating Invasion & Metastasis | FAK (Integrin-binding) | Phosphorylation at Ser732 regulates focal adhesions and EMT. | Breast | [116] |
| Talin (Integrin-binding) | Phosphorylation at Ser425 modulates integrin activation and cell migration. | Colorectal | [117,118] | |
| Vimentin (Cytoskeleton) | Phosphorylation at Ser56 remodels intermediate filaments to increase cell motility. | Melanoma | [119] | |
| DLC1 (Tumor Suppressor) | Phosphorylation enhances its Rho-GAP activity, exerting context-dependent anti-oncogenic effects. | NSCLC | [120] | |
| Deregulating Cellular Metabolism | G6PD (Metabolic Enzyme) | Phosphorylation activates the pentose phosphate pathway for redox balance. | Breast | [121] |
| Drp1 (Mitochondrial Fission) | Phosphorylation at the conserved Ser616-equivalent site drives mitochondrial fission and creates a therapeutic vulnerability. | Colorectal, Hepatocellular | [122,123] | |
| ACSS2 (Metabolic Enzyme) | Phosphorylation at Ser267 stabilizes the enzyme, driving acetyl-CoA production from acetate. | Glioblastoma | [124] | |
| Avoiding Immune Destruction | MHC-I (Antigen Presentation) | Astrocyte-induced downregulation of surface MHC-I enables T-cell evasion. | Breast (Brain Metastasis) | [125] |
| PD-L1 (Immunosuppression) | Context-dependent regulation: promotes degradation (Hepatocellular) or stabilization (Lung). | Hepatocellular, Lung | [126,127] | |
| Genome Instability & Mutation | p53 (Tumor Suppressor) | Dual regulation: pro-apoptotic in some contexts; may contribute to DDR dysregulation in others. | (General) | [128] |
| Nonmutational Epigenetic Reprogramming | HDAC1 (Epigenetic Modifier) | Inhibition of deacetylase activity (in pathological contexts) leads to aberrant gene expression and DNA damage. | (General) | [129] |
| mSds3 (HDAC Complex Component) | Phosphorylation at Ser228 modulates HDAC complex and histone acetylation. | (General) | [130] | |
| DNMT1 (Epigenetic Modifier) | Phosphorylation at Ser154 shown in vitro; in vivo oncogenic relevance requires validation. | (General) | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, Y.V.; Kawauchi, T. The Duality of Cdk5: A Master Regulator in Neurodevelopment and a Hijacked Oncogene in Cancer. Cells 2025, 14, 1876. https://doi.org/10.3390/cells14231876
Nishimura YV, Kawauchi T. The Duality of Cdk5: A Master Regulator in Neurodevelopment and a Hijacked Oncogene in Cancer. Cells. 2025; 14(23):1876. https://doi.org/10.3390/cells14231876
Chicago/Turabian StyleNishimura, Yoshiaki V., and Takeshi Kawauchi. 2025. "The Duality of Cdk5: A Master Regulator in Neurodevelopment and a Hijacked Oncogene in Cancer" Cells 14, no. 23: 1876. https://doi.org/10.3390/cells14231876
APA StyleNishimura, Y. V., & Kawauchi, T. (2025). The Duality of Cdk5: A Master Regulator in Neurodevelopment and a Hijacked Oncogene in Cancer. Cells, 14(23), 1876. https://doi.org/10.3390/cells14231876







