Biosynthesis of Ribose-5-Phosphate—Metabolic Regulator of Escherichia coli Viability
Abstract
1. Introduction
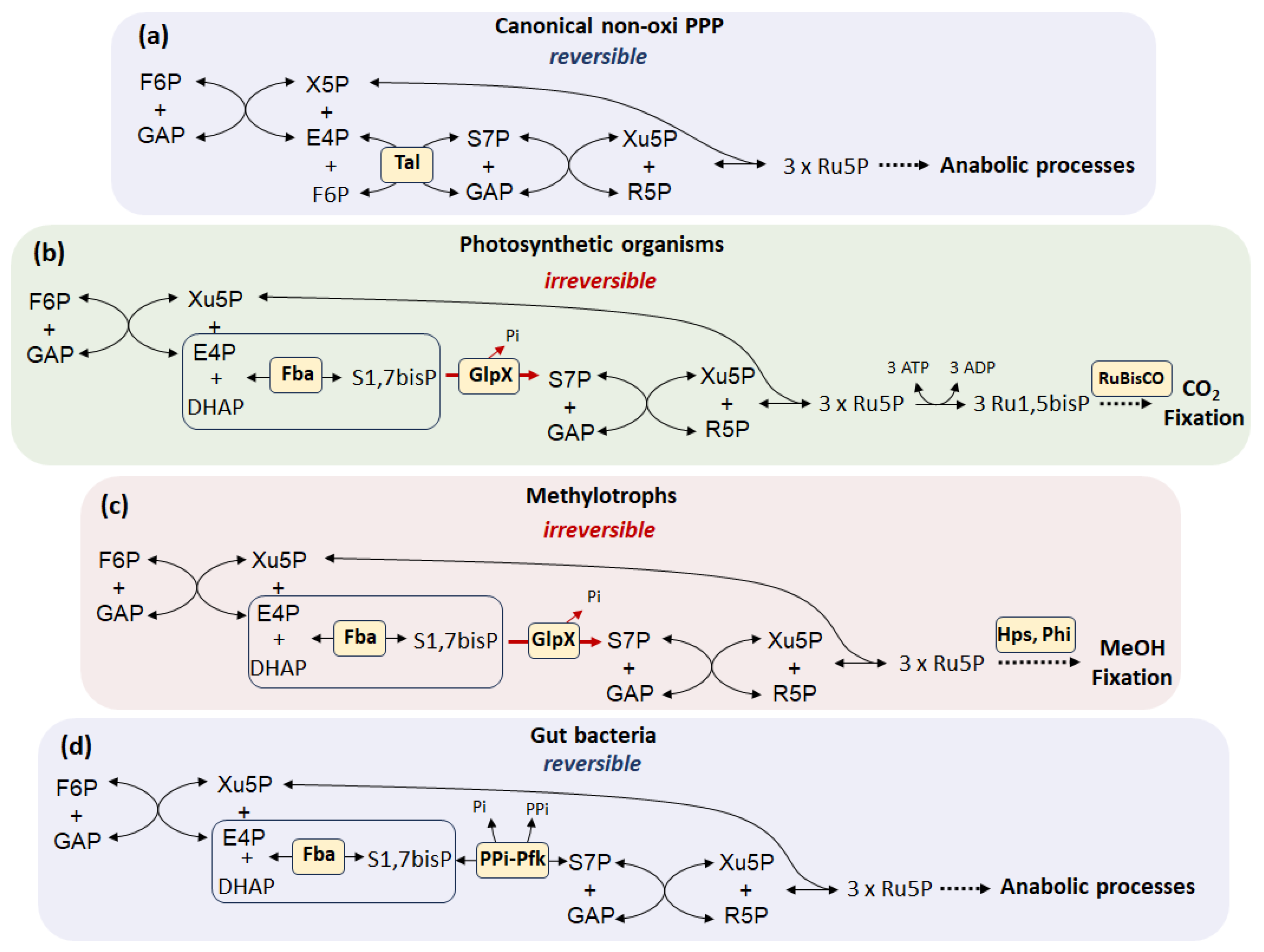
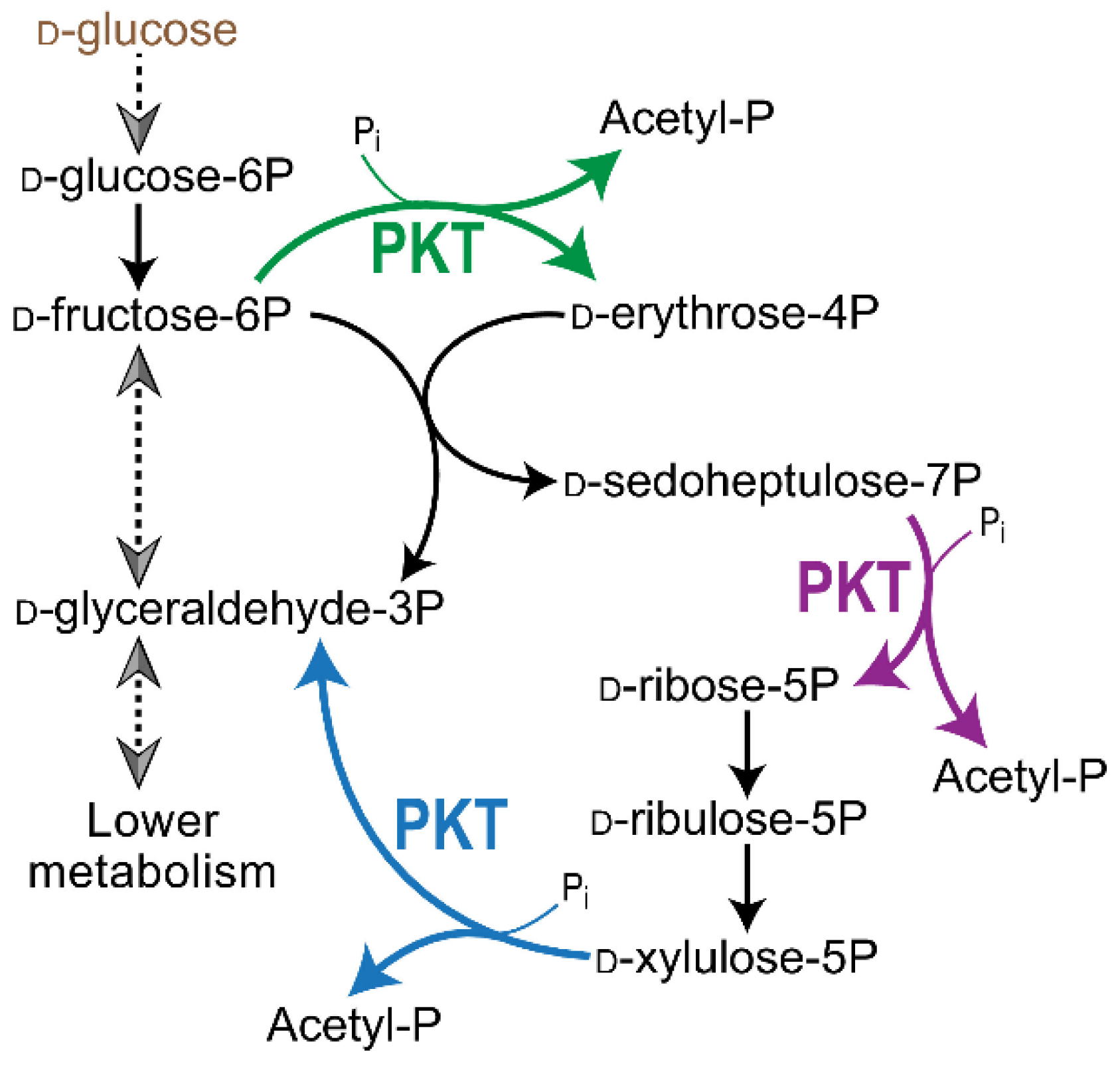
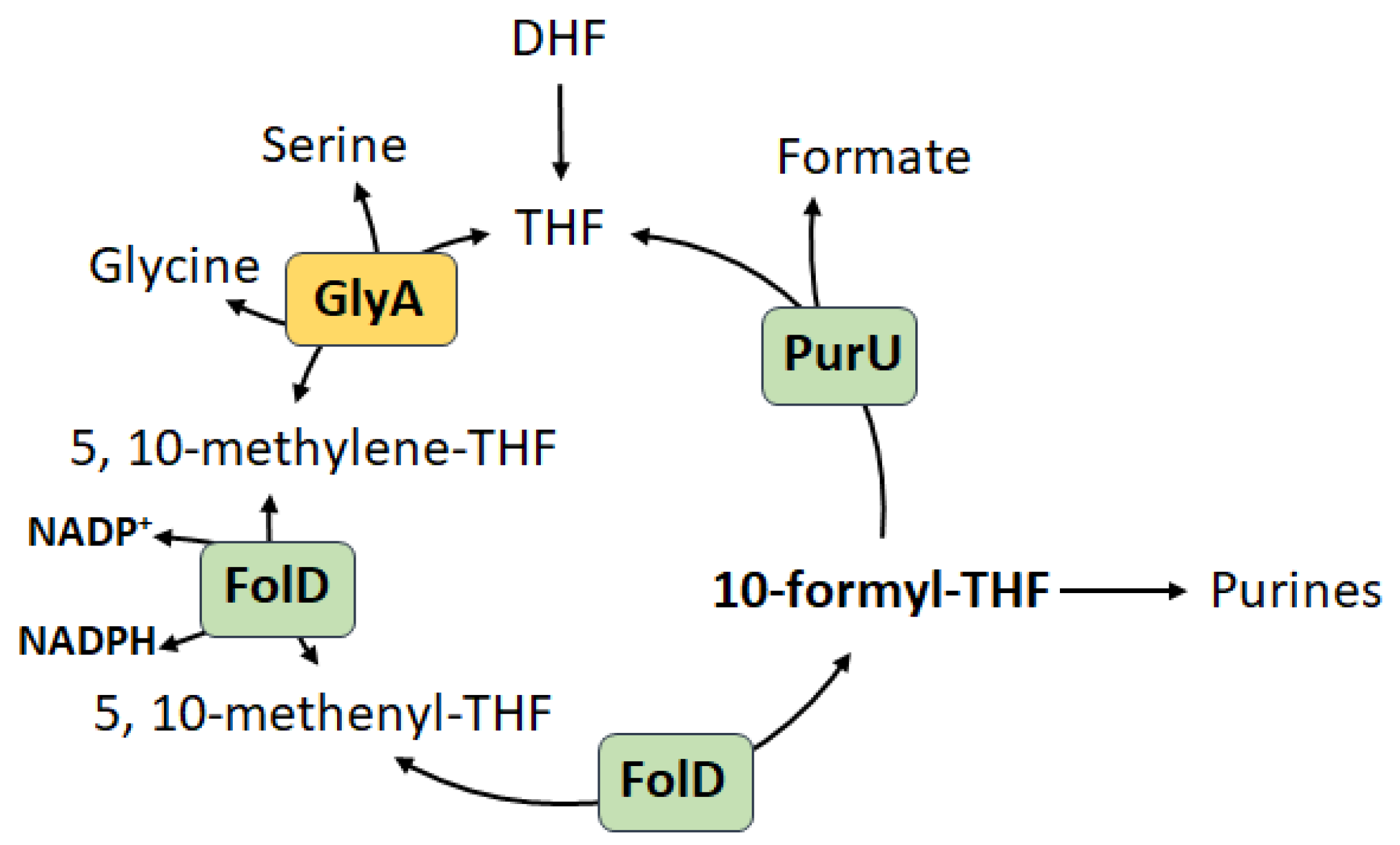
2. Diversity of R5P Biosynthetic Pathways
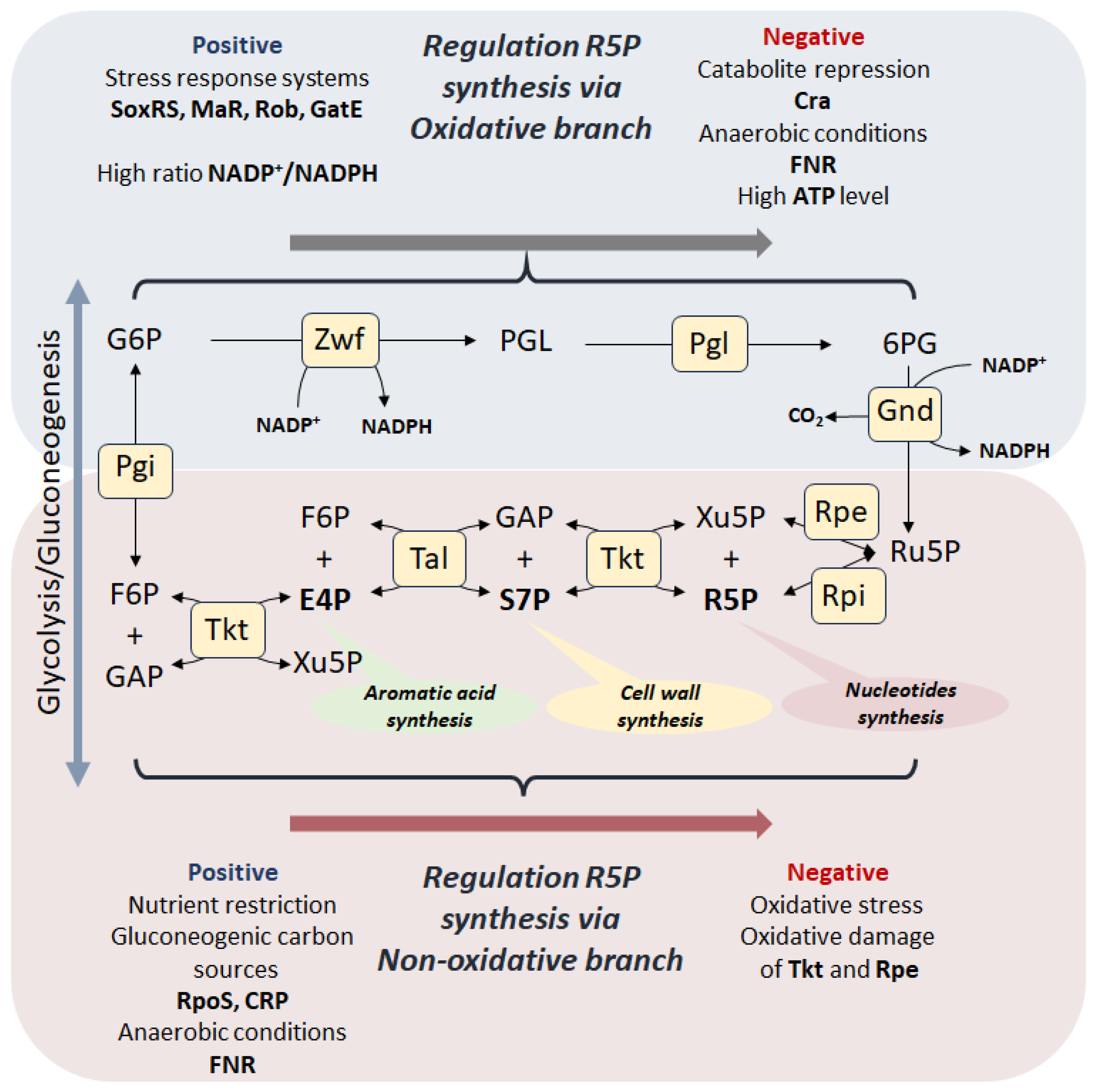
3. Regulation of PPP
3.1. Regulation of the Oxidative Branch
3.2. Regulation of the Non-Oxidative Branch
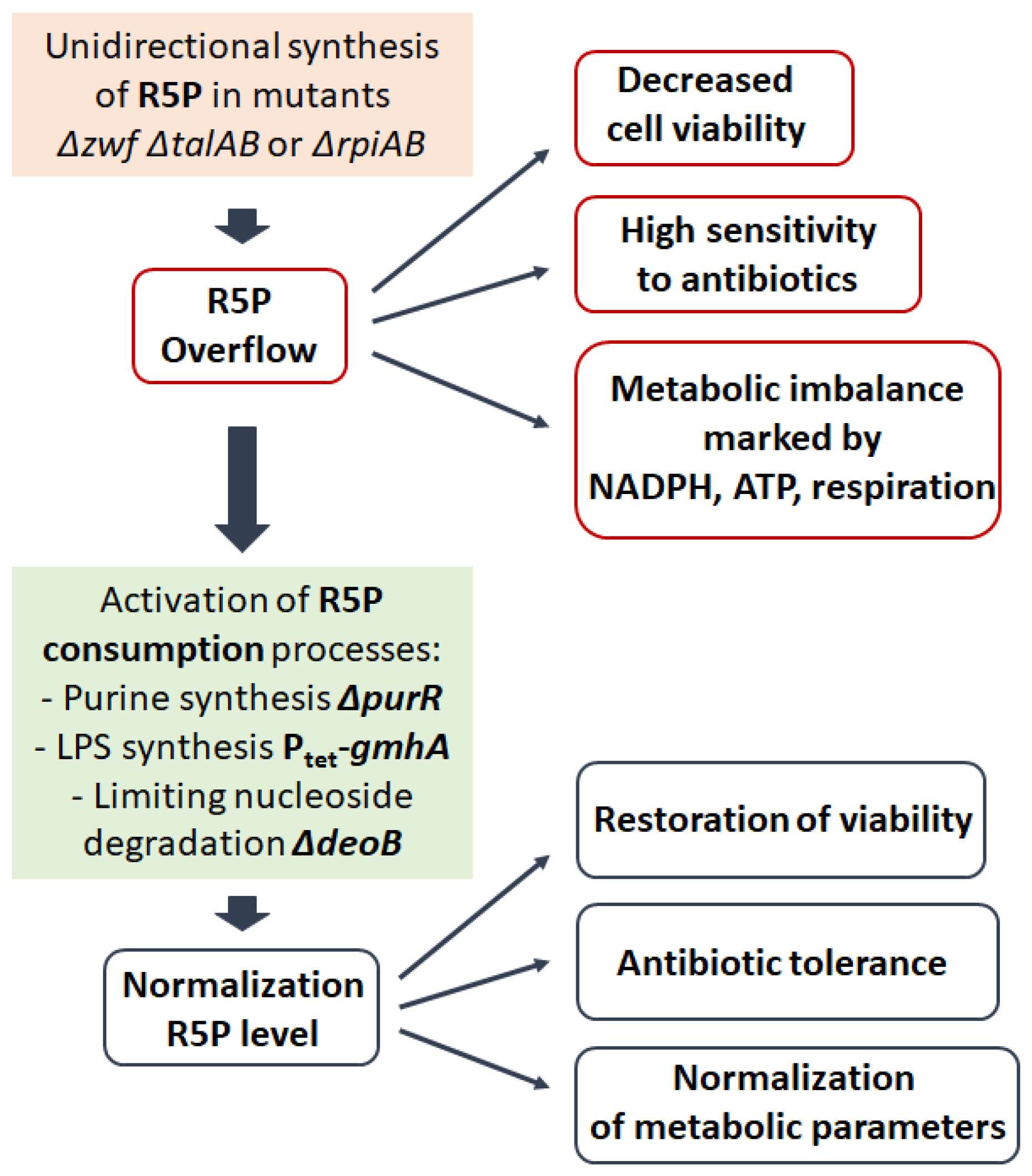
4. Genetic Modification of PPP and Consequences for the Cell
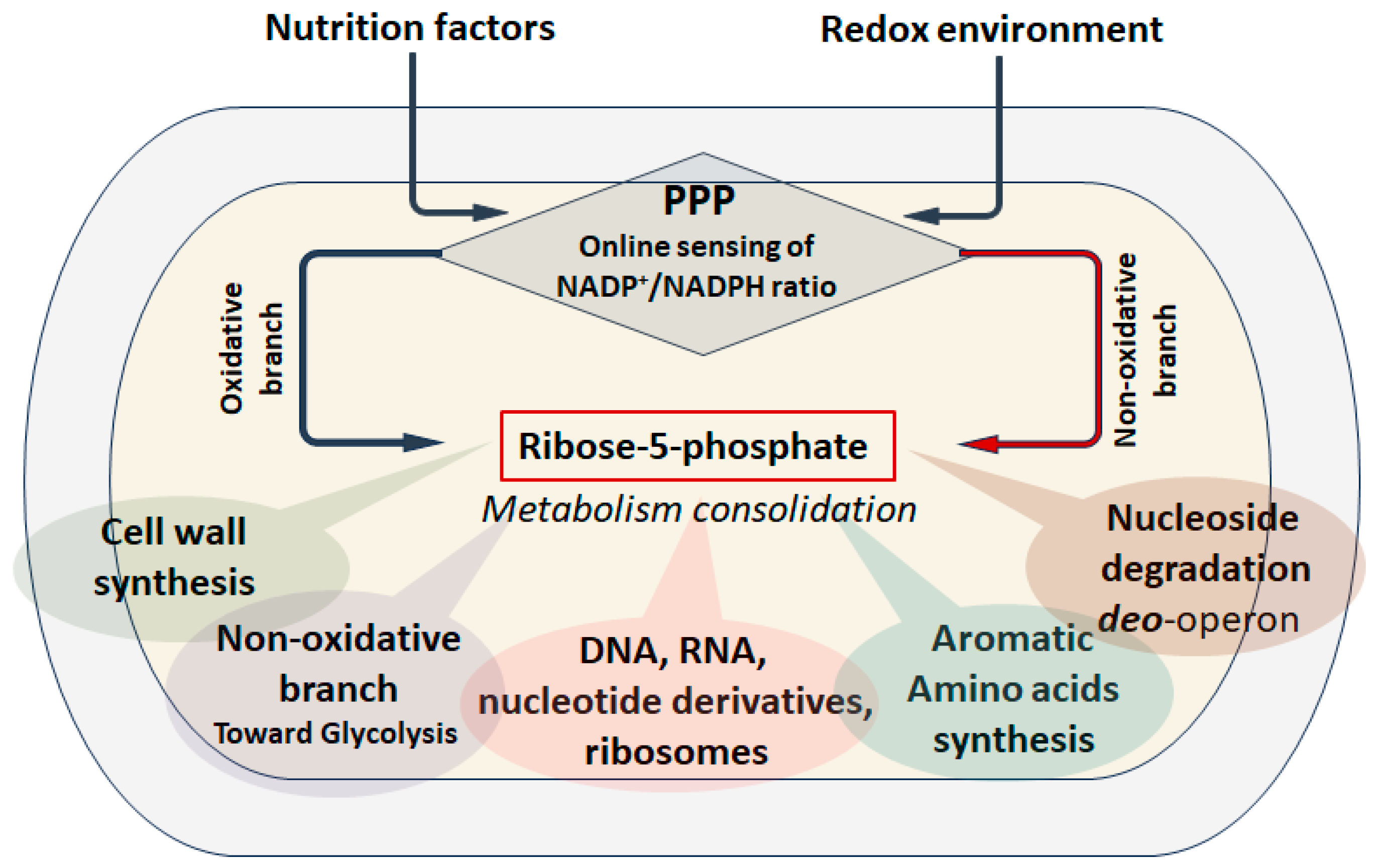
5. PPP as an Anabolic Sensor
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Wamelink, M.M.C.; Struys, E.A.; Jakobs, C. The Biochemistry, Metabolism and Inherited Defects of the Pentose Phosphate Pathway: A Review. J. Inherit. Metab. Dis. 2008, 31, 703–717. [Google Scholar] [CrossRef]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The Pentose Phosphate Pathway in Health and Disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.-L.; Norambuena, J.; Boyd, J.M.; Parker, D. Impact of the Pentose Phosphate Pathway on Metabolism and Pathogenesis of Staphylococcus Aureus. PLoS Pathog. 2023, 19, e1011531. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gao, M.; Suástegui, M.; Mei, Y.; Shao, Z. Building Microbial Factories for the Production of Aromatic Amino Acid Pathway Derivatives: From Commodity Chemicals to Plant-Sourced Natural Products. Metab. Eng. 2020, 58, 94–132. [Google Scholar] [CrossRef]
- Wulf, P.D.; Vandamme, E.J. Production of D-Ribose by Fermentation. Appl. Microbiol. Biotechnol. 1997, 48, 141–148. [Google Scholar] [CrossRef]
- Moon, S.J.; Dong, W.; Stephanopoulos, G.N.; Sikes, H.D. Oxidative Pentose Phosphate Pathway and Glucose Anaplerosis Support Maintenance of Mitochondrial NADPH Pool Under Mitochondrial Oxidative Stress. Bioeng. Transl. Med. 2020, 5, e10184. [Google Scholar] [CrossRef]
- Hurbain, J.; Thommen, Q.; Anquez, F.; Pfeuty, B. Quantitative Modeling of Pentose Phosphate Pathway Response to Oxidative Stress Reveals a Cooperative Regulatory Strategy. iScience 2022, 25, 104681. [Google Scholar] [CrossRef]
- Loopmans, S.; Rohlenova, K.; van Brussel, T.; Stockmans, I.; Moermans, K.; Peredo, N.; Carmeliet, P.; Lambrechts, D.; Stegen, S.; Carmeliet, G. The Pentose Phosphate Pathway Controls Oxidative Protein Folding and Prevents Ferroptosis in Chondrocytes. Nat. Metab. 2025, 7, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Reyes, J.S.; Figueroa, J.D.; Davies, M.J.; López-Alarcón, C. The Enzymes of the Oxidative Phase of the Pentose Phosphate Pathway as Targets of Reactive Species: Consequences for NADPH Production. Biochem. Soc. Trans. 2023, 51, 2173–2187. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics Induce Redox-Related Physiological Alterations as Part of Their Lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Seregina, T.A.; Shakulov, R.S.; Sklyarova, S.A.; Mironov, A.S. Disruptions of rpiAB Genes Encoding Ribose-5-Phosphate Isomerases in E. coli Increases Sensitivity of Bacteria to Antibiotics. Cells 2024, 13, 1915. [Google Scholar] [CrossRef]
- Seregina, T.; Shakulov, R.; Quarta, G.; Shatalin, K.; Sklyarova, S.; Petrushanko, I.; Fedulov, A.P.; Ivanov, A.V.; Mitkevich, V.; Makarov, A.; et al. Ribose-5-Phosphate Metabolism Protects E. coli from Antibiotic Lethality. mBio 2025, 16, e00654-25. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of Life: The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Bada, J.L.; Korenaga, J. Exposed Areas Above Sea Level on Earth >3.5 Gyr Ago: Implications for Prebiotic and Primitive Biotic Chemistry. Life 2018, 8, 55. [Google Scholar] [CrossRef]
- Banfalvi, G. Ribose Selected as Precursor to Life. DNA Cell Biol. 2020, 39, 177–186. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; Montero-Gómez, N.; Montero, F. From Prebiotic Chemistry to Cellular Metabolism—The Chemical Evolution of Metabolism before Darwinian Natural Selection. J. Theor. Biol. 2008, 252, 505–519. [Google Scholar] [CrossRef]
- Cronin, L.; Walker, S.I. Beyond Prebiotic Chemistry. Science 2016, 352, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.A.; Turchyn, A.V.; Ralser, M. Non-enzymatic Glycolysis and Pentose Phosphate Pathway-like Reactions in a Plausible Archean Ocean. Mol. Syst. Biol. 2014, 10, 725. [Google Scholar] [CrossRef]
- Sharkey, T.D. Pentose Phosphate Pathway Reactions in Photosynthesizing Cells. Cells 2021, 10, 1547. [Google Scholar] [CrossRef]
- Sarwar, A.; Lee, E.Y. Methanol-Based Biomanufacturing of Fuels and Chemicals Using Native and Synthetic Methylotrophs. Synth. Syst. Biotechnol. 2023, 8, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Garschagen, L.S.; Franke, T.; Deppenmeier, U. An Alternative Pentose Phosphate Pathway in Human Gut Bacteria for the Degradation of C5 Sugars in Dietary Fibers. FEBS J. 2021, 288, 1839–1858. [Google Scholar] [CrossRef] [PubMed]
- Basen, M.; Kurrer, S.E. A Close Look at Pentose Metabolism of Gut Bacteria. FEBS J. 2021, 288, 1804–1808. [Google Scholar] [CrossRef]
- Soderberg, T. Biosynthesis of Ribose-5-Phosphate and Erythrose-4-Phosphate in Archaea: A Phylogenetic Analysis of Archaeal Genomes. Archaea 2005, 1, 314760. [Google Scholar] [CrossRef]
- Siebers, B.; Zaparty, M.; Raddatz, G.; Tjaden, B.; Albers, S.-V.; Bell, S.D.; Blombach, F.; Kletzin, A.; Kyrpides, N.; Lanz, C.; et al. The Complete Genome Sequence of Thermoproteus Tenax: A Physiologically Versatile Member of the Crenarchaeota. PLoS ONE 2011, 6, e24222. [Google Scholar] [CrossRef]
- Grochowski, L.L.; Xu, H.; White, R.H. Ribose-5-Phosphate Biosynthesis in Methanocaldococcus Jannaschii Occurs in the Absence of a Pentose-Phosphate Pathway. J. Bacteriol. 2005, 187, 7382–7389. [Google Scholar] [CrossRef]
- Yasueda, H.; Kawahara, Y.; Sugimoto, S.-I. Bacillus Subtilis yckG and yckF Encode Two Key Enzymes of the Ribulose Monophosphate Pathway Used by Methylotrophs, and yckH Is Required for Their Expression. J. Bacteriol. 1999, 181, 7154–7160. [Google Scholar] [CrossRef]
- Reizer, J.; Reizer, A.; Saier, M.H. Is the Ribulose Monophosphate Pathway Widely Distributed in Bacteria? Microbiology 1997, 143, 2519–2520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, K.; Shirai, T.; Vavricka, C.J.; Matsuda, M.; Kondo, A.; Hasunuma, T. Dark Accumulation of Downstream Glycolytic Intermediates Initiates Robust Photosynthesis in Cyanobacteria. Plant Physiol. 2023, 191, 2400–2413. [Google Scholar] [CrossRef]
- Shinde, S.; Zhang, X.; Singapuri, S.P.; Kalra, I.; Liu, X.; Morgan-Kiss, R.M.; Wang, X. Glycogen Metabolism Supports Photosynthesis Start through the Oxidative Pentose Phosphate Pathway in Cyanobacteria1. Plant Physiol. 2020, 182, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, G.A. Genetics of Pentose-Phosphate Pathway Enzymes of Escherichia coli K-12. Arch. Microbiol. 1995, 164, 324–330. [Google Scholar] [CrossRef]
- Phégnon, L.; Pérochon, J.; Uttenweiler-Joseph, S.; Cahoreau, E.; Millard, P.; Létisse, F. 6-Phosphogluconolactonase Is Critical for the Efficient Functioning of the Pentose Phosphate Pathway. FEBS J. 2024, 291, 4459–4472. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Weiss, B. Two-Stage Induction of the soxRS (Superoxide Response) Regulon of Escherichia coli. J. Bacteriol. 1992, 174, 3915–3920. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Fawcett, W.P.; Wolf, R.E. Genetic Definition of the Escherichia coli Zwf “Soxbox,” the DNA Binding Site for SoxS-Mediated Induction of Glucose 6-Phosphate Dehydrogenase in Response to Superoxide. J. Bacteriol. 1995, 177, 1742–1750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jair, K.W.; Martin, R.G.; Rosner, J.L.; Fujita, N.; Ishihama, A.; Wolf, R.E. Purification and Regulatory Properties of MarA Protein, a Transcriptional Activator of Escherichia coli Multiple Antibiotic and Superoxide Resistance Promoters. J. Bacteriol. 1995, 177, 7100–7104. [Google Scholar] [CrossRef]
- Ariza, R.R.; Li, Z.; Ringstad, N.; Demple, B. Activation of Multiple Antibiotic Resistance and Binding of Stress-Inducible Promoters by Escherichia coli Rob Protein. J. Bacteriol. 1995, 177, 1655–1661. [Google Scholar] [CrossRef]
- Chetri, S.; Das, B.J.; Bhowmik, D.; Chanda, D.D.; Chakravarty, A.; Bhattacharjee, A. Transcriptional Response of Mar, Sox and Rob Regulon against Concentration Gradient Carbapenem Stress within Escherichia coli Isolated from Hospital Acquired Infection. BMC Res. Notes 2020, 13, 168. [Google Scholar] [CrossRef]
- Chubiz, L.M.; Glekas, G.D.; Rao, C.V. Transcriptional Cross Talk within the Mar-Sox-Rob Regulon in Escherichia coli Is Limited to the Rob and marRAB Operons. J. Bacteriol. 2012, 194, 4867–4875. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial Response to Acid Stress: Mechanisms and Applications. Appl. Microbiol. Biotechnol. 2019, 104, 51–65. [Google Scholar] [CrossRef]
- Saint Martin, C.; Caccia, N.; Darsonval, M.; Gregoire, M.; Combeau, A.; Jubelin, G.; Dubois-Brissonnet, F.; Leroy, S.; Briandet, R.; Desvaux, M. Spatially Localised Expression of the Glutamate Decarboxylase gadB in Escherichia coli O157:H7 Microcolonies in Hydrogel Matrices. npj Sci. Food 2023, 7, 55. [Google Scholar] [CrossRef]
- Kumar, R.; Shimizu, K. Transcriptional Regulation of Main Metabolic Pathways of cyoA, cydB, Fnr, and Fur Gene Knockout Escherichia coli in C-Limited and N-Limited Aerobic Continuous Cultures. Microb. Cell Factories 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, T.M. Cra and the Control of Carbon Flux via Metabolic Pathways. Res. Microbiol. 1996, 147, 489–493. [Google Scholar] [CrossRef]
- Josephson, B.L.; Fraenkel, D.G. Sugar Metabolism in Transketolase Mutants of Escherichia coli. J. Bacteriol. 1974, 118, 1082–1089. [Google Scholar] [CrossRef]
- Olavarría, K.; Valdés, D.; Cabrera, R. The Cofactor Preference of Glucose-6-Phosphate Dehydrogenase from Escherichia coli– Modeling the Physiological Production of Reduced Cofactors. FEBS J. 2012, 279, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Mori, H.; Tomita, M.; Yoshino, M. Inhibitory Effect of Phosphoenolpyruvate on Glycolytic Enzymes in Escherichia coli. Res. Microbiol. 2007, 158, 159–163. [Google Scholar] [CrossRef] [PubMed]
- PARR, C.W. Inhibition of Phosphoglucose Isomerase. Nature 1956, 178, 1401. [Google Scholar] [CrossRef]
- Blackburn, M.N.; Noltmann, E.A. Physical Studies on the Subunit Structure of Rabbit Muscle Phosphoglucose Isomerase. J. Biol. Chem. 1972, 247, 5668–5674. [Google Scholar] [CrossRef]
- Bonsignore, A.; Pontremoli, S.; Grazi, E.; Horecker, B.L. Effect of Orthophosphate on the Transaldolase Reaction. J. Biol. Chem. 1960, 235, 1888–1890. [Google Scholar] [CrossRef]
- Venkataraman, R.; Racker, E. Mechanism of Action of Transaldolase: II. The Substrate-Enzyme Intermediate. J. Biol. Chem. 1961, 236, 1883–1886. [Google Scholar] [CrossRef]
- Vaseghi, S.; Baumeister, A.; Rizzi, M.; Reuss, M. In Vivo Dynamics of the Pentose Phosphate Pathway in Saccharomyces Cerevisiae. Metab. Eng. 1999, 1, 128–140. [Google Scholar] [CrossRef]
- Barcia-Vieitez, R.; Ramos-Martínez, J.I. The Regulation of the Oxidative Phase of the Pentose Phosphate Pathway: New Answers to Old Problems. IUBMB Life 2014, 66, 775–779. [Google Scholar] [CrossRef]
- Avigad, G. Inhibition of Glucose 6-Phosphate Dehydrogenase by Adenosine 5′-Triphosphate. Proc. Natl. Acad. Sci. USA 1966, 56, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.N.; Anderson, W.B.; Nordlie, R.C. Glucose Dehydrogenase Activity of Yeast Glucose 6-Phosphate Dehydrogenase. Inhibition by Adenosine 5′-Triphosphate and Other Nucleoside 5′-Triphosphates and Diphosphates. Biochemistry 1970, 9, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Aledo, J.C.; del Valle, A.E. The ATP Paradox Is the Expression of an Economizing Fuel Mechanism. J. Biol. Chem. 2004, 279, 55372–55375. [Google Scholar] [CrossRef]
- Sprenger, G.A.; Schörken, U.; Sprenger, G.; Sahm, H. Transketolase a of Escherichia coli K12. Eur. J. Biochem. 1995, 230, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Teshiba, S.; Mizobuchi, K. Identification and Characterization of the tktB Gene Encoding a Second Transketolase in Escherichia coli K-12. J. Bacteriol. 1993, 175, 5375–5383. [Google Scholar] [CrossRef]
- Shaw, J.A.; Henard, C.A.; Liu, L.; Dieckman, L.M.; Vázquez-Torres, A.; Bourret, T.J. Salmonella Enterica Serovar Typhimurium Has Three Transketolase Enzymes Contributing to the Pentose Phosphate Pathway. J. Biol. Chem. 2018, 293, 11271–11282. [Google Scholar] [CrossRef]
- Iwata, H.; Kobayashi, Y.; Mizushima, D.; Watanabe, T.; Ogihara, J.; Kasumi, T. Complementary Function of Two Transketolase Isoforms from Moniliella Megachiliensis in Relation to Stress Response. AMB Express 2017, 7, 45. [Google Scholar] [CrossRef]
- Hu, C.-W.; Chang, Y.-L.; Chen, S.J.; Kuo-Huang, L.-L.; Liao, J.C.; Huang, H.-C.; Juan, H.-F. Revealing the Functions of the Transketolase Enzyme Isoforms in Rhodopseudomonas Palustris Using a Systems Biology Approach. PLoS ONE 2011, 6, e28329. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Klauck, E.; Hengge-Aronis, R. The Response Regulator RssB, a Recognition Factor for σS Proteolysis in Escherichia coli, Can Act like an Anti-σS Factor. Mol. Microbiol. 2000, 35, 657–666. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Miyagawa, H.; Nakamura-Tsuruta, S.; Takaya, N.; Ogino, C.; Kondo, A. Enhanced Phenyllactic Acid Production in Escherichia coli Via Oxygen Limitation and Shikimate Pathway Gene Expression. Biotechnol. J. 2019, 14, 1800478. [Google Scholar] [CrossRef]
- Vimala, A.; Harinarayanan, R. Transketolase Activity Modulates Glycerol-3-Phosphate Levels in Escherichia coli. Mol. Microbiol. 2016, 100, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.C.; Dalby, P.A. Novel Insights into Transketolase Activation by Cofactor Binding Identifies Two Native Species Subpopulations. Sci. Rep. 2019, 9, 16116. [Google Scholar] [CrossRef]
- Littlechild, J.; Turner, N.; Hobbs, G.; Lilly, M.; Rawas, A.; Watson, H. Crystallization and Preliminary X-Ray Crystallographic Data with It Escherichia coli Transketolase. Acta Crystallogr. Sect. D 1995, 51, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.K.; Woodley, J.M.; Lilly, M.D. Escherichia coli Transketolase-Catalyzed Carbon-Carbon Bond Formation: Biotransformation Characterization for Reactor Evaluation and Selection. Enzym. Microb. Technol. 1998, 22, 64–70. [Google Scholar] [CrossRef]
- Saha, A.; Connelly, S.; Jiang, J.; Zhuang, S.; Amador, D.T.; Phan, T.; Pilz, R.B.; Boss, G.R. Akt Phosphorylation and Regulation of Transketolase Is a Nodal Point for Amino Acid Control of Purine Synthesis. Mol. Cell 2014, 55, 264–276. [Google Scholar] [CrossRef]
- Thorell, S.; Gergely, P.; Banki, K.; Perl, A.; Schneider, G. The Three-Dimensional Structure of Human Transaldolase. FEBS Lett. 2000, 475, 205–208. [Google Scholar] [CrossRef]
- Jung, I.L.; Phyo, K.H.; Kim, I.G. RpoS-Mediated Growth-Dependent Expression of the Escherichia coli Tkt Genes Encoding Transketolases Isoenzymes. Curr. Microbiol. 2005, 50, 314–318. [Google Scholar] [CrossRef]
- Samland, A.K.; Sprenger, G.A. Transaldolase: From Biochemistry to Human Disease. Int. J. Biochem. Cell Biol. 2009, 41, 1482–1494. [Google Scholar] [CrossRef]
- Macek, B.; Gnad, F.; Soufi, B.; Kumar, C.; Olsen, J.V.; Mijakovic, I.; Mann, M. Phosphoproteome Analysis of E. Coli Reveals Evolutionary Conservation of Bacterial Ser/Thr/Tyr Phosphorylation. Mol. Cell. Proteom. 2008, 7, 299–307. [Google Scholar] [CrossRef]
- Schneider, S.; Sandalova, T.; Schneider, G.; Sprenger, G.A.; Samland, A.K. Replacement of a Phenylalanine by a Tyrosine in the Active Site Confers Fructose-6-Phosphate Aldolase Activity to the Transaldolase of Escherichia coli and Human Origin. J. Biol. Chem. 2008, 283, 30064–30072. [Google Scholar] [CrossRef]
- Lv, G.-Y.; Guo, X.-G.; Xie, L.-P.; Xie, C.-G.; Zhang, X.-H.; Yang, Y.; Xiao, L.; Tang, Y.-Y.; Pan, X.-L.; Guo, A.-G.; et al. Molecular Characterization, Gene Evolution, and Expression Analysis of the Fructose-1, 6-Bisphosphate Aldolase (FBA) Gene Family in Wheat (Triticum aestivum L.). Front. Plant Sci. 2017, 8, 1030. [Google Scholar] [CrossRef]
- Kim, D.; Seo, S.W.; Gao, Y.; Nam, H.; Guzman, G.I.; Cho, B.-K.; Palsson, B.O. Systems Assessment of Transcriptional Regulation on Central Carbon Metabolism by Cra and CRP. Nucleic Acids Res. 2018, 46, 2901–2917. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.-Y.; Fu, Y.; Shen, J.-L.; Yin, B.-C.; You, D.; Ye, B.-C. A Dual Program for CRP-Mediated Regulation in Bacterial Alarmone (p)ppGpp. mBio 2024, 15, e02430-24. [Google Scholar] [CrossRef]
- Roos, A.K.; Mariano, S.; Kowalinski, E.; Salmon, L.; Mowbray, S.L. D-Ribose-5-Phosphate Isomerase B from Escherichia coli Is Also a Functional d-Allose-6-Phosphate Isomerase, While the Mycobacterium Tuberculosis Enzyme Is Not. J. Mol. Biol. 2008, 382, 667–679. [Google Scholar] [CrossRef]
- Kroner, G.M.; Wolfe, M.B.; Freddolino, P.L. Escherichia coli Lrp Regulates One-Third of the Genome via Direct, Cooperative, and Indirect Routes. J. Bacteriol. 2019, 201, e00411-18. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Rathod, J.; Chiu, Y.-C.; Chen, J.-W.; Tsai, P.-J.; Huang, I.-H. The Transcriptional Regulator Lrp Contributes to Toxin Expression, Sporulation, and Swimming Motility in Clostridium Difficile. Front. Cell. Infect. Microbiol. 2019, 9, 356. [Google Scholar] [CrossRef]
- Skinner, A.J.; Cooper, R.A. The Regulation of Ribose-5-Phosphate Isomerisation in Escherichia coli K12. FEBS Lett. 1971, 12, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Moreno, L.; Molina-Henares, M.A.; Ramos-González, M.I.; Espinosa-Urgel, M. Role of the Transcriptional Regulator ArgR in the Connection Between Arginine Metabolism and C-Di-GMP Signaling in Pseudomonas Putida. Appl. Environ. Microbiol. 2022, 88, e00064-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kang, Z.; Guo, X.; Guo, S.; Xiao, D.; Liu, Y.; Ma, C.; Xu, P.; Gao, C. Regulation of Glutarate Catabolism by GntR Family Regulator CsiR and LysR Family Regulator GcdR in Pseudomonas Putida KT2440. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef]
- Sobota, J.M.; Imlay, J.A. Iron Enzyme Ribulose-5-Phosphate 3-Epimerase in Escherichia coli Is Rapidly Damaged by Hydrogen Peroxide but Can Be Protected by Manganese. Proc. Natl. Acad. Sci. USA 2011, 108, 5402–5407. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The Pentose Phosphate Pathway and Cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Banki, K.; Perl, A. Cell Type-Specific Regulation of the Pentose Phosphate Pathway During Development and Metabolic Stress-Driven Autoimmune Diseases: Relevance for Inflammatory Liver, Renal, Endocrine, Cardiovascular and Neurobehavioral Comorbidities, Carcinogenesis, and Aging. Autoimmun. Rev. 2025, 24, 103781. [Google Scholar] [CrossRef]
- Sandoval, J.M.; Arenas, F.A.; Vásquez, C.C. Glucose-6-Phosphate Dehydrogenase Protects Escherichia coli from Tellurite-Mediated Oxidative Stress. PLoS ONE 2011, 6, e25573. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.T.; Monach, P.; Chou, J.H.; Josephy, P.D.; Demple, B. Positive Control of a Global Antioxidant Defense Regulon Activated by Superoxide-Generating Agents in Escherichia coli. Proc. Natl. Acad. Sci. USA 1990, 87, 6181–6185. [Google Scholar] [CrossRef]
- Zhao, J.; Baba, T.; Mori, H.; Shimizu, K. Global Metabolic Response of Escherichia coli to Gnd or Zwf Gene-Knockout, Based on 13C-Labeling Experiments and the Measurement of Enzyme Activities. Appl. Microbiol. Biotechnol. 2004, 64, 91–98. [Google Scholar] [CrossRef]
- Fraenkel, D.G. Selection of Escherichia coli Mutants Lacking Glucose-6-Phosphate Dehydrogenase or Gluconate-6-Phosphate Dehydrogenase. J. Bacteriol. 1968, 95, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Singer, A.; Gooding, J.R.; Xu, X.; Dong, A.; Cui, H.; Campagna, S.R.; Savchenko, A.; Yakunin, A.F.; et al. Riboneogenesis in Yeast. Cell 2011, 145, 969–980. [Google Scholar] [CrossRef]
- Nakahigashi, K.; Toya, Y.; Ishii, N.; Soga, T.; Hasegawa, M.; Watanabe, H.; Takai, Y.; Honma, M.; Mori, H.; Tomita, M. Systematic Phenome Analysis of Escherichia coli Multiple-Knockout Mutants Reveals Hidden Reactions in Central Carbon Metabolism. Mol. Syst. Biol. 2009, 5, 306. [Google Scholar] [CrossRef]
- Satanowski, A.; Dronsella, B.; Noor, E.; Vögeli, B.; He, H.; Wichmann, P.; Erb, T.J.; Lindner, S.N.; Bar-Even, A. Awakening a Latent Carbon Fixation Cycle in Escherichia coli. Nat. Commun. 2020, 11, 5812. [Google Scholar] [CrossRef]
- Krüsemann, J.L.; Lindner, S.N.; Dempfle, M.; Widmer, J.; Arrivault, S.; Debacker, M.; He, H.; Kubis, A.; Chayot, R.; Anissimova, M.; et al. Artificial Pathway Emergence in Central Metabolism from Three Recursive Phosphoketolase Reactions. FEBS J. 2018, 285, 4367–4377. [Google Scholar] [CrossRef]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic Rerouting of the Carbohydrate Flux Is Key to Counteracting Oxidative Stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef]
- Christodoulou, D.; Kuehne, A.; Estermann, A.; Fuhrer, T.; Lang, P.; Sauer, U. Reserve Flux Capacity in the Pentose Phosphate Pathway by NADPH Binding Is Conserved Across Kingdoms. iScience 2019, 19, 1133–1144. [Google Scholar] [CrossRef]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol. Cell 2015, 59, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Miller, C.G.; Grossmann, J.; Szyrwiel, L.; Schwecke, T.; Demichev, V.; Mikecin Drazic, A.-M.; Mayakonda, A.; Lutsik, P.; Veith, C.; et al. The GAPDH Redox Switch Safeguards Reductive Capacity and Enables Survival of Stressed Tumour Cells. Nat. Metab. 2023, 5, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Marino, A.; Morabito, R.; Remigante, A. Interplay Between Metabolic Pathways and Increased Oxidative Stress in Human Red Blood Cells. Cells 2024, 13, 2026. [Google Scholar] [CrossRef] [PubMed]
- Britt, E.C.; Lika, J.; Giese, M.A.; Schoen, T.J.; Seim, G.L.; Huang, Z.; Lee, P.Y.; Huttenlocher, A.; Fan, J. Switching to the Cyclic Pentose Phosphate Pathway Powers the Oxidative Burst in Activated Neutrophils. Nat. Metab. 2022, 4, 389–403. [Google Scholar] [CrossRef]
- Pisano, M.; Cocco, P.; Cherchi, R.; Onnis, R.; Cherchi, P. Glucose-6-Phosphate Dehydrogenase Deficiency and Lung Cancer: A Hospital Based Case-Control Study. Tumori 1991, 77, 12–15. [Google Scholar] [CrossRef]
- Cocco, P.; Dessí, S.; Avataneo, G.; Picchiri, G.; Heinemann, E. Glucose-6-Phosphate Dehydrogenase Deficiency and Cancer in a Sardinian Male Population: A Case-Control Study. Carcinogenesis 1989, 10, 813–816. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.; Shlomi, T.; Thompson, C.; Rabinowitz, J. Quantitative Flux Analysis Reveals Folate-Dependent NADPH Production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Roell, M.-S.; Weber, A.P.M. Mechanistic Understanding of Photorespiration Paves the Way to a New Green Revolution. New Phytol. 2019, 223, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Rashida, Z.; Laxman, S. The Pentose Phosphate Pathway and Organization of Metabolic Networks Enabling Growth Programs. Curr. Opin. Syst. Biol. 2021, 28, 100390. [Google Scholar] [CrossRef]
- Eggleston, L.V.; Krebs, H.A. Regulation of the Pentose Phosphate Cycle. Biochem. J. 1974, 138, 425–435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seregina, T.A.; Shakulov, R.S.; Petrushanko, I.Y.; Lobanov, K.V.; Mironov, A.S. Biosynthesis of Ribose-5-Phosphate—Metabolic Regulator of Escherichia coli Viability. Cells 2025, 14, 1775. https://doi.org/10.3390/cells14221775
Seregina TA, Shakulov RS, Petrushanko IY, Lobanov KV, Mironov AS. Biosynthesis of Ribose-5-Phosphate—Metabolic Regulator of Escherichia coli Viability. Cells. 2025; 14(22):1775. https://doi.org/10.3390/cells14221775
Chicago/Turabian StyleSeregina, Tatyana A., Rustem S. Shakulov, Irina Yu. Petrushanko, Konstantin V. Lobanov, and Alexander S. Mironov. 2025. "Biosynthesis of Ribose-5-Phosphate—Metabolic Regulator of Escherichia coli Viability" Cells 14, no. 22: 1775. https://doi.org/10.3390/cells14221775
APA StyleSeregina, T. A., Shakulov, R. S., Petrushanko, I. Y., Lobanov, K. V., & Mironov, A. S. (2025). Biosynthesis of Ribose-5-Phosphate—Metabolic Regulator of Escherichia coli Viability. Cells, 14(22), 1775. https://doi.org/10.3390/cells14221775





