Transient Early Mechanical Loading Induces Hypertrophic Chondrocyte Differentiation of Human Mesenchymal Stromal Cells
Highlights
- Mechanical stimulation induces hypertrophic differentiation of naïve MSCs.
- Short early stimulation is as efficient as prolonged stimulation.
- Our findings could guide future in vivo studies exploring the effect of mechanical stimulation on healing outcomes.
- Our data could provide in vitro support for the development of smart implants.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cellularized Hydrogel Preparation
2.1.1. Cell Isolation, Expansion
2.1.2. Hydrogel Preparation
2.1.3. Cell Encapsulation
2.2. Mechanical Stimulation
- P2ce (cycle equivalent): 168 cycles were condensed into 42 min (5 s strain + 10 s pause per cycle), after which samples were maintained in free swelling until day 14.
- P2te (time equivalent): Continuous stimulation for 14 days with 5 s strain + 10 s pause per cycle, resulting in a total of 80,640 cycles.
2.3. Cell Characterization
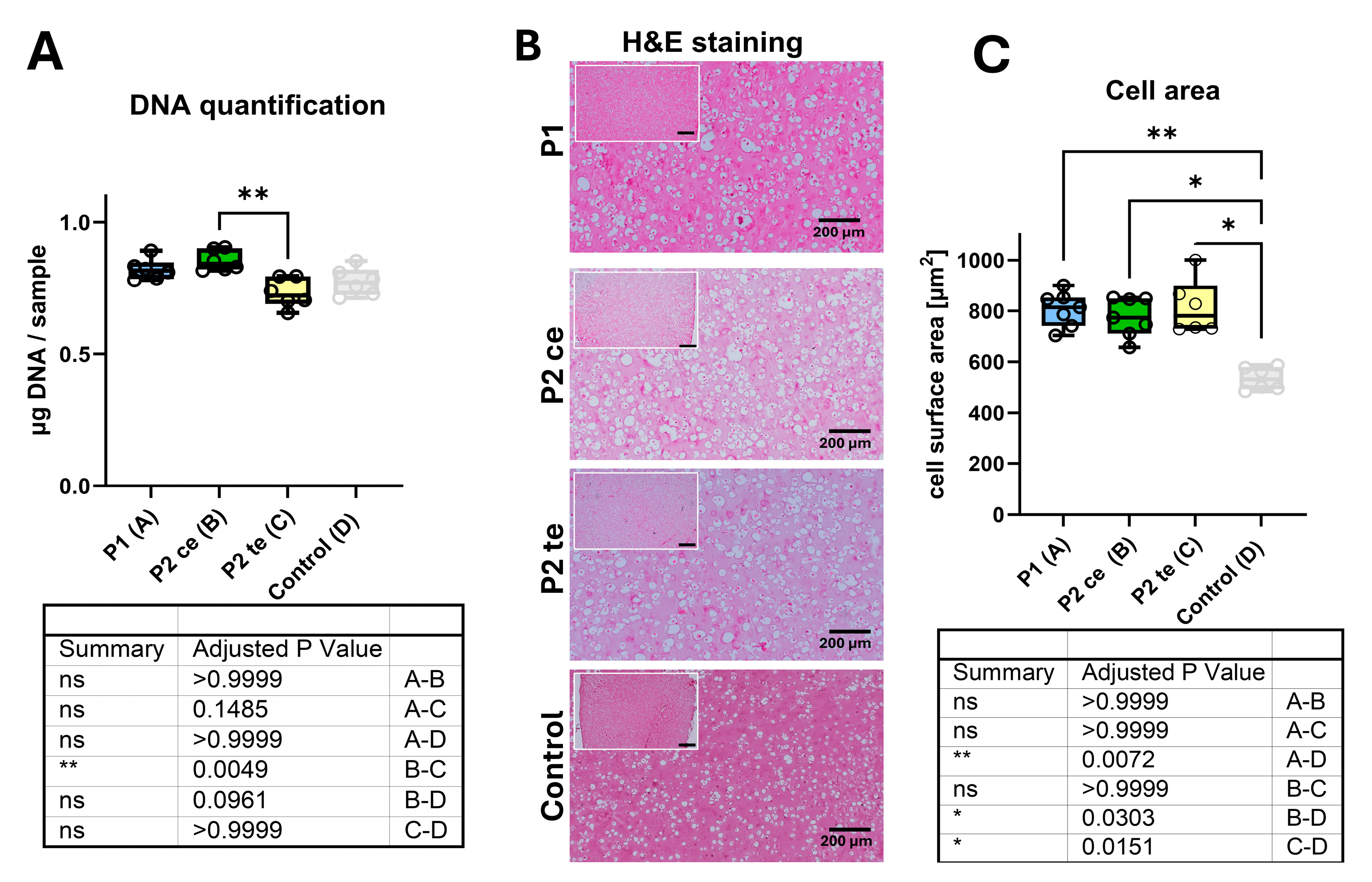
2.3.1. DNA Content
2.3.2. Cell Morphology
2.3.3. Gene Expression
2.4. Extracellular Matrix Characterization
2.4.1. Paraffin Embedding and Sectioning
2.4.2. Histology Staining
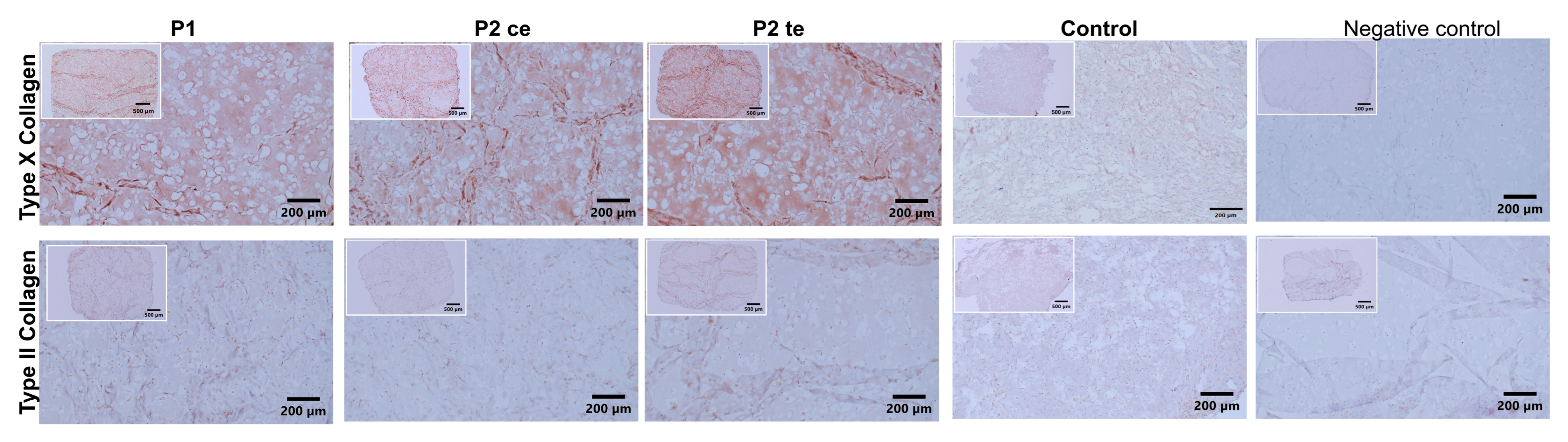
2.4.3. Immunohistochemistry
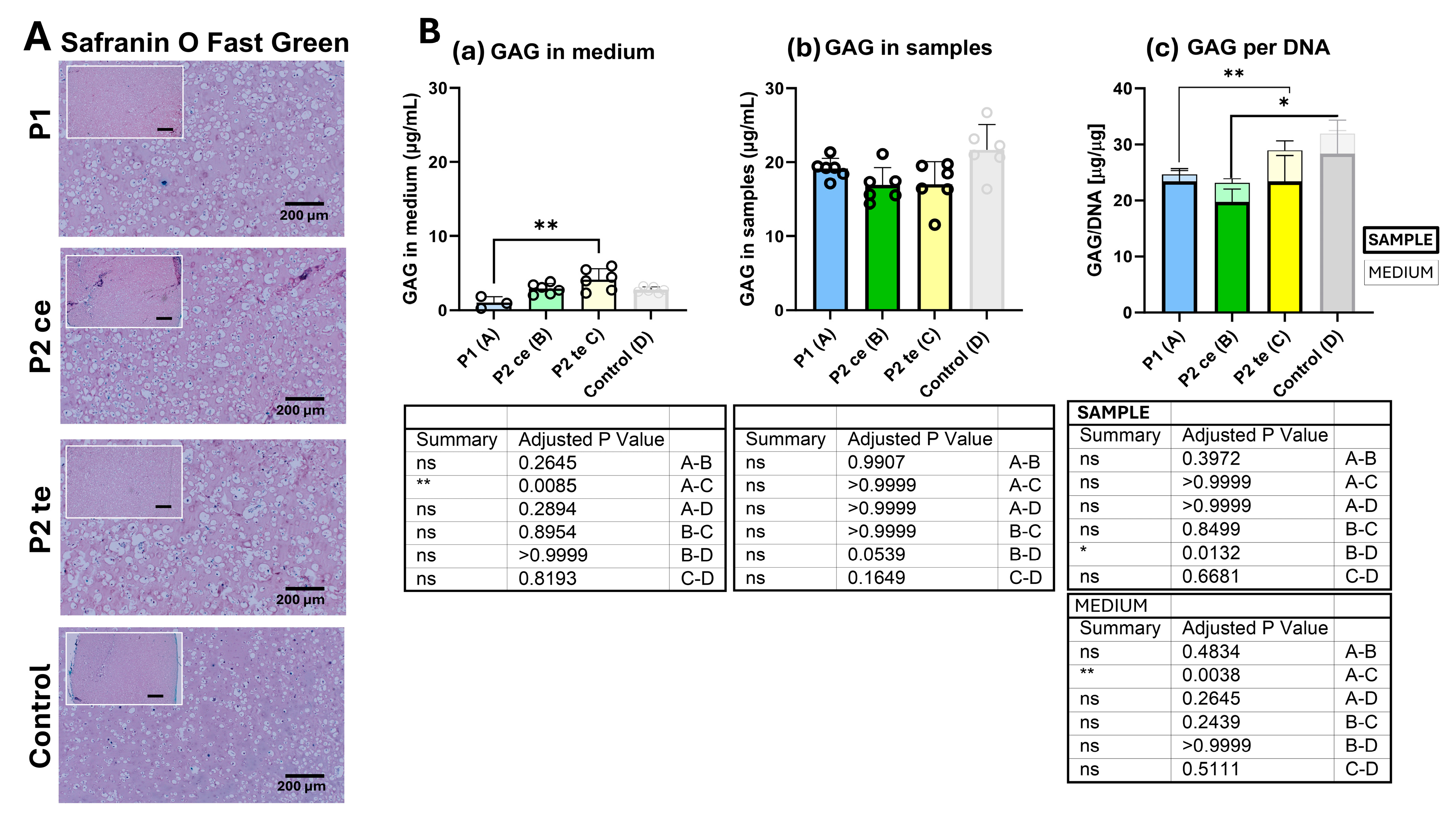
2.4.4. Glycosaminoglycan (GAG) Deposition
2.5. Statistical Analysis
3. Results
3.1. Samples Characterization
3.2. Extra Cellular Matrix Characteristics
3.2.1. Glycosaminoglycans (GAG) Synthesis Analysis
3.2.2. Type II and Type X Collagen Immunostaining
3.2.3. Gene Expression Analysis
4. Discussion
4.1. Mechanical Stimulation Drives Hypertrophic Differentiation of Naïve MSCs
4.2. Influence of Specific Mechanical Loading Protocols
4.3. Implications for Clinical Translation
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augat, P.; Hollensteiner, M.; von Rüden, C. The role of mechanical stimulation in the enhancement of bone healing. Injury 2021, 52 (Suppl. S2), S78–S83. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front. Physiol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Claes, L.; Augat, P.; Suger, G.; Wilke, H.J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J. Orthop. Res. 1997, 15, 577–584. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Perren, S.M.; Cordey, J. The concept of interfragmentary strain. In Current Concepts of Internal Fixation of Fractures; Uhthoff, H.K., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1980; pp. 63–77. [Google Scholar]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Hente, R.; Perren, S.M. Mechanical Stimulation of Fracture Healing—Stimulation of Callus by Improved Recovery [Mechanická stimulace hojení zlomenin—stimulace svalku prodloužením fáze zotavení]. Acta Chir. Orthop. Traumatol. Cech. 2018, 85, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Barcik, J.; Ernst, M.; Buchholz, T.; Constant, C.; Mys, K.; Epari, D.R.; Zeiter, S.; Windolf, M. The absence of immediate stimulation delays bone healing. Bone 2023, 175, 116834. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Ernst, M.; Schwyn, R.; Arens, D.; Zeiter, S. The relation between fracture activity and bone healing with special reference to the early healing phase—A preclinical study. Injury 2021, 52, 71–77. [Google Scholar] [CrossRef]
- Glatt, V.; Bartnikowski, N.; Quirk, N.; Schuetz, M.; Evans, C. Reverse Dynamization: Influence of Fixator Stiffness on the Mode and Efficiency of Large-Bone-Defect Healing at Different Doses of rhBMP-2. J. Bone Jt. Surgery. Am. Vol. 2016, 98, 677–687. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Gockelmann, M.; Schoen, M.; Ignatius, A.; Willie, B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Stoddart, M.J. Regenerative rehabilitation: The role of mechanotransduction in orthopaedic regenerative medicine. J. Orthop. Res. 2019, 37, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.L.; Gottsauner-Wolf, F.; Palmer, J.; Aro, H.T.; Chao, E.Y. Effects of axial dynamization on bone healing. J. Trauma. 1993, 34, 185–192. [Google Scholar] [CrossRef]
- Gardner, M.J.; Ricciardi, B.F.; Wright, T.M.; Bostrom, M.P.; van der Meulen, M.C.H. Pause insertions during cyclic in vivo loading affect bone healing. Clin. Orthop. Relat. Res. 2008, 466, 1232–1238. [Google Scholar] [CrossRef]
- Goodship, A.E.; Cunningham, J.L.; Kenwright, J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S105–S115. [Google Scholar] [CrossRef]
- Hofmann-Fliri, L.; Epari, D.R.; Schwyn, R.; Zeiter, S.; Windolf, M. Biphasic Plating—In vivo study of a novel fixation concept to enhance mechanobiological fracture healing. Injury 2020, 51, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, P.; Tavakoli, A.; Dlaska, C.; Neumann, M.; Shanker, M.; Saifzadeh, S.; Steck, R.; Schuetz, M.; Epari, D. Early mechanical stimulation only permits timely bone healing in sheep. J. Orthop. Res. 2018, 36, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Duan, Z.W.; Lu, H. Effect of Mechanical Strain on Cells Involved in Fracture Healing. Orthop. Surg. 2021, 13, 369–375. [Google Scholar] [CrossRef]
- Betts, D.C.; Muller, R. Mechanical regulation of bone regeneration: Theories, models, and experiments. Front. Endocri. 2014, 5, 211. [Google Scholar] [CrossRef]
- Castillo, A.B.; Jacobs, C.R. Mesenchymal stem cell mechanobiology. Curr. Osteoporos. Rep. 2010, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Jacobs, C.R. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res. Ther. 2013, 4, 107. [Google Scholar] [CrossRef]
- Ehrlich, P.J.; Lanyon, L.E. Mechanical strain and bone cell function: A review. Osteoporos. Int. 2002, 13, 688–700. [Google Scholar] [CrossRef]
- Jörimann, T.; Fullemann, P.; Jose, A.; Matthys, R.; Wehrle, E.; Stoddart, M.J.; Verrier, S. In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naive MSCs by Strain. Cells 2024, 14, 25. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Herlofsen, S.R.; Kuchler, A.M.; Melvik, J.E.; Brinchmann, J.E. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng. Part A 2011, 17, 1003–1013. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kenwright, J.; Goodship, A.E. Controlled mechanical stimulation in the treatment of tibial fractures. Clin. Orthop. Relat. Res. 1989, 241, 36–47. [Google Scholar] [CrossRef]
- Jagodzinski, M.; Krettek, C. Effect of mechanical stability on fracture healing—An update. Injury 2007, 38 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Narita, T.; Ito, H. Experimental study on the effect of mechanical stimulation on the early stage of fracture healing. J. Nippo Med. Sch. 2004, 71, 252–262. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Y.; Li, F.; Qiao, L.; Wang, Q.; Li, N.; Borgia, J.A.; Deng, Y.; Lei, G.; Zheng, Q. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014, 5, e1469. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. The hypertrophic chondrocyte: To be or not to be. Histol. Histopathol. 2021, 36, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.L.; Yu, S.N.; Park, H.; Estes, B.T.; Moutos, F.T.; Bloomquist, C.J.; Wu, P.B.; Welter, J.F.; Langer, R.; Guilak, F.; et al. Chondrogenic, hypertrophic, and osteochondral differentiation of human mesenchymal stem cells on three-dimensionally woven scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Shapiro, F.D. Expression of bone-specific genes by hypertrophic chondrocytes: Implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J. Cell. Biochem. 1996, 62, 1–9. [Google Scholar] [CrossRef]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Kirschner, M.W.; Tabin, C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype-Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Füllermann, P.; Jörimann, T.; Wehrle, E.; Matthys, R.; Stoddart, M.; Verrier, S. Divergent effects of mechanical loading and TGFβ1 stimuli on the Hypertrophic-Chondrocyte Differentiation of Naïve MSCs. In Proceedings of the ORS 2025, Phoenix, AR, USA, 7–11 February 2025. [Google Scholar]
- Jörimann, T.; Füllermann, P.; Matthys, R.; Stoddart, M.; Verrier, S. Strain induces hypertrophic differentiation of naïve human MSCs in a 3D in vitro model. In Proceedings of the ORS, Tampa, FL, USA, 4–8 February 2022. [Google Scholar]
- Chen, N.; Wu, R.W.H.; Lam, Y.; Chan, W.C.W.; Chan, D. Hypertrophic chondrocytes at the junction of musculoskeletal structures. Bone Rep. 2023, 19, 101698. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, G.; Morello, R.; Chen, Y.; Garcia-Rojas, X.; Lee, B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 2003, 162, 833–842. [Google Scholar] [CrossRef]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte Hypertrophy in Osteoarthritis: Mechanistic Studies and Models for the Identification of New Therapeutic Strategies. Cells 2022, 11, 4034. [Google Scholar] [CrossRef]
- Jaabar, I.L.; Foley, B.; Mezzetti, A.; Pillier, F.; Berenbaum, F.; Landoulsi, J.; Houard, X. Unraveling the Mechanisms of Hypertrophy-Induced Matrix Mineralization and Modifications in Articular Chondrocytes. Calcif. Tissue Int. 2024, 115, 269–282. [Google Scholar] [CrossRef]
- Jung, Y.K.; Park, H.R.; Cho, H.J.; Jang, J.A.; Lee, E.J.; Han, M.S.; Kim, G.W.; Han, S. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 2019, 9, 15846. [Google Scholar] [CrossRef]
- Ma, S.K.Y.; Chan, A.S.F.; Rubab, A.; Chan, W.C.W.; Chan, D. Extracellular Matrix and Cellular Plasticity in Musculoskeletal Development. Front. Cell Dev. Biol. 2020, 8, 781. [Google Scholar] [CrossRef]
- Mwale, F.; Stachura, D.; Roughley, P.; Antoniou, J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. 2006, 24, 1791–1798. [Google Scholar] [CrossRef]
- Solheim, K. The glycosaminoglycans in callus during fracture repair. Calcif. Tissue Res. 1970, 4, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016, 2016, 2470351. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surgery. Am. Vol. 1998, 80, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Vuoristo, J.T.; Larson, B.L.; Prockop, D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 4397–4402. [Google Scholar] [CrossRef]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef]
- Gardinier, J.D. The Diminishing Returns of Mechanical Loading and Potential Mechanisms that Desensitize Osteocytes. Curr. Osteoporos. Rep. 2021, 19, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Hente, R.; Füchtmeier, B.; Schlegel, U.; Ernstberger, A.; Perren, S.M. The influence of cyclic compression and distraction on the healing of experimental tibial fractures. J. Orthop. Res. 2004, 22, 709–715. [Google Scholar] [CrossRef]
- Srinivasan, S.; Weimer, D.A.; Agans, S.C.; Bain, S.D.; Gross, T.S. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Min. Res. 2002, 17, 1613–1620. [Google Scholar] [CrossRef]
- Wang, C.; Fu, R.; Yang, H. Toward a clear relationship between mechanical signals and bone adaptation. Mechanobiol. Med. 2025, 3, 100115. [Google Scholar] [CrossRef]
- Turner, C.H.; Pavalko, F.M. Mechanotransduction and functional response of the skeleton to physical stress: The mechanisms and mechanics of bone adaptation. J. Orthop. Sci. 1998, 3, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef]
- Sittichokechaiwut, A.; Edwards, J.H.; Scutt, A.M.; Reilly, G.C. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur. Cell Mater. 2010, 20, 45–57. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef]
- Miller, D.L.; Goswami, T. A review of locking compression plate biomechanics and their advantages as internal fixators in fracture healing. Clin. Biomech. 2007, 22, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Feliciano, S.; Martin, I.; de Wild, M.; Wendt, D. Novel Perfused Compression Bioreactor System as an in vitro Model to Investigate Fracture Healing. Front. Bioeng. Biotechnol. 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.; Didiano, D.M.; Chu, C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthr. Cartil. 2010, 18, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Herrmann, M.; Hildebrand, M.; Menzel, U.; Fahy, N.; Alini, M.; Lang, S.; Benneker, L.; Verrier, S.; Stoddart, M.J.; Bara, J.J. Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head. Int. J. Mol. Sci. 2019, 20, 3454. [Google Scholar] [CrossRef]


| Assay on demand | Assay ID | ||
| OAZI | Hs00427923_m1 | ||
| ALP | Hs00758162_m1 | ||
| COL2A1 (type II collagen) | Hs00264051_m1 | ||
| COMP | Hs00164359_m1 | ||
| SOX9 | Hs00165814_m1 | ||
| Mycrosynth | Forward | Reverse | Probe |
| RPLP0 | 5’-TGG GCA AGA ACA CCA TGA TG-3’ | 5’-CGG ATA TGA GGC AGC AGT TTC-3’ | 5’-AGG GCA CCT GGA AAA CAA CCC AGC-3’ |
| COL10A1 (type X collagen) | 5’-ACG CTG AAC GAT ACC AAA TG-3’ | 5’-TGC TAT ACC TTT ACT CTT TAT GGT GTA-3’ | 5’-ACT ACC CAA CAC CAA GAC ACA GTT CTT CAT TCC-3’ |
| ACAN | 5’-AGT CCT CAA GCC TCC TGT ACT CA-3’ | 5’-CGG GAA GTG GCG GTA ACA-3’ | 5’CCG GAA TGG AAA CGT GAA TCA GAA TCA ACT-3’ |
| MMP13 | 5’-CGG CCA CTC CTT AGG TCT TG-3’ | 5’-TTT TGC CGG TGT AGG TGT AGA TAG-3’ | 5’-CTC CAA GGA CCC TGG AGC ACT CAT GT-3’ |
| RUNX2 | 5’-AGC AAG GTT CAA CGA TCT GAG AT-3’ | 5’TTT GTG AAG ACG GTT ATG GTC AA-3’ | 5’-TGA AAC TCT TGC CTC GTC CAC TCC G-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enzmann, S.; Klaus, A.N.; Matthys, R.; Wehrle, E.; Stoddart, M.J.; Verrier, S. Transient Early Mechanical Loading Induces Hypertrophic Chondrocyte Differentiation of Human Mesenchymal Stromal Cells. Cells 2025, 14, 1773. https://doi.org/10.3390/cells14221773
Enzmann S, Klaus AN, Matthys R, Wehrle E, Stoddart MJ, Verrier S. Transient Early Mechanical Loading Induces Hypertrophic Chondrocyte Differentiation of Human Mesenchymal Stromal Cells. Cells. 2025; 14(22):1773. https://doi.org/10.3390/cells14221773
Chicago/Turabian StyleEnzmann, Sina, Aline N. Klaus, Romano Matthys, Esther Wehrle, Martin J. Stoddart, and Sophie Verrier. 2025. "Transient Early Mechanical Loading Induces Hypertrophic Chondrocyte Differentiation of Human Mesenchymal Stromal Cells" Cells 14, no. 22: 1773. https://doi.org/10.3390/cells14221773
APA StyleEnzmann, S., Klaus, A. N., Matthys, R., Wehrle, E., Stoddart, M. J., & Verrier, S. (2025). Transient Early Mechanical Loading Induces Hypertrophic Chondrocyte Differentiation of Human Mesenchymal Stromal Cells. Cells, 14(22), 1773. https://doi.org/10.3390/cells14221773








