Efficacy of Repeated Administration of Cultured Human CD34+ Cells Against Streptozotocin-Induced Diabetic Nephropathy in Rats
Highlights
- Human cultured CD34+ cells improved urinary protein excretion and pathological damage in rats with diabetic nephropathy.
- Human cultured CD34+ cells upregulated the expression of anti-inflammatory and angiogenesis-related genes in rat kidney tissues.
- Human CD34+ cells have a regenerative effect and the potential to improve diabetic nephropathy.
- The results of this study support the validity of future clinical trials using CD34+ cells to treat diabetic nephropathy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Preparation
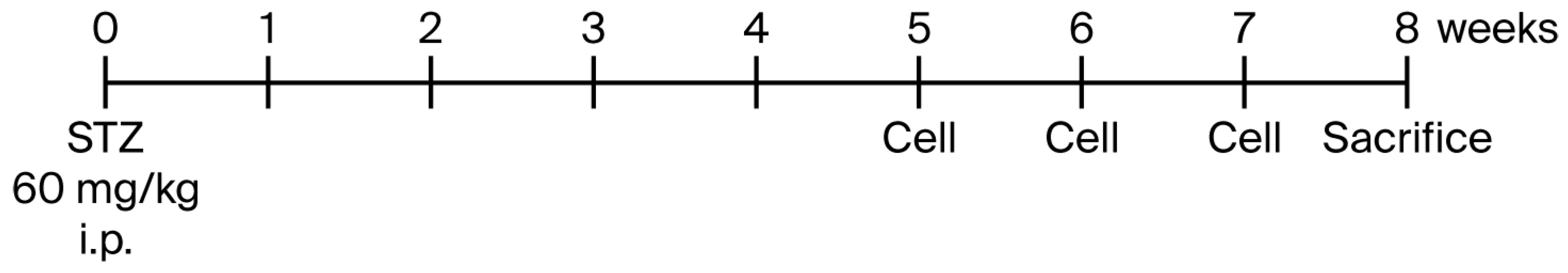
2.2. In Vivo Experiment
2.3. Kidney Function and Pathological Evaluation
2.4. CD31 and F4/80 Immunohistochemistry
2.5. RNA Extraction and Library Preparation
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Blood Glucose and Body Weight
3.2. Urinary Albumin Excretion and Kidney Function
3.3. Pathological Findings
3.4. Transcriptome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CKD | Chronic kidney disease |
| EPC | Endothelial progenitor cell |
| FDR | False discovery rate |
| MSC | Mesenchymal stromal cell |
| STZ | Streptozotocin |
| UACR | Urinary albumin/creatinine ratio |
References
- Bikbov, B.; Perico, N.; Remuzzi, G.; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, N.; Abe, M.; Joki, N.; Ogawa, T.; Kanda, E.; Kikuchi, K.; Goto, S.; Taniguchi, M.; Nakai, S.; Naganuma, T.; et al. Annual dialysis data report 2019, JSDT Renal Data Registry. Ren. Replace. Ther. 2023, 9, 47. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerie, H.J.; Broedl, U.C.; Zinman, B. Enpagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomized clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Jongs, N.; Chertow, G.M.; Langkilde, A.M.; McMurrey, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjostrom, C.D.; Stefansson, B.V.; Toto, R.D.; et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Anavekar, N.S.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Packer, M.; Shi, M.; et al. Angiotensin-Neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020, 142, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.T.; Sun, C.K. Stem cell therapy against ischemic heart disease. Int. J. Mol. Sci. 2024, 25, 3778. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.P.; Liu, Z.J.; Velazquez, O.C. A molecular and clinical review of stem cell therapy in critical limb ischemia. Stem Cells Int. 2017, 2017, 750829. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.; Nih, L.R.; Liberale, L.; Yin, H.; EL Amki, M.; Ong, L.K.; Zlokovic, B.V. Brain repair mechanisms after cell therapy for stroke. Brain 2024, 147, 3286–3305. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunnath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Itaba, S.; Salybekov, A.A.; Sheng, Y.; Sato, T.; Yanai, M.; Imagawa, M.; Fujii, S.; Kumagai, H.; Harata, M.; et al. Repetitive administration of cultured human CD34 positive cells improve adenine-induced kidney injury in mice. World J. Stem Cells 2023, 15, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, C. Mesenchymal stem cell therapy: Therapeutic opportunities and challenges for diabetic kidney disease. Int. J. Mol. Sci. 2024, 25, 10540. [Google Scholar] [CrossRef] [PubMed]
- Patchan, D.; Schwarze, K.; Henze, E.; Becker, J.U.; Patchan, S.; Müller, G.A. eEOC-mediated modulation of endothelial autophagy, senescence, and EnMT in murine diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2014, 307, F686–F694. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Nandula, S.R.; Asico, L.D.; Fakhri, M.; Banerjee, J.; Jose, P.A.; Sen, S. Transplantation of apoptosis-resistant endothelial progenitor cells improve renal function in diabetic kidney disease. J. Am. Heart Assoc. 2021, 10, e019365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuen, D.A.; Advani, A.; Thai, K.; Advani, S.L.; Kepecs, D.; Kabir, M.G.; Connelly, K.A.; Gilbert, R.E. Early-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetes. Diabetes 2012, 61, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Sato, T.; Tsukiyama, T.; Muraoka, S.; Mitomo, S.; Maruyama, H.; Yamano, M.; Mochida, Y.; Ishioka, K.; Oka, M.; et al. Preliminary evidence of kidney function improvement in chronic progressive kidney disease using autologous CD34+ cell therapy: A clinical trial. World J. Stem Cells 2024, 16, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Rafikova, O.; O’Connor, P.M.; Sullivan, J.C. Greater high-morbidity group box 1 in male compared with female spontaneously hypertensive rats worsens renal ischemia-reperfusion injury. Clin. Sci. 2020, 134, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog Liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced. diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012, 61, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Haruna, Y.; Kondeti, V.K.; Kanwar, Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, M.J.; Pasricha, S.V.; Nauth, A.; Ward, M.R.; Connely, K.A. Endothelial progenitor cells for diabetic cardiac and kidney disease. Stem Cells Transl. Med. 2024, 13, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Kobayashi, S. EPC in kidney disease. In Endothelial Progenitor Cells in Health and Disease; Salybekov, A.A., Kobayashi, S., Asahara, T., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022; pp. 137–163. [Google Scholar]
- Salybekov, A.A.; Kawaguchi, A.T.; Masuda, H.; Vorateera, K.; Okada, C.; Asahara, T. Regeneration-associated cells improve recovery from myocardial infarction through enhanced vasculogenesis, anti-inflammation, and cardiomyogenesis. PLoS ONE 2018, 13, e0203244. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, Y.; Zhou, Z.; Li, Q.; Chen, X.; Wang, W.; Jin, Y.; Hu, Z.; Chen, G.; Deng, Q.; et al. Human expandable pancreatic progenitor-derived ß cells ameliorate diabetes. Sci. Adv. 2022, 8, eabk1826. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Kobayashi, S.; Slavin, S.; Mochida, Y.; Ishioka, K.; Moriya, H.; Hidaka, S.; Matsuura, R.; Sumida, M.; Katagiri, D.; et al. Human peripheral blood mononuclear cells incubated in vasculogenic conditioning medium dramatically improve ischemia-reperfusion acute kidney injury in mice. Cell Transplant. 2018, 27, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ohtake, T.; Tsukiyama, T.; Morota, M.; Ishioka, K.; Moriya, H.; Mochida, Y.; Hidaka, S.; Sato, T.; Asahara, T.; et al. Acute kidney injury successfully treated with autologous granulocyte colony-stimulating factor-mobilized peripheral blood CD34-positive cell transplantation: A first-in-human report. Stem Cells Transl. Med. 2021, 10, 1253–1257. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtake, T.; Salybekov, A.A.; Sato, T.; Okamura, S.; Yazawa, M.; Yano, Y.; Hassanpour, M.; Yanai, M.; Imagawa, M.; Asahara, T.; et al. Efficacy of Repeated Administration of Cultured Human CD34+ Cells Against Streptozotocin-Induced Diabetic Nephropathy in Rats. Cells 2025, 14, 1766. https://doi.org/10.3390/cells14221766
Ohtake T, Salybekov AA, Sato T, Okamura S, Yazawa M, Yano Y, Hassanpour M, Yanai M, Imagawa M, Asahara T, et al. Efficacy of Repeated Administration of Cultured Human CD34+ Cells Against Streptozotocin-Induced Diabetic Nephropathy in Rats. Cells. 2025; 14(22):1766. https://doi.org/10.3390/cells14221766
Chicago/Turabian StyleOhtake, Takayasu, Amankeldi A. Salybekov, Tsutomu Sato, Shigeaki Okamura, Masaki Yazawa, Yuki Yano, Mehdi Hassanpour, Mitsuru Yanai, Makoto Imagawa, Takayuki Asahara, and et al. 2025. "Efficacy of Repeated Administration of Cultured Human CD34+ Cells Against Streptozotocin-Induced Diabetic Nephropathy in Rats" Cells 14, no. 22: 1766. https://doi.org/10.3390/cells14221766
APA StyleOhtake, T., Salybekov, A. A., Sato, T., Okamura, S., Yazawa, M., Yano, Y., Hassanpour, M., Yanai, M., Imagawa, M., Asahara, T., & Kobayashi, S. (2025). Efficacy of Repeated Administration of Cultured Human CD34+ Cells Against Streptozotocin-Induced Diabetic Nephropathy in Rats. Cells, 14(22), 1766. https://doi.org/10.3390/cells14221766







