Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts and Sample Material
2.2. Isolation of cmiRNAs and Tumor RNA
2.3. Library Preparation and Sequencing
2.4. Bioinformatics
2.5. Statistical Analyses
2.6. Pathway Enrichment Analysis
3. Results
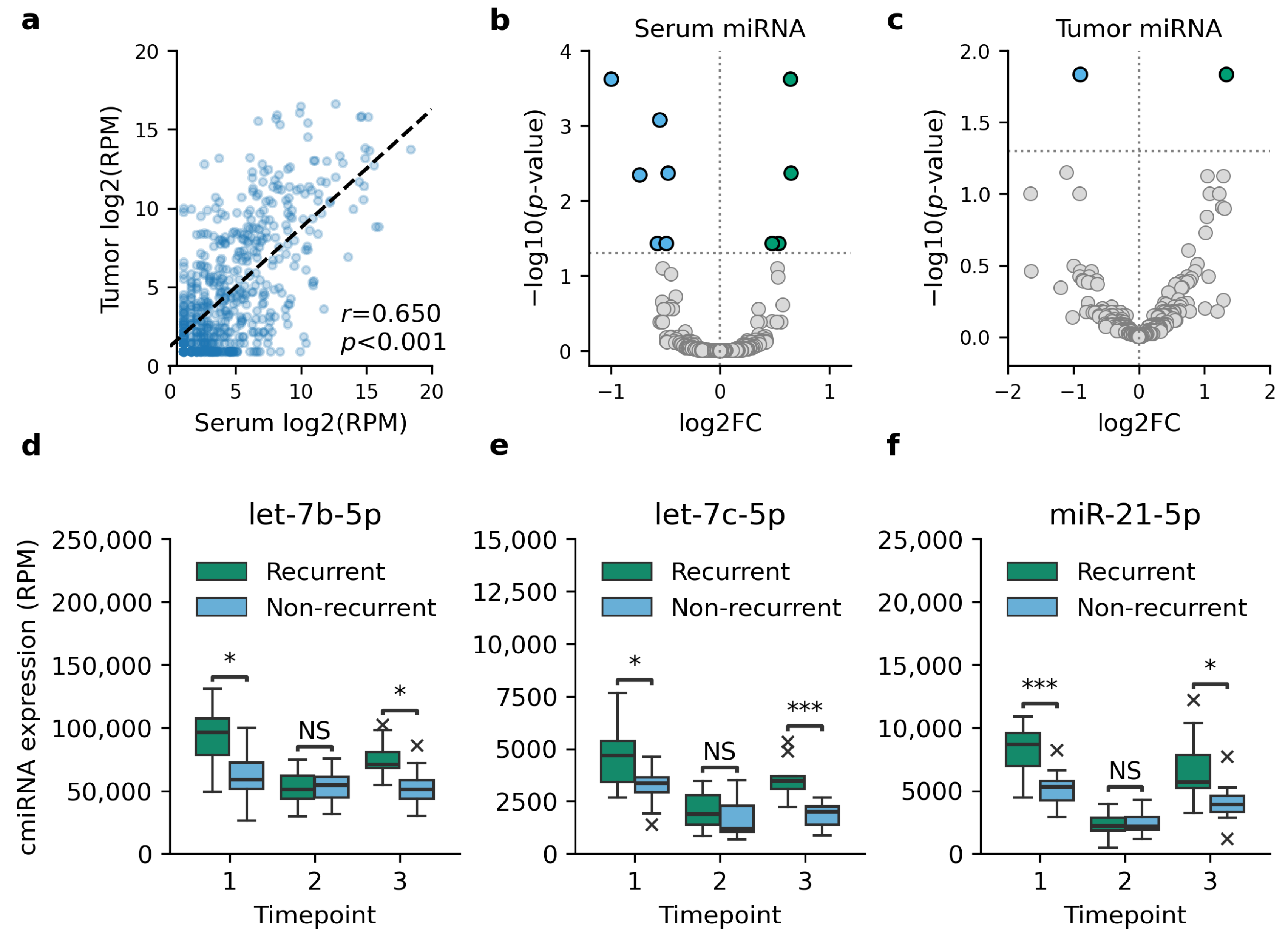
3.1. Recurrent TNBC Is Characterized by Ten DE cmiRNAs
3.2. DE cmiRNAs Are Associated with RFS
3.3. DE cmiRNAs Are Associated with Poor Tumor Characteristics
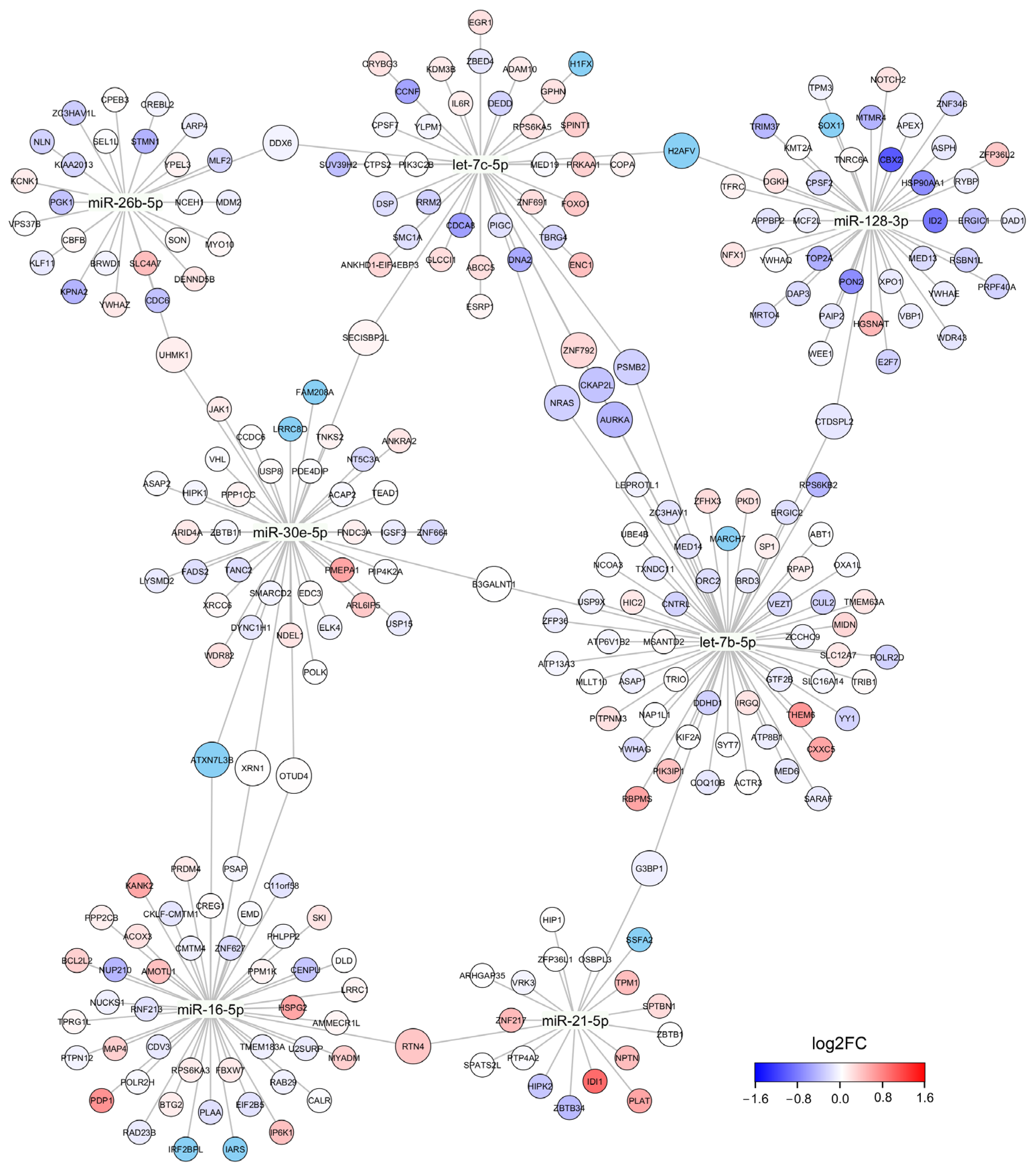
3.4. DE cmiRNAs Are Associated with Cancer-Associated Pathways
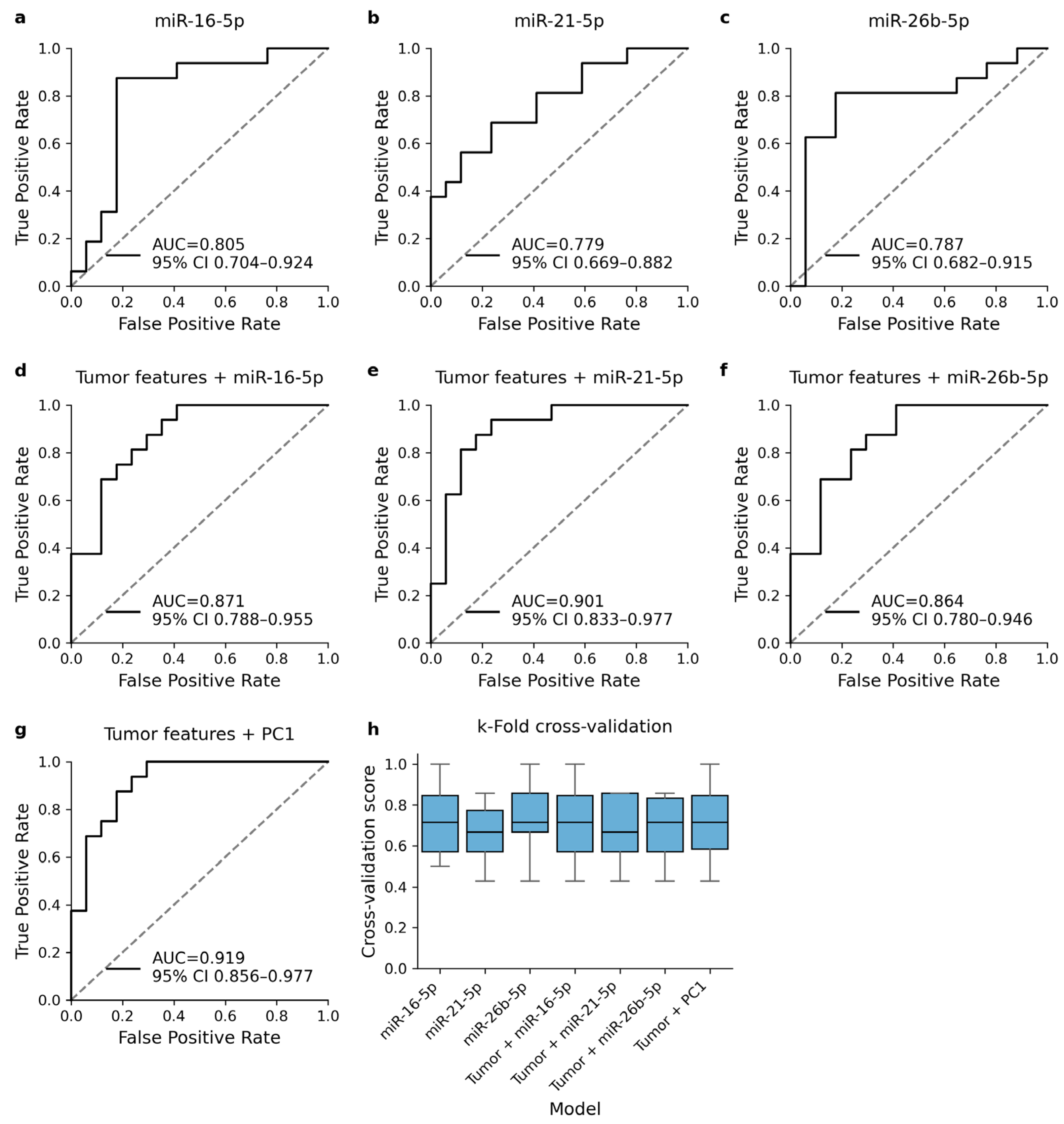
3.5. DE cmiRNAs Improve the Performance of Logistic Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyle, P. Triple-Negative Breast Cancer: Epidemiological Considerations and Recommendations. Ann. Oncol. 2012, 23 (Suppl. S6), vi7–vi12. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S. Triple-Negative Breast Cancer: Epidemiology and Management Options. Drugs 2010, 70, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus Nab-Paclitaxel as First-Line Treatment for Unresectable, Locally Advanced or Metastatic Triple-Negative Breast Cancer (IMpassion130): Updated Efficacy Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Steward, L.; Conant, L.; Gao, F.; Margenthaler, J.A. Predictive Factors and Patterns of Recurrence in Patients with Triple Negative Breast Cancer. Ann. Surg. Oncol. 2014, 21, 2165–2171. [Google Scholar] [CrossRef]
- Li, S.-Y.; Li, Y.-W.; Ma, D.; Shao, Z.-M. Prediction of Axillary Lymph Node Metastasis in Triple-Negative Breast Cancer by Multi-Omics Analysis and an Integrated Model. Ann. Transl. Med. 2022, 10, 623. [Google Scholar] [CrossRef]
- Devi, N.L.; Dhall, A.; Patiyal, S.; Raghava, G.P.S. Transcriptomics Based Prediction of Metastasis in TNBC Patients: Challenges in Cross-Platforms Validation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chen, X.; Thomas, K.; Folkert, M.R.; Kim, D.N.; Rahimi, A.S.; Zhou, Z.; Wang, J. Predicting Recurrence in Triple Negative Breast Cancer Patients from Clinical Parameters Using a Multi-Objective Classifier. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E53. [Google Scholar] [CrossRef]
- Gilson, P.; Merlin, J.-L.; Harlé, A. Deciphering Tumour Heterogeneity: From Tissue to Liquid Biopsy. Cancers 2022, 14, 1384. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as Potential Cancer Biomarkers: The Advantage and Disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef]
- Davey, M.G.; McGuire, A.; Casey, M.C.; Waldron, R.M.; Paganga, M.; Holian, E.; Newell, J.; Heneghan, H.M.; McDermott, A.M.; Keane, M.M.; et al. Evaluating the Role of Circulating MicroRNAs in Predicting Long-Term Survival Outcomes in Breast Cancer: A Prospective, Multicenter Clinical Trial. J. Am. Coll. Surg. 2023, 236, 317–327. [Google Scholar] [CrossRef]
- Patellongi, I.; Amiruddin, A.; Massi, M.N.; Islam, A.A.; Pratama, M.Y.; Sutandyo, N.; Latar, N.H.M.; Faruk, M. Circulating miR-221/222 Expression as microRNA Biomarker Predicting Tamoxifen Treatment Outcome: A Case–Control Study. Ann. Med. Surg. 2023, 85, 3806. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Ciniselli, C.M.; Pizzamiglio, S.; Cappelletti, V.; Silvestri, M.; El-Abed, S.; Izquierdo, M.; Bajji, M.; Nuciforo, P.; Huober, J.; et al. End-of-Neoadjuvant Treatment Circulating microRNAs and HER2-Positive Breast Cancer Patient Prognosis: An Exploratory Analysis from NeoALTTO. Front. Oncol. 2023, 12, 1028825. [Google Scholar] [CrossRef]
- Pellikainen, M.J.; Pekola, T.T.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.-M. p21WAF1 Expression in Invasive Breast Cancer and Its Association with P53, AP-2, Cell Proliferation, and Prognosis. J. Clin. Pathol. 2003, 56, 214–220. [Google Scholar] [CrossRef]
- Hartikainen, J.M.; Tuhkanen, H.; Kataja, V.; Dunning, A.M.; Antoniou, A.; Smith, P.; Arffman, A.; Pirskanen, M.; Easton, D.F.; Eskelinen, M.; et al. An Autosome-Wide Scan for Linkage Disequilibrium–Based Association in Sporadic Breast Cancer Cases in Eastern Finland: Three Candidate Regions Found. Cancer Epidemiol. Biomark. Prev. 2005, 14, 75–80. [Google Scholar] [CrossRef]
- Kauppinen, J.M.; Kosma, V.-M.; Soini, Y.; Sironen, R.; Nissinen, M.; Nykopp, T.K.; Kärjä, V.; Eskelinen, M.; Kataja, V.; Mannermaa, A. ST14 Gene Variant and Decreased Matriptase Protein Expression Predict Poor Breast Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2133–2142. [Google Scholar] [CrossRef]
- Kärkkäinen, E.; Heikkinen, S.; Tengström, M.; Kosma, V.-M.; Mannermaa, A.; Hartikainen, J.M. The Debatable Presence of PIWI-Interacting RNAs in Invasive Breast Cancer. Cancer Med. 2021, 10, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Köster, J.; Rahmann, S. Snakemake—A Scalable Bioinformatics Workflow Engine. Bioinformatics 2018, 34, 3600. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Heger, A.; Sudbery, I. UMI-Tools: Modeling Sequencing Errors in Unique Molecular Identifiers to Improve Quantification Accuracy. Genome Res. 2017, 27, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z.; et al. Rfam 14: Expanded Coverage of Metagenomic, Viral and microRNA Families. Nucleic Acids Res. 2021, 49, D192–D200. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 8 November 2020).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinforma. Oxf. Engl. 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R Package Rsubread Is Easier, Faster, Cheaper and Better for Alignment and Quantification of RNA Sequencing Reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-Learn: Machine Learning in Python. arXiv 2018, arXiv:1201.0490v4. [Google Scholar]
- Tastsoglou, S.; Skoufos, G.; Miliotis, M.; Karagkouni, D.; Koutsoukos, I.; Karavangeli, A.; Kardaras, F.S.; Hatzigeorgiou, A.G. DIANA-miRPath v4.0: Expanding Target-Based miRNA Functional Analysis in Cell-Type and Tissue Contexts. Nucleic Acids Res. 2023, 51, W154–W159. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A Decade-Long Collection of Experimentally Supported miRNA–Gene Interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed]
- Sondka, Z.; Bamford, S.; Cole, C.G.; Ward, S.A.; Dunham, I.; Forbes, S.A. The COSMIC Cancer Gene Census: Describing Genetic Dysfunction across All Human Cancers. Nat. Rev. Cancer 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Federico, A.; Monti, S. hypeR: An R Package for Geneset Enrichment Workflows. Bioinformatics 2020, 36, 1307–1308. [Google Scholar] [CrossRef]
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast Cancer Survival by Molecular Subtype: A Population-Based Analysis of Cancer Registry Data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef]
- Cocco, S.; Piezzo, M.; Calabrese, A.; Cianniello, D.; Caputo, R.; Di Lauro, V.; Fusco, G.; di Gioia, G.; Licenziato, M.; de Laurentiis, M. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4579. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Green, B.B.; Seigne, J.D.; Schned, A.R.; Marsit, C.J. MicroRNA Molecular Profiling from Matched Tumor and Bio-Fluids in Bladder Cancer. Mol. Cancer 2015, 14, 194. [Google Scholar] [CrossRef]
- Zedan, A.H.; Hansen, T.F.; Assenholt, J.; Pleckaitis, M.; Madsen, J.S.; Osther, P.J.S. microRNA Expression in Tumour Tissue and Plasma in Patients with Newly Diagnosed Metastatic Prostate Cancer. Tumor Biol. 2018, 40, 1010428318775864. [Google Scholar] [CrossRef]
- Cojocneanu, R.; Braicu, C.; Raduly, L.; Jurj, A.; Zanoaga, O.; Magdo, L.; Irimie, A.; Muresan, M.-S.; Ionescu, C.; Grigorescu, M.; et al. Plasma and Tissue Specific miRNA Expression Pattern and Functional Analysis Associated to Colorectal Cancer Patients. Cancers 2020, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-H.; Tsao, C.-J. Emerging Role of microRNA-21 in Cancer (Review). Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Mao, X.; Shi, P.; He, B.; Xu, K.; Zhang, S.; Wang, J. MicroRNAs in the Prognosis of Triple-Negative Breast Cancer. Medicine 2017, 96, e7085. [Google Scholar] [CrossRef]
- Yan, L.-X.; Huang, X.-F.; Shao, Q.; Huang, M.-Y.; Deng, L.; Wu, Q.-L.; Zeng, Y.-X.; Shao, J.-Y. MicroRNA miR-21 Overexpression in Human Breast Cancer Is Associated with Advanced Clinical Stage, Lymph Node Metastasis and Patient Poor Prognosis. RNA 2008, 14, 2348–2360. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, X.; Ji, J.; Chen, L.; Cao, J.; Luo, J.; Zhang, S. High Expression Levels of miR-21 and miR-210 Predict Unfavorable Survival in Breast Cancer: A Systemic Review and Meta-Analysis. Int. J. Biol. Markers 2015, 30, e347–e358. [Google Scholar] [CrossRef]
- Yan, L.-X.; Liu, Y.-H.; Xiang, J.-W.; Wu, Q.-N.; Xu, L.-B.; Luo, X.-L.; Zhu, X.-L.; Liu, C.; Xu, F.-P.; Luo, D.-L.; et al. PIK3R1 Targeting by miR-21 Suppresses Tumor Cell Migration and Invasion by Reducing PI3K/AKT Signaling and Reversing EMT, and Predicts Clinical Outcome of Breast Cancer. Int. J. Oncol. 2016, 48, 471–484. [Google Scholar] [CrossRef]
- Fang, H.; Xie, J.; Zhang, M.; Zhao, Z.; Wan, Y.; Yao, Y. miRNA-21 Promotes Proliferation and Invasion of Triple-Negative Breast Cancer Cells through Targeting PTEN. Am. J. Transl. Res. 2017, 9, 953–961. [Google Scholar]
- Prvanović, M.; Nedeljković, M.; Tanić, N.; Tomić, T.; Terzić, T.; Milovanović, Z.; Maksimović, Z.; Tanić, N. Role of PTEN, PI3K, and mTOR in Triple-Negative Breast Cancer. Life 2021, 11, 1247. [Google Scholar] [CrossRef]
- Huo, D.; Clayton, W.M.; Yoshimatsu, T.F.; Chen, J.; Olopade, O.I. Identification of a Circulating microRNA Signature to Distinguish Recurrence in Breast Cancer Patients. Oncotarget 2016, 7, 55231–55248. [Google Scholar] [CrossRef]
- Feliciano, A.; González, L.; Garcia-Mayea, Y.; Mir, C.; Artola, M.; Barragán, N.; Martín, R.; Altés, A.; Castellvi, J.; Benavente, S.; et al. Five microRNAs in Serum Are Able to Differentiate Breast Cancer Patients From Healthy Individuals. Front. Oncol. 2020, 10, 586268. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Chen, J.; Wang, H.; Yang, L.; Chen, F.; Fan, S.; Wang, J.; Shao, B.; Yin, D.; et al. A Serum microRNA Signature Predicts Trastuzumab Benefit in HER2-Positive Metastatic Breast Cancer Patients. Nat. Commun. 2018, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, H.; Lv, X.; Liu, Y.; Wang, X.; Zhang, M.; Zhang, X.; Li, Y.; Lou, Q.; Li, S.; et al. MicroRNA-16-5p Overexpression Suppresses Proliferation and Invasion as Well as Triggers Apoptosis by Targeting VEGFA Expression in Breast Carcinoma. Oncotarget 2017, 8, 72400–72410. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A Review on the Role of Mir-16-5p in the Carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wei, H.; Wang, C.; Han, J.; Chen, X.; Li, Y. MiR-26b-5p Inhibits Cell Proliferation and EMT by Targeting MYCBP in Triple-Negative Breast Cancer. Cell. Mol. Biol. Lett. 2021, 26, 52. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in Cancer Biology, Tumour Microenvironment and Angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Bang-Christensen, S.R.; Jørgensen, A.M.; Løppke, C.; Spliid, C.B.; Sand, N.T.; Clausen, T.M.; Salanti, A.; Agerbæk, M.Ø. The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis. Front. Cell Dev. Biol. 2020, 8, 749. [Google Scholar] [CrossRef]
- Godard, P.; van Eyll, J. Pathway Analysis from Lists of microRNAs: Common Pitfalls and Alternative Strategy. Nucleic Acids Res. 2015, 43, 3490–3497. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef]
- Matias-Garcia, P.R.; Wilson, R.; Mussack, V.; Reischl, E.; Waldenberger, M.; Gieger, C.; Anton, G.; Peters, A.; Kuehn-Steven, A. Impact of long-term storage and freeze-thawing on eight circulating microRNAs in plasma samples. PLoS ONE 2020, 15, e0227648. [Google Scholar] [CrossRef]
| Variable | Grouping | Non-Recurrent (n = 19) | Recurrent (n = 14) |
|---|---|---|---|
| Age (years) | ≤39 | 4 (21.1) | 1 (7.1) |
| 40–49 | 3 (15.8) | 2 (14.3) | |
| 50–59 | 6 (31.6) | 5 (35.7) | |
| 60–69 | 2 (10.4) | 2 (14.3) | |
| ≥70 | 4 (21.1) | 4 (28.6) | |
| Tumor grade | II | 1 (5.3) | 3 (21.4) |
| III | 18 (94.7) | 11 (78.6) | |
| Tumor size | T1 | 10 (52.6) | 5 (35.7) |
| T2 | 8 (42.1) | 5 (35.7) | |
| T3 | 1 (5.3) | 4 (28.6) | |
| Lymph node status | N0 | 14 (73.7) | 6 (42.9) |
| N1 | 5 (26.3) | 7 (50.0) | |
| N2 | 0 (0.0) | 1 (7.1) | |
| Chemotherapy | Yes | 5 (26.3) | 3 (21.4) |
| No | 14 (73.7) | 11 (78.6) | |
| Radiotherapy | Yes | 9 (47.4) | 4 (28.6) |
| No | 10 (52.6) | 10 (71.4) | |
| Hormonal therapy | Yes | 3 (15.8) | 6 (42.9) |
| No | 16 (84.2) | 8 (57.1) |
| miRNA | Accession ID | Serum | Tumor | ||
|---|---|---|---|---|---|
| log2FC | FDR | log2FC | FDR | ||
| hsa-let-7b-5p | MIMAT0000063 | 0.54 | 0.037 | 0.43 | 0.661 |
| hsa-let-7c-5p | MIMAT0000064 | 0.48 | 0.037 | 1.33 | 0.015 |
| hsa-miR-16-5p | MIMAT0000069 | −0.48 | 0.004 | −0.01 | 0.993 |
| hsa-miR-21-5p | MIMAT0000076 | 0.65 | 2.38 × 10−4 | 0.09 | 0.952 |
| hsa-miR-26b-5p | MIMAT0004500 | −0.55 | 8.39 × 10−4 | −0.33 | 0.706 |
| hsa-miR-30e-5p | MIMAT0000692 | −0.50 | 0.037 | 0.25 | 0.844 |
| hsa-miR-128-3p | MIMAT0000424 | −0.74 | 0.004 | −0.90 | 0.015 |
| hsa-miR-146a-5p | MIMAT0000449 | −0.57 | 0.037 | 0.54 | 0.762 |
| hsa-miR-199a-5p | MIMAT0000231 | −1.00 | 2.38 × 10−4 | 0.646 | 0.453 |
| hsa-miR-3614-3p | MIMAT0017993 | 0.65 | 0.004 | NA | NA |
| miRNA | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Z | p | HR | 95% CI | p | |
| hsa-let-7b-5p | 1.61 | 0.204 | 1.23 | 0.70–2.16 | 0.301 |
| hsa-let-7c-5p | 0.62 | 0.423 | 1.16 | 0.65–2.04 | 0.616 |
| hsa-miR-16-5p | 19.96 | 7.91 × 10−6 | 0.53 | 0.30–0.95 | 0.032 |
| hsa-miR-21-5p | 16.82 | 4.12 × 10−5 | 1.87 | 1.06–3.30 | 0.030 |
| hsa-miR-26b-5p | 19.57 | 9.61 × 10−6 | 0.52 | 0.29–0.91 | 0.023 |
| hsa-miR-30e-5p | 0.70 | 0.404 | 0.93 | 0.53–1.62 | 0.787 |
| hsa-miR-128-3p | 3.55 | 0.060 | 0.77 | 0.44–1.36 | 0.375 |
| hsa-miR-146a-5p | 4.21 | 0.040 | 0.75 | 0.43–1.32 | 0.322 |
| hsa-miR-199a-5p | 4.67 | 0.031 | 0.76 | 0.43–1.34 | 0.341 |
| hsa-miR-3614-3p | 3.06 | 0.080 | 1.25 | 0.71–2.21 | 0.433 |
| KEGG Pathway Name | DIANA-miRPath 1 | HypeR 2 |
|---|---|---|
| Ubiquitin mediated proteolysis | 3.97 × 10−12 | 0.130 |
| Protein processing in endoplasmic reticulum | 9.14 × 10−12 | NA |
| Pathways in cancer | 1.39 × 10−11 | 0.240 |
| Shigellosis | 1.39 × 10−11 | NA |
| Adherens junction | 6.75 × 10−11 | 1.000 |
| Autophagy-animal | 7.51 × 10−11 | NA |
| Proteoglycans in cancer | 1.10 × 10−10 | NA |
| Cell cycle | 4.46 × 10−10 | 0.001 |
| FoxO signaling pathway | 1.64 × 10−8 | NA |
| Hepatitis B | 2.02 × 10−8 | NA |
| p53 signaling pathway | 2.02 × 10−8 | 0.860 |
| Neurotrophin signaling pathway | 8.63 × 10−8 | 0.006 |
| Hippo signaling pathway | 9.16 × 10−8 | NA |
| TGF-beta signaling pathway | 9.16 × 10−8 | 0.260 |
| Prostate cancer | 1.00 × 10−7 | 0.260 |
| Focal adhesion | 2.00 × 10−7 | 1.000 |
| Salmonella infection | 3.85 × 10−7 | NA |
| Tight junction | 2.40 × 10−6 | 0.860 |
| Rap1 signaling pathway | 2.40 × 10−6 | NA |
| Oocyte meiosis | 2.94 × 10−6 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujala, J.; Tengström, M.; Heikkinen, S.; Taipale, M.; Kosma, V.-M.; Hartikainen, J.M.; Mannermaa, A. Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer. Cells 2024, 13, 1884. https://doi.org/10.3390/cells13221884
Kujala J, Tengström M, Heikkinen S, Taipale M, Kosma V-M, Hartikainen JM, Mannermaa A. Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer. Cells. 2024; 13(22):1884. https://doi.org/10.3390/cells13221884
Chicago/Turabian StyleKujala, Jouni, Maria Tengström, Sami Heikkinen, Mari Taipale, Veli-Matti Kosma, Jaana M. Hartikainen, and Arto Mannermaa. 2024. "Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer" Cells 13, no. 22: 1884. https://doi.org/10.3390/cells13221884
APA StyleKujala, J., Tengström, M., Heikkinen, S., Taipale, M., Kosma, V.-M., Hartikainen, J. M., & Mannermaa, A. (2024). Circulating Micro-RNAs Predict the Risk of Recurrence in Triple-Negative Breast Cancer. Cells, 13(22), 1884. https://doi.org/10.3390/cells13221884








