1. Introduction
Skeletal muscle fiber formation is a highly complex process that occurs during embryonic development and postnatal life and involves several regulatory networks [
1]. While the molecular and cellular mechanisms by which different signaling pathways regulate muscle development have been deeply analyzed in the last years, the interplay between specific cellular organelles, such as lysosomes, and signaling networks during myogenesis have been less studied.
Lysosomes are membrane-bound organelles that can vary considerably in size, shape, subcellular positioning, luminal pH, enzyme content, cargos, and function [
2,
3]. There are different lysosomal subpopulations in cells that vary considerably depending on the cell type [
4]. Our understanding of the role of lysosomes in eukaryotic cells has considerably changed in the last years. Lysosomes are now seen as key components in the regulation of cell signaling, in addition to their well-established role in the degradation of molecules [
5]. Both functions are highly intricate since the sequestration and degradation of signaling molecules can inhibit or activate specific signaling pathways, such as the Wnt/beta-catenin pathway. It has been reported that glycogen synthase kinase 3 (GSK3) can be sequestered from the cytosol into lysosomes, which activates the canonical Wnt/beta-catenin pathway [
6]. In the non-activated state of the canonical Wnt/beta-catenin signaling pathway, GSK3 phosphorylates beta-catenin, triggering its degradation [
7]. Thus, sequestration of GSK3 into lysosomes inhibits GSK3 activity leading to stabilization and activation of beta-catenin and TCF/LEF-dependent gene transcription, which activates the pathway.
Despite the recent increase in the understanding of the role of lysosomes in cell signaling, little is known about their role during skeletal muscle differentiation. Here, we studied morphological and functional features of lysosomes in muscle and non-muscle cells in chick myogenic cell cultures. Our results show the involvement of the canonical Wnt/beta-catenin pathway in the regulation of the positioning, size, and function of lysosomes in muscle cells.
2. Materials and Methods
2.1. Antibodies and Probes
Rabbit polyclonal antibody against the major lysosomal membrane protein LAMP2 (#PA1-655) was from ThermoFisher (São Paulo, Brazil). Mouse monoclonal antibodies against alpha-tubulin (clone DM1a) and rabbit polyclonal antibodies against beta-catenin (#C2206) were from Sigma-Aldrich (São Paulo, Brazil). Mouse monoclonal anti-Lamin A/C (#4777) was from Cell Signaling Technology (Danvers, MA, USA). NucSpot live cell nuclear stain was from Biotium Inc. (Fremont, CA, USA). DNA-binding probe 4,6-Diamino-2-phenylindole dihydrochloride (DAPI), Hoechst nuclear stain (#33342), Alexa Fluor 488-goat anti-rabbit IgG antibodies, and Alexa Fluor 546-goat anti-mouse IgG antibodies were from Molecular Probes (Eugene, OR, USA).
2.2. Cell Cultures
The primary cultures of myogenic cells were prepared from breast muscles of 11-day-old chicken embryos as previously described [
8]. Chicken embryos were obtained from Granja Tolomei (Rio de Janeiro, Brazil) and handled according to the Institutional Animal Care and Use Committee, under protocol number 081/22. Briefly, fragments of pectoral muscle were incubated at 37 °C for 10 min in a calcium–magnesium-free solution (CMF, Sigma-Aldrich) containing 0.025% trypsin (Sigma-Aldrich). After removal of the trypsin solution, cells were dispersed by repeated pipetting in 8–1–0.5 culture medium (minimum essential medium with 10% horse serum, 0.5% chick embryo extract, 1% L-glutamine, and 1% penicillin/streptomycin, all from Invitrogen, Brazil). The resulting suspension was filtered, and cells were plated at an initial density of 7.5 × 10
5 cells/35 mm culture dishes in 2 mL of medium. Cells were cultured on 22 mm-Aclar plastic coverslips (Pro-Plastics Inc., Linden, NJ, USA) previously coated with rat tail collagen. Cells were grown in a humidified 5% CO
2 atmosphere at 37 °C. After the first 24 h, cultures were fed daily with fresh 8–1–0.5 cultured medium.
2.3. Cell Treatments
For lysosomal inhibition, 24 h chick myogenic cells were treated with the chloroquine Lys05 (Sigma-Aldrich, dissolved in 0.1% DMSO) at a final concentration of 1 μM; or with 0.1% DMSO, both for 24 h. For the activation of the Wnt/beta-catenin pathway, 24-h cells were treated with 5 µM 6-bromoindirubin-30-oxime (BIO, Sigma-Aldrich) or conditioned media (50% v/v) enriched in Wnt3a (obtained from L-Wnt3a cells, ATCC, Manassas, VA, USA). For acidic cellular organelle labeling, cells were stained with 2 ng/mL acridine orange solution for 10 min at 37 °C and 5% CO2. The cytoskeleton interfering drugs Taxol (1 µM), Colcemid (0.05 µM), and Cytochalasin B (0.1 µM), all from Sigma-Aldrich, were used in 24 h cells.
2.4. LAMP2 Knockdown
siRNA sequences specific for LAMP2 of Gallus gallus (mRNA sequence obtained from NCBI reference sequence NM_001030295) or enhanced green fluorescent protein (eGFP) were from Integrated DNA Technologies (Coralville, IA, USA). The Gallus gallus sequences of siRNA that were selected to target LAMP2 or eGFP are:
eGFP from Gallus gallus;
5′ rArArGrCrUrGrArCrCrCrUrGrArArGrUrUrCrArUrCrUrGCA 3′ (strand);
5′ rUrGrCrArGrArUrGrArArCrUrUrCrArGrGrGrUrCrArGrCrUrUrGrC 3′ (passenger);
LAMP2 from Gallus gallus (seq 1467-1492);
sense 5′ rGrArArUrUrGrGrArArArUrUrUrArCrUrUrGrArArGrCrUAC 3′;
antisense 5′ rGrUrArGrCrUrUrCrArArGrUrArArArUrUrUrCrCrArArUrUrCrArU 3′.
2.5. Wnt/Beta-Catenin Gene Reporter Assay
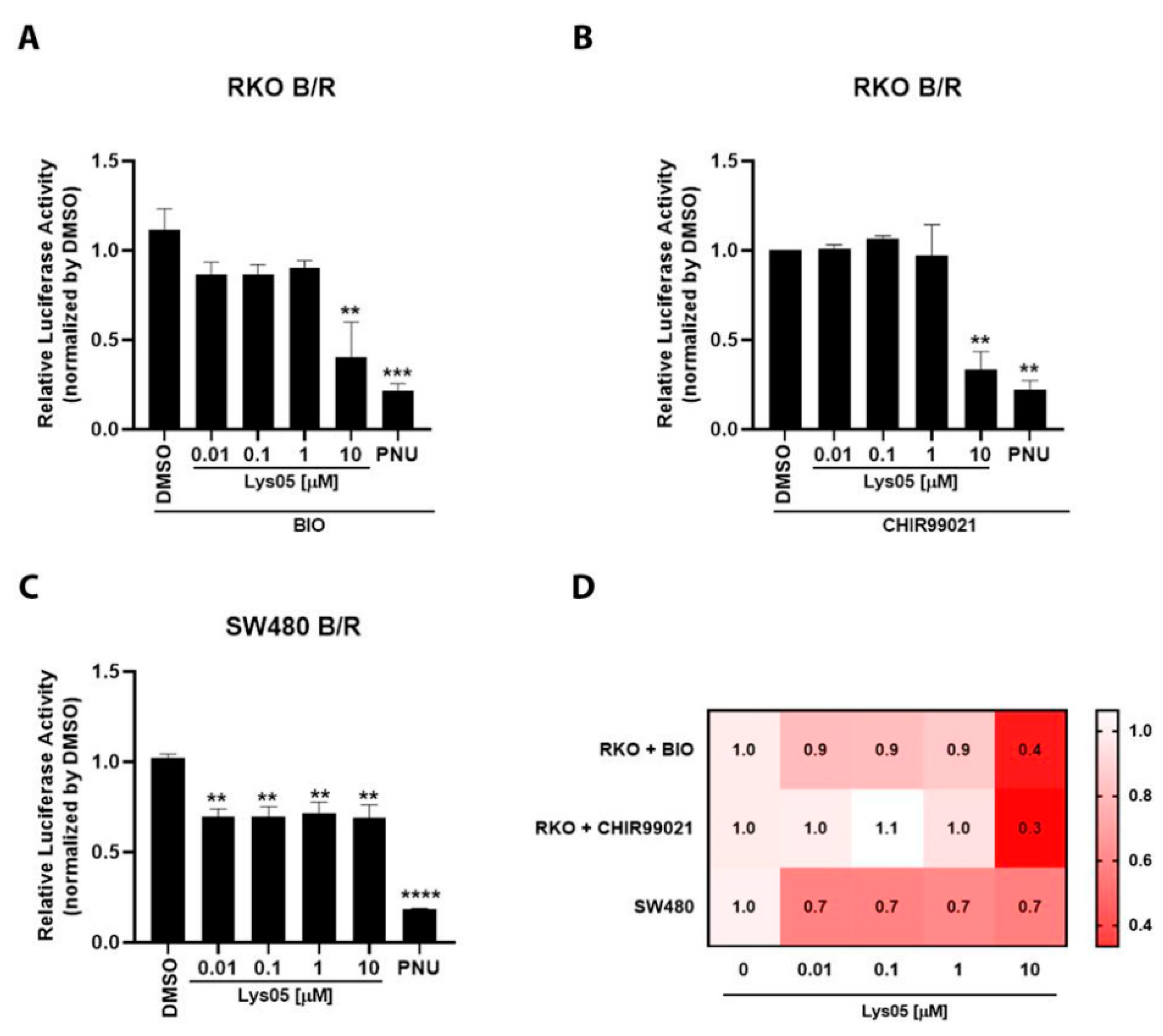
RKO-pBAR/Renilla (RKO B/R) or SW480-pBAR/Renilla (SW480 B/R) cells were seeded into 96-well plates with 2.0 × 104 cells per well. Cells were treated with increased concentrations of Lys05 (0.01, 0.1, 1, and 10 µM) for 24 h. After treatment, cells were lysed with passive lysis buffer (Promega, Madison, WI, USA) and assayed for Firefly and Renilla luciferase activity using the dual-luciferase reporter assay (Promega #E1960). Luminescence was measured using the Modulus IITM Microplate Multimode Reader (Promega). Control media (DMEM F-12) were used as negative controls for Wnt/beta-catenin reporter stimulation by BIO or CHIR99021 in RKO B/R. All experiments were performed in triplicate and repeated at least three times.
2.6. Immunofluorescence and Digital Image Acquisition
Chick myogenic cells were rinsed with PBS and fixed in absolute methanol at −20 °C for 5 min. Then, they were permeabilized and blocked in PBS with 0.1% saponin, 1% BSA, and 5% horse serum for 30 min. Cells were incubated overnight at 4 °C with primary antibodies (all diluted 1:50 in the permeabilization and blocking solution). After incubation, cells were washed for 30 min in the permeabilization and blocking solutions and incubated for 1 h at 37 °C with Alexa Fluor-conjugated secondary antibodies (all diluted 1:100 in the permeabilization and blocking solution). Nuclei were labeled with DAPI, Hoechst, or NucSpot. Cells were mounted in Prolong Gold (Molecular Probes) and examined with either an Axiovert 100 microscope (Carl Zeiss, Jena, Germany) coupled to an Olympus DP71 high-resolution camera, a Leica TCS SPE laser scanning confocal microscope (Leica, Wetzlar, Germany), or a DSU Spinning Disk confocal scanner mounted on an inverted fluorescent microscope (Olympus, Shinjuku, Japan). Control experiments with no primary antibodies showed only a faint background staining (data not shown). Image processing was performed using Fiji software, version 1.53q [
9] and figure plates were mounted with Adobe Photoshop software, version 7.0.1 (Adobe Systems Inc., Mountain View, CA, USA).
2.7. Quantification of Chick Cell Cultures
The nuclear localization of beta-catenin, the diameter of myotubes, the diameter and area of lysosomes, as well as the distance between lysosomes and the nuclei, were quantified using Fiji software,
https://imagej.net/software/fiji/ (accessed on 25 August 2022). We measured the total area occupied by lysosomes in the three phenotypes of cultured cells (myoblasts, myotubes, and fibroblasts) by analyzing images of Lysotracker (lysosomal marker), Hoechst (nuclear marker), and phase contrast microscopy (whole cell image). The limits of cells were accessed by phase contrast microscopy. The area occupied by lysosomes per cell was measured concerning the total cell area (area of lysosomes (%) ÷ total cell area (%)). DAPI and desmin double staining enables the identification of myoblasts through the presence of desmin and fibroblasts through differences in nuclear morphology and fluorescence intensity [
10]. More specifically, muscle fibroblasts show large, flattened, and pale nuclei and negative cytoplasm staining for desmin, whereas myoblasts are desmin-positive cells with small, round, and bright nuclei [
10]. All data were quantified from at least fifty randomly chosen microscopic fields collected from three independent experiments.
2.8. Nuclear and Cytoplasmic Fractions
Nuclear and cytoplasmic fractions were prepared based on a previously described method [
11]. Cultured chicken myogenic cells were scraped off the dishes with 500 μL of culture medium, transferred to Eppendorf tubes, and centrifuged at 4000 rpm for 10 min. The supernatant was discarded, and cells were washed in sterile PBS, then centrifuged at 4000 rpm for 10 min. Then, cells were resuspended in 500 μL of hypotonic buffer solution (20 mM Tris-HCl pH 7.4, 10 mM KCl, 2 mM MgCl
2, 1 mM EGTA, 0.5 mM DTT, and 0.05% sodium azide) containing 0.1% NP-40 and protease inhibitor cocktail (#P2714, Sigma-Aldrich), vortexed for 5 s and incubated for 3 min on ice cold. Then, cells were transferred to a Dounce-type homogenizer and macerated 20 times. Subsequently, the cell extract was centrifuged at 13,400 rpm for 10 min, fractionating the nucleus (pellet) and cytoplasm (supernatant). The pellet was resuspended in hypotonic buffer solution, and then the pellet and the supernatant were centrifuged again at 13,400 rpm for 10 min, and the cycle was repeated three times. For immunoblot analysis, 100 μL of RIPA buffer (150 mM sodium chloride, 50 mM Tris-HCl pH 8.0, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), protease inhibitor cocktail, and DNAse were added to each sample.
2.9. Immunoblotting
The protein concentration in cytoplasmic and nuclear fractions was determined with the Bradford assay and denatured with a Laemmli buffer at 95 °C for 5 min. Samples were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto 0.45 μm polyvinylidene fluoride (PVDF) membranes (Millipore, Burlington, MA, USA, #IEVH85R). After the blockade of nonspecific protein signals in 2% (w/v) polyvinylpyrrolidone (PVP) (Sigma-Aldrich, #P0930) in Tris-buffered saline with 0.01% Tween-20 (TBST) for 1 h, PVDF membranes were incubated overnight at 4 °C with primary antibodies against α-tubulin (1:2000, Sigma-Aldrich, #T9026), beta-catenin (1:2000, BD Bioscience, #610154), Lamin A/C (1:500, Cell Signaling Technology, #4777) and desmin (1:2000, Sigma-Aldrich, #D8281). The next day, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Invitrogen, #31460 and #31430) and antibody-bound bands were visualized with the SuperSignal™ West Pico PLUS Chemiluminescent Substrate using a ChemiDoc™ XRS+ System with Image Lab™ Software, version 6.1 (Bio-Rad Laboratories, Hercules, CA, USA #1708265).
2.10. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism software version 8. The results of at least three independent experiments were compared. Statistical analysis was performed with a one-way ANOVA followed by a Tukey’s multiple comparison test, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. Error bars denote mean ± SEM.
4. Discussion
The lysosomal system has been implicated in several aspects of muscle structure and physiology, such as the control of myofiber homeostasis and muscle mass [
15]. However, the molecular and cellular mechanisms involved in the interplay between lysosomes and signaling pathways during myogenesis are not known. Here, we aimed to explore different mechanisms that could modulate the positioning, size, and function of lysosomes during myogenesis, including the Wnt/beta-catenin pathway. We used a primary muscle cell culture system to study the morphological and functional features of lysosomes. These cell cultures were obtained from the pectoral skeletal muscle of 11-day-old chick embryos (
Gallus gallus), and they harbor three main cell phenotypes: myoblasts, myotubes, and fibroblasts [
10,
13]. The chick myogenic cell culture is a robust model of myogenesis, in which myotubes can harbor hundreds of nuclei.
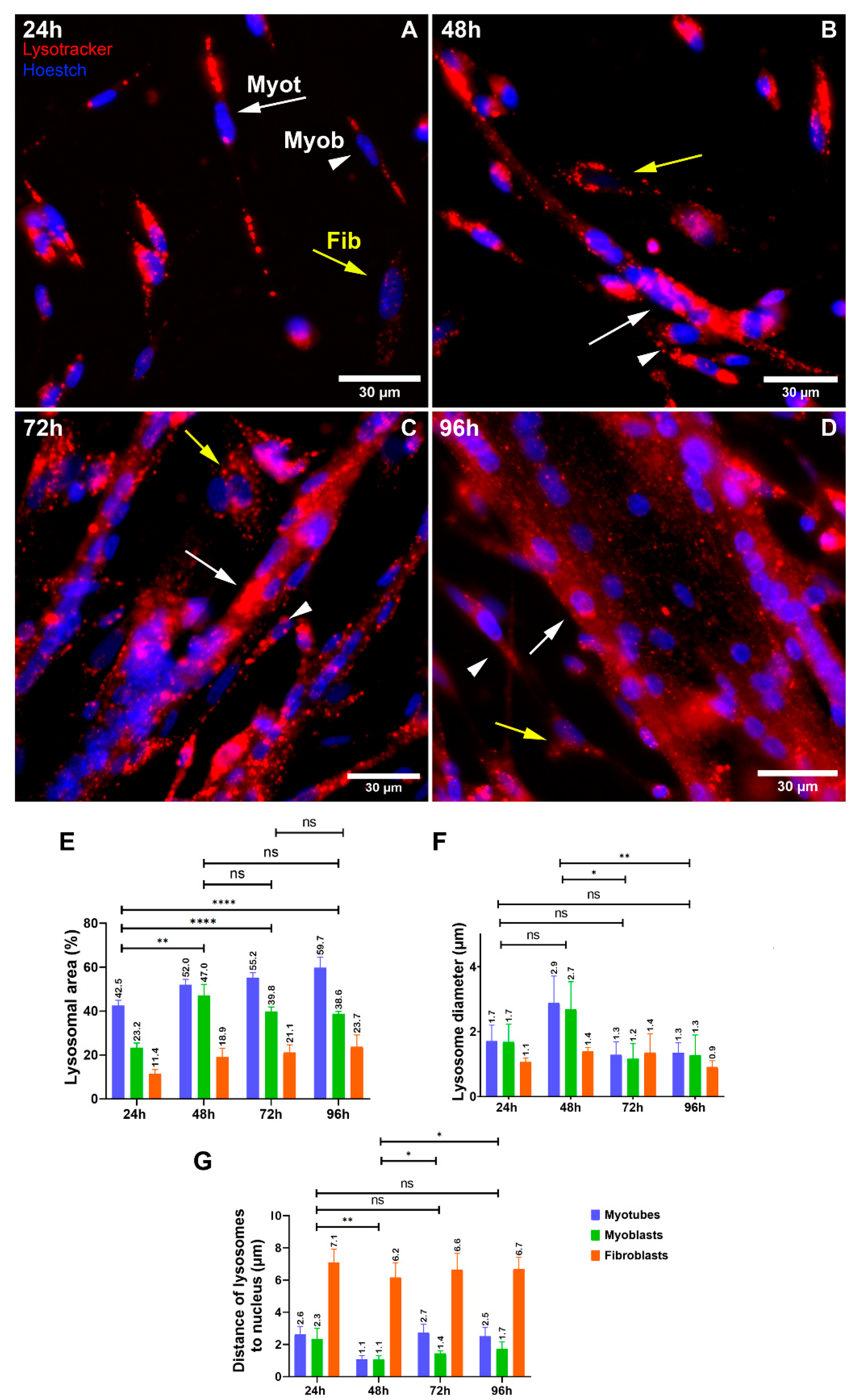
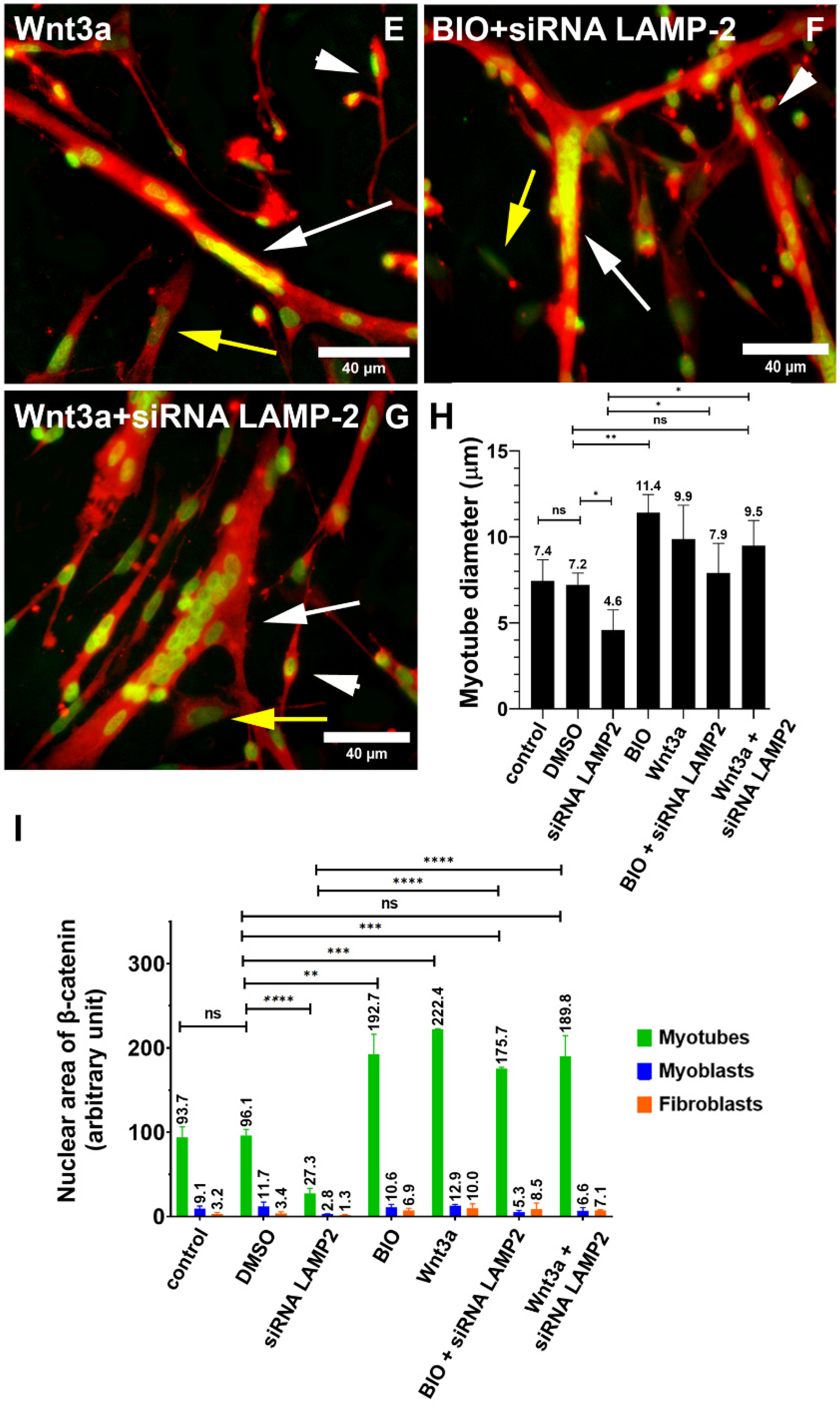
The collection of our results shows that (i) lysosomal size distribution and its distribution size are different when comparing muscle and versus non-muscle cells, (ii) lysosomes are found in spherical structures in myoblasts and fibroblasts and tubular structures in myotubes, (iii) lysosomes are found close to the plasma membrane in fibroblasts and close to the nucleus in myoblasts and myotubes, (iv) lysosomal distribution and size are dependent on the integrity of microtubules and microfilaments in myogenic cells, (v) alterations in lysosomal function, expression of LAMP2, and Wnt/beta-catenin signaling pathway affects the distribution and size of lysosomes in myogenic cells, (vi) the effects of the knockdown of LAMP2 on myogenesis can be rescued by the activation of the Wnt/beta-catenin pathway, and (vii) the chloroquine Lys05 is a potent inhibitor of both the Wnt/beta-catenin pathway and lysosomal function.
Lysosomes are generally described as small spherical organelles, but they may form several microns-long tubules that interconnect as a network [
12,
16,
17]. It has been shown that spherical lysosomes can spontaneously elongate into tubules in certain conditions [
18]. Chemical imaging revealed that tubular lysosomes differ from vesicular ones in terms of their luminal pH, calcium, and proteolytic activity [
18]. Here, we found lysosomes as spherical structures in myoblasts and fibroblasts and as tubular structures in myotubes. We can hypothesize that during muscle differentiation, the vesicular lysosomes found in myoblasts elongate and fuse into tubules in myotubes and that this morphological change is accompanied by a change in their function.
Lysosomal size is tightly controlled in cells and is intimately correlated with lysosomal function [
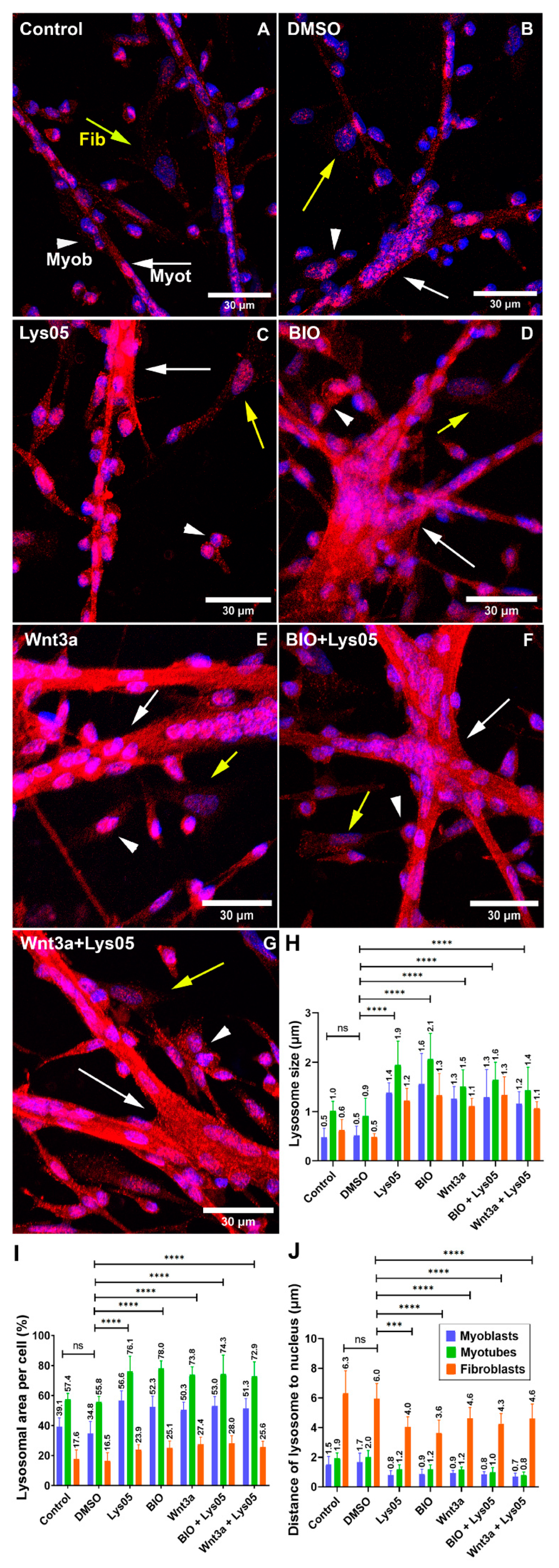
2]. Lysosomal diseases are frequently accompanied by alterations in lysosomal size. The enlarged lysosomes that we detected in chick muscle cells after the inhibition of lysosomal function with Lys05 are probably related to acidification defects that promote the accumulation of undegradable cargo in catabolically inactive lysosomes, as previously described [
2]. Interestingly, we found an increase in the diameter of lysosomes in myoblasts from 48 h-cultures, which could be related to the peak of myoblast fusion in chick muscle cultures. These results agree with previous data indicating that most chick myoblast fusion occurs between 48 and 65 h in vitro [
10,
19].
Lysosomes were found close to the plasma membrane in fibroblasts, whereas they were close to the nucleus in myoblasts and myotubes. It has been reported that the intracellular positioning of lysosomes correlates with their luminal pH and function [
20]. Since peripheral lysosomes (near the plasma membrane) have been associated with reduced acidification and impaired proteolytic activity [
20], we can infer that chick myoblasts and myotubes have lysosomes with increased proteolytic activity, as compared to muscle fibroblasts. Furthermore, lysosomes have been implicated in the regulation of different signaling pathways, such as mTOR and Wnt/beta-catenin [
3]. Regarding the relationship between lysosomes and the mTOR pathway, in the presence of amino acids, mTORC1 is located on peripheral lysosomes close to its upstream signaling elements, whereas during starvation mTORC1 and lysosomes are preferentially clustered in the perinuclear area, facilitating fusion of lysosomes with autophagosomes [
3].
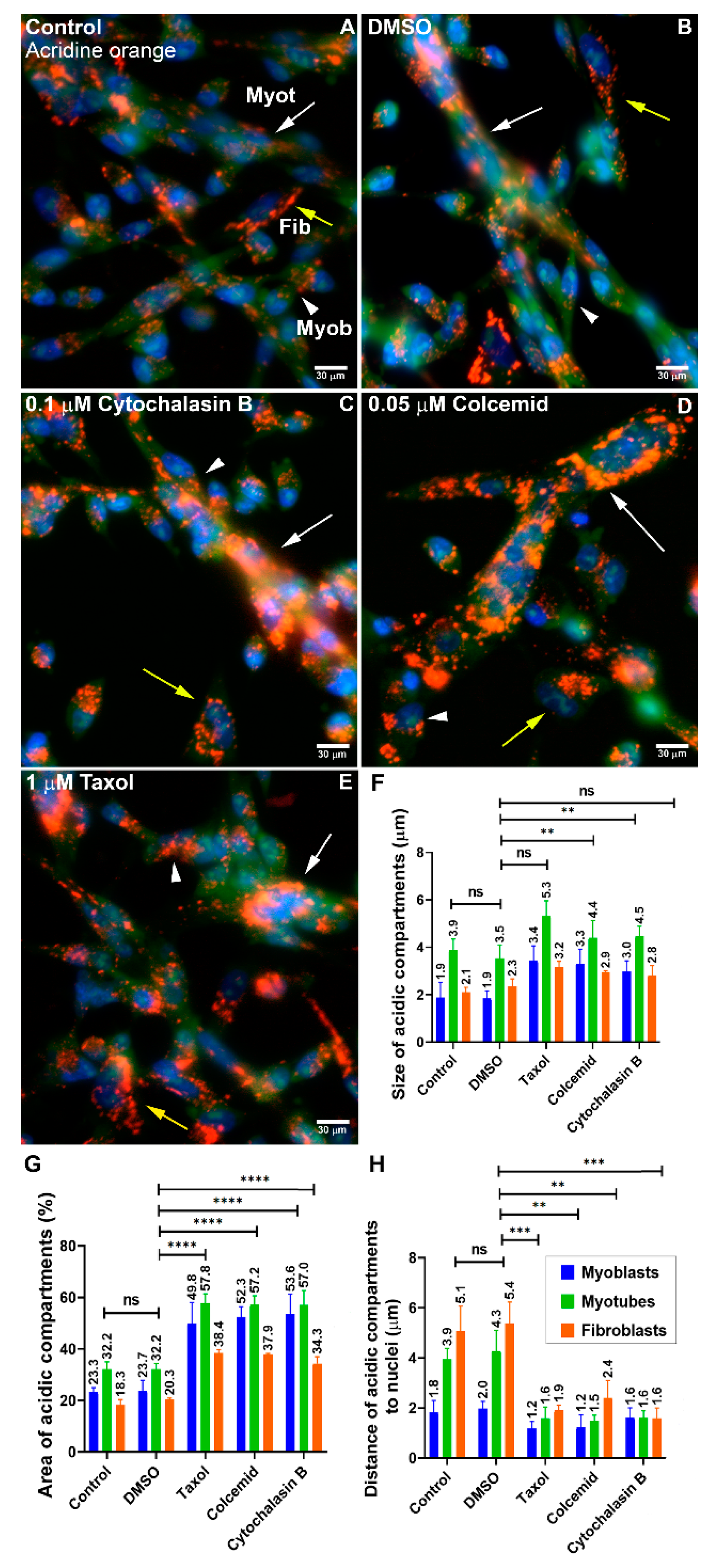
The intracellular positioning of lysosomes is dependent on their movement within the cell which has been associated with microtubules [
12,
21]. Here, we showed that the distribution of lysosomes in myogenic cells was altered in the presence of agents that interfere with the polymerization or stability of microtubules and microfilaments. Interestingly, these cytoskeletal interfering drugs induced an increase in the size of lysosomes suggesting that the cytoskeleton regulates the movement, size, and function of lysosomes in muscle cells. Importantly, it has been reported that nocodazole, but not Colchicine or Taxol, alters lysosome acidity and induces lysosomal disruption [
22]. Therefore, the effects of Taxol and Colcemid on lysosomes from muscle cells that we described here need to be further explored.
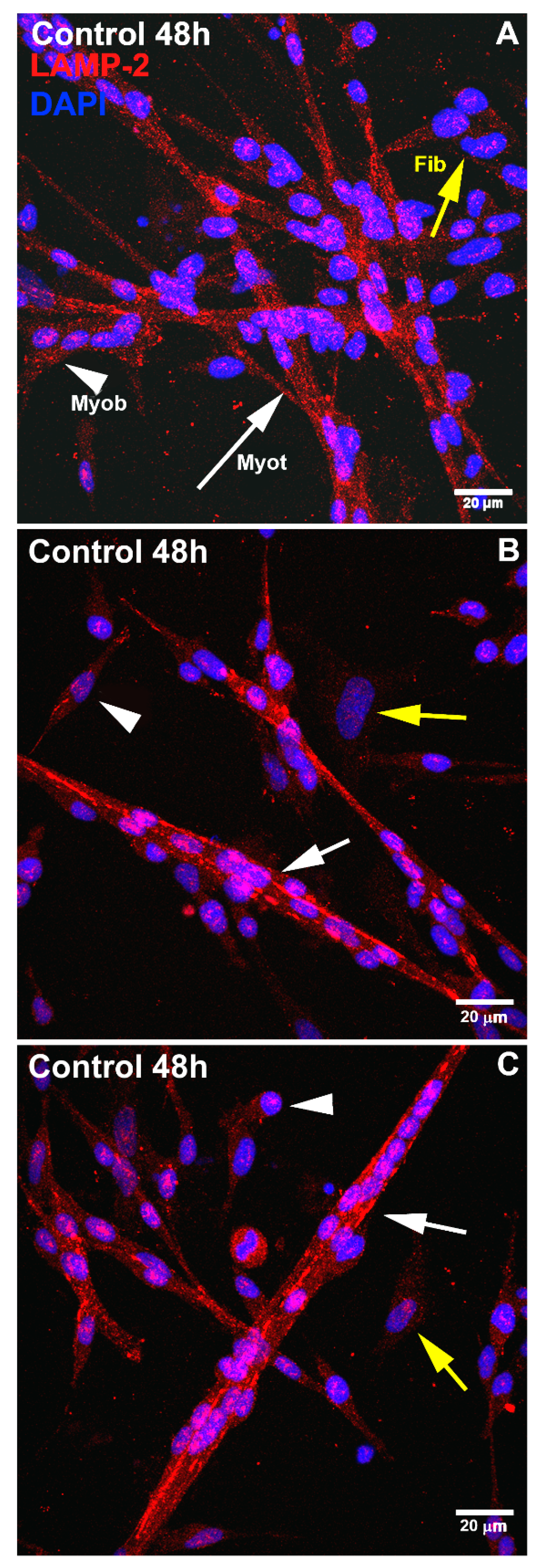
LAMP1 and LAMP2 are major lysosomal membrane proteins involved in lysosomal biogenesis and in the maintenance of the structural integrity of the lysosomal compartment [
23]. Our data show that the knockdown of LAMP2 by siRNA inhibited the formation of myotubes and altered the distribution and size of lysosomes in chick muscle cell cultures. These results agree with previous data showing that LAMP1 or LAMP2 depletion by siRNA impaired the differentiation of C2C12 mouse myoblasts, reduced the diameter of C2C12 myotubes, and decreased the expression levels of myogenic regulatory factors, MyoD, and myogenin [
24].
Importantly, our results show the involvement of the Wnt/beta-catenin pathway in the regulation of the lysosomal morphology and function during chick myogenesis. Two activators of the Wnt/beta-catenin pathway, Wnt3a and BIO, were able to rescue the deleterious effects of the knockdown of LAMP2 in the formation of muscle fibers. Different mechanisms could be involved in the interplay between lysosomes and the Wnt pathway. It has been reported that GSK3 can be sequestered from the cytosol into lysosomes, which activates the canonical Wnt/beta-catenin pathway [
6]. Further studies are necessary to understand the molecular mechanism involved in the Wnt/beta-catenin regulation of lysosomal dynamics in muscle cells.