Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review
Abstract
1. Introduction
2. Hormones
2.1. Sex Steroids and Gonadotropins
2.1.1. Androgen (Testosterone)
2.1.2. Estrogen
2.1.3. Progesterone
2.1.4. Gonadotropic Hormones
2.2. Calciotropic Hormones
2.2.1. Vitamin D
2.2.2. Parathyroid Hormone
2.2.3. Calcitonin
2.3. Circadian Physiology-Associated Hormones
2.3.1. Glucocorticoids
2.3.2. Melatonin
2.4. Thyroid Hormone
2.5. Growth Hormone
3. Metabolic Disorders of Energy Substrates
3.1. Bioenergetics of Bone Cells
3.2. Clinical Relevance
3.2.1. Glucose Metabolism
3.2.2. Lipid Metabolism
Dyslipidemia with Osteoporosis and Periodontitis
FAs Profile with Osteoporosis and Periodontitis
3.2.3. Amino Acid Metabolism
4. Lifestyle
4.1. Smoking
4.2. Alcohol Consumption
5. Psychological Stress
Neurogenic Factors in Osteoporosis and Periodontitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Kim, J.; Go, M.; Lee, S.Y. Early estrogen-induced gene 1 facilitates osteoclast formation through the inhibition of interferon regulatory factor 8 expression. FASEB J. 2020, 34, 12894–12906. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontol. 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Moon, R.J.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin. Med. 2015, 15, s92–s96. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Penoni, D.C.; Leão, A.T.T.; Fernandes, T.M.; Torres, S.R. Possible links between osteoporosis and periodontal disease. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 270–273. [Google Scholar] [CrossRef]
- Chin, K.Y. The Relationship between Follicle-stimulating Hormone and Bone Health: Alternative Explanation for Bone Loss beyond Oestrogen? Int. J. Med. Sci. 2018, 15, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.; Mawhinney, M. Endocrinology of sex steroid hormones and cell dynamics in the periodontium. Periodontol. 2000 2013, 61, 69–88. [Google Scholar] [CrossRef]
- Akcalı, A.; Akcalı, Z.; Batool, F.; Petit, C.; Huck, O. Are Sex Steroid Hormones Influencing Periodontal Conditions? A Systematic Review. Curr. Oral Health Rep. 2018, 5, 33–38. [Google Scholar] [CrossRef]
- Steffens, J.P.; Wang, X.; Starr, J.R.; Spolidorio, L.C.; Van Dyke, T.E.; Kantarci, A. Associations Between Sex Hormone Levels and Periodontitis in Men: Results From NHANES III. J. Periodontol. 2015, 86, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Shiau, H.J.; Aichelmann-Reidy, M.E.; Reynolds, M.A. Influence of sex steroids on inflammation and bone metabolism. Periodontol. 2000 2014, 64, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef]
- Syed, F.; Khosla, S. Mechanisms of sex steroid effects on bone. Biochem. Biophys. Res. Commun. 2005, 328, 688–696. [Google Scholar] [CrossRef]
- Seifert-Klauss, V.; Prior, J.C. Progesterone and bone: Actions promoting bone health in women. J. Osteoporos. 2010, 2010, 845180. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malmstrom, H.; Abduljabbar, T.; Vohra, F.; Kellesarian, T.V.; Javed, F.; Romanos, G.E. “Low Testosterone Levels in Body Fluids Are Associated With Chronic Periodontitis”. Am. J. Mens. Health 2017, 11, 443–453. [Google Scholar] [CrossRef]
- Steffens, J.P.; Coimbra, L.S.; Ramalho-Lucas, P.D.; Rossa, C., Jr.; Spolidorio, L.C. The effect of supra- and subphysiologic testosterone levels on ligature-induced bone loss in rats--a radiographic and histologic pilot study. J. Periodontol. 2012, 83, 1432–1439. [Google Scholar] [CrossRef]
- Wu, Y.X.; Si, S.S.; Zhang, X.; Lian, K.Q. Effect and mechanism of testosterone level on inflammatory bone resorption in periodontitis with mice. Shanghai Kou Qiang Yi Xue 2020, 29, 380–385. [Google Scholar] [PubMed]
- de Paiva Goncalves, V.; Ortega, A.A.C.; Steffens, J.P.; Spolidorio, D.M.P.; Rossa, C.; Spolidorio, L.C. Long-term testosterone depletion attenuates inflammatory bone resorption in the ligature-induced periodontal disease model. J. Periodontol. 2018, 89, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.P.; Coimbra, L.S.; Rossa, C., Jr.; Kantarci, A.; Van Dyke, T.E.; Spolidorio, L.C. Androgen receptors and experimental bone loss—An in vivo and in vitro study. Bone 2015, 81, 683–690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brusca, M.I.; Verdugo, F.; Amighini, C.; Albaina, O.; Moragues, M.D. Anabolic steroids affect human periodontal health and microbiota. Clin. Oral Investig. 2014, 18, 1579–1586. [Google Scholar] [CrossRef]
- Steffens, J.P.; Valenga, H.M.; Santana, L.C.L.; Albaricci, M.; Kantarci, A.; Spolidorio, L.C. Role of testosterone and androgen receptor in periodontal disease progression in female rats. J. Periodontol. 2020, 91, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ayed, M.S.; Alsharif, A.F.; Divakar, D.D.; Jhugroo, C.; Alosaimi, B.; Mustafa, M. Evaluating the possible association between systemic osteoporosis and periodontal disease progression in postmenopausal women. Dis. Mon. 2019, 65, 193–215. [Google Scholar] [CrossRef]
- Cannarella, R.; Barbagallo, F.; Condorelli, R.A.; Aversa, A.; La Vignera, S.; Calogero, A.E. Osteoporosis from an Endocrine Perspective: The Role of Hormonal Changes in the Elderly. J. Clin. Med. 2019, 8, 1564. [Google Scholar] [CrossRef]
- Shu, L.; Guan, S.M.; Fu, S.M.; Guo, T.; Cao, M.; Ding, Y. Estrogen modulates cytokine expression in human periodontal ligament cells. J. Dent. Res. 2008, 87, 142–147. [Google Scholar] [CrossRef]
- Tang, X.L.; Meng, H.X.; Zhang, L.; Hou, J.X.; Han, J. Effect of 17-beta estradiol on the expression of receptor activator of nuclear factor kappaB ligand and osteoprotegerin of human periodontal ligament cells during their osteogenic differentiation. Beijing Da Xue Xue Bao Yi Xue Ban 2007, 39, 50–53. [Google Scholar]
- Macari, S.; Ajay Sharma, L.; Wyatt, A.; Knowles, P.; Szawka, R.E.; Garlet, G.P.; Grattan, D.R.; Dias, G.J.; Silva, T.A. Osteoprotective Effects of Estrogen in the Maxillary Bone Depend on ERalpha. J. Dent. Res. 2016, 95, 689–696. [Google Scholar] [CrossRef]
- Guan, X.; Guan, Y.; Shi, C.; Zhu, X.; He, Y.; Wei, Z.; Yang, J.; Hou, T. Estrogen deficiency aggravates apical periodontitis by regulating NLRP3/caspase-1/IL-1beta axis. Am. J. Transl. Res. 2020, 12, 660–671. [Google Scholar] [PubMed]
- Silva de Araujo Figueiredo, C.; Gonçalves Carvalho Rosalem, C.; Costa Cantanhede, A.L.; Abreu Fonseca Thomaz, É.B.; Fontoura Nogueira da Cruz, M.C. Systemic alterations and their oral manifestations in pregnant women. J. Obstet. Gynaecol. Res. 2017, 43, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Cai, C.; Dai, J.; Liu, Y.; Zhang, R.; Dai, Y.; Wen, L.; Ding, Y. Progesterone modulates the proliferation and differentiation of human periodontal ligament cells. Calcif. Tissue Int. 2010, 87, 158–167. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bhardwaj, S.V. Effect of androgens, estrogens and progesterone on periodontal tissues. J. Orofac. Res. 2012, 2, 165–170. [Google Scholar] [CrossRef]
- Lapp, C.A.; Lohse, J.E.; Lewis, J.B.; Dickinson, D.P.; Billman, M.; Hanes, P.J.; Lapp, D.F. The effects of progesterone on matrix metalloproteinases in cultured human gingival fibroblasts. J. Periodontol. 2003, 74, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ji, Y.; Liu, S.; Bian, Z. Follicle-stimulating hormone enhances alveolar bone resorption via upregulation of cyclooxygenase-2. Am. J. Transl. Res. 2016, 8, 3861–3871. [Google Scholar]
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17. [Google Scholar] [CrossRef]
- Sun, D.; Bai, M.; Jiang, Y.; Hu, M.; Wu, S.; Zheng, W.; Zhang, Z. Roles of follicle stimulating hormone and its receptor in human metabolic diseases and cancer. Am. J. Transl. Res. 2020, 12, 3116–3132. [Google Scholar]
- Qian, H.; Jia, J.; Yang, Y.; Bian, Z.; Ji, Y. A Follicle-Stimulating Hormone Exacerbates the Progression of Periapical Inflammation Through Modulating the Cytokine Release in Periodontal Tissue. Inflammation 2020, 43, 1572–1585. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Troen, B.R. Calciotropic hormones. In Osteoporosis in Older Persons; Springer: Berlin/Heidelberg, Germany, 2016; pp. 43–58. [Google Scholar]
- Krawiec, M.; Dominiak, M. The role of vitamin D in the human body with a special emphasis on dental issues: Literature review. Dent. Med. Probl. 2018, 55, 419–424. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proenca, L.; Mendes, J.J.; Botelho, J. Vitamin D and Periodontitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Ketharanathan, V.; Torgersen, G.R.; Petrovski, B.; Preus, H.R. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D(3) in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Chen, J.; Wu, J.; Li, J.; Wang, L.; Goltzman, D.; Miao, D. 1,25-dihydroxyvitamin D deficiency accelerates alveolar bone loss independent of aging and extracellular calcium and phosphorus. J. Periodontol. 2018, 89, 983–994. [Google Scholar] [CrossRef]
- Hong, H.H.; Hong, A.; Wang, C.C.; Huang, E.W.; Chiang, C.C.; Yen, T.H.; Huang, Y.F. Calcitriol exerts a mineralization-inductive effect comparable to that of vitamin C in cultured human periodontium cells. Am. J. Transl. Res. 2019, 11, 2304–2316. [Google Scholar] [PubMed]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 16030. [Google Scholar] [CrossRef]
- Bi, C.S.; Li, X.; Qu, H.L.; Sun, L.J.; An, Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. Calcitriol inhibits osteoclastogenesis in an inflammatory environment by changing the proportion and function of T helper cell subsets (Th2/Th17). Cell Prolif. 2020, 53, e12827. [Google Scholar] [CrossRef] [PubMed]
- Menzel, L.P.; Ruddick, W.; Chowdhury, M.H.; Brice, D.C.; Clance, R.; Porcelli, E.; Ryan, L.K.; Lee, J.; Yilmaz, O.; Kirkwood, K.L.; et al. Activation of vitamin D in the gingival epithelium and its role in gingival inflammation and alveolar bone loss. J. Periodontal Res. 2019, 54, 444–452. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Hou, J. Activity of 25-hydroxylase in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e52053. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, K.; Meng, H. Preliminary investigation of the vitamin D pathway in periodontal connective tissue cells. J. Periodontol. 2018, 89, 294–302. [Google Scholar] [CrossRef]
- De Filippis, A.; Fiorentino, M.; Guida, L.; Annunziata, M.; Nastri, L.; Rizzo, A. Vitamin D reduces the inflammatory response by Porphyromonas gingivalis infection by modulating human beta-defensin-3 in human gingival epithelium and periodontal ligament cells. Int. Immunopharmacol. 2017, 47, 106–117. [Google Scholar] [CrossRef]
- Meghil, M.M.; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, K.; Hou, J. Extending the vitamin D pathway to vitamin D3 and CYP27A1 in periodontal ligament cells. J. Periodontol. 2021, 92, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N.; Kronenberg, H.M. Regulation of Bone Remodeling by Parathyroid Hormone. Cold. Spring. Harb. Perspect. Med. 2018, 8, a031237. [Google Scholar] [CrossRef]
- Stutz, C.; Batool, F.; Petit, C.; Strub, M.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Huck, O. Influence of parathyroid hormone on periodontal healing in animal models: A systematic review. Arch. Oral Biol. 2020, 120, 104932. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. Teriparatide and osseous regeneration in the oral cavity. N. Engl. J. Med. 2010, 363, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Keller, H.; Leupin, O.; Kneissel, M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol. Metab. 2010, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Tsuchiya, K.; Burr, D.B.; Minner, D.E.; Condon, K.W.; Miller, C.A.; Satoh, S.; Inaba, M.; Nakayama, T.; Tanizawa, T.; et al. Osteocytic perilacunar/canalicular turnover in hemodialysis patients with high and low serum PTH levels. Bone 2018, 113, 68–76. [Google Scholar] [CrossRef]
- Al-Dujaili, S.A.; Koh, A.J.; Dang, M.; Mi, X.; Chang, W.; Ma, P.X.; McCauley, L.K. Calcium Sensing Receptor Function Supports Osteoblast Survival and Acts as a Co-Factor in PTH Anabolic Actions in Bone. J. Cell Biochem. 2016, 117, 1556–1567. [Google Scholar] [CrossRef]
- Wang, J.; Gilchrist, A.; Stern, P.H. Antagonist minigenes identify genes regulated by parathyroid hormone through G protein-selective and G protein co-regulated mechanisms in osteoblastic cells. Cell Signal 2011, 23, 380–388. [Google Scholar] [CrossRef]
- Lossdorfer, S.; Stier, S.; Gotz, W.; Jager, A. Maturation-state dependent response of human periodontal ligament cells to an intermittent parathyroid hormone exposure in vitro. J. Periodontal Res. 2006, 41, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lossdorfer, S.; Gotz, W.; Jager, A. Parathyroid hormone modifies human periodontal ligament cell proliferation and survival in vitro. J. Periodontal Res. 2006, 41, 519–526. [Google Scholar] [CrossRef]
- Marques, M.R.; dos Santos, M.C.; da Silva, A.F.; Nociti, F.H., Jr.; Barros, S.P. Parathyroid hormone administration may modulate periodontal tissue levels of interleukin-6, matrix metalloproteinase-2 and matrix metalloproteinase-9 in experimental periodontitis. J. Periodontal Res. 2009, 44, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Saito, M.; Ngan, P.; Lanese, R.; Shanfeld, J.; Davidovitch, Z. Effects of parathyroid hormone and cytokines on prostaglandin E synthesis and bone resorption by human periodontal ligament fibroblasts. Arch. Oral Biol. 1990, 35, 845–855. [Google Scholar] [CrossRef]
- Mehta, N.M.; Malootian, A.; Gilligan, J.P. Calcitonin for osteoporosis and bone pain. Curr. Pharm. Des. 2003, 9, 2659–2676. [Google Scholar] [CrossRef]
- Ankam, A.; Koduganti, R.R. Calcitonin receptor gene polymorphisms at codon 447 in patients with osteoporosis and chronic periodontitis in South Indian population—An observational study. J. Indian Soc. Periodontol. 2017, 21, 107–111. [Google Scholar] [CrossRef]
- Huebner, A.K.; Schinke, T.; Priemel, M.; Schilling, S.; Schilling, A.F.; Emeson, R.B.; Rueger, J.M.; Amling, M. Calcitonin deficiency in mice progressively results in high bone turnover. J. Bone Miner. Res. 2006, 21, 1924–1934. [Google Scholar] [CrossRef]
- Wei, Y.; Ye, Q.; Tang, Z.; Tian, G.; Zhu, Q.; Gao, H.; Wang, D.; Cao, Z. Calcitonin induces collagen synthesis and osteoblastic differentiation in human periodontal ligament fibroblasts. Arch. Oral Biol. 2017, 74, 114–122. [Google Scholar] [CrossRef]
- Wada, S.; Yasuda, S. [Appropriate clinical usage of calcitonin escape phenomenon and intermittent v.s. daily administration of calcitonin]. Clin. Calcium. 2001, 11, 1169–1175. [Google Scholar] [CrossRef]
- Zhang, Z.; Neff, L.; Bothwell, A.L.; Baron, R.; Horne, W.C. Calcitonin induces dephosphorylation of Pyk2 and phosphorylation of focal adhesion kinase in osteoclasts. Bone 2002, 31, 359–365. [Google Scholar] [CrossRef]
- Davey, R.A.; Findlay, D.M. Calcitonin: Physiology or fantasy? J. Bone Miner. Res. 2013, 28, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, J.; Kim, B.; Lu, C.; Zhang, Z.; Liu, H.; Kang, H.; Sun, Y.; Guan, H.; Fang, Z.; et al. Insights into the Role of Circadian Rhythms in Bone Metabolism: A Promising Intervention Target? Biomed. Res. Int. 2018, 2018, 9156478. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Sousa, L.H.; Moura, E.V.; Queiroz, A.L.; Val, D.; Chaves, H.; Lisboa, M.; Furlaneto, F.; Brito, G.A.; Goes, P. Effects of glucocorticoid-induced osteoporosis on bone tissue of rats with experimental periodontitis. Arch. Oral Biol. 2017, 77, 55–61. [Google Scholar] [CrossRef]
- Decker, A.; Askar, H.; Tattan, M.; Taichman, R.; Wang, H.L. The assessment of stress, depression, and inflammation as a collective risk factor for periodontal diseases: A systematic review. Clin. Oral Investig. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Desmet, S.J.; De Bosscher, K. Glucocorticoid receptors: Finding the middle ground. J. Clin. Investig. 2017, 127, 1136–1145. [Google Scholar] [CrossRef]
- Lu, H.; Xu, M.; Wang, F.; Liu, S.; Gu, J.; Lin, S.; Zhao, L. Chronic stress accelerates ligature-induced periodontitis by suppressing glucocorticoid receptor-alpha signaling. Exp. Mol. Med. 2016, 48, e223. [Google Scholar] [CrossRef]
- Carpentieri, A.R.; Peralta Lopez, M.E.; Aguilar, J.; Sola, V.M. Melatonin and periodontal tissues: Molecular and clinical perspectives. Pharmacol. Res. 2017, 125, 224–231. [Google Scholar] [CrossRef]
- Li, T.; Jiang, S.; Lu, C.; Yang, W.; Yang, Z.; Hu, W.; Xin, Z.; Yang, Y. Melatonin: Another avenue for treating osteoporosis? J. Pineal Res. 2019, 66, e12548. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, S.; Chen, G.; Zheng, W.; Peng, J.; Huang, X.; Chen, L. Insight into the roles of melatonin in bone tissue and bone-related diseases (Review). Int. J. Mol. Med. 2021, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Balaji, T.M.; Varadarajan, S.; Jagannathan, R.; Gupta, A.A.; Raj, A.T.; Patil, S.; Fageeh, H.I.; Fageeh, H.N. Melatonin levels in periodontitis vs. the healthy state: A systematic review and meta-analysis. Oral Dis. 2022, 28, 284–306. [Google Scholar] [CrossRef]
- Zhen, Y.; Yue, H.; Xiao, Y.; Liu, Q.; Zhao, M. Efficacy of melatonin supplementation in the treatment of periodontitis: A systematic review and meta-analysis. Int. J. Dent. Oral Health 2021, 2021, 362. [Google Scholar] [CrossRef]
- Koyama, H.; Nakade, O.; Takada, Y.; Kaku, T.; Lau, K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.S.; Malaiappan, S. Role of melatonin in periodontal disease—A systematic review. Indian J. Dent. Res. 2020, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, X.; Zhu, C.L.; He, Z.Y.; Liang, J.P.; Song, Z.C. Melatonin Receptor Agonists as the “Perioceutics” Agents for Periodontal Disease through Modulation of Porphyromonas gingivalis Virulence and Inflammatory Response. PLoS ONE 2016, 11, e0166442. [Google Scholar] [CrossRef]
- Al-Otaibi, A.M.; Al-Gebaly, A.S.; Almeer, R.; Albasher, G.; Al-Qahtani, W.S.; Abdel Moneim, A.E. Melatonin pre-treated bone marrow derived-mesenchymal stem cells prompt wound healing in rat models. Biomed. Pharmacother. 2022, 145, 112473. [Google Scholar] [CrossRef]
- Renn, T.Y.; Huang, Y.K.; Feng, S.W.; Wang, H.W.; Lee, W.F.; Lin, C.T.; Burnouf, T.; Chen, L.Y.; Kao, P.F.; Chang, H.M. Prophylactic supplement with melatonin successfully suppresses the pathogenesis of periodontitis through normalizing RANKL/OPG ratio and depressing the TLR4/MyD88 signaling pathway. J. Pineal Res. 2018, 64, e12464. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Shindo, S.; Ozaki, K.; Matsuo, T. Melatonin Inhibits CXCL10 and MMP-1 Production in IL-1beta-Stimulated Human Periodontal Ligament Cells. Inflammation 2016, 39, 1520–1526. [Google Scholar] [CrossRef]
- Kose, O.; Arabaci, T.; Kizildag, A.; Erdemci, B.; Ozkal Eminoglu, D.; Gedikli, S.; Ozkanlar, S.; Zihni, M.; Albayrak, M.; Kara, A.; et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J. Periodontal Res. 2017, 52, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of Melatonin on Oxidative Stress Index and Alveolar Bone Loss in Diabetic Rats With Periodontitis. J. Periodontol. 2016, 87, e82–e90. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Doria, C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Monea, A.M.; Csinszka, K.-I.A.; Coşarcă, A.; Bereşescu, G. Oral health status in patients with thyroid disorders. Med. Sci. 2015, 4, 1–3. [Google Scholar]
- Shcherba, V.; Krynytska, I.; Marushchak, M.; Korda, M. Does thyroid dysfunction influence inflammatory mediators in experimental periodontitis? Endocr. Regul. 2021, 55, 131–141. [Google Scholar] [CrossRef]
- Shcherba, V.; Miz, A.; Kyryliv, M.; Bekus, I.; Krynytska, I.; Korda, M. Correlative link ages between indices of bone metabolism and thyroid hormones in rats with periodontitis. J. Educ. Health Sport 2017, 7, 184–196. [Google Scholar]
- Tuchendler, D.; Bolanowski, M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res. 2014, 7, 12. [Google Scholar] [CrossRef]
- Monfoulet, L.E.; Rabier, B.; Dacquin, R.; Anginot, A.; Photsavang, J.; Jurdic, P.; Vico, L.; Malaval, L.; Chassande, O. Thyroid hormone receptor beta mediates thyroid hormone effects on bone remodeling and bone mass. J. Bone Miner. Res. 2011, 26, 2036–2044. [Google Scholar] [CrossRef]
- Shcherba, V.; Yaroshenko, T.Y.; Kubant, R.; Korda, M. The influence of thyroid hormones on protein oxidative modification in case of experimental periodontitis. Med. Clin. Chem. 2019, 4, 52–59. [Google Scholar] [CrossRef]
- Shcherba, V.; Krynytska, I.Y.; Cherkashyn, S.; Machohan, V.; Stoikevych, H.; Korda, M. The state of peroxide lipid oxidation in rats with periodontitis on the background of hyper-and hypothyroidism. World Med. Biol. 2018, 14, 185–189. [Google Scholar] [CrossRef]
- Shcherba, V.; Vydoinyk, O.; Posolenyk, L.; Korda, M. The influence of thyroid hormones on mitochondrial mechanisms of blood neutrophils’ apoptosis in case of experimental periodontitis. Arch. Balk. Med. Union 2019, 54, 64–71. [Google Scholar] [CrossRef]
- Shcherba, V.; Havrylenko, Y.; Krynytska, I.; Marushchak, M.; Korda, M. A comparative study of oral microbiocenosis structure in experimental comorbidity-free periodontitis and in periodontitis combined with thyroid dysfunction. Pol. Merkur. Lekarski. 2020, 48, 32–38. [Google Scholar] [PubMed]
- Al-Hindawi, S.H.; Luaibi, N.M.; Al-Ghurabi, B.H. Estimation of Alkaline Phosphatase level in the Serum and Saliva of Hypothyroid Patients with and without Periodontitis. Res. J. Pharm. Technol. 2018, 11, 2993–2996. [Google Scholar] [CrossRef]
- Yerke, L.; Levine, M.; Cohen, R. MON-616 Potential Relationship between Hypothyroidism and Periodontal Disease Severity. J. Endocr. Soc. 2019, 3, 616. [Google Scholar] [CrossRef]
- Aldulaijan, H.A.; Cohen, R.E.; Stellrecht, E.M.; Levine, M.J.; Yerke, L.M. Relationship between hypothyroidism and periodontitis: A scoping review. Clin. Exp. Dent. Res. 2020, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Padova, G.; Borzì, G.; Incorvaia, L.; Siciliano, G.; Migliorino, V.; Vetri, M.; Tita, P. Prevalence of osteoporosis and vertebral fractures in acromegalic patients. Clin. Cases Miner. Bone Metab. 2011, 8, 37–43. [Google Scholar] [PubMed]
- Frost, H.M. Growth hormone and osteoporosis: An overview of endocrinological and pharmacological insights from the Utah paradigm of skeletal physiology. Horm. Res. 2000, 54, 36–43. [Google Scholar] [CrossRef]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell Endocrinol. 2021, 519, 111052. [Google Scholar] [CrossRef]
- Litsas, G. Growth Hormone and Craniofacial Tissues. An update. Open Dent. J. 2015, 9, 1–8. [Google Scholar] [CrossRef]
- BAŞÇIL, S.; İyidir, Ö.T.; Bayraktar, N.; Ertörer, M.E.; TÜTÜNCÜ, N.B. Severe chronic periodontitis is not common in Acromegaly: Potential protective role of gingival BMP-2. Turk. J. Med. Sci. 2021, 51, 1172–1178. [Google Scholar] [CrossRef]
- Haase, H.R.; Ivanovski, S.; Waters, M.J.; Bartold, P.M. Growth hormone regulates osteogenic marker mRNA expression in human periodontal fibroblasts and alveolar bone-derived cells. J. Periodontal Res. 2003, 38, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, Y.; Keceli, H.G.; Helvaci, N.; Erbas, T.; Nohutcu, R.M. The tendency of reduced periodontal destruction in acromegalic patients showing similar inflammatory status with periodontitis patients. Endocrine 2019, 66, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Araujo, I.M.P.; Albuquerque-Souza, E.; Aguiar-Oliveira, M.H.; Holzhausen, M.; Oliveira-Neto, L.A.; Salvatori, R.; Saraiva, L.; Mayer, M.P.A.; Pannuti, C.M.; Ribeiro, A.O.; et al. Immunological and microbiological periodontal profiles in isolated growth hormone deficiency. J. Periodontol. 2018, 89, 1351–1361. [Google Scholar] [CrossRef]
- Tritos, N.A.; Klibanski, A. Effects of Growth Hormone on Bone. Prog. Mol. Biol. Transl. Sci. 2016, 138, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Doga, M.; Frara, S.; Maffezzoni, F.; Porcelli, T.; Cerri, L.; Maroldi, R.; Giustina, A. Incidence of morphometric vertebral fractures in adult patients with growth hormone deficiency. Endocrine 2016, 52, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.P.; Herrera, B.S.; Coimbra, L.S.; Stephens, D.N.; Rossa, C., Jr.; Spolidorio, L.C.; Kantarci, A.; Van Dyke, T.E. Testosterone regulates bone response to inflammation. Horm. Metab. Res. 2014, 46, 193–200. [Google Scholar] [CrossRef]
- Junior, C.G.; de Arruda Amorim, J.P.; Welter, R.W.; Machado, M.A.; de Almeida Chuffa, L.G.; Amorim, E.M.P. Testosterone Deficiency Associated with Periodontal Disease Increases Alveolar Bone Resorption and Changes the Thickness of the Gingival Epithelium. J. Adv. Med. Med. Res. 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Cheng, C.L.; de Groat, W.C. Effect of orchiectomy and testosterone replacement on lower urinary tract function in anesthetized rats. Am. J. Physiol. Renal. Physiol. 2016, 311, F864–F870. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Kang, C.; Xie, Q.; Zhang, B.; Li, Y. Effects of estrogen deficiency on microstructural changes in rat alveolar bone proper and periodontal ligament. Mol. Med. Rep. 2015, 12, 3508–3514. [Google Scholar] [CrossRef][Green Version]
- Lee, D.J.; Wu, L.; Shimono, M.; Piao, Z.; Green, D.W.; Lee, J.M.; Jung, H.S. Differential Mechanism of Periodontitis Progression in Postmenopause. Front. Physiol. 2018, 9, 1098. [Google Scholar] [CrossRef]
- Lerner, U.H. Bone remodeling in post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R.; Saville, P.D.; Heaney, R.P. Effect of estrogens and calcium carbonate on bone loss in postmenopausal women. Ann. Intern. Med. 1977, 87, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Anbarcioglu, E.; Kirtiloglu, T.; Ozturk, A.; Kolbakir, F.; Acikgoz, G.; Colak, R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2019, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Cagetti, M.G.; Wolf, T.G.; Tennert, C.; Camoni, N.; Lingstrom, P.; Campus, G. The Role of Vitamins in Oral Health. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2020, 17, 938. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Ballyram, R.; Jadwat, Y.; Fourie, J.; Lemmer, J.; Feller, L. Vitamin D Deficiency as It Relates to Oral Immunity and Chronic Periodontitis. Int. J. Dent. 2018, 2018, 7315797. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proenca, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Bi, C.S.; Wang, J.; Qu, H.L.; Li, X.; Tian, B.M.; Ge, S.; Chen, F.M. Calcitriol suppresses lipopolysaccharide-induced alveolar bone damage in rats by regulating T helper cell subset polarization. J. Periodontal Res. 2019, 54, 612–623. [Google Scholar] [CrossRef]
- Li, H.; Zhong, X.; Li, W.; Wang, Q. Effects of 1,25-dihydroxyvitamin D3 on experimental periodontitis and AhR/NF-κB/NLRP3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019, 27, e20180713. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, Y.; Song, D.; Zhu, Z.; Zhou, C.; Dai, L.; Xu, X. Tyrosine-protein phosphatase non-receptor type 2 inhibits alveolar bone resorption in diabetic periodontitis via dephosphorylating CSF1 receptor. J. Cell Mol. Med. 2019, 23, 6690–6699. [Google Scholar] [CrossRef]
- Blufstein, A.; Behm, C.; Kubin, B.; Gahn, J.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Transcriptional activity of vitamin D receptor in human periodontal ligament cells is diminished under inflammatory conditions. J. Periodontol. 2021, 92, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Abuduwali, N.; Lossdorfer, S.; Winter, J.; Kraus, D.; Guhlke, S.; Wolf, M.; Jager, A. Functional characterization of the parathyroid hormone 1 receptor in human periodontal ligament cells. Clin. Oral Investig. 2014, 18, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Jager, A.; Abuduwali, N.; Gotz, W.; Lossdorfer, S. Continuous PTH modulates alkaline phosphatase activity in human PDL cells via protein kinase C dependent pathways in vitro. Ann. Anat. 2013, 195, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Lossdorfer, S.; Gotz, W.; Rath-Deschner, B.; Jager, A. Parathyroid hormone(1-34) mediates proliferative and apoptotic signaling in human periodontal ligament cells in vitro via protein kinase C-dependent and protein kinase A-dependent pathways. Cell Tissue Res. 2006, 325, 469–479. [Google Scholar] [CrossRef]
- Kraus, D.; Jäger, A.; Abuduwali, N.; Deschner, J.; Lossdörfer, S. Intermittent PTH(1-34) signals through protein kinase A to regulate osteoprotegerin production in human periodontal ligament cells in vitro. Clin. Oral Investig. 2012, 16, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Pirih, F.Q.; Koh, A.J.; Michalski, M.; Eber, M.R.; Ritchie, K.; Sinder, B.; Oh, S.; Al-Dujaili, S.A.; Lee, J.; et al. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. J. Biol. Chem. 2013, 288, 6814–6825. [Google Scholar] [CrossRef]
- von Wowern, N.; Klausen, B.; Olgaard, K. Steroid-induced mandibular bone loss in relation to marginal periodontal changes. J. Clin. Periodontol. 1992, 19, 182–186. [Google Scholar] [CrossRef]
- Breivik, T.; Opstad, P.K.; Gjermo, P.; Thrane, P.S. Effects of hypothalamic-pituitary-adrenal axis reactivity on periodontal tissue destruction in rats. Eur. J. Oral Sci. 2000, 108, 115–122. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Gjermo, P.; Opstad, P.K. Glucocorticoid receptor antagonist RU 486 treatment reduces periodontitis in Fischer 344 rats. J. Periodontal Res. 2000, 35, 285–290. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Osmundsen, H.; Fonnum, F.; Opstad, P.K. Neonatal dexamethasone and chronic tianeptine treatment inhibit ligature-induced periodontitis in adult rats. J. Periodontal Res. 2006, 41, 23–32. [Google Scholar] [CrossRef]
- Gomaa, N.; Tenenbaum, H.; Glogauer, M.; Quinonez, C. The Biology of Social Adversity Applied to Oral Health. J. Dent. Res. 2019, 98, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Konecna, B.; Chobodova, P.; Janko, J.; Banasova, L.; Babickova, J.; Celec, P.; Tothova, L. The Effect of Melatonin on Periodontitis. Int. J. Mol. Sci. 2021, 22, 2390. [Google Scholar] [CrossRef] [PubMed]
- Vaseenon, S.; Chattipakorn, N.; Chattipakorn, S.C. Effects of melatonin in wound healing of dental pulp and periodontium: Evidence from in vitro, in vivo and clinical studies. Arch. Oral Biol. 2021, 123, 105037. [Google Scholar] [CrossRef]
- Rahangdale, S.I.; Galgali, S.R. Periodontal status of hypothyroid patients on thyroxine replacement therapy: A comparative cross-sectional study. J. Indian Soc. Periodontol. 2018, 22, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Britto, I.M.; Aguiar-Oliveira, M.H.; Oliveira-Neto, L.A.; Salvatori, R.; Souza, A.H.; Araujo, V.P.; Corraini, P.; Pannuti, C.M.; Romito, G.A.; Pustiglioni, F.E. Periodontal disease in adults with untreated congenital growth hormone deficiency: A case-control study. J. Clin. Periodontol. 2011, 38, 525–531. [Google Scholar] [CrossRef]
- Serinsöz, H.; Ertörer, M.E.; Başcıl, S.; Bakıner, O.; Bozkırlı, E.; Tütüncü, N.B. Low Prevalence of Periodontitis in Acromegaly: Growth Hormone May Exert a Protective Effect. Turk. J. Endocrinol. Metab. 2015, 19, 42–48. [Google Scholar] [CrossRef]
- Koffi, K.A.; Doublier, S.; Ricort, J.M.; Babajko, S.; Nassif, A.; Isaac, J. The Role of GH/IGF Axis in Dento-Alveolar Complex from Development to Aging and Therapeutics: A Narrative Review. Cells 2021, 10, 1181. [Google Scholar] [CrossRef]
- Yang, J.; Ueharu, H.; Mishina, Y. Energy metabolism: A newly emerging target of BMP signaling in bone homeostasis. Bone 2020, 138, 115467. [Google Scholar] [CrossRef]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef]
- Mohsin, S.; Baniyas, M.M.; AlDarmaki, R.S.; Tekes, K.; Kalász, H.; Adeghate, E.A. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin. Biol. Ther. 2019, 19, 937–948. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sugimoto, T. Advanced Glycation End Products, Diabetes, and Bone Strength. Curr. Osteoporos. Rep. 2016, 14, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Serni, L.; Monami, M.; Caliri, M.; Barbato, L.; Cairo, F.; Mannucci, E. Type 1 diabetes and periodontitis: Prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020, 57, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, T.; Cai, L.; Kong, C.; He, J. Current Knowledge Regarding the Interaction Between Oral Bone Metabolic Disorders and Diabetes Mellitus. Front. Endocrinol. 2020, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shrestha, A.; Zhang, H.; Li, L.; Li, D.; Fu, T.; Song, J.; Ji, P.; Huang, Y.; Chen, T. Impact of diabetes mellitus simulations on bone cell behavior through in vitro models. J. Bone Miner. Metab. 2020, 38, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yamaguchi, T.; Kanazawa, I.; Sugimoto, T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun. 2015, 461, 193–199. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Yang, B.; Shi, Y.X.; Zhang, W.L.; Liu, F.; Zhao, W.; Yang, M.W. High glucose downregulates the effects of autophagy on osteoclastogenesis via the AMPK/mTOR/ULK1 pathway. Biochem. Biophys. Res. Commun. 2018, 503, 428–435. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Zhou, Y.; Chen, W.; Luo, G.; Zhang, Z.; Wang, H.; Zhang, Y.; Xu, D.; Sheng, P. Advanced glycation end products biphasically modulate bone resorption in osteoclast-like cells. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E355–E366. [Google Scholar] [CrossRef]
- Karner, C.M.; Long, F. Glucose metabolism in bone. Bone 2018, 115, 2–7. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, S.; Chen, G.; Ding, Y.; Wu, Y.; Tian, W. Hyperglycemia Induces Osteoclastogenesis and Bone Destruction Through the Activation of Ca(2+)/Calmodulin-Dependent Protein Kinase II. Calcif. Tissue Int. 2019, 104, 390–401. [Google Scholar] [CrossRef]
- Malta, F.S.; Garcia, R.P.; Azarias, J.S.; Ribeiro, G.; Miranda, T.S.; Shibli, J.A.; Bastos, M.F. Impact of hyperglycemia and treatment with metformin on ligature-induced bone loss, bone repair and expression of bone metabolism transcription factors. PLoS ONE 2020, 15, e0237660. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, L.; Wang, B.; Xu, B.; Zhang, J. GLP-1 inhibits PKCbeta2 phosphorylation to improve the osteogenic differentiation potential of hPDLSCs in the AGE microenvironment. J. Diabetes Complicat. 2020, 34, 107495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, T.; Sun, W.; Yuan, Z.; Yu, M.; Chen, G.; Guo, W.; Xiao, J.; Tian, W. DNA Demethylation Rescues the Impaired Osteogenic Differentiation Ability of Human Periodontal Ligament Stem Cells in High Glucose. Sci. Rep. 2016, 6, 27447. [Google Scholar] [CrossRef] [PubMed]
- Panezai, J.; Altamash, M.; Engstrm, P.E.; Larsson, A. Association of Glycated Proteins with Inflammatory Proteins and Periodontal Disease Parameters. J. Diabetes Res. 2020, 2020, 6450742. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, D.L.; Britten, T.M.; Storch, D.L.; Calderon, N.L.; Sorenson, H.L.; Wallet, S.M. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013, 19, 303–312. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Chen, Y.Y.; Liu, Q.; Chen, Y.; Tan, L.; Zhang, S.H.; Gao, Z.R.; Zhou, Y.H.; Zhang, G.Y.; et al. miR-124-3p increases in high glucose induced osteocyte-derived exosomes and regulates galectin-3 expression: A possible mechanism in bone remodeling alteration in diabetic periodontitis. FASEB J. 2020, 34, 14234–14249. [Google Scholar] [CrossRef]
- Akram, Z.; Alqahtani, F.; Alqahtani, M.; Al-Kheraif, A.A.; Javed, F. Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type-2 diabetes mellitus. J. Periodontol. 2020, 91, 396–402. [Google Scholar] [CrossRef]
- Sakamoto, E.; Kido, J.I.; Takagi, R.; Inagaki, Y.; Naruishi, K.; Nagata, T.; Yumoto, H. Advanced glycation end-product 2 and Porphyromonas gingivalis lipopolysaccharide increase sclerostin expression in mouse osteocyte-like cells. Bone 2019, 122, 22–30. [Google Scholar] [CrossRef]
- Kang, J.; Boonanantanasarn, K.; Baek, K.; Woo, K.M.; Ryoo, H.M.; Baek, J.H.; Kim, G.S. Erratum: Institutions, Correspondence, Figures & Legends Correction. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J. Periodontal Implant. Sci. 2015, 45, 156–159. [Google Scholar] [CrossRef]
- Graves, D.T.; Alshabab, A.; Albiero, M.L.; Mattos, M.; Corrêa, J.D.; Chen, S.; Yang, Y. Osteocytes play an important role in experimental periodontitis in healthy and diabetic mice through expression of RANKL. J. Clin. Periodontol. 2018, 45, 285–292. [Google Scholar] [CrossRef]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontology 2000 2020, 83, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Chien, L.Y.; Chong, L.Y.; Kuo, Y.P.; Hsiao, J.K. Glycated matrix up-regulates inflammatory signaling similarly to Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2013, 48, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Nie, L.; Zhao, P.; Zhou, X.; Ding, Y.; Chen, Q.; Wang, Q. Diabetes fuels periodontal lesions via GLUT1-driven macrophage inflammaging. Int. J. Oral Sci. 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yue, Z.; Nie, L.; Zhao, Z.; Wang, Q.; Chen, J.; Wang, Q. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J. Clin. Periodontol. 2021, 48, 1379–1392. [Google Scholar] [CrossRef]
- Morran, M.P.; Alexander, L.A.; Slotterbeck, B.D.; McInerney, M.F. Dysfunctional innate immune responsiveness to Porphyromonas gingivalis lipopolysaccharide in diabetes. Oral Microbiol. Immunol. 2009, 24, 331–339. [Google Scholar] [CrossRef]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- XU, D.; WANG, K.; WU, J. Analysis of the relationship between complement and lipid metabolism and bone mineral density in elderly population. Chin. J. Lab. Med. 2019, 12, 1020–1024. [Google Scholar]
- Chen, Y.Y.; Wang, W.W.; Yang, L.; Chen, W.W.; Zhang, H.X. Association between lipid profiles and osteoporosis in postmenopausal women: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1–9. [Google Scholar] [CrossRef]
- Sivas, F.; Alemdaroglu, E.; Elverici, E.; Kulug, T.; Ozoran, K. Serum lipid profile: Its relationship with osteoporotic vertebrae fractures and bone mineral density in Turkish postmenopausal women. Rheumatol. Int. 2009, 29, 885–890. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.M.; Miranda, M.; Figueroa, C.; Rodriguez, J.P.; Rosen, C.J. Qualitative Aspects of Bone Marrow Adiposity in Osteoporosis. Front. Endocrinol. 2016, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xie, F.; Zhou, B.; Wang, J.; Wu, B.; Li, L.; Kang, Y.; Dai, R.; Jiang, Y. Differentially expressed proteins identified by TMT proteomics analysis in bone marrow microenvironment of osteoporotic patients. Osteoporos. Int. 2019, 30, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, R.; Pigossi, S.C.; Finoti, L.S.; Orrico, S.R.P.; Cirelli, J.A.; Barros, S.P.; Offenbacher, S.; Scarel-Caminaga, R.M. Serum lipid levels in patients with periodontal disease: A meta-analysis and meta-regression. J. Clin. Periodontol. 2017, 44, 1192–1207. [Google Scholar] [CrossRef]
- Kırzıoğlu, F.Y.; Fentoğlu, Ö.; Bulut, M.T.; Doğan, B.; Özdem, M.; Özmen, Ö.; Çarsancaklı, S.A.; Ergün, A.G.; Orhan, H. Is a Cholestrol-Enriched Diet a Risk Factor for Alveolar Bone Loss? J. Periodontol. 2016, 87, 529–538. [Google Scholar] [CrossRef]
- Silva, N.L.C.; Motta, N.A.V.; Soares, M.A.; Araujo, O.M.O.; Espíndola, L.C.P.; Colombo, A.P.V.; Lopes, R.T.; Brito, F.C.F.; Miranda, A.L.P.; Tributino, J.L.M. Periodontal status, vascular reactivity, and platelet aggregation changes in rats submitted to hypercholesterolemic diet and periodontitis. J. Periodontal Res. 2020, 55, 453–463. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, C. Effects of Hyperlipidemia and Cardiovascular Diseases on Proliferation, Differentiation and Homing of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 377–387. [Google Scholar] [CrossRef]
- You, L.; Sheng, Z.Y.; Tang, C.L.; Chen, L.; Pan, L.; Chen, J.Y. High cholesterol diet increases osteoporosis risk via inhibiting bone formation in rats. Acta Pharmacol. Sin. 2011, 32, 1498–1504. [Google Scholar] [CrossRef]
- Kim, H.; Oh, B.; Park-Min, K.H. Regulation of Osteoclast Differentiation and Activity by Lipid Metabolism. Cells 2021, 10, 89. [Google Scholar] [CrossRef]
- Herrmann, M. Marrow Fat-Secreted Factors as Biomarkers for Osteoporosis. Curr. Osteoporos. Rep. 2019, 17, 429–437. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Leite, G.A.; Carmo-Silva, C.C.; Taira, T.M.; Neves, K.B.; Colon, D.F.; da Silva, L.A.; Salvador, S.L.; Tostes, R.C.; Cunha, F.Q.; et al. Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice. J. Bone Miner. Res. 2017, 32, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.; Han, Q.; Tu, Q.; Zhang, L.; Yu, L.; Murry, D.; Tu, T.; Tang, Y.; Lian, J.B.; Stein, G.S.; et al. Epigenetic Modulation in Periodontitis: Interaction of Adiponectin and JMJD3-IRF4 Axis in Macrophages. J. Cell Physiol. 2016, 231, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Baque, V.; Serino, M.; Vergnes, J.N.; Riant, E.; Loubieres, P.; Arnal, J.F.; Gourdy, P.; Sixou, M.; Burcelin, R.; Kemoun, P. High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: Protective action of estrogens. PLoS ONE 2012, 7, e48220. [Google Scholar] [CrossRef]
- Cavagni, J.; de Macedo, I.C.; Gaio, E.J.; Souza, A.; de Molon, R.S.; Cirelli, J.A.; Hoefel, A.L.; Kucharski, L.C.; Torres, I.L.; Rosing, C.K. Obesity and Hyperlipidemia Modulate Alveolar Bone Loss in Wistar Rats. J. Periodontol. 2016, 87, e9–e17. [Google Scholar] [CrossRef]
- Montalvany-Antonucci, C.C.; Zicker, M.C.; Ferreira, A.V.M.; Macari, S.; Ramos-Junior, E.S.; Gomez, R.S.; Pereira, T.S.F.; Madeira, M.F.M.; Fukada, S.Y.; Andrade, I., Jr.; et al. High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J. Nutr. Biochem. 2018, 59, 93–103. [Google Scholar] [CrossRef]
- Chen, S.; Lin, G.; You, X.; Lei, L.; Li, Y.; Lin, M.; Luo, K.; Yan, F. Hyperlipidemia causes changes in inflammatory responses to periodontal pathogen challenge: Implications in acute and chronic infections. Arch. Oral Biol. 2014, 59, 1075–1084. [Google Scholar] [CrossRef]
- Sobaniec, H.; Sobaniec-Lotowska, M.E. Morphological examinations of hard tissues of periodontium and evaluation of selected processes of lipid peroxidation in blood serum of rats in the course of experimental periodontitis. Med. Sci. Monit. 2000, 6, 875–881. [Google Scholar]
- Fentoğlu, Ö.; Kırzıoğlu, F.Y.; Bulut, M.T.; Kumbul Doğuç, D.; Kulaç, E.; Önder, C.; Günhan, M. Evaluation of lipid peroxidation and oxidative DNA damage in patients with periodontitis and hyperlipidemia. J. Periodontol. 2015, 86, 682–688. [Google Scholar] [CrossRef]
- Fentoğlu, Ö.; Tözüm Bulut, M.; Doğan, B.; Kırzıoğlu, F.Y.; Kemer Doğan, E.S. Is the relationship between periodontitis and hyperlipidemia mediated by lipoprotein-associated inflammatory mediators? J. Periodontal Implant. Sci. 2020, 50, 135–145. [Google Scholar] [CrossRef]
- Nagahama, Y.; Obama, T.; Usui, M.; Kanazawa, Y.; Iwamoto, S.; Suzuki, K.; Miyazaki, A.; Yamaguchi, T.; Yamamoto, M.; Itabe, H. Oxidized low-density lipoprotein-induced periodontal inflammation is associated with the up-regulation of cyclooxygenase-2 and microsomal prostaglandin synthase 1 in human gingival epithelial cells. Biochem. Biophys. Res. Commun. 2011, 413, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Garfinkel, A.; Demer, L.L. Role of lipids in osteoporosis. Arter. Thromb. Vasc. Biol. 2000, 20, 2346–2348. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, A.M. Diabetic periodontitis: Possible lipid-induced defect in tissue repair through alteration of macrophage phenotype and function. Oral Dis. 1995, 1, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Fentoglu, O.; Bozkurt, F.Y. The Bi-Directional Relationship between Periodontal Disease and Hyperlipidemia. Eur. J. Dent. 2008, 2, 142–146. [Google Scholar] [CrossRef]
- Ohgi, K.; Kajiya, H.; Goto, T.K.; Okamoto, F.; Yoshinaga, Y.; Okabe, K.; Sakagami, R. Toll-like receptor 2 activation primes and upregulates osteoclastogenesis via lox-1. Lipids Health Dis. 2018, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Kim, H.J.; Joo, J.Y.; Lee, J.Y.; Lee, J.H.; Park, H.R. Periodontal Pathogens Promote Foam Cell Formation by Blocking Lipid Efflux. J. Dent. Res. 2021, 100, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Kudo, Y.; Ishimaru, N.; Funaki, M. Potential Role of Free Fatty Acids in the Pathogenesis of Periodontitis and Primary Sjögren’s Syndrome. Int. J. Mol. Sci. 2017, 18, 836. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zhang, X.; Yu, H.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Metabolic syndrome exacerbates inflammation and bone loss in periodontitis. J. Dent. Res. 2015, 94, 362–370. [Google Scholar] [CrossRef]
- Muluke, M.; Gold, T.; Kiefhaber, K.; Al-Sahli, A.; Celenti, R.; Jiang, H.; Cremers, S.; Van Dyke, T.; Schulze-Späte, U. Diet-Induced Obesity and Its Differential Impact on Periodontal Bone Loss. J. Dent. Res. 2016, 95, 223–229. [Google Scholar] [CrossRef]
- Antona, M.E.; Ramos, C.; Stranges, A.; Monteiro, A.F.; Gonzales Chaves, M.M.; Mandalunis, P.; Zago, V.; Friedman, S.M.; Macri, E.V. Fish oil diet effects on alveolar bone loss, in hypercholesterolemic rats. Arch. Oral Biol. 2020, 109, 104553. [Google Scholar] [CrossRef]
- Qi, T.; Li, L.; Weidong, T. The Role of Sphingolipid Metabolism in Bone Remodeling. Front. Cell Dev. Biol. 2021, 9, 752540. [Google Scholar] [CrossRef]
- Alsahli, A.; Kiefhaber, K.; Gold, T.; Muluke, M.; Jiang, H.; Cremers, S.; Schulze-Spate, U. Palmitic Acid Reduces Circulating Bone Formation Markers in Obese Animals and Impairs Osteoblast Activity via C16-Ceramide Accumulation. Calcif. Tissue Int. 2016, 98, 511–519. [Google Scholar] [CrossRef]
- van Heerden, B.; Kasonga, A.; Kruger, M.C.; Coetzee, M. Palmitoleic Acid Inhibits RANKL-Induced Osteoclastogenesis and Bone Resorption by Suppressing NF-kappaB and MAPK Signalling Pathways. Nutrients 2017, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Albertazzi, P.; Coupland, K. Polyunsaturated fatty acids. Is there a role in postmenopausal osteoporosis prevention? Maturitas. 2002, 42, 13–22. [Google Scholar] [CrossRef]
- Symmank, J.; Chorus, M.; Appel, S.; Marciniak, J.; Knaup, I.; Bastian, A.; Hennig, C.L.; Döding, A.; Schulze-Späte, U.; Jacobs, C.; et al. Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts. Sci. Rep. 2020, 10, 15706. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Brinson, C.W.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. CD36 is upregulated in mice with periodontitis and metabolic syndrome and involved in macrophage gene upregulation by palmitate. Oral Dis. 2017, 23, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Hirasawa, A.; Ichimura, A.; Kimura, I.; Tsujimoto, G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J. Pharm. Sci. 2011, 100, 3594–3601. [Google Scholar] [CrossRef]
- Shikama, Y.; Kudo, Y.; Ishimaru, N.; Funaki, M. Possible Involvement of Palmitate in Pathogenesis of Periodontitis. J. Cell Physiol. 2015, 230, 2981–2989. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, G.H.; Long, M.T.; Cheung, C.L.; Vasan, R.S.; Hsu, Y.H.; Kiel, D.P.; Liu, C.T. Metabolomics Insights into Osteoporosis Through Association With Bone Mineral Density. J. Bone Miner. Res. 2021, 36, 729–738. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Chan, R.; Kwok, T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 2019, 129, 115082. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, H.; He, B.; He, J.; Tian, Y. Relationship Between Serum Amino Acid Levels and Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 763538. [Google Scholar] [CrossRef] [PubMed]
- Ohata, M.; Fujita, T.; Orimo, H.; Yoshikawa, M. Amino acid metabolism in osteoporosis. J. Am. Geriatr. Soc. 1970, 18, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pernow, Y.; Thorén, M.; Sääf, M.; Fernholm, R.; Anderstam, B.; Hauge, E.M.; Hall, K. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone 2010, 47, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated with Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence From Discordant Monozygotic Twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Iwata, J. Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone 2021, 146, 115881. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Rasmussen, H.M.; Dallal, G.E. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos. Int. 2007, 18, 955–961. [Google Scholar] [CrossRef] [PubMed]
- El Refaey, M.; Watkins, C.P.; Kennedy, E.J.; Chang, A.; Zhong, Q.; Ding, K.H.; Shi, X.M.; Xu, J.; Bollag, W.B.; Hill, W.D.; et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol. Cell Endocrinol. 2015, 410, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, M.; Hollak, C.E.M. Bone health in patients with inborn errors of metabolism. Rev. Endocr. Metab. Disord. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R. Human osteopenic bone-derived osteoblasts: Essential amino acids treatment effects. Artif. Cells Blood. Substit. Immobil. Biotechnol. 2003, 31, 35–46. [Google Scholar] [CrossRef]
- Visser, J.J.; Hoekman, K. Arginine supplementation in the prevention and treatment of osteoporosis. Med. Hypotheses 1994, 43, 339–342. [Google Scholar] [CrossRef]
- Civitelli, R.; Villareal, D.T.; Agnusdei, D.; Nardi, P.; Avioli, L.V.; Gennari, C. Dietary L-lysine and calcium metabolism in humans. Nutrition 1992, 8, 400–405. [Google Scholar] [PubMed]
- Ling, C.W.; Miao, Z.; Xiao, M.L.; Zhou, H.; Jiang, Z.; Fu, Y.; Xiong, F.; Zuo, L.S.; Liu, Y.P.; Wu, Y.Y.; et al. The Association of Gut Microbiota With Osteoporosis Is Mediated by Amino Acid Metabolism: Multiomics in a Large Cohort. J. Clin. Endocrinol. Metab. 2021, 106, e3852–e3864. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.K.; Ohara-Nemoto, Y. Exopeptidases and gingipains in Porphyromonas gingivalis as prerequisites for its amino acid metabolism. Jpn. Dent. Sci. Rev. 2016, 52, 22–29. [Google Scholar] [CrossRef]
- Uematsu, H.; Hoshino, E. Degradation of arginine and other amino acids by Eubacterium nodatum ATCC 33099. Microbial. Ecol. Health Dis. 1996, 9, 305–311. [Google Scholar] [CrossRef][Green Version]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Kanazawa, I.; Sugimoto, T. Diabetes Mellitus-induced Bone Fragility. Intern. Med. 2018, 57, 2773–2785. [Google Scholar] [CrossRef]
- van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sato, M.; Dehle, F.C.; Brnabic, A.J.; Weston, A.; Burge, R. Lifestyle-Related Metabolic Disorders, Osteoporosis, and Fracture Risk in Asia: A Systematic Review. Value Health Reg. Issues 2016, 9, 49–56. [Google Scholar] [CrossRef]
- Collier, B.; Dossett, L.A.; May, A.K.; Diaz, J.J. Glucose control and the inflammatory response. Nutr. Clin. Pract. 2008, 23, 3–15. [Google Scholar] [CrossRef]
- Li, H.; Zhong, X.; Chen, Z.; Li, W. Suppression of NLRP3 inflammasome improves alveolar bone defect healing in diabetic rats. J. Orthop. Surg. Res. 2019, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, D.; Wu, Y.; Yan, L.; Liu, J.; Pan, C.; Pan, Y. The expression of inducible nitric oxide synthase in the gingiva of rats with periodontitis and diabetes mellitus. Arch. Oral Biol. 2020, 112, 104652. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuang, S.; Zhang, M.; Huang, X.; Chen, J.; Guan, M.; Qin, W.; Xu, H.H.K.; Lin, Z. Inhibition of CCL2 by bindarit alleviates diabetes-associated periodontitis by suppressing inflammatory monocyte infiltration and altering macrophage properties. Cell Mol. Immunol. 2021, 18, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wang, S.; Zhu, C.; Guo, J.; Li, K.; Li, A. Complement 3 mediates periodontal destruction in patients with type 2 diabetes by regulating macrophage polarization in periodontal tissues. Cell Prolif. 2020, 53, e12886. [Google Scholar] [CrossRef]
- Huang, Z.; Pei, X.; Graves, D.T. The Interrelationship Between Diabetes, IL-17 and Bone Loss. Curr. Osteoporos. Rep. 2020, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Fernandes, T.; Zagalo, L.; Gonçalves, L. Uncovering the links between osteoporosis and diabetes. Ann. Med. 2019, 51, 50. [Google Scholar] [CrossRef]
- Kanazawa, I. Interaction between bone and glucose metabolism [Review]. Endocr. J. 2017, 64, 1043–1053. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Chew, C.K.; Monroe, D.G.; Farr, J.N.; Atkinson, E.J.; Geske, J.R.; Eckhardt, B.; Thicke, B.; Ruan, M.; Tweed, A.J.; et al. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 2020, 11, 87. [Google Scholar] [CrossRef]
- Fernandes, T.A.P.; Gonçalves, L.M.L.; Brito, J.A.A. Relationships between Bone Turnover and Energy Metabolism. J. Diabetes Res. 2017, 2017, 9021314. [Google Scholar] [CrossRef]
- Tang, Q.-Q. Lipid Metabolism and Diseases; Springer: Berlin/Heidelberg, Germany, 2016; Volume 61, pp. 1471–1472. [Google Scholar]
- Hill, M.F.; Bordoni, B. Hyperlipidemia. Available online: https://www.statpearls.com/search/sitesearch/ (accessed on 20 July 2022).
- Gomes-Filho, I.S.; Oliveira, M.T.; Cruz, S.S.D.; Cerqueira, E.M.M.; Trindade, S.C.; Vieira, G.O.; Couto Souza, P.H.; Adan, L.F.F.; Hintz, A.M.; Passos-Soares, J.S.; et al. Periodontitis is a factor associated with dyslipidemia. Oral Dis. 2021, 28, 813–823. [Google Scholar] [CrossRef]
- Fu, Y.W.; Li, X.X.; Xu, H.Z.; Gong, Y.Q.; Yang, Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: A randomized controlled trial. Clin. Oral Investig. 2016, 20, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Wilcox, W.R.; Cederbaum, S.D. Amino acid metabolism. Emery Rimoin’s Essent. Med. Genet. 2013, 372, 1–42. [Google Scholar]
- Balci, N.; Kurgan, Ş.; Çekici, A.; Çakır, T.; Serdar, M.A. Free amino acid composition of saliva in patients with healthy periodontium and periodontitis. Clin. Oral Investig. 2021, 25, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Michalowska, M.; Znorko, B.; Kaminski, T.; Oksztulska-Kolanek, E.; Pawlak, D. New insights into tryptophan and its metabolites in the regulation of bone metabolism. J. Physiol. Pharmacol. 2015, 66, 779–791. [Google Scholar] [PubMed]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino. Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Prince, R.L. Lifestyle and osteoporosis. Curr. Osteoporos. Rep. 2015, 13, 52–59. [Google Scholar] [CrossRef]
- Yang, C.Y.; Cheng-Yen Lai, J.; Huang, W.L.; Hsu, C.L.; Chen, S.J. Effects of sex, tobacco smoking, and alcohol consumption osteoporosis development: Evidence from Taiwan biobank participants. Tob. Induc. Dis. 2021, 19, 52. [Google Scholar] [CrossRef]
- Cusano, N.E. Skeletal Effects of Smoking. Curr. Osteoporos. Rep. 2015, 13, 302–309. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Ravidà, A.; Troiano, G.; Qazi, M.; Saleh, M.H.A.; Saleh, I.; Borgnakke, W.S.; Wang, H.L. Dose-dependent effect of smoking and smoking cessation on periodontitis-related tooth loss during 10–47 years periodontal maintenance-A retrospective study in compliant cohort. J. Clin. Periodontol. 2020, 47, 1132–1143. [Google Scholar] [CrossRef]
- Ueno, M.; Ohara, S.; Sawada, N.; Inoue, M.; Tsugane, S.; Kawaguchi, Y. The association of active and secondhand smoking with oral health in adults: Japan public health center-based study. Tob. Induc. Dis. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Joehanes, R.; Just, A.C.; Marioni, R.E.; Pilling, L.C.; Reynolds, L.M.; Mandaviya, P.R.; Guan, W.; Xu, T.; Elks, C.E.; Aslibekyan, S.; et al. Epigenetic Signatures of Cigarette Smoking. Circ. Cardiovasc. Genet. 2016, 9, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, K.J.; Tang, Z.Q.; Liu, R.H.; Zeng, F.; Cheng, M.Y.; Lian, Q.S.; Wu, H.K. Effects of nicotine on the metabolism and gene expression profile of Sprague-Dawley rat primary osteoblasts. Mol. Med. Rep. 2018, 17, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Rocha, I.; Fernandes, M.H. Complex osteoclastogenic inductive effects of nicotine over hydroxyapatite. J. Cell Physiol. 2018, 233, 1029–1040. [Google Scholar] [CrossRef]
- Park, R.; Madhavaram, S.; Ji, J.D. The Role of Aryl-Hydrocarbon Receptor (AhR) in Osteoclast Differentiation and Function. Cells 2020, 9, 2294. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Hanieh, H.; Nakahama, T.; Kishimoto, T. The roles of aryl hydrocarbon receptor in immune responses. Int. Immunol. 2013, 25, 335–343. [Google Scholar] [CrossRef]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellström, D.; Andersson, E.M. Smoking-Induced Risk of Osteoporosis Is Partly Mediated by Cadmium From Tobacco Smoke: The MrOS Sweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef]
- Chahal, G.S.; Chhina, K.; Chhabra, V.; Chahal, A. Smoking and its effect on periodontium–Revisited. Indian J. Dent. Sci. 2017, 9, 44. [Google Scholar] [CrossRef]
- Robo, I.; Heta, S.; Papa, P.; Sadiku, E.; Alliu, N. The impact of smoking on the health of periodontal tissue. Radioecology 2017, 2, 228–230. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, F.; Wang, L.; Zhu, B.; Chen, Y.; Yu, Y.; Wang, X. Inflammation has synergistic effect with nicotine in periodontitis by up-regulating the expression of α7 nAChR via phosphorylated GSK-3β. J. Cell Mol. Med. 2020, 24, 2663–2676. [Google Scholar] [CrossRef]
- Wu, L.Z.; Duan, D.M.; Liu, Y.F.; Ge, X.; Zhou, Z.F.; Wang, X.J. Nicotine favors osteoclastogenesis in human periodontal ligament cells co-cultured with CD4(+) T cells by upregulating IL-1beta. Int. J. Mol. Med. 2013, 31, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Scott, D.A. Tobacco-induced suppression of the vascular response to dental plaque. Mol. Oral Microbiol. 2018, 33, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Lallier, T.E.; Moylan, J.T.; Maturin, E. Greater Sensitivity of Oral Fibroblasts to Smoked Versus Smokeless Tobacco. J. Periodontol. 2017, 88, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yuan, S.; Zhou, Z.; Wu, L.; Wang, L.; Wu, X.; Wang, X. A preliminary study on the autophagy level of human periodontal ligament cells regulated by nicotine. Hua Xi Kou Qiang Yi Xue Za Zhi 2017, 35, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.; Boșca, A.B.; Sorițău, O.; Băciuț, M.; Lucaciu, O.; Virag, P.; Ilea, A.; Dirzu, N.; Câmpian, R.S. Nicotine cytotoxicity on the mesenchymal stem cells derived from human periodontium. Rom. Biotechnol. Lett. 2016, 21, 11763. [Google Scholar]
- Ng, T.K.; Huang, L.; Cao, D.; Yip, Y.W.; Tsang, W.M.; Yam, G.H.; Pang, C.P.; Cheung, H.S. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci. Rep. 2015, 5, 7828. [Google Scholar] [CrossRef]
- Monnouchi, S.; Maeda, H.; Yuda, A.; Serita, S.; Wada, N.; Tomokiyo, A.; Akamine, A. Benzo[a]pyrene/aryl hydrocarbon receptor signaling inhibits osteoblastic differentiation and collagen synthesis of human periodontal ligament cells. J. Periodontal Res. 2016, 51, 779–788. [Google Scholar] [CrossRef]
- Tura-Ceide, O.; Lobo, B.; Paul, T.; Puig-Pey, R.; Coll-Bonfill, N.; Garcia-Lucio, J.; Smolders, V.; Blanco, I.; Barbera, J.A.; Peinado, V.I. Cigarette smoke challenges bone marrow mesenchymal stem cell capacities in guinea pig. Respir. Res. 2017, 18, 50. [Google Scholar] [CrossRef]
- Yilmaz Sastim, C.; Gursoy, M.; Kononen, E.; Kasurinen, A.; Norvio, S.; Gursoy, U.K.; Dogan, B. Salivary and serum markers of angiogenesis in periodontitis in relation to smoking. Clin. Oral Investig. 2021, 25, 1117–1126. [Google Scholar] [CrossRef]
- Hanioka, T.; Morita, M.; Yamamoto, T.; Inagaki, K.; Wang, P.L.; Ito, H.; Morozumi, T.; Takeshita, T.; Suzuki, N.; Shigeishi, H.; et al. Smoking and periodontal microorganisms. Jpn. Dent. Sci. Rev. 2019, 55, 88–94. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Cheng, L.; Li, M. The Impact of Smoking on Subgingival Microflora: From Periodontal Health to Disease. Front. Microbiol. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Kilsgard, O.; Akerman, S.; Klinge, B.; Demmer, R.T.; Malmstrom, J.; Jonsson, D. The Human Salivary Antimicrobial Peptide Profile according to the Oral Microbiota in Health, Periodontitis and Smoking. J. Innate Immun. 2019, 11, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Gundersen, Y.; Gjermo, P.; von Hörsten, S.; Opstad, P.K. Nicotinic acetylcholine receptor activation mediates nicotine-induced enhancement of experimental periodontitis. J. Periodontal Res. 2009, 44, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Han, M.L.; Teng, N.C.; Lee, C.Y.; Huang, W.T.; Lin, C.T.; Huang, Y.K. Cigarette Smoking Aggravates the Activity of Periodontal Disease by Disrupting Redox Homeostasis- An Observational Study. Sci. Rep. 2018, 8, 11055. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, Z.; Doosti-Irani, A.; Almasi-Hashiani, A.; Baigi, V.; Mansournia, N.; Etminan, M.; Mansournia, M.A. The effect of alcohol on osteoporosis: A systematic review and meta-analysis. Drug Alcohol Depend. 2019, 197, 197–202. [Google Scholar] [CrossRef]
- Jang, H.D.; Hong, J.Y.; Han, K.; Lee, J.C.; Shin, B.J.; Choi, S.W.; Suh, S.W.; Yang, J.H.; Park, S.Y.; Bang, C. Relationship between bone mineral density and alcohol intake: A nationwide health survey analysis of postmenopausal women. PLoS ONE 2017, 12, e0180132. [Google Scholar] [CrossRef] [PubMed]
- Gay, I.C.; Tran, D.T.; Paquette, D.W. Alcohol intake and periodontitis in adults aged ≥30 years: NHANES 2009-2012. J. Periodontol. 2018, 89, 625–634. [Google Scholar] [CrossRef]
- Baumeister, S.E.; Freuer, D.; Nolde, M.; Kocher, T.; Baurecht, H.; Khazaei, Y.; Ehmke, B.; Holtfreter, B. Testing the association between tobacco smoking, alcohol consumption, and risk of periodontitis: A Mendelian randomization study. J. Clin. Periodontol. 2021, 48, 1414–1420. [Google Scholar] [CrossRef]
- Hyeong, J.-H.; Lee, Y.-H. Gender-Specific association between average volume of alcohol consumption, binge drinking, and periodontitis among Korean adults: The Korea National health and nutrition examination survey, 2013~2014. J. Dent. Hyg. Sci. 2016, 16, 339–348. [Google Scholar] [CrossRef][Green Version]
- Liberman, D.N.; Pilau, R.M.; Gaio, E.J.; Orlandini, L.F.; Rösing, C.K. Low concentration alcohol intake may inhibit spontaneous alveolar bone loss in Wistar rats. Arch. Oral Biol. 2011, 56, 109–113. [Google Scholar] [CrossRef]
- de Souza, D.M.; Rodrigues, V.A.; Silva, A.A.; Gonsalves, V.S.; Pereira, K.A.; Nishioka, R.S.; de Carvalho, C. Influence of different alcohol intake frequencies on alveolar bone loss in adult rats: A sem study. J. Clin. Exp. Dent. 2018, 10, e852–e857. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.M.; Da Rocha, R.F. Influence of variable concentration of ethanol intake on alveolar bone loos in rats periodontitis model. Rev. Odonto Ciência 2014, 29, 76–80. [Google Scholar] [CrossRef][Green Version]
- Souza, D.M.d.; Rocha, R.F.d. Low caloric value of ethanol itself increases alveolar bone loss in ligature-induced periodontitis in male rats. Braz. Oral Res. 2009, 23, 460–466. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.M.; Pazmino, V.F.C.; Novaes, V.C.N.; Bomfim, S.R.M.; Nagata, M.J.H.; Oliveira, F.L.P.; Matheus, H.R.; Ervolino, E. Chronic consumption of alcohol increases alveolar bone loss. PLoS ONE 2020, 15, e0232731. [Google Scholar] [CrossRef] [PubMed]
- Surkin, P.N.; Ossola, C.Á.; Mohn, C.E.; Elverdin, J.C.; Fernández-Solari, J. Chronic alcohol consumption alters periodontal health in rats. Alcohol. Clin. Exp. Res. 2014, 38, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Yoon, M.-S.; Lim, Y.-H.; Lee, S.-R.; Kim, S.-Y.; Park, S.-J.; Shin, S.-J. The association between types of smoking and periodontal disease according to the survey year using the fourth and fifth Korea National Health and Nutrition Examination Surveys. J. Dent. Hyg. Sci. 2017, 17, 487–494. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Baake, S.; Pedersen, L.D.; Scheutz, F.; López, R. Impact of Smoking Cessation on Periodontitis: A Systematic Review and Meta-analysis of Prospective Longitudinal Observational and Interventional Studies. Nicotine Tob. Res. 2019, 21, 1600–1608. [Google Scholar] [CrossRef]
- Freitag-Wolf, S.; Munz, M.; Wiehe, R.; Junge, O.; Graetz, C.; Jockel-Schneider, Y.; Staufenbiel, I.; Bruckmann, C.; Lieb, W.; Franke, A. Smoking modifies the genetic risk for early-onset periodontitis. J. Dent. Res. 2019, 98, 1332–1339. [Google Scholar] [CrossRef]
- Ilacqua, A.; Emerenziani, G.P.; Aversa, A.; Guidetti, L.; Baldari, C. Lifestyle and Osteoporosis Risk in Men (Physical Activity, Diet, Alcohol Abuse). In Male Osteoporosis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 109–115. [Google Scholar]
- Chen, Y.; Gao, H.; Yin, Q.; Chen, L.; Dong, P.; Zhang, X.; Kang, J. ER stress activating ATF4/CHOP-TNF-α signaling pathway contributes to alcohol-induced disruption of osteogenic lineage of multipotential mesenchymal stem cell. Cell. Physiol. Biochem. 2013, 32, 743–754. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, X.; Chen, C.; Yu, W.; Su, Y.; Kim, Y.; Shi, S.; Liu, Y. Chronic High Dose Alcohol Induces Osteopenia via Activation of mTOR Signaling in Bone Marrow Mesenchymal Stem Cells. Stem Cells 2016, 34, 2157–2168. [Google Scholar] [CrossRef]
- Eby, J.M.; Sharieh, F.; Callaci, J.J. Impact of Alcohol on Bone Health, Homeostasis and Fracture repair. Curr. Pathobiol. Rep. 2020, 8, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Yan, J.; Liu, T.; Pan, G.; Yang, H.; Pei, M.; He, F. Alcohol Induces Cellular Senescence and Impairs Osteogenic Potential in Bone Marrow-Derived Mesenchymal Stem Cells. Alcohol Alcohol. 2017, 52, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Naruo, M.; Iijima, O.; Igarashi, T.; Katsuyama, M.; Maruyama, M.; Akimoto, T.; Ohno, Y.; Haseba, T. The Contribution of Alcohol Dehydrogenase 3 to the Development of Alcoholic Osteoporosis in Mice. J. Nippon Med. Sch. 2018, 85, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.F.; Fausti, K.A.; Carlos, A.S. Ethanol inhibits human osteoblastic cell proliferation. Alcohol. Clin. Exp. Res. 1996, 20, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.B.; Osborn, M.L.; Robertson, A.C.; Williams, A.E.; Watt, J.; Denys, A.; Schröder, K.; Ronis, M.J. Chronic Ethanol Feeding in Mice Decreases Expression of Genes for Major Structural Bone Proteins in a Nox4-Independent Manner. J. Pharmacol. Exp. Ther. 2020, 373, 337–346. [Google Scholar] [CrossRef]
- Naruo, M.; Negishi, Y.; Okuda, T.; Katsuyama, M.; Okazaki, K.; Morita, R. Alcohol consumption induces murine osteoporosis by downregulation of natural killer T-like cell activity. Immun. Inflamm. Dis. 2021, 9, 1370–1382. [Google Scholar] [CrossRef]
- Perrien, D.S.; Brown, E.C.; Fletcher, T.W.; Irby, D.J.; Aronson, J.; Gao, G.G.; Skinner, R.A.; Hogue, W.R.; Feige, U.; Suva, L.J.; et al. Interleukin-1 and tumor necrosis factor antagonists attenuate ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis. J. Pharmacol. Exp. Ther. 2002, 303, 904–908. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Liu, Y.; Chen, H.; Shi, S.; Liu, Y. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol. Life Sci. 2017, 74, 4443–4453. [Google Scholar] [CrossRef]
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16. [Google Scholar] [CrossRef]
- Rachdaoui, N.; Sarkar, D.K. Pathophysiology of the Effects of Alcohol Abuse on the Endocrine System. Alcohol. Res. 2017, 38, 255–276. [Google Scholar]
- Cheng, M.; Tan, B.; Wu, X.; Liao, F.; Wang, F.; Huang, Z. Gut Microbiota Is Involved in Alcohol-Induced Osteoporosis in Young and Old Rats through Immune Regulation. Front. Cell Infect. Microbiol. 2021, 11, 636231. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lages, E.J.; Costa, F.O.; Cortelli, S.C.; Cortelli, J.R.; Cota, L.O.; Cyrino, R.M.; Lages, E.M.; Nobre-Franco, G.C.; Brito, J.A.; Gomez, R.S. Alcohol consumption and periodontitis: Quantification of periodontal pathogens and cytokines. J. Periodontol. 2015, 86, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Khocht, A.; Schleifer, S.; Janal, M.; Keller, S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J. Int. Acad. Periodontol. 2013, 15, 68–74. [Google Scholar]
- Katyal, R.; Saroch, N.; Bhushan, A.B. Alcohol and periodontal health in adolescence. SRM J. Res. Dent. Sci. 2012, 3, 257. [Google Scholar] [CrossRef]
- Warren, K.R.; Postolache, T.T.; Groer, M.E.; Pinjari, O.; Kelly, D.L.; Reynolds, M.A. Role of chronic stress and depression in periodontal diseases. Periodontology 2000 2014, 64, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.S.; Chin, K.Y. Potential mechanisms linking psychological stress to bone health. Int. J. Med. Sci. 2021, 18, 604–614. [Google Scholar] [CrossRef]
- Coelho, J.M.F.; Miranda, S.S.; da Cruz, S.S.; Trindade, S.C.; Passos-Soares, J.d.S.; Cerqueira, E.d.M.; Costa, M.d.C.N.; Figueiredo, A.C.M.; Hintz, A.M.; Barreto, M.L. Is there association between stress and periodontitis? Clin. Oral Investig. 2020, 24, 2285–2294. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Christiansen, B.A.; Giannone, A.A.; Genetos, D.C. The role of nerves in skeletal development, adaptation, and aging. Front. Endocrinol. 2020, 11, 646. [Google Scholar] [CrossRef]
- Yu, X.; Lv, L.; Zhang, J.; Zhang, T.; Xiao, C.; Li, S. Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J. Mol. Histol. 2015, 46, 195–203. [Google Scholar] [CrossRef]
- Akcali, A.; Huck, O.; Tenenbaum, H.; Davideau, J.L.; Buduneli, N. Periodontal diseases and stress: A brief review. J. Oral Rehabil. 2013, 40, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Feng, L.; Zhu, M.L.; Yang, Z.M.; Wu, T.Y.; Xu, J.; Liu, Y.; Lin, W.P.; Lo, J.H.T.; Zhang, J.F.; et al. Vasoactive Intestinal Peptide Stimulates Bone Marrow-Mesenchymal Stem Cells Osteogenesis Differentiation by Activating Wnt/beta-Catenin Signaling Pathway and Promotes Rat Skull Defect Repair. Stem Cells Dev. 2020, 29, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, A.; Emingil, G.; Nizam, N.; Doğanavşargil, B.; Sezak, M.; Kütükçüler, N.; Atilla, G. Therapeutic efficacy of vasoactive intestinal peptide in escherichia coli lipopolysaccharide-induced experimental periodontitis in rats. J. Periodontol. 2009, 80, 1655–1664. [Google Scholar] [CrossRef]
- Yan, K.; Lin, Q.; Tang, K.; Liu, S.; Du, Y.; Yu, X.; Li, S. Substance P participates in periodontitis by upregulating HIF-1alpha and RANKL/OPG ratio. BMC Oral Health 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Hanns, P.; Paczulla, A.M.; Medinger, M.; Konantz, M.; Lengerke, C. Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress 2019, 3, 221–235. [Google Scholar] [CrossRef]
- Khoury, R.D.; Prado, R.F.D.; Matos, F.S.; Meireles, B.R.; Cardoso, F.; Oliveira, L.D.; Carvalho, C.A.T.; Valera, M.C. The influence of adrenergic blockade in rats with apical periodontitis under chronic stress conditions. Arch. Oral Biol. 2020, 110, 104590. [Google Scholar] [CrossRef]
- Martins, L.G.; Spreafico, C.S.; Tanobe, P.G.; Tavares, T.A.A.; Castro, M.L.; Franco, G.C.N.; do Prado, R.F.; Anbinder, A.L. Influence of Adrenergic Neuromodulation during Induction of Periodontitis in Rats. J. Int. Acad. Periodontol. 2017, 19, 80–88. [Google Scholar]
- Okada, Y.; Hamada, N.; Kim, Y.; Takahashi, Y.; Sasaguri, K.; Ozono, S.; Sato, S. Blockade of sympathetic b-receptors inhibits Porphyromonas gingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Arch. Oral Biol. 2010, 55, 502–508. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Opstad, P.K.; Fonnum, F. Chemical sympathectomy inhibits periodontal disease in Fischer 344 rats. J. Periodontal Res. 2005, 40, 325–330. [Google Scholar] [CrossRef]
- Roberts, A.; Matthews, J.B.; Socransky, S.S.; Freestone, P.P.; Williams, P.H.; Chapple, I.L. Stress and the periodontal diseases: Growth responses of periodontal bacteria to Escherichia coli stress-associated autoinducer and exogenous Fe. Oral Microbiol. Immunol. 2005, 20, 147–153. [Google Scholar] [CrossRef]
- Lundy, F.T.; Linden, G.J. neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit. Rev. Oral Biol. Med. 2004, 15, 82–98. [Google Scholar] [CrossRef] [PubMed]
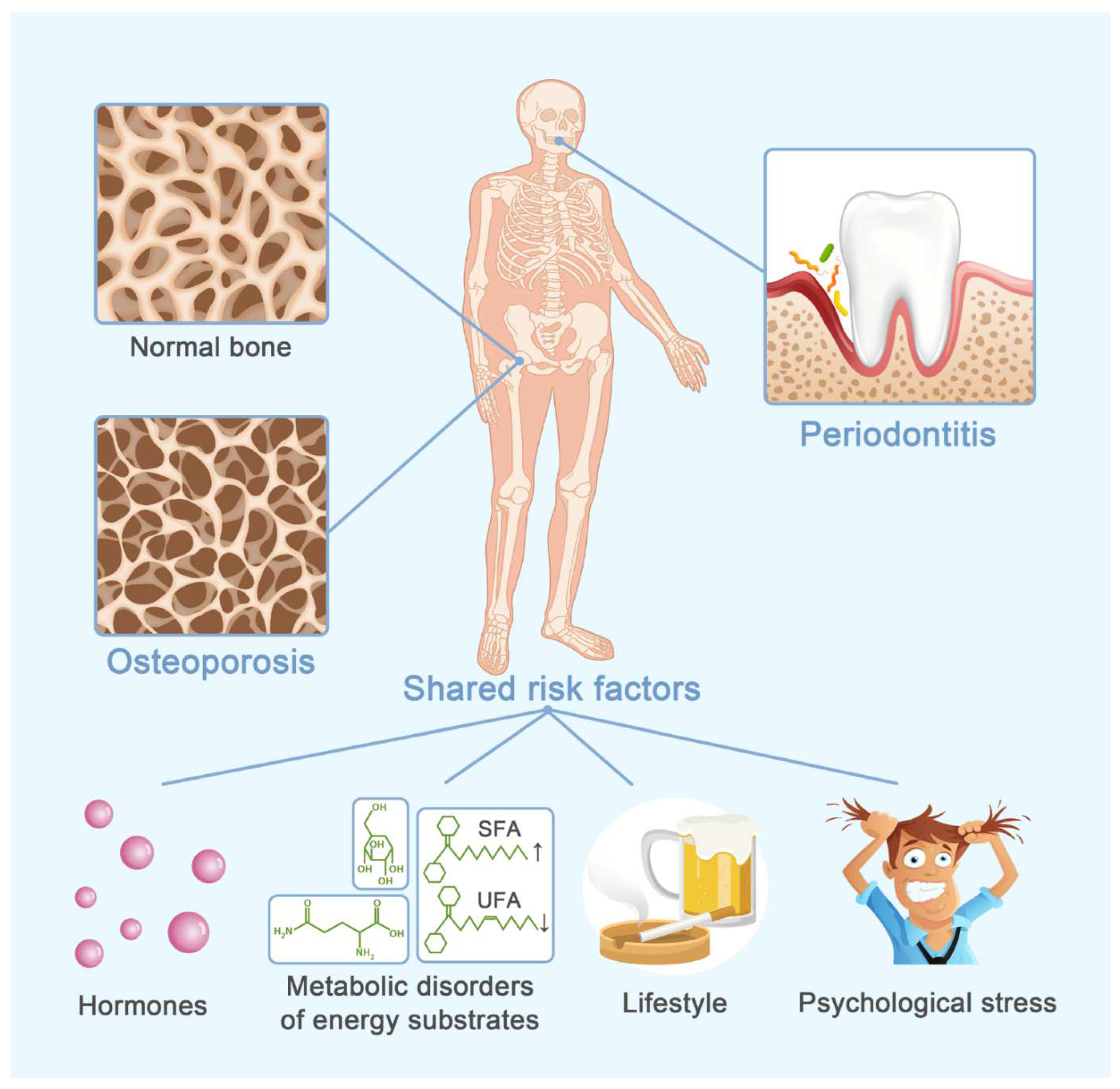
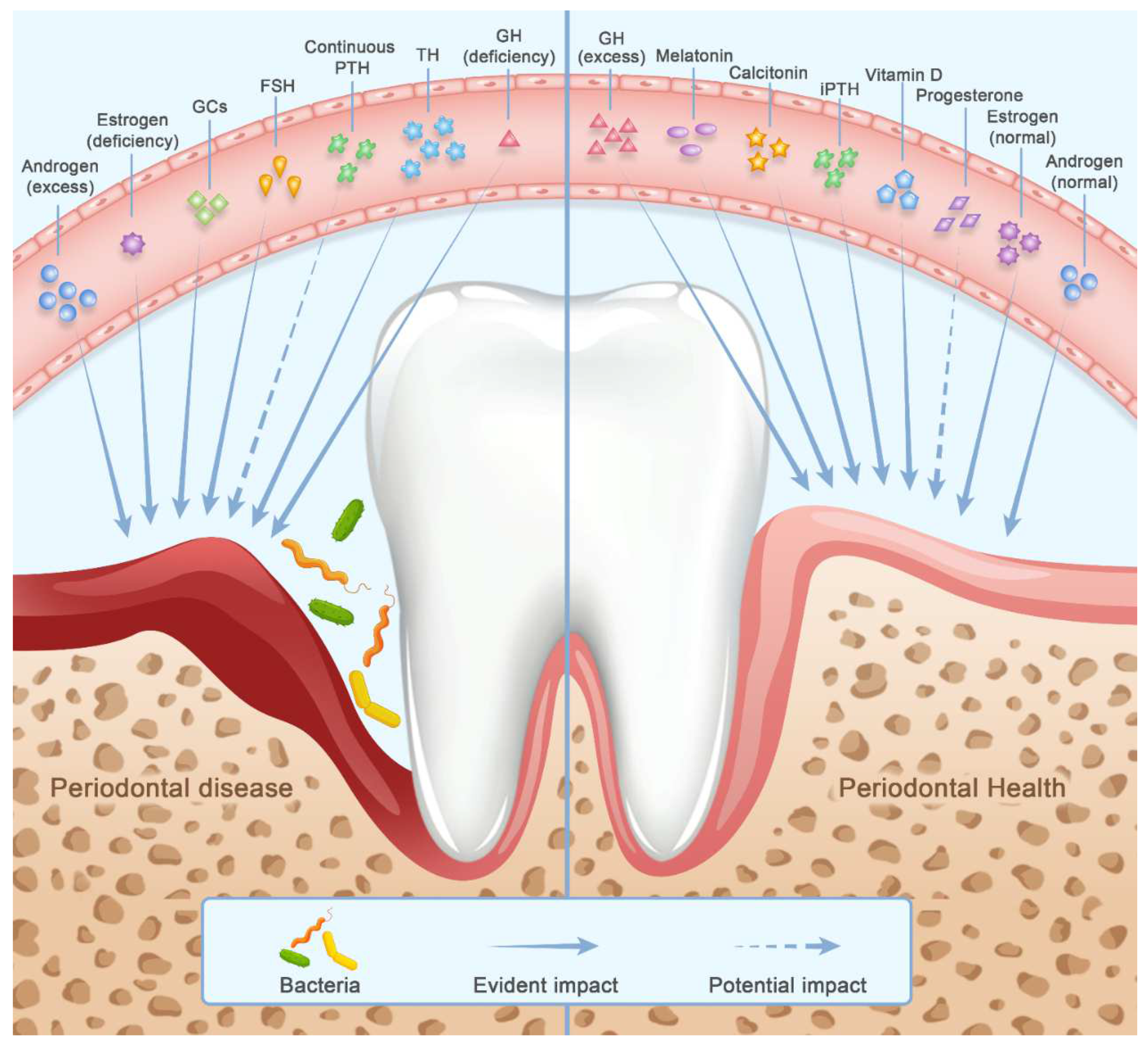

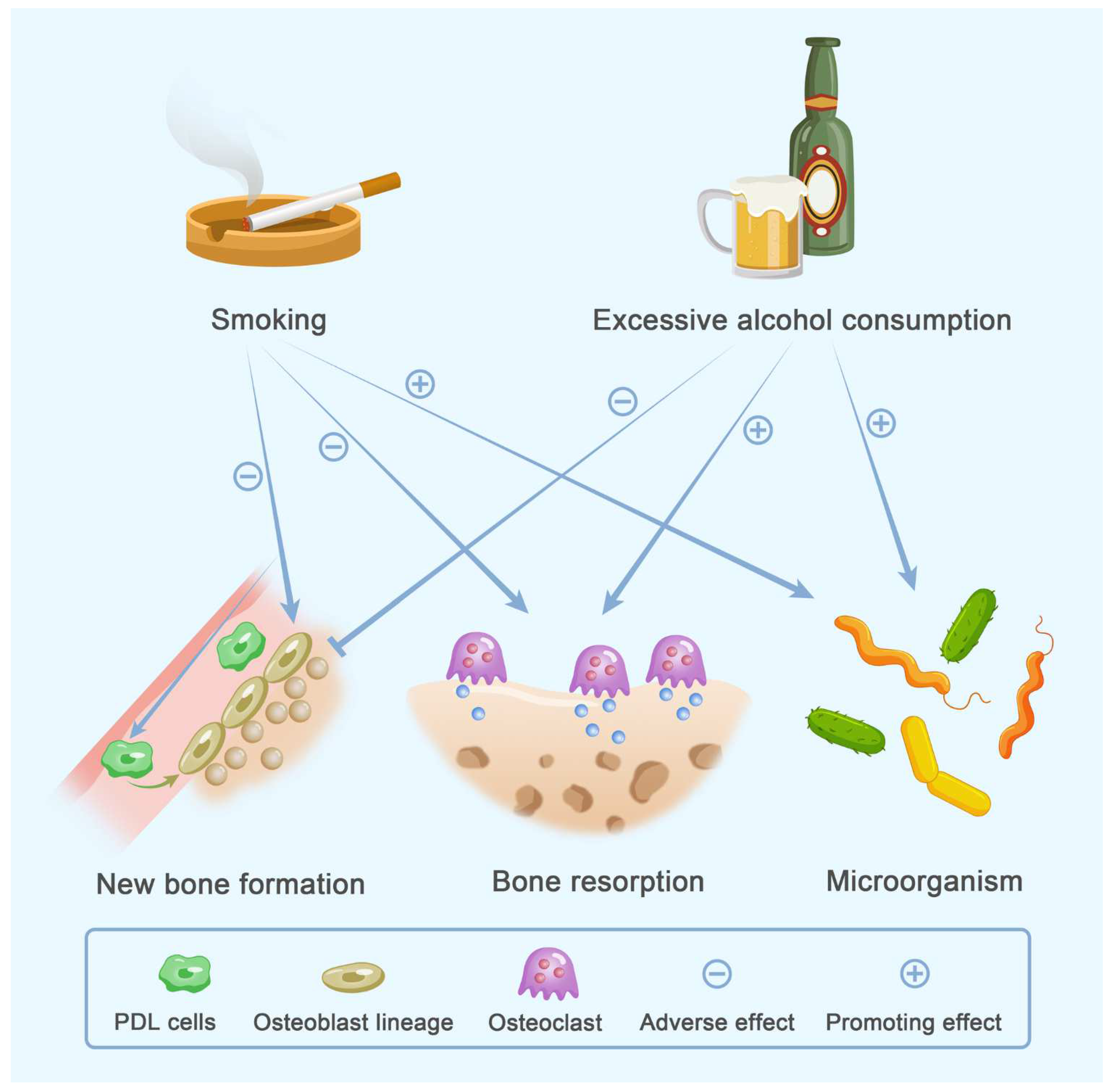
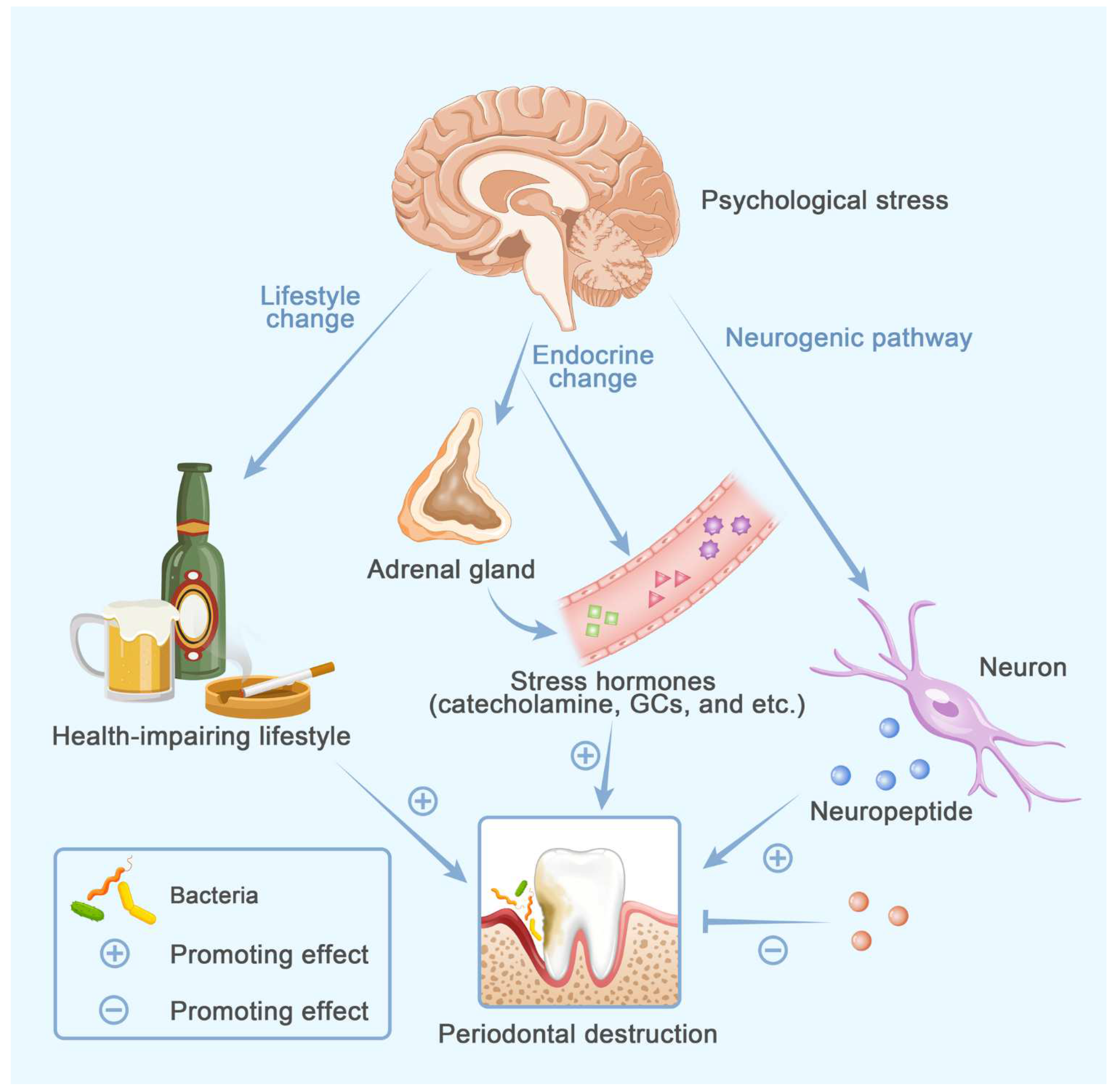
| Hormone | Osteoporosis | Periodontitis | |||||
|---|---|---|---|---|---|---|---|
| Overall | Bone Cells | Resident Cells (PDL Cells) | Others | ||||
| Androgen [1,14,15,16,17,18,19,20,21,22,23,24,25] | Normal | Normal level↓ Others unclear | ↓ | OBs↑ Osteocytes↑ | Unclear | Pro-inflammatory cytokines (IL-1β, IL-6)↓ | |
| High | ↑ | OCs↑ | Unclear | Biofilm pathogenity↑ Pro-inflammatory cytokines (IL-1β)↑ | |||
| Estrogen [26,27,28,29,30,31] | Sufficient | ↓ | ↓ | OBs↑ OCs↑ | Osteoblastogenesis↑ | Pro-inflammatory cytokines (TNF-α, IL-1, IL-6, RANKL, PGE2, IL-8)↓ | |
| Deficient | ↑ | ↑ | OCs↑ | Unclear | Pro-inflammatory cytokines (IL-33, TNF-α, IL-1β)↑ | ||
| Progesterone [18,32,33,34,35] | ↓ | Unclear | OBs↑ OCs unclear | Osteoblastogenesis↑ | P. gingivalis↑ Pro-inflammatory cytokines (IL-6↓, PGE2↑) | ||
| FSH [11,36,37,38,39] | ↑ | ↑ | OCs↑ OBs unclear | Unclear | Pro-inflammatory cytokines (IL-1β, IL-6, TNF-α)↑ | ||
| Vitamin D [40,41,42,43,44,45,46,47,48,49,50,51,52,53] | ↓ | ↓ | OBs↑ OCs unclear | Mineralization activity↑ | Bacterial loads↓ Degeneration-associated factor (MMP)↓ Cytotoxic T lymphocytes↓ Pro-inflammatory cytokines (IL-4, IL-6, IL-8, IL-10, TNF-α, MCP-1)↓ Anti-microbial properties↑ Anti-inflammatory cytokine (IL-17)↑ | ||
| PTH | Intermittent PTH [54,55,56,57,58,59,60,61,62,63,64] | ↓ | ↓ | OBs↑ OCs↑ Osteocytes↓ | Immature PDL cells: cell number↓ osteogenesis↑ | Degeneration-associated factors (IL-6, MMP-2, MMP-9)↓ | |
| Mature PDL cells: cell number↑ osteogenesis↓ | |||||||
| Continuous PTH [54,57,65,66] | ↑ | Unclear | OCs↑ Osteocytes↓ | Osteoblastogenesis↑ (transient) | Pro-inflammatory cytokines (PGE2 transiently↓, then↑) | ||
| Calcitonin [67,68,69,70,71,72,73,74] | ↓ | ↓ | OBs↓ OCs↓ | Osteoblastogenesis and secretion activity↑ | Bone remodeling coupling↑ Pro-inflammatory cytokines (PGE2)↓ | ||
| GCs [75,76,77,78,79,80] | ↑ | ↑ | OBs↓ OCs↑ (transient) | Unclear | Immunosuppressive property: Th1 response↓ Th2 response↑ Anti-inflammatory property: LPS signaling↓ Receptor for GCs↓ | ||
| Melatonin [81,82,83,84,85,86,87,88,89,90,91,92,93] | ↓ | ↓ | OBs↑ OCs↓ | Unclear | Pathogenicity and formation of biofilm↓ Pro-inflammatory cytokines↓ Th1 cells migration↓ Oxidative stress↓ Angiogenesis↑ Glucose homeostasis and circadian rhythms↑ | ||
| TH | Hyperthyroidism [94,95,96,97,98,99,100,101,102,103] | ↑ | ↑ | OBs↑ OCs↑ | Unclear | Periodontal flora imbalance↑ Oxidative stress and ROS↑ Neutrophil apoptosis↑ Inflammatory cytokines (IL-6, TNF-α)↑ | |
| Hypothyroidism [94,98,99,100,101,102,103,104,105,106] | Unclear | ↑ | |||||
| GH | Excess [107,108,109,110,111,112,113,114] | ↑ | ↓ | OBs↑ OCs↑ | Osteogenic potential↑ | Inflammatory response and collage metabolism (IL-1, IL-10, carboxyterminal telopeptide of type I)↓ | |
| Deficiency [108,109,110,111,112,113,114,115,116] | ↑ | ↑ | Inflammatory mediators (CRP, MMP-8 and IL-8)↑ | ||||
| Metabolic Disorders of Energy Substrates | Osteoporosis | Periodontitis | ||||
|---|---|---|---|---|---|---|
| Overall | Bone Cells | Resident Cells | Others | |||
| Glucose (hyperglycemia) [151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177] | ↑ | ↑ | OBs↓ OCs Unclear Osteocytes↓ | Osteogenic differentiation and proliferation of PDL stem cells↓ | Energy fueling Collagen brittleness↑ Local pathogen loads↑ immune-inflammatory response: (1) Immune cells: Inflammation infiltration↑ Senescence, pyroptosis, immunocompromise and M1 polarization of macrophages↑ (2) Cytokines: Pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-4, IL-10, IL-17)↑ Anti-inflammatory cytokines (IL-10, FGF-21, MCP-1, TNF-β)↑ | |
| Lipid | Hyperlipidemia [178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207] | ↑ | ↑ | OBs↓ OCs↑ | Unclear | Alternative energy source Pathogen loads↑ Inflammatory cytokines and cells↑ Lipid oxidation and oxidative damage↑ |
| High SFA/UFA ratio [178,183,191,205,208,209,210,211,212,213,214,215,216,217,218,219] | Survival and osteogenic differentiation of PDL fibroblasts↓ | Inflammatory cytokines↑ Local immune cells (Neutrophil, monocyte, lymphocyte)↑ | ||||
| AAs | BCAAs, aromatic AAs [220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236] | ↓ | Unclear | OBs↑ | Unclear | Alternative energy source for bone cells Prime energy substrates for some bacteria |
| Sulphur-containing AAs [221] | ↑ | OBs↓ OCs↑ | ||||
| Lifestyle | Osteoporosis | Periodontitis | ||||
|---|---|---|---|---|---|---|
| Overall | Bone Cells | Resident Cells | Others | |||
| Smoking [261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287] | ↑ | ↑ | OBs↓ OCs↑ | Viability and osteogenic potential of MSCs and PDL cells↓ | Subgingival microbial dysbiosis↑ Innate and adaptive host immune defense: compromised Salivary antimicrobial peptides↓ | |
| Alcohol Consumption [288,289,290,291,292,293,294,295,296,297,298] | Low/ moderate | Unclear | ↓ | Unclear | Unclear | Unclear |
| Heavy | ↑ | ↑ | OBs↓ OCs↑ | Subgingival microbial dysbiosis↑ Disturbed redox homeostasis | ||
| Psychological Stress | Osteoporosis | Periodontitis | ||||
|---|---|---|---|---|---|---|
| Overall | Bone Cells | Resident Cells | Others | |||
| Behavioral factors | ↑ | As shown in Table 3 | ||||
| Physiological factors | Endocrine factors | As shown in Table 1 | ||||
| Neurogenic factors [322,323,324,325,326,327,328,329,330,331,332,333,334] | ↑ | OBs↓ OCs↑ | Unclear | Modify immuno-inflammatory response Dysbacteriosis↑ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhou, C.; Chen, S.; Huang, D.; Jiang, Y.; Lan, Y.; Zou, S.; Li, Y. Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review. Cells 2022, 11, 3380. https://doi.org/10.3390/cells11213380
Zhu L, Zhou C, Chen S, Huang D, Jiang Y, Lan Y, Zou S, Li Y. Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review. Cells. 2022; 11(21):3380. https://doi.org/10.3390/cells11213380
Chicago/Turabian StyleZhu, Li, Chenchen Zhou, Shuo Chen, Danyuan Huang, Yukun Jiang, Yuanchen Lan, Shujuan Zou, and Yuyu Li. 2022. "Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review" Cells 11, no. 21: 3380. https://doi.org/10.3390/cells11213380
APA StyleZhu, L., Zhou, C., Chen, S., Huang, D., Jiang, Y., Lan, Y., Zou, S., & Li, Y. (2022). Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review. Cells, 11(21), 3380. https://doi.org/10.3390/cells11213380




