Abstract
Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) predominantly infects the respiratory system, several investigations have shown the involvement of the central nervous system (CNS) along the course of the illness, with encephalitis being one of the symptoms. The objective of this systematic review was to evaluate the characteristics (clinical, neuro-radiological aspects, and laboratory features) and outcomes of encephalitis in COVID-19 patients. PubMed, Scopus, and Google Scholar databases were searched from 1 December 2019 until 21 July 2022 to identify case reports and case series published on COVID-19 associated with encephalitis. The quality of the included studies was assessed by the Joanna Briggs Institute critical appraisal checklists. This systematic review included 79 studies, including 91 COVID-19 patients (52.7% male) experiencing encephalitis, where 85.6% were adults (49.3 ± 20.2 years), and 14.4% were children (11.2 ± 7.6 years). RT-PCR was used to confirm 92.2% of the COVID-19 patients. Encephalitis-related symptoms were present in 78.0% of COVID-19 patients at the time of diagnosis. In these encephalitis patients, seizure (29.5%), confusion (23.2%), headache (20.5%), disorientation (15.2%), and altered mental status (11.6%) were the most frequently reported neurologic manifestations. Looking at the MRI, EEG, and CSF findings, 77.6%, 75.5%, and 64.1% of the patients represented abnormal results. SARS-CoV-2-associated or -mediated encephalitis were the most common type observed (59.3%), followed by autoimmune encephalitis (18.7%). Among the included patients, 66.7% were discharged (37.8% improved and 28.9% fully recovered), whereas 20.0% of the reported COVID-19-positive encephalitis patients died. Based on the quality assessment, 87.4% of the studies were of high quality. Although in COVID-19, encephalitis is not a typical phenomenon, SARS-CoV-2 seems like a neuropathogen affecting the brain even when there are no signs of respiratory illness, causing a high rate of disability and fatality.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus attacks the respiratory system. According to the World Health Organization’s most recent data, over 550 million individuals have been infected, with over 6 million deaths globally [1]. As the coronavirus disease 2019 (COVID-19) pandemic continues, there is growing evidence that this virus also affects the central nervous system (CNS), exhibiting its potential neurotropic and neuroinvasive properties [2,3]. Besides systematic, respiratory, and gastrointestinal symptoms, [4,5,6], neurological manifestations are increasingly recognized in patients with COVID-19, including headache, smell dysfunction, taste disorder, and seizure [7,8,9]. Published data have been suggesting that encephalitis is one of the most fatal neurologic manifestations of COVID-19 involving both adult and pediatric patients [10,11,12]. While the exact mechanism of CNS invasion is still being investigated, possibilities have included both direct viral invasion and indirect damages via inflammatory and autoimmune pathways [13,14].
Encephalitis is an inflammation of the brain parenchyma, exerting serious neurological dysfunction, which is majorly caused by viruses characterized by clinical manifestations such as confusion, reduced or alternative levels of consciousness, fever, headache, seizures, and movement disorder. Diagnosis of encephalitis is usually a combinational approach of laboratory, neuroimaging, and electrophysiologic findings, including blood tests, bronchoalveolar lavage or sputum, urine and stool tests, computed tomography (CT) scan, X-ray, electroencephalogram (EEG), lumbar puncture, and magnetic resonance imaging (MRI) [15,16].
The first case of COVID-19-associated meningoencephalitis was confirmed in a 24-year-old male with severe febrile confusion and generalized tonic-clonic seizure in February 2020 [17]. A recent multicenter retrospective study conducted by the Spanish Society of Neurology reported that encephalitis was present in 2.2% of the COVID-19 patients with neurological symptoms [18]. Previous studies have reported several clinical and laboratory features of SARS-CoV-2-mediated encephalitis. As there has been an escalating number of incidents of encephalitis in COVID-19 patients, with alarming morbidity and mortality rates, this study aimed to systematically evaluate the characteristics (clinical, neuro-radiological aspects, and laboratory features) and outcomes of encephalitis in COVID-19 patients, as well as the possible causative mechanisms of CNS damage.
2. Methods
2.1. Study Guideline
This systematic review (PROSPERO registration number CRD42022354224) implemented the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline [19].
2.2. Search Strategies
We used the advanced and expert options of PubMed, Scopus, and Google Scholar databases, searching for journal articles published between 1 December 2019 and 21 July 2022, combining appropriate keywords associated with COVID-19 and encephalitis (Table S1).
2.3. Eligibility Criteria
We considered only case reports or case series published in the English language. In this systematic review, cases with confirmed encephalitis or meningoencephalitis were considered eligible; however, cases reporting only meningitis were excluded.
2.4. Study Screening and Selection
Following the removal of duplicate studies using EndNote X8 software (Clarivate Analytics, Philadelphia, PA, USA), titles, abstracts, and full texts were screened independently by two authors (MAI and SSA) to identify the eligible studies. The reference lists of the included studies were also reviewed to identify any potentially eligible studies. Any disagreements on whether a study should be included or excluded were discussed with the third author (CC) and resolved subsequently.
2.5. Data Extraction
From each study, all the important data and information was retrieved by two authors (MAI and SSA), including study ID (first author’s last name and year of publication), country of origin of the patient, number, age and gender of the patient, SARS-CoV-2 confirmatory test, past medical history, severity of COVID-19, onset of encephalitis from COVID-19 presentation, neurological and psychiatric symptoms, type of suspected or confirmed encephalitis, patients’ outcome, leukocyte types present, opening pressure when performing lumbar puncture, serum blood glucose value, IgG index, brain computerized tomography (CT) scan, magnetic resonance imaging (MRI) and electroencephalogram (EEG) results, white blood cells (WBCs) level, total protein and glucose concentration, status of SARS-CoV-2, and investigation of other pathogens. All the extracted data were verified by another author (CC).
2.6. Quality Assessment
The Joanna Briggs Institute (JBI) critical appraisal checklist for assessing case reports and case series were used to evaluate the methodological quality of the included studies. Two authors (SSA and SK) independently assessed the quality of each of the included studies, and discrepancies were resolved by discussing with the third author (MAI). Studies receiving scores of <50, 50–70, or >70% were classified as low quality (high risk of bias), moderate quality (moderate risk of bias), or high quality (low risk of bias) [9].
3. Results
3.1. Study Selection
After excluding review articles (n = 23), non-human research (n = 64), and duplicate studies (n = 310) from our initial search results (n = 540), 143 papers were evaluated for eligibility, and 79 studies were eventually included in this systematic review (Figure 1).
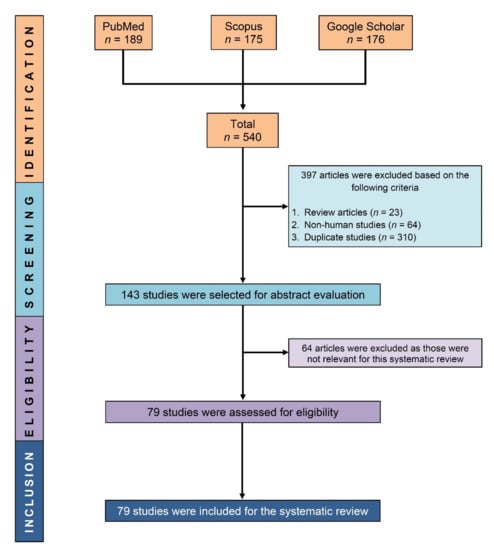
Figure 1.
PRISMA flow diagram showing the process of selecting eligible studies.
3.2. General Characteristics of the Included Studies
Major characteristics of the included studies are presented in Table 1. In brief, among the included 91 patients, 52.7% were male, 85.6% were adults (aged 49.3 ± 20.2 years), and 14.4% were pediatric patients (aged 11.2 ± 7.6 years), where a majority of the patients’ ages ranged between 41 and 70 years (Figure 2). The majority of the patients’ SARS-CoV-2 (92.2%) were confirmed by using the RT-PCR method.

Table 1.
Major characteristics of the included studies.
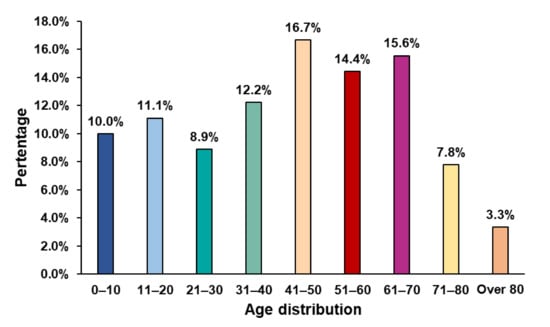
Figure 2.
Age distribution of COVID-19 patients with encephalitis.
A majority of the studies were reported on European (37.2%), followed by Asian (30.8%) and North American (23.1%), COVID-19 patients. Of these patients, 31.9 were healthy, without any past medical history of illness. Among the reported levels of severity of the COVID-19 patients, 11.7%, 38.3%, 11.7%, and 38.3% of the patients were denoted as asymptomatic, mild, moderate, and severe, respectively. Interestingly, the onset of encephalitis-associated symptoms was manifested in 78.0% of the COVID-19 patients as an initial presentation. Seizure (29.5%), confusion (23.2%), headache (20.5%), disorientation (15.2%), and altered mental status (11.6%) were the most frequently reported neurologic manifestations. Among the types of diagnosed encephalitis, a majority were confirmed to be SARS-CoV-2-associated or -mediated encephalitis (59.3%), followed by autoimmune encephalitis (18.7%). Among the included patients, 66.7% were discharged (37.8% improved, 28.9% fully recovered, and 12.2% patients were discharged without reporting their outcomes), whereas 20.0% of the reported COVID-19-positive encephalitis patients died. Looking at the MRI, EEG, and CT scan findings, 77.6%, 75.5%, and 64.1% of the patients presented with abnormal results, respectively. All the diagnostic features of the COVID-19 patients with encephalitis are presented in Table 2 and S2.

Table 2.
Diagnostic features of encephalitis in patients with COVID-19.
3.3. Evidence-Based Analyses
3.3.1. Infant to Children with COVID-19
Seizures were frequently present, and prognosis was often poor. Neurologic involvement in a term neonate with prenatal exposure to SARS-CoV-2 was described for the first time in 2022 [62]. The main and first symptoms of this baby’s infection were respiratory distress and ground glass pneumonia. She manifested seizure thereafter, and neurological deficit only at 49 days of disease following extubating and respiratory improvement. The neurological deficit corresponded to documented MRI lesions. Only IG therapy showed some benefit. A fatal case of acute hemorrhagic necrotizing encephalitis (AHNE) affected a 2-month-old boy, who presented with fever and general symptoms, but had cardiac arrest followed by brain death within a few days [66]. Another young patient was a 9-month-old infant [84]. The initial symptoms were fever and profuse vomiting for two days. Then, he manifested convulsive seizure and consciousness alteration. CT scan indicated severe hydrocephalus, and a high protein titer in the CSF suggested aseptic meningoencephalitis (Table 1). Despite antibiotic, antiviral, anticonvulsant, and anti-edematous medication, the child had a cardiac arrest and had to be resuscitated and intubated. Due to migrating to another country, the rest of the history of the infant remained unclear. A 34-month boy also had a poor prognosis [35]. He manifested with fever, seizure, and upward gaze, with progressive worsening, and was admitted to intensive care unit due to recurrent seizures and consciousness compromission. His brain MRI documented scattered foci of altered signal together with hemorrhagic foci. Therapy based on antibiotics, antiviral, and antiepileptic drug (AED) failed; he had a mild improvement after dexamethasone, hydroxychloroquine, and intravenous immunoglobulins (IVIG). He was finally in a vegetative state. Six more children—three girls, one aged 23 months [31] and two others aged 7 years [20,55], and two boys aged 7 and 11 years [11,46]—were described with febrile and manifesting epileptic seizures. Ahsan’s and McAbee’s [11,20] cases were with status epilepticus. Burr’s [31] child also showed neurological deficits with language disorders and involuntary movements. Neurological impairment occurred up to nine days after the onset of symptoms; an MRI of the brain revealed no abnormalities. Anti-NMDAR receptor antibodies were detected both in serum and CSF [31]. In this case, the initial treatment with IV methylprednisolone was unsuccessful; therefore, IV immunoglobulin (IVIG) was given instead. The case described by Ahsan et al. [20] tells a similar story, with status epilepticus and aphasia following sporadic self-limited seizures in the prior week and neurologic worsening one week later. This girl’s MRI documented alteration in the perirolandic and posterior area of the parietal lobe, with cortical edema. Following the investigation of various autoantibodies, anti-myelin oligodendrocyte glycoprotein (MOG) antibodies were detected. Following that, IVIG therapy was given, and the child improved over the following five days before being sent home; mild dysarthria persisted at follow-up. In addition, the boy described by Ferdosian et al. [46] had fever and aphasia, with consciousness alteration, for three days, and a history of recurrent seizures in the last five months. His MRI documented diffuse brain edema and CSF contained SARS-CoV-2 RNA. He improved initially with therapy with antibiotics, antiviral, and AED, but did not improve after therapy with IVIG, remdesivir, and AED.
The child described by McAbee et al. [11], who developed status epilepticus and fever after two days of generalized weakness, achieved a better prognosis. He was treated with anti-epileptic drugs (AED), and recovery was completed in six days. A girl, described by Kahwagi et al. [55], was the only child manifesting respiratory symptoms. She had a cough, headache, and fever for six days, followed on day six by multiple generalized tonic-clonic seizures. Her MRI was unremarkable. She experienced gait and behavioral issues, confusional syndrome, and osteotendinous hyperreflexia on day nine. Her EEG background displayed slowness and superimposed pseudoperiodic complexes. This girl was treated with only AED; her seizures diminished, and behavior disorders disappeared over the next two months. Finally, a five-year-old girl manifested with respiratory symptoms, low-grade fever, and neck edema with lymphadenopathy [87]. In a few days, she became lethargic; her EEG was characterized by slow-wave rhythm, and brain oedema with altered signal of splenium of corpus callosum and subcortical parietal lobes was detected in brain MR. She improved and was discharged after two weeks, after having been treated with antibiotics, antivirals, and steroids.
3.3.2. Adolescents with COVID-19
Seven teenagers suffered from encephalitis attributed to COVID-19 infection. Other than respiratory symptoms, headache, seizures, mood, and conscious level alteration were the main neurologic manifestations. Natarajan et al. [10] reported the case of a 13-year-old girl with a fluctuant history of fever, headache, tonic seizures, and status epilepticus. Although she did not manifest respiratory symptoms, her chest CT scan revealed patchy peripheral ground glass opacities. She was treated with levetiracetam and ceftriaxone, and improved in 48 h. Ground glass opacities in the lungs were also detected in the 16-year-old boy mentioned by Bhavsar et al. [29]. This boy suffered from pharyngitis, headaches, fever, and generalized weakness. On day 11, his neurologic status worsened, with progressive somnolence, confusion, incoherent speech, and walking. After that, he had seizures that required benzodiazepine and AED. Laboratory investigations revealed plasmatic and urinary alterations consistent with the syndrome of inappropriate antidiuretic hormone secretion (SIADH), and his EEG had a slow background. He was treated with antibiotics, and improvement of hyponatremia was accomplished, although he exhibited persistent neurologic disfunction. After one week, he developed an infrapopliteal deep venous thrombosis, and therapy with low-molecular-weight heparin was started. He was discharged to his home on day 15.
An 18-year-old girl was also infected with ground glass pneumonia [21]. She also manifested with seizures and mood disorders. A brain edema and the presence of anti-NMDA receptor antibodies were causative alterations. Steroids and IVIG determined a good prognosis. Two girls with learning disabilities worsened both intellectually/psychically and with neurological deficit. The 19-year-old described by Kasturiachi et al. [57] had progressive encephalopathy, epileptic EEG alteration without seizures, and multiple MR brain lesions. This case was complicated due to coexisting thrombotic thrombocytopenic purpura and other serologic poliautoimmunity; however, her prognosis was good after therapies with plasmapheresis, steroids, and rituximab.
A controversial case of a 16-year-old girl have been described by Gaughan et al. [48]. She had a history of mild learning needs; she complained of sore throat, fever, and psychotic behavior. After five days of IVIG therapy, the condition worsened, presenting mutism, little to no motor activity, incontinence, and being fed via nasogastric tube. Chest X-ray, MRI, and CSF were unremarkable. Two weeks later, following IVIG therapy, anti-GAD antibodies and extractable nuclear antigen appeared in the serum, transitorily. Repeated EEG showed slow activity. The girl improved 4 weeks after initial presentation and was discharged on day 48. The follow-up visit after six months revealed that she is still experiencing memory difficulties and fatigue.
Another 18-year-old girl, whose case has been described by Ayatollahi et al. [23], had fever, fatigue, malaise, and loss of appetite for a week, which progressed to drowsiness and confusion, and finally urinary retention and repeated generalized tonic-clonic seizure. Both CT brain scans and MRI were normal. In the following days, she had new seizures and fluctuating mood alterations, which were resistant to several therapies. Later on, a new MRI showed altered signals in the claustrum, external capsules, and some areas of the adjacent anterior insular cortex. Thereafter, therapy with intravenous methylprednisolone was started, and after three days, she was discharged in good condition. At follow-up, after one month, MRI showed nearly complete resolution of the previously described signal alterations. Seizures did not recur, although the memory deficit persisted. The last young girl mainly had retinal involvement, with subsequent encephalitis of the temporal lobes. Apart from fever, body pain, headache, and nausea with vomiting, the only neurologic symptom was drowsiness, and the prognosis was good [77].
3.3.3. Adults with COVID-19
Initial Presentation with Neurological and Respiratory Symptoms
Most cases manifested respiratory symptoms as an early manifestation of SARS-CoV-2 infection. Six cases also manifested neurological symptoms from the beginning of their clinical history [26,27,42,49,69,93]. Several cases developed neurological impairment following respiratory symptoms. The latency between the onset of the infection with respiratory manifestations and the onset of neurological complications varied between 3 days and 41 days [12,24,27,28,30,32,34,36,37,43,45,51,56,58,64,65,70,76,80,81,82,83,85,86,89,90,91,93,94]. Overall, 13 patients were intubated and mechanically ventilated due to respiratory distress; in these cases, the detection of neurological impairment happened when sedation was interrupted, with latency ranging between 4 and 38 days depending on the case [27,32,34,76,80,82,85,93]. In patients whose respiratory distress was less evident, neurological manifestation appeared after a few days in most cases [27,28,30,37,45,56,58,65,89,91,93,94]. In some cases, the latency ranged from over two weeks to 60 days [12,24,27,64,90]. Three patients began their illness with symptoms other than respiratory and developed neurological impairments between 5 and 17 days after the onset of the initial symptoms [22,47,50]. Their clinical presentations consisted of fatigue and malaise for two weeks in one case [47]; progressive diffuse arthralgia and sore throat, followed by fever [50]; and gastrointestinal manifestations in the third case [22]. An onset with neurological presentation was described in a number of reports [17,25,30,33,38,39,41,44,52,53,54,56,59,60,61,68,71,72,73,74,75,78,79,92]. Respiratory complications came later and were symptomatic in three cases [61,75,78]. Even though several patients did not have subjective respiratory difficulties or changes in arterial oxygen saturation, most of them had lung alterations on thorax CT and/or chest X-ray, which were typical of COVID-19 interstitial pneumonia, primarily as ground glass opacities and areas of parenchymal consolidation.
Neurological and Psychiatric Manifestations
Sleep disturbances, progressive altered mental status, psychiatric behavioural symptoms, and hallucination have been described by some authors [22,26,37,47,51,52,56,58,63,72,73,75,78,81,86,89,94]; most of these cases are associated with EEG representations [22,26,37,63,74,75], and some with brain neuroimaging alteration [51,52,56,78,81,94]. Neurological deficit referred to language deficit, cognitive deficits, akinetic syndrome with mutism, signs of cortical impairment, seizures, stroke, cerebellar signs, chorea, paralysis, coma, and signs of brain death [17,25,33,36,37,38,39,47,49,51,52,53,58,59,60,64,65,71,72,74,76,78,79,89,92].
Few cases presented with severe epileptic manifestations. Epileptic manifestations occurred from the first phase of infection, evolving into status epilepticus in some patients. Two of these patients died. The first developed intracranial hypertension with diffuse cerebral edema, and cerebellar herniation occurred [79]. The second case manifested a tonic-clonic seizure, followed by cardiac arrest, and was successfully resuscitated, intubated, and transferred to the intensive care unit. In this case, CT and MRI also showed brain edema and cerebellar herniation [45]. A third patient developed status epilepticus that required the patient to be intubated and mechanically ventilated. Chest CT showed interstitial pneumonia. He had an opening high pressure when lumbar puncture was performed, and SARS-CoV-2 RNA was detected in his CSF, while MRI revealed ventriculitis and encephalitis, mainly in the right mesial lobe and hippocampus [17]. The last case reported early onset of drug-refractory epilepsy; MRI and spectroscopy were suggestive for high-grade glioma; after lobectomy, histopathologic diagnosis was of encephalitis [41]. A late-onset status epilepticus with intracranial hypertension ion caused the death of another patient [12]. In this case, the disease had a two-phase evolution. He had a first respiratory phase, with recovery in 2–3 weeks. After 41 days, the fever reappeared, together with severe headache and vomiting. A brain CT scan and MRI showed unilateral hemispheric vasogenic edema with shift of the medial cerebral structures.
Particular Case of COVID-Linked Encephalitis
Some cases were marked by identification of specific neural antibodies. Three patients suffered from NMDA encephalitis [68,73,88]. Clinical manifestations were psychotic symptoms and seizures only. All of them improved with immuno-therapies, including IV methylprednisolone, IVIG, therapeutic plasma exchange. Only in the case described by Valadez-Calderon et al. [88], in which anti-NMDAR and anti-GAD65 antibodies were co-expressed, were EEG (slow rhythm) and MRI (MR signal alteration anterior cingulated cortex and temporal lobes bilaterally) both altered.
One patient developed an MOG antibody-associated encephalitis. He had minor clinical manifestations, but mulptiple MRI signal alterations: T2 and FLAIR, mainly with cortical distribution. This patient also improved after IV steroids [40]. Bickerstaff brainstem encephalitis involved three patients: two women and a man. Their main manifestation was truncal and cerebellar disfunction. In the case with anti-GD1 IgG antibodies [25], MRI images were altered (alterations in the caudal vermis and right flocculus of the cerebellum, and contrast enhancement in the floor of the IV ventricle). Improvement and resolution of symptoms were obtained with IV methylprednisolone. In the case with anti-gangliosides (GQ1b, GT1a, and GM1/GT1a), both EEG and MRI were normal. The patient improved after therapy with IV steroids, IVIG, but mainly after plasma exchange. The third case had serum onconeural antibodies against amphiphysin, and MR of brainstem encephalitis; this man also improved after steroidal therapy [59].
Another case of autoimmune encephalitis was described by Grimaldi et al. [50]. This man presented with progressive diffuse arthralgia and sore throat and interstitial pneumonia, and after 17 days, progressive action tremor, cerebellar syndrome, and diffuse myoclonus. EEG showed diffuse slowing, and MRI was normal, but PET with F-FDG showed putaminal, cerebellum, and diffuse cortical hypometabolism, confirmed by whole-brain voxel-based SPM quantification. Antibodies against the nuclei of Purkinje cell, striatal neurons, and hippocampal neurons were found in both serum and CSF. The patient was then treated with IVIG without clinical improvement. He improved after therapy with IV methylprednisolone and clonazepam.
Other particular cases of encephalitis were described. Three case reports described limbic encephalitis [43,76,92]. All of these patients had severe pneumonia, seizures or consciousness alteration, and anti-typical MRI alterations. All of them improved after having been treated with steroids, only in one case with IVIG added. A 67-year-old woman had an acute disseminated encephalitis (ADEM), with drowsiness; she had bilateral pneumonia and respiratory distress, was treated with IV steroids and IVIG, and unfortunately died after 4 weeks [44]. Two cases of mild encephalitis with reversible splenial lesion syndrome (RESLES) were described [42,54]. Both of them had interstitial pneumonia. The first patient [42] was afebrile, and presented a short loss of consciousness, cough, and headache. CSF and brain CT scan were normal, so he was discharged from the hospital. Nine days later, he manifested persistent headache, vertigo and intermittent disturbance of consciousness, myalgia, tiredness and persistent bibasilar rales, psychomotor slowing, and vestibular syndrome. MRI documented a signal alteration on the splenium of the corpus callosum. He was treated with analgesics and antibiotics. The patient improved gradually over the next few days. At one-month follow-up, lung complications were reduced, and MRI normalized. The second patient [54] showed marked dysmetria and mild ataxic gate. MRI documented abnormal alteration of the splenium of the corpus callosum, with a suspicion of RESLES. After a few hours, they manifested fever and hypoxemia. Treated with antiviral, antibiotics, and steroids, they neurologically improved but died at day 12 due to respiratory failure. Seven cases had AHNE [49,69,70,90]. This is a severe condition with poor prognosis. Neurological manifestations appeared after several days but were abrupt. Only four cases had pulmonary compromission [69,70,90]. All seven adults with AHNE died within 3 weeks.
Managing Severe Cases
Severe respiratory impairment was the main feature of some cases and required intubation. Benameur et al. [27] described three cases that manifested neurological complications after sedation was discontinued. In one case, cerebral edema with intracranial hypertensive signs was detected with MRI and CSF examination, consistent with encephalomyelitis and superimposed hypoxic ischemia. This woman died when life support was withdrawn. In the second case, profound encephalopathy with multifocal myoclonus was detected, and the patient was neurologically comatose. At lumbar puncture, there was a high opening pressure, suggesting intracranial hypertension. MRI disclosed a signal alteration at the splenium of the corpus callosum. The third case also had a profound encephalopathy without brainstem reflexes. He was treated with hydroxychloroquine, which caused myoclonus that disappeared at the cessation of this therapy. MRI showed signal alteration involving the temporal lobe. In this last case, the IgG anti-S1 receptor-bindle domain was present in serum, and a mild IgM level for SARS-CoV-2 S1 was found in CSF. In these cases, inflammatory molecules have been dosed in CSF, and in one case (the third), IL-8, IL-10, IP-10, and TNFα were increased. In the case series by Cao et al. [32], all five patients were intubated due to acute respiratory distress syndrome. When sedation was withdrawn, one patient was comatose, and the other four were in an unresponsive wakefulness syndrome (vegetative state). Four of them had high IL-6 level in serum, and one patient also had a slight increase of IL-6 in CSF. MRI showed several lesions in supratentorial deep white matter, in the pons, with several multiple small haemorrhagic lesions. Three out of five patients rapidly improved after therapy with intravenous steroids and therapeutic plasma exchange; one patient died. In addition, in the case by Svedung Wettervik et al. [85], the patient was comatose at the wake-up test after intubation. She had brain edema, and microhemorrhages with basal ganglia involvement, in line with acute hemorrhagic leukoencephalitis. An external ventricular drain was positioned, and CSF had fluctuating pressures, with a large amount of white and red cells. IL-6 was high in both plasma and CSF. This patient underwent therapeutic plasma exchange, after which she improved both clinically and in neuroimaging. Chalil et al.’s case developed respiratory distress two weeks from onset [34]. She was intubated, and severe lung complications were observed in chest CT. Therapy was hydroxychloroquine and tocilizumab. Due to the detection of very high D-dimer and ferritin levels, increased CRP, and severe hypoxemia, full anticoagulation started. Extensive bilateral parieto-occipital intraparenchymal hemorrage were observed with intraventricular extension and acute hydrocephalus. On day 15, brainstem reflexes were absent. Heparin was stopped, and external ventricular drain positioned. The authors interpreted this case as a post-infectious acute necrotizing hemorrhagic encephalopathy, due to thalamic involvement, suggested as typical for acute necrotizing hemorragic encephalopathy [34]. The patient was finally extubated, but severe neurologic deficit persisted.
The most prescribed drugs were antibiotics and antiviral agents. Quite often, hydroxychloroquine and high-dose methylprednisolone have been used. Until 2021, therapeutical plasmapheresis and immunosuppressive drugs were rarely prescribed. After the first period of the COVID-19 pandemic, immunomodulating agents, in particular IVIG and tocilizumab, plasmapheresis, and convalescent’s serum, have been used more often, with good response [21,51,59,60,70,81,83,88], except for the most severe cases, like in ANHE.
3.4. Quality Assessment
Among the included studies in this systematic review, based on the JBI critical appraisal tools assessing case reports and case series, 87.3% of the studies were of high quality, followed by 11.4% moderate- and 1.3% low-quality studies (Tables S3 and S4).
4. Discussion
In this systematic review, we comprehensively investigated the clinical and laboratory features, as well as the outcomes of COVID-19 patients with encephalitis. COVID-19 encephalitis can present before, together, or after respiratory manifestation of the illness. The most frequent neurological manifestations of encephalitis are seizure, confusion, headache, disorientation, status epilepticus, and altered mental status, and the degree of the condition is dependent mostly upon the severity of COVID-19 infection, entity of respiratory damage, and entity of neuronal damage and its site [91,95]. Severity of respiratory impairment and neuronal damage are the factors that seem to determine the worst prognosis. The knowledge of possible para- or post-infectious SARS-CoV-2 encephalitis, and of possible inflammatory or immune mechanisms of neuronal damage, can drive research for better therapies to improve outcomes [27,96].
In most cases, preforming a lumbar puncture can suggest the presence of encephalitic involvement. Brain CT scan in an emergency can be useful only in those cases that manifest with very acute neurological compromission, and can reveal brain edema, endocranial hypertension, massive necrosis, and or hemorrhagic lesions. A rapid intervention, acting to reduce intracranial hypertension, can help give the patient a chance to survive and receive the proper therapeutical efforts [97,98]. In those cases with psychiatric or epileptic presentation, CT scan can be unremarkable. MRI is also useful to detect small lesions, which can sometimes be transitory. In some cases, the only way to reveal neuronal disfunction is to use a neurological functional test, such as PET [99,100]. EEG is mostly aspecific, with slow background activity. Sometime EEG can also reveal epileptic activity of the non-convulsive type [101].
In the case of COVID-19, confirmed or suspected, it is important to consider possible neurological involvement. The clinician should consider any behavioral changes or alterations of consciousness. These can be due to hypoxic damage, hematochemical alteration, or circulatory problems, or they can be COVID-19-related. However, it can be a manifestation of neuronal involvement. The precocity of a specific intervention is essential to preserve neurons, and in case of any suspicion, proper tests and exams should be performed. Sometimes, neurological damage progresses slowly or tardively, and neuro-clinical symptoms can appear several day or weeks after the onset of the first symptoms. In these cases, an autoimmune mechanism of damage can be supposed, and this should drive the therapeutical approach [102,103].
Although the exact pathogenesis of the SARS-CoV-2 virus’s access to the CNS and triggering of various neurological manifestations is still under investigation, researchers have suggested a few mechanisms. SARS-CoV-2 can exert neurological manifestations by two main mechanisms. The first mechanism is the direct CNS infection, where SARS-CoV-2 gain access to the CNS from the peripheral nervous system, as RNA of the SARS-CoV-2 virus has been detected in the CSF of the COVID-19 patients. As in mature human olfactory nerves, ACE2 has not been reported, and ACE2 is most likely present at low levels in neurons [104,105]. SARS-CoV-2 possibly uses other facilitator(s) to access the CNS. One of the plausible routes for SARS-CoV-2 entry in the CNS could be that upon nasal infection, SARS-CoV-2 enters the CNS through the olfactory bulb, facilitated by neuropilin-1 (retrograde axonal transport), the only part of the CNS not protected by dura, causing inflammation and demyelination [106,107,108]. The second mechanism involves the disruption of the blood–brain barrier via SARS-CoV-2-ACE2 receptor-mediated vascular damage following viraemia. In this inflammation-mediated autoimmune-associated mechanism, both innate and adaptive immune systems play a role via upregulated inflammatory mediators exerting cytokine storm syndrome, which may result in acute hemorrhagic necrotizing encephalitis via perivascular demyelination [109,110,111].
Neurological symptoms seem to be the initial sign of illness in certain cases of COVID-19. A few cases, however, had already developed antibodies against SARS-CoV-2, suggesting a previous asymptomatic or pauci-symptomatic COVID-19 disease. That is why it is important, in case of neurological manifestation, to perform both the nasopharyngeal swab for SARS-CoV-2 and the serum test for antibodies against the virus. It is speculated that SARS-CoV-2-mediated encephalitis may present inflammatory injury, edema, and alterations in consciousness in patients with COVID-19 [91,96]. Although viral encephalitis confirmation requires virus isolation in the CSF, due to transient dissemination of SARS-CoV-2 virus and low CSF titre, it becomes extremely difficult to confirm viral encephalitis in COVID-19 [91]. For the management of encephalitis in COVID-19, we observed that IVIG therapy, plasma exchange, and corticosteroids may be useful in the treatment of COVID-19-related encephalitis.
There are some notable strengths of our study. First, this is the first systematic analysis based on the published case reports and series that was represented in a comprehensive way. Second, the search strategy was strong, as we used multiple databases with robust search strategies. Third, from our quality assessment, a majority of the studies were of high quality; therefore, the outcome of this systematic review is reliable, and it is based on high-methodological-quality studies. Nevertheless, there are some notable limitations. First, due to the study pattern, we could not analyze the data through a meta-analysis approach. Second, due to exploring the clinical, laboratory, and neuro-imaging data in a comprehensive way, we only included case reports and case series, and we did not consider any observational studies. Third, we could not retrieve our data of interest from all of the studies, which leads to incomplete data for some of the cases. However, since there is presently very little published information on encephalitis in COVID-19 patients, our analysis provided a strong early insight into the clinical, laboratory, and neuro-imaging characteristics of encephalitis in COVID-19 patients.
In the future, larger sample sizes would aid in determining if the neurological aspects, particularly the relationship with encephalitis, are purely coincidental, or whether there are phenotypes and associations particular to SARS-CoV-2.
5. Conclusions
COVID-19 patients with acute characteristic neurological signs such as seizure, confusion, headache, disorientation, status epilepticus, and altered mental status should be evaluated for viral encephalitis immediately, given the present condition of the COVID-19 pandemic. Patients with COVID-19 who are suspected of having encephalitis should have further testing, such as a brain MRI scan, long-term EEG monitoring, and lumbar puncture. The lack of a characteristic CSF profile of viral meningitis/encephalitis, as well as the negative PCR for SARS-CoV-2 virus in CSF, makes the diagnosis of encephalitis caused by the SARS-CoV-2 virus less evident, pointing to a possible autoimmune neuropathogenesis. In the post-acute phase of SARS-CoV-2 infection, it is critical to evaluate the neurological consequences. In this phase, encephalitis should be diagnosed only if there are clinical signs of brain inflammation, such as pleocytosis in the CSF, imaging alterations, focal seizures, or histological alterations. Even if the virus is found in the CSF, encephalitis should not be diagnosed until brain inflammation is present.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11162575/s1, Table S1: Search strategies, Table S2: Laboratory features of encephalitis patients with SARS-Cov-2 infection, Table S3: Quality assessment of the included case reports, Table S4: Quality assessment of the included case series.
Author Contributions
Conceptualization, M.A.I. and C.C.; methodology, M.A.I., S.S.A., and S.K.; validation, C.C., M.A.I., S.S.A., and F.R.; formal analysis, C.C. and M.A.I.; investigation, M.A.I., C.C., S.S.A., and S.K.; resources, M.A.I., C.C., and F.R.; data curation, C.C., M.A.I., F.R., and S.S.A.; writing—original draft preparation, C.C. and M.A.I. writing—review and editing, M.A.I., C.C., M.A.K., and F.R.; project administration, M.A.I. and F.R.; funding acquisition, M.A.I. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. WHO COVID-19 Dashboard. 2022. Available online: https://covid19.who.int (accessed on 9 August 2022).
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Kundu, S.; Alam, S.S.; Hossan, T.; Kamal, M.A.; Hassan, R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients. PLoS ONE 2021, 16, e0249788. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, F.; Fahriani, M.; Mamada, S.S.; Frediansyah, A.; Abubakar, A.; Maghfirah, D.; Fajar, J.K.; Maliga, H.A.; Ilmawan, M.; Bin Emran, T.; et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: A systematic review and meta-analysis. F1000Research 2021, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Cares-Marambio, K.; Montenegro-Jiménez, Y.; Torres-Castro, R.; Vera-Uribe, R.; Torralba, Y.; Alsina-Restoy, X.; Vasconcello-Castillo, L.; Vilaró, J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chronic Respir. Dis. 2021, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saniasiaya, J.; Islam, A.; Abdullah, B. Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients. Otolaryngol. Neck Surg. 2020, 165, 33–42. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Islam, A.; Abdullah, B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope 2020, 131, 865–878. [Google Scholar] [CrossRef]
- Islam, A.; Alam, S.S.; Kundu, S.; Hossan, T.; Kamal, M.A.; Cavestro, C. Prevalence of Headache in Patients with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of 14,275 Patients. Front. Neurol. 2020, 11, 562634. [Google Scholar] [CrossRef]
- Natarajan, S.; Ganesh, R.; Palaniappan, N.; Kannan, L. SARS-CoV-2 Encephalitis in an Adolescent Girl. Indian Pediatr. 2020, 57, 1186–1187. [Google Scholar] [CrossRef]
- McAbee, G.N.; Brosgol, Y.; Pavlakis, S.; Agha, R.; Gaffoor, M. Encephalitis Associated with COVID-19 Infection in an 11-Year-Old Child. Pediatr. Neurol. 2020, 109, 94. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Renisi, G.; Signorini, L.; Battaglia, L.; Ferrara, M.; Rasulo, F.A.; Panciani, P.P.; Fontanella, M.M. Delayed onset of fatal encephalitis in a COVID-19 positive patient. Int. J. Neurosci. 2021, 1–4. [Google Scholar] [CrossRef]
- Espíndola, O.M.; Brandão, C.O.; Gomes, Y.C.P.; Siqueira, M.; Soares, C.N.; Lima, M.A.S.D.; Leite, A.C.C.B.; Torezani, G.; Araujo, A.Q.C.; Silva, M.T.T. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int. J. Infect. Dis. 2020, 102, 155–162. [Google Scholar] [CrossRef]
- Bodnar, B.; Patel, K.; Ho, W.; Luo, J.J.; Hu, W. Cellular mechanisms underlying neurological/neuropsychiatric manifestations of COVID-19. J. Med. Virol. 2020, 93, 1983–1998. [Google Scholar] [CrossRef]
- Venkatesan, A.; Geocadin, R.G. Diagnosis and management of acute encephalitis: A practical approach. Neurol. Clin. Pract. 2014, 4, 206–215. [Google Scholar] [CrossRef]
- Ellul, M.; Solomon, T. Acute encephalitis–diagnosis and management. Clin. Med. 2018, 18, 155. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Abildúa, A.; Atienza, S.; Monteiro, G.; Aguirre, M.; Aguayo, L.; Álvarez, E.; García-Azorín, D.; Montesinos, I.; Lezama, L.; Pérez, M. Encephalopathy and encephalitis during acute SARS-CoV-2 infection. Spanish Society of Neurology COVID-19 Registry. Neurologia 2021, 36, 127–134. [Google Scholar] [CrossRef]
- Page, M.J.; E McKenzie, J.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemio. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Ahsan, N.; Jafarpour, S.; Santoro, J.D. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin. Exp. Pediatr. 2021, 64, 310–312. [Google Scholar] [CrossRef]
- Allahyari, F.; Hosseinzadeh, R.; Nejad, J.H.; Heiat, M.; Ranjbar, R. A case report of simultaneous autoimmune and COVID-19 encephalitis. J. Neurovirol. 2021, 27, 504–506. [Google Scholar] [CrossRef]
- Andrea, M.; Christian, M.; Lorenzo, M.; Francesco, D.; Walter, A.; Marco, M.; Andreina, B.; Maria, G.A.; Paolo, G.; Daniela, D.G.; et al. Unusual Presentation of COVID-19: Encephalitis and Syndrome of Inappropriate Anti-Diuretic Hormone Secretion. Int. J. Clin. Med. 2020, 11, 559–564. [Google Scholar] [CrossRef]
- Ayatollahi, P.; Tarazi, A.; Wennberg, R. Possible autoimmune encephalitis with claustrum sign in case of acute SARS-CoV-2 infection. Can. J. Neurol. Sci. 2021, 48, 430–432. [Google Scholar] [CrossRef]
- Ayuningtyas, T.; Natadidjaja, R.I.; Octaviani, C.; Sahli, F.; Adlani, H. Confirmed severe acute respiratory syndrome coronavirus 2 encephalitis in cerebrospinal fluid: A case report. J. Med Case Rep. 2022, 16, 1–4. [Google Scholar] [CrossRef]
- Ayuso, L.L.; Rubio, P.T.; Rosário, R.F.B.D.; Arroyo, M.L.G.; Sierra-Hidalgo, F. Bickerstaff encephalitis after COVID-19. J. Neurol. 2020, 268, 2035–2037. [Google Scholar] [CrossRef]
- Babar, A.; Lewandowski, U.; Capin, I.; Khariton, M.; Venkataraman, A.; Okolo, N.; Sharma, D. SARS-CoV-2 encephalitis in a 20-year old healthy female. Pediatr. Infect. Dis. J. 2020, 39, 320–321. [Google Scholar] [CrossRef]
- Benameur, K.; Agarwal, A.; Auld, S.C.; Butters, M.P.; Webster, A.S.; Ozturk, T.; Howell, J.C.; Bassit, L.C.; Velasquez, A.; Schinazi, R.F.; et al. Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020, 26, 2016–2021. [Google Scholar] [CrossRef]
- Bernard-Valnet, R.; Pizzarotti, B.; Anichini, A.; Demars, Y.; Russo, E.; Schmidhauser, M.; Cerutti-Sola, J.; Rossetti, A.O.; Du Pasquier, R. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur. J. Neurol. 2020, 27, 43–44. [Google Scholar] [CrossRef]
- Bhavsar, S.M.; Agarwal, S.; Lewis, R.; Ganta, A.; Roshchina, Y.S.; Clouser, K.N.; Baer, A.Z.; Gliksman, F.; Piwoz, J.A. COVID-19 Infection Associated With Encephalitis in an Adolescent. Neurol. Clin. Pract. 2020, 11, e189–e192. [Google Scholar] [CrossRef]
- Bodro, M.; Compta, Y.; Llansó, L.; Esteller, D.; Doncel-Moriano, A.; Mesa, A.; Rodríguez, A.; Sarto, J.; Martínez-Hernandez, E.; Vlagea, A. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2–associated encephalitis. Neurology 2020, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burr, T.; Barton, C.; Doll, E.; Lakhotia, A.; Sweeney, M. N-Methyl-d-Aspartate Receptor Encephalitis Associated With COVID-19 Infection in a Toddler. Pediatr. Neurol. 2020, 114, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Rohaut, B.; Le Guennec, L.; Saheb, S.; Marois, C.; Altmayer, V.; Carpentier, V.T.; Nemlaghi, S.; Soulie, M.; Morlon, Q.; et al. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain 2020, 143, e102. [Google Scholar] [CrossRef] [PubMed]
- Casez, O.; Willaume, G.; Grand, S.; Nemoz, B.; Lupo, J.; Kahane, P.; Brion, J.-P. Teaching NeuroImages: SARS-CoV-2–Related Encephalitis: MRI Pattern of Olfactory Tract Involvement. Neurology 2021, 96, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Chalil, A.; Baker, C.S.; Johnston, R.B.; Just, C.; Debicki, D.B.; Mayich, M.S.; Bosma, K.J.; Steven, D.A. Acute Hemorrhagic Encephalitis Related to COVID-19. Neurol. Clin. Pract. 2020, 11, e147–e151. [Google Scholar] [CrossRef] [PubMed]
- Cheraghali, F.; Tahamtan, A.; Hosseini, S.A.; Gharib, M.H.; Moradi, A.; Razavi Nikoo, H.; Tabarraei, A. Case report: Detection of SARS-CoV-2 from cerebrospinal fluid in a 34-month-old child with encephalitis. Front. Pediatrics 2021, 9, 565778. [Google Scholar] [CrossRef]
- Dahshan, A.; Abdellatef, A.A. Autoimmune encephalitis as a complication of COVID-19 infection: A case report. Egypt. J. Intern. Med. 2022, 34, 1–3. [Google Scholar] [CrossRef]
- Dono, F.; Carrarini, C.; Russo, M.; De Angelis, M.V.; Anzellotti, F.; Onofrj, M.; Bonanni, L. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: A case report. Neurol. Sci. 2021, 42, 35–38. [Google Scholar] [CrossRef]
- Duong, L.; Xu, P.; Liu, A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 2020, 87, 33. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef]
- Durovic, E.; Bien, C.; Bien, C.G.; Isenmann, S. MOG antibody-associated encephalitis secondary to Covid-19: Case report. BMC Neurol. 2021, 21, 1–5. [Google Scholar] [CrossRef]
- Efe, I.E.; Aydin, O.U.; Alabulut, A.; Çelik, O.; Aydin, K. COVID-19−Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020, 140, 46–48. [Google Scholar] [CrossRef]
- El Aoud, S.; Sorial, D.; Selmaoui, A.; Menif, I.; Lazard, M.; Hocine, M.S.; Thomas, L. A first case of Mild Encephalitis with Reversible Splenial Lesion (MERS) as a presenting feature of SARS-CoV-2. Rev. Neurol. 2021, 177, 139–141. [Google Scholar] [CrossRef]
- Elmouhib, A.; Benramdane, H.; Ahsayen, F.Z.; El Haddad, I.A.; El Ghalet, A.; Laaribi, I.; Bkiyar, H.; Nasri, S.; Skiker, I.; Housni, B. A case of limbic encephalitis associated with severely COVID-19 infection. Ann. Med. Surg. 2022, 74, 103274. [Google Scholar] [CrossRef]
- Esmaeili, S.; Abbasi, M.H.; Mojtahed, M.; Emamikhah, M.; Makiani, M.J.; Nazarian, H.; Mirzaasgari, Z. Acute disseminated encephalitis (ADEM) as the first presentation of COVID-19; a case report. Ann. Med. Surg. 2022, 77, 103511. [Google Scholar] [CrossRef]
- Etemadifar, M.; Salari, M.; Murgai, A.A.; Hajiahmadi, S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurol. Sci. 2020, 41, 3027–3029. [Google Scholar] [CrossRef]
- Ferdosian, F.; Mohsenolhoseini, Z.; Fallah, R. Encephalitis Associated With COVID-19 in A 7-year-old Boy: A Case Report. Case Rep. Clin. Pract. 2022, 6, 246–250. [Google Scholar] [CrossRef]
- Freire-Álvarez, E.; Guillén, L.; Lambert, K.; Baidez, A.; García-Quesada, M.; Andreo, M.; Alom, J.; Masiá, M.; Gutiérrez, F. COVID-19-associated encephalitis successfully treated with combination therapy. Clin. Infect. Pract. 2020, 7–8, 100053. [Google Scholar] [CrossRef]
- Gaughan, M.; Connolly, S.; O’Riordan, S.; Tubridy, N.; McGuigan, C.; Kinsella, J.A. Pediatric Parainfectious Encephalitis Associated With COVID-19. Neurology 2021, 96, 541–544. [Google Scholar] [CrossRef]
- Ghosh, R.; Dubey, S.; Finsterer, J.; Chatterjee, S.; Ray, B.K. SARS-CoV-2-Associated Acute Hemorrhagic, Necrotizing Encephalitis (AHNE) Presenting with Cognitive Impairment in a 44-Year-Old Woman without Comorbidities: A Case Report. Am. J. Case Rep. 2020, 21, e925641. [Google Scholar] [CrossRef]
- Grimaldi, S.; Lagarde, S.; Harlé, J.-R.; Boucraut, J.; Guedj, E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: Insight from 18F-FDG PET imaging and neuronal autoantibodies. J. Nucl. Med. 2020, 61, 1726–1729. [Google Scholar] [CrossRef]
- Gunawardhana, C.; Nanayakkara, G.; Gamage, D.; Withanage, I.; Bandara, M.; Siriwimala, C.; Senaratne, N.; Chang, T. Delayed presentation of postinfectious encephalitis associated with SARS-CoV-2 infection: A case report. Neurol. Sci. 2021, 42, 3527–3530. [Google Scholar] [CrossRef]
- Haider, A.; Siddiqa, A.; Ali, N.; Dhallu, M. COVID-19 and the Brain: Acute Encephalitis as a Clinical Manifestation. Cureus 2020, 12, e10784. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Syed, F.; Ali, L.; Rajput, H.M.; Faisal, F.; Shahzad, W.; Badshah, M. Chorea as a presentation of SARS-CoV-2 encephalitis: A clinical case report. J. Mov. Disord. 2021, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sahashi, Y.; Baba, Y.; Okura, H.; Shimohata, T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J. Neurol. Sci. 2020, 415, 116941. [Google Scholar] [CrossRef] [PubMed]
- Kahwagi, J.; Diagne, R.; Fall, M.; Basse, A.; Ndiaye, M.; Diop, A. Post infectious encephalitis at Covid19: About one pediatric observation and review of the literature. Rev. Neurol. 2020, 177, 132–134. [Google Scholar] [CrossRef]
- Kamal, Y.M.; Abdelmajid, Y.; Al Madani, A.A.R. Cerebrospinal fluid confirmed COVID-19-associated encephalitis treated successfully. BMJ Case Rep. 2020, 13, e237378. [Google Scholar] [CrossRef]
- Kasturiarachi, B.M.; Alsbrook, D.L.; Crook, J.; Shah, N. An Immunologic Storm: A Case of Encephalitis and Thrombotic Thrombocytopenic Purpura With Underlying Likely Sjogren’s Syndrome Induced by a COVID-19 Immune Response. Neurohospitalist 2022, 12, 529–535. [Google Scholar] [CrossRef]
- Khoo, A.; McLoughlin, B.; Cheema, S.; Weil, R.S.; Lambert, C.; Manji, H.; Zandi, M.S.; Morrow, J.M. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1013–1014. [Google Scholar]
- Kimura, M.; Hashiguchi, S.; Tanaka, K.; Hagiwara, M.; Takahashi, K.; Miyaji, Y.; Joki, H.; Doi, H.; Koga, M.; Takeuchi, H.; et al. Case Report: Takotsubo Cardiomyopathy in Bickerstaff Brainstem Encephalitis Triggered by COVID-19. Front. Neurol. 2021, 12, 822247. [Google Scholar] [CrossRef]
- Koh, S.; Kim, Y.S.; Kim, M.H.; Choi, Y.H.; Choi, J.Y.; Kim, T.-J. Encephalitis with status epilepticus and stroke as complications of non-severe COVID-19 in a young female patient: A case report. BMC Neurol. 2022, 22, 1–5. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Kumar, A.; Pati, B.K.; Kumar, A.; Singh, C.; Sarfraz, A. Acute necrotizing encephalitis as a probable association of covid-19. Indian J. Crit. Care 2020, 24, 991–994. [Google Scholar] [CrossRef]
- Kumar, V.H.; Natarajan, C.; Siddharth, M.; Shivabalan, S.; Gopinath, C.; Shyam, A.; Jean, M. Post covid pneumonia pulmonary fibrosis and encephalitis in a term neonate with prenatal exposure to SARS CoV-2: A case report. IDCases 2022, 27, e01414. [Google Scholar] [CrossRef]
- Marques, L.M.; Marques, S.R.; Costa, O.; Freitas, E.; Machado, Á. COVID-19-Associated Encephalitis: Two Case Reports. Cureus 2022, 14, e23243. [Google Scholar] [CrossRef]
- Mekheal, E.; Mekheal, M.; Roman, S.; Mikhael, D.; Mekheal, N.; Manickam, R. A Case Report of Autoimmune Encephalitis: Could Post-COVID-19 Autoimmunity Become a Lethal Health Issue? Cureus 2022, 14, e25910. [Google Scholar] [CrossRef]
- Meshref, M.; Hewila, I.M.; Mageed, S.A.; Morra, M.E. COVID-19 Associated With Encephalitis: Case Report and Review of Literature. Neurologist 2021, 26, 268. [Google Scholar] [CrossRef]
- Mierzewska-Schmidt, M.; Baranowski, A.; Szymanska, K.; Ciaston, M.; Kuchar, E.; PLoSki, R.; Kosinska, J.; Pagowska-Klimek, I. The case of fatal acute hemorrhagic necrotizing encephalitis in a two-month-old boy with Covid-19. Int. J. Infect. Dis. 2021, 116, 151–153. [Google Scholar] [CrossRef]
- Miqdad, M.A.; Enabi, S.; Alshurem, M.; Al-Musawi, T.; Alamri, A. COVID-19–Induced Encephalitis: A Case Report of a Rare Presentation With a Prolonged Electroencephalogram. Cureus 2021, 13, e14476. [Google Scholar] [CrossRef]
- Monti, G.; Giovannini, G.; Marudi, A.; Bedin, R.; Melegari, A.; Simone, A.M.; Santangelo, M.; Pignatti, A.; Bertellini, E.; Trenti, T.; et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure 2020, 81, 18–20. [Google Scholar] [CrossRef]
- Morvan, A.-C.; Kerambrun, H. Fatal necrotizing encephalitis associated with COVID-19: A case report. Neurology 2021, 11, 214–215. [Google Scholar]
- Mullaguri, N.; Sivakumar, S.; Battineni, A.; Anand, S.; Vanderwerf, J. COVID-19 Related Acute Hemorrhagic Necrotizing Encephalitis: A Report of Two Cases and Literature Review. Cureus 2021, 13, e14236. [Google Scholar] [CrossRef]
- Oosthuizen, K.; Steyn, E.C.; Tucker, L.; Ncube, I.V.; Hardie, D.; Marais, S. SARS-CoV-2 encephalitis presenting as a clinical cerebellar syndrome: A case report. Neurology 2021, 97, 27–29. [Google Scholar] [CrossRef]
- Orsini, M.; Porto, F.H.D.G.; Nascimento, J.F.D. Neuropsychiatric presentation of Covid-19-related encephalitis: Case report. Psychiatry Res. Commun. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Panariello, A.; Bassetti, R.; Radice, A.; Rossotti, R.; Puoti, M.; Corradin, M.; Moreno, M.; Percudani, M. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: A case report. Brain, Behav. Immun. 2020, 87, 179–181. [Google Scholar] [CrossRef]
- Picod, A.; Dinkelacker, V.; Savatovsky, J.; Trouiller, P.; Guéguen, A.; Engrand, N. SARS-CoV-2-associated encephalitis: Arguments for a post-infectious mechanism. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef]
- Pilotto, A.; Odolini, S.; Masciocchi, S.S.; Comelli, A.; Volonghi, I.; Gazzina, S.; Nocivelli, S.; Pezzini, A.; Focà, E.; Caruso, A.; et al. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. Ann. Neurol. 2020, 88, 423–427. [Google Scholar] [CrossRef]
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav. Immun. Health 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Poursadeghfard, M.; Sharifian-Dorche, M.; Nemati, A.; Mowla, A. Simultaneous Encephalitis and Neuroretinitis After COVID-19 in a Young Adult: A Case Report. J. Neurol. Res. 2021, 11, 102–107. [Google Scholar] [CrossRef]
- Rebeiz, T.; Lim-Hing, K.; Khazanehdari, S.; Rebeiz, K. Behavioral Changes Without Respiratory Symptoms as a Presenting Sign of COVID-19 Encephalitis. Cureus 2020, 12, e10469. [Google Scholar] [CrossRef]
- Reddy, R. Status epilepticus in a young woman with suspected SARS-CoV-2 encephalitis. Crit. Care Med. 2021, 49, 107. [Google Scholar] [CrossRef]
- Sangare, A.; Dong, A.; Valente, M.; Pyatigorskaya, N.; Cao, A.; Altmayer, V.; Zyss, J.; Lambrecq, V.; Roux, D.; Morlon, Q.; et al. Neuroprognostication of Consciousness Recovery in a Patient with COVID-19 Related Encephalitis: Preliminary Findings from a Multimodal Approach. Brain Sci. 2020, 10, 845. [Google Scholar] [CrossRef]
- Sarmast, S.T.; Mohamed, A.S.; Amar, Z.; Sarwar, S.; Ahmed, Z. A Case of Acute Encephalitis in COVID-19 Patient: A Rare Complication. Cureus 2021, 13, e15636. [Google Scholar] [CrossRef]
- Sattar, S.B.A.; Haider, M.A.; Zia, Z.; Niazi, M.; Iqbal, Q.Z. Clinical, Radiological, and Molecular Findings of Acute Encephalitis in a COVID-19 Patient: A Rare Case Report. Cureus 2020, 12, e10650. [Google Scholar] [CrossRef]
- Sharma, R.; Nalleballe, K.; Shah, V.; Haldal, S.; Spradley, T.; Hasan, L.; Mylavarapu, K.; Vyas, K.; Kumar, M.; Onteddu, S.; et al. Spectrum of Hemorrhagic Encephalitis in COVID-19 Patients: A Case Series and Review. Diagnostics 2022, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Sofijanova, A.; Bojadzieva, S.; Duma, F.; Superlishka, E.; Murtezani, A.; Jordanova, O. Severe Encephalitis in Infant with COVID-19: A Case Report. Open Access Maced. J. Med Sci. 2020, 8, 514–517. [Google Scholar] [CrossRef]
- Wettervik, T.S.; Kumlien, E.; Rostami, E.; Howells, T.; von Seth, M.; Velickaite, V.; Lewén, A.; Enblad, P. Intracranial Pressure Dynamics and Cerebral Vasomotor Reactivity in Coronavirus Disease 2019 Patient With Acute Encephalitis. Crit. Care Explor. 2020, 2, e0197. [Google Scholar] [CrossRef] [PubMed]
- Tee, T.Y.; Thabit, A.A.M.; Khoo, C.S.; Shahrom, H.M.; Chan, E.Z.; Marzukie, M.M.; Kamaruddin, Z.A.C.; Thayan, R.; Chidambaram, S.K. Acute encephalitis associated with SARS-CoV-2 confirmed in cerebrospinal fluid: First case in Malaysia. J. Clin. Neurol. 2021, 17, 490. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Distefano, M.G.; Cambula, G.; Colomba, A.I.; Nuzzo, D.; Picone, P.; Giacomazza, D.; Sicurella, L. The case of encephalitis in a COVID-19 pediatric patient. Neurol. Sci. 2021, 43, 105–112. [Google Scholar] [CrossRef]
- Valadez-Calderon, J.; Navarro, A.O.; Rodriguez-Chavez, E.; Vera-Lastra, O. Co-expression of anti-NMDAR and anti-GAD65 antibodies. A case of autoimmune encephalitis in a post-COVID-19 patient. Neurologia 2022, 37, 503. [Google Scholar] [CrossRef]
- Vandervorst, F.; Guldolf, K.; Peeters, I.; Vanderhasselt, T.; Michiels, K.; Berends, K.J.; Van Laethem, J.; Pipeleers, L.; Vincken, S.; Seynaeve, L. Encephalitis associated with the SARS-CoV-2 virus: A case report. Interdiscip. Neurosurg. 2020, 22, 1–3. [Google Scholar] [CrossRef]
- Woldie, I.L.; Brown, I.G.; Nwadiaro, N.F.; Patel, A.; Jarrar, M.; Quint, E.; Khokhotva, V.; Hugel, N.; Winger, M.; Briskin, A. Autoimmune Hemolytic Anemia in a 24-Year-Old Patient With COVID-19 Complicated by Secondary Cryptococcemia and Acute Necrotizing Encephalitis: A Case Report and Review of Literature. J. Med. Cases 2020, 11, 362–365. [Google Scholar] [CrossRef]
- Ye, M.; Ren, Y.; Lv, T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020, 88, 945–946. [Google Scholar] [CrossRef]
- Zambreanu, L.; Lightbody, S.; Bhandari, M.; Hoskote, C.; Kandil, H.; Houlihan, C.F.; Lunn, M.P. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1229–1230. [Google Scholar] [CrossRef]
- Zandifar, S.; Zandifar, Z. Acute Viral Encephalitis Associated with SARS-CoV-2. Ann. Clin. Case Rep. 2020, 5, 1–3. [Google Scholar]
- Zuhorn, F.; Omaimen, H.; Ruprecht, B.; Stellbrink, C.; Rauch, M.; Rogalewski, A.; Klingebiel, R.; Schäbitz, W.-R. Parainfectious encephalitis in COVID-19: “The Claustrum Sign”. J. Neurol. 2020, 268, 2031–2034. [Google Scholar] [CrossRef]
- Pilotto, A.; Masciocchi, S.; Volonghi, I.; Crabbio, M.; Magni, E.; De Giuli, V.; Caprioli, F.; Rifino, N.; Sessa, M.; Gennuso, M. Clinical presentation and outcomes of SARS-CoV-2 related encephalitis: The ENCOVID multicentre study. J. Infect. Dis. 2020, 223, 28–37. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K.; Gupta, A. Encephalopathy in patients with COVID-19: A review. J. Med. Virol. 2020, 93, 206–222. [Google Scholar] [CrossRef]
- Kas, A.; Soret, M.; Pyatigoskaya, N.; Habert, M.-O.; Hesters, A.; Le Guennec, L.; Paccoud, O.; Bombois, S.; Delorme, C. The cerebral network of COVID-19-related encephalopathy: A longitudinal voxel-based 18F-FDG-PET study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2543–2557. [Google Scholar] [CrossRef]
- Guo, K.; Wei, Y.; Yuan, M.; Wei, L.; Lu, J. Identifying the characteristics of brain glucose metabolism using normal 18F-FDG PET database in patients with temporal lobe epilepsy. Neurol. Sci. 2020, 41, 3219–3226. [Google Scholar] [CrossRef]
- Cani, I.; Barone, V.; D’Angelo, R.; Pisani, L.; Allegri, V.; Spinardi, L.; Malpassi, P.; Fasano, L.; Rinaldi, R.; Fanti, S.; et al. Frontal encephalopathy related to hyperinflammation in COVID-19. J. Neurol. 2020, 268, 16–19. [Google Scholar] [CrossRef]
- Hosseini, A.A.; Shetty, A.K.; Sprigg, N.; Auer, D.P.; Constantinescu, C.S. Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav. Immun. 2020, 88, 68–70. [Google Scholar] [CrossRef]
- Umapathi, T.; Quek, W.M.J.; Yen, J.M.; Khin, H.S.W.; Mah, Y.Y.; Chan, C.Y.J.; Ling, L.M.; Yu, W.-Y. Encephalopathy in COVID-19 patients; viral, parainfectious, or both? Eneurologicalsci 2020, 21, 100275. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef]
- Lersy, F.; Anheim, M.; Willaume, T.; Chammas, A.; Brisset, J.-C.; Cotton, F.; Kremer, S. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J. Neuroradiol. 2021, 48, 141–146. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Klingenstein, M.; Klingenstein, S.; Neckel, P.H.; Mack, A.F.; Wagner, A.P.; Kleger, A.; Liebau, S.; Milazzo, A. Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs 2021, 209, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.-Y.; Tsang, J.O.-L.; Huang, X. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).