Intraocular RGD-Engineered Exosomes and Active Targeting of Choroidal Neovascularization (CNV)
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
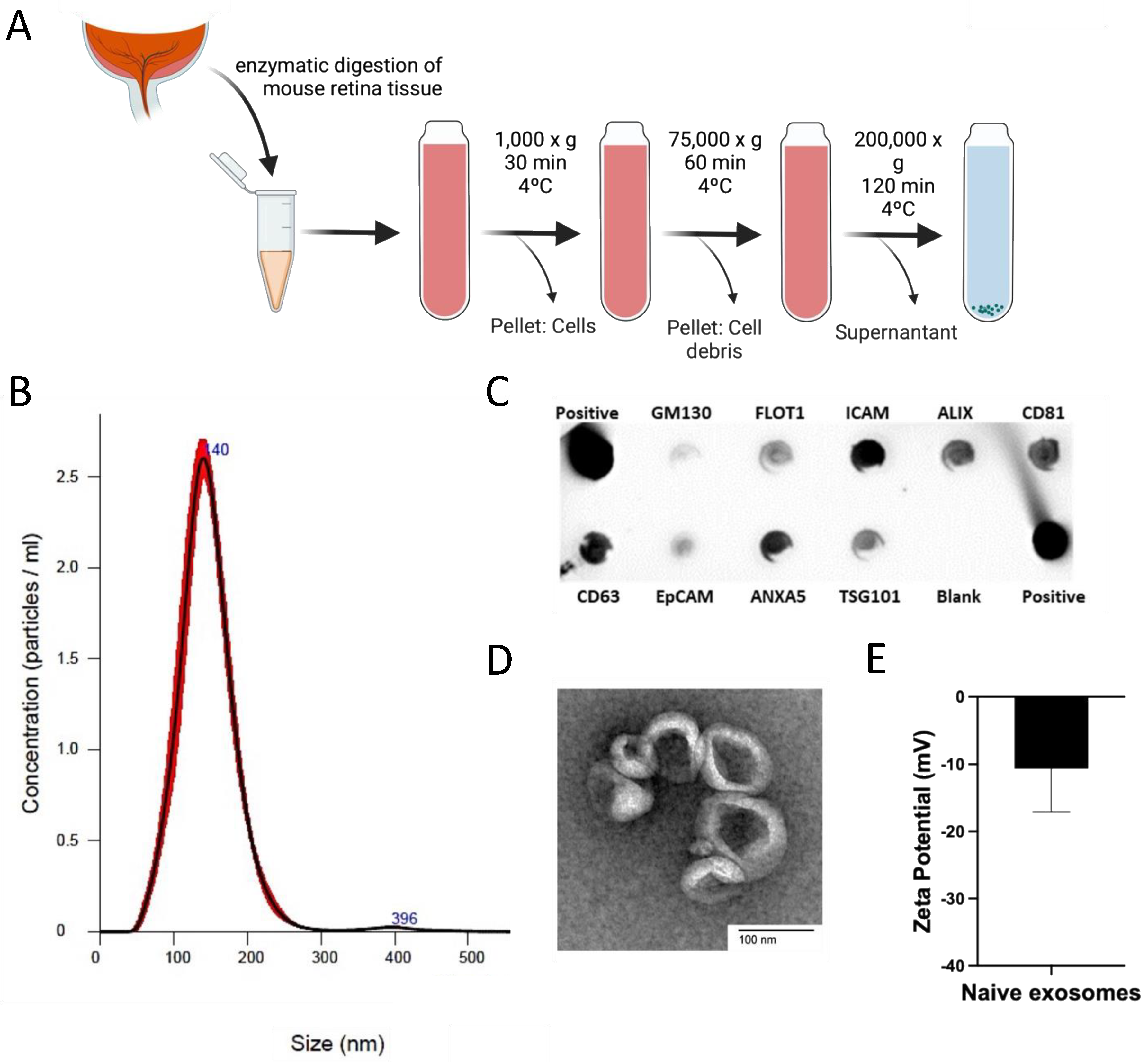
2.2. Exosome Isolation and Characterization
2.3. Fluorescent Labeling of Exosomes
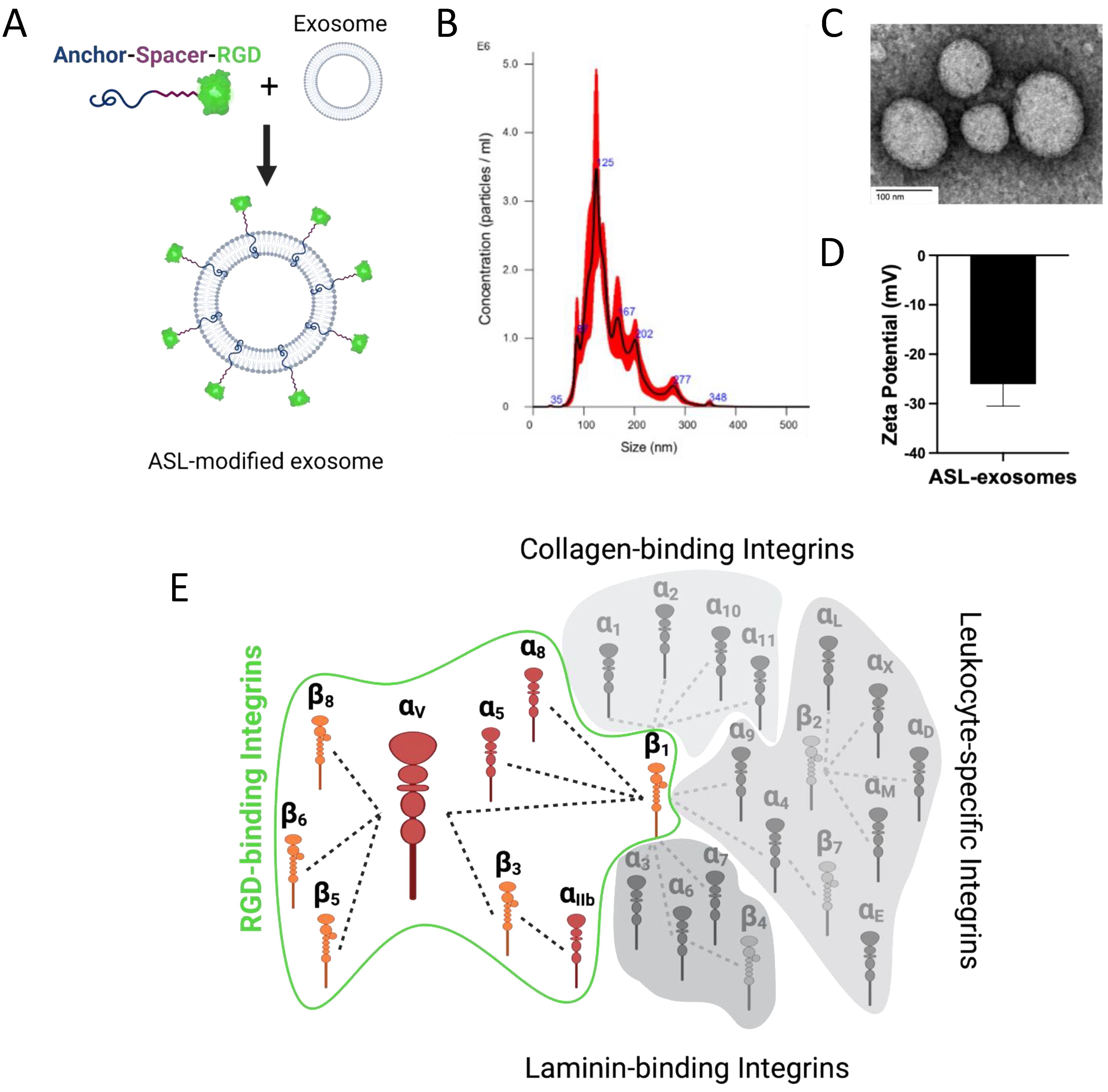
2.4. Bioengineering of ASL Exosomes
2.5. In Vitro Exosome Uptake
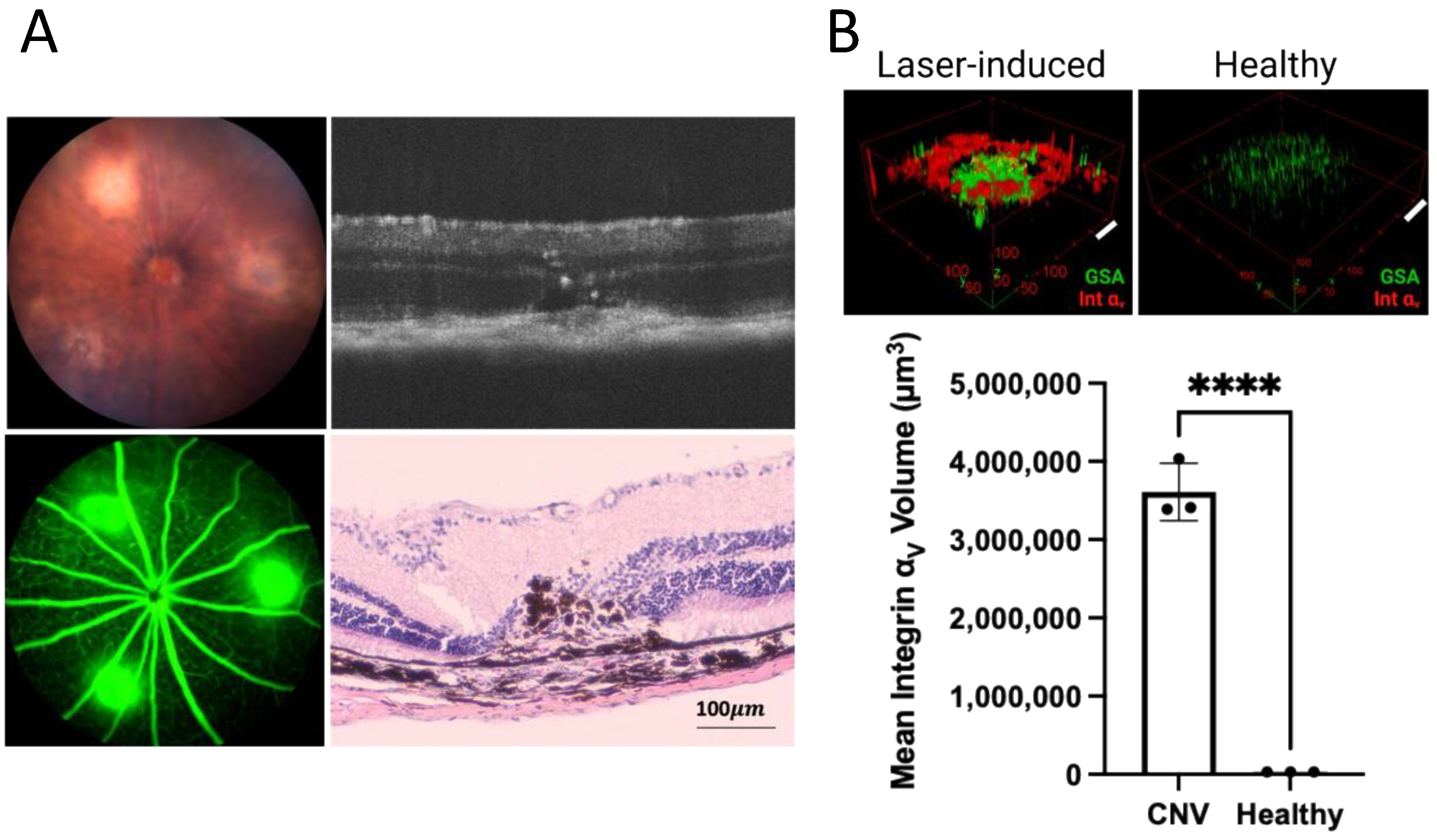
2.6. Laser-Induced Choroidal Neovascularization Mouse Model
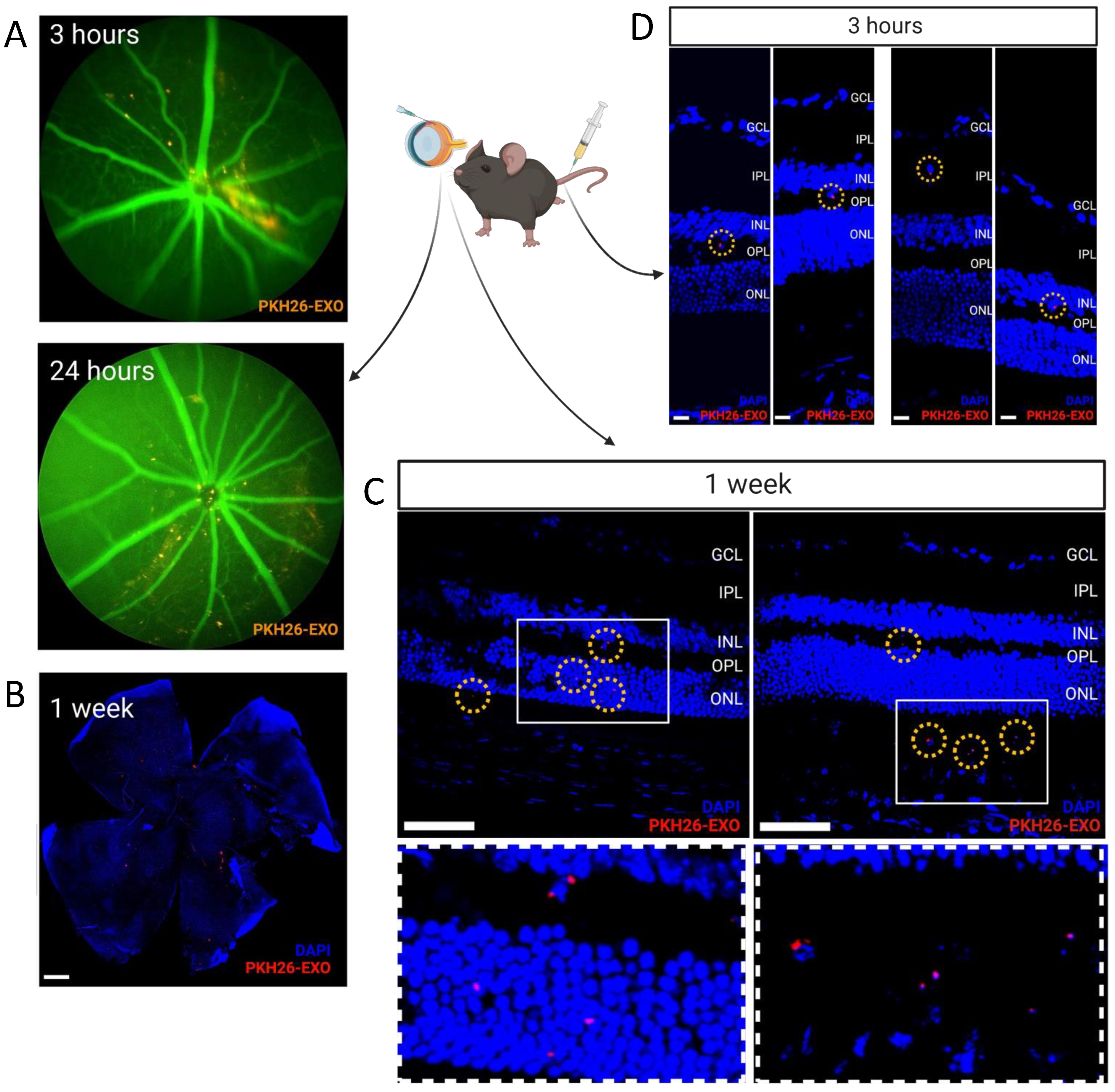
2.7. Intravitreal Injection of Exosomes
2.8. Intravenous Injection of Exosomes
2.9. In Vivo Imaging Analysis
2.10. Histological Analysis
2.11. Image Analysis
2.12. Statistical Analysis
3. Results
3.1. Exosome Recovery and Characterization
3.2. Retinal Uptake of Intravitreally Delivered Exosomes and BRB crossing of Systemically Delivered Exosomes
3.3. Engineered Anchor, Spacer, and RGD Ligand Modified ASL Exosomes
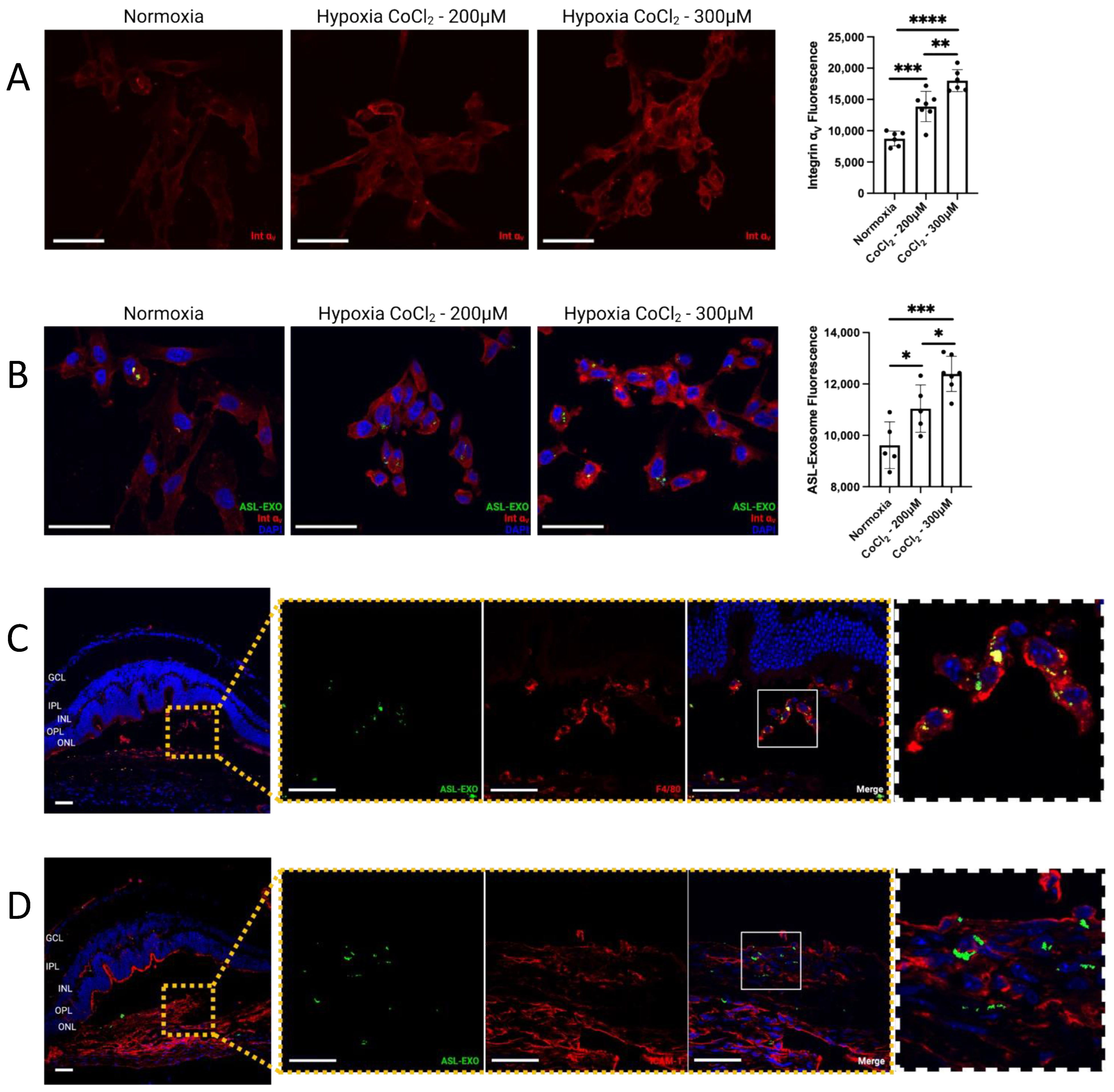
3.4. Increased Integrin v Expression in Choroidal Neovascularization
3.5. Active Uptake of ASL Exosomes to CNV Sites
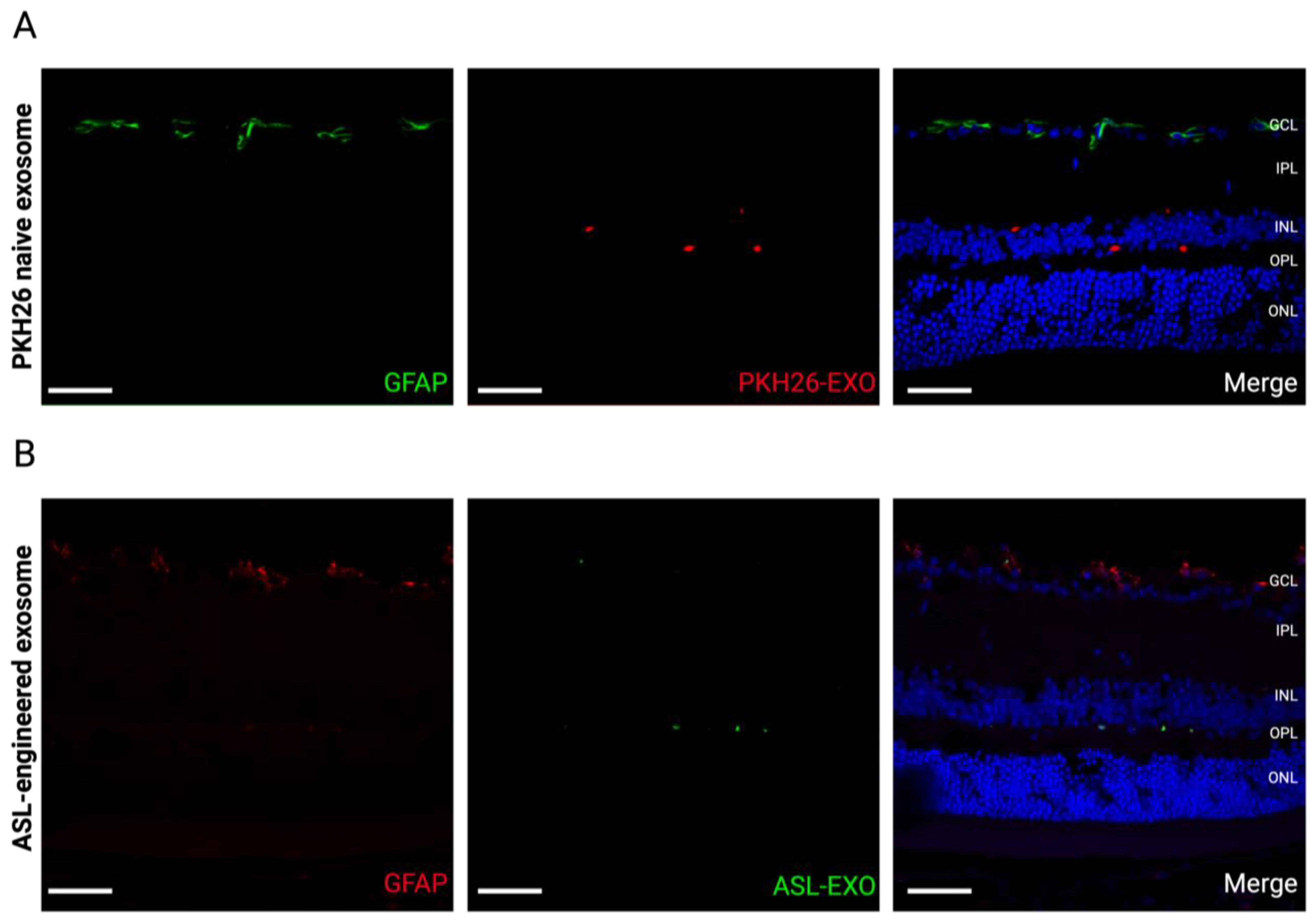
3.6. No Reactive Retinal Gliosis after Intravitreal Injection of ASL Exosomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Quah, B.J.; O’Neill, H.C. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol. Dis. 2005, 35, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Zhao, C.; He, X.; Gong, Y.; Ren, C.; Ge, L.; Zeng, Y.; Li, Q.; Chen, M.; Weng, C.; et al. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J. Extracell. Vesicles 2020, 9, 1748931. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Z.; Gong, Y.; Zheng, Z. Human umbilical cord mesenchymal stem cell-derived exosomal miR-27b attenuates subretinal fibrosis via suppressing epithelial–mesenchymal transition by targeting HOXC6. Stem Cell Res. Ther. 2021, 12, 24. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020, 8, 586130. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Khanani, A.M.; Skelly, A.; Bezlyak, V.; Griner, R.; Torres, L.R.; Sagkriotis, A. SIERRA-AMD: A Retrospective, Real-World Evidence Study of Patients with Neovascular Age-Related Macular Degeneration in the United States. Ophthalmol. Retina 2020, 4, 122–133. [Google Scholar] [CrossRef]
- Hutton-Smith, L.A.; Gaffney, E.A.; Byrne, H.M.; Maini, P.K.; Gadkar, K.; Mazer, N.A. Ocular Pharmacokinetics of Therapeutic Antibodies Given by Intravitreal Injection: Estimation of Retinal Permeabilities Using a 3-Compartment Semi-Mechanistic Model. Mol. Pharm. 2017, 14, 2690–2696. [Google Scholar] [CrossRef]
- Teo, K.Y.C.; Joe, A.W.; Nguyen, V.; Invernizzi, A.; Arnold, J.J.; Barthelmes, D.; Gillies, M. Prevalence and Risk Factors for the Development of Physician-Graded Subretinal Fibrosis in Eyes Treated for Neovascular Age-Related Macular Degeneration. Retina 2020, 40, 2285–2295. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from Retinal Astrocytes Contain Antiangiogenic Components That Inhibit Laser-induced Choroidal Neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef]
- Kang, C.; Han, P.; Lee, J.S.; Lee, D.; Kim, D. Anchor, Spacer, and Ligand-Modified Engineered Exosomes for Trackable Targeted Therapy. Bioconjug. Chem. 2020, 31, 2541–2552. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Reardon, D.A.; Nabors, L.B.; Stupp, R.; Mikkelsen, T. Cilengitide: An integrin-targeting arginine–glycine–aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin. Investig. Drugs 2008, 17, 1225–1235. [Google Scholar] [CrossRef]
- Shan, D.; Li, J.; Cai, P.; Prasad, P.; Liu, F.; Rauth, A.M.; Wu, X.Y. RGD-conjugated solid lipid nanoparticles inhibit adhesion and invasion of αvβ3 integrin-overexpressing breast cancer cells. Drug Deliv. Transl. Res. 2015, 5, 15–26. [Google Scholar] [CrossRef]
- Wang, C.; Bao, C.; Liang, S.; Fu, H.; Wang, K.; Deng, M.; Liao, Q.; Cui, D. RGD-conjugated silica-coated gold nanorods on the surface of carbon nanotubes for targeted photoacoustic imaging of gastric cancer. Nanoscale Res. Lett. 2014, 9, 264. [Google Scholar] [CrossRef]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A.; Chang, S.; Cheresh, D.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef]
- Nair, G.K.G.; Pollalis, D.; Wren, J.D.; Georgescu, C.; Sjoelund, V.; Lee, S.Y. Proteomic Insight into the Role of Exosomes in Proliferative Vitreoretinopathy Development. J. Clin. Med. 2022, 11, 2716. [Google Scholar] [CrossRef]
- Saishin, Y.; Saishin, Y.; Takahashi, K.; Silva, R.L.E.; Hylton, D.; Rudge, J.S.; Wiegand, S.J.; Campochiaro, P.A. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the bloodretinal barrier. J. Cell. Physiol. 2003, 195, 241–248. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Emam, S.E.; Lila, A.S.A.; Elsadek, N.E.; Ando, H.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.S.; Ishida, T. Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues. Eur. J. Pharm. Biopharm. 2019, 145, 27–34. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Torres, L.; Gamboa Acha, L.; Tran, S.; Liu, A.; Patel, R.; Chennakesavalu, M.; Aneesh, A.; Huang, C.C.; Feinstein, D.; et al. Uptake and Distribution of Administered Bone Marrow Mesenchymal Stem Cell Extracellular Vesicles in Retina. Cells 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin, RGD peptide and du-al-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bohner, A.; Bhuvanagiri, S.; Uehara, H.; Upadhyay, A.K.; Emerson, L.L.; Bondalapati, S.; Muddana, S.K.; Fang, D.; Li, M.; et al. Targeted Intraceptor Nanoparticle for Neovascular Macular Degeneration: Preclinical Dose Optimization and Toxicology Assessment. Mol. Ther. 2017, 25, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Muddana, S.K.; Zhang, X.; Das, S.K.; Bhuvanagiri, S.; Liu, J.; Wu, Y.; Choi, S.; Carroll, L.S.; Archer, B.; et al. Targeted Delivery of FLT-Morpholino Using Cyclic RGD Peptide. Transl. Vis. Sci. Technol. 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Zhu, J.; Zhao, L.; Mao, K.; Gu, Q.; Li, D.; Zhao, J.; Wu, X. RGD-modified multifunctional nanoparticles encapsulating salvianolic acid A for targeted treatment of choroidal neovascularization. J. Nanobiotechnol. 2021, 19, 196. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollalis, D.; Kim, D.; Nair, G.K.G.; Kang, C.; Nanda, A.V.; Lee, S.Y. Intraocular RGD-Engineered Exosomes and Active Targeting of Choroidal Neovascularization (CNV). Cells 2022, 11, 2573. https://doi.org/10.3390/cells11162573
Pollalis D, Kim D, Nair GKG, Kang C, Nanda AV, Lee SY. Intraocular RGD-Engineered Exosomes and Active Targeting of Choroidal Neovascularization (CNV). Cells. 2022; 11(16):2573. https://doi.org/10.3390/cells11162573
Chicago/Turabian StylePollalis, Dimitrios, Dongin Kim, Gopa Kumar Gopinadhan Nair, Changsun Kang, Arjun V. Nanda, and Sun Young Lee. 2022. "Intraocular RGD-Engineered Exosomes and Active Targeting of Choroidal Neovascularization (CNV)" Cells 11, no. 16: 2573. https://doi.org/10.3390/cells11162573
APA StylePollalis, D., Kim, D., Nair, G. K. G., Kang, C., Nanda, A. V., & Lee, S. Y. (2022). Intraocular RGD-Engineered Exosomes and Active Targeting of Choroidal Neovascularization (CNV). Cells, 11(16), 2573. https://doi.org/10.3390/cells11162573





