Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress
Abstract
1. Introduction
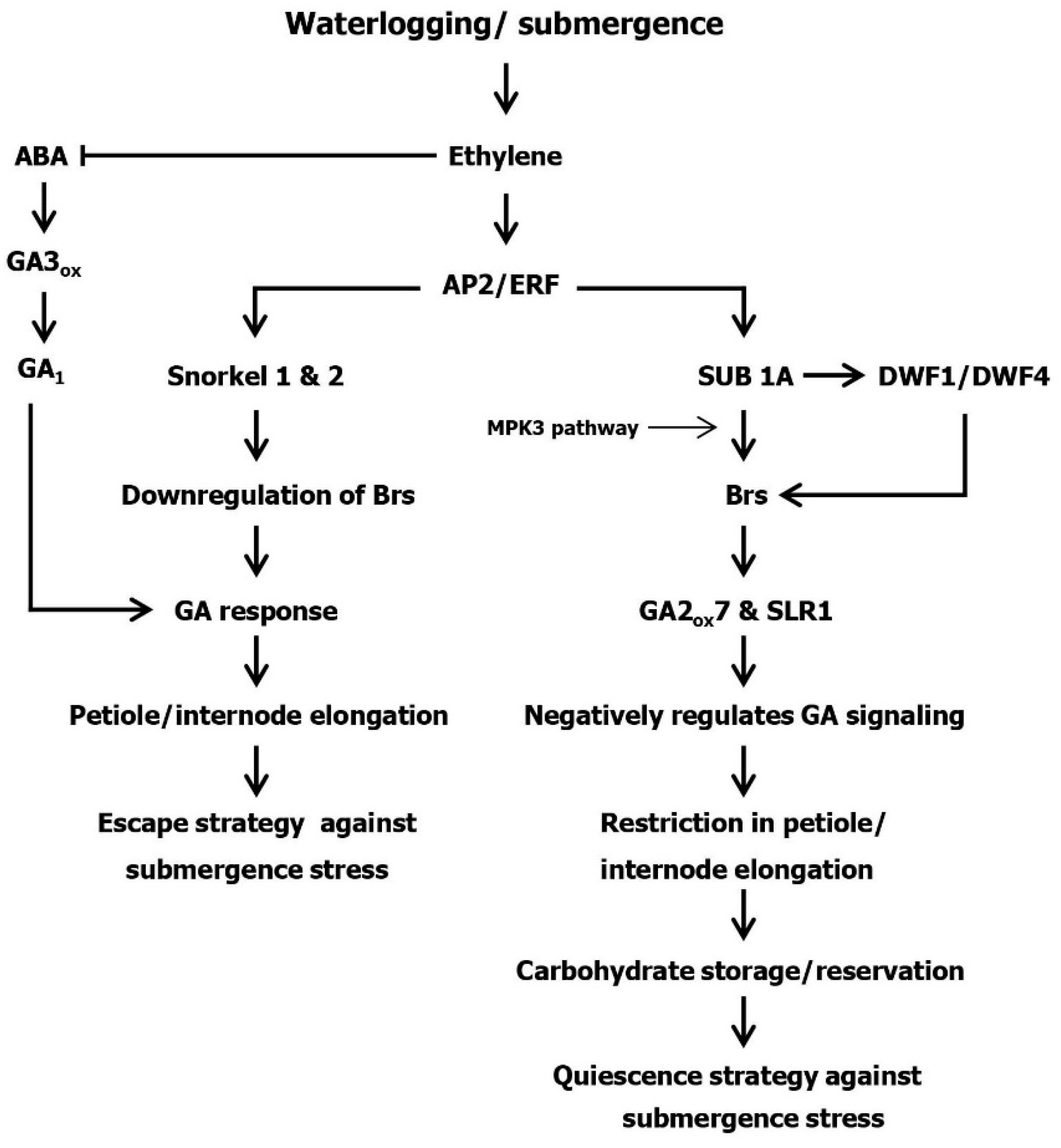
2. Ethylene, GA and ABA Interactions in Plants under Submergence Stress
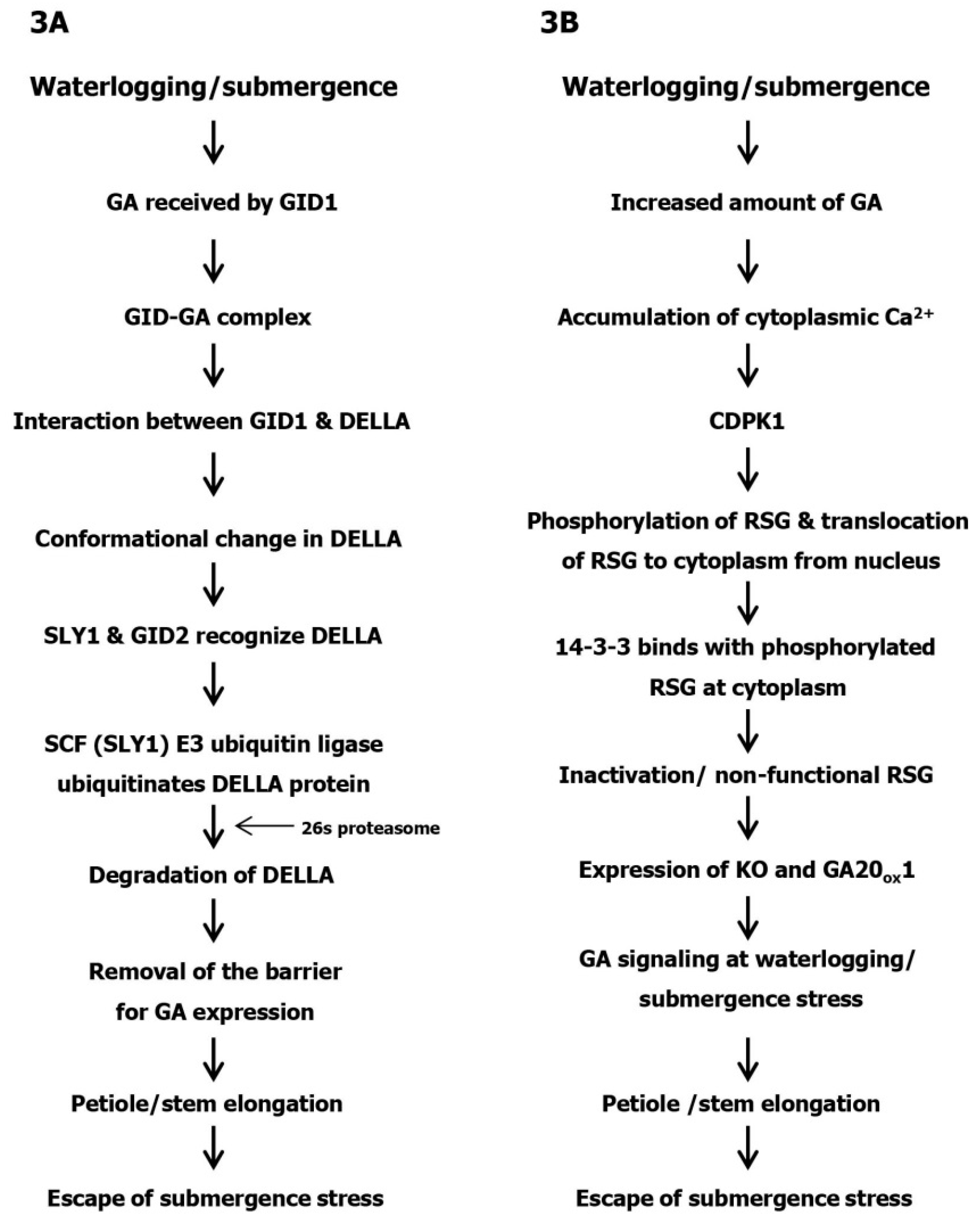
3. DELLA-Dependent GA Expression under Submergence Stress
4. DELLA Independent GA Expression under Submergence Stress
5. ABA in Plants under Waterlogging Stress
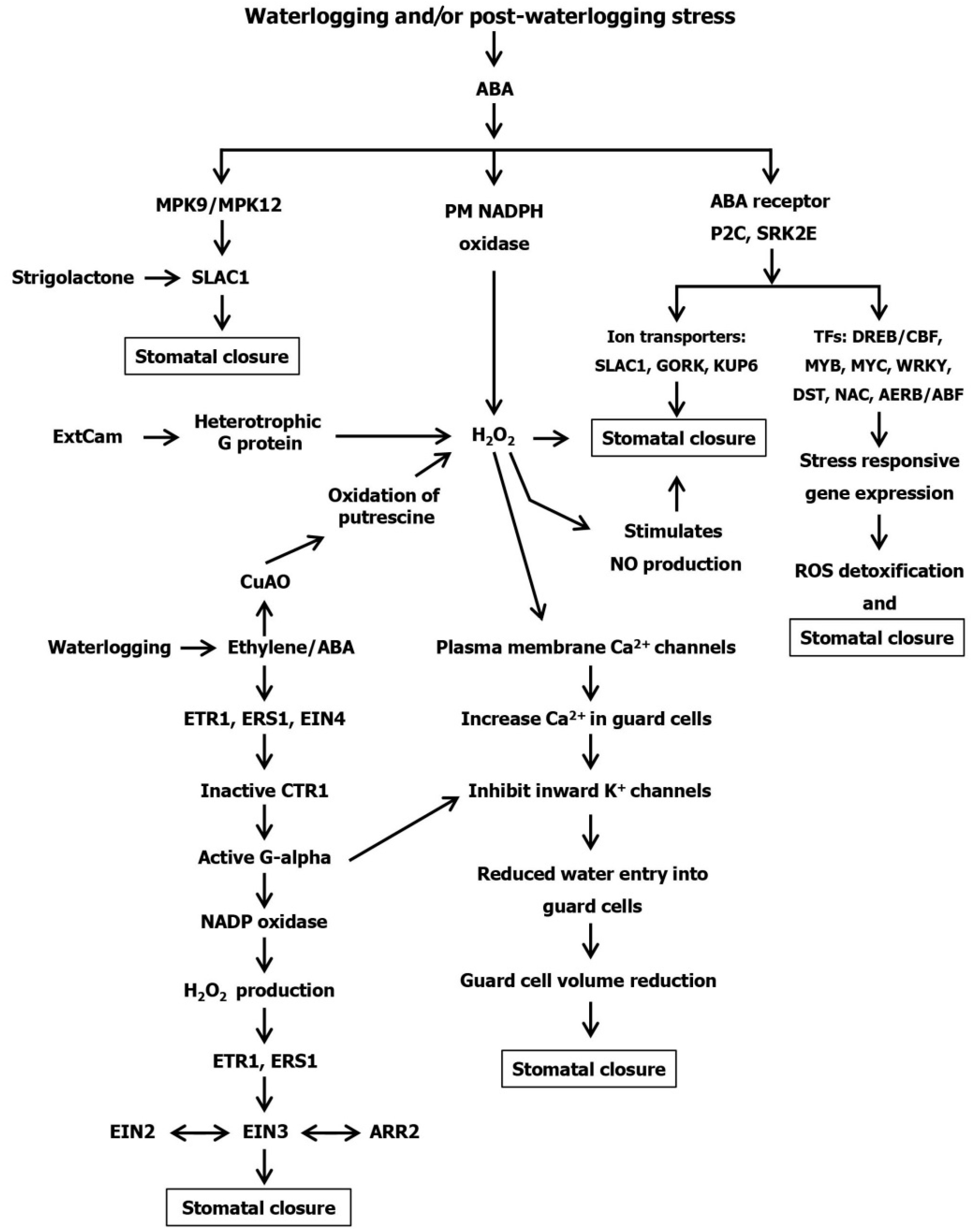
6. Stomatal Regulation at Waterlogging Stress
Hormonal Regulation in Stomatal Closing of Plants under Waterlogging Stress
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Verboven, P.; Pedersen, O.; Quang, T.H.; Nicolai, B.M.; Colmer, T.D. The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant Cell Environ. 2014, 37, 2433–2452. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant biol. 2018, 1, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging–flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef]
- Lin, I.; Wu, Y.; Chen, C.; Chen, G.; Hwang, S.; Jauh, G.; Tzen, J.T.C.; Yang, C. AtRBOH I confers submergence tolerance and is involved in auxin-mediated signaling pathways under hypoxic stress. Plant Growth Regul. 2017, 83, 277–285. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2015, 36, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Li, Z.; Lin, H.; Chen, H.; Zhang, J.; Zhu, D. Transcriptomic analysis of gibberellin- and paclobutrazol-treated rice seedlings under submergence. Int. J. Mol. Sci. 2017, 18, 2225. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef]
- Göring, H.; Koshuchowa, S.; Deckert, C. Influence of gibberellic acid on stomatal movement. Biochem. Physiol. Pflanzen 1990, 186, 367–374. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Life in the balance: A signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 2010, 13, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adach, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.C.J.; Dongen, J.T.V. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xiong, L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Seo, Y.; Walia, H.; Cao, P.; Fukao, T.; Canlas, P.E.; Amonpant, F.; Bailey-Serres, J.; Ronald, P.C. The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol. 2010, 152, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sinha, A.K. A positive feedback loop governed by SUB1A1 interaction with Mitogen-activated protein kinase3 imparts submergence tolerance in rice. Plant Cell. 2016, 28, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.M.; Gregory, A.B., Jr.; Sathnur, S.; Larive, C.K.; Bailey-Serres, J. Rice SUB1A constrains remodeling of the transcriptome and metabolome during submergence to facilitate post-submergence recovery. Plant Cell Environ. 2018, 41, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. PNAS 2008, 105, 16814–16819. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012, 160, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.J.; Folsom, J.J.; Jikamaru, Y.; Ronald, P.; Walia, H. SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol. 2013, 198, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; El-kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.; Rothstein, S.J. The APETALA-2-Like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Q.; Zhang, X.; Mattson, N.S.; Ren, H.; Cao, J.; Wang, Y.; Yao, D.; Xu, Y. Genome-wide transcriptional analysis of submerged lotus reveals cooperative regulation and gene responses. Sci. Rep. 2018, 8, 918. [Google Scholar] [CrossRef]
- Benschop, J.J.; Jackson, M.B.; Guhl, K.; Vreeburg, R.A.M.; Croker, S.J.; Peeters, A.J.; Voesenek, L.A.C.J. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 2005, 44, 756–768. [Google Scholar] [CrossRef]
- Benschop, J.J.; Bou, J.; Peeters, A.J.; Wagemaker, N.; Gühl, K.; Ward, D.; Hedden, P.; Moritz, T.; Voesenek, L.A.C.J. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin. Plant Physiol. 2006, 141, 1644–1652. [Google Scholar] [CrossRef]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8-hydroxylase in rice. Plant Cell Physiol. 2007, 48, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Perata, P. The rice SUB1A gene: Making adaptation to submergence and post-submergence possible. Plant Cell Environ. 2018, 41, 717–720. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.; Hsing, Y.C.; Kitano, H.; Yamaguchi, I.; et al. Gibberellin insensitive dwarf1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.; et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef]
- Ariizumi, T.; Lawrence, P.K.; Steber, C.M. The role of two F-box proteins, SLEEPY1 and SNEEZY, in Arabidopsis gibberellin signaling. Plant Physiol. 2011, 155, 765–775. [Google Scholar] [CrossRef]
- Okada, K.; Ito, T.; Fukazawa, J.; Takahashi, Y. Gibberellin induces an increase in cytosolic Ca2+ via a DELLA-independent signaling pathway. Plant Physiol. 2017, 175, 1536–1542. [Google Scholar] [CrossRef]
- Ito, T.; Okada, K.; Fukazawa, J.; Takahashi, Y. DELLA-dependent and -independent gibberellin signaling. Plant Signal Behav. 2018, 13, e1445933. [Google Scholar] [CrossRef]
- Ishida, S.; Yuasa, T.; Nakata, M.; Takahashi, Y. A tobacco calcium-dependent protein kinase, CDPK1, regulates the transcription factor REPRESSION OF SHOOT GROWTH in response to gibberellins. Plant Cell 2008, 20, 3273–3288. [Google Scholar] [CrossRef]
- Ishida, S.; Fukazawa, J.; Yuasa, T.; Takahashi, Y. Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator REPRESSION OF SHOOT GROWTH by gibberellins. Plant Cell 2004, 16, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Sakai, T.; Ishida, S.; Yamaguchi, I.; Kamiya, Y.; Takahashi, Y. Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 2000, 12, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Nakata, M.; Ito, T.; Yamaguchi, S.; Takahashi, Y. The transcription factor RSG regulates negative feedback of NtGA20ox1 encoding GA 20-oxidase. Plant J. 2010, 62, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nakata, M.; Fukazawa, J.; Ishida, S.; Takahashi, Y. Phosphorylation-independent binding of 14-3-3 to NtCDPK1 by a new mode. Plant Signal. Behav. 2014, 9, e977721. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishida, S.; Oe, S.; Fukazawa, J.; Takahashi, Y. Autophosphorylation affects substrate-binding affinity of tobacco Ca2+-dependent protein kinase1. Plant Physiol. 2017, 174, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Morello, L.; Giani, S.; Breviario, D. The Influence of anaerobiosis on membrane-associated rice (O. sativa L.) protein kinase activities. J. Plant Physiol. 1994, 144, 500–504. [Google Scholar] [CrossRef]
- Igarashi, D.; Ishida, S.; Fukazawa, J.; Takahashi, Y. 14-3-3 proteins regulate intracellular localization of the bZIP transcriptional activator RSG. Plant Cell 2001, 13, 2483–2497. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Garnica, M.; Fuentes, M.; Casanova, E.; Zamarreño, A.M.; Iriarte, J.C.; Etayo, D.; Ederra, I.; et al. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant Physiol. 2015, 169, 2587–2596. [Google Scholar] [CrossRef]
- Nagel, O.W.; Konings, H.; Lambers, H. Growth-rate, plant development and water relations of the ABA-deficient tomato mutant sitiens. Physiol. Plant. 1994, 92, 102–108. [Google Scholar] [CrossRef]
- Thompson, A.J.; Andrews, J.; Mullholland, B.J.; McKee, J.M.T.; Hilton, H.W.; Horridge, J.S.; Farquhar, G.D.; Smeeton, R.C.; Smillie, I.R.A.; Black, C.R.; et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007, 143, 1905–1917. [Google Scholar] [CrossRef]
- Bai, T.; Yin, R.; Li, C.; Ma, F.; Yue, Z.; Shu, H. Comparative analysis of endogenous hormones in leaves and roots of two contrasting Malus species in response to hypoxia stress. J. Plant Growth Regul. 2011, 30, 119–127. [Google Scholar] [CrossRef]
- Olivella, C.; Biel, C.; Vendrell, M.; Save´, R. Hormonal and physiological responses of Gerbera jamesonii to flooding stress. HortScience 2000, 35, 222–225. [Google Scholar]
- Nan, R.; Carman, J.G.; Salisbury, F.B. Water stress, CO2 and photoperiod influence hormone levels in wheat. J. Plant Physiol. 2002, 159, 307–312. [Google Scholar] [CrossRef]
- Cowan, I.R.; Troughton, J.H. The relative role of stomata in transpiration and assimilation. Planta 1971, 97, 325–336. [Google Scholar] [CrossRef]
- Raschke, K.; Zeevaart, J.A. Abscisic acid content, transpiration, and stomatal conductance as related to leaf age in plants of Xanthium strumarium L. Plant Physiol. 1976, 58, 169–174. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, Z.; Zhang, Z.; Yu, X.; Zhang, X.; Du, K. Molecular and physiological responses in roots of two full-sib poplars uncover mechanisms that contribute to differences in partial submergence tolerance. Sci. Rep. 2018, 8, 12829. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol. Plant 2007, 29, 535–541. [Google Scholar] [CrossRef]
- Du, H.; Chang, Y.; Huang, F.; Xiong, L. GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice. J. Integr. Plant Biol. 2015, 57, 954–968. [Google Scholar] [CrossRef]
- Blanke, M.M.; Cooke, D.T. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004, 42, 153–160. [Google Scholar] [CrossRef]
- Yetisir, H.; Caliskan, M.E.; Soylu, S.; Sakar, M. Some physiological and growth responses of watermelon [Citrullus lanatus (Thumb.) Matsum. And Nakai] grafted onto Langenaria siceraria to flooding. Environ. Exper. Bot. 2006, 58, 1–8. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Harrison-Murray, R.S.; Taylor, J.M. Rapid-flood induced stomatal closure accompanies xylem sap transportation of root-derived acetaldehyde and ethanol in Forsythia. Environ. Exp. Bot. 2008, 64, 196–205. [Google Scholar] [CrossRef]
- Ruiz-Sa´nchez, M.C.; Domingo, R.; Morales, D.; Torrecillas, A. Water relations of Fino lemon plants on two rootstocks under flooded conditions. Plant Sci. 1996, 120, 119–125. [Google Scholar] [CrossRef]
- Domingo, R.; Pe´rez-Pastor, A.; Ruiz-Sa´nchez, C. Physiological responses of apricot plants grafted on two different rootstocks to flooding conditions. J. Plant Physiol. 2002, 159, 725–732. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Dell’Amico, J.; Alarcón, J.J. The effect of short-term flooding on the sap flow, gas exchange and hydraulic conductivity of young apricot trees. Trees 2005, 19, 51–57. [Google Scholar] [CrossRef]
- Eisenach, C.; Angeli, A.D. Ion transport at the vacuole during stomatal movements. Plant Physiol. 2017, 174, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Loreti, E.; Cardarelli, F.; Novi, G.; Parlanti, S.; Pucciariello, C.; Bassolino, L.; Banti, V.; Licausi, F.; Perata, P. Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat. Plants 2015, 1, 15151. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ma, L.; He, S.; Hao, F. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response in Arabidopsis. Plant Signal. Behav. 2018, 13, e1513300. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Uzunova, A.N.; Popova, L.P. Effects of short-term soil flooding on stomata behavior and leaf gas exchange in barley plants. Biol. Plant. 2005, 49, 317–319. [Google Scholar] [CrossRef]
- Zang, J.; Zang, X. Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J. Exp. Bot. 1994, 45, 1335–1342. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Miao, L.F.; Yang, F.; Han, C.Y.; Pu, Y.J.; Ding, Y.; Zhang, L.J. Sex-specific responses to winter flooding, spring waterlogging and post-flooding recovery in Populus deltoides. Sci. Rep. 2017, 7, 2534. [Google Scholar] [CrossRef] [PubMed]
- Vantoai, T.T.; Bolles, C.S. Postanoxic injury in soybean (Glycine max) seedlings. Plant Physiol. 1991, 97, 588–592. [Google Scholar] [CrossRef]
- Kalashnikov, Y.E.; Balakhnina, T.I.; Zakrzhevsky, D.A. Effect of soil hypoxia on activation of oxygen and the system of protection from oxidative destruction in roots and leaves of Hordeum vulgare. Russ. J. Plant Physiol. 1994, 41, 583–588. [Google Scholar]
- Yan, B.; Da, Q.; Liu, X.; Huang, S.; Wang, Z. Flooding induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 1996, 179, 261–268. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Mock, H.P.; Grimm, B. Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ. 2000, 23, 135–144. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Coelho, S.M.; Taylor, A.R.; Ryan, K.P.; Sousa-Pinto, I.; Brown, M.T.; Brownlee, C. Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell 2002, 14, 2369–2381. [Google Scholar] [CrossRef]
- Fairley-Grenot, K.; Assmann, S.M. Evidence for G-protein regulation of inward K+ channel current in guard-cells of fava-bean. Plant Cell 1991, 3, 1037–1044. [Google Scholar] [CrossRef]
- Wu, W.H.; Assmann, S.M. A membrane-delimited pathway of G protein regulation of the guard-cell inward K+ channel. Proc. Natl. Acad. Sci. USA 1994, 91, 6310–6314. [Google Scholar] [CrossRef]
- Fischer, R.A. Stomatal opening: Role of potassium uptake by guard cells. Science 1968, 160, 784–785. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Song, X.G.; She, X.P.; Yue, M.; Liu, Y.E.; Wang, Y.X.; Zhu, X.; Huang, A.X. Involvement of Copper Amine Oxidase (CuAO)-Dependent Hydrogen Peroxide Synthesis in Ethylene-Induced Stomatal Closure in Vicia faba. Russ. J. Plant Physiol. 2014, 61, 390–396. [Google Scholar] [CrossRef]
- Bashar, K.K. Hormone dependent survival mechanisms of plants during post-waterlogging stress. Plant Signal Behav. 2018, 13, e1529522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, R.; Xiao, Y.; Lü, P.; Chen, J.; Wang, X. Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiol. 2004, 136, 4096–4103. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Steffens, B.; Sauter, M. Heterotrimeric G protein signaling is required for epidermal cell death in rice. Plant Physiol. 2009, 151, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.M.; Cai, H.L.; Lei, X.; Zhou, X.; Yue, M.; He, J.M. Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J. 2015, 82, 138–150. [Google Scholar] [CrossRef]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-Y.; VanToai, T.T. Abscisic acid induces anaerobiosis tolerance in corn. Plant Physiol. 1991, 97, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talón, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Abscisic acid and hypoxic induction of anoxia tolerance in roots of lettuce seedlings. J. Exp. Bot. 2000, 51, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Dennis, E.S.; Peacock, W.J. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Phyisol. 1999, 119, 57–64. [Google Scholar] [CrossRef]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.M. Sensing and signaling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Hocking, T.J.; Hillman, J.R.; Wilkins, M.B. Movement of abscisic acid in phaseolus vulgaris plants. Nat. New Biol. 1972, 235, 124–125. [Google Scholar] [CrossRef]
- Jackson, M.B.; Hall, K.C. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant Cell Environ. 1987, 10, 121–130. [Google Scholar] [CrossRef]
- Rodriguez-Gamir, J.; Ancillo, G.; Gonzalez-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signaling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. and Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashar, K.K.; Tareq, M.Z.; Amin, M.R.; Honi, U.; Tahjib-Ul-Arif, M.; Sadat, M.A.; Hossen, Q.M.M. Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress. Agronomy 2019, 9, 43. https://doi.org/10.3390/agronomy9020043
Bashar KK, Tareq MZ, Amin MR, Honi U, Tahjib-Ul-Arif M, Sadat MA, Hossen QMM. Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress. Agronomy. 2019; 9(2):43. https://doi.org/10.3390/agronomy9020043
Chicago/Turabian StyleBashar, Kazi Khayrul, Md. Zablul Tareq, Md. Ruhul Amin, Ummay Honi, Md. Tahjib-Ul-Arif, Md. Abu Sadat, and Quazi Md. Mosaddeque Hossen. 2019. "Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress" Agronomy 9, no. 2: 43. https://doi.org/10.3390/agronomy9020043
APA StyleBashar, K. K., Tareq, M. Z., Amin, M. R., Honi, U., Tahjib-Ul-Arif, M., Sadat, M. A., & Hossen, Q. M. M. (2019). Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress. Agronomy, 9(2), 43. https://doi.org/10.3390/agronomy9020043





