Abstract
Drought stress is one of the major agronomic concerns that lead towards a sharp decline in sugarcane yield. An urgent demand to overcome drought is critical to ensure sugarcane production. Mutation breeding is one of the promising tools available to produce stress-resistant plants, with the induction of new alleles due to point mutation within existing sugarcane germplasm. The current study was directed to chemically mutagenize the calli of two sugarcane cultivars (ROC22 and FN39) via 0.1% EMS, with focus on inducing mutations in their genome. The 1644 regenerated plants of ROC22 and 1398 of FN39 were exposed to 28% PEG-6000 stimulated osmotic stress. Eighteen plants of ROC22 and 2 plants of FN39, that survived after in vitro osmotic stress treatment, were then subjected to preliminary greenhouse pot trials to confirm drought tolerance by analyzing them using various physiological parameters, including photosystem II (PSII) photochemical efficiency (Fv/Fm), leaf chlorophyll content, and photosynthetic rate. The genetic diversity among drought-resistant mutant lines was further assessed by 15 pairs of simple sequence repeat (SSR) markers amplification and CEL (Celery) I endonuclease digestion, to investigate the mutated sites. Mutant lines of ROC22 (i.e., MR22-15 and MR22-20) were found to be promising for future drought resistance breeding, due to better physiological adaptation under drought stress.
1. Introduction
Globally, sugarcane (Saccharum officinarum L.) is the most promising crop for renewable energy [1] and sugar. It is the main source of biomass for bioelectricity and second-generation bioethanol, as well [2]. Globally, sugarcane contributes to 75% of the total sugar production, with 90% occurring in China [3]. Exposure of sugarcane plants, to suboptimal or limiting environmental conditions during the growing season, not only causes a drastic reduction in cane yield, but also sugar content [4]. Among abiotic stresses, drought is a major factor which negatively affects the tillering and main growth phases which, in turn, causes a severe reduction in annual cane yield and sucrose content [5,6]. Sugarcane is commonly cultivated in hot plain areas, so growers of sugarcane cannot avoid annual drought seasons, due to the cane maturation period extending across 12 to 18 months [1]. Drought stress triggered a variety of morpho-physiological responses, depending upon plant growth stage and environmental conditions. For instance, reductions in leaf gaseous exchange, total biomass, leaf elongation, and stomatal conductance, are the major upshots of drought stress [7]. Drought limits the photosynthetic performance of sugarcane by altering the biochemical and photochemical reactions, i.e., physiological responses [8,9]. Sugarcane plants also reduces leaf area (LA) in order to prevent water loss, as well as further dehydration [10]. In the future, drought is expected to increase, due to climate change in most parts of the world [11,12]. Hence, it is an urgent need, at this time, to breed cultivars with enhanced drought tolerance and high water-use efficiency, which can be achieved by employing both conventional plant breeding and genetic engineering [13]. Several attempts have been made since past decades, in order to improve sugarcane germplasm by adopting these breeding techniques. However, conventional plant breeding techniques are hampered, due to extreme complexity of sugarcane genome, narrow genetic base, and its high ploidy level, as well as lack of fertile seed production. Even though genetic engineering has the potential to improve desired traits via transgenic plant development approach, genetically modified organisms (GMOs) are usually subjected to legal restriction and unfavorable public acceptance. On the other hand, mutation breeding is a promising tool to produce stress-tolerant plants by the inducing genetic diversity within the existing sugarcane germplasm [14]. In this regard, chemical mutagenesis provides an easy and cost-effective way to produce a high density of novel nucleotide diversity within plants having a complex genome. Ethyl methanesulfonate (EMS) is widely used as a chemical mutagen, in order to induce hundreds to thousands of heritable single base mutations in a single plant line [15]. After mutagenesis, EMS-mutated plant populations can be screened against environmental stresses on a phenotypic and molecular basis [16]. The best way to assess genetic diversity through mutagenesis is to use molecular markers, among them, simple sequence repeats (SSRs) are important [17,18,19], due to their high polymorphism, co-dominant inheritance, extensive genome coverage, high reproducibility, and ease of use [20,21,22]. Utilization of TILLING technology is a new high-throughput and cost-effective reverse genetics approach for the detection of chemically induced point mutation in a target region. In addition, it produces high-frequency point mutation, and effectively combines with high-throughput detection assays and PCR screening technologies. The key enzyme of TILLING technology “CEL-I” (isolated from celery) is the first eukaryotic nuclease which is known to cleave DNA heteroduplexes with high specificity at DNA distortions and base mismatch sites [23]. The enzyme requires Mg2+ and Zn2+ for activity at neutral pH with significant purification to achieve apparent homogeneity.
Since a few decades ago, ROC22 has been a widely grown commercial cultivar in China with good quality traits, such as drought resistance, high cane yield, and sucrose content, amongst others. However, the fine characters of the cultivar are degenerating rapidly after long-term cultivation, including its drought resistance [16]. There is no doubt that FN39 is a sugarcane variety with high yield and high sucrose content, but with poor drought tolerance. Hence, the current study was directed to chemically mutagenize the calli of two sugarcane cultivars (ROC22 and FN39) with the aim of generating genetic diversity in their genome. Mutant calli were screened, in vitro, by PEG-6000-stimulated drought stress. Drought resistant mutant lines surviving the in vitro osmotic stress were then subjected to preliminary greenhouse pot trials, in order to confirm drought tolerance, by analyzing them using various physiological, molecular, and enzymatic tools.
2. Materials and Methods
2.1. Plant Source and In Vitro Callus Regeneration
Two sugarcane cultivars (FN39 and ROC22) were utilized to regenerate their calli. Sugarcane tips with sheath were rinsed with 95% ethanol, followed by aseptic isolation of basal part of the inner leaf roll from the explant, which were then sliced into approximately 3–5 mm thick discs and cultured on Murashige and Skoog Medium (MS) supplemented with 3 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 30 g/L sucrose, and 8.5 g/L agar at pH 5.8 [24]. After 15 to 30 days of callus induction, the proliferated callus was aseptically transferred to differentiation media containing 2.5 mg/L 6-benzyladenine (BA), 0.5 mg/L kinetin (KT), 0.2 mg/L naphthaleneacetic acid (NAA), 30 g/L sucrose, and 8.5 g/L agar solution for embryo germination and development. Plantlets were transferred to rooting medium. After root formation, surviving mutants were shifted to pots in a greenhouse, for hardening and further screening for drought tolerance at morpho-physiological and molecular levels.
2.2. Measurement of Optimal EMS Concentration and Mutagenic Treatment
EMS solution was prepared as 0.1 M in phosphate buffer at pH 7.0. One hundred embryogenic calli of FN39 and ROC22 were harvested and treated with different concentrations of EMS (0%, 0.05%, 0.1%, 0.2%, and 0.5%) at different time intervals (5, 10, 15, 20, and 25 h) [20]. Then, callus tissues were cut at a uniform diameter (3 mm), immersed into EMS phosphate buffer, and further placed on a shaker at 20 °C, 130 rpm/min. After incubation, calli were rinsed 4–5 times with sterile water to remove residual EMS, followed by air-drying on sterile filter paper for 5 min in a laminar flow cabinet. Treated calli were subsequently transferred to induction medium and incubated for 4 weeks. Data, regarding size and numbers of callus clumps where embryos germinate, were collected. Embryogenic calli were then shifted to differentiation media for regeneration. After 6 weeks of regeneration, plantlets were moved to rooting media for root formation. The number of phenotypically off-type (green and white) plantlets, as well as shoot length, were recorded.
2.3. Measurement of Optimal In Vitro PEG-6000.Stimulated Osmotic Stress
PEG-6000 solid medium was prepared according to the method described by van der Weele et al. [25]. In order to lower water potential, rooting medium was prepared by the addition of different concentrations of PEG-6000 formulated as 0%, 8%, 16%, 24%, and 32%, for 15 days. All PEG-6000 solutions were sterilized using a 0.22 µm microporous membrane. PEG-diffused agar medium (20 mL) was poured into sterile bottles and kept for 18 h, allowing PEG-6000 to fully penetrate into solid medium. After 18 h, the agar medium was further used for experiments, after measuring the water potential, to determine optimal osmotic stress regimes for screening mutant plantlets. These calli were also cultured on nutrient agar medium without PEG-6000 as a well-watered control medium. Growth and development parameters of calli and plantlets were recorded after every 2 weeks.
2.4. Physiological Index Detection
After in vitro mutagenesis and screening against osmotic stress, surviving mutant clones (ROC22, FN39), along with wild-type clones, were planted in pots under greenhouse conditions, with two replicates. Initially, pots were irrigated with the same quantity of water. At this stage, there were no significant differences among wild-type and treated plants of both cultivars. Four weeks after plantation, plants were subjected to drought stress i.e., irrigated only once after every 10 days. Physiological data was collected from mutated plants that outlived the checks (ROC22 and FN39) or were recovered when re-watered after the period of drought stress, and were considered as potentially drought-tolerant mutants. Physiological responses of wild-type and mutant clones were evaluated via measuring chlorophyll a, leaf chlorophyll content, and photosynthetic rate.
2.4.1. Chlorophyll a
Chlorophyll a fluorescence characteristics were estimated on upper leaves (Atago Pocket Refractometer PAL-1, Tokyo, Japan). On each assessment date, before fluorescence measurements, at least four leaves per pot were dark-adapted for 30 min by using leaf clips (FL-DC, Opti-Science, Tyngsboro, MA, USA). The Fv/Fm ratio was estimated according to Maxwell and Johnson [26].
2.4.2. Leaf Chlorophyll Content
Leaf chlorophyll content was determined using a chlorophyll meter (SPAD-502). This index has been considered as a reliable and non-destructive tool for rapid screening against drought tolerance in sugarcane [27]. The average of five measurements was recorded from the middle part of upper leaves of different plants in every pot.
2.4.3. Photosynthetic Rate
The photosynthetic rate was measured with the help of a Portable Photosynthesis System (LI-6400/XT, Gene ecotek, Beijing, China). Measurements were recorded from the middle part of the first four leaves of every replicate of each mutant and wild-type clone. The photosynthetic rate was calculated according to LI-6400/XT using the following equation;
A = F (CO2 r − CO2 s (1000 − H2O r)/(1000 − H2O s))/100S
An understanding of data recorded for these physiological indexes could help to explore the response of mutant clones to drought stress, and their adaption to the current breeding program.
2.5. DNA Isolation
Fresh young leaves were collected from wild-type and mutant lines that survived under greenhouse screening against drought, and ground to a fine powder in liquid nitrogen. Genomic DNA was isolated from check and treated sugarcane leaves using Kit DP3111 (Bioteke Corporation; Beijing, China) according to manufacturer’s instruction. The quality of DNA was verified by electrophoresis using a 1.5% agarose gel. Genomic DNA was also quantified spectrophotometrically by measuring its absorbance at 260 nm. DNA was then diluted to make a working concentration for SSR marker analysis.
2.6. SSR Marker PCR Amplification and CELL-1 Enzyme Digestion
Fifteen pairs of SSR primers were used for the amplification of polymorphisms using polymerase chain reaction (PCR). The polymerization of PCR product of different pairs of SSR primers was detected using 9% polyacrylamide gel electrophoresis (PAGE), and visualized in a gel documentation system. Detailed information about the SSR primers used for this purpose is given in Table 1.

Table 1.
Information about simple sequence repeat (SSR) primers used for mutant analysis.
Pairs of primers that amplified the target genes were subjected to PCR amplification, again, using Celery (CEL-I) enzyme, in order to detect the presence of point mutations in the heteroduplexed DNA. Heteroduplexed DNA was subjected to an incubation period at 45 °C for 15 min with purified CEL-I enzyme. CEL-I digestion product was then analyzed by PAGE, and also sent to the company Sangon Biotechnology Co., Ltd. (Shanghai, China) for capillary electrophoresis to identify mutated sites in the genome induced by EMS treatment in mutants that withstand drought stress and to verify if they are real mutants.
3. Results
3.1. Effect of Various Concentrations of EMS on Sugarcane Calli Differentiation
Determination of the right dose of EMS and calli processing time is critical for mutagenic treatment. Hence, based on the median lethal dose, the optimal EMS dose concentration and time were determined for further processing of sugarcane calli for mutagenic treatment. For this purpose, calli of ROC22 and FN39 were subjected to different EMS concentrations and time intervals in differentiation media. Influence of different EMS treatments on the sugarcane calli revealed that the relative differentiation rate decreased with the increase in concentration and processing time of EMS exposure. Moreover, the ability of ROC22 to withstand EMS hazard was found to be stronger than for FN39, as shown in Table 2. Based on the median lethal dose, the selection criteria determined for mutagenic treatment of ROC22 calli was 0.1% EMS for 17 h while, for FN39 calli, it was 0.1% EMS for 14 h. Additionally, the effect of 0.1% EMS treatments on the relative differentiation rate of ROC22 and FN39 has also been shown in Figure 1 and Figure 2, respectively.

Table 2.
Effects of EMS treatments on the relative differentiation rate of the sugarcane calli.
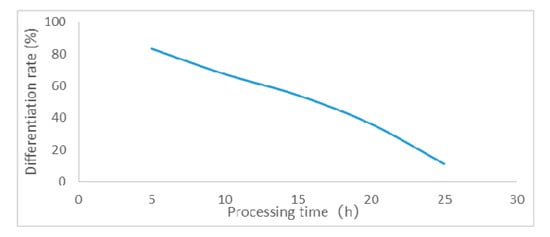
Figure 1.
Effect of 0.1% EMS treatment on the relative differentiation rate of ROC22 calli.
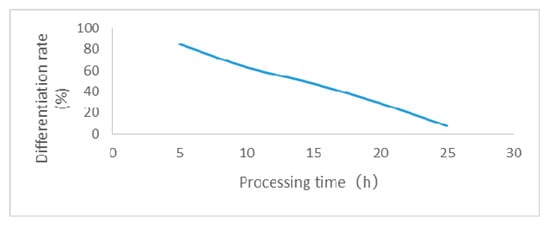
Figure 2.
Effect of 0.1% EMS treatment on the relative differentiation rate of FN39 calli.
3.2. Effect of Various Concentrations of PEG-6000-Stimulated Stress on Seedling Growth
Treatment of calli regenerated plantlets at different concentrations of PEG-6000, was conducted at 0%, 8%, 16%, 24%, and 32%. Few plantlets could survive at 24% PEG-6000 media, whereas no plantlets survived at 32% PEG-6000 concentration (Figure 3 and Figure 4). More precise tests of PEG-6000 concentrations at 24%, 26%, 28%, 30%, and 32% showed no root development of plantlets at 28% PEG-6000-stimulated stress. Hence, 28% PEG-6000 was selected as the minimum lethal concentration for further selection and screening of drought-resistant mutants. The 1644 EMS-regenerated plants of ROC22 and 1398 of FN39 were subjected to drought stress screening with 28% PEG provided in the rooting medium. Eighteen EMS-regenerated plants of ROC22 and two plants of FN39 survived after in vitro osmotic stress treatment.

Figure 3.
Effect of PEG on the growth of calli regenerated plantlets of ROC22 (20 days).

Figure 4.
Effect of PEG on the growth of calli regenerated plantlets of FN39 (20 days).
3.3. Physiological Analysis
Eighteen plants of ROC22 and two plants of FN39, obtained after in vitro screening, were planted in the greenhouse along with wild-type plants, and physiologically analyzed to determine their efficiency to withstand water stress. The collected data for every physiological parameter was subjected to an analysis of variance (ANOVA) using least significance difference test (LSD; p < 0.05, p < 0.01). The ANOVA revealed no significant differences between wild-type and treated plants of both cultivars under well-watered conditions. When data after every stress treatment were analyzed, there was still no significant difference between wild-type (FN39) and treated plants of FN39 (FN39-1, FN39-2) for PSII photochemical efficiency (Fv/Fm), photosynthetic rate, as well as chlorophyll content before and after treatment, as shown in Table 3. While, on average, different mutant genotypes of ROC22 show a different response to Fv/Fm, as well as difference in photosynthetic rates. For PSII photochemical efficiency, there was a highly significant difference between wild-type (ROC22) and treated plants of ROC22 (MR22-15 and MR22-20) at p < 0.05 and p < 0.01 (Table 4). Additionally, these two mutants also differ significantly from wild-type for chlorophyll content. Chlorophyll content was found to decrease with the increase in water stress. However, these two mutants manage to survive significantly under water stress, as compared to wild-type lines of ROC22. For the photosynthetic rate, 6 mutants from ROC22 (namely, MR22-2, MR22-7, MR22-9, MR22-11, MR22-15, MR22-20) were significantly different from parent clone at p < 0.05, while MR22-2 and MR22-9 show no significant difference at p < 0.01, as shown in Table 5.

Table 3.
Significance analysis of field mutagenic plants of ROC22, FN39.

Table 4.
Chlorophyll a fluorescence (Fv/Fm) for ROC22 and mutagenic plants of ROC22.

Table 5.
Photosynthetic rate for ROC22 and mutagenic plants of ROC22.
3.4. SSR Analysis
DNA samples of 22 check and mutagenic plants that outlived after in vitro screening were amplified through 15 pairs of SSR primers. The polymerization of PCR products of different pairs of primers was detected through PAGE gel. Out of 15 pairs of primers, PAGE results only for primer pair 6, namely, sep-23, show amplification for polymorphic genes of MR22-15 and MR22-20 compared with the wild-type. Hence, the PCR product of MR22-15 and MR22-20 was further digested with CEL-I enzyme, labeled with fluorescence dye. After CEL-I incubation, PCR reaction was repeated using the sep-23 pair of primers for respective DNA samples. CEL-I digestion product was then analyzed by PAGE, and finally sent to the company (Sangon Biotech Co., Ltd., Shanghai, China) for CE (capillary electrophoresis) analysis. Results of capillary electrophoresis (CE) of primer sep-23 for ROC22-15 and ROC22-20 DNA (P = control, N = treated) has been shown in Table 6. Additionally, peak map results of capillary electrophoresis for control and treated DNA samples of MR22-15 and MR22-20 have been shown in Figure 5, Figure 6, Figure 7 and Figure 8. Mutant MR22-15 showed polymorphic bands that were significantly different from check, and marked with arrows in Figure 6. The band B disappeared when treated by CEL-I, and three new bands, C, D, and E, appeared. The bands B and C disappeared in Figure 8 after treatment with CEL-I, and two new bands D and E were raised in comparison with those in Figure 7. CEL-I is a key enzyme to identify and digest the sites mutated by EMS. Hence, the two plants, MR22-15 and MR22-20, were real mutants which had good trait resistant to osmotic stress.

Table 6.
Results of capillary electrophoresis (CE) of primer Sep-23 for MR22-15 and MR22-20 DNA (P = control, N = treated).
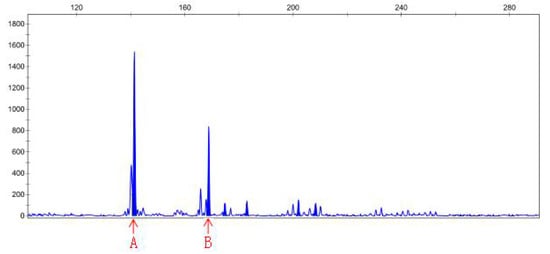
Figure 5.
Peak map result of CE for primer Sep-23, MR22-15 DNA (control).
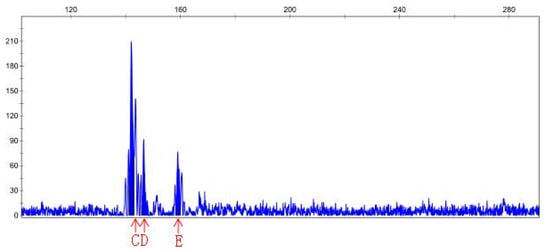
Figure 6.
Peak map result of CE for primer Sep-23, MR22-15 DNA (treated). Note: peak B and C disappeared, and new peaks D and E appeared.
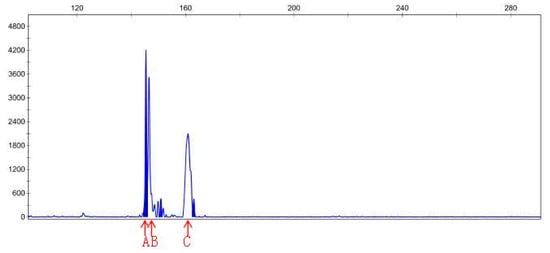
Figure 7.
Peak map result of CE for primer Sep-23, MR22-20 DNA (control).
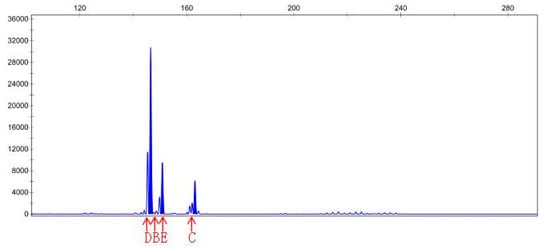
Figure 8.
Peak map result of CE for primer Sep-23, MR22-20 DNA (treated).
4. Discussion
In previous studies, EMS-induced mutagenesis has been shown to induce non-lethal point mutations in a number of plant species, creating genetic diversity in their genome [14,28,29]. Utilization of EMS mutagenesis, followed by in vitro, in vivo, and molecular screening of drought-resistant mutant clones, improved the selection efficiency, but has not gained much attention so far. Various sugarcane researchers have induced mutations in order to enhance tolerance against disease, drought, and yield traits, but they did not characterize mutant clones molecularly for the further selection of potential mutants [14,28,30,31,32,33]. The “EMS-induced sugarcane mutants” evaluated in previous studies were “putative” sugarcane mutants [14,28,34]. No scientific evidence was provided to prove that the mentioned “sugarcane mutants” were true mutants. It is well known that true mutants are heritable, so the mutagenesis sites are certainly in the genome. Therefore, in the current study, we screened drought-tolerant mutant clones in vitro, and then characterized them molecularly rather than only physiologically. The present study is the first to use the SSR-PCR method combined with CEL-I endonuclease digestion analysis to identify putative sugarcane mutant clones, which provides a new molecular method for identifying true sugarcane mutations induced by EMS at the DNA level. Of course, it is still somewhat difficult to find mutated functional genes in the genome of sugarcane mutants, although the new molecular approach has successfully identified true sugarcane mutants.
We tried to isolate the bands digested by CEL-I endonuclease to identify gene names, since the sizes were known. However, the bands could not be sequenced successfully because of technical difficulties. It is known that several bands are produced, generally, in one PCR using a single pair of SSR primers. Hence, sequences of the 5′ end and 3′ end of some bands were unknown, and the bands could not be identified for their sequence and functions. For treatment of EMS to sugarcane, the optimum mutagenic treatment reported for the production of salt-, drought-, and herbicide-tolerant plants from calli, was 40 mM EMS for 2.5 h, 20 mM EMS, and 16 mM EMS for 4 h, respectively [14,31,35]. Due to the difference in sensitivity and mutagenic treatment, the frequency of mutagenesis may be different for other sugarcane cultivars. For the current experiment, the highest variable concentration of 0.1% EMS dose was considered best for the mutagenic treatment and sugarcane calli differentiation, for a period of 17 and 14 h, for ROC22 and FN39, respectively, when subjected to 28% PEG-stimulated osmotic stress for in vitro screening. The eighteen and two mutant clones from ROC22 and FN39, respectively, that survived during in vitro osmotic stress, were evaluated for various physiological parameters.
Generally, drought stress is concerned with the timing, duration, and severity of drought period. Sugarcane, being a C4 plant, during its growth period mostly experienced physiological changes, such as changes in photosystem II efficiency, photosynthetic rate, and chlorophyll content under drought stress conditions [36,37]. Extension of the drought period over weeks helps to identify mutant clones with enhanced tolerance traits, due to physiological changes that occur in plants in order to accommodate the stress. In previous findings, a negative correlation has been found between drought and drought-related physiological parameters (i.e., efficiency of photosynthetic system II, photosynthetic rate, and chlorophyll content) depending upon various plant developmental stages and the duration of drought stress by various researchers [27,34,38,39]. In our study, significant reductions in all these parameters have also been noted, with the increase in drought stress. However, the cultivar ROC22 was found to be more tolerant to osmotic stress as compared to FN39. Eighteen mutant clones from ROC22 that survived during in vitro osmotic stress also managed to survive under preliminary greenhouse trials with improved physiological traits. However, we found that the two elite sugarcane mutants, MR22-15 and MR22-20, proved during molecular analysis that they were actual potential mutants that could be a source of drought-resistant sugarcane breeding material for the future studies. We utilized SSR markers for molecular characterization of mutant clones because of their good discriminatory power for analysis of genetic diversity among mutant clones. Fifteen pairs of SSR primers, developed on sugarcane expressed sequence tags (EST), used in this study, were provided by the Key Laboratory of Sugarcane Biology and Genetic Breeding (Fujian), Fuzhou, China. These primers could be used in fingerprint analysis and had a high degree of polymorphism. Since the EMS-induced mutation sites are uncertain, we tried to use SSR primers with a high degree of polymorphism to verify them so that, under the action of CEL-I enzyme, it is easier to detect potential mutated lines, such as the MR22-15 and MR22-20 lines identified in our study.
5. Conclusions
To sum up, an in-depth understanding of chemically induced mutagenesis is needed to control the direction and nature of mutations, to further enhance our knowledge, the research area regarding high-throughput mutation screening technology, and to improve a mutant’s efficiency under various circumstances. Screening for drought resistance is an effective method of selection, but whether the drought resistance can be maintained in later generations of mutants still needs further studies.
Author Contributions
Conceptualization, C.P. and F.K.; Methodology, F.K.; Software, X.N.; Validation, C.P., X.N. and M.T.; Formal Analysis, X.N.; Investigation, C.P.; Resources, C.P.; Data Curation, X.N.; Writing—Original Draft Preparation, F.K.; Writing—Review & Editing, M.T.; Visualization, C.P.; Supervision, C.P.; Project Administration, C.P.; Funding Acquisition, C.P.
Acknowledgments
This work was supported by the National Natural Science Foundation, P. R. China (KF2015080, KF2015118, and KFA17263A).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gentile, A.; Ferreira, T.H.; Mattos, R.S.; Dias, L.I.; Hoshino, A.A.; Carneiro, M.S.; Souza, G.M.; Calsa, T.; Nogueira, R.M.; Endres, L.; et al. Effects of drought on the microtranscriptome of field-grown sugarcane plants. Planta 2013, 237, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane Water Stress Tolerance Mechanisms and Its Implications on Developing Biotechnology Solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.R.; Yang, L.-T.T. Sugarcane agriculture and sugar industry in China. Sugar Tech 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Guo, J.; Ling, H.; Wu, Q.; Xu, L.; Que, Y. The choice of reference genes for assessing gene expression in sugarcane under salinity and drought stresses. Sci. Rep. 2014, 4, 7042. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.R.; Andrade Dias Brito da Cunha, B.; Martins, P.K.; Martins, M.T.B.; Alekcevetch, J.C.; Chalfun-Júnior, A.Ô.; Andrade, A.C.; Ribeiro, A.P.; Qin, F.; Mizoi, J.; et al. Induced over-expression of AtDREB2A CA improves drought tolerance in sugarcane. Plant Sci. 2014, 221–222, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P. Effect of different levels of drought during the formative phase on growth parameters and its relationship with dry matter accumulation in sugarcane. J. Agron. Crop Sci. 2000, 185, 83–89. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; MacHado, R.S.; MacHado, E.C.; MacHado, D.F.S.P.; Magalhães Filho, J.R.; Landell, M.G.A. Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp. Agric. 2013, 49, 212–224. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Ribeiro, R.V.; Silveira, J.A.G.; Machado, E.C.; Martins, M.O.; Lagôa, A.M.M.A. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013, 73, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of Drought Stress on Anatomical Structure and Chloroplast Ultrastructure in Leaves of Sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L.; van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, M.; Islam, W.; Khalil, F.; Ziqin, P.; Caifang, Z.; Arafat, Y.; Hui, L.; Rizwan, M.; Ahmad, K.; Waheed, S.; et al. Biochar: An efficient way to manage low water availability in plants. Appl. Ecol. Environ. Res. 2018, 16, 2565–2583. [Google Scholar] [CrossRef]
- Khalil, F.; Rauf, S.; Monneveux, P.; Anwar, S.; Iqbal, Z. Genetic analysis of proline concentration under osmotic stress in sunflower (Helianthus annuus L.). Breed. Sci. 2016, 66, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Al-Khayri, J.M.; Zaharieva, M.; Monneveux, P.; Khalil, F. Breeding strategies to enhance drought tolerance in crops. Adv. Plant Breed. Strateg. Agron. Abiot. Biot. Stress Traits 2016, 2, 397–445. [Google Scholar] [CrossRef]
- Masoabi, M.; Lloyd, J.; Kossmann, J.; van der Vyver, C. Ethyl Methanesulfonate Mutagenesis and In Vitro Polyethylene Glycol Selection for Drought Tolerance in Sugarcane (Saccharum spp.). Sugar Tech 2018, 20, 50–59. [Google Scholar] [CrossRef]
- Jankowicz-Cieslak, J.; Till, B.J. Chemical Mutagenesis of Seed and Vegetatively Propagated Plants Using EMS. Curr. Protoc. Plant Biol. 2016, 1, 617–635. [Google Scholar]
- WEI, C.L.; Chang-lian, W.E.I. Analysis and countermeasures of the degradation status of sugarcane variety ROC22 in Guangxi. J. South. Agric. 2012, 43, 2113–2117. [Google Scholar]
- Agarwal, P.; Jaiswal, V.; Kumar, S.; Balyan, H.S.; Gupta, P.K. Chromosome mapping of four novel mutants in bread wheat (Triticum aestivum L.). Acta Physiol. Plant. 2015, 37, 66. [Google Scholar] [CrossRef]
- Rastogi, J.; Siddhant, P.B.; Sharma, B.L. Somaclonal Variation: A new dimension for sugarcane improvement. GERF Bull. Biosci. 2015, 6, 5–10. [Google Scholar]
- Mallick, M.; Bharadwaj, C.; Srivastav, M.; Sharma, N.; Awasthi, O.P. Molecular characterization of Kinnow mandarin clones and mutants using cross genera SSR markers. Indian J. Biotechnol. 2017, 16, 244–249. [Google Scholar]
- Nybom, H.; Weising, K.; Rotter, B. DNA fingerprinting in botany: Past, present, future. Investig. Genet. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, S.; Kumar, P.; Singh, R.K.; Verma, K.S.; Bhatt, R.; Sharma, M.; Kachhwaha, S.; Kothari, S.L. Assessment of Functional EST-SSR Markers (Sugarcane) in Cross-Species Transferability, Genetic Diversity among Poaceae Plants, and Bulk Segregation Analysis. Genet. Res. Int. 2016, 2016, 7052323. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Schwartz, B.M.; Kim, C.; Adhikari, J.; Rainville, L.K.; Auckland, S.A.; Paterson, A.H. Cross-taxon application of sugarcane EST-SSR to genetic diversity analysis of bermudagrass (Cynodon spp.). Genet. Resour. Crop Evol. 2017, 64, 2059–2070. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Chmielewska, B.; Gajewska, P.; Szarejko, I. Mutation detection by analysis of DNA heteroduplexes in TILLING populations of diploid species. In Biotechnologies for Plant Mutation Breeding: Protocols; Jankowicz-Cieslak, J., Tai, T., Kumlehn, J., Till, B., Eds.; Springer: Cham, Switzerland, 2016; pp. 281–303. ISBN 9783319450216. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Van der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.A.; Jifon, J.L.; Da Silva, J.A.G.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef]
- Jamil, S.; Shahzad, R.; Talha, G.M.; Sakhawat, G.; Sajid-Ur-Rahman; Sultana, R.; Iqbal, M.Z. Optimization of Protocols for in Vitro Regeneration of Sugarcane (Saccharum officinarum). Int. J. Agron. 2017, 2017, 2089381. [Google Scholar] [CrossRef]
- Serrat, X.; Esteban, R.; Guibourt, N.; Moysset, L.; Nogués, S.; Lalanne, E. EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 2014, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Naz, S.; Alam, S.S.; Iqbal, J. In vitro induced mutation for screening of red rot (Colletotrichum falcatum) resistance in sugarcane (Saccharum officinarum). Pak. J. Bot. 2007, 39, 1979–1994. [Google Scholar]
- Kenganal, M.; Hanchinal, R.R.; Nadaf, H.L. Ethyl methanesulfonate (EMS) induced mutation and selection for salt tolerance in sugarcane in vitro. Indian J. Plant Physiol. 2008, 13, 405–410. [Google Scholar]
- Nikam, A.A.; Devarumath, R.M.; Shitole, M.G.; Ghole, V.S.; Tawar, P.N.; Suprasanna, P. Gamma radiation, in vitro selection for salt (NaCl) tolerance, and characterization of mutants in sugarcane (Saccharum officinarum L.). In Vitro Cell. Dev. Biol. Plant 2014, 50, 766–776. [Google Scholar] [CrossRef]
- Oloriz, M.I.; Gil, V.; Rojas, L.; Veitía, N.; Höfte, M.; Jiménez, E. Selection and characterisation of sugarcane mutants with improved resistance to brown rust obtained by induced mutation. Crop Pasture Sci. 2012, 62, 1037–1044. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.C.; Ramgareeb, S.; Rutherford, R.S.; Snyman, S.J.; Watt, M.P. An in vitro mutagenesis protocol for the production of sugarcane tolerant to the herbicide imazapyr. In Vitro Cell. Dev. Biol. Plant 2012, 48, 417–427. [Google Scholar] [CrossRef]
- De Almeida Silva, M.; Jifon, J.L.; Sharma, V.; da Silva, J.A.G.; Caputo, M.M.; Damaj, M.B.; Guimarães, E.R.; Ferro, M.I.T. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 2011, 13, 191. [Google Scholar] [CrossRef]
- Da Graça, J.P.; Rodrigues, F.A.; Farias, J.R.B.; de Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S.M. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Dinh, T.H.; Watanabe, K.; Takaragawa, H.; Nakabaru, M.; Kawamitsu, Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).