Abstract
The ecological interface between grasslands and farmlands forms a critical landscape component, significantly contributing to the stability and functioning of ecosystems within the agro-pastoral transition zone of northern China. Nevertheless, the variation patterns and interactions between soil physicochemical attributes and microbial community diversity at this interface remain poorly understood. In this study, we investigated nine sites located within 50 m of the grassland–farmland boundary in the Songnen Plain, northeastern China. We assessed the soil’s physicochemical properties and the composition of bacterial and fungal communities across these sites. Results indicated a declining gradient in soil physicochemical characteristics from grassland to farmland, except for pH and total phosphorus (TP). The composition of bacterial and fungal communities differed notably in response to contrasting land-use types across the ecological interface. Soil environmental variables were closely aligned with shifts observed in bacterial and fungal assemblages. Concentrations of total nitrogen (TN), available phosphorus (AP), alkali-hydrolyzable nitrogen (AN), and available potassium (AK) exhibited inverse correlations with both bacterial and fungal populations. Alterations in microbial community composition were significantly linked to TN, TP, total potassium (TK), AN, AP, AK, and soil pH levels. Variability in soil properties, as well as microbial biomass and diversity, was evident across the grassland–cropland boundary. Long-term utilization and conversion of grassland into cultivated land altered the soil’s physicochemical environment, thereby indirectly shaping the structure of microbial communities, including both bacteria and fungi. These findings provide a valuable basis for understanding the ecological implications of land-use transitions and inform microbial-based indicators for assessing soil health in agro-pastoral ecotones.
1. Introduction
Ecotone, first conceptualized by Clements in the early 20th century, refers to a climate-mediated transitional zone where different vegetation communities overlap [1]. Among global ecotones, the agro-pastoral type spans a vast geographic extent, making it one of the most spatially continuous interfaces worldwide [2]. In northern China, this ecotone acts as a transitional buffer integrating crop farming and livestock production systems [3]. Its ecosystem is structurally diverse and functionally dynamic, shaped by both anthropogenic and natural disturbances. Distinct land-use types across this ecotone produce considerable variation in soil physicochemical conditions [4,5], which subsequently alter nutrient cycling, productivity, and microbial community structure [6].
The Songnen Plain represents a characteristic ecotonal zone situated at the eastern margin of China’s agro-pastoral transition region [7]. Due to the expansion of cultivated land, formerly contiguous grasslands have fragmented into patches, creating a complex grassland–farmland boundary [8]. Such fragmentation disrupts ecosystem equilibrium, driving changes in the functioning of the agro-pastoral mosaic landscape [9]. This includes spatial divergence in species composition, soil properties, and microbial diversity. Previous studies in this region have highlighted notable ecological consequences, including increased heterogeneity and significant shifts in microbial abundance, moisture regimes, nutrient availability, and thermal profiles along the interface [10,11,12].
Soil nutrients—particularly total nitrogen (TN), total phosphorus (TP), and total potassium (TK)—are key determinants of soil fertility, plant productivity, and microbial metabolism in ecotonal systems. In agro-pastoral ecotones similar to Songnen Plain, Inner Mongolia, spatial distribution of soil C:N:P:K stoichiometry has been documented, revealing that grassland and farmland display different nutrient ratios influenced by fertilization and environmental factors (e.g., mean annual temperature) [13]. In the Songnen Plain, salt-tolerant vegetation has been shown to enhance soil nutrient levels—including TN and TK—while modifying microbial community structure, particularly in saline–alkali environments [14]. Such heterogeneity in soil nutrients across land-use interfaces is expected to significantly shape microbial assemblages, although integrated studies in the Songnen ecotone remain scarce.
Soil microbes play a pivotal role in terrestrial ecosystem regulation and are central agents in nutrient turnover and soil biochemical processes, regulating and mediating various subtle dynamics within the soil system. They also serve as sensitive biological indicators for assessing soil health and functionality [15]. The structure and diversity of soil microbial communities are typically influenced by the physical, chemical, and biological attributes of the soil environment [16]. To sustain critical soil functions, it is essential to comprehensively understand microbial dynamics at ecological transition zones, particularly within the ecotone [17]. However, due to limited research focused on the grassland–farmland interface in the agro-pastoral ecotone [18,19], it remains unclear how spatial changes at this interface influence the composition of microbial communities and soil physicochemical properties [20].
Many studies have shown that grassland fragmentation and salinization exert substantial and often lasting impacts on both soil physicochemical attributes and microbial community structure [21,22]. Alterations in these soil conditions have been linked to shifts in fungal and bacterial community structures [23,24,25]. Variations in soil properties are also known to influence aboveground plant diversity through changes in nutrient dynamics and habitat conditions [26]. However, the factors influencing microbial community shifts within the grassland–agricultural ecotone of the Songnen Plain remain unclear. Previous research indicates that soil microbial communities exhibited pronounced responses to land-use shifts, especially in proximity to ecotonal boundaries, with responses distinct from those observed in other landscapes [27,28,29]. Gaining insights into these transformation patterns is essential for optimizing soil functionality and enhancing agricultural productivity [30,31].
This study focused on assessing the variations in soil physicochemical traits and microbial community composition along the grassland–farmland boundary of the agro-pastoral ecotone in the Songnen Plain. We compared the physicochemical environment and microbial profiles across grassland, farmland, and their transitional zones within the Songnen Plain and analyzed the patterns of change and interrelationships among them.
2. Materials and Methods
2.1. Field Description
The study area was conducted in the interlaced grassland and farmland area around Lindian County, Daqing City, in the northern part of the Songnen Plain (124°48′27″ E, 46°46′30″ N) (Figure 1). The type of grassland is temperate grassland, the dominant species is Leymus chinensis, and the associated species are Phragmites australis (Cav.) Trin. ex Steud, Cyperus rotundus L, and forbs. Farmland is reclaimed from the former grassland and has been under cultivation for more than a decade. It is distributed in patches in the study area and the main crops are corn and soybean. The climate type of the region is a north temperate continental monsoon climate, with an average annual temperature of 4 °C, an average annual sunshine of 2900 h, and an average annual precipitation of 400 mm [32].
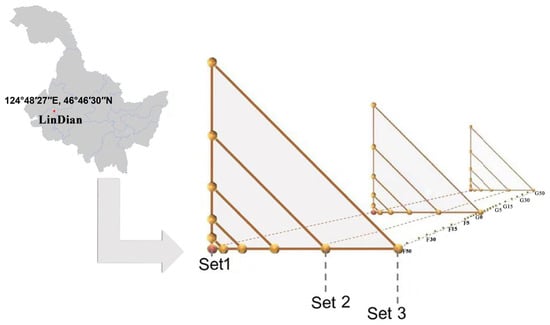
Figure 1.
Study model map. The study area is noted as G0 for the grassland–farmland interface, F for farmland, and G for grassland. Set 1: Repeat 1, Set 2: Repeat 2, Set 3: Repeat 3.
2.2. Experimental Design
The experiment was carried out in a relatively uniform landscape comprising adjacent grassland and farmland. The fence separating these land uses served as the interface, and three sampling transects were established perpendicular to the fence, extending 50 m into both directions. Each transect was spaced at least 50 m apart. The intersection of the transect and fence was designated as the origin (0 m), denoted as G0. Grassland sampling points were labeled G (e.g., G5, G15, G30), and farmland points were labeled F (e.g., F5, F15, F30), with G50 and F50 indicating the furthest distances from the fence.
Sampling plots of 0.5 × 0.5 m were placed at five distances (0, 5, 15, 30, 50 m) along each transect, yielding a total of three replicates per distance per land use, resulting in 27 soil samples (Figure 1). At each sampling point, soil was extracted from a depth of 0–20 cm using a 5 cm diameter auger. The samples were cleared of coarse materials such as stones and roots, homogenized, and divided into two portions. One portion was air-dried in the laboratory, sieved through a 1 mm mesh, and used for analysis of physicochemical properties. The other portion was preserved on dry ice and transported to the laboratory, where it was stored at −80 °C for subsequent microbial community assessments. This field experiment was conducted in July 2018 in the Songnen agro-pastoral ecotone of Northeast China.
2.3. Soil Sampling and Analysis
2.3.1. Soil Physicochemical Properties
Soil pH was determined using a combination pH meter with a combination electrode (PHS-3C, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China) in a 2.5:1 soil-to-water ratio (v/w). Soil organic matter (OM) was measured by the Walkley–Black dichromate oxidation method using an automatic titrator (ZDJ-4B, Shanghai INESA Scientific Instrument Co., Ltd., China). Total nitrogen (TN) was determined by the semi-micro Kjeldahl digestion method using a Kjeldahl nitrogen analyzer (K9840, Hanon Instruments, Jinan, China), and alkali-hydrolyzable nitrogen (AN) was determined by the alkaline hydrolysis diffusion method. Total phosphorus (TP) was measured after H2SO4–HClO4 digestion by the molybdenum–antimony anti-colorimetric method at 880 nm using a UV–Vis spectrophotometer (UV-2550, Shimadzu Corporation, Kyoto, Japan). Total potassium (TK) and available potassium (AK) were measured by flame photometry (FP6410, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China) after NaOH fusion or 1 mol/L NH4OAc extraction, respectively. Available phosphorus (AP) was determined based on the Olsen extraction protocol. Soil water content (WC) was determined gravimetrically by oven-drying at 105 °C to constant weight using a forced-air drying oven (DHG-9140A, Shanghai Yiheng Scientific Instruments Co., Ltd., Shanghai, China). All chemical analyses were conducted following standard procedures described in Methods of Soil Analysis. Part 3—Chemical Methods [33].
2.3.2. Soil DNA Extraction
The extraction of nucleic acids was carried out using the TGuide S96 (TIANGEN Biotech (Beijing) Co., Ltd., Beijing, China) bead-based soil/manure genomic DNA extraction kit following the standard procedure provided by the kit manufacturer. The isolated DNA was quantified with enzymatic assays and then subjected to amplification; PCR amplicons were evaluated for integrity through 1.8% agarose gel electrophoresis (manufacturer: Beijing Bomifuxin Technology Co., Ltd., Beijing, China). The PCR reaction system targeting specific regions was assembled from quality-checked samples, followed by purification and amplification, then gel extraction and fragment recovery. The purified DNA was stored at −80 °C until further analysis.
2.3.3. Amplification, Sequencing, and Sequence Processing of 16S rRNA and ITS Data
The extracted high-quality DNA was used for amplification of bacterial and fungal target regions. For bacteria, the V3–V4 hypervariable regions of the 16S rRNA gene were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [34]. For fungi, the ITS1 region was amplified using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) [35]. PCR reactions were performed in triplicate using TransStart® FastPfu DNA Polymerase (TransGen Biotech, Beijing, China). The thermal cycling conditions for the V3–V4 region were as follows: initial denaturation at 95 °C for 5 min; followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 40 s; and a final extension at 72 °C for 7 min. For ITS1, the conditions were as follows: initial denaturation at 95 °C for 5 min; followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 40 s; and a final extension at 72 °C for 7 min. Sequencing libraries were prepared using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) and sequenced on the Illumina HiSeq 2500 platform (paired-end 250 bp).
2.4. Statistical Analysis
The raw data regarding soil physicochemical characteristics were organized in Excel 2019, and descriptive statistics (means and standard deviations) were computed. One-way analysis of variance (ANOVA) was conducted to assess variations in soil physicochemical indices (pH, OM, TP, TN, TK, AN, AP, AK, water content) and microbial Shannon diversity. These analyses were executed using SPSS software (version 19.0; IBM, Armonk, NY, USA). Duncan’s multiple range test was applied to evaluate significance at p < 0.05. To assess compositional dissimilarities, non-metric multidimensional scaling (NMDS) was conducted based on Bray–Curtis distance matrices. Variation in soil bacterial and fungal communities was further analyzed using principal coordinates analysis (PCoA) with the ‘vegan’ package [36] in R (v3.6.2), and correlation structures were explored via the ‘corrplot’ package [37]. The BMK cloud platform (https://www.biocloud.net/) was used for processing soil microbial diversity data.
3. Results
3.1. Soil Physicochemical Analysis
In the direction from grassland to farmland, soil moisture exhibited an initial rise followed by a subsequent decline (Table 1), with values ranging between 8.46% and 22.72%. G5, G0, and F5 recorded notably elevated soil moisture levels compared to other locations. Each farmland site had lower moisture content than grassland sites, with the exception of G5 (p ≤ 0.05). Soil pH values spanned from 7.02 to 8.21 across all sampling sites, gradually increasing from grassland to farmland. Soil pH in farmland samples was consistently higher than that in grassland samples (p ≤ 0.05), and values fluctuated markedly among G5, G0, and F5. Organic matter content gradually decreased from grassland to farmland (Table 1), with F5 exhibiting significantly lower values than other farmland sites (p ≤ 0.05). Organic matter also showed fluctuating growth with depth from G0 to the grassland, peaking at G50 (79.60 g/kg). Total nitrogen displayed an upward pattern initially, followed by a downward trend, with G5, G0, and F5 showing higher concentrations than other sites. The contents of total phosphorus and potassium generally increased first, then declined thereafter, especially between G5 and F5, where their values were significantly higher (p ≤ 0.05) than at other sites. Alkali-hydrolyzable nitrogen in soil showed a declining pattern, a rise, and then dropped again from grassland to farmland, with G5, G0, and F5 having elevated levels compared to all farmland sites (p ≤ 0.05). Rapidly available phosphorus in the soil first increased and then decreased along the grassland–farmland gradient, with G5, G0, and F5 displaying notably higher concentrations than other locations (p ≤ 0.05).

Table 1.
Analysis of soil physical and chemical properties of grassland–farmland interface.
3.2. Soil Bacterial Diversity and Correlation Analysis
The α-diversity analysis was conducted at the grassland–farmland interface. The Shannon–Wiener index was lower at sample site G0 than at other sample sites, and the peak bacterial diversity occurred at sampling location F5 (Figure 2A). Across grassland, interface (G0), and farmland zones, a total of 2306 OTUs were shared, while the number of unique OTUs was 0, 1, and 4, respectively (Figure 2B). The farmland and interface (G0) shared the highest number of microorganisms and were the most similar of the three areas (Figure 2B). There was a significant change in bacterial composition in the grassland–farmland interface (Figure 2C). The abundance of Acidobacteriota was higher in the grassland at sample points G50, G30, and G15 than in the farmland, and it was significantly higher at sample point G0 than at G5 and F5, showing a significant interface effect. Actinobacteriota exhibited markedly elevated values compared to those at G5 and F5 locations, which also showed a significant interfacial effect (Figure 2C). The bacterial community structure at the grassland–farmland interface was also explored. All sample sites except the F5 sample site exhibited |βNTI| > 2, indicating that bacterial community changes at the grassland–farmland interface belong to natural selection processes (Figure 2D). Principal component analysis (PCA) revealed similarities in microbial structural composition as well as physicochemical properties between soil samples, where G0, G5, and F5 sample sites differed significantly from other sample sites, showing obvious ecotone effects (Figure 3A). The RDA of OTU in the bacterial community showed that the first canonical axis explained 40.66% of the variation, while the second canonical axis explained 14.84% of the total variation (Figure 3B). Soil moisture, total nitrogen, total potassium, and total phosphorus had a significant role in influencing the structure of bacterial assemblages in the soil (p ≤ 0.01) (Figure 3B). Bacterial community composition was highly significantly correlated (p ≤ 0.001) with soil physicochemical parameters pH, alkali-hydrolyzable nitrogen, fast-acting phosphorus, and fast-acting potassium (Figure 3B). Furthermore, among these factors, alkali-hydrolyzable nitrogen and fast-acting potassium had the highest negative effect (p ≤ 0.001), while pH had the highest positive effect on the bacterial community (p ≤ 0.001, Figure 3B). The genus names shown in Figure 3B correspond to the top five most abundant bacterial taxa across all samples, included to highlight dominant community members and assist in interpreting the RDA ordination.
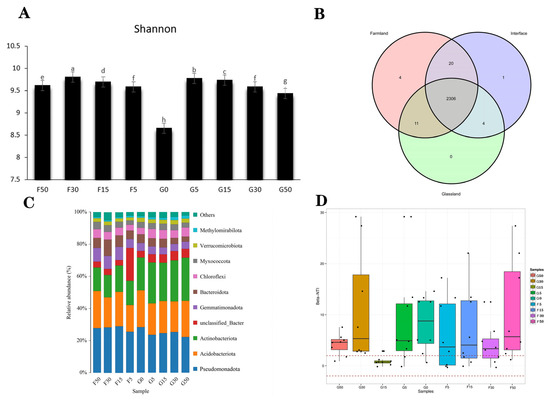
Figure 2.
Bacterial Shannon indices (A) and Venn diagrams showing core and unique bacterial community composition (B) for sample sites at different grassland–farmland interfaces, as well as analysis of the proportion of bacterial taxa identified at each location (C) and the organizational profile of bacterial assemblages (D). G50: Grassland interface 50 m from origin; G30: Grassland interface 30 m from origin; G15: Grassland interface 15 m from origin; G5: Grassland interface 5 m from origin; G0: Interface point 0; F5: Farmland interface 5 m from origin; F15: Farmland interface 15 m from origin; F30: Farmland interface 30 m from origin; F50: Farmland interface 50 m from origin. Different lowercase letters in (A) indicate significant differences among treatments at p < 0.05. In (B), red, purplec, and green represent farmland, interface, and grassland, respectively. In (C), each color corresponds to a bacterial phylum as indicated in the legend. In (D), different colors represent sample sites as shown in the legend.
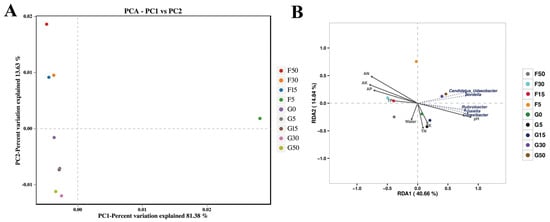
Figure 3.
PCA reflecting the structure of bacterial populations in soil samples at each sample site (A). Redundancy analysis (RDA) of operational taxonomic units of the bacterial community explained by environmental factors (B). G50: Grassland interface 50 m from origin; G30: Grassland interface 30 m from origin; G15: Grassland interface 15 m from origin; G5: Grassland interface 5 m from origin; G0: Interface point 0; F5: Farmland interface 5 m from origin; F15: Farmland interface 15 m from origin; F30: Farmland interface 30 m from origin; F50: Farmland interface 50 m from origin. Abbreviation list: TK: total potassium; TP: total phosphorus; TN: total nitrogen; AK: fast-acting potassium; AP: fast-acting phosphorus; AN: alkali-hydrolyzable nitrogen; Water: moisture. Names in (B) indicate the top 5 most abundant bacterial genera across all samples.
The bacterial network consisted of 80 nodes linked through 987 edges with an average clustering coefficient of 0.666. Among the bacterial networks, Sphingomonadaceae and Gemmatimonadaceae were the major components, showing relative proportions of 0.076 and 0.061, respectively (Figure 4).
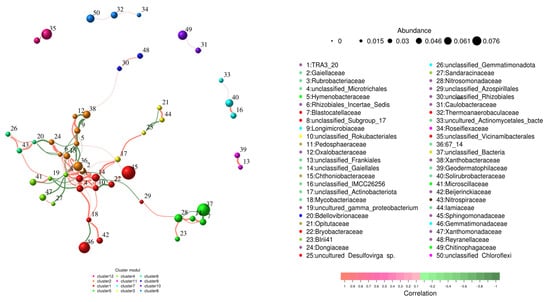
Figure 4.
Co-occurrence networks and hypothetical key connectors for bacterial communities at the OTU level. Positive associations are denoted by red edges, while green lines indicate negative ones; line thickness reflects the strength of association (p < 0.05).
3.3. Soil Fungal Diversity and Correlation Analysis
We also used the Shannon–Wiener to evaluate alpha diversity within fungal communities based on the Shannon–Wiener, and the Shannon–Wiener of sample sites G0, G5 was significantly different from other sample sites and the index of sample site F15 was the highest (Figure 5A). Fungal communities in the grassland, interface (G0), and farmland regions shared 943 common OTUs, while they had 89, 8, and 98 unique OTUs, respectively, with grassland and farmland having the highest similarity among the three regions, sharing 425 OTUs that were not present in the interface (G0) (Figure 5B). The major fungal phyla found in the grassland–farmland interface included Ascomycota, Basidiomycota, and Glomeromycota (Figure 5C), where Ascomycota was significantly less abundant in G0 than in G5, F5 sample sites, showing a clear interface effect, and Basidiomycota was more abundant in farmland than in grassland (Figure 5C). Fungal community structure changes in farmland except for the F50 sample point showed |βNTI| < 2, indicating that the fungal community structure changes in farmland belonged to a random process, while in grassland and interface (G0) showed |βNTI| > 2 except for the G50 sample point, indicating that the fungal community changes in grassland belonged to a natural selection process (Figure 5D).
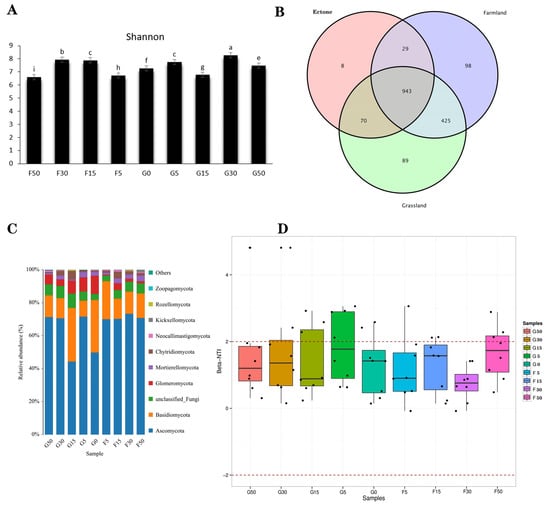
Figure 5.
Fungi Shannon indices (A) and Venn diagrams showing core and unique fungi community composition (B) for sample sites at different grassland–farmland interfaces, as well as analysis of the proportional representation of fungal taxa across sites (C) and the structural composition of fungi communities (D). G50: Grassland interface 50 m from origin; G30: Grassland interface 30 m from origin; G15: Grassland interface 15 m from origin; G5: Grassland interface 5 m from origin; G0: Interface point 0; F5: Farmland interface 5 m from origin; F15: Farmland interface 15 m from origin; F30: Farmland interface 30 m from origin; F50: Farmland interface 50 m from origin. Different lowercase letters in (A) indicate significant differences among treatments at p < 0.05. In (B), red, purplec, and green represent farmland, interface, and grassland, respectively. In (C), each color corresponds to a fungal phylum as indicated in the legend. In (D), different colors represent sample sites as shown in the legend.
Fungal community principal component analysis (PCA) accounted for 62.03% of the variation in the dataset, where the G0, G5, and F5 sample sites differed significantly from the other sample sites with obvious interface effects (Figure 6A). In the fungal community RDA biplot, the combination of variables explained 40.38% of the variance in the fungal community (Figure 6B). Soil pH, total potassium, and total phosphorus were positively correlated with bacterial community composition, with pH having the highest positive effect (p ≤ 0.01) (Figure 6B). The structure of the community was inversely associated with soil moisture, alkali-hydrolyzable nitrogen, fast-acting phosphorus, total nitrogen, and fast-acting potassium, with the highest negative effects for alkali-hydrolyzable nitrogen, fast-acting potassium among these factors (p ≤ 0.001, Figure 6B). The genus names shown in Figure 6B represent the top five most abundant fungal taxa across all samples, included to highlight dominant community members and facilitate interpretation of the RDA ordination.
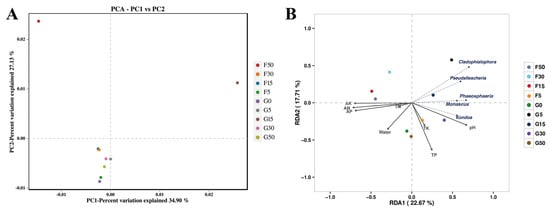
Figure 6.
PCA was used to assess the structure of soil fungal communities at each sample site (A). Redundancy analysis (RDA) of operational taxonomic units of the bacterial community explained by environmental factors (B). G50: Grassland interface 50 m from origin; G30: Grassland interface 30 m from origin; G15: Grassland interface 15 m from origin; G5: Grassland interface 5 m from origin; G0: Interface point 0; F5: Farmland interface 5 m from origin; F15: Farmland interface 15 m from origin; F30: Farmland interface 30 m from origin; F50: Farmland interface 50 m from origin. Abbreviations: TK: total potassium; TP: total phosphorus; TN: total nitrogen; AK: fast-acting potassium; AP: fast-acting phosphorus; AN: alkali-hydrolyzable nitrogen; Water: moisture. Names in (B) indicate the top five most abundant fungal genera across all samples.
The fungal network consisted of 80 nodes connected by 358 edges with an average clustering coefficient of 0.447. Within the fungal network, Fusarium and Geopora were the main components with relative abundances of 0.065 and 0.049 (Figure 7).
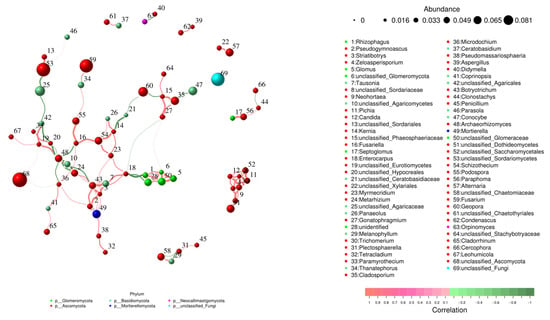
Figure 7.
Co-occurrence networks and hypothetical key connectors for fungi communities at the OTU level. Red edges indicate positive relationships, green lines indicate negative ones, and line width reflects the magnitude of correlation (p < 0.05).
4. Discussion
4.1. Changes in Soil Physicochemical Properties at the Grassland–Farmland Interface
The physical and chemical characteristics of soil are spatially variable at the scale of the ecological ecotone and this variation is mainly influenced by factors such as composition of plant community [38], soil microbial diversity, and fertiliser application [39]. Previous research has demonstrated that soil chemical and physical indicators like pH, total nitrogen, total phosphorus, and fast-acting potassium vary strongly around the ecological interface, while other functional areas do not vary significantly. The findings of this research suggest that the trend of soil physicochemical properties in grassland and farmland is relatively gradual, while at the G0, G5, and F5 sample points, the variation is high, which is in agreement with previous findings. Many previous studies have pointed out that the root distribution of different types of cover vegetation and the apoplastic material they produce are the underlying causes responsible for the distinct variations in soil chemical and physical traits [40,41,42,43]. In this study, the dominant cover vegetation in grassland plots consisted mainly of Leymus chinensis (Trin.) Tzvelev, with associated species including Phragmites australis (Cav.) Trin. ex Steud, Cyperus rotundus L., and various forbs [30], whereas farmland plots were primarily cultivated with Zea mays L. and Glycine max (L.) Merr. In the ecotonal zones, the vegetation cover was more complex, comprising a mixture of grassland species interspersed with crop residues. The vegetation cover in different areas of this study was different, with grass and farmland cover vegetation types being relatively homogeneous [44,45], while the vegetation cover types in the G0, G5 and F5 sample sites were relatively complex and the soil indicators of TN, TK and TP varied dramatically in the G0, G5 and F5 sample sites. This is consistent with the findings on soil ecotone in areas with different types of cover vegetation [46].
It was observed that the total nitrogen content in the soil, phosphorus, and potassium were lower in the G50, G30, and G15 sample points than in the F50, F30, F15, and F5 sample points of the farmland. Numerous earlier studies have indicated that the nutrients in the soil were different in the farmland compared to the grassland due to the heavy application of nitrogen, phosphorus, and potassium fertilisers during long-term agricultural production, where total nitrogen and phosphorus were higher than in the grassland, except for organic matter [47,48]. A previous study has suggested that the presence of horizontal ecological flows causes soil nutrients to converge at the ecotone [49], resulting in a greater magnitude of change in soil physicochemical properties at the ecological ecotone, which aligns with the outcomes observed in this study.
4.2. Shift in Bacterial Community Composition
The drivers of bacterial community composition at the grassland–farmland interface in the Songnen Plain are mainly the autocorrelated total potassium, total nitrogen, and pH. Numerous prior findings corroborate the observed association between soil pH and bacterial community structure [50,51,52]. Soil pH influences bacterial composition by regulating the proliferation of specific taxa, whose physiological traits are best suited to narrow pH intervals [53,54]. Prior research identified soil pH as the dominant physicochemical factor most strongly associated with bacterial community structure, serving as a primary indicator of its variation [55,56,57,58]. In this study, soil pH had the highest positive correlation with the soil bacterial community.
Significant differences in bacterial community composition among interface (G0), grassland, and farmland could be attributed to differences in recalcitrant root microorganisms accumulation in the topsoil in lands of different use patterns [59,60,61]. This has also been confirmed by the findings of bacterial communities in grassland–agricultural ecotone in Ethiopia [62]. Distinct vegetation forms exert marked influences on soil bacterial communities, as root-associated microbial assembly is shaped by plant species identity and localized rhizosphere conditions [63,64,65].
Soil microbial diversity is influenced not only by vegetation type, but also by soil pH and other factors such as soil nutrient quality and fertiliser application [66,67]. Soil nutrient parameters, including total nitrogen, phosphorus, and potassium, signify the nutrient utilization capacity of microbial communities and serve as key metrics of bacterial diversity [68,69]. Our results revealed marked differences in bacterial communities across the grassland, farmland, and interface (G0) zones. Acidobacteriota, Proteobacteriota, and other major component bacteria in the farmland were higher than in the grassland area but lower than in the interface (G0). This may be due to the fact that crops have been grown in the farmland area for a long time and a large amount of nitrogen, phosphorus and potassium fertilisers have been added, resulting in higher soil total nitrogen, potassium and phosphorus contents and higher soil bacterial metabolism and increased diversity, which verifies that previous findings [70,71,72].
4.3. Shift in Fungal Community Composition
We found that changes in fungal community composition in the grassland–farmland interface of the Songnen Plain vary markedly across regions with different land use practices. Similar to bacteria, fungal assemblages exhibited associations with soil pH and nutrient availability, distinguishing grassland, ecotone, and farmland [73,74]. Again, soil pH has been widely recognized as a primary determinant explaining the variation in fungal communities in previous studies. This is consistent with the results of this study [75,76].
Fungal diversity was markedly greater in farmland compared to grassland in our analysis. Previous studies have revealed that biological drivers have been implicated in shaping fungal composition, including plant inter-root secretions, resulting in different fungal community composition in different vegetation cover regions, aligning with the experimental observations of this study [73,77,78].
Similar to bacterial communities, soil fungal community diversity is influenced by vegetation type, soil pH, and other factors such as soil nutrient quality [79]. Evidence suggests that soil fungi are central agents in organic matter decomposition and can break down most of the organic matter in the soil to support their metabolic processes [80]. In this experiment, farmland had notably lower organic matter content but higher fungal diversity relative to the grassland, supporting prior reports [81,82,83].
5. Conclusions
Different areas at the grassland–farmland interface in the Songnen Plain significantly affect the characteristics of soil chemistry and the diversity of microbial taxa. The microbial species diversity of soils in grassland areas was lower in diversity compared to soils in farmland areas where crops were grown year-round. Most bacterial and fungal species diversity was correlated with pH, total nitrogen, total potassium, and total phosphorus, while moisture, total nitrogen, nitrogen, phosphorus, and potassium contents were notably elevated in ecotonal soils relative to other regions, showing significant interfacial effects. These results suggest that key physicochemical properties of soil and ecotone in different areas indirectly affect soil bacterial and fungal biomass. Therefore, our study shows how soil environmental factors contribute to shaping microbial communities in the Songnen Plain grassland–farmland interface and its change pattern. These findings not only enhance our understanding of soil–microbe interactions in the Songnen agro-pastoral ecotone but also provide a methodological framework applicable to other ecotones, such as grassland–cropland or wetland–cropland interfaces. The approach and results may support evidence-based land management and ecological restoration strategies in similar transitional ecosystems worldwide.
Author Contributions
Conceptualization, H.L. and L.Q.; methodology, H.L.; validation, X.S.; formal analysis, H.L., Z.H., X.S. and Z.L.; investigation, H.L., J.L., Z.L. and C.F.; resources, H.X.; data curation, J.L. and W.Z.; visualization, H.L. and W.Z.; writing—original draft preparation, H.L.; writing—review and editing, J.L., Z.H., W.Z., L.Q. and L.M.; supervision, L.Q. and L.M.; project administration, L.M.; funding acquisition, L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of Heilongjiang Province (Grant No. YQ2023C013) and the National Natural Science Foundation of China (Grant No. 32271770). The APC was funded by the same sources.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gosz, J.R. Fundamental Ecological Characteristics of Landscape Boundaries. In Ecotones; Holland, M.M., Risser, P.G., Naiman, R.J., Eds.; Springer: Boston, MA, USA, 1991; pp. 8–30. ISBN 978-1-4615-9688-2. [Google Scholar]
- Zhai, X.; Huang, D.; Tang, S.; Li, S.; Guo, J.; Yang, Y.; Liu, H.; Li, J.; Wang, K. The Emergy of Metabolism in Different Ecosystems under the Same Environmental Conditions in the Agro-Pastoral Ecotone of Northern China. Ecol. Indic. 2017, 74, 198–204. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, B.; He, C.; Shao, R. Detecting Vegetation Variations and Main Drivers over the Agropastoral Ecotone of Northern China through the Ensemble Empirical Mode Decomposition Method. Remote Sens. 2019, 11, 1860. [Google Scholar] [CrossRef]
- Kerns, B.K.; Powell, D.C.; Mellmann-Brown, S.; Carnwath, G.; Kim, J.B. Effects of Projected Climate Change on Vegetation in the Blue Mountains Ecoregion, USA. Clim. Serv. 2018, 10, 33–43. [Google Scholar] [CrossRef]
- You, G.; Liu, B.; Zou, C.; Li, H.; McKenzie, S.; He, Y.; Gao, J.; Jia, X.; Arain, M.A.; Wang, S.; et al. Sensitivity of Vegetation Dynamics to Climate Variability in a Forest-Steppe Transition Ecozone, North-Eastern Inner Mongolia, China. Ecol. Indic. 2021, 120, 106833. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Li, X.; Bian, Y.; Wang, F.; Yang, X.; Gu, C.; Jiang, X. Long-Term Plastic Greenhouse Cultivation Changes Soil Microbial Community Structures: A Case Study. J. Agric. Food Chem. 2018, 66, 8941–8948. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Jiang, Q.; Chen, S.; Ma, J.-Y.; Sun, W. Carbon Isotope Composition of Nighttime Leaf-Respired CO2 in the Agricultural-Pastoral Zone of the Songnen Plain, Northeast China. PLoS ONE 2015, 10, e0137575. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Crusciol, C.A.; Leite, M.F.; Merloti, L.F.; Moretti, L.G.; Pascoaloto, I.M.; Kuramae, E.E. Modulation of the Soil Microbiome by Long-Term ca-Based Soil Amendments Boosts Soil Organic Carbon and Physicochemical Quality in a Tropical No-till Crop Rotation System. Soil Biol. Biochem. 2021, 156, 108188. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, S.; Qin, Y.; Sun, Y.; Shangguan, D.; Meng, B.; Li, M.; Zhang, J. Effects of Patchiness on Surface Soil Moisture of Alpine Meadow on the Northeastern Qinghai-Tibetan Plateau: Implications for Grassland Restoration. Remote Sens. 2020, 12, 4121. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Zhang, H.; Jiang, L.; Zhang, A.; Pan, T. Effect of Farmland Expansion on Drought over the Past Century in Songnen Plain, Northeast China. J. Geogr. Sci. 2020, 30, 439–454. [Google Scholar] [CrossRef]
- Chang, C.; Tian, L.; Tian, Z.; McLaughlin, N.; Tian, C. Change of Soil Microorganism Communities under Saline-sodic Land Degradation on the Songnen Plain in Northeast China#. J. Plant Nutr. Soil Sci. 2022, 185, 297–307. [Google Scholar] [CrossRef]
- Shi, C.Q.; Li, Y.; Yu, S.P.; Hu, B.Z.; Guo, H.; Jin, L.; Cong, D.L.; Meng, B.; Ding, J.N.; Liang, X.W. Saline-Alkaline Soil Bacterial Community Structure and Diversity Analysis under Different Patterns of Land-Use in a Lake Wetland in Songnen Plain, China. Appl. Ecol. Environ. Res. 2021, 19, 1337–1352. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Han, L.; Yang, J.; Zhao, X.; Qu, J.; Li, L.; Bai, Y.; Yan, D.; Hou, G. Spatial Distribution Characteristics of Soil C:N:P:K Eco-Stoichiometry of Farmland and Grassland in the Agro-Pastoral Ecotone in Inner Mongolia, China. Agronomy 2024, 14, 346. [Google Scholar] [CrossRef]
- Song, J.; Guan, X.; Cui, H.; Liu, L.; Li, Y.; Li, Y.; Ma, S. The Impact of Salt-Tolerant Plants on Soil Nutrients and Microbial Communities in Soda Saline-Alkali Lands of the Songnen Plain. Front. Microbiol. 2025, 16, 1592834. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wu, X.; Duan, C.; Smith, A.R.; Jones, D.L. Traits of Dominant Species and Soil Properties Co-Regulate Soil Microbial Communities across Land Restoration Types in a Subtropical Plateau Region of Southwest China. Ecol. Eng. 2020, 153, 105897. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Zheng, W.; Hou, F.; Hu, Y.; Guo, S. Converting Croplands to Orchards Changes Soil Microbial Community Composition and Co-occurrence Patterns. Land Degrad. Dev. 2021, 32, 2509–2519. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Alive and Kicking: Why Dormant Soil Microorganisms Matter. Soil Biol. Biochem. 2018, 116, 419–430. [Google Scholar] [CrossRef]
- Lu, Z.; Lei, G.; Guo, Y.; Ma, Q. Changes of Land Use Intensity in the Songnen Plain of Different Spatial Scales and Their Effects on Climatic Factors. Acta Ecol. Sin. 2021, 41, 1894–1906. [Google Scholar] [CrossRef]
- Wu, P.; Xie, Y.; Chi, Y.; Kang, C.; Sun, L.; Wei, Z.; Zhang, M.; Zhang, Y. Loess Accumulation in Harbin with Implications for Late Quaternary Aridification in the Songnen Plain, Northeast China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 570, 110365. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Jiang, M.; Lu, X. Vegetation Change and Its Response to Climate Change between 2000 and 2016 in Marshes of the Songnen Plain, Northeast China. Sustainability 2020, 12, 3569. [Google Scholar] [CrossRef]
- Brunel, C.; Da Silva, A.-M.F.; Gros, R. Environmental Drivers of Microbial Functioning in Mediterranean Forest Soils. Microb. Ecol. 2020, 80, 669–681. [Google Scholar] [CrossRef]
- Shao, J.-L.; Lai, B.; Jiang, W.; Wang, J.-T.; Hong, Y.-H.; Chen, F.-B.; Tan, S.-Q.; Guo, L.-X. Diversity and Co-Occurrence Patterns of Soil Bacterial and Fungal Communities of Chinese Cordyceps Habitats at Shergyla Mountain, Tibet: Implications for the Occurrence. Microorganisms 2019, 7, 284. [Google Scholar] [CrossRef]
- Canini, F.; Geml, J.; D’Acqui, L.P.; Buzzini, P.; Turchetti, B.; Onofri, S.; Ventura, S.; Zucconi, L. Fungal Diversity and Functionality Are Driven by Soil Texture in Taylor Valley, Antarctica. Fungal Ecol. 2021, 50, 101041. [Google Scholar] [CrossRef]
- Díaz, M.; Quiroz-Moreno, C.; Jarrín-V, P.; Piquer-Esteban, S.; Monfort-Lanzas, P.; Rivadeneira, E.; Castillejo, P.; Arnau, V.; Díaz, W.; Sangari, F.J.; et al. Soil Bacterial Community along an Altitudinal Gradient in the Sumaco, a Stratovolcano in the Amazon Region. Front. For. Glob. Change 2022, 5, 738568. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Lu, J.; Chen, W.; Wei, G.; Lin, Y. Topography Affects the Soil Conditions and Bacterial Communities along a Restoration Gradient on Loess-Plateau. Appl. Soil Ecol. 2020, 150, 103471. [Google Scholar] [CrossRef]
- Ma, J.; Nergui, S.; Han, Z.; Huang, G.; Li, H.; Zhang, R.; Zhu, L.; Liao, J. The Variation of the Soil Bacterial and Fungal Community Is Linked to Land Use Types in Northeast China. Sustainability 2019, 11, 3286. [Google Scholar] [CrossRef]
- Plassart, P.; Prévost-Bouré, N.C.; Uroz, S.; Dequiedt, S.; Stone, D.; Creamer, R.; Griffiths, R.I.; Bailey, M.J.; Ranjard, L.; Lemanceau, P. Soil Parameters, Land Use, and Geographical Distance Drive Soil Bacterial Communities along a European Transect. Sci. Rep. 2019, 9, 605. [Google Scholar] [CrossRef]
- Sui, X.; Zeng, X.; Li, M.; Weng, X.; Frey, B.; Yang, L.; Li, M. Influence of Different Vegetation Types on Soil Physicochemical Parameters and Fungal Communities. Microorganisms 2022, 10, 829. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Wang, Z.; Zhang, Y.; Wang, K. Effects of Grassland Converted to Cropland on Soil Microbial Biomass and Community from Agro-pastoral Ecotone in Northern China. Grassl. Sci. 2022, 68, 36–43. [Google Scholar] [CrossRef]
- Aredehey, G.; Berhe Zenebe, G.; Gebremedhn, A. Land Use Impacts on Physicochemical and Microbial Soil Properties across the Agricultural Landscapes of Debrekidan, EasternTigray, Ethiopia. Cogent Food Agric. 2019, 5, 1708683. [Google Scholar] [CrossRef]
- Hota, S.; Mishra, V.; Mourya, K.K.; Giri, K.; Kumar, D.; Jha, P.K.; Saikia, U.S.; Prasad, P.V.; Ray, S.K. Land Use, Landform, and Soil Management as Determinants of Soil Physicochemical Properties and Microbial Abundance of Lower Brahmaputra Valley, India. Sustainability 2022, 14, 2241. [Google Scholar] [CrossRef]
- Shuo, L.; Yunping, C.; Dongmei, H.; Yuanyun, X.; Chunguo, K.; Peng, W. Evolution of Summer Monsoon in Songnen Plain since Middle Pleistocene: Magnetic Susceptibility, Geochemistry and Total Organic Carbon Records from Harbin Loess. Chin. J. Geol. 2021, 56, 1279–1298. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. (Eds.) Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-866-7. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.9. 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 August 2025).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘Corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Akhtaruzzaman, M.D.; Roy, S.; Mahmud, M.S.; Shormin, T. Soil Properties under Different Vegetation Types in Chittagong University Campus, Bangladesh. J. For. Environ. Sci. 2020, 36, 133–142. [Google Scholar]
- Zhang, Z.; Wang, J.; Feng, Y. Linking the Reclaimed Soils and Rehabilitated Vegetation in an Opencast Coal Mining Area: A Complex Network Approach. Environ. Sci. Pollut. Res. 2019, 26, 19365–19378. [Google Scholar] [CrossRef]
- Dong, L.; Li, J.; Zhang, Y.; Bing, M.; Liu, Y.; Wu, J.; Hai, X.; Li, A.; Wang, K.; Wu, P.; et al. Effects of Vegetation Restoration Types on Soil Nutrients and Soil Erodibility Regulated by Slope Positions on the Loess Plateau. J. Environ. Manag. 2022, 302, 113985. [Google Scholar] [CrossRef]
- Ferrari, F.R.; Schaefer, C.E.; Pereira, A.B.; Thomazini, A.; Schmitz, D.; Francelino, M.R. Coupled Soil-Vegetation Changes along a Topographic Gradient on King George Island, Maritime Antarctica. Catena 2021, 198, 105038. [Google Scholar] [CrossRef]
- Szymański, W.; Maciejowski, W.; Ostafin, K.; Ziaja, W.; Sobucki, M. Impact of Parent Material, Vegetation Cover, and Site Wetness on Variability of Soil Properties in Proglacial Areas of Small Glaciers along the Northeastern Coast of Sørkappland (SE Spitsbergen). Catena 2019, 183, 104209. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fu, Z.; Wang, K. Effects of Vegetation Restoration on Soil Properties along an Elevation Gradient in the Karst Region of Southwest China. Agric. Ecosyst. Environ. 2021, 320, 107572. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Gao, H.; Nie, N. Climate Change and Anthropogenic Impacts on Wetland and Agriculture in the Songnen and Sanjiang Plain, Northeast China. Remote Sens. 2018, 10, 356. [Google Scholar] [CrossRef]
- hua An, F.; tao Yang, H.; Yang, F.; chun Wang, Z. Soil Degradation and Its Relation to Soil Properties in Songnen Plain, Northeast China. Feb Fresenius Environ. Bull. 2021, 30, 1304–1311. [Google Scholar]
- Liu, R.; Pan, Y.; Bao, H.; Liang, S.; Jiang, Y.; Tu, H.; Nong, J.; Huang, W. Variations in Soil Physico-Chemical Properties along Slope Position Gradient in Secondary Vegetation of the Hilly Region, Guilin, Southwest China. Sustainability 2020, 12, 1303. [Google Scholar] [CrossRef]
- Li, Y.-B.; Li, P.; Wang, S.-H.; Xu, L.-Y.; Deng, J.-J.; Jiao, J.-G. Effects of Organic Fertilizer Application on Crop Yield and Soil Properties in Rice-Wheat Rotation System: A Meta-Analysis. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2021, 32, 3231–3239. [Google Scholar]
- Tan, G.; Wang, H.; Xu, N.; Junaid, M.; Liu, H.; Zhai, L. Effects of Biochar Application with Fertilizer on Soil Microbial Biomass and Greenhouse Gas Emissions in a Peanut Cropping System. Environ. Technol. 2021, 42, 9–19. [Google Scholar] [CrossRef]
- Parker, S.S.; Seabloom, E.W.; Schimel, J.P. Grassland Community Composition Drives Small-Scale Spatial Patterns in Soil Properties and Processes. Geoderma 2012, 170, 269–279. [Google Scholar] [CrossRef]
- Csontos, P.; Mucsi, M.; Ragályi, P.; Tamás, J.; Kalapos, T.; Pápay, G.; Mjazovszky, Á.; Penksza, K.; Szili-Kovács, T. Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures. Agronomy 2021, 11, 1652. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Tigabu, M.; Cai, L.; Meng, F.; Liu, A.; Ma, X. Changes in Soil Physico-Chemical Properties Following Vegetation Restoration Mediate Bacterial Community Composition and Diversity in Changting, China. Ecol. Eng. 2019, 138, 171–179. [Google Scholar] [CrossRef]
- Weng, X.; Li, J.; Sui, X.; Li, M.; Yin, W.; Ma, W.; Yang, L.; Mu, L. Soil Microbial Functional Diversity Responses to Different Vegetation Types in the Heilongjiang Zhongyangzhan Black-Billed Capercaillie Nature Reserve. Ann. Microbiol. 2021, 71, 26. [Google Scholar] [CrossRef]
- Bottos, E.M.; Laughlin, D.C.; Herbold, C.W.; Lee, C.K.; McDonald, I.R.; Cary, S.C. Abiotic Factors Influence Patterns of Bacterial Diversity and Community Composition in the Dry Valleys of Antarctica. FEMS Microbiol. Ecol. 2020, 96, fiaa042. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Sun, X.; Tong, J.; Fu, Z.; Li, J. Sustainability of Urban Soil Management: Analysis of Soil Physicochemical Properties and Bacterial Community Structure under Different Green Space Types. Sustainability 2019, 11, 1395. [Google Scholar] [CrossRef]
- Cai, L.; Guo, Z.; Zhang, J.; Gai, Z.; Liu, J.; Meng, Q.; Liu, X. No Tillage and Residue Mulching Method on Bacterial Community Diversity Regulation in a Black Soil Region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Li, Z.; Bu, L.; Jin, Z.; Wei, G.; Li, Z. Fertile Islands Lead to More Conspicuous Spatial Heterogeneity of Bacteria than Soil Physicochemical Properties in a Desert Ecosystem. Catena 2021, 206, 105526. [Google Scholar] [CrossRef]
- Zhang, N.; Zhong, B.; Zhao, C.; Wang, E.; Wang, Y.; Chen, D.; Shi, F. Change of Soil Physicochemical Properties, Bacterial Community and Aggregation during Desertification of Grasslands in the Tibetan Plateau. Eur. J. Soil Sci. 2021, 72, 274–288. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Y.; Chen, W.; Guo, Y.; Wu, M.; Wang, Y.; Li, H. Soil Type and pH Mediated Arable Soil Bacterial Compositional Variation across Geographic Distance in North China Plain. Appl. Soil Ecol. 2022, 169, 104220. [Google Scholar] [CrossRef]
- Du, H.-D.; Wang, S.; Nie, W.-J.; Song, S.-J. Soil Properties and Bacterial Community Dynamics in a Coal Mining Subsidence Area: Active versus Passive Revegetation. J. Soil Sci. Plant Nutr. 2021, 21, 2573–2585. [Google Scholar] [CrossRef]
- Hutengs, C.; Eisenhauer, N.; Schädler, M.; Lochner, A.; Seidel, M.; Vohland, M. VNIR and MIR Spectroscopy of PLFA-Derived Soil Microbial Properties and Associated Soil Physicochemical Characteristics in an Experimental Plant Diversity Gradient. Soil Biol. Biochem. 2021, 160, 108319. [Google Scholar] [CrossRef]
- Waymouth, V.; Miller, R.E.; Kasel, S.; Ede, F.; Bissett, A.; Aponte, C. Soil Bacterial Community Responds to Land-Use Change in Riparian Ecosystems. Forests 2021, 12, 157. [Google Scholar] [CrossRef]
- Delelegn, Y.T.; Purahong, W.; Sandén, H.; Yitaferu, B.; Godbold, D.L.; Wubet, T. Transition of Ethiopian Highland Forests to Agriculture-Dominated Landscapes Shifts the Soil Microbial Community Composition. BMC Ecol. 2018, 18, 58. [Google Scholar] [CrossRef]
- Li, X.; Pang, H.; Zhao, Y.; Sun, M.; Zhang, X.; Xu, N.; He, G.; Zhang, H.; Sun, G. Shifts in the Bacterial Community Structure and Function along a Vegetation Gradient in the Great Xing’an Mountains. Scand. J. For. Res. 2018, 33, 103–113. [Google Scholar] [CrossRef]
- Xiang, X.; Gibbons, S.M.; Li, H.; Shen, H.; Chu, H. Proximate Grassland and Shrub-Encroached Sites Show Dramatic Restructuring of Soil Bacterial Communities. PeerJ 2019, 7, e7304. [Google Scholar] [CrossRef]
- Xiao, R.; Man, X.; Duan, B.; Cai, T.; Ge, Z.; Li, X.; Vesala, T. Changes in Soil Bacterial Communities and Nitrogen Mineralization with Understory Vegetation in Boreal Larch Forests. Soil Biol. Biochem. 2022, 166, 108572. [Google Scholar] [CrossRef]
- Li, P.; Kong, D.; Zhang, H.; Xu, L.; Li, C.; Wu, M.; Jiao, J.; Li, D.; Xu, L.; Li, H.; et al. Different Regulation of Soil Structure and Resource Chemistry under Animal- and Plant-Derived Organic Fertilizers Changed Soil Bacterial Communities. Appl. Soil Ecol. 2021, 165, 104020. [Google Scholar] [CrossRef]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-Term Fertilisation Form, Level and Duration Affect the Diversity, Structure and Functioning of Soil Microbial Communities in the Field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Liu, H.; Xu, W.; Li, J.; Yu, Z.; Zeng, Q.; Tan, W.; Mi, W. Short-Term Effect of Manure and Straw Application on Bacterial and Fungal Community Compositions and Abundances in an Acidic Paddy Soil. J. Soils Sediments 2021, 21, 3057–3071. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Shi, J.; Wang, S.; White, P.J.; Shi, L.; Xu, F. Effect of Balanced Application of Boron and Phosphorus Fertilizers on Soil Bacterial Community, Seed Yield and Phosphorus Use Efficiency of Brassica napus. Sci. Total Environ. 2021, 751, 141644. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; Li, S.; Zheng, X.; Zhang, J.; Sun, L.; Lv, W. Effects of Application Rates of Poly-γ-Glutamic Acid on Vegetable Growth and Soil Bacterial Community Structure. Appl. Soil Ecol. 2020, 147, 103405. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J.; Peng, F.; Gao, P. Long-Term Combined Application of Manure and Chemical Fertilizer Sustained Higher Nutrient Status and Rhizospheric Bacterial Diversity in Reddish Paddy Soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P.; Zeng, Z. Dynamics of Bacterial Communities in a 30-Year Fertilized Paddy Field under Different Organic–Inorganic Fertilization Strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
- Canini, F.; Zucconi, L.; Pacelli, C.; Selbmann, L.; Onofri, S.; Geml, J. Vegetation, pH and Water Content as Main Factors for Shaping Fungal Richness, Community Composition and Functional Guilds Distribution in Soils of Western Greenland. Front. Microbiol. 2019, 10, 2348. [Google Scholar] [CrossRef]
- Ding, T.; Yan, Z.; Zhang, W.; Duan, T. Green Manure Crops Affected Soil Chemical Properties and Fungal Diversity and Community of Apple Orchard in the Loess Plateau of China. J. Soil Sci. Plant Nutr. 2021, 21, 1089–1102. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and Nutrients Shape the Vertical Distribution of Microbial Communities in an Alpine Wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Xu, M.-P.; Wang, J.-Y.; Zhu, Y.-F.; Han, X.-H.; Ren, C.-J.; Yang, G.-H. Plant Biomass and Soil Nutrients Mainly Explain the Variation of Soil Microbial Communities during Secondary Succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Dumack, K.; Liu, W.; Zhang, Q.; He, Y.; Di, H.; Bonkowski, M.; Xu, J.; Li, Y. Protists Modulate Fungal Community Assembly in Paddy Soils across Climatic Zones at the Continental Scale. Soil Biol. Biochem. 2021, 160, 108358. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Ye, Z.; Li, J.; Feng, Y.; Lu, Q. Relationships between Fungal and Plant Communities Differ between Desert and Grassland in a Typical Dryland Region of Northwest China. Front. Microbiol. 2018, 9, 2327. [Google Scholar] [CrossRef]
- Li, S.; Shakoor, A.; Wubet, T.; Zhang, N.; Liang, Y.; Ma, K. Fine-Scale Variations of Fungal Community in a Heterogeneous Grassland in Inner Mongolia: Effects of the Plant Community and Edaphic Parameters. Soil Biol. Biochem. 2018, 122, 104–110. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of Organic Substitution Rates on Soil Quality and Fungal Community Composition in a Tea Plantation with Long-Term Fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.; Biasi, C.; Heinonsalo, J. Interaction between Tannins and Fungal Necromass Stabilizes Fungal Residues in Boreal Forest Soils. New Phytol. 2019, 223, 16–21. [Google Scholar] [CrossRef]
- Das, S.; Lee, J.G.; Cho, S.R.; Song, H.J.; Kim, P.J. Silicate Fertilizer Amendment Alters Fungal Communities and Accelerates Soil Organic Matter Decomposition. Front. Microbiol. 2019, 10, 2950. [Google Scholar] [CrossRef]
- Kohout, P.; Sudová, R.; Brabcová, V.; Vosolsobě, S.; Baldrian, P.; Albrechtová, J. Forest Microhabitat Affects Succession of Fungal Communities on Decomposing Fine Tree Roots. Front. Microbiol. 2021, 12, 541583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).